Abstract
Exosomes are nanosized extracellular vesicles secreted by all cell types, including canine adipose-derived stem cells (cADSCs). By mediating intercellular communication, exosomes modulate the biology of adjacent and distant cells by transferring their cargo. In the present work after isolation and characterization of exosomes derived from canine adipose tissue, we treated the same canine donors affected by hepatopathies with the previously isolated exosomes. We hypothesize that cADSC-sourced miRNAs are among the factors responsible for a regenerative and anti-inflammatory effect in the treatment of hepatopathies in dogs, providing the clinical veterinary field with an effective and innovative cell-free therapy. Exosomes were isolated and characterized for size, distribution, surface markers, and for their miRNomic cargo by microRNA sequencing. 295 dogs affected with hepatopathies were treated and followed up for 6 months to keep track of their biochemical marker levels. Results confirmed that exosomes derived from cADSCs exhibited an average diameter of 91 nm, and positivity to 8 known exosome markers. The administration of exosomes to dogs affected by liver-associated inflammatory pathologies resulted in the recovery of the animal alongside the normalization of biochemical parameters of kidney function. In conclusion, cADSCs-derived exosomes are a promising therapeutic tool for treating inflammatory disorders in animal companions.
Keywords: extracellular nanovesicles, exosomes, miRNA, liver diseases, regenerative medicine.
Introduction
Exosomes are extracellular vesicles that range in size from 30 nm up to 150 nm in diameter, comprised in a lipid bilayer and secreted by multiple cell lines. They can be found in a variety of biological fluids (including urine, plasma, and colostrum), and can also be isolated from the conditioned media of cells cultured in vitro 1-3. Exosomes play a major role in cell-cell communication both at a paracrine and systemic level. They are involved in either physiological processes (i.e., immune response, cell proliferation, and differentiation) or pathological ones (i.e., cancer progression, cardiovascular diseases) 1,4,5. Exosomes contain nucleic acids, proteins, lipids, and other metabolites that are secreted by donor cells and taken up by recipient cells.
In particular, exosomal microRNAs (miRNAs) have recently gained attention because of their ability to regulate gene expression 6-8. miRNAs are a class of single-stranded, non-coding RNA of approximately 22 nucleotides 6,9. miRNAs are mostly transcribed from DNA, processed into precursor miRNAs, and finally into mature miRNAs 6, 10. They are responsible for the alteration and degradation of a target mRNA transcript via binding with its 3' UTR, leading to the suppression of biological functions 11, 12. miRNAs expression can be correlated to several physio-pathological conditions 13,14.
In human medicine, great importance is currently given to the miRNA cargo of exosomes employed as diagnostic and prognostic biomarkers to diagnose a condition or to monitor its development before and after treatment - mainly concerning neoplastic malignancies 15. By contrast, veterinary medicine still requires data supporting the biomedical application of exosomes 16. Recent studies have shown how exosomes can be adopted in the framework of oncology and orthopedic diseases with positive results in animals carrying liver diseases 17,18, however, to our knowledge, literature is still lacking exosome application as a treatment for inflammatory diseases such as liver diseases in companion animals.
In the present study, liver hepatopathies were selected because the liver represents the organ that mostly shows great perspectives for the use of exosomes as regenerative strategy 19. As it is well known indeed, in healthy conditions liver cell turnover is very slow, but at the same time, after 70% partial hepatectomy the liver is the only organ whose size returns to the original extent in about two weeks 20. Despite this, in chronic and severe acute liver disease, this regenerative and replicative ability of the hepatic stem cells fails to regenerate the liver, as diffuse inflammation is present inducing increased production of matrix proteins, decreased matrix remodeling, progression to fibrosis, increase in the proportion of senescent hepatocytes (i.e., hepatocytes arrested at G1/S transition of the cell cycle) and hepatocyte death 21. In this context, dogs primarily present with parenchymal pathologies such as hepatitis with an estimated frequency of up to 12% in the general canine population 21-23. Considering this, in the present work we focused our attention on liver-inflamed diseases, to shift our previous study, on which we demonstrated that the use of mesenchymal stem cells in such pathology is a winning strategy 24, from a cell-based therapy to a cell-free therapy. To reach this aim we focused our research on the characterization of canine exosomes from adipose-derived mesenchymal stem cells (cADSC-exos) and the efficacy of their clinical application on dogs suffering from inflammatory conditions in the liver.
Material and methods
Ethics statement
The present study follows the guideline published in the Official Gazette the October 17, 2013 agreement “Guidelines concerning the minimum health requirements for the use of stem cells in veterinary medicine” (OJ General Series No. 277, 11-26-2013). The guideline applies to MSC Multipotent Stromal Cells that have not undergone relevant manipulation, prepared on a non-repetitive basis, for a product intended exclusively for autologous use, in a specific animal owned by legal and/or natural persons, subjected to their informed consent. To this view, only the informed consent was needed.
Isolation and culture of canine ADSCs
Cells were isolated from the fat tissue of canine specimens. Briefly, biopsies were washed with PBS (Phosphate buffered saline, Aurogene, Rome, Italy), dissected, and digested with collagenase type II (Sigma-Aldrich, St. Louis, Missouri, USA) in Hanks' balanced salts solution (HBSS) with calcium and magnesium (EuroClone, Pero, Milan, Italy) for 3 hours at room temperature with intense shaking. The cellular fraction was pelleted and rinsed with PBS.
Cells were cultured in DMEM Dulbecco′s Modified Eagle′s Medium high glucose (Sigma-Aldrich, St. Louis, Missouri, USA) supplemented with 2% Antibiotic-Antimycotic Solution 100× (Sigma-Aldrich, St. Louis, Missouri, USA) and 10% Fetal Bovine Serum (FBS) (Thermo Fisher Scientific, Waltham, Massachusetts, USA) at 37 °C and 5% CO2. Cellular growth was observed via the Evos XL Core microscope (Invitrogen, Waltham, Massachusetts, USA).
