Abstract
BACKGROUND
The ventral intermediate nucleus (Vim) of the thalamus is a surgical target for treating various types of tremor. Because it is difficult to visualize the Vim using standard magnetic resonance imaging, the structure is usually targeted based on the anterior and posterior commissures. This standard targeting method is practical in most patients but not in those with thalamic asymmetry. The authors examined the usefulness of quantitative susceptibility mapping (QSM) and transformed Vim atlas images to estimate the Vim localization in a patient with tremor and significant thalamic hypertrophy.
OBSERVATIONS
A 51-year-old right-handed female had experienced a predominant left-hand action tremor for 6 years. Magnetic resonance imaging showed significant hypertrophy of the right thalamus and caudal shift of the thalamic ventral border. The authors referred to the QSM images to localize the decreased susceptibility area within the lateral ventral thalamic nuclei to target the Vim. In addition, the nonlinearly transformed Vim atlas images complemented the imaging-based targeting. The radiofrequency thalamotomy at the modified Vim target relieved the tremor completely.
LESSONS
A combination of QSM and nonlinear transformation of the thalamic atlas can be helpful in the targeting method of the Vim for tremor patients with thalamic asymmetry.
Keywords: tremor, ventral intermediate nucleus, caudal zona incerta, quantitative susceptibility mapping, case report
ABBREVIATIONS: 4D = four-dimensional, AC = anterior commissure, CRST = Clinical Rating Scale for Tremor, CT = computed tomography, cZI = caudal zona incerta, MRI = magnetic resonance imaging, PC = posterior commissure, QSM = quantitative susceptibility mapping, QUEST = Quality of Life in Essential Tremor Questionnaire, RF = radiofrequency, RN = red nucleus, STN = subthalamic nucleus, SWI = susceptible weighted imaging, Vc = ventrocaudal nucleus, Vim = ventral intermediate nucleus, Vo = ventro-oral nucleus, Voa = ventralis oralis anterior, Vop = ventralis oralis posterior
The ventral intermediate nucleus (Vim) of the thalamus is a surgical target to treat essential and other types of tremor.1,2 Because it is difficult to identify the Vim on standard magnetic resonance imaging (MRI), the Vim target is usually placed by referring to the anterior commissure (AC) and posterior commissure (PC), the third ventricular wall, and the lateral thalamic border.2–4 The Schaltenbrand and Wahren atlas5 can also guide the location of the Vim. In most cases, AC-PC–based targeting can provide practical Vim coordinates; however, when the thalamus is asymmetrical, surgeons may need to modify the AC-PC–based Vim coordinates.
Advanced MRI techniques may indicate the Vim position visually. Proton density imaging,6,7 white-matter nullification,8,9 and susceptible weighted imaging (SWI)10 can enhance the contrast of the Vim with other ventral thalamic nuclei. Furthermore, 3-T7 and 7-T10,11 MRI systems also provide high-resolution Vim images. In addition to these refinements of structural MRI, tractography based on diffusion-weighted MRI can predict the location of the Vim by showing the thalamocortical12,13 and cerebellothalamic pathways,14,15 as well as the pyramidal and spinothalamic tracts adjacent to the Vim.16
Herein, we report a rare tremor case with a unilaterally hypertrophic thalamus using two advanced targeting methods. The patient presented with a severe left-hand tremor with significant hypertrophy of the right thalamus. To target the Vim within the hypertrophic thalamus, we referred to quantitative susceptibility mapping (QSM) images17–19 and the atlas-to-patient warped Vim thalamus images.20,21 The patient underwent radiofrequency (RF) thalamotomy using the modified right Vim target.
