Highlights
-
•
Secondary amenorrhea should be evaluated with SLCT on the differential.
-
•
Indications for familial genetic testing include individuals with an associated neoplasm or a family history of DICER1 mutation with any childhood tumor.
-
•
Surveillance recommendations can be considered in individuals identified to have a DICER1 germline mutation.
Abstract
Sertoli-Leydig cell tumors (SLCT) are a rare form of sex cord stromal tumors. DICER1 germline mutations have been identified in a portion of these cases. We report a 15-year-old individual who presented to a well-child visit with secondary amenorrhea and subjective observations of a deepening voice and broadening shoulders. Elevations were noted in serum testosterone, inhibin B, androstenedione, and DHEA. Pelvic ultrasound and magnetic resonance imaging (MRI) revealed a left ovarian complex lesion measuring 5.8 x 5.5 x 4.6 cm. A laparoscopic unilateral salpingo-oophorectomy was performed with negative pelvic washings and a diagnosis of stage 1A, poorly differentiated/grade 3 SLCT of the ovary. Somatic and germline testing both demonstrated DICER1 pathologic variations. Adjuvant chemotherapy with cisplatin/etoposide/ifosfamide (PEI) was completed under the care of pediatric oncology, and this patient is now undergoing surveillance with no signs of recurrence. DICER1 Syndrome is associated with multiple tumors, including SLCT, pleuropulmonary blastoma (PPB), cystic sarcomas, and Wilms tumor among others. Patients with SLCT found to have a DICER1 mutation should undergo genetic testing and cancer screening, which may help to identify neoplasms associated with the DICER1 mutation at an early stage. This case will serve as a useful addition to the literature and review suggested pre-operative, operative, and surveillance guidelines.
1. Introduction
Sertoli-Leydig cell tumors (SLCT) are mixed sex cord-stromal tumors that comprise < 0.2% of ovarian neoplasms and typically present in the first two decades of life. Approximately 50% are associated with DICER1 mutations (Haley et al., 2019); (Schultz et al., 2017); (Seidler et al., 2020). DICER1 syndrome is an autosomal dominant pleiotropic tumor predisposition syndrome (Haley et al., 2019); (Schultz et al., 2017); (Schultz et al., 2017); (Stewart et al., 2019). We report a case of a SLCT in a patient who was subsequently identified to have a germline DICER1 mutation.
2. Case
A 15-year-old individual presented to her primary care provider for a well-child visit and reported a history consistent with secondary amenorrhea. She experienced menarche at the age of 10 years and she initially had irregular cycles, however she experienced complete cycle cessation several years prior to this visit. Upon further questioning, her adoptive mother noted that her voice had recently deepened and her shoulders had broadened.
Her past medical history and surgical history were non-contributory. She took no medications. Her family history was limited as she was adopted along with her biological sister. The only known oncologic history was her maternal uncle who died at age 36 of pancreatic cancer.
