Key Points
Question
In patients with early-stage triple-negative breast cancer (TNBC) treated with locoregional therapy but without adjuvant or neoadjuvant chemotherapy, is a higher abundance of tumor-infiltrating lymphocytes (TIL) in breast cancer tissue associated with better survival?
Findings
In this retrospective analysis of 1966 participants with early-stage TNBC treated with locoregional therapy but without adjuvant or neoadjuvant chemotherapy, survival rates were 90% for patients with a TIL level of 50% or greater, compared with 72% for patients with a TIL level of less than 30% at 5-year follow-up.
Meaning
In patients with early-stage TNBC treated with locoregional therapy only, higher TIL levels in breast cancer tissue were associated with improved survival.
Abstract
Importance
The association of tumor-infiltrating lymphocyte (TIL) abundance in breast cancer tissue with cancer recurrence and death in patients with early-stage triple-negative breast cancer (TNBC) who are not treated with adjuvant or neoadjuvant chemotherapy is unclear.
Objective
To study the association of TIL abundance in breast cancer tissue with survival among patients with early-stage TNBC who were treated with locoregional therapy but no chemotherapy.
Design, Setting, and Participants
Retrospective pooled analysis of individual patient-level data from 13 participating centers in North America (Rochester, Minnesota; Vancouver, British Columbia, Canada), Europe (Paris, Lyon, and Villejuif, France; Amsterdam and Rotterdam, the Netherlands; Milan, Padova, and Genova, Italy; Gothenburg, Sweden), and Asia (Tokyo, Japan; Seoul, Korea), including 1966 participants diagnosed with TNBC between 1979 and 2017 (with follow-up until September 27, 2021) who received treatment with surgery with or without radiotherapy but no adjuvant or neoadjuvant chemotherapy.
Exposure
TIL abundance in breast tissue from resected primary tumors.
Main Outcomes and Measures
The primary outcome was invasive disease-free survival [iDFS]. Secondary outcomes were recurrence-free survival [RFS], survival free of distant recurrence [distant RFS, DRFS], and overall survival. Associations were assessed using a multivariable Cox model stratified by participating center.
Results
This study included 1966 patients with TNBC (median age, 56 years [IQR, 39-71]; 55% had stage I TNBC). The median TIL level was 15% (IQR, 5%-40%). Four-hundred seventeen (21%) had a TIL level of 50% or more (median age, 41 years [IQR, 36-63]), and 1300 (66%) had a TIL level of less than 30% (median age, 59 years [IQR, 41-72]). Five-year DRFS for stage I TNBC was 94% (95% CI, 91%-96%) for patients with a TIL level of 50% or more, compared with 78% (95% CI, 75%-80%) for those with a TIL level of less than 30%; 5-year overall survival was 95% (95% CI, 92%-97%) for patients with a TIL level of 50% or more, compared with 82% (95% CI, 79%-84%) for those with a TIL level of less than 30%. At a median follow-up of 18 years, and after adjusting for age, tumor size, nodal status, histological grade, and receipt of radiotherapy, each 10% higher TIL increment was associated independently with improved iDFS (hazard ratio [HR], 0.92 [0.89-0.94]), RFS (HR, 0.90 [0.87-0.92]), DRFS (HR, 0.87 [0.84-0.90]), and overall survival (0.88 [0.85-0.91]) (likelihood ratio test, P < 10e-6).
Conclusions and Relevance
In patients with early-stage TNBC who did not undergo adjuvant or neoadjuvant chemotherapy, breast cancer tissue with a higher abundance of TIL levels was associated with significantly better survival. These results suggest that breast tissue TIL abundance is a prognostic factor for patients with early-stage TNBC.
This study of patients with early-stage triple-negative breast cancer not treated with adjuvant or neoadjuvant chemotherapy analyzes the association between tumor-infiltrating lymphocyte levels, cancer recurrence, and survival.
Introduction
Patients with triple-negative breast cancer (TNBC) have higher recurrence and mortality rates following tumor resection compared with patients who have hormone receptor–positive or ERBB2 (formerly HER2)-positive breast cancer.1 Because of this, adjuvant or neoadjuvant chemotherapy is recommended for most patients with early-stage TNBC.2,3 The type and number of chemotherapy drugs are selected based on tumor size and the presence or absence of lymph node metastases. Biomarkers guiding optimal systemic treatment, while avoiding overtreatment, have not been identified. A prognostic marker associated with a lower risk of TNBC recurrence could help identify patients who can achieve high cure rates with less intensive therapy.
Tumor-infiltrating lymphocyte (TIL) levels are markers of an active antitumor immune response. Patients with early-stage TNBC and high TIL levels in breast cancer tissue have longer survival following adjuvant chemotherapy4 and higher rates of pathologic complete response following neoadjuvant chemotherapy, compared with those who have lower TIL levels.5 Studies of less than 500 patients have suggested that higher TIL levels are associated with improved survival, even without adjuvant or neoadjuvant chemotherapy.6,7,8 However, associations of TNBC TIL abundance with breast cancer recurrence and mortality rates among women who do not undergo chemotherapy remain unclear.6,7,8
This study evaluated the association of breast cancer TIL levels with survival among patients with early-stage TNBC treated with locoregional therapy who had no exposure to adjuvant or neoadjuvant chemotherapy.
Methods
Patients
We analyzed individual patient data from 1966 women with stages I to III TNBC who received locoregional therapy but no neoadjuvant or adjuvant chemotherapy at one of 13 centers in North America (Rochester, Minnesota; Vancouver, British Columbia, Canada), Europe (Paris, Lyon, and Villejuif, France; Amsterdam and Rotterdam, the Netherlands; Milan, Padova, and Genova, Italy; Gothenburg, Sweden) and Asia (Tokyo, Japan; Seoul, Korea) between January 1979 and November 2017. Final follow-up occurred on September 27, 2021. Participants included 1099 patients from 6 centers (Rochester, Minnesota; Paris and Villejuif, France; Amsterdam, the Netherlands; Milan, Italy; and Seoul, Korea) in 3 previously reported smaller studies,6,7,8 and previously unreported data from 867 patients from 7 additional centers (Vancouver, British Columbia, Canada; Lyon, France; Rotterdam, Netherlands; Padova and Genova, Italy; Gothenburg, Sweden; and Tokyo, Japan). This study was approved by the Gustave Roussy’s institutional review board (IRB), the French Committee for Data Protection, and local IRBs. Informed consent was waived by local IRBs given that this study was retrospective and noninterventional.
