INTRODUCTION
Bipolar disorder (BD) is among the most significant health problems for adults in America today, causing great morbidity, mortality, and over $40 billion in annual healthcare expenditures, we know far less about BD in children and adolescents 1. Recent data indicate that pediatric BD (PBD) is a growing health problem whose incidence has risen 40-fold in the past decade, now accounting for 20% of all minors discharged from psychiatric hospitals 2;3. It remains unknown if this increase represents greater awareness of a serious psychiatric disorder once thought not to exist in children or adolescents, or if it represents the diagnosis being too broadly applied. Determining which of these possibilities is correct is further complicated by the fact that there is no lab test for BD or any other psychiatric disorder, whether in children or adults. Instead, establishing the diagnosis of BD (or any other psychiatric disorder) is based entirely upon detailed clinical history that is often more difficult to elicit from minors than from adults. Thus, there is a pressing need to identify bio-behavioral markers of BD that could augment clinical history in the diagnostic and treatment process, especially for children and adolescents.
Towards that end, clinicians and researchers alike have been using the language of neuroscience to describe PBD patients as having “affect regulation problems.” While our understanding of PBD has advanced thanks to such neurobiological research, some have suggested that thinking of BD youth as suffering from affect regulation problems may be an oversimplification, akin to stating that a febrile child is suffering from “temperature regulation problems”—i.e., factually true, but not illuminating the situation sufficiently to render a diagnosis and a treatment 4. Clinicians and parents may wonder “does affect regulation improve what we know about the diagnosis and treatment of PBD, or does it further complicate matters?”
In a word, our answer is “yes”: while adding complexity and depth, we believe affect regulation does improve what we know about PBD. Affect regulation provides a framework for conducting biological research to identify the underlying mechanisms of BD, ultimately improving our care for these youth. While numerous books, manuscripts, and presentations have begun to address this topic, our present manuscript is intended to provide a concise and current guide to affect regulation in PBD, incorporating the latest in research as well as highlighting goals for the future. First, we will review the basics of affect regulation 5. Then, we will discuss what is known about the three important affects in BD: irritability, euphoria, and depression. Finally, we review what is known about affect regulation in PBD.
AFFECT REGULATION BASICS
What is affect regulation? Clinicians and researchers alike often report BD youth as suffering from “affect regulation problems”, including having abnormally high highs, low lows, and frequently shifting between the two, without maintaining much stability. Moreover, the pharmaceutical industry has seized upon this notion by using the term “mood stabilizer” to refer to medications used to treat BD, despite the fact that such medications are incredibly heterogeneous in terms of their pharmacological mechanisms 6;7.
Further complicating matters is the fact that the terms “emotion”, “feeling”, “mood”, and “affect” are used interchangeably. Clinically, mental health professionals routinely distinguish between “mood” and “affect” when evaluating and treating patients, whether child or adult, in their formal mental status examination. In this context, the term “mood” usually refers to the patient’s self-assessment of their emotional state (e.g., happy, sad, worried), whereas “affect” usually refers to the mental health professional’s objective, observable assessment of the patient’s emotional state and responsiveness (e.g., emotional state: depressed, euphoric, euthymic; emotional responsiveness: blunted, flat, expansive). The term “feeling” is often used similarly to “mood” as describing an individual’s conscious awareness of their emotional state (e.g., I feel happy, sad, worried). Yet, there is no universal agreement among clinicians or researchers on the distinction between the terms “emotion” and “affect”. Some use these terms interchangeably. In contrast, others suggest that “affect” is a biological, innate, instinctive response and “emotion” is the individual’s memory of prior affects, which was summarized by Nathanson as affect is biology, feeling is psychology, and emotion is biography 8. Given the lack of consensus on distinctions between “affect” and “emotion” and to align with the field of studying the neurobiology of emotion, known as “affective neuroscience”, we will preferentially use the term “affect” but recognize that the two terms may be used similarly.
