Abstract
Purpose
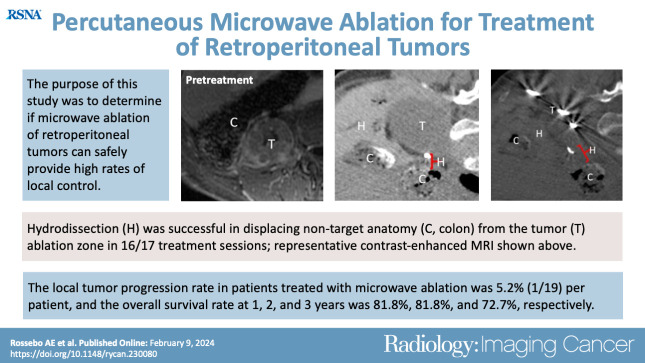
To determine if microwave ablation (MWA) of retroperitoneal tumors can safely provide high rates of local tumor control.
Materials and Methods
This retrospective study included 19 patients (median age, 65 years [range = 46–78 years]; 13 [68.4%] men and six [31.6%] women) with 29 retroperitoneal tumors treated over 22 MWA procedures. Hydrodissection (0.9% saline with 2% iohexol) was injected in 17 of 22 (77.3%) procedures to protect nontarget anatomy. The primary outcomes evaluated were local tumor progression (LTP) and complication rates. Oncologic outcomes, including overall survival (OS), progression-free survival (PFS), and treatment-free interval (TFI), were examined as secondary outcome measures.
Results
Median follow-up was 18 months (range = 0.5–113). Hydrodissection was successful in displacing nontarget anatomy in 16 of 17 (94.1%) procedures. The LTP rate was 3.4% (one of 29; 95% CI: 0.1, 17.8) per tumor and 5.3% (one of 19; 95% CI: 0.1, 26.0) per patient. The overall complication rate per patient was 15.8% (three of 19), including two minor complications and one major complication. The OS rate at 1, 2, and 3 years was 81.8%, 81.8%, and 72.7%, respectively, with a median OS estimated at greater than 7 years. There was no evidence of a difference in OS (P = .34) and PFS (P = .56) between patients with renal cell carcinoma (six of 19 [31.6%]) versus other tumors (13 of 19 [68.4%]) and patients treated with no evidence of disease (15 of 22 [68.2%]) versus patients with residual tumors (seven of 22 [31.8%]). Median TFI was 18 months (range = 0.5–108).
Conclusion
Treatment of retroperitoneal tumors with MWA combined with hydrodissection provided high rates of local control, prolonged systemic therapy−free intervals, and few serious complications.
Keywords: Ablation Techniques (ie, Radiofrequency, Thermal, Chemical), Retroperitoneum, Microwave Ablation, Hydrodissection
© RSNA, 2024
Keywords: Ablation Techniques (ie, Radiofrequency, Thermal, Chemical); Retroperitoneum; Microwave Ablation; Hydrodissection
Summary
Microwave ablation for oligometastatic disease in the retroperitoneum provided effective local control, prolonged systemic therapy−free intervals after treatment, and few serious complications when combined with hydrodissection.
Key Points
■ The local tumor progression rate in patients with retroperitoneal tumors treated with microwave ablation was 3.4% (one of 29) per tumor and 5.3% (one of 19) per patient.
■ Hydrodissection was successful in displacing nontarget anatomy in 16 of 17 (94.1%) total procedures.
■ The overall survival rate at 1, 2, and 3 years was 81.8%, 81.8%, and 72.7%, respectively, with a median overall survival estimated at greater than 7 years.
Introduction
Aggressive local treatment of oligometastatic disease is an important emerging concept in oncology (1–5). While originally reserved for special circumstances in a limited number of primary tumors, such as colorectal metastases to the liver (6), improvements in systemic therapy and more effective, less invasive treatment options have increased interest in treating a wider variety of tumors (2,4,5,7,8). The retroperitoneum is a frequent site of oligometastatic disease but is a particularly challenging anatomic location for local therapies due to the proximity of bowel, pancreas, major blood vessels, and lymphatics (9). Recent single-center and phase 2 radiation therapy studies demonstrated a potential improvement in progression-free survival (PFS), even in tumor types in which treatment of metastases had previously been considered futile (2–5,7,8). However, radiation therapy in the retroperitoneum can be associated with high levels of gastrointestinal toxicity (10–12). Other local therapies being investigated for use in treating retroperitoneal tumors include surgery and thermal ablation (13–17).
Radiofrequency ablation (RFA) and cryoablation are the most established thermal ablation modalities, and both have been in use for several decades worldwide (15,16). More recently, interest in microwave ablation (MWA) is increasing due to faster heating and higher applied tissue temperatures, which may increase local tumor control (13,14,17). This increase may be particularly relevant in the retroperitoneum where the substantial heat sink effect created by the aorta and inferior vena cava can increase local tumor progression (LTP) after RFA and cryoablation (18,19). A single prior study of MWA in the retroperitoneum performed without hydrodissection (injection of fluid to displace and protect adjacent structures) resulted in acceptable local control, but at the cost of complications and prolonged hospitalizations (20). Thus, two important questions remain: (a) Can the thermal and oncologic benefits of MWA demonstrated in other organs be translated to the retroperitoneum to achieve high rates of local tumor control with an acceptable safety profile when combined with hydrodissection, and (b) is there an oncologic benefit of treating retroperitoneal metastases with thermal ablation?