Illustrative Case
Presentation
A 51-year-old right-handed female had experienced left-hand tremor for 6 years. The tremor worsened and impaired her activities of daily living. She had a surgical history of pituitary adenoma but no history of tic, seizure, or epilepsy. She did not have a family history of tremor. Upon examination, she had left-hand postural and intentional tremor with no resting tremor. The head postural tremor and right-hand postural and intentional tremor were mild. The left-hand tremor deteriorated her spiral and line drawing significantly (Fig. 1A). She had moderate and severe difficulties in drinking, hygiene, dressing, working, and social activities because of her left-hand tremor. The Clinical Rating Scale for Tremor (CRST) Parts A, B, and C scores were 8, 15, and 19, respectively. The Quality of Life in Essential Tremor Questionnaire (QUEST) score was 70.
FIG. 1.
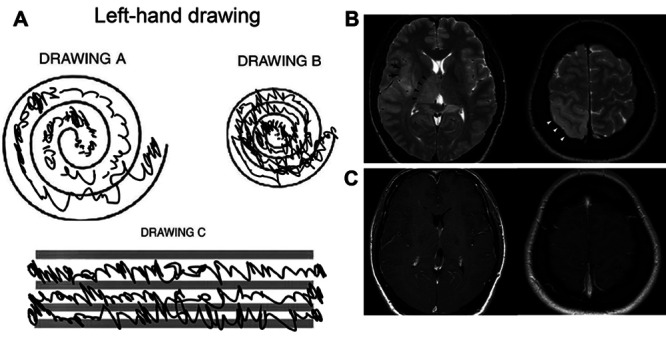
Preoperative left-hand Archimedes spiral and line drawings (A). Preoperative MRI showing T2-weighted hyperintense hypertrophy of the right thalamus (arrowheads, B) and cortical thickening of the insula (arrows, B) and parietal lobe (white arrowheads, B) with no gadolinium enhancement (C).
Preoperative T2-weighted MRI revealed hyperintense hypertrophy of the right thalamus (Fig. 1B) and hyperintense cortical thickening in the right insula and parietal lobe, including the right postcentral gyrus. Gadolinium injection did not enhance these lesions (Fig. 1C). These radiological findings suggested thalamic hypertrophy with focal cortical dysplasia or grade 2 gliomatosis cerebri.
Because the patient preferred to treat her left-hand tremor without device implantation and to have a biopsy for the right thalamic hypertrophy, she consented to RF Vim thalamotomy for tremor and stereotactic biopsy of the right thalamus rather than opting for the thalamic deep brain stimulation or focused ultrasound thalamotomy.
QSM Image Acquisition and Processing
The patient underwent QSM imaging with a single-head-orientation, multiecho, gradient-echo protocol (8 echoes were received; the first echo time was 4.5 msec, Δt was 5 msec) on a 3-T MRI unit (Ingenia Elition X, Philips) 2 days before surgery. The 8 magnitude and 8 phase data were integrated into four-dimensional (4D) MATLAB data using the original code developed in MATLAB 2021a (MathWorks). The STI Suite (UC Berkeley) pipeline17 processed the 4D MATLAB data into STAR QSM images.