Data were collected by each institution using medical record review and included the following information: breast cancer diagnosis date; surgery type and date; age at diagnosis; menopausal status; tumor histology, grade, and size; number of involved axillary lymph nodes; estrogen receptor and progesterone receptor expression (reported as percent of tumor cell nuclei staining positive using immunohistochemistry); ERBB2 status; stromal TIL levels in the resected primary breast tumor (reported as percent); receipt of radiotherapy; last follow-up or death dates; overall survival status at last follow-up; and occurrence of locoregional and/or distant recurrence, contralateral breast cancer, or second malignancy.
TIL Levels and Pathological Analysis
TIL levels were measured in breast cancer tissue using hematoxylin and eosin–stained slides by pathologists trained in TIL assessment following the International Immuno-Oncology Biomarker Working Group guidelines,9 who were unaware of clinical outcomes. Briefly, on a resected breast tumor slide, the stromal area was identified (ie, area occupied by noncancer cells). TIL levels were quantified within the stromal area and reported as 0% to 100% of stromal tissue. The denominator used to determine the percent of stromal TIL levels was the area of stromal tissue (ie, area occupied by TIL levels within the total stromal area). Among 32 trained pathologists, this method had intraclass correlation coefficients between 0.81 and 0.93 for defining a TIL threshold of 30% and between 0.90 and 0.94 for defining a TIL threshold of 75% in a previously reported concordance analysis.10 Given that optimal TIL thresholds have not been defined, TIL levels were analyzed both as a continuous variable and according to prespecified TIL thresholds of less than 30% or greater than or equal to 30% andless than 50% or greater than or equal to 50% (Section VI: Statistical Methods in Supplement 1). In addition, we evaluated a TIL threshold of less than 75% or greater than or equal to 75% in a posthoc and exploratory manner.
Tumor size, grade, and histology were assessed at each center according to institutional guidelines. Anatomical stage was assessed according to the AJCC Staging Manual, 8th ed.11 Tumors negative for estrogen receptor and progesterone receptor were defined as having less than 1% of tumor cell nuclei staining positive using immunohistochemistry, except for 3 institutions that used a threshold of less than 10% (eTable 1A in Supplement 2). Only ERBB2–negative tumors were eligible (defined as immunohistochemistry 0, 1+, or 2+ and in situ hybridization–negative; eTable 1B in Supplement 2). For patients treated prior to routine clinical ERBB2 testing and for whom ERBB2 information was not available in the clinical record, ERBB2 expression was directly evaluated in archival tumor tissue and confirmed to be negative prior to inclusion in the study (Section 1: Study Population in Supplement 2).
Outcomes
The primary outcome was invasive disease-free survival (iDFS). Secondary outcomes included recurrence-free survival (RFS), distant disease-free survival (DDFS), distant relapse-free survival (DRFS) and overall survival (Sections 3A and 3B in Supplement 1). Outcomes followed the Standardized Definitions for Efficacy End Points (STEEP) criteria (version 2.0) in adjuvant breast cancer clinical trials (eTable 3A in Supplement 2).12 iDFS events include the occurrence of invasive local, regional, and distant recurrences; contralateral breast cancer; second nonbreast primary cancer; and death from any cause (excluding noninvasive in situ cancers). RFS events include the same events but exclude contralateral breast cancer or second nonbreast primary cancers. DDFS and DRFS events include distant recurrences and death from any cause, but DRFS excludes second nonbreast primary cancers. Overall survival includes death from any cause.
Statistical Analysis
Patients who did not experience one of the prespecified outcomes were censored at last follow-up.
Cox regression models, stratified by institution, were used to test the independent association of TIL levels (adjusted for age, tumor size, histologic grade, lymph node metastases, and radiotherapy) with all prespecified outcomes, using likelihood ratio tests. Adjusted hazard ratios (HRs [95% CIs]) and 2-sided P values were calculated, with P values of less than .05 being considered statistically significant. Schoenfeld residuals were plotted to investigate violation of the proportional-effects assumption in the Cox model (eFigure 11A, eFigure 11B, and eTable 11 in Supplement 2). Heterogeneity across institutions was evaluated using the Cochran Q test and the I2 statistic (<25% defined as low heterogeneity) (Section 6 in Supplement 1).
The Kaplan-Meier method was used to estimate survival probabilities, with 95%CIs calculated using a percentile bootstrap method. Pairwise correlation was performed between the variables of interest using Spearman rank correlation and Kendall τ rank correlation. Multiple imputation using a multilevel model was used to calculate adjustments for missing data. Discrimination of the multivariable models was evaluated by the area under the time-dependent receiver operating characteristic curve (area under the curve [AUC]) at 5 years for all survival outcomes (eTable 9A and eTable 9B in Supplement 2). Calibration plots were constructed using leave-one-study-out cross validation (eFigures 10A-10E in Supplement 2).13 Because patients were treated over 4 decades (1979-2017), differences were evaluated in the association of TIL levels with clinical outcomes before vs after 1998, when the Saint Gallen Consensus Conference first recommended adjuvant chemotherapy for node-negative TNBC.14 To evaluate the association of TIL levels with the occurrence of second primary cancers, distant recurrence, or death, a competing risk analysis was conducted using the prespecified thresholds of 30% and 50% TIL levels.