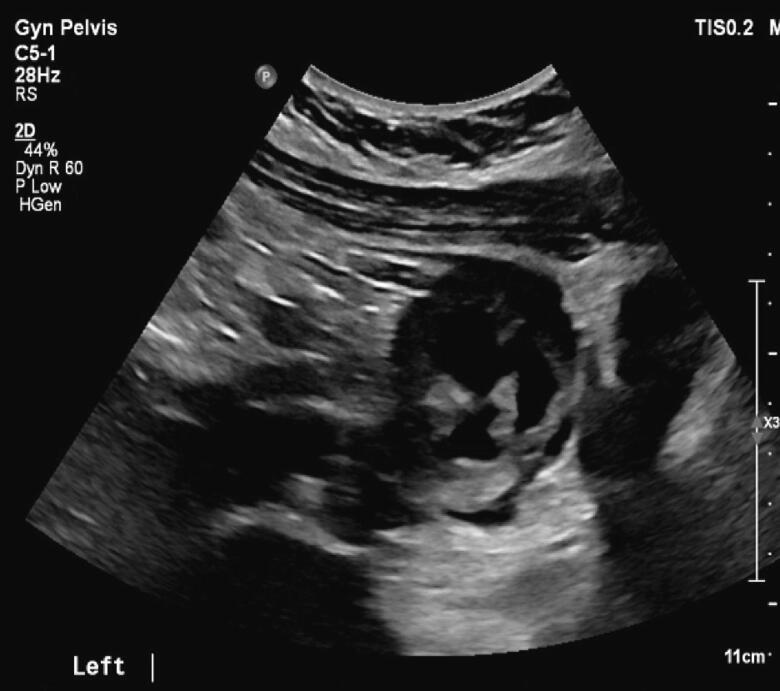
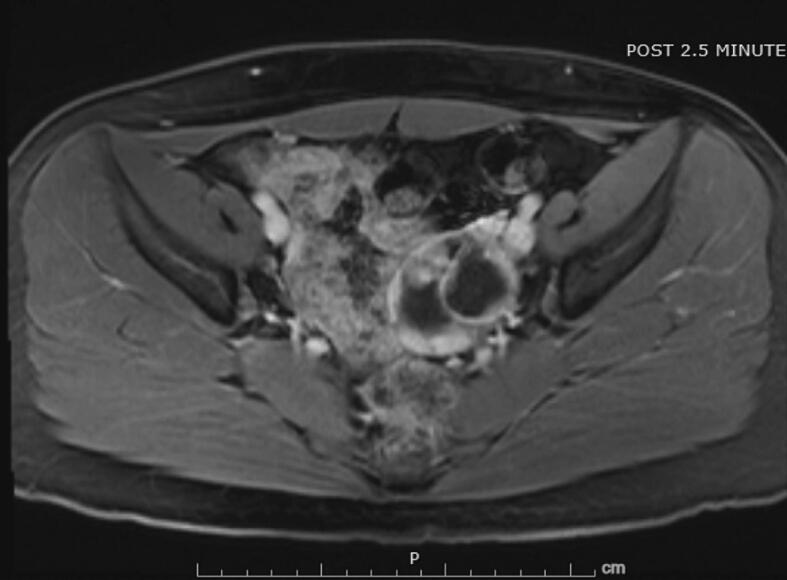
Physical examination revealed normal breast and pubic hair development. Laboratory evaluation revealed elevated serum testosterone (326 ng/dL), inhibin B (392 pg/mL), androstenedione (4.3 ng/mL), and DHEA (13.2 ng/mL). AFP and prolactin levels were within normal limits. A pelvic ultrasound demonstrated a normal uterus with 3 mm endometrial stripe, a normal right ovary, and a left ovary with a 5.8 x 5.5 x 4.6 cm, complex, multilocular, cystic lesion with thickened septations without internal flow (Fig. 1). A subsequent pelvic MRI confirmed a 4.7 x 6.1 x 5.4 cm, complex, solid and cystic ovarian mass with irregular enhancing septations and multiple enhancing soft tissue nodules with minimal macroscopic fat (Fig. 2).
Fig. 1.
This transvaginal ultrasound (TVUS) demonstrates complex, multilocular, cystic lesion with thickened septations.
Fig. 2.
This MRI demonstrates complex, solid and cystic ovarian mass with irregular enhancing separations and multiple enhancing soft tissue nodules with minimal macroscopic fat.
This patient was referred to a general obstetrician/gynecologist and subsequently a gynecologic oncologist who performed a fertility sparing, laparoscopic left salpingo-oophorectomy with pelvic washings. Intraoperative findings included a complex left ovarian mass with a smooth surface and normal appearing left fallopian tube. In addition, there were no visible implants on the peritoneal surfaces or omentum and no free fluid was noted. Clinical staging and pathology were determined to be IA grade 3 Sertoli-Leydig cell tumor of the left ovary with negative pelvic washings. Molecular profile analysis identified DICER1 DNA tumor pathologic variants c.307+1G>A and p.D1709N. Germline testing also demonstrated DICER1 gene mutation at c.307+1G>A.