Flow cytometry of cADSCs
Cells growing in adherence were detached and resuspended in flow cytometry staining buffer (R&D Systems, Minneapolis, Minnesota, USA) to a final concentration of 1x106 cells/mL, as in our previous work.22 cADSCs were incubated with the fluorescent antibodies CD90-APC, CD44-FITC, CD14-PE, CD45-FITC (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Flow cytometry was carried out with Attune™ NxT Acoustic Focusing Cytometer (Life Technologies, Carlsbad, California, USA), and data were analyzed using the Attune NxT Soft-ware version 2.5.
Exosome isolation
Cells were cultured in a suitable medium consisting of DMEM Dulbecco′s Modified Eagle′s Medium high glucose (Sigma-Aldrich, St. Louis, Missouri, USA) supplemented with 2% Antibiotic-Antimycotic Solution 100× (Sigma-Aldrich, St. Louis, Missouri, USA) and 10% exosome-depleted Fetal Bovine Serum (Thermo Fisher Scientific, Waltham, Massachusetts, USA) to avoid serum lipoprotein contamination. After 72 hours, exosomes were isolated using the Amicon® Ultra-15 Centrifugal Filter Units with Ultracel-100 regenerated cellulose membrane (cat. no. UFC910024, Millipore, Burlington, Massachusetts, USA); conditioned medium was centrifuged at 2000 rcf for 30 minutes at +4 °C, followed by washing with PBS at 2000 rcf for 30 minutes at +4 °C; eventually, exosomes retained by the filter were collected and stored at -20 °C.
Tunable Resistive Pulse Sensing (TRPS) measurements
Exosomes were characterized in size and concentration via the qNano Gold (IZON Science LTD, Christchurch, New Zealand). Measurements were performed at a pressure of 20 mbar and normalized using 100 nm calibration particles. Data were analyzed and plotted with the qNano Control Suite Software.
Scanning Electron Microscopy (SEM)
Samples were fixed with 2% glutaraldehyde in phosphate buffer at +4 °C, dehydrated with ethanol, and eventually sputter-coated with gold/palladium. Imaging was performed with the SEM Zeiss EVO 40 microscope (Zeiss, Oberkochen, Germany) at the Centro di Microscopia Elettronica (University of Ferrara, Ferrara, Italy).
Protein quantification
Exosome protein quantification was performed using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA), as per instructions of the manufacturer. Briefly, bovine serum albumin (BSA) was used to obtain a 9-points standard curve. 10 μL of each sample were pipetted, in duplicate, into a 96-well Falcon® culture microplate (Corning Inc., New York, USA). 200 µL of working reagent solution provided in the kit were added and incubated for 30 minutes at 37 °C. Finally, a multilabel plate reader (Victor 3, Perkin Elmer, Milan, Italy) was used to read the absorbance. Data are expressed as means ± SEM.
Exosomes antibody array
The ELISA test of exosome-specific markers was performed with the Exo-Check™ Exosome Antibody Array (Systems Biosciences, Palo Alto, USA) according to the manufacturer's instructions. 8 known exosome markers were included: CD81, CD63, FLOT1, ALIX, ANXA5, EpCAM, TSG101 and ICAM1.
Fluorescent Labeling and image acquisition
cADSC-exos were stained with the PKH26 Red Fluorescent Cell Linked Midi Kit for General Cell Membrane Labeling (Sigma-Aldrich, Saint Louis, USA). Exosomes were incubated with a staining solution of diluent C and PKH26 dye, followed by blocking with an equal volume of exosome-depleted FBS; to remove the excess staining, 10 mL of PBS were added and the solution was centrifuged at 37400 rcf for 30 minutes with an Ultracentrifuge Optima L-70 (Beckman Coulter Inc., Brea, USA), type 70 Ti rotor. The supernatant was removed, and the pellet was resuspended in 200 µL PBS. 20000 cADSCs were seeded on 13 mm glass slides and treated for 24 hours with the PKH26-stained cADSC-exos. Specimens were then fixed with PFA 4%, blocked with BSA 1%, and incubated with Hoechst fluorescent dye (Sigma-Aldrich, St. Louis, USA) to stain cell nuclei and Alexa Fluor™ 488 Phalloidin (Sigma-Aldrich, St. Louis, USA) to stain actin filaments. Images were acquired with the Zeiss Axiovert 200M Fluorescence Microscope (Zeiss, Oberkochen, Germany) equipped with a 63x oil objective.
Exosomal RNA extraction
Exosomal RNA was extracted using the Cell Culture Media Exosome Purification and RNA Isolation Mini Kit (cat. no. 60700, Norgen Biotek Corp., Thorold, Canada). RNA samples were stored at -80°C.
microRNA (miRNA) sequencing
miRNA-seq libraries were generated using the QIAseq miRNA Library Kit (Qiagen; Hilden, Germany) and sequenced using NovaSeq 6000 (Illumina, San Diego, USA) in 2x150 paired-end mode. miRNAs found in the specimens were identified using the QIAseq miRNA-NGS data analysis software with single-end read as read type and 75 cycles in Read 1. Datasets from miRNA sequencing were analyzed with the Qiagen Ingenuity Pathway Analysis (IPA) software.
Statistical Analyses
All experiments were performed in triplicate, and each experiment was performed independently three times. In vitro results were expressed as mean ± standard deviation (SD). In vivo results were expressed as mean ± SEM.
Blood sample collection and detection of blood biochemical markers
Blood samples were obtained from the jugular vein of dogs of the Maltese, Akita, Basset Hound, Beagle, Bernese Mountain Dogs, Cavalier King Charles Spaniel, Chihuahua, Shih tzu, Siberian Husky, Mastiff, Cane Corso, and Miniature Schnauzer dog breeds. The blood of canine donors was collected before the injection and after 30 and 180 days, then stored at -20°C for analysis using commercial sandwich ELISA kits (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Alanine Aminotransferase (AST), Gamma-Glutamyl Transferase (GGT), Total Serum Bile Acid (TSBA), Blood Urea Nitrogen (BUN). Optical density values at 405 nm were measured using a multilabel plate reader.
Injection of autologous cADSCs-derived exosomes
295 dogs with severe degenerative hepatopathy were included in this study. Dogs with cancer in the liver or other organs were excluded. Thrombosis of the hepatic vein was another criterion of exclusion. Dogs were selected following the American College of Veterinary Internal Medicine (ACVIM) consensus statement on the diagnosis and treatment of chronic hepatitis 25. For the injection, exosomes (0.2 mg of protein/mL per kg) suspended in 2 mL PBS were transfused through the peripheral vein. Dogs underwent two injections of autologous exosomes at 30 and 180 days.
