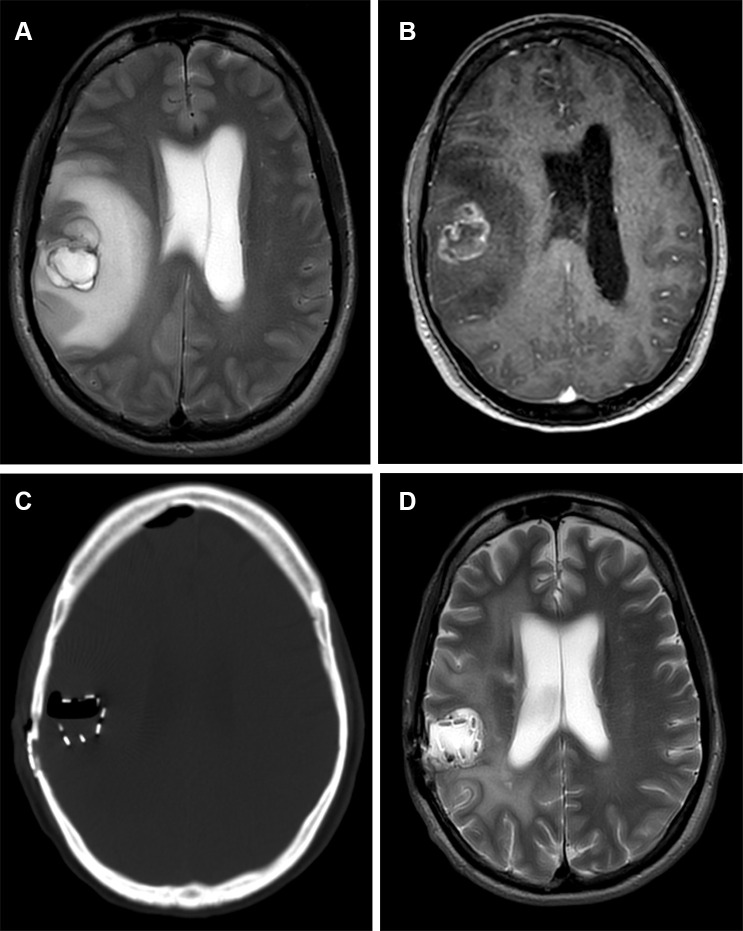
A 55-year-old female patient with IDH-wildtype right parietal glioblastoma underwent tumor resection with subsequent GammaTile (GT Medical Technologies) placement for brachytherapy (Figure).
Preoperative and postoperative images in a 55-year-old female patient with IDH-wildtype glioblastoma. (A) Axial T2-weighted preoperative image and (B) T1-weighted postcontrast image demonstrate a large necrotic mass with surrounding T2-weighted fluid-attenuated inversion recovery hyperintense signal abnormality in the right parietal lobe with mass effect causing leftward midline shift. (C) Axial postoperative bone window CT scan and (D) axial T2-weighted image show evenly spaced radiation seeds (GammaTiles) outlining the resection cavity.
GammaTile is a brachytherapy platform consisting of four titanium encapsulated cesium-131 seeds evenly spaced within a resorbable collagen tile matrix approximately the size of a postage stamp (2 × 2 × 0.4 cm) (1,2). It was recently approved by the U.S. Food and Drug Administration to treat new and malignant recurrent brain tumors, regardless of their histologic type. GammaTile is surgically implanted at the time of maximal safe tumor resection, and the radiation is emitted over approximately 6 weeks, reaching a target depth of 5–8 mm (1).
Due to the unique resorbable collagen housing, GammaTile therapy results in fewer adverse effects compared with traditional brachytherapy. Nonhoused brachytherapy seeds are subject to nontarget radiation and supratherapeutic dosing of immediately adjacent tissue. The collagen housing creates a fixed distance between target parenchyma and the cesium-131 seeds, as well as a fixed interseed distance to optimize uniform dose delivery. The bioresorbable matrix maintains its shape for approximately four half-lives, 3–4 months, adding structure to both the implant and resection cavity. This design approach suggests improved rates of radiation necrosis and nontarget radiation (3).
The increasing use of GammaTile requires radiologist familiarity for effective treatment-related communication. Complications and long-term appearances are not well documented, given its recent introduction. Continuous monitoring is crucial for understanding potential implant changes.
Footnotes
Authors declared no funding for this work.
Disclosures of conflicts of interest: D.C. No relevant relationships. J.H. No relevant relationships. M.A. No relevant relationships. N.M. No relevant relationships. R.M. No relevant relationships.
Keywords: Radiation Therapy, Neuro-Oncology, MR-Imaging, CT, Radiation Therapy/Oncology, CNS, Brain/Brain Stem, Neoplasms-Primary
References
- 1. Gessler DJ , Ferreira C , Dusenbery K , Chen CC . GammaTile®: Surgically targeted radiation therapy for glioblastomas . Future Oncol 2020. ; 16 ( 30 ): 2445 – 2455 . [DOI] [PubMed] [Google Scholar]
- 2. Ekhator C , Nwankwo I , Rak E , Homayoonfar A , Fonkem E , Rak R . GammaTile: Comprehensive Review of a Novel Radioactive Intraoperative Seed-Loading Device for the Treatment of Brain Tumors . Cureus 2022. ; 14 ( 10 ): e29970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Odia Y , Gutierrez AN , Kotecha R . Surgically targeted radiation therapy (STaRT) trials for brain neoplasms: A comprehensive review . Neuro Oncol 2022. ; 24 ( Suppl 6 ): S16 – S24 . [DOI] [PMC free article] [PubMed] [Google Scholar]