Analyses were performed using R software version 3.2.3.15
Results
Patient Characteristics
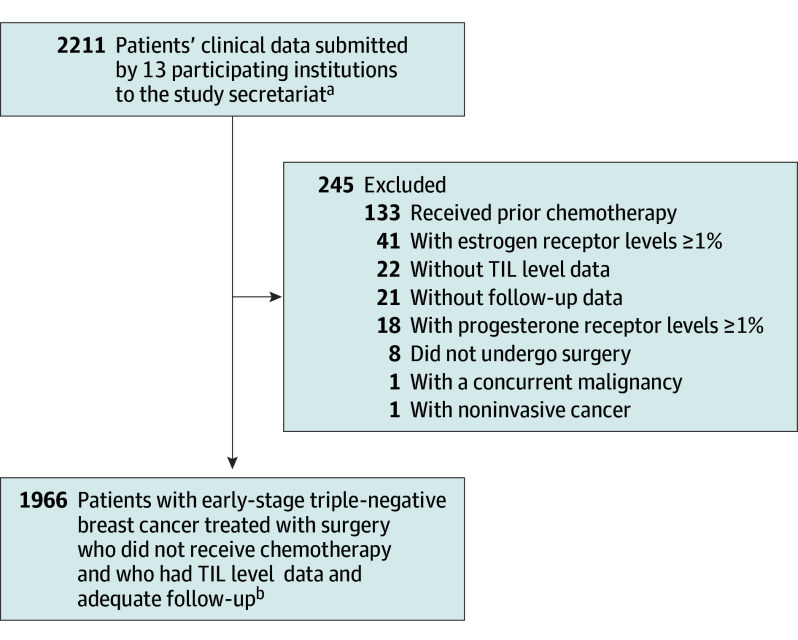
Data were obtained for 2211 patients. Of these, 1966 met eligibility criteria and were included in the analysis, with a median follow-up of 18 (95% CI, 15-20) years (Figure 1; eTable 1C in Supplement 2). A total of 173 (9%) were missing data on at least 1 variable (74 [4%] missing tumor size, 60 [3%] lymph node status, 29 [1%] tumor grade, and 12 [1%] radiotherapy history; eTables 3D-F and eFigures 3A-C in Supplement 2). The median age at diagnosis was 56 years (IQR, 39-71), and 41% were younger than 50 years. Most tumors were T1 (60%) or T2 (36%), node-negative (87%), and stage I (55%). Among patients with stage I TNBC, 65% had T1cN0 tumors. Sixty-one percent had breast-conserving surgery and radiotherapy (Table; eTable 1C and eFigures 1A-E in Supplement 2).
Figure 1. Flow of Patients.
aPatients were women with triple-negative breast cancer who received locoregional therapy but no chemotherapy from 13 participating institutions. Data were submitted from the 13 participating centers, which were located in North America (Rochester, Minnesota, USA; Vancouver, British Columbia, Canada), Europe (Paris, Lyon, and Villejuif, France; Amsterdam and Rotterdam, the Netherlands; Milan, Padova, and Genova, Italy; Gothenburg, Sweden), and Asia (Tokyo, Japan; Seoul, Korea).
bNumber of cases included for each tumor-infiltrating lymphocyte (TIL) level: less than 30%, 1300 (66%); 30% to 49%, 249 (13%); 50% to 74%, 249 (13%); and 75% or greater, 168 (9%).
Table. Characteristics of the Study Population at Baseline.
| Characteristics | Stromal TIL percentage | |||
|---|---|---|---|---|
| <30 (n = 1300) | 30-49 (n = 249) | 50-74 (n = 249) | ≥75 (n = 168) | |
| Age, median (IQR), y | 59 (41-72) | 57 (43-70) | 48 (37-68) | 38 (35-55) |
| Age <50 y, No. (%) | 467 (36) | 84 (34) | 130 (52) | 119 (71) |
| Premenopausal, No./total No. (%) | 198/929 (21) | 51/192 (27) | 39/139 (28) | 20/55 (36) |
| Tumor size, cm | ||||
| Median (IQR) | 2.0 (1.2-2.5) | 2 (1.2-2.8) | 2 (1.3-3.0) | 1.8 (1.3-2.3) |
| Mean (SD) | 2.1 (1.6) | 2.2 (1.4) | 2.4 (1.6) | 1.9 (1.1) |
| Pathologic tumor size, No./total No. (%)a | ||||
| <2 cm (pT1) | 782/1278 (61) | 142/249 (57) | 126/247 (51) | 109/166 (66) |
| 2-5 cm (pT2) | 448/1278 (35) | 97/249 (39) | 105/247 (43) | 55/166 (33) |
| >5 cm (pT3) or any size with chest wall or skin extension (pT4)b | 48/1278 (4) | 10/249 (4) | 16/247 (7) | 2/166 (1) |
| ALN metastases, No./total No. (%)c | ||||
| None (pN0) | 1075/1252 (86) | 205/243 (84) | 211/244 (87) | 160/167 (96) |
| 1-3 ALN (pN1) | 117/1252 (9) | 24/243 (10) | 22/244 (9) | 3/167 (2) |
| 4-9 ALN (pN2) | 41/1252 (3) | 7/243 (3) | 10/244 (4) | 4/167 (2) |
| ≥10 ALN (pN3) | 19/1252 (2) | 7/243 (3) | 1/244 (0.4) | 0/167 (0) |
| AJCC anatomic stage, No./total No. (%)d | ||||
| I | 689/1230 (56) | 124/243 (51) | 114/242 (47) | 107/165 (65) |
| II | 470/1230 (38) | 105/243 (43) | 114/242 (47) | 54/165 (33) |
| III | 71/1230 (6) | 14/243 (6) | 14/242 (6) | 4/165 (2) |
| pT1 N0 subgroups, No./total No. (% of stage I) | ||||
| pT1mi N0 tumor, ≤0.1 cm | 21/675 (3) | 3/122 (3) | 9/108 (8) | 0/102 (0) |
| pT1a N0 tumor, 0.2-0.5 cm | 66/675 (10) | 16/122 (13) | 17/108 (16) | 12/102 (12) |
| pT1b N0 tumor, 0.6-1.0 cm | 146/675 (22) | 31/122 (25) | 19/108 (18) | 18/102 (18) |
| pT1c N0 tumor, 1.1-2.0 cm | 442/675 (65) | 72/122 (59) | 63/108 (58) | 72/102 (71) |
| Histological subtype, No./total No. (%) | ||||
| Invasive breast carcinomae | 937/1225 (77) | 186/239 (78) | 186/240 (78) | 118/166 (71) |
| Medullary | 32/1225 (3) | 21/239 (9) | 30/240 (13) | 37/166 (22) |
| Lobular carcinomaf | 35/1225 (3) | 2/239 (1) | 2/240 (1) | 2/166 (1) |
| Metaplastic carcinomaf | 45/1225 (4) | 3/239 (1) | 10/240 (4) | 4/166 (2) |
| Mucinous adenocarcinoma | 8/1225 (0.7) | 0/239 | 1/240 (0.4) | 0/166 |
| Tubular carcinoma | 2/1225 (0.2) | 1/239 (0.4) | 0/240 | 0/166 |
| Otherg | 166/1225 (14) | 26/239 (11) | 11/240 (5) | 5/166 (3) |
| Histological grade, No./total No. (%)h | ||||
| Low | 85/1285 (7) | 7/242 (3) | 2/245 (1) | 1/165 (1) |
| Intermediate | 384/1285 (30) | 44/242 (18) | 30/245 (12) | 15/165 (9) |
| High | 816/1285 (64) | 191/242 (79) | 213/245 (87) | 149/165 (90) |
| Stromal TIL levels, %i | ||||
| TIL level, median (IQR), % | 5 (4-15) | 35 (30-40) | 60 (55-70) | 85 (80-90) |
| TIL level, mean (SD), % | 9 (7) | 36 (6) | 61 (7) | 85 (7) |
| Primary surgery, No./total No. (%) | ||||
| Breast conservation | 789/1297 (61) | 147/249 (59) | 141/248 (57) | 124/165 (75) |
| Mastectomy | 508/1297 (39) | 102/249 (41) | 107/248 (43) | 41/165 (25) |
| Adjuvant radiotherapy | 793/1292 (61) | 146/248 (59) | 148/246 (60) | 124/168 (74) |
Abbreviations: AJCC, American Joint Committee on Cancer; ALN, axillary lymph node; TIL, tumor-infiltrating lymphocyte.