Our institution does not allow treatment of patients under 18 years of age in the adult infusion center, so this patient was referred to the affiliated pediatric hematology/oncology service for adjuvant chemotherapy. Due to the rare occurrence of this tumor, the international DICER1 registry was consulted regarding adjuvant therapy. The recommendation was for systemic chemotherapy using a multi-agent regimen with PEI (cisplatin, etoposide, ifosfamide) or BEP (bleomycin, etoposide, cisplatin). The patient and her mother were counseled that approximately 50% of patients with poorly differentiated Sertoli Leydig tumors recur without chemotherapy, and the decision was made to proceed with 4 cycles of a PEI regimen.
During treatment, this patient was maintained on continuous norethindrone to suppress menstruation. This was discontinued after completion of her chemotherapy, and she had a return of menses. A post-treatment computerized tomography (CT) abdomen and pelvis showed no residual or recurrent mass. Plans were made for the patient to follow up in three months for surveillance of her ovarian cancer. This patient’s biological sister also underwent germline testing for a DICER1 mutation which was not identified. Thus far, two months after chemotherapy, the patient is doing well. For DICER1 related surveillance, it was recommended this patient follow with the pediatric cancer predisposition clinic.
3. Discussion
SLCTs have been reported in patients ages 2–76 years (Schultz et al., 2017); (Eoh et al., 2021); (Nef and Huber, 2021); (Wang et al., 2021); (Young and Scully, 1985). They present with varying compositions of Sertoli and Leydig cells that can produce testosterone. These tumors provoke symptoms and signs including abdominal pain and swelling, amenorrhea, menstrual irregularity, postmenopausal bleeding, hirsutism, and virilization. Androgen excess manifests in at least 40–50% of cases (Nef and Huber, 2021); (Young and Scully, 1985). Most cases are identified at stage I and intermediate differentiation (grade 2).
Preoperative evaluation recommendations for non-epithelial ovarian tumors vary across consensus groups and societies. Many guidelines recommend a variety of imaging modalities and tumor markers. A summary of several society recommendations compiled from Margioula-Siarkou et al.’s guidelines review for ovarian malignant tumors in adolescents and the National Comprehensive Cancer Network is detailed in Table 1 (Margioula-Siarkou et al., 2023, Network and Clinical Practice Guidelines, 2024).
Table 1.
Review of society peri-operative evaluations for adolescents with suspected non-epithelial ovarian tumors.
| Society | Guidelines Issued Year - Title | Preoperative Imaging Recommendations | Peri-operative Tumor Marker Recommendations (B-hCG, AFP, LDH, CA125) |
|---|---|---|---|
| National Comprehensive Cancer Network (NCCN) | 2024 - Clinical Practice Guidelines in Oncology: Ovarian cancer including fallopian tube cancer and primary peritoneal cancer | Abdominal-pelvic ultrasound or abdominal-pelvic CT or abdominal-pelvic MRI | Specified to clinical presentation |
| L’Observatoire des Tumeurs Malignes Rares Gynécologiques (Centres Experts TMRG) | 2022 - Les tumeurs malignes rares gynécologiques -- Référentiels | Abdominal-pelvic ultrasound Endometrial thickness evaluation (for suspected hormone-producing tumors) Abdominal-pelvic MRI (for suspected Germ Cell Tumors) PET scan (in selected cases) |
Pre- and post-operative measurement |
| European Cooperative Study Group for Pediatric Rare Tumors as part of the Paediatric Rare Tumours Network-European Registry (EXPeRT/PARTNER) | 2021 - Consensus recommendations from the EXPeRT/PARTNER groups for the diagnosis and therapy of sex cord stromal tumors in children and adolescents | Abdominal-pelvic ultrasound Chest X-ray or low-dose chest CT Abdominal-pelvic MRI |
Recommended |
| European Society of Gynecological Oncology and the European Society for Paediatric Oncology ESGO/EIOPE | 2020 - Non-epithelial ovarian cancers in adolescents and young adults | Endometrial thickness evaluation (for suspected hormone-producing tumors) Thoracic CT scan Abdominal-pelvic MRI |