Fusion of QSM and Nonlinearly Transformed Vim Atlas Images
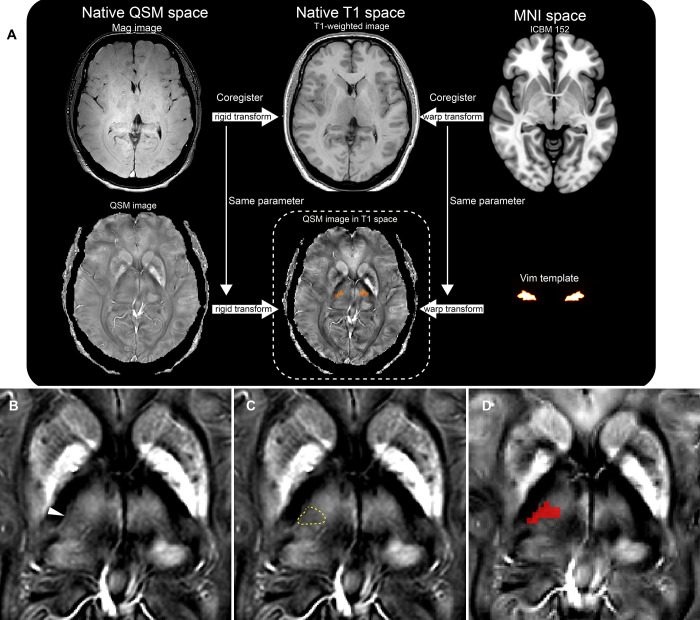
Affine parameters of SPM 12 (Wellcome Centre for Human Neuroimaging) coregistered the magnitude images of the QSM data to the native T1 space. After that, the same affine parameters coregistered the native QSM images to the native T1 space (Fig. 2A). Then, the inverted parameter of the normalization nonlinearly transformed and superimposed the ICBM 152 template brain20,22 on the coregistered QSM images (Fig. 2A). The QSM images demonstrated that the Vim as a decreased susceptibility area between the hyperintense ventro-oral (Vo) and ventrocaudal (Vc) nuclei of the thalamus (Fig. 2B and C) in the right ventrolateral thalamic nuclei. To have the atlas-to-patient warped right Vim images, the right Vim area of the template brain20,22 was also nonlinearly transformed and superimposed on the QSM images (Fig. 2D).
FIG. 2.
A flowchart (A) merging the images of QSM and T1-weighted MRI of the patient native space. The ICBM 152 templates are transformed into the native space. The same warp transformation is applied to the atlas images of the Vim of the thalamus. A QSM image of the right hypertrophic thalamus. A slightly decreased susceptibility area in the ventrolateral thalamus indicates the Vim (arrowhead, B; dotted line, C). A warped Vim atlas image (red, D) overlays the Vim area. MNI = Montreal Neurological Institute.
Target Determination
The AC-PC–based Vim coordinates were 10.5 mm from the third ventricular wall and 6.0 mm (25% of the AC-PC distance) anterior to the PC on the AC-PC plane on the navigation system (Stealth Station 7, Medtronic; Fig. 3A). However, the right Vim target was 2.3 mm above the ventral border of the hypertrophic right thalamus. Therefore, we visually inspected the ventral border of the T2-hyperintense right thalamus (Fig. 3B) and the superior border of the right subthalamic nucleus (STN) to guide the right Vim target. We also referred to the Vim atlas image overlaid on the T1-weighted magnetic resonance and QSM images. In addition, we adjusted the trajectory to cross the Vim atlas and to reach the superior part of the posterior subthalamic area, including the caudal zona incerta (cZI), which was posterior to the STN and lateral to the red nucleus (RN).23 The modified right Vim target was placed inferior and anterior to the AC-PC–based Vim target: 10.5 mm from the third ventricular wall, 8.9 mm anterior to the PC, and 2.7 mm below the AC-PC plane. The extended trajectory reached the right cZI. We confirmed that the trajectory did not cross the lateral ventricle and blood vessels.
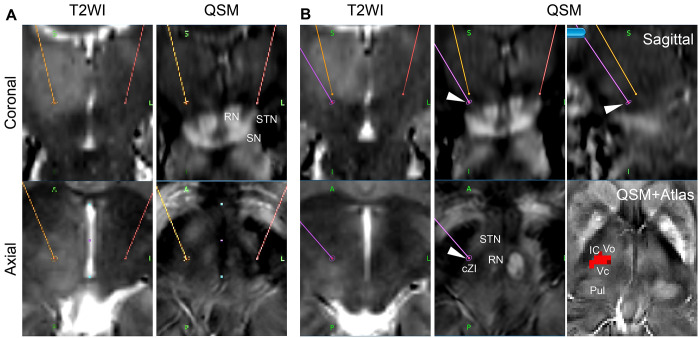
FIG. 3.