The primary purpose of this retrospective, single-center study was to determine if MWA of retroperitoneal tumors results in a low incidence of LTP with few severe complications. Oncologic outcomes such as overall survival (OS), PFS, and treatment-free interval (TFI) were also examined as secondary outcome measures.
Materials and Methods
This retrospective study was conducted under an institutional review board–approved protocol that allows for the retrospective review of diagnostic imaging and image-guided procedures. The institutional review board included a waiver of informed consent in accordance with 45 CR 46.116 (f)(3) to access an ablation database and de-identify patient information for this Health Insurance Portability and Accountability Act–compliant, single-center, retrospective study. Electronic medical records and picture archiving and communication systems were used to extract relevant patient data. A separate report on the treatment of renal cell carcinoma (RCC) metastases included 12 of the 29 tumors reported in this study (21).
Patient Selection
Patients with retroperitoneal tumors treated with MWA between 2011 and 2021 were identified from an institutional ablation database. All eligible patients were included. The final study sample consisted of 19 patients with 29 retroperitoneal tumors treated over 22 MWA procedures. Patients were selected for treatment by a multidisciplinary group of physicians in consensus (urology, radiology, oncology) based on tumor biology, location, and technical feasibility.
MWA Procedure
All procedures were performed in a dedicated interventional CT suite (Optima 580 16 W; GE HealthCare) under general anesthesia (18 of 22 [81.8%] procedures) or conscious sedation (four of 22 [18.2%] procedures) by a multidisciplinary team of radiologists and urologists with 1–25 years of ablation experience. After 2018, all procedures were performed under general anesthesia using high-frequency jet ventilation to minimize respiratory motion (22). A combination of CT fluoroscopy, US (LOGIQ E9 or E10; GE HealthCare), and an electromagnetic navigation system (CT-Navigation; IMACTIS) were used to place microwave antennas. Hydrodissection (0.9% saline with 2% iohexol; GE HealthCare) was performed as necessary to temporarily displace nontarget anatomy away from the ablation zone (Figs 1, 2) (23).
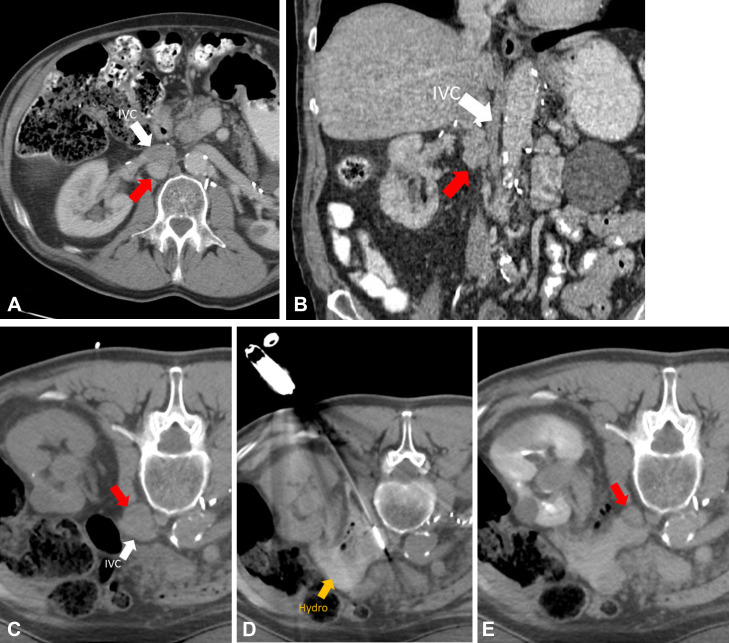
Figure 1:
CT scans in a 67-year-old male patient with metastatic pheochromocytoma to the retroperitoneum. (A, B) Preprocedural CT scans demonstrate metastatic lymph node (red arrows, 2.4 × 2.0 × 3.7 cm) immediately posterior to the inferior vena cava (IVC; white arrows). (C) Patient in the prone treatment position demonstrating nodal target (red arrow). (D) One of two microwave ablation antennas in place after hydrodissection fluid was placed in the retroperitoneum (Hydro; yellow arrow). Treatment was performed for 5 minutes at 65 W. (E) Immediate postprocedural scan with intravenous contrast material. Note shrinkage of node (red arrow) after microwave ablation due to tissue dehydration.