Refers to tumor size on surgical specimen.
Data for pT3 and pT4 were combined into a single category given that only tumor size information was available. Information on characteristics defining T4 tumors (extension to chest wall, ulceration, skin nodules, inflammatory changes) was not available.
Pathologic nodal stage refers to the number of lymph nodes affected by metastases.
Categories indicate AJCC 8th edition anatomic stages: I, less advanced disease with a pT1 tumor and no lymph node involvement (pN0) or microscopic lymph node involvement only; II, more advanced disease with either macroscopic lymph node involvement in 1 to 3 lymph nodes (pN1) or a larger tumor (pT2-3 without nodal involvement); and III, more extensive nodal involvement (N2-3) or a T4 tumor regardless of extent of nodal involvement.
Indicates no special type of invasive breast carcinoma.
Indicates subtype category was not otherwise specified.
Other histologic subtypes included apocrine carcinoma, papillary carcinoma, anaplastic carcinoma, neuroendocrine carcinoma, secretory carcinoma, and mixed ductal and lobular carcinoma.
Histological grade describes the degree of tumor differentiation, with grade 1 tumors being well-differentiated, grade 2 tumors being moderately differentiated, and grade 3 tumors being poorly differentiated. In general, the higher the grade, the more aggressive the biology of the tumor.
Stromal TIL levels refer to the percentage of the tumor stromal area occupied by mononuclear inflammatory cells in breast tumor tissue (reported as 0%-100%), as assessed using hematoxylin and eosin–stained tissue sections according to the International Immuno-Oncology Biomarker Working Group Guidelines.
Stromal TIL Levels
Overall, the median percentage of TIL levels was 15% (IQR, 5-40). Thirty-four percent had TIL levels of 30% or greater, and 21% had TIL levels of 50% or greater (Table; eTable 2A, eFigure 2A, and eFigure 2B in Supplement 2). TIL distribution showed no significant heterogeneity across institutions (eFigure 2B and eTable 2A in Supplement 2). Higher TIL levels were associated with younger age (median age, 59 [IQR, 41-72] years for patients with TIL levels less than 30%; median age, 41 [IQR, 36-63] years for patients with TIL levels of 50% or greater; Table) and higher tumor grade (P ≤ 10−6) but not with tumor size or number of involved lymph nodes (eTable 2B, and eFigure 2B, and eFigure 2C in Supplement 2). For patients aged 50 years or older, 4% had TIL levels of 75% or greater (Figure 2).
Figure 2. Clinical Outcomes According to TIL Thresholds in the Overall Study Population and According to Stage.
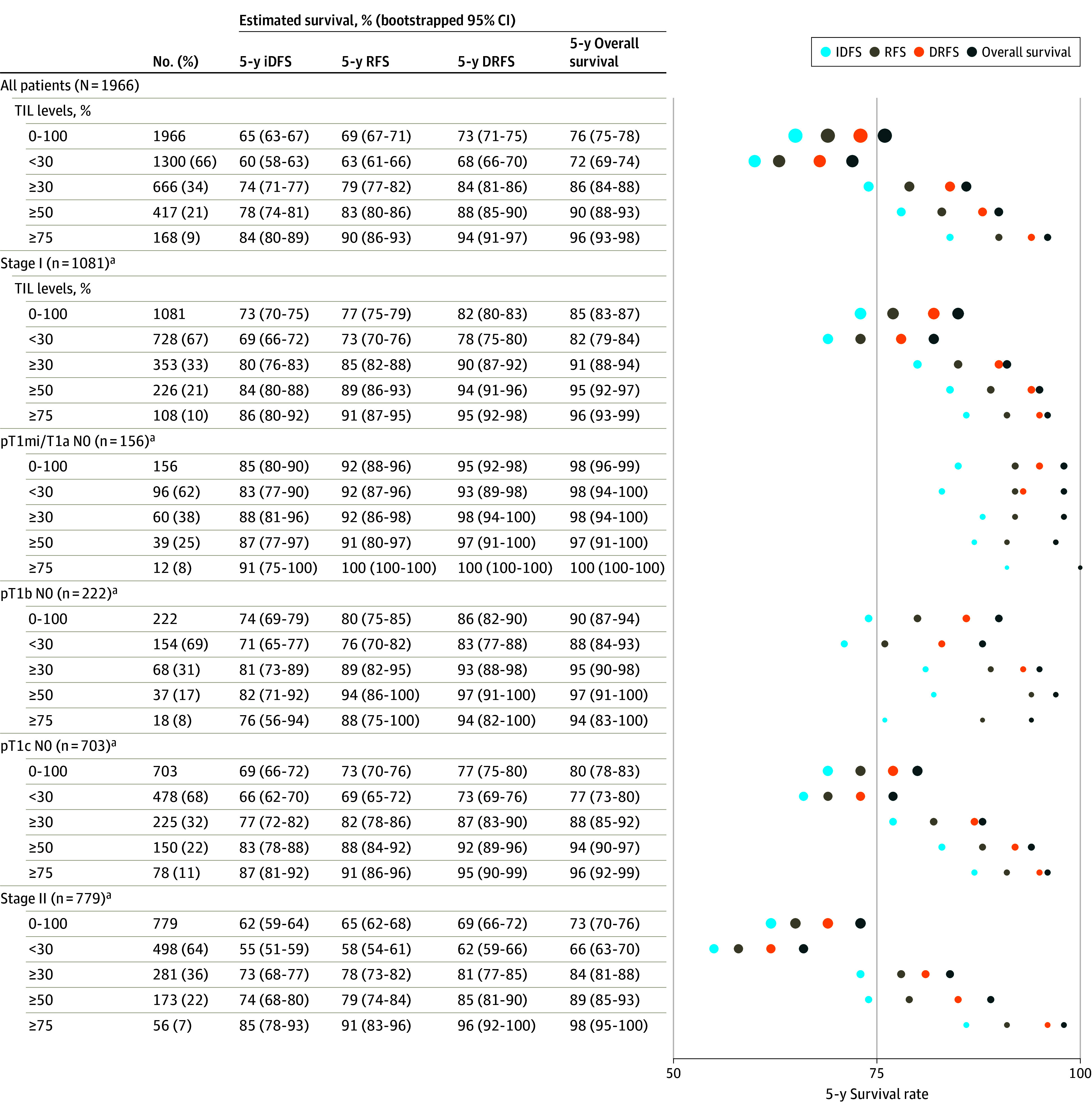
aIndicates sample sizes after multilevel multiple imputation for missing variables, as described in Methods.