Pre- and post-operative measurement |
| British Society for Paediatric & Adolescent Gynaecology (BritSPAG) | 2018 - Guidelines for the management of ovarian cysts in children and adolescents | Abdominal-pelvic ultrasound (initial imaging) | Recommended |
| European Society for Medical Oncology (ESMO) | 2018 - Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up | Abdominal-pelvic ultrasound Endometrial curettage for adults Chest X-ray Abdominal-pelvic CT PET scan (in selected cases) |
Pre- and post-operative measurement |
Surgery alone is a common treatment for well-differentiated tumors (grade 1). Peritoneal spread is the most common route of sex cord stromal tumor metastasis. Thus, surgical staging with peritoneal samplings, including fluid examination/washings, infracolic omentectomy, and random peritoneal biopsies (diaphragmatic peritoneum, paracolic gutters, pelvic peritoneum), may be included for complete surgical staging of these tumors. A lymphadenectomy is not commonly performed due to low risk of nodal spread or recurrence. In many cases, peritoneal staging is not performed during a primary surgery because tumor pathology is not identified until the final pathology report is finalized postoperatively (Negri et al., 2022). In this reported case, only pelvic washings were completed as the mass was not grossly suspicious, and the case was completed at an outside facility with limited ability to assess a frozen section. No peritoneal or omental implants were noted, the contralateral ovary was normal in appearance, and no ascites was present. In retrospect, it would have been reasonable to complete peritoneal staging with peritoneal and omental biopsies.
Multimodal treatment with surgery and adjuvant chemotherapy is frequently implemented in intermediate and poorly differentiated tumors; however, data are scarce regarding adjuvant therapy. Regimens documented in the literature include PEI, BEP, TP (paclitaxel, cisplatin), VIP (ifosfamide, etoposide, cisplatin), PVB (cisplatin, vincristine, bleomycin), and PAC (cisplatin, epirubicin, cyclophosphamide) (Wang et al., 2021). Relapse rates may approach 33%, with 95% of these relapses occurring within the first five years (Nef and Huber, 2021).
In patients with SLCT, some studies demonstrate that > 90% are affected by DICER1 when accounting for both germline and tumor DNA mutations (Schultz et al., 2017). DICER1 is located on chromosome 14q32.13 and encodes a RNase endoribonuclease that produces active microRNA. Mutations produce altered expression of microRNAs and an increased risk of neoplasms. RNaseIIIb mutations are a common product of DICER1 associated loss of function/mutation (Schultz et al., 2017); (Robertson et al., 2018). DICER1 mutated SLCT are more likely present with adrenergic signs and symptoms compared to DICER1 wild type SLCT (Karnezis et al., 2019). Additionally, mutated SLCT are more likely to be moderately and poorly differentiated rather than well-differentiated (de Kock et al., 2017).
DICER1 syndrome is an autosomal dominant pleiotropic tumor predisposition syndrome also associated with pleuropulmonary blastomas (PPB), cystic nephromas, renal sarcomas, Wilms’ tumors, other sex cord stromal tumors, thyroid neoplasms, pituitary blastomas, pineoblastomas, and gastrointestinal polyps (Schultz et al., 2017); (Schultz et al., 2017); (Stewart et al., 2019). Individuals with germline mutations present with neoplasms at younger ages than those with only tumor-limited mutations. Adhering to proper genetic testing and subsequent cancer screening guidelines can facilitate identification of neoplasms in early stages and is attributed to improved outcomes (Schultz et al., 2017). DICER1 gene testing is suggested in any patient with an associated neoplasm or a family history of DICER1 mutation with any childhood tumor. Suggestions of screening guidelines are detailed in Table 2.