Specimen collection and cytopathological examinations
Hepatic cytology specimens were obtained by ultrasound-guided fine-needle capillary sampling without aspiration before the first exosome injections and 30 days after the second treatment. Smears were air-dried, stained using the Diff-quick stain (Merck, Darmstadt, Germany), and then imaged under a light microscope.
Results
Characterization of canine ADSCs
Canine ADSCs (cADSCs) were isolated and maintained in culture in tissue culture flasks, exhibiting their characteristic fusiform, fibroblastic-like morphology (Figure 1). cADSCs were positive for MSC-selective surface markers CD90-APC and CD44-FITC (Figure 2A and Figure 2B), and negative for CD14-PE and CD45-FITC (Figure 2C and Figure 2D).
Figure 1.
Canine ADSC characterization. cADSCs showed their typical elongated morphology. Scale bar is 20 µm. Images were taken with the Evos XL Core microscope (Invitrogen, Waltham, Massachusetts, USA) equipped with a 4x objective.
Figure 2.
Flow cytometry analysis of surface markers in cADSCs. (A-B) cADSCs are positive for CD90-APC and CD44-FITC. (C-D) cADSCs are negative for CD14-PE and CD45-FITC. Measurements were taken with the Attune™ NxT Acoustic Focusing Cytometer (Life Technologies, Carlsbad, California, USA).
Characterization of canine ADSC-derived exosomes (cADSC-exos)
The supernatant was collected from canine adipose-derived mesenchymal stem cells (cADSCs), and exosomes were isolated with a commercial kit as described in the Material and methods section. Exosomes were characterized using the Tunable Resistive Pulse Sensing (TRPS) technique, a single-particle technology that allows a precise and accurate measurement of exosome diameter. Exosome concentration was 6.62x108 particles/mL, with an average diameter of 91 nm (Figure 3A). Exosome size was further confirmed by scanning electron microscopy (SEM) (Figure 3B). According to the BCA assay, the mean protein concentration of cADSC-exos is estimated at 483.9 µg/mL (Figure 3C). Protein content evaluation confirmed cADSC-exos positivity to CD81 and CD63, which are surface markers characteristic of MSC-derived exosomes (Figure 3D). Taken together, these data are in line with the Minimal information for studies of extracellular vesicles (MISEV) 26 and demonstrate that the conditioned medium of cADSCs is a source of exosomes.
Figure 3.
cADSC-exo characterization. (A) The histogram shows the size distribution of cADSC-exos evaluated by Tunable resistive pulse sensing. The mean diameter is 91 nm (St. Dev ± 28.7), while the average concentration is 6.62x108 particles/mL. (B) SEM images confirm the exosome diameter. Images were taken with the SEM Zeiss EVO 40 microscope (Zeiss, Oberkochen, Germany) at a 20.000x magnification. (C) cADSC-exos show a protein concentration of 483.9µg/mL (St. Dev ± 18.3). Results are the mean of 3 replicates. (D) cADSC-exos are positive for the specific markers MSC-derived exosomes CD81 and CD63. Results are expressed as percentages compared to positive control. Analyses were performed in triplicate.
cADSC-exos interact with and are internalized by cADSCs
cADSC-exos were stained with the PKH26 membrane dye, and after a 24-hour treatment, their interaction with cADSCs was evaluated via fluorescent microscopy. As shown in Figure 4, exosomes (highlighted in red and inside the circles) were taken up by cADSCs (whose nuclei are in blue and actin filaments in green) and detected in the cytoplasm.
Figure 4.
Internalization of cADSC-exos by cADSCs. PKH26-stained exosomes have been taken up by cADSCs and appeared in the cytoplasmic region of cells as red spots. Images were acquired with the Zeiss Axiovert 200M Fluorescence Microscope (Zeiss, Oberkochen, Germany) equipped with a 63x oil objective. Cell nuclei are stained in blue; actin filaments are stained in green; exosomes are stained in red. Scale bar is 20 µm.
miRNA profile of cADSC-sourced exosomes and most abundant miRNAs
A total of 26 miRNAs was identified after microRNA sequencing (miRNA-seq) and the alignment of the individual transcripts to the Canis lupus familiaris genome (Table 1). The 10 most abundant miRNAs represent more than 80% of all detected miRNAs in cADSC-exos (Figure 5A) and are respectively: cfa-miR-199, cfa-let-7b, cfa-miR-21, cfa-let-7a, cfa-miR-143, cfa-miR-16, cfa-miR-8859a, cfa-miR-125b, cfa-miR-29a, cfa-miR-27b (Figure 5B).
Table 1.
Summary of miRNAs detected in exosomes derived from cADSCs. The reads are the average of three replicates. “cfa”: Canis lupus familiaris.
| miRNA | Mean reads | miRNA | Mean reads | |
|---|---|---|---|---|
| cfa-miR-199 | 320 | cfa-miR-221 | 22 | |
| cfa-let-7b | 319 | cfa-miR-378 | 21 | |
| cfa-miR-21 | 96 | cfa-miR-148a | 18 | |
| cfa-let-7a | 94 | cfa-let-7c | 16 | |
| cfa-miR-143 | 69 | cfa-miR-122 | 16 | |
| cfa-miR-16 | 63 | cfa-miR-193a | 16 | |
| cfa-miR-8859a | 51 | cfa-miR-25 | 14 | |
| cfa-miR-125b | 47 | cfa-miR-320 | 13 | |
| cfa-miR-29a | 42 | cfa-miR-145 | 12 | |
| cfa-miR-27b | 38 | cfa-miR-28 | 12 | |
| cfa-miR-423a | 28 | cfa-miR-26b | 11 | |
| cfa-let-7f | 25 | cfa-miR-203 | 9 | |
| cfa-let-7e | 24 | cfa-miR-196a | 7 |
Figure 5.
miRNA cargo of cADSC-exos. (A) The 10 most abundant miRNAs represent the 83% of the total miRNA cargo in cADSC-exos. (B) Distribution of the 10 top-ranked miRNAs. Analysis was performed in triplicate.
miRNAs expressed in mesenchymal stem cell-derived exosomes hold regenerative properties
Literature review revealed that human MSC-sourced miR-199, let-7b, miR-21, miR-125b, and mir-29a are responsible for regenerative effects by positively modulating angiogenesis, cell, proliferation, and tissue remodeling in several inflammatory conditions (Table 2).