Right panel illustrates point estimates for each survival end point in all patients, according to stage and tumor-infiltrating lymphocyte (TIL) level. Area of the circles is proportional to the sample size for each TIL subgroup. iDFS indicates invasive disease-free survival; RFS, recurrence-free survival; and DRFS, survival free of distant recurrence.
Follow-Up and Outcomes
The number of events for each end point were as follows: for iDFS, 1074 (55%); RFS, 940 (48%); DDFS, 894 (46%); DRFS, 832 (42%) (eTable 3B in Supplement 2); and deaths, 803 (41%) (eTable 3C in Supplement 2). One-hundred forty (13%) of 1074 iDFS events consisted of a new contralateral breast cancer, and 110 (10%) consisted of a second nonbreast primary malignancy. Survival rates according to TIL levels are shown in Figure 2, Figure 3, and eFigures 5A-R, and eTable 5A in Supplement 2. In a multivariable Cox model including age, tumor size, nodal status, histological grade, and receipt of radiotherapy, higher TIL levels were associated with improved iDFS, RFS, DDFS, DRFS, and overall survival (all likelihood ratio P values <10−6; eTables 4A-F in Supplement 2). Each 10%-higher TIL level was associated with an 8% lower risk of an iDFS event (HR, 0.92 [95% CI, 0.89-0.94]), a 10% lower risk of an RFS event (HR, 0.90 [95% CI, 0.87-0.92]), a 13% lower risk of a DDFS event (HR, 0.87 [95% CI, 0.85-0.90]), a 13% lower risk of a DRFS event (HR, 0.87 [95% CI, 0.84-0.90]), and a 12% lower risk of death (HR, 0.88 [95% CI, 0.85-0.91]).
Figure 3. Cumulative Rates of Cancer and Mortality Events for the Entire Population According to Prespecified TIL Thresholds.
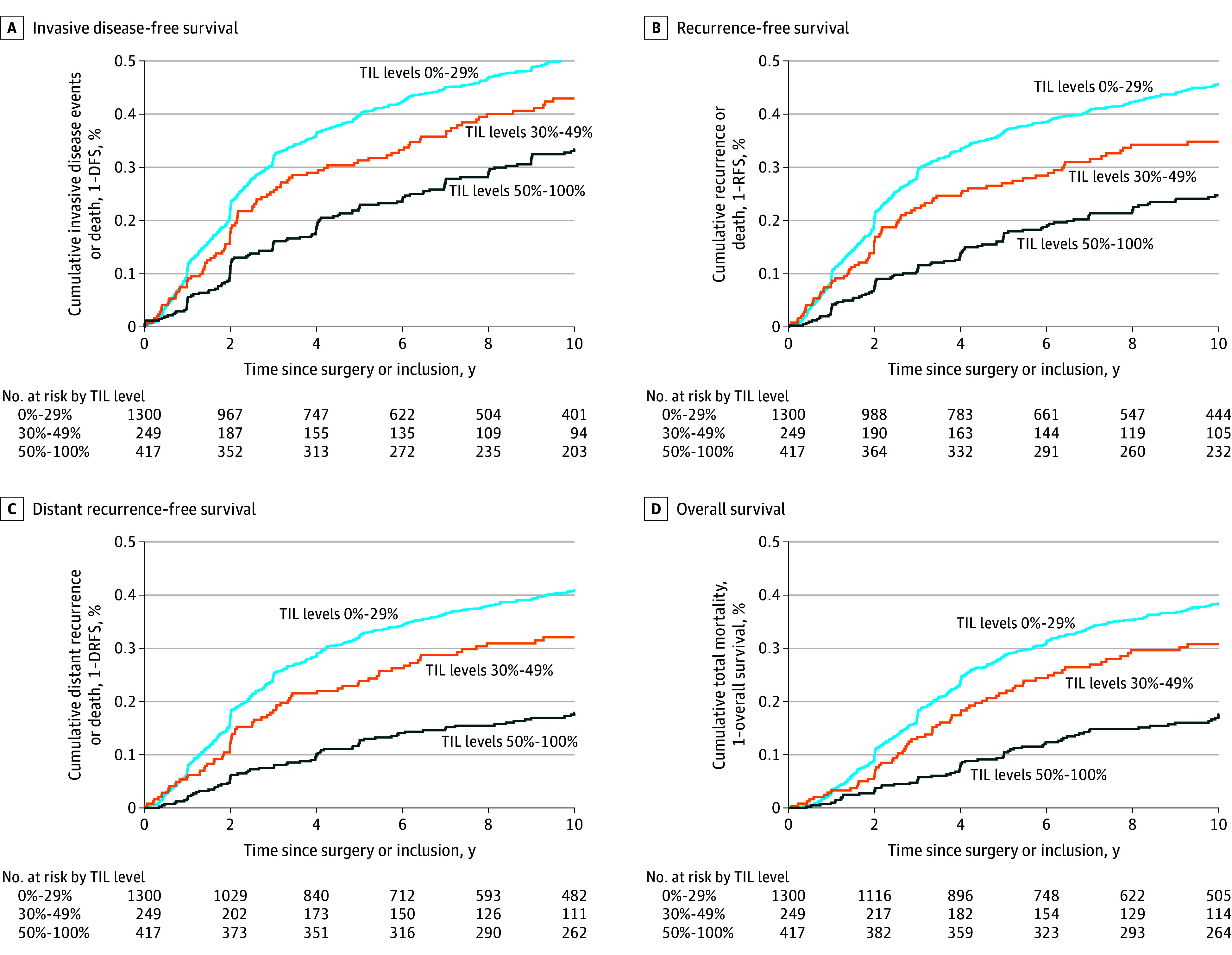
The median was reached at 9.71 years (95% CI, 8.16-11.2) years. TIL indicates tumor-infiltrating leukocyte; DFS, disease-free survival; RFS, recurrence-free survival; and DRFS, survival free of distant recurrence.