Table 2.
Cancer Screening Suggestions for Individuals with DICER1 Mutation. These cancer screening recommendations are drawn from an expert review and working group on DICER1 associated conditions (Bakhuizen et al., 2021); (Schultz et al., 2018).
| Cancer Type | Initial Screening Recommendation | Subsequent Screening Recommendation |
|---|---|---|
|
Lung Pleuropulmonary Blastoma Pulmonary Blastoma |
If diagnosed prenatally, ultrasound during 3rd trimester of pregnancy Chest radiograph (CXR) at birth Chest CT between 3 and 6 months of age |
Chest CT between 2.5 and 3 years CXR every 4–6 months until 8 years and then annually until 12 years |
|
Renal Cystic Nephroma Wilms Tumor Renal Sarcoma |
Abdominal US at birth | Abdominal ultrasound every 6 months until 9 years, then annually until 12 years |
|
Brain Pineoblastoma Pituitary blastoma |
Physical exam Dilated ophthalmologic exam at 3 years |
Annual dilated ophthalmologic exam until 10 years Urgent brain MRI if symptoms of intracranial pathology manifest; surveillance is controversial due to rarity of these tumors |
|
Gonadal Sertoli Leydig Cell Tumor Gynandroblastoma Cervical embryonal rhabdomyosarcoma |
For females: pelvic US beginning at 8–10 years | Pelvic and abdominal ultrasound every 6--12 months until at least 40 years of age |
|
Thyroid Multinodular Goiter Differentiated Thyroid Cancer |
If diagnosed prenatally, monitor thyroid function during pregnancy Thyroid/regional adenopathy ultrasound at 8 years |
Thyroid/renal adenopathy ultrasound every 3 years until 40 years of age |
|
Ear/Nose/Throat & Gastrointestinal Polyps |
As needed evaluation with nasal endoscopy/colonoscopy for persistent symptoms of nasal obstruction or intestinal obstruction |
In summary, this case reports a SLCT with associated somatic and germline DICER1 pathologic variations. Many patients with DICER1 mutations will be identified following surgical resection and pathologic review of related tumors. Education reinforcing universal testing for these mutations in patients with SLCT may facilitate referrals to appropriate cancer predisposition programs. There is a paucity of data regarding appropriate adjuvant chemotherapeutic regimens for these patients. The current PEI regimen in this case was well tolerated, and the patient is without evidence of disease albeit a short follow-up time interval has passed. This patient was registered into the International PPB/DICER1 Registry which is currently recruiting eligible participants to clarify outcome data on incidence of neoplasms, response to chemotherapy regimens, event free survival, overall survival, and quality of life. The registry can be found at https://clinicaltrials.gov/study/NCT03382158 and additional information at https://www.ppbregistry.org/about-the-registry/.