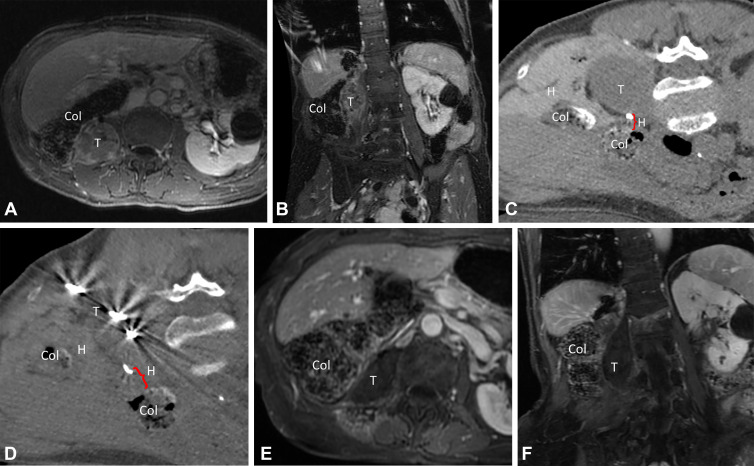
Figure 2:
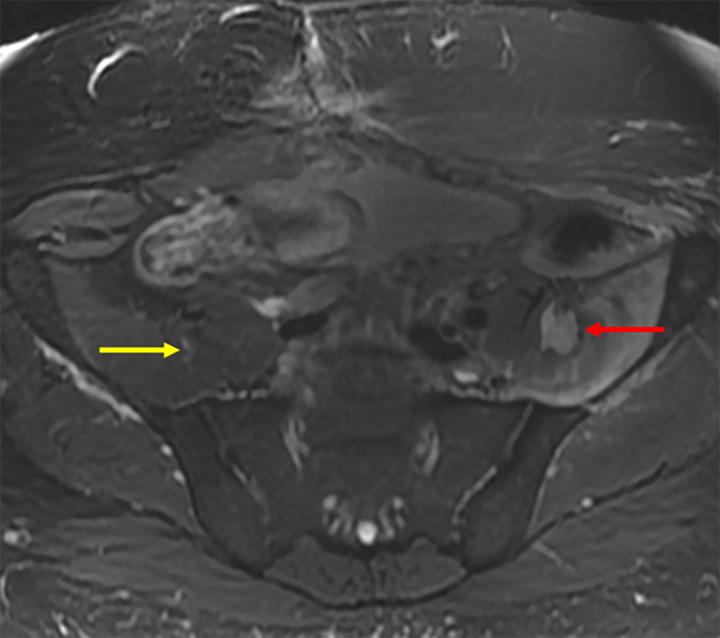
Images in a 73-year-old female patient with a 4.5-cm metastatic adrenal oncocytic neoplasm to the retroperitoneum. (A, B) Pretreatment contrast-enhanced MR images in the (A) axial and (B) coronal planes. Note the proximity of the colon to the tumor, putting the patient at risk for colonic injury with thermal ablation without displacement. (C) Intraprocedural CT image in patient in prone treatment position before antenna placement. The colon has been partially displaced from the tumor by the injection of hydrodissection fluid (0.9% saline mixed with 2% iohexol) under US guidance. (D) Intraprocedural CT image obtained after placement of three microwave ablation antennas and injection of more hydrodissection fluid. Note the further displacement of the colon away from the tumor, increasing the safety of thermal ablation. (E, F) Nine-month posttreatment contrast-enhanced MR images in the (E) axial and (F) coronal planes demonstrate no residual tumor enhancement or tumor shrinkage and no evidence of colonic injury. Col = colon, H = hydrodissection, T = metastatic adrenal oncocytic neoplasm.
A multiprobe, gas-cooled, in-phase, 2.45-GHz MWA system was used for all procedures (NeuWave Microwave Ablation System; Ethicon). Treatment time, power, and number of probes were determined by the operating physician based on real-time monitoring, tumor size, and distance to other anatomic structures.
Data Collection
Electronic medical records, picture archiving and communication systems, and an institutional ablation database were searched for procedure specifics, oncologic history, and complications. Ablation metrics were reported using established criteria (24). Additional information collected for each patient included the following: age; sex; diagnosis; standard cancer metrics for ablation studies, including LTP, OS, PFS, and TFI; tumor size and number; presence or absence of distant metastatic disease; procedural adjunctive maneuvers; postablation treatments; and pre- or postablation systemic therapies (25). All times were reported from the date of the ablation procedure (24).
Complications
All adverse events within 30 days of the procedure were included and graded according to the Society of Interventional Radiology guidelines for adverse event classification by outcome (26).
Statistical Analysis
OS was defined as the time from treatment to death due to any cause. PFS was defined as the time from treatment to death, new or progressing metastatic disease, LTP, or latest imaging follow-up (25). TFI was defined as the time from the ablation to the initiation of systemic therapy or death (21). Kaplan-Meier curves were plotted for OS and PFS to analyze survival for patients with RCC, patients with non-RCC, and the presence or absence of residual tumor. The log-rank test was used to compare event rates between groups. All analyses were performed using R software, version 4.02 (Foundation for Statistical Computing), with the survival package for survival analysis. P < .05 was considered statistically significant.
Results
Patient Characteristics
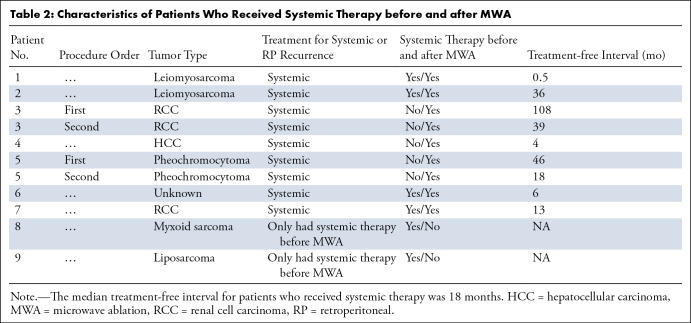
The study sample comprised 29 tumors (27 metastases and two primary tumor recurrences in the retroperitoneum) treated over 22 procedures in 19 patients (median age, 65 years [range = 46–78 years]; 13 [68.4%] men and six [31.6%] women) (Table 1). The median follow-up was 18 months (range = 0.5–113 months). Patients had primary tumors in various locations, with the most common cell types being RCC (12 of 29 [41.4%]), liposarcoma (four of 29 [13.8%]), leiomyosarcoma (four of 29 [13.8%]), and hepatocellular carcinoma (three of 29 [10.3%]). Metastatic status was confirmed with biopsy in 65.5% (19 of 29) of treated tumors. Nine of 19 patients (47.4%) received neoadjuvant or adjuvant systemic therapy (Table 2). The mean tumor diameter was 2.6 cm (range = 0.5–4.5 cm). The number of tumors ablated per procedure included one tumor (17 of 22 [77.3%]), two tumors (four of 22 [18.2%]), and four tumors (one of 22 [4.5%]). The median time from initial cancer diagnosis to time of ablation was 100 months (range = 14–276 months).