A: A coronal T2-weighted image (T2WI) shows a hyperintense hypertrophic right thalamus. A coronal QSM image shows the caudal shift of the RN, STN, and substantia nigra (SN). AC-PC–based targeting of the left (red dot) and right (orange dot) Vim. B: The right modified Vim target (arrowheads) and its trajectory (purple line) are shown. The QSM and atlas images show the Vim (red pixels). The trajectory is designed to reach the caudal zona incerta (cZI). IC = internal capsule; Pul = pulvinar thalamus; Vo = ventro-oral nucleus; Vc = ventro-caudal nucleus.
Surgery
On the morning of surgery, the patient had a Leksell G frame (Elekta, Stockholm, Sweden) placed on the head while under local anesthesia and underwent a computed tomography (CT). We coregistered the CT data to the preoperative MRI data to finalize the stereotactic coordinates. After a trephine and dural incision, we inserted a thermocoagulation electrode into the target. Instead of using microelectrode recording, we performed intraoperative stimulation at the target site to verify tremor suppression with no adverse effects. Stimulation was achieved by using a 1-mm diameter, 2-mm long electrode, with a pulse duration of 100 μsec, a frequency of 133 Hz, and an intensity ranging from 0.5 to 3 mA. Subsequently, the application of radiofrequency at a temperature of 64°C for a duration of 30 seconds, followed by a temperature of 70°C for another 30 seconds, resulted in the creation of a thalamotomy lesion. The initial RF lesioning of the Vim and cZI, performed 2.7 mm below the AC-PC plane, resulted in near-complete resolution of tremor in the left hand (Fig. 4A). An additional RF lesion 2 mm above the target along the trajectory augmented the tremor suppression (Fig. 4B). The patient had not reported any sensory disturbance. Subsequently, a stereotactic biopsy of the T2-weighted hyperintense dorsal thalamus was performed.
FIG. 4.
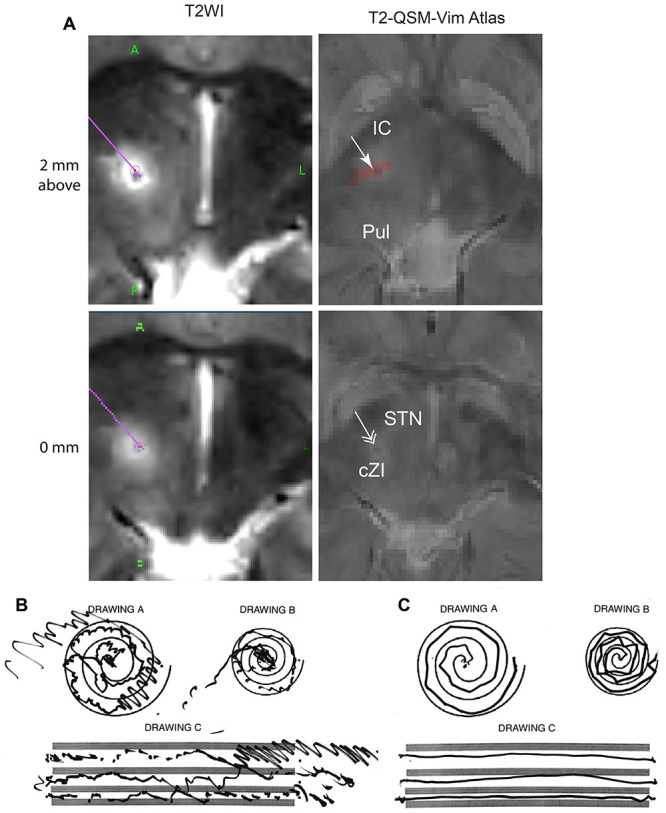
Postoperative T2-weighted MRI (T2WI; A) shows the subthalamic and thalamic RF lesions along the trajectory (purple). The postoperative T2WI with preoperative QSM and Vim atlas (T2-QSM-Vim Atlas) images identify the lesion (arrow) within the Vim area (red pixels) and the cZI (double arrow). Preoperative (B) and postoperative (C) left-hand spiral and line drawings show marked improvement after thalamotomy and subthalamotomy.