Informed Consent
Written informed consent was obtained from the patient’s guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Shae N. Jansen: Writing – review & editing, Writing – original draft, Project administration, Investigation. Samantha L. McCarty: Writing – original draft, Project administration, Investigation. Lisa M. Landrum: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bakhuizen J.J., Hanson H., van der Tuin K., Lalloo F., Tischkowitz M., Wadt K., et al. Surveillance recommendations for DICER1 pathogenic variant carriers: a report from the SIOPE Host Genome Working Group and CanGene-CanVar Clinical Guideline Working Group. Fam Cancer. 2021;20(4):337–348. doi: 10.1007/s10689-021-00264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kock L., Terzic T., McCluggage W.G., Stewart C.J.R., Shaw P., Foulkes W.D., et al. DICER1 Mutations Are Consistenntly Present in Moderately and Poorly Differentiated Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2017;41(9):1178–1187. doi: 10.1097/PAS.0000000000000895. [DOI] [PubMed] [Google Scholar]
- Eoh K.J., Park J., Kim H.M., Lee M., Kim Y.T. Comparison of the Prognostic Outcome between High-Grade Ovarian Sertoli-Leydig Cell Tumors (SLCTs) and Low-Grade SLCTs. Yonsei Med. J. 2021;62(4):366–369. doi: 10.3349/ymj.2021.62.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley M., Bindal P., McAuliffe A., Vredenburgh J. A family with Sertoli-Leydig cell tumour, multinodular goiter, and DICER1 mutation. Curr. Oncol. 2019;26(3):183–185. doi: 10.3747/co.26.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis A.N., Wang Y., Keul J., Tessier-Cloutier B., Magrill J., Kommoss S., et al. DICER1 and FOXL2 Mutation Status Correlates With Clinicopathologic Features in Overian Sertoli-Leydig Cell Tumors. Am. J. Surg. Pathol. 2019;43(5):628–638. doi: 10.1097/PAS.0000000000001232. [DOI] [PubMed] [Google Scholar]
- Margioula-Siarkou C., Petousis S., Margioula-Siarkou G., Mavromatidis G., Chatzinikolaou F., Hatzipantelis E., et al. Therapeutic Management and Prognostic Factors for Ovarian Malignant Tumors in Adolescents: A Comprehensive Review of Current Guidelines. Diagnostics. 2023;13(6):1080. doi: 10.3390/diagnostics13061080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef J., Huber D.E. Ovarian Sertoli-Leydig cell tumours: A systematic review of relapsed cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;263:261–274. doi: 10.1016/j.ejogrb.2021.06.036. [DOI] [PubMed] [Google Scholar]
- Negri S., Grassi T., Fruscio R. Use of staging for sex cord stromal tumours. Curr. Opin. Oncol. 2022;34(5):504–510. doi: 10.1097/CCO.0000000000000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Ovarian Cancer Including Fallopian Tube and Primary Peritoneal Cancer. 2024. p. MS-9-MS-12.
- Robertson J.C., Jorcyk C.L., Oxford J.T. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers. 2018;10(5) doi: 10.3390/cancers10050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K.A.P., Harris A.K., Finch M., Dehner L.P., Brown J.B., Gershenson D.M., et al. DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: Clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol. Oncol. 2017;147(3):521–527. doi: 10.1016/j.ygyno.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K.A.P., Rednam S.P., Kamihara J., Doros L., Achatz M.I., Wasserman J.D., et al. PTEN, DICER1, FH, and Their Associated Tumor Susceptibility Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin. Cancer Res. 2017;23(12):e76–e82. doi: 10.1158/1078-0432.CCR-17-0629. [DOI] [PubMed] [Google Scholar]
- Schultz K.A.P., Williams G.M., Kamihara J., Stewart D.R., Harris A.K., Bauer A.J., et al. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin. Cancer Res. 2018;24(10):2251–2261. doi: 10.1158/1078-0432.CCR-17-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler S.J., Huber A., Nef J., Huber D.E. Sertoli-Leydig Cell Ovarian Tumors: Is Fertility or Endocrine-Sparing Surgery an Option upon Relapse? Case Rep. Oncol. 2020;13(2):935–940. doi: 10.1159/000508532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D.R., Best A.F., Williams G.M., Harney L.A., Carr A.G., Harris A.K., et al. Neoplasm Risk Among Individuals With a Pathogenic Germline Variant in DICER1. J. Clin. Oncol. 2019;37(8):668–676. doi: 10.1200/JCO.2018.78.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Zhang R., Li C., Chen A. Characteristics and outcomes analysis of ovarian Sertoli-Leydig cell tumors (SLCTs): analysis of 15 patients. J. Ovar. Res. 2021;14(1):150. doi: 10.1186/s13048-021-00909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.H., Scully R.E. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am. J. Surg. Pathol. 1985;9(8):543–569. doi: 10.1097/00000478-198508000-00001. [DOI] [PubMed] [Google Scholar]