Table 1:
Characteristics of Study Patients
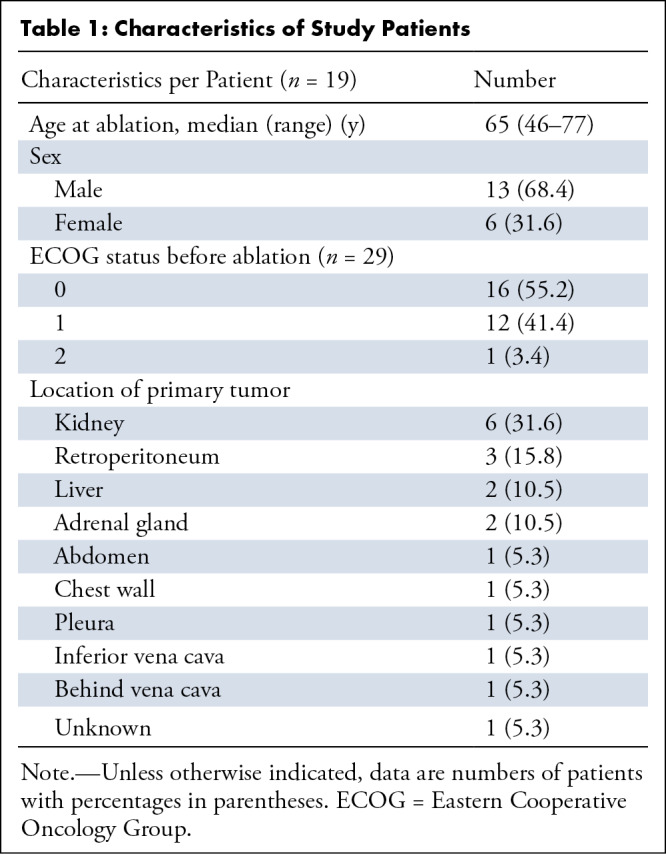
Table 2:
Characteristics of Patients Who Received Systemic Therapy before and after MWA
Ablation Procedure, Technical Success, and Residual Tumor
Technical success was achieved in 100% (29 of 29) of treatments, as confirmed with immediate CT follow-up imaging. Mean ablation time was 5.1 minutes with a mean power of 64.9 W (range = 35–95 W). Operating physicians used one to three probes per tumor (one probe, 19 of 29 [65.5%]; two probes, six of 29 [20.7%]; three probes, four of 29 [13.8%]). Hydrodissection (mean volume = 555 mL [range = 60–3000 mL]) was used for 77.3% (17 of 22) of procedures, with success in 94.1% (16 of 17) for achieving displacement from vulnerable structures. No cases were aborted due to an inability to adequately displace the tumor from vulnerable nontarget anatomy (primarily bowel). No evidence of disease status was obtained in 15 of 22 (68.2%) procedures after the planned treatment course, (12 of 22 [54.5%] procedures after single-procedure MWA treatment and an additional three [13.6%] procedures after a planned second-stage treatment to nonretroperitoneal sites of disease).
Complications
The overall complication rate was 15.8% (three of 19). There were minor complications for two patients: two cases of urinary retention requiring Foley catheter placement (grade 1), with one patient additionally having a small stable pneumothorax with no intervention required (grade 1). One expected major complication (grade 2) occurred due to the femoral nerve being immediately adjacent to the target tumor, which could not be displaced by hydrodissection (Fig 3). This complication resulted in chronic quadriceps weakness requiring a knee brace for stabilization, with ambulation and neuropathic pain well controlled with gabapentin. There were no cases of bowel injury, pancreatic injury, or bleeding, and no patients died within 30 days of the MWA procedure.
Figure 3:
Axial T2-weighted MR image in a 46-year-old male patient with a post–microwave ablation femoral nerve injury. The femoral nerve on the treated side could not be separated from the tumor (red arrow). The location of the femoral nerve on the contralateral side (yellow arrow) is noted for comparison.
Local Tumor Progression
The LTP rate was 3.4% (one of 29; 95% CI: 0.1, 17.8) per tumor and 5.3% (one of 19; 95% CI: 0.1, 26.0) per patient. The single local recurrence was not retreated due to concurrent evidence of multifocal metastatic disease.