From an affective neuroscience perspective, “affect” may be defined as “an evoked response to an environmental stimulus with motivational salience” 9. Motivational salience may be parsed into two orthogonal dimensions: (1) “valence” and (2) “arousal”. Valence refers to whether a stimulus is appetitively rewarding [positive valence] or aversive [negative valence]. Arousal refers to the amount of an organism’s resources, such as energy, that are mobilized in response to a stimulus. In turn, “affect regulation” has been defined by Thompson as the “extrinsic and intrinsic processes responsible for monitoring, evaluating, and modifying emotional reactions, especially their intensive and temporal features, to accomplish one’s goals” 10. To amplify several aspects of this definition, affect regulation is not just dampening or suppression of negative emotion (such as anger), but rather the dynamic balance between positively- and negatively-valenced emotions which can involve increasing or decreasing the elicited arousal 11. Moreover, affect regulation involves processes within the individual, both conscious and unconscious, as well as external influences, such as input from caretakers and experience from prior experiences with others. Lastly, affect regulation is not a solitary process that follows a one-way sequence from emotion to its regulation 12. Instead, affect regulation requires the interplay between many separable sub-processes, such as attention and processing of emotional stimuli, memory for prior events, response to rewards and punishments, and decision-making.
To exemplify these constructs consider the scenario of a child tasting some chocolate ice cream (stimulus). The child finds the ice cream very positively rewarding (affect; moderate arousal, positive valence). The child is aware that the ice cream tastes good (feeling), and s/he giggles remembering the last time s/he had ice cream (emotion). The child then goes to obtain more ice cream (affect regulation).
It is beyond the scope of this manuscript to fully describe all of the brain/behavior interactions underlying the sub-processes of affect regulation, as innumerable manuscripts, presentations, and entire books have attempted this. However, we would like to discuss several that seem germane to BD.
By definition, affect regulation begins with the detection of an environmental stimulus and categorizing it. For example, is that a lion that I should avoid, or a housecat that I should pet? Is mom or dad happy … or angry? Making these determinations of object identity involves the fusiform gyrus, occipital cortex, and superior temporal gyrus among others 13. These regions are categorize stimuli as animate or inanimate, and moving or not moving. Importantly, these regions seem especially geared to process faces, which are among the most important emotional stimuli in humans 14–18.
The recognition of facial emotions is another sub-process of affect regulation. It can be probed using computerized behavioral tasks on their own, or in conjunction with magnetic resonance imaging (MRI), whereby participants must identify the emotion (e.g., happy, sad, angry) displayed by an actor. In addition to the above regions involved in stimulus detection and categorization, the amygdala has also been implicated in processing facial emotions. Many studies have linked the amygdala to negatively-valenced emotionally stimuli. For example, surgical resection of the amygdala results in impaired recognition of facial expressions of negatively-valenced emotions, such as fear and sadness, but not of positively-valenced emotions, such as happiness 19;20. Compared to adults, adolescents have greater amygdala activation to negatively-valenced emotional expressions, including fear, vs. neutral stimuli 21–23. Moreover, there is a significant association between increased activation to fearful or threatening faces and the short variant of the serotonin transporter gene which is thought to convey susceptibility for depression and anxiety 24. Nevertheless, studies have also linked the amygdala to the processing of positively-valenced emotional stimuli. For example, functional MRI studies have demonstrated increased amygdala activation to happy vs. neutral faces in adult controls 25.