Postoperative Course and Pathological Diagnosis
The postoperative T2-weighted images merged with the preoperative QSM and Vim atlas images demonstrated that the lesion went through the right Vim and extended to the right cZI (Fig. 4A). The lesion may have involved part of the right ventralis oralis anterior (Voa)/ventralis oralis posterior (Vop), as the anteroposterior target coordinate was 8.9 mm anterior to the PC. The patient experienced left-hand tremor reduction (Fig. 4B, C) with no significant adverse effects on language, sensory function, and gait function. The postoperative 3-month CRST Parts A, B, and C and QUEST scores were 0, 1, 1, and 19, respectively. The patient had no recurrence of left-hand tremor for 12 months after the surgery.
The biopsied tissue had low cellular density with no cellular atypia. Immunohistochemistry showed that the p53-positive cells were less than 10%, and mutations in isocitrate dehydrogenase 1 R132H and ATP-dependent helicase were negative. These results weakly suggested evidence of gliomatosis cerebri.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Thalamic Hypertrophy and Tremor
Thalamic hypertrophy or enlargement can be associated with gliomatosis cerebri,24 low-grade glioma,25 diffuse midline,26 and epilepsy.27 An association between focal cortical dysplasia and thalamic hypertrophy is unknown, while its association with thalamic hypotrophy has been reported.28 Thalamic hypertrophy and associated tremor have been reported only in a case with gliomatosis.29 Thalamic hypertrophy of unknown etiology and essential tremor, as seen in the present case, is a rare clinical constellation.
Asymmetrical Thalamus and Vim Targeting
In the present case, significant thalamic hypertrophy shifted the ventral border of the thalamus below the AC-PC plane. The caudal shift of the STN and RN on the QSM images supported the thalamic border shift. Therefore, we placed the Vim target below the AC-PC plane. A dim hypointense band within the ventrolateral thalamic nuclei indicated the Vim. The warped Vim atlas also suggested the location of the Vim. In case of a targeting failure, we placed the tip of the electrode beneath the AC-PC plane and posterior to the STN, which corresponded to the upper part of the cZI, as the cZI is a valuable target to control tremor.30 In the present case, the right Vim and cZI lesioning significantly suppressed the patient’s left-hand tremor.
The 7-T SWI may indicate the Vim as a hypointense area.10,31 As an advanced imaging technique of SWI, QSM may also indicate the Vim as a hypointense area.11 However, the predictive value of a SWI/QSM-hypointense Vim remains unknown, as the magnetic susceptibility of the Vim may vary among patients with tremor.11,31 To complement the accuracy of the QSM-based Vim localization, we utilized the atlas-to-patient nonlinear transformation of the Vim atlas.20,22
Because the present study is a single case report, our findings on the Vim localization in the hypertrophic thalamus are limited in accuracy and efficacy. In further cases, it is necessary to examine the delineation accuracy of the SWI and QSM and the complementary effect of combining QSM and warped atlas images. Our study also lacks a technical comparison of the present combined technique with other advanced MRI protocols such as high-resolution proton density imaging,6,7 white-matter nullification,8,9 and diffusion tensor imaging–based Vim targeting methods.12–16 Therefore, the technical feasibility of a QSM adjunct with atlas warping cannot be compared with other imaging methods.
Lessons
A combination of the QSM and nonlinearly transformed Vim atlas imaging can be helpful to target the Vim, especially in patients with tremor and a deformed thalamus. The QSM may indicate the Vim as a hypointense band along the lateral ventral thalamic nuclei, and the warped atlas can indicate the area of the Vim reflecting the individual anatomical feature of the thalamus.