OS and PFS
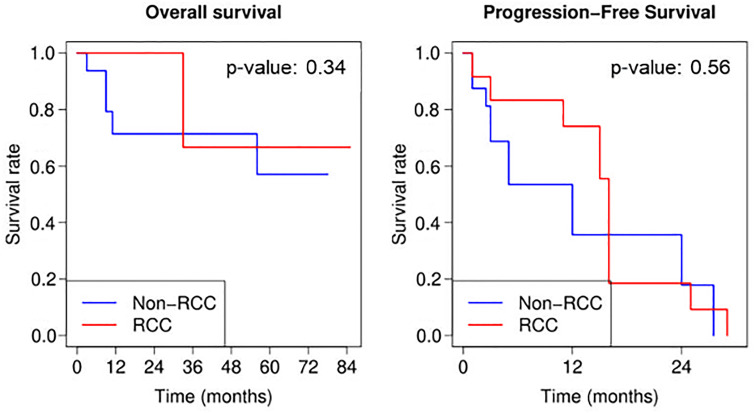
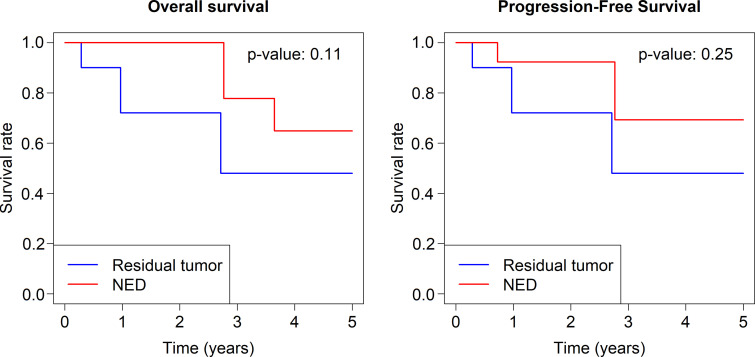
OS rates at 1, 2, and 3 years were 81.8%, 81.8%, and 72.7%, respectively. The median OS was greater than 7 years, but the precise value was inestimable because fewer than half the patients had died at the end of follow-up. PFS rates at 1, 2, and 3 years were 53.3%, 20.0%, and 0%, respectively (Fig 4). There was no evidence of a difference in OS (P = .34) or PFS (P = .56) rates for RCC (12 of 29 [41.4%]) compared with other types of malignant neoplasms (17 of 29 [58.6%]). There was also no evidence of a difference in OS (P = .11) or PFS (P = .25) rates for patients with no evidence of disease after MWA treatment (15 of 22 [68.2%]) versus those with residual tumor (seven of 22 [31.8%]) (Fig 5).
Figure 4:
Graphs of Kaplan-Meier curves for overall survival and progression-free survival for all study patients, patients with renal cell carcinoma (RCC), and patients without RCC. Treatment day = day 0. P values are based on the log-rank test; non-RCC appears to have lower overall and progression-free survival rates early on, but the differences with RCC are not significant.
Figure 5:
Graphs of Kaplan-Meier curves for overall and progression-free survival by no evidence of disease (NED) and residual tumor.
Treatment-free Interval
Nine of 19 (47.4%) patients received either neoadjuvant or adjuvant systemic therapy (Table 2). Six of 19 (31.6%) patients had systemic therapy before ablation, with four (66.7%) of these patients receiving repeat therapy after ablation. Three of nine (33.3%) patients who received initial systemic therapy after ablation had systemic disease progression, not recurrence of retroperitoneal masses. The overall median TFI was 18 months (range = 0.5–108 months). The patient who had a 0.5-month TFI resumed systemic therapy after ablation due to preexisting distant disease in the liver, lungs, and skin. This patient did not have evidence of LTP during the follow-up period.
Discussion
This study demonstrates that percutaneous MWA of retroperitoneal tumors can be performed in a single procedure with minimal morbidity and high rates of local control, particularly when paired with hydrodissection. Importantly, patients did not experience the serious adverse events previously reported with retroperitoneal MWA (without hydrodissection) nor the gastrointestinal complications observed with radiation to the retroperitoneum (10–12,27). Patients did not die of progressive retroperitoneal disease and experienced a long systemic therapy−free interval after MWA, primarily due to high rates of local control. The single grade 2 complication in this study, an injury to the femoral nerve, was discussed with the patient before the procedure and anticipated due to the proximity of the nerve to the target tumor. MWA was chosen over cryoablation due to the known high local control rates for MWA in other tumor types, as well as a postcryoablation nerve injury in an almost identical patient at the study center 12 months prior (13,14). Neural monitoring was used but remained normal until only approximately 5 seconds before the end of the procedure.
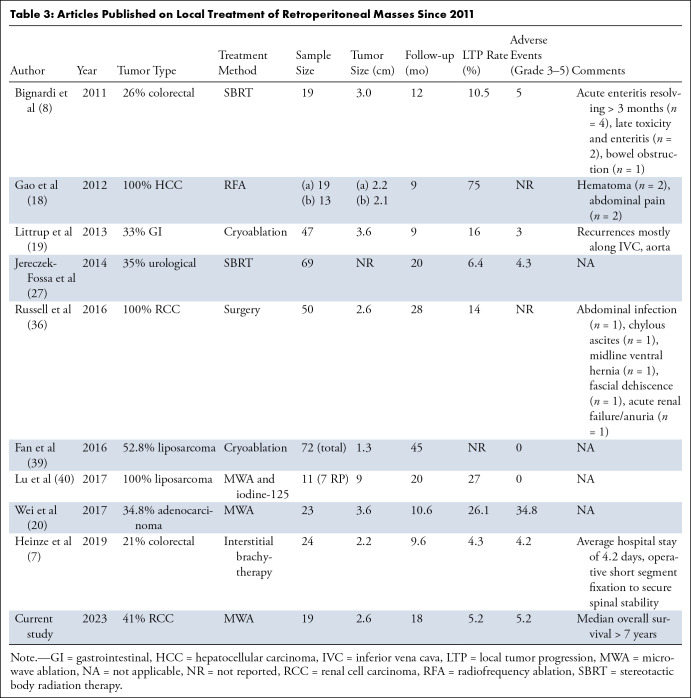
The rationale for using MWA to treat retroperitoneal tumors was to leverage the rapid heating and high tissue temperatures to overcome the heat sink effect of the aorta and inferior vena cava, which are inevitably near the target tumor (28,29). When compared with prior studies on RFA and cryoablation, the high local control rates in this study appeared to confirm the advantage of MWA to overcome vascular mediated cooling (13,14,28). For example, Gao et al (18) reported a post-RFA local control rate in the retroperitoneum of only 41.7% at 10 months. The largest cryoablation study of retroperitoneal tumors reported a 16% LTP rate—with almost all recurrences adjacent to the aorta or inferior vena cava (30). The single prior MWA study for retroperitoneal tumors was performed without hydrodissection and demonstrated a 23.1% local recurrence rate, substantially higher than the current study (20). In addition to protecting surrounding structures from collateral damage, hydrodissection enables a more aggressive ablation, which likely contributed to the high control rates in this study.