Table 2.
Studies demonstrating the positive outcome of exosomal miRNA transfer in inflammatory conditions. The table summarizes the major findings on miR-199, let-7b, miR-21, miR-125b, and mir-29a in the treatment of inflammatory conditions.
| Intervention field | Outcome | Major findings | miRNA transferred | References |
|---|---|---|---|---|
| Liver regeneration | Liver regeneration and hepatocyte proliferation | ↑ hepatocyte proliferation ↓ hepatocyte apoptosis |
miR-199 | 27 |
| Renal inflammation | Renal protection | ↑ immunosuppressive M2 phenotype in renal macrophages ↓ production of inflammatory cytokines |
let-7b | 28 |
| Acute inflammation at the site of injury | Stem cell niche maintenance | ↑ multiple cellular proliferation ↑ blood vessel formation at the site of injury |
miR-21 | 29,30 |
| Renal inflammation | Renal protection | ↓ NF-kB activity ↓ reduced maturation and function of infiltrating dendritic cells |
miR-21 | 31,32 |
| Ischemic acute kidney injury |
Tubular epithelial cells maintenance | ↓ tubular epithelial cells apoptosis by repressing p53 expression | miR-125b | 33 |
| Unilateral Ureteral Obstruction | Kidney damage attenuation | ↓ renal fibrosis | miR-29a | 34 |
miRNA transcripts with regenerative properties are conserved between human and canine genome
The “microRNAviewer” web server was employed to investigate the homology of miRNA families among species.35 This tool showed that the miRNA transcripts of miR-199, let-7b, miR-21, miR-125b, and mir-29a are conserved between the human and canine (“cfa”) genomes, with a degree of conservation close or equal to 1 (Table 3).
Table 3.
Conservation of miRNA transcripts between canine and the human genome. The conservation grade among canine (“cfa”, Canis lupus familiaris) and human (Homo sapiens) genomes for the miRNA of interest is between 0.98 and 1. The analysis was performed with the online tool microRNAviewer (miRviewer).
|
Canis lupus familiaris genome |
Degree of conservation |
|---|---|
| cfa-miR-199 | 0.98 |
| cfa-let-7b | 0.98 |
| cfa-miR-21 | 1 |
| cfa-let-7a | 0 |
| cfa-miR-143 | 1 |
| cfa-miR-16 | 0 |
| cfa-miR-8859a | No degree |
| cfa-miR-125b | 1 |
| cfa-miR-29a | 1 |
| cfa-miR-27b | 0.99 |
In vivo injection of autologous exosomes in dogs ameliorates clinical signs of liver disease
The selection of the dogs and the diagnostic tests, the treatment procedure, and the conduction of clinical research in hepatology followed the consensus statement on chronic hepatitis (CH) in dogs were based on the expert opinion of 7 specialists with extensive experience in CH 25. This is because the panel that identifies the diagnosis and treatment of CH in dogs is a complex process that requires the integration of clinical signs with clinical pathology, diagnostic imaging, and hepatic biopsy (Figure S1).
Clinical signs reported for dogs with CH are summarized in Table 4. Clinical signs typically are not specific - such as loss of appetite and lethargy. As reported in Table 4, the first injection of exosomes induces the recovery of two important clinical signs - decreased appetite and depression.
Table 4.
Clinical signs of dogs suffering from CH before and 30 days after autologous exosome treatment. CH is mainly characterized by non-specific symptoms; autologous treatment injection resulted in the recovery of such symptoms, notably decreased appetite and lethargy/depression after 30 days.
| Clinical signs before exosome treatment |
Percentage (%) of dogs |
Clinical signs 30 days after treatment |
Percentage (%) of dogs |
|---|---|---|---|
| Decreased appetite | 68 | Recovery | 96 |
| Lethargy/Depression | 63 | Recovery | 98 |
| Icterus | 37 | Recovery | 65 |
| Ascites | 35 | Recovery | 34 |
| Vomiting | 27 | Recovery | 73 |
| Diarrhea | 21 | Recovery | 12 |
| Hepatic Encephalopathy | 8 | Recovery | 81 |
| Melena | 5 | Recovery | 45 |
| Abdominal Pain | 2 | Recovery | 56 |
In addition to clinical sign, cytological analysis in a small (12) group of dogs before the first exosome injections and 30 days after the second injection was performed. As reported in the guidelines, the key features of liver biopsy specimen interpretation include evaluation of inflammation (granulomatous, neutrophilic, lymphocytic, suppurative inflammation). Moreover, it is mandatory to analyze the extent and distribution of cell death (i.e., individual, massive, multifocal, centrilobular, and periportal cells), characterized by vacuolar shift. Often, fibrosis of the connective tissue is also present as septa formation and nodular regeneration of hepatocytes. Before exosome injections, hepatocytes show a round central nucleus, and the presence of inflammation process is demonstrated by the presence of neutrophils, small and medium activated lymphocytes, and foamy macrophages (Figure 6A). After the second injection of exosomes (after 30 days), cytological images show that liver inflammation is declined, as well as the presence of spindle cells; furthermore, no sign of fibrosis is evident (Figure 6B).
Figure 6.
Diff-quick stain of cytological samples before and 30 days after the second exosome injection after 30 days. (A) Before exosome treatment, inflammatory processes are occurring with foamy macrophages and hepatic debris; red cells are also visible. (B) After exosome treatment, fibrosis and inflammation are strongly reduced.
We were cautious since the primary concern for any hepatic sampling technique is post-procedural haemorrhage due to coagulation abnormalities related to the alteration of normal liver function. Risks of hepatic biopsy other than haemorrhage include anaesthetic complications, air embolism and pneumothorax (with laparoscopy), and infection. Hence, we used ultrasound to define the shift of portal hypertension. As reported in Table 5, portal hypertension strongly improves after exosome injections.
Table 5.