Acknowledgments
We thank Hideaki Inoue, Tsukasa Mishima, Taro Nishi, Megumi Hamano, and Miki Ohshima for their technical help in obtaining the QSM image.
This work was funded by a grant from the Japan Society for the Promotion of Science (JSPS) (grant number 21K09116) to Hiroki Toda.
Author Contributions
Conception and design: Toda, Sawada. Acquisition of data: Toda, Ohtsuki, Sawada, Yoshizaki. Analysis and interpretation of data: Toda, Ohtsuki, Sawada, Yoshizaki. Drafting the article: Toda, Ohtsuki, Sawada, Sawamoto. Critically revising the article: Toda, Yoshizaki, Sawamoto, Fushimi. Reviewed submitted version of manuscript: Toda, Sawada, Yoshizaki, Ishimori, Sawamoto, Fushimi. Approved the final version of the manuscript on behalf of all authors: Toda. Administrative/technical/material support: Ishimori. Study supervision: Toda, Ishimori.
References
- 1. Jankovic J, Cardoso F, Grossman RG, Hamilton WJ. Outcome after stereotactic thalamotomy for parkinsonian, essential, and other types of tremor. Neurosurgery. 1995;37(4):680–687. doi: 10.1227/00006123-199510000-00011. [DOI] [PubMed] [Google Scholar]
- 2. Hamani C, Dostrovsky JO, Lozano AM. The motor thalamus in neurosurgery. Neurosurgery. 2006;58(1):146–158. doi: 10.1227/01.neu.0000192166.62017.c1. [DOI] [PubMed] [Google Scholar]
- 3. Hirai T, Ohye C, Nagaseki Y, Matsumura M. Cytometric analysis of the thalamic ventralis intermedius nucleus in humans. J Neurophysiol. 1989;61(3):478–487. doi: 10.1152/jn.1989.61.3.478. [DOI] [PubMed] [Google Scholar]
- 4. Guiot G, Arfel G, Derôme P. Stereotaxic surgery for rest and attitude tremors. Medical Gazette of France. 1968;75:4029–4056. [Google Scholar]
- 5.Schaltenbrand G. Atlas for Stereotaxy of the Human Brain. Georg Thieme Publishers; 1977. [Google Scholar]
- 6. Spiegelmann R, Nissim O, Daniels D, Ocherashvilli A, Mardor Y. Stereotactic targeting of the ventrointermediate nucleus of the thalamus by direct visualization with high-field MRI. Stereotact Funct Neurosurg. 2006;84(1):19–23. doi: 10.1159/000092683. [DOI] [PubMed] [Google Scholar]
- 7. Wakim AA, Sioda NA, Zhou JJ, Lambert M, Evidente VGH, Ponce FA. Direct targeting of the ventral intermediate nucleus of the thalamus in deep brain stimulation for essential tremor: a prospective study with comparison to a historical cohort. J Neurosurg. 2021;136(3):662–671. doi: 10.3171/2021.2.JNS203815. [DOI] [PubMed] [Google Scholar]
- 8. Su JH, Choi EY, Tourdias T, et al. Improved Vim targeting for focused ultrasound ablation treatment of essential tremor: a probabilistic and patient-specific approach. Hum Brain Mapp. 2020;41(17):4769–4788. doi: 10.1002/hbm.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sudhyadhom A, Haq IU, Foote KD, Okun MS, Bova FJ. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR) Neuroimage. 2009;47(suppl 2):T44–T52. doi: 10.1016/j.neuroimage.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 10. Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery. 2010;67(6):1745–1756, discussion 1756. doi: 10.1227/NEU.0b013e3181f74105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR. Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage. 2013;65:299–314. doi: 10.1016/j.neuroimage.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 12. Klein JC, Barbe MT, Seifried C, et al. The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology. 2012;78(11):787–795. doi: 10.1212/WNL.0b013e318249f702. [DOI] [PubMed] [Google Scholar]
- 13. Pouratian N, Zheng Z, Bari AA, Behnke E, Elias WJ, Desalles AA. Multi-institutional evaluation of deep brain stimulation targeting using probabilistic connectivity-based thalamic segmentation. J Neurosurg. 2011;115(5):995–1004. doi: 10.3171/2011.7.JNS11250. [DOI] [PubMed] [Google Scholar]