In contrast to thermal ablation, radiation therapy does not suffer from a vulnerability to heat sinks. However, radiation lacks the precision of ablation, resulting in nontarget structures such as intestine being included in the treatment field. For example, one study reported a high local control rate (95%) but two acute grade 3 and one grade 4 toxicity in 69 patients (27). A different study reported a relatively low LTP rate of 10.5%, but 21% of patients experienced acute enteritis persisting for over 3 months, 10.5% had late toxicity and enteritis, and one patient had a grade 3 complication (subocclusive bowel obstruction) (8). A few reports of bowel displacement before radiation therapy to the retroperitoneum have been published, but this is not yet standard and suffers from both complexity and conversion of a noninvasive technique into a minimally invasive one (30,31). Oncologic comparisons between ablative modalities and radiation remain difficult due to differences in tumor types and patient populations and will require larger studies with similar tumor types.
The retroperitoneum and retroperitoneal nodes are an important first metastatic station for many tumor types (9,32). Locally treating oligometastatic disease, including in the retroperitoneum, is increasingly accepted within the oncology community (33). While not the primary outcome of this study, the oncologic outcomes (particularly the OS of greater than 7 years) appear promising. In addition to tumor control and survival, TFI is an important metric for quality-of-life considerations (34,35). The overall median TFI for this study was 18 months, which is roughly comparable to values previously reported in the RCC literature, despite the high proportion of patients without RCC (13 of 19) (21,35,36). Patients with RCC were of specific interest to this study because the retroperitoneum is a common metastatic site, and there is increasing acceptance of local treatments for selected RCC metastases (21,34,35). The current study includes only one patient with RCC who received post-MWA systemic therapy, so it was not possible to compare the TFI between patients with and without RCC. Importantly, in all patients treated with systemic therapy after MWA, treatment was for tumors outside the retroperitoneum, not due to failure of local control.
The translation of high local control rates into overall better disease-specific survival is difficult to elucidate from a single-center retrospective study of this size; however, there is indirect evidence of a benefit of complete pathologic response. Patients with hepatocellular carcinoma bridged to transplant by local-regional therapies present a unique opportunity to examine the importance of complete pathologic response due to the availability of organs treated by local-regional therapies (37). Individuals with a complete pathologic response have better overall cancer outcomes, including higher disease-specific survival rates; however, the ability of local-regional therapy to create a complete pathologic response varies substantially between modalities, highlighting the importance of treatment approach and technology (37,38). For metastatic RCC, the most common single tumor type included in this study, the importance of complete tumor removal has been demonstrated in surgical series (34,36).
This study had several limitations. The MWA device in this study was an in-phase multiantenna system, which may produce ablation zones different from single-antenna MWA systems (13,14,28). The single-center, retrospective design with no comparison arm makes it difficult to compare MWA to other treatment modalities (Table 3). Because of the heterogeneity in treated tumor types, oncologic outcomes are interesting but not definitive. While no evidence of statistical differences between OS and PFS were found between patients with and without RCC, these results need to be viewed with caution due to the small sample size in each cohort. Oligometastatic disease to the retroperitoneum treatable by local means is uncommon, so while the number of patients in this study was low, it is in line with other reports (Table 3).
Table 3:
Articles Published on Local Treatment of Retroperitoneal Masses Since 2011
In summary, our study suggests that MWA for oligometastatic disease provides effective local control, prolonged systemic therapy−free intervals after treatment, and few serious complications when combined with hydrodissection. The posttreatment OS appears promising but is limited by the small sample size and variety of primary tumors. Further studies with larger treatment groups are warranted to determine if there is an improvement in oncologic end points, such as OS and PFS.
Supported by the Clinical and Translational Science Award program, through the National Institutes of Health National Center for Advancing Translational Sciences (grant UL1TR002373). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: A.E.R. No relevant relationships. A.M.Z. No relevant relationships. E.A.K. No relevant relationships. L.M. No relevant relationships. A.B.C. No relevant relationships. T.J.Z. Contracts from Ethicon (research funding to institution for clinical trial unrelated to this study), HistoSonics (research funding to institution), and NIH (research funding to institution); consulting fees from Elephas; speakers bureau payment from Canon; Data Safety Monitoring Board for Elephas; stock in HistoSonics. J.L.H. Paid consultant for Neuwave Medical (unrelated to this manuscript); stockholder for Elucent Medical, HistoSonics, and Accure (unrelated to this manuscript). E.J.A. No relevant relationships. M.G.L. Spouse is consultant for Elephas Bio; Igor Laufer Visiting Professor for Society of Abdominal Radiology (supported by Bracco). E.M.K.K. Consultant for Boston Scientific. S.A.W. Research relationship with GE HealthCare and Siemens Healthineers; consulting relationship with Ethicon and Cook Medical. L.M.S. Presentation honoraria from Varian; shareholder in Elucent Medical. P.F.L. Contract with HistoSonics and Siemens Healthineers; consulting fees from Elucent Medical, HistoSonics, and J&J/Ethicon/NeuWave Medical; stock/stock options in HistoSonics, Elucent Medical, McGinley Orthopedics, and RevOps Medical. F.T.L. Research support from Ethicon and HistoSonics; royalties from Medtronic; consulting fees from Ethicon; medical advisory board for Canon; support for travel to HistoSonics board of directors meetings; Wisconsin Alumni Research Foundation; participation on Canon board; participation on HistoSonics board of directors; stock/stock options in HistoSonics; research equipment from HistoSonics.