Clinical signs related to portal hypertension. All the parameters are improved 30 and 180 days after treatment with autologous exosomes.
| Clinical signs of portal hypertension |
Percentage (%) of dogs | Improvement (%) 30 days after treatment |
Improvement (%) 180 days after treatment |
|---|---|---|---|
| Decreased velocity of portal vein blood flow | 83 | 25 | 42 |
| Hepatofugal flow in the portal vein | 76 | 21 | 65 |
| Portal vein/aorta ratio ≤ 0.65 in the absence of a single congenital portosystemic shunt | 81 | 27 | 64 |
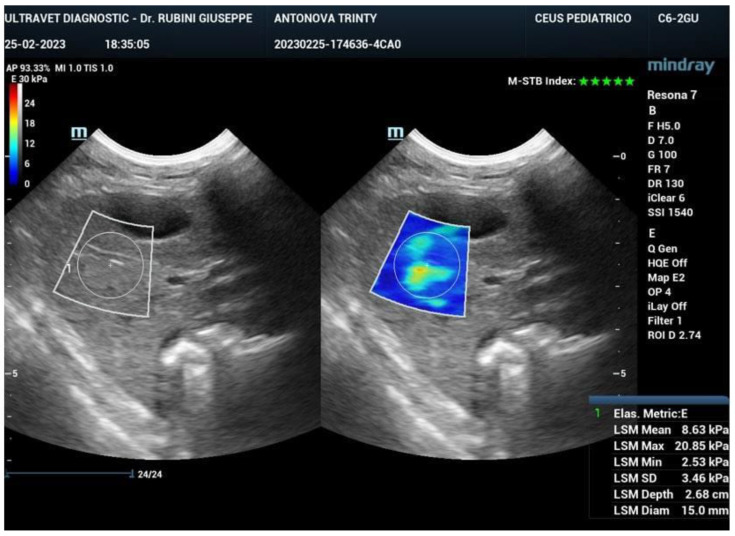
Ultrasound elastography (also known as sonoelastography) was used to support the diagnosis and management of diffuse liver disease. Elastography can identify early-stage and advanced liver fibrosis and thus is a major application in clinical liver care. In addition, elastographic characterization of focal liver lesions and evaluation of clinically significant portal hypertension can potentially be clinically useful and are areas of active clinical research. Currently, the accepted liver fibrosis staging reference standard is the histopathologic evaluation of non-focal liver biopsy specimens. However, liver biopsy has some limitations: 1) liver biopsy is invasive, 2) liver biopsy is costly, and 3) inter-observer variability limits the clinical interpretability of liver biopsy samples. To this end, 34 dogs treated with exosomes were followed also by sonoelastography to evaluate the presence and reduction of fibrosis after 180 days from the first injection of exosomes. As reported in Figure 7, fibrotic tissue with alteration of blood flow (highlighted in the circle) is visible before the treatment. Figure 8 shows that the fibrotic event is no more present and the portal vein blood flow has recovered after 180 days from the first injection of exosomes.
Figure 7.
Sonoelastography of the canine liver before injection of autologous exosomes. Fibrotic tissue and altered portal vein blood flow are shown.
Figure 8.
Sonoelastography of the canine liver 180 days after injection of autologous exosomes. No fibrotic tissue is visible, and the portal vein blood flow is restored.
Serum enzymology was performed to detect serum liver enzyme activities focalizing our attention on alanine transaminase (ALT) activity - which is the earliest indicator of CH, and alkaline phosphatase (ALP) activity - that occurs later in CH. Since the liver is the largest synthetic reserve for albumin synthesis, hypoalbuminemia has been detected. Together with these markers, gamma-glutamyl transferase (GGT), total serum bile acid (TSBA), blood urea nitrogen (BUN), and cholesterol levels were assessed. As reported in Table 6, all biochemical blood markers recovered 180 days after exosome treatment.
Table 6.
Biochemical blood markers panel of serum enzymes in liver disease. The variation of parameters before, and after exosome treatment (30 and 180 days) is reported in comparison to data from literature.
| Parameter | Percent (%) change in literature | Variation before exosome treatment | Variation 30 days after treatment | Variation 180 days after treatment | Number of dogs |
|---|---|---|---|---|---|
| Increased ALT | 85+/-16 | 81+/-15 | 74+/-12 | 44+/-15 | 63 |
| Increased ALP | 84+/-19 | 87+/-13 | 72+/-14 | 42+/-12 | 63 |
| Increased AST | 78+/-10 | 69+/-14 | 52+/-9 | 46+/-7 | 45 |
| Increased GGT | 61+/-12 | 66+/-15 | 56+/-18 | 32+/-11 | 45 |
| Increased TSBA | 75+/-14 | 72+/-11 | 62+/-13 | 54+/-13 | 63 |
| Decreased BUN | 40+/-29 | 36+/-12 | 31+/-10 | 21+/-14 | 23 |
| Decreased Albumin | 49+/-19 | 62+/-18 | 51+/-15 | 22+/-19 | 94 |
| Decreased Cholesterol | 40+/-12 | 36+/-23 | 32+/-13 | 18+/-11 | 23 |
Abbreviations: ALT, Alanine Transaminase; ALP, Alkaline Phosphatase; AST, Alanine Aminotransferase; GGT, Gamma-Glutamyl Transferase; TSBA, Total Serum Bile Acid; BUN, Blood Urea Nitrogen.
Discussion
Mesenchymal stem cells (MSCs) are multipotent stem cells crucial for tissue regeneration and repair that can be isolated from different sources, including adipose tissue 36. In the last decade, MSCs have drawn attention because they possess some peculiar properties: 1) self-renewal, 2) multilineage differentiation, 3) secretion of trophic and growth factors, 4) immunomodulation, and 5) ease of amplification in vitro 36,37. Concerning dogs carrying liver diseases, adipose tissue can be easily collected during well-controlled routine procedures such as sterilization, without exposing the animal to additional surgeries. We already demonstrated that autologous adipose-derived mesenchymal stem cells (ADSCs) can be successfully employed as a treatment for hepatic disorders in the veterinary field 24, however, exosomes could represent an alternative cell-free therapy as they retain the same features of the cells they originate from. Assessing the proper isolation of cADSCs was a fundamental first step in our research, and we also confirmed the results obtained from our previous study 24. Indeed, flow cytometry confirmed the positive expression of CD90-APC and CD44-FITC surface markers and negativity to CD14-PE and CD45-FITC in the isolated cADSCs (Figure 2).