Competing risk analyses display the cumulative incidence of second primary (nonbreast) cancers, distant relapse, or death according to TIL levels (eTables 8A-E and eFigures 8A-D in Supplement 2). At 10 years, patients with a greater abundance of TILs had a lower cumulative incidence of distant relapse or death (21% [95% CI, 17%-24%] for TIL levels ≥30% compared with 38% [95% CI 35%-41%] for TIL levels <30% [eFigure 8A in Supplement 2]; and 16% [95% CI, 12%-20%] for TIL levels ≥50% compared with 37% [95% CI, 34%-40%] for TIL levels <50%) [eFigure 8B in Supplement 2]). The 10-year cumulative incidence of second primary (nonbreast) cancers was 3.7% (95% CI, 2.3%-5.6%) for TIL levels ≥30% compared with 7.3% (95% CI, 5.6%-9.2%) for TIL levels <30% [eFigure 8A in Supplement 2]; and 2.8% (95% CI, 1.5%-5.0%) for TIL levels ≥50% compared with 7.0% (95% CI, 5.6%-8.8%) for TIL levels <50% [eFigure 8B in Supplement 2]. Similar results were observed in patients who did not have lymph node involvement with breast cancer (eFigure 8C in Supplement 2).
TIL Levels and Outcomes in Node-Negative and Stage I TNBC
Overall, patients with stage I TNBC had a 5-year RFS of 77% (95% CI, 75%-79%), DRFS of 82% (95% CI, 80%-83%), and overall survival of 85% (95% CI, 83%-87%) (Figure 2). Among stage I patients with TIL levels of 50% or greater, 5-year RFS was 89% (95% CI, 86%-93%), DRFS was 94% (95% CI, 91%-96%), and overall survival was 95% (95% CI, 92%-97%); while for patients with TIL levels of less than 30%, 5-year RFS was 73% (95% CI, 70%-76%), DRFS was 78% (95% CI, 75%-80%), and overall survival was 82% (95% CI, 79%-84%) (Figure 2; eFigures 5S-X, and eTable 7A and eTable 7B in Supplement 2). Cumulative rates of cancer and mortality events are shown in Figure 4.
Figure 4. Cumulative Rates of Cancer and Mortality Events for the Subset of Patients With Stage I Triple-Negative Breast Cancer According to Prespecified TIL Thresholds.
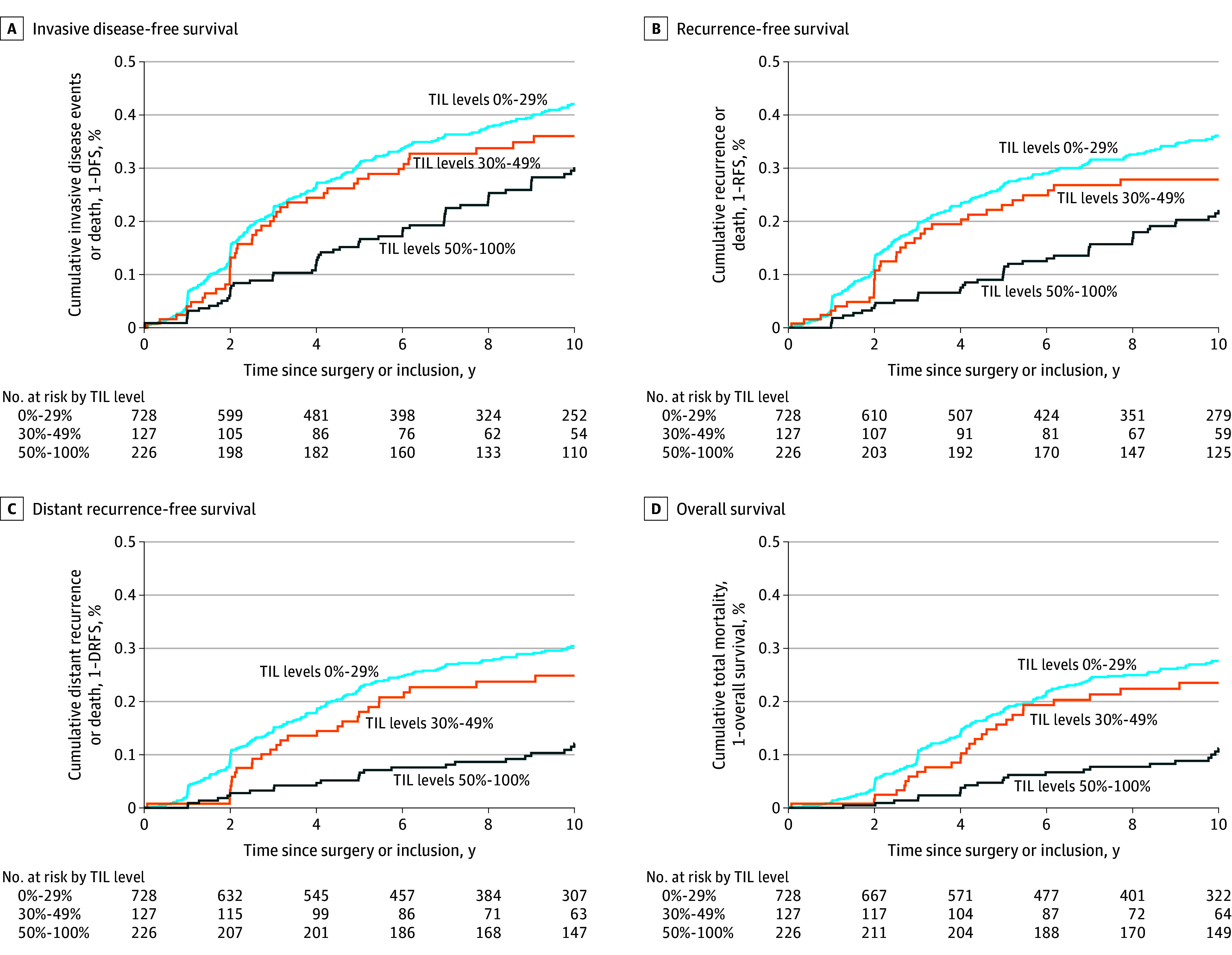
TIL indicates tumor-infiltrating leukocyte; DFS, disease-free survival; RFS, recurrence-free survival; and DRFS, survival free of distant recurrence.