Abbreviations:
- LTP
- local tumor progression
- MWA
- microwave ablation
- OS
- overall survival
- PFS
- progression-free survival
- RCC
- renal cell carcinoma
- RFA
- radiofrequency ablation
- TFI
- treatment-free interval
References
- 1. Lievens Y , Guckenberger M , Gomez D , et al . Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document . Radiother Oncol 2020. ; 148 : 157 – 166 . [DOI] [PubMed] [Google Scholar]
- 2. Sutera P , Clump DA , Kalash R , et al . Initial results of a multicenter Phase 2 trial of stereotactic ablative radiation therapy for oligometastatic cancer . Int J Radiat Oncol Biol Phys 2019. ; 103 ( 1 ): 116 – 122 . [DOI] [PubMed] [Google Scholar]
- 3. Palma DA , Olson R , Harrow S , et al . Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET Phase II randomized trial . J Clin Oncol 2020. ; 38 ( 25 ): 2830 – 2838 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zayed S , Correa RJM , Palma DA . Radiation in the treatment of oligometastatic and oligoprogressive disease: Rationale, recent data, and research questions . Cancer J 2020. ; 26 ( 2 ): 156 – 165 . [DOI] [PubMed] [Google Scholar]
- 5. Ning MS , Gomez DR , Heymach JV , Swisher SG . Stereotactic ablative body radiation for oligometastatic and oligoprogressive disease . Transl Lung Cancer Res 2019. ; 8 ( 1 ): 97 – 106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knott EA , Ziemlewicz TJ , Lubner SJ , et al . Microwave ablation for colorectal cancer metastasis to the liver: a single-center retrospective analysis . J Gastrointest Oncol 2021. ; 12 ( 4 ): 1454 – 1469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Heinze C , Omari J , Manig M , et al . Efficacy and safety of percutaneous computed tomography-guided high-dose-rate interstitial brachytherapy in treatment of oligometastatic lymph node metastases of retroperitoneal space . J Contemp Brachytherapy 2019. ; 11 ( 5 ): 436 – 442 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bignardi M , Navarria P , Mancosu P , et al . Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases . Int J Radiat Oncol Biol Phys 2011. ; 81 ( 3 ): 831 – 838 . [DOI] [PubMed] [Google Scholar]
- 9. Luo CH , Zou B . Retroperitoneal Lymph Node Metastases . In: Luo CH , ed. Retroperitoneal Tumors . Springer; , 2018. ; 273 – 276 . [Google Scholar]
- 10. Burkoň P , Oberreiterová S , Kazda T , et al . Stereotactic body radiotherapy of lymph node oligometastases . Klin Onkol 2020. ; 33 ( 2 ): 114 – 122 . [DOI] [PubMed] [Google Scholar]
- 11. Yuce Sari S , Cengiz M , Dauletkazin A , et al . Hypofractionated radiotherapy for non-metastatic bone and soft tissue sarcomas . Cancer Radiother 2019. ; 23 ( 8 ): 853 – 859 . [DOI] [PubMed] [Google Scholar]
- 12. Ost P , Jereczek-Fossa BA , Van As N , et al . Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences . Clin Oncol (R Coll Radiol) 2016. ; 28 ( 9 ): e115 – e120 . [DOI] [PubMed] [Google Scholar]
- 13. Lubner MG , Brace CL , Ziemlewicz TJ , Hinshaw JL , Lee FT Jr . Microwave ablation of hepatic malignancy . Semin Intervent Radiol 2013. ; 30 ( 1 ): 56 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lubner MG , Brace CL , Hinshaw JL , Lee FT Jr . Microwave tumor ablation: mechanism of action, clinical results, and devices . J Vasc Interv Radiol 2010. ; 21 ( 8 Suppl ): S192 – S203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lencioni R , Cioni D , Crocetti L , et al . Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation . Radiology 2005. ; 234 ( 3 ): 961 – 967 . [DOI] [PubMed] [Google Scholar]
- 16. Mirza AN , Fornage BD , Sneige N , et al . Radiofrequency ablation of solid tumors . Cancer J 2001. ; 7 ( 2 ): 95 – 102 . [PubMed] [Google Scholar]
- 17. Sonntag PD , Hinshaw JL , Lubner MG , Brace CL , Lee FT Jr . Thermal ablation of lung tumors . Surg Oncol Clin N Am 2011. ; 20 ( 2 ): 369 – 387, ix . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao F , Gu Y , Huang J , Zhao M , Wu P . Radiofrequency ablation of retroperitoneal metastatic lymph nodes from hepatocellular carcinoma . Acad Radiol 2012. ; 19 ( 8 ): 1035 – 1040 . [DOI] [PubMed] [Google Scholar]
- 19. Littrup PJ , Bang HJ , Currier BP , et al . Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes . J Vasc Interv Radiol 2013. ; 24 ( 12 ): 1817 – 1825 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei Z , Ye X , Yang X , et al . The efficacy and safety of microwave ablation in patients with retroperitoneal metastases . Int J Hyperthermia 2018. ; 34 ( 7 ): 1053 – 1060 . [DOI] [PubMed] [Google Scholar]