Adipose-derived mesenchymal stem cells (ADSCs) are not only easy to expand in vitro, but they also secrete a considerable amount of exosomes 15,37. Furthermore, the immunomodulatory and regenerative properties of MSCs are preserved in MSC-derived exosomes 37, making these cells an ideal source to obtain exosomes. Vesicle characterization is essential to univocally identify exosomes. In our work, cADSC-exos were analyzed through an ELISA kit (Figure 3). Additionally, exosome size distribution was measured via the TRPS approach and SEM imaging (Figure 3).
Exosomes act as messengers in the process of intercellular communication at a paracrine and endocrine level 5, interacting with cells (as established in Figure 4) and transferring their cargo to recipient cells - thus changing their phenotype or regulating gene expression 14. The exosome loading is sorted by ESCRT-dependent and independent mechanisms, and it is influenced by the parental cell and environmental characteristics 14,38,39. Nevertheless, the final content of exosomes includes proteins, lipids, nucleic acids, and other metabolites 2.
Concerning nucleic acids, we focused on the miRNome of exosomes, as this family of molecules plays a role in pathophysiological conditions, targeting specific messenger RNAs (mRNAs) and determining their degradation or translation repression. miRNA-seq from cADSC-derived exosomes determined that 26 miRNAs were associated with the Canis lupus familiaris genome, as identified by the prefix “cfa” (Table 1). As reported in the “Results” section, the 10 top-ranked miRNAs accounted for 83% of all the miRNAs detected (Figure 5).
To put our findings into context, literature research was conducted (Table 2). It is acknowledged that exosomes derived from mesenchymal stem cells reduce inflammatory processes while providing a positive impact on collagen deposition, angiogenesis, and cell proliferation in a plethora of inflammatory conditions 40-42. In particular, miR-199 promotes liver regeneration by enhancing hepatocyte proliferation while decreasing their apoptosis 27. MSC-sourced let-7b decreased renal inflammation through the polarization of renal macrophages to the M2 phenotype while reducing inflammatory cytokine production 28. miR-21 plays a role in controlling the stem cell niche at the site of injury, promoting cell proliferation and angiogenesis through the activation of protein kinase B and extracellular signal-regulated kinase 29,30. Moreover, miR-21 mediates the maturation and migration of infiltrating dendritic cells and decreases inflammation through the NF-kB pathway in kidney inflammation 31,32. miR-125b plays a role in renal angiogenesis by partially inhibiting p53 signaling, improving tubular epithelial cell proliferation, and limiting their apoptosis 33. Wang and colleagues demonstrated that exosomal miR-29a limits renal fibrosis in a unilateral ureteral obstruction mice model via the downregulation of the fibrotic-related proteins YY1, TGF-β1, and TGF-β3 34. Overall, MSC-derived exosome miRNome shows immunosuppressive and protective features that relieve inflammatory conditions (Table 2) 40.
Interestingly, miR-199, let-7b, miR-21, miR-125b, and miR-29a fall under the category of mitomiRs, which are miRNA transcripts specifically associated with mitochondrial metabolism or enriched in this distinct organelle. Zheng and colleagues analyzed the expression of mitomiRs in purified mitochondrial fractions of MSC and from whole extracts of MSC, identifying several mitomiRs which regulate several mitochondrial functions 43.
Surprisingly, many software and online tools used for functional analysis of OMICs data or literature research are not equipped with databases other than human. To overcome this issue, we investigated the homology between the 10 most abundant miRNAs in the dog (“cfa”, Canis lupus familiaris) with the corresponding miRNA of the human species (“hsa”, Homo sapiens), sure in the knowledge that many miRNA families, as well as their targets, are conserved among different animal species 44-46. The miRviewer software confirmed the conservation between dog and human of cfa-miR-199, cfa-let-7b, cfa-miR-21, cfa-miR-143, cfa-miR-125b, cfa-miR-29a, and cfa-miR-27b, with a degree of conservation close or equal to 1 (Table 3).
Taken together, data from the current research suggested that cADSC-exos could be used as a cell-free therapy for the treatment of CH in dogs. Such disease is well characterized in human medicine from a pathological and clinical perspective 24,45,47. Noteworthy, Volk and colleagues emphasized that humans share similarities with dogs more than other animal species in terms of anatomy, physiology, and similar disease onset 24,48. Consequently, human liver diseases resemble the ones occurring in animal species and thus results from human research may be translated to the veterinary field 24,48,49.
In light of these considerations, 295 dogs affected with hepatopathies were treated with autologous exosomes following the recommendation of the ACVIM consensus guideline 25. Fibrosis, inflammation, and liver function activity levels were first defined through histology, imaging technology, and biochemical analyses; then, the progress of clinical signs and biochemical markers was assessed 30 and 180 days after the first injection of exosomes. As reported, the first positive clinical evidence is represented by the recovery of appetite and depression state of dogs (Table 4), followed by the recovery of fibrosis, inflammation, and liver parameters such as ALT, AST, ALP, GGT, and TSBA (Table 6).
Conclusion
Treatment of hepatopathies and CH in dogs should target the causative agent, unfortunately, this liver disease is often idiopathic. Concerning autologous exosome treatment, it can be indicated with non-specific hepatoprotective agents with or without a trial of immunosuppressive treatment as also suggested by ACVIM consensus. In this view, exosomes derived from canine adipose-derived stem cells have proven to be a valid therapeutic tool for the treatment of inflammatory liver disease in dogs, and possibly in the veterinary field in general.
Acknowledgments
The authors would like to acknowledge that sequencing of all miRNAs was carried out by ARGO Open Lab Platform for Genome sequencing of Area Science Park (ASP, Trieste, Italy), and that Scanning Electron Microscopy imaging was carried out by Centro di Microscopia Elettronica (University of Ferrara, Ferrara, Italy).
Funding statement
This project was financed by the call “Covid rapid detection by novel bioactivated biosensing nanostructures.” by Area Science Park and Sistema Argo.