TIL Levels and Outcomes According to Institution and Inclusion Period
The distribution of tumor size, nodal status, and stage did not differ according to institution (eFigures 1A-E in Supplement 2). Cochran Q test values were associated with a P value of greater than .05, and I2 values were 0 to 22 for comparisons by institution or inclusion year for all outcomes (section 6 in Supplement 1). Patients with higher TIL levels had significantly better outcomes than those with lower TIL levels, regardless of inclusion period (1999-2017 vs 1979-1988). However, in a multivariable model adjusted for clinicopathological factors and TIL levels but not stratified by institution, there was a significant effect of inclusion period on outcomes, with those treated between 1999 and 2017 exhibiting more favorable outcomes for RFS (HR, 0.67 [95% CI, 0.55-0.80]; P < 10−4) and for overall survival (HR, 0.54 [95% CI, 0.44-0.66]; P < 10−6). For patients with TIL levels of 50% or greater between 1999 and 2017, the 5-year RFS was 81% (95% CI, 74-87), and overall survival was 89% (95% CI, 84-94), compared with RFS of 84% (95% CI, 81-88) and overall survival of 91% (95% CI, 88-94) between 1979 and 1998. For patients with TNBC and TIL levels of less than 30% between 1999 and 2017, the 5-year RFS was 69% (95% CI, 65-72), and overall survival was 77% (95% CI, 74-80), compared with RFS of 60% (95% CI, 57-63) and overall survival of 68% (95% CI, 65-71) between 1979 and 1998. Rates for other survival outcomes and additional TIL thresholds are reported in eTable 12, eFigure 12A, and eFigure 12B in Supplement 2.
Incorporating TIL levels to the clinicopathological model improved the AUC at 5 and 10 years for all outcomes. At 10 years, incorporation of TIL levels improved the AUC for iDFS (from 0.64[0.62-0.67] to 0.68[0.65-0.71]), for RFS (from 0.68[0.65-0.71] to 0.72[0.69-0.74]), for DRFS (from 0.71[0.68-0.74] to 0.75[0.72-0.78]), and for overall survival (from 0.71[0.68-0.74] to 0.75[0.72-0.78]) (Figure 4; eTable 9A, eTable 9B, and eFigures 10A-E in Supplement 2).
Discussion
Among 1966 TNBC patients who did not receive adjuvant/neoadjuvant chemotherapy, higher breast tissue TIL levels were associated with improved clinical outcomes (iDFS, RFS, DRFS, DDFS, and overall survival) at a median follow-up of 18 years, independent of age, tumor size, nodal status, and histologic grade. While the association of TIL levels with clinical outcomes in early-stage TNBC has been previously reported,4,5,16 in most studies, patients were exposed to chemotherapy, making it challenging to assess whether TIL levels were independently associated with prognosis or simply associated with greater chemotherapy responsiveness. This study adds to prior evidence6,7,8 by demonstrating that higher TIL levels were associated with better survival in patients with TNBC who had no exposure to adjuvant/neoadjuvant chemotherapy.
Current guidelines recommend considering adjuvant/neoadjuvant multiagent chemotherapy for T1b-cN0 TNBC, and omitting chemotherapy for T1aN0 TNBC.2,3 In this study, the survival of patients with T1b-cN0 TNBC differed according to TIL levels. Analysis from this study has shown that using TIL levels of greater than or equal to 50% identified patients with 5-year RFS, DRFS, and overall survival rates approaching or exceeding 90%, even without chemotherapy. These data suggest that current multiagent chemotherapy regimens may have a limited effect on further reducing recurrence and mortality in this population. However, further study is needed. Similar to the approach of using genomic profiles to identify patients with hormone receptor–positive breast cancer who can avoid chemotherapy,17 future studies may investigate whether patients with T1b-cN0 TNBC and high TIL levels may achieve high cure rates with less intensive chemotherapy regimens than currently recommended. For patients with T1mi and T1aN0 TNBC, 5-year RFS, DRFS and overall survival exceeded 90% regardless of TIL infiltration, findings that support current recommendations to omit chemotherapy for these patients.2,3
While the primary end point of this study was iDFS, this end point included new contralateral breast cancers and death from other causes. Some of the outcomes included in iDFS do not reflect a return of the initial breast cancer but rather newly formed tumors, and their development would not be affected by the administration (or not) of chemotherapy for a preceding malignancy.12 Perioperative chemotherapy aims to eradicate microscopic residual cancer cells that may persist after surgery and for which regrowth would lead to malignancy recurrence. Patients with previously cured TNBC may still be at risk of developing new contralateral breast cancers (newly transformed from healthy contralateral breast cells, rather than regrowth of the original malignancy) or subsequent nonbreast primary tumors (new cancers arising in nonbreast organs, rather than regrowth or spread of the original breast cancer), particularly in the context of cancer predisposition gene variants. In data reported here, 23% of the first iDFS events were events other than breast cancer recurrence. For example, 140 (13%) were new contralateral invasive breast cancers, and 110 (10%) were second nonbreast primary tumors. Because some patients were diagnosed as early as 1979, germline breast and ovarian cancer predisposition gene variants (eg, BRCA variants) may not have been diagnosed, and prophylactic breast and/or ovarian surgery may not have been performed. This could account for the high rate of contralateral breast cancers and subsequent nonbreast primary cancers observed in this cohort. End points that exclude events that do not represent a recurrence of the original cancer should be considered in future studies to accurately measure the effect of using less intensive chemotherapy on the risk of breast cancer recurrences.12
Consistent with other studies,4,8 younger age was associated with higher TIL levels. Despite this, TIL levels remained independently associated with survival in a multivariable model adjusting for age. Lower TIL levels in older patients may relate to age-dependent changes in immune phenotype and function,18 and/or from greater TIL infiltration observed in high-grade tumors , which are more common in younger patients.4,7,19 Additional investigation to improve understanding of the interactions between age, immunity, and clinical outcomes is needed.