- 21. Maciolek KA , Abel EJ , Best SL , et al . Percutaneous microwave ablation for local control of metastatic renal cell carcinoma . Abdom Radiol (NY) 2018. ; 43 ( 9 ): 2446 – 2454 . [DOI] [PubMed] [Google Scholar]
- 22. Denys A , Lachenal Y , Duran R , Chollet-Rivier M , Bize P . Use of high-frequency jet ventilation for percutaneous tumor ablation . Cardiovasc Intervent Radiol 2014. ; 37 ( 1 ): 140 – 146 . [DOI] [PubMed] [Google Scholar]
- 23. Patel SR , Hinshaw JL , Lubner MG , Lee FT Jr , Nakada SY , Hedican SP . Hydrodissection using an iodinated contrast medium during percutaneous renal cryoablation . J Endourol 2012. ; 26 ( 5 ): 463 – 466 . [DOI] [PubMed] [Google Scholar]
- 24. Ahmed M , Solbiati L , Brace CL , et al . Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update . Radiology 2014. ; 273 ( 1 ): 241 – 260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Puijk RS , Ahmed M , Adam A , et al . Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: Results of the SIO and DATECAN initiative . Radiology 2021. ; 301 ( 3 ): 533 – 540 . [DOI] [PubMed] [Google Scholar]
- 26. Khalilzadeh O , Baerlocher MO , Shyn PB , et al . Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee . J Vasc Interv Radiol 2017. ; 28 ( 10 ): 1432 – 1437.e3 . [Published correction appears in J Vasc Interv Radiol 2018;29(1):146.] [DOI] [PubMed] [Google Scholar]
- 27. Jereczek-Fossa BA , Piperno G , Ronchi S , et al . Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer . Am J Clin Oncol 2014. ; 37 ( 3 ): 227 – 233 . [DOI] [PubMed] [Google Scholar]
- 28. Hinshaw JL , Lubner MG , Ziemlewicz TJ , Lee FT Jr , Brace CL . Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? RadioGraphics 2014. ; 34 ( 5 ): 1344 – 1362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhardwaj N , Strickland AD , Ahmad F , Atanesyan L , West K , Lloyd DM . A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver . Pathology 2009. ; 41 ( 2 ): 168 – 172 . [DOI] [PubMed] [Google Scholar]
- 30. Maybody M , Soliman MM , Yamada Y , et al . Temporary organ displacement to escalate radiation dose to retroperitoneal tumors and decrease toxicity to organs at risk . J Vasc Interv Radiol 2020. ; 31 ( 10 ): 1578 – 1586 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reid J , Smith R , Borg M , et al . Feasibility of spacers to facilitate postoperative radiotherapy for retroperitoneal sarcomas . J Med Imaging Radiat Oncol 2017. ; 61 ( 6 ): 812 – 818 . [DOI] [PubMed] [Google Scholar]
- 32. Wittekind C . Diagnosis and staging of lymph node metastasis . In: Schlag PM , Veronesi U , eds. Lymphatic Metastasis and Sentinel Lymphonodectomy . Recent Results in Cancer Research , vol 157 . Springer; , 2000. ; 20 – 28 . [DOI] [PubMed] [Google Scholar]
- 33. Weichselbaum RR , Hellman S . Oligometastases revisited . Nat Rev Clin Oncol 2011. ; 8 ( 6 ): 378 – 382 . [DOI] [PubMed] [Google Scholar]
- 34. Dabestani S , Marconi L , Hofmann F , et al . Local treatments for metastases of renal cell carcinoma: a systematic review . Lancet Oncol 2014. ; 15 ( 12 ): e549 – e561 . [DOI] [PubMed] [Google Scholar]
- 35. Tang C , Msaouel P , Hara K , et al . Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial . Lancet Oncol 2021. ; 22 ( 12 ): 1732 – 1739 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russell CM , Lue K , Fisher J , et al . Oncological control associated with surgical resection of isolated retroperitoneal lymph node recurrence of renal cell carcinoma . BJU Int 2016. ; 117 ( 6B ): E60 – E66 . [DOI] [PubMed] [Google Scholar]
- 37. Couillard AB , Knott EA , Zlevor AM , et al . Microwave Ablation as Bridging to Liver Transplant for Patients with Hepatocellular Carcinoma: A Single-Center Retrospective Analysis . J Vasc Interv Radiol 2022. ; 33 ( 9 ): 1045 – 1053 . [DOI] [PubMed] [Google Scholar]
- 38. Agopian VG , Morshedi MM , McWilliams J , et al . Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients . Ann Surg 2015. ; 262 ( 3 ): 536 – 545 ; discussion 543–545. [DOI] [PubMed] [Google Scholar]
- 39. Fan W , Niu L , Wang Y , et al . Percutaneous computed tomography-guided cryoablation for recurrent retroperitoneal soft tissue sarcoma: a study of safety and efficacy . Oncotarget 2016. ; 7 ( 27 ): 42639 – 42649 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu M , Yao W , Zhang T , et al . Feasibility and efficacy of microwave ablation combined with iodine-125 seed implantation in local control of recurrent retroperitoneal liposarcomas: initial clinical experience . Oncologist 2017. ; 22 ( 12 ): 1500 – 1505 . [DOI] [PMC free article] [PubMed] [Google Scholar]