Authors contribution
Conceptualization, B.Z.; methodology, E.T., F.Z., T.P.; software, D.L., M.D.; validation, M.T., F.Z., L.D.; formal analysis, M.T., F.Z., E.T., I.Z.; investigation, I.Z., M.T., F.Z., E.T., T.P., L.F.; resources, A.R., G.R., F.B.; data curation, L.F., L.L., M.T.; writing—original draft preparation, I.Z., M.T., E.T., F.Z., T.P., B.Z.; writing—review and editing, I.Z., M.T., E.T., F.Z., B.Z.; supervision, B.Z.; project administration L.L., B.Z.; funding acquisition, B.Z. All authors have read and agreed to the published version of the manuscript.
Data availability statement
Data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval statement
The owners gave written consent to have their animals participate in this research study. Ethical review and approval were waived for this study due to the use of autologous exosomes in veterinary medicine disciplined by (OJ General Series No. 277 of 26-11-2013).
Abbreviations
- ACVIM
American College of Veterinary Internal Medicine
- ALIX
ALG-2-Interacting protein X
- ALP
Alkaline Phosphatase
- ALT
Alanine Transaminase
- ANOVA
Analysis Of Variance
- ANXA5
Annexin A5
- AST
Alanine Aminotransferase
- BSA
Bovine Serum Albumin
- BUN
Blood Urea Nitrogen
- cADSCs
canine Adipose-Derived Stem Cells
- cADSCs-exos
canine exosomes from Adipose-Derived mesenchymal Stem Cells
- CH
Chronic Hepatitis
- DMEM
Dulbecco′S Modified Eagle′S Medium
- EpCAM
Epithelial Cell Adhesion Molecule
- FBS
Fetal Bovine Serum
- FLOT1
Flotillin 1
- GGT
Gamma-Glutamyl Transferase
- GM130
cis-Golgi Matrix protein 130kD
- HBSS
Hanks' Balanced Salts Solution
- ICAM
Intercellular Adhesion Molecule 1
- IPA
Ingenuity Pathway Analysis
- miRNA(s)
microRNA(s)
- miRNA-seq
microRNA sequencing
- MISEV
Minimal Information for Studies of Extracellular Vesicles
- PBS
Phosphate Buffered Saline
- SEM
Scanning Electron Microscopy
- TSBA
Total Serum Bile Acid
- TSG101
Tumor Susceptibility Gene 101
- TRPS
Tunable Resistive Pulse Sensing
Supplementary Material
Supplementary figures.
References
- 1.Diomaiuto E, Principe V, Luca AD, Laperuta F, Alterisio C, Loria AD. Exosomes in dogs and cats: an innovative approach to neoplastic and non-neoplastic diseases. Pharmaceuticals. 2021. 14. [DOI] [PMC free article] [PubMed]
- 2.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020. 367. [DOI] [PMC free article] [PubMed]
- 3.Brunello G, Zanotti F, Trentini M, Zanolla I, Pishavar E, Favero V. et al. Exosomes derived from dental pulp stem cells show different angiogenic and osteogenic properties in relation to the age of the donor. Pharmaceutics. 2022;14:908. doi: 10.3390/pharmaceutics14050908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zheng S, Ma C, Chen X, Li X, Li S, Research progress on exosomes in podocyte injury associated with diabetic kidney disease. Front Endocrinol. 2023. 14. [DOI] [PMC free article] [PubMed]
- 5.Zanotti F, Zanolla I, Trentini M, Tiengo E, Pusceddu T, Licastro D. et al. Mitochondrial metabolism and EV cargo of endothelial cells is affected in presence of EVs derived from MSCs on which HIF is activated. Int J Mol Sci. 2023;24:6002. doi: 10.3390/ijms24066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trentini M, Zanolla I, Zanotti F, Tiengo E, Licastro D, Dal Monego S. et al. Apple derived exosomes improve collagen type I production and decrease MMPs during aging of the skin through downregulation of the NF-κB pathway as mode of action. Cells. 2022;11:3950. doi: 10.3390/cells11243950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trentini M, Zanotti F, Tiengo E, Camponogara F, Degasperi M, Licastro D. et al. An apple a day keeps the doctor away: potential role of miRNA 146 on macrophages treated with exosomes derived from apples. Biomedicines. 2022;10:415. doi: 10.3390/biomedicines10020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Li T, Zheng Y, Xu X, Sun R, Zhan S. et al. Evaluating adipose-derived stem cell exosomes as miRNA drug delivery systems for the treatment of bladder cancer. Cancer Med. 2022;11:3687–99. doi: 10.1002/cam4.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CL, Li W, Pu GZ, Wu JW, Qin F. Exosomes derived from miR-338-3p-modified adipose stem cells inhibited inflammation injury of chondrocytes via targeting RUNX2 in osteoarthritis. J Orthop Surg Res. 2022;17:567. doi: 10.1186/s13018-022-03437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weis A, Marquart L, Calvopina D, Genz B, Ramm G, Skoien R. Serum microRNAs as biomarkers in hepatitis C: preliminary evidence of a microrna panel for the diagnosis of hepatocellular carcinoma. IJMS. 2019;20:864. doi: 10.3390/ijms20040864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira-da-Silva T, Napoleão P, Costa MC, Gabriel AF, Selas M, Silva F, Circulating miRNAs are associated with the systemic extent of atherosclerosis: novel observations for miR-27b and miR-146. Diagnostics. 2021. 11. [DOI] [PMC free article] [PubMed]
- 13.Ro WB, Kang MH, Song DW, Kim HS, Lee GW, Park HM. Identification and characterization of circulating microRNAs as novel biomarkers in dogs with heart diseases. Front Vet Sci. 2021. 8. [DOI] [PMC free article] [PubMed]
- 14.Wei H, Chen Q, Lin L, Sha C, Li T, Liu Y. et al. Regulation of exosome production and cargo sorting. Int J Biol Sci. 2021;17:163. doi: 10.7150/ijbs.53671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Zhang L. Circulating exosomal miRNA as diagnostic biomarkers of neurodegenerative diseases. Front Mol Neurosci. 2020. 13. [DOI] [PMC free article] [PubMed]