- 14. Akram H, Dayal V, Mahlknecht P, et al. Connectivity derived thalamic segmentation in deep brain stimulation for tremor. Neuroimage Clin. 2018;18:130–142. doi: 10.1016/j.nicl.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coenen VA, Mädler B, Schiffbauer H, Urbach H, Allert N. Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: a concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery. 2011;68(4):1069–1076. doi: 10.1227/NEU.0b013e31820a1a20. [DOI] [PubMed] [Google Scholar]
- 16. Sammartino F, Krishna V, King NK, et al. Tractography-based ventral intermediate nucleus targeting: novel methodology and intraoperative validation. Mov Disord. 2016;31(8):1217–1225. doi: 10.1002/mds.26633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li W, Avram AV, Wu B, Xiao X, Liu C. Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed. 2014;27(2):219–227. doi: 10.1002/nbm.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C, Li W, Tong KA, Yeom KW, Kuzminski S. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. J Magn Reson Imaging. 2015;42(1):23–41. doi: 10.1002/jmri.24768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fushimi Y, Nakajima S, Sakata A, Okuchi S, Otani S, Nakamoto Y. Value of quantitative susceptibility mapping in clinical neuroradiology. J Magn Reson Imaging. 2023 doi: 10.1002/jmri.29010. [DOI] [PubMed] [Google Scholar]
- 20. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 21. Chakravarty MM, Sadikot AF, Germann J, Bertrand G, Collins DL. Towards a validation of atlas warping techniques. Med Image Anal. 2008;12(6):713–726. doi: 10.1016/j.media.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 22. Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage. 2018;170:271–282. doi: 10.1016/j.neuroimage.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 23. Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129(Pt 7):1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 24. del Carpio-O’Donovan R, Korah I, Salazar A, Melançon D. Gliomatosis cerebri. Radiology. 1996;198(3):831–835. doi: 10.1148/radiology.198.3.8628879. [DOI] [PubMed] [Google Scholar]
- 25. Waqar M, Hanif S, Rathi N, et al. Diagnostic challenges, management and outcomes of midline low-grade gliomas. J Neurooncol. 2014;120(2):389–398. doi: 10.1007/s11060-014-1563-6. [DOI] [PubMed] [Google Scholar]
- 26. Meyronet D, Esteban-Mader M, Bonnet C, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19(8):1127–1134. doi: 10.1093/neuonc/now274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Della Marca G, Vollono C, Ferraro D, et al. Left thalamomegaly in a patient with partial epilepsy. Clin Neurol Neurosurg. 2008;110(3):298–301. doi: 10.1016/j.clineuro.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 28. Rezayev A, Feldman HA, Levman J, Takahashi E. Bilateral thalamocortical abnormalities in focal cortical dysplasia. Brain Res. 2018;1694:38–45. doi: 10.1016/j.brainres.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 29. Nevin S. Thalamic hypertrophy or gliomatosis of the optic thalamus. J Neurol Psychiatry. 1938;1(4):342–358. doi: 10.1136/jnnp.1.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plaha P, Khan S, Gill SS. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J Neurol Neurosurg Psychiatry. 2008;79(5):504–513. doi: 10.1136/jnnp.2006.112334. [DOI] [PubMed] [Google Scholar]
- 31. Najdenovska E, Tuleasca C, Jorge J, et al. Comparison of MRI-based automated segmentation methods and functional neurosurgery targeting with direct visualization of the Ventro-intermediate thalamic nucleus at 7T. Sci Rep. 2019;9(1):1119. doi: 10.1038/s41598-018-37825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]