Breast cancer biomarker guidelines (eg, European Society for Medical Oncology Early Breast Cancer guidelines3 and the World Health Organization/International Agency for Research on Cancer Anatomic Pathology Breast Cancer Classification20) recommend reporting TIL levels as part of pathology reports. Currently, College of American Pathologists guidelines do not recommend reporting TIL levels. TIL measurement is inexpensive and requires only visual assessment by a trained pathologist using a routine hematoxylin and eosin–stained slide (the same slide used to diagnose breast cancer and describe pathologic features). TIL levels could be measured in low-resource settings, as they require minimal time and are inexpensive to perform. However, assessment reproducibility and lack of standardization are barriers to widespread clinical implementation. To overcome this, the International Immuno-Oncology Working Group has led efforts to standardize TIL assessment, train pathologists worldwide, and demonstrate their analytical validity, reproducibility, and concordance.10 Future clinical trials should consider including TIL levels as a biomarker to help evaluate whether less toxic chemotherapy could replace current multiagent chemotherapy regimens for early-stage TNBC and high TIL levels.
Our study had several strengths. First, this study had relatively low levels of missing data and a long duration of follow-up. Second, this study included 13 international participating centers. Third, this study used standardized TIL assessment following the International Immuno-Oncology Working Group guidelines,9 with pathologists who were unaware of clinical outcomes.
Limitations
This study has several limitations. First, analyses were retrospective and require prospective validation. Second, because data are observational, no causal inferences can be made. Third, younger age was strongly associated with higher TIL levels, and residual and unmeasured confounding cannot be excluded. Fourth, lack of central TIL review may have introduced interobserver variability. Fifth, lack of germline cancer predisposition mutation data may have influenced the frequency of risk-reducing prophylactic surgeries, which could have affected the risk of second breast and nonbreast primary tumors. Sixth, data from patients treated nearly 45 years ago may be less relevant to clinical practice today. Seventh, data were not available for race or ethnicity. Eighth, the association of TIL levels with prognosis was linear, and the prespecified thresholds were selected arbitrarily. Ninth, prospective evaluation in clinical trials is necessary before TIL quantification can be used to guide decision-making about adjuvant/neoadjuvant chemotherapy.
Conclusion
In patients with early-stage TNBC who did not undergo adjuvant or neoadjuvant chemotherapy, breast cancer tissue with a higher abundance of TIL levels was associated with significantly better survival. These results suggest that breast tissue TIL abundance is a prognostic factor for patients with early-stage TNBC.
Trial Protocol
eTables and eFigures
Data Sharing Statement
References
- 1.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463-5472. doi: 10.1002/cncr.27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gradishar WJ, Moran MS, Abraham J, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691-722. doi: 10.6004/jnccn.2022.0030 [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Kyriakides S, Ohno S, et al. ; ESMO Guidelines Committee . Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194-1220. doi: 10.1093/annonc/mdz173 [DOI] [PubMed] [Google Scholar]
- 4.Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559-569. doi: 10.1200/JCO.18.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40-50. doi: 10.1016/S1470-2045(17)30904-X [DOI] [PubMed] [Google Scholar]
- 6.Leon-Ferre RA, Polley MY, Liu H, et al. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2018;167(1):89-99. doi: 10.1007/s10549-017-4499-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JH, Jonas SF, Bataillon G, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30(12):1941-1949. doi: 10.1093/annonc/mdz395 [DOI] [PubMed] [Google Scholar]
- 8.de Jong VMT, Wang Y, Ter Hoeve ND, et al. Prognostic value of stromal tumor-infiltrating lymphocytes in young, node-negative, triple-negative breast cancer patients who did not receive (neo)adjuvant systemic therapy. J Clin Oncol. 2022;40(21):2361-2374. doi: 10.1200/JCO.21.01536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salgado R, Denkert C, Demaria S, et al. ; International TILs Working Group 2014 . The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259-271. doi: 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kos Z, Roblin E, Kim RS, et al. ; International Immuno-Oncology Biomarker Working Group . Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer. 2020;6(1):17. doi: 10.1038/s41523-020-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin MB, Edge SB, Greene FL, et alet al. AJCC Cancer Staging Manual. 8th ed. Springer; 2017. [Google Scholar]
- 12.Tolaney SM, Garrett-Mayer E, White J, et al. Updated standardized definitions for efficacy end points (STEEP) in adjuvant breast cancer clinical trials: STEEP version 2.0. J Clin Oncol. 2021;39(24):2720-2731. doi: 10.1200/JCO.20.03613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uno H, Cai T, Tian L, Wei LJ. Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc. 2007;102(478):527-537. doi: 10.1198/016214507000000149 [DOI] [Google Scholar]
- 14.Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst. 1998;90(21):1601-1608. doi: 10.1093/jnci/90.21.1601 [DOI] [PubMed] [Google Scholar]
- 15.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 16.Sharma P, Stecklein SR, Yoder R, et al. Clinical and biomarker results of neoadjuvant phase II study of pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer (TNBC)(NeoPACT). Am J Clin Oncol. 2022;40(suppl 16). 513. doi: 10.1200/JCO.2022.40.16_suppl.513 [DOI] [Google Scholar]
- 17.Andre F, Ismaila N, Allison KH, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(16):1816-1837. doi: 10.1200/JCO.22.00069 [DOI] [PubMed] [Google Scholar]
- 18.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20(2):89-106. doi: 10.1038/s41568-019-0222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Boo L, Cimino-Mathews A, Lubeck Y, et al. Tumour-infiltrating lymphocytes (TILs) and BRCA-like status in stage III breast cancer patients randomised to adjuvant intensified platinum-based chemotherapy versus conventional chemotherapy. Eur J Cancer. 2020;127:240-250. doi: 10.1016/j.ejca.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 20.Tan PH, Ellis I, Allison K, et al. ; WHO Classification of Tumours Editorial Board . The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181-185. doi: 10.1111/his.14091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTables and eFigures
Data Sharing Statement