- 16.Moccia V, Sammarco A, Cavicchioli L, Castagnaro M, Bongiovanni L, Zappulli V. Extracellular vesicles in veterinary medicine. Animals. 2022;12:2716. doi: 10.3390/ani12192716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu R, Li H, Sun C, Liu J, Chen D, Yu H. et al. Exosome-based strategy for degenerative disease in orthopedics: recent progress and perspectives. J Orthop Translat. 2022;36:8–17. doi: 10.1016/j.jot.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Lin Y, Zhang X, Shan C. Research progress of exosomes in orthopedics. Front Genet. 2022;13:915141. doi: 10.3389/fgene.2022.915141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J, Wang J, Chen J. Exosomes as therapeutic vehicles in liver diseases. Ann Transl Med. 2021;9:735. doi: 10.21037/atm-20-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi S, Hirata M, Miyachi Y, Uemoto S. Liver regeneration after hepatectomy and partial liver transplantation. Int J Mol Sci. 2020;21:8414. doi: 10.3390/ijms21218414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayem AA, Song K, Lee S, Kim A, Cho SG. New therapeutic approach with extracellular vesicles from stem cells for interstitial cystitis/bladder pain syndrome. BMB Reports. 2022;55:205–12. doi: 10.5483/BMBRep.2022.55.5.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho H, Yang S, Suh G, Choi J. Correlating two-dimensional shear wave elastography of acute pancreatitis with Spec cPL in dogs. J Vet Sci. 2022;23:e79. doi: 10.4142/jvs.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gori E, Lippi I, Guidi G, Perondi F, Pierini A, Marchetti V. Acute pancreatitis and acute kidney injury in dogs. Vet J. 2019;245:77–81. doi: 10.1016/j.tvjl.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Gardin C, Ferroni L, Bellin G, Rubini G, Barosio S, Zavan B. Therapeutic potential of autologous adipose-derived stem cells for the treatment of liver disease. IJMS. 2018. 19. [DOI] [PMC free article] [PubMed]
- 25.Webster CRL, Center SA, Cullen JM, Penninck DG, Richter KP, Twedt DC. et al. ACVIM consensus statement on the diagnosis and treatment of chronic hepatitis in dogs. J Vet Intern Med. 2019;33:1173–200. doi: 10.1111/jvim.15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Ye B, Wei J, Wang Q, Xu C, Yu G. MiR-199a-5p regulates rat liver regeneration and hepatocyte proliferation by targeting TNF-α TNFR1/TRADD/CASPASE8/CASPASE3 signalling pathway. Artif Cells Nanomed Biotechnol. 2019;47:4110–8. doi: 10.1080/21691401.2019.1683566. [DOI] [PubMed] [Google Scholar]
- 28.Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J. et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:1–14. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N. et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM. et al. The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Sci Rep. 2018 8:1. 2018 Jan;8(1):1–12. doi: 10.1038/s41598-018-19581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song N, Zhang T, Xu XL, Lu Z, Yu X, Fang Y. et al. miR-21 protects against ischemia/reperfusion-induced acute kidney injury by preventing epithelial cell apoptosis and inhibiting dendritic cell maturation. Front Physiol. 2018;9:790. doi: 10.3389/fphys.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F. et al. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18:1–16. doi: 10.1186/s12964-020-00650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao JY, Wang B, Tang TT, Wen Y, Li ZL, Feng ST. et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248–66. doi: 10.7150/thno.54550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Wang J, He W, Zhao Y, Zhang A, Liu Y. et al. Exogenous miR-29a attenuates muscle atrophy and kidney fibrosis in unilateral ureteral obstruction mice. Hum Gene Ther. 2020;31:367. doi: 10.1089/hum.2019.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiezun A, Artzi S, Modai S, Volk N, Isakov O, Shomron N. miRviewer: a multispecies microRNA homologous viewer. BMC Res Notes. 2012;5:92. doi: 10.1186/1756-0500-5-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen C, Xie L, Hu C. Roles of mesenchymal stem cells and exosomes in interstitial cystitis/bladder pain syndrome. J Cellular Molecular Medi. 2022;26:624–35. doi: 10.1111/jcmm.17132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yaghoubi Y, Movassaghpour AA, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sciences. 2019;233:116733. doi: 10.1016/j.lfs.2019.116733. [DOI] [PubMed] [Google Scholar]
- 38.Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A. et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36:328–34. doi: 10.1016/j.biotechadv.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Villatoro AJ, Martín-Astorga M del C, Alcoholado C, Becerra J. Canine colostrum exosomes: characterization and influence on the canine mesenchymal stem cell secretory profile and fibroblast anti-oxidative capacity. BMC Vet Res. 2020. 16. [DOI] [PMC free article] [PubMed]
- 40.Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021;10:1959. doi: 10.3390/cells10081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, Wilk R, Hudecki J, Los MJ. Novel trends in application of stem cells in skin wound healing. Eur J Pharmacol. 2019;843:307–15. doi: 10.1016/j.ejphar.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018. 9. [DOI] [PMC free article] [PubMed]
- 43.Zheng H, Liu J, Yu J, McAlinden A. Expression profiling of mitochondria-associated microRNAs during osteogenic differentiation of human MSCs. Bone. 2021. 151. [DOI] [PMC free article] [PubMed]
- 44.Ha M, Pang M, Agarwal V, Chen ZJ. Interspecies regulation of MicroRNAs and their targets. Biochimica et biophysica acta. 2008;1779:735. doi: 10.1016/j.bbagrm.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong P, Schneider RF, Hulsey CD, Meyer A, Franchini P. Conservation and novelty in the microRNA genomic landscape of hyperdiverse cichlid fishes. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-50124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warnefors M, Liechti A, Halbert J, Valloton D, Kaessmann H. Conserved microRNA editing in Mammalian evolution, development and disease. Genome Biol. 2014;15:1–14. doi: 10.1186/gb-2014-15-6-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7:125. doi: 10.1186/s13287-016-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volk SW, Theoret C. Translating stem cell therapies: the role of companion animals in regenerative medicine: stem cell therapies in veterinary medicine. Wound Repair Regen. 2013;21:382–94. doi: 10.1111/wrr.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arzi B, Mills-Ko E, Verstraete FJM, Kol A, Walker NJ, Badgley MR. et al. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl Med. 2016;5:75–86. doi: 10.5966/sctm.2015-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.
Data Availability Statement
Data generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.