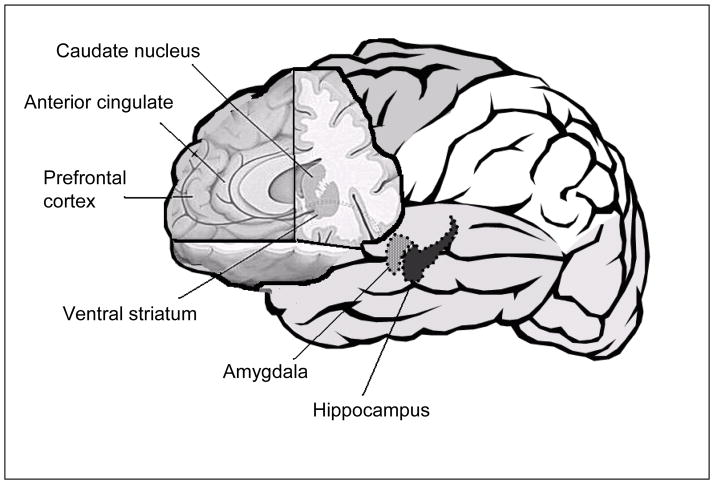
Also by definition, affect regulation involves response to rewards (appetitive, pleasurable, positively valenced stimuli) and punishments (aversive, painful, negatively-valenced stimuli). For example, do I finish my homework after I arrive home from school because it will make me happy? My parents happy? To get the 50 cents I was promised? To avoid getting my parents mad? If I do not do my homework, and my parents get mad, will I learn and tomorrow complete my homework when I arrive home from school? From an affective neuroscience perspective, response to rewards and punishment is integral to our understanding of BD, given clinical symptoms that potentially suggest mania to be a hyper-hedonic period involving excessive goal-directed activity, involvement in pleasurable activities with high potential for painful consequences, and grandiosity, whereas depression may be a hypo-hedonic period involving anhedonia, worthlessness, and hopelessness. It appears that these behavioral/emotional manifestations of mood disorders are linked to three functionally connected brain regions involved in processing rewards and punishments: (1) the prefrontal cortex (PFC), (2) the amygdala, and (3) the striatum 26–29.
One example of the myriad sub-processes of affect regulation that involve response to rewards and punishment is cognitive flexibility, meaning the ability to adapt one’s thinking and behavior in response to changing environmental conditions, including rewards 30;31. In the lab, cognitive flexibility may be tested using reversal learning tasks, whereby participants try to win as many points possible by first learning, through trial-and-error, which of two objects is rewarded (wins points) and which is punished (loses points) in order to stick with the rewarded stimulus. Then, without warning, the stimulus-reward association is reversed—i.e., the previously rewarded stimulus is now punished, and the previously punished stimulus is now rewarded. For example, if a dog and cat are presented, through trial-and-error, the child figures out that the dog is rewarded initially. After a number of trials of successfully winning points by picking the dog, the child switches to now win points by picking the cat once the dog starts losing points.
Reversal learning is mediated by a distributed circuit encompassing: (1) the prefrontal cortex (PFC), including the orbitofrontal cortex, which mediates the reversal of stimulus-reward relationships 30–32, (2) the striatum, including the accumbens area, caudate, and putamen, which transforms concrete stimulus-exemplar information into motor responses 30;33, and (3) the amygdala, which encodes the salience of rewards and punishments 27;34. Moreover, Blair et al. suggest that reversal learning and the OFC tap into the regulation of aggression 35–38. In particular, the OFC has been linked to social response reversal, whereby people react to others’ negatively-valenced emotional states, such as anger, by displaying aggression, including frustration and irritability.
Two additional and related sub-processes of affect regulation are attentional control and conflict monitoring. To illustrate these related processes, do I direct my attention to the teacher at the head of the class, or the student whispering next to me? Attentional control refers to the top-down process of devoting attentional resources and planning to stimuli and is most often linked to the dorsolateral PFC (DLPFC) 39–41. Conflict monitoring refers to the process of adjusting behavior to override one response in favor of another and is most often associated with the anterior cingulate cortex (ACC) 42. Attentional control can be tested by tasks involving planning, problem-solving, and executive function. Conflict monitoring can be tested by tasks such as the Stroop paradigm, whereby participants have more difficulty with incongruous stimuli (e.g., reading the word “RED” printed in blue ink) than control items (e.g., the word “RED” printed in red ink) 43. Go/No-go paradigms are also used to probe conflict monitoring and response inhibition, whereby participants are instructed to press a button as fast as they can if they see a word (e.g., “ZEBRA”) but to not press if they see a non-word (e.g., “QWERT”). Most trials on a go/no-go task are go responses (words), so that pressing the button becomes the “prepotent” response, with not pressing (to non-words) requires response inhibition.
AFFECT REGULATION IN TYPICAL DEVELOPMENT
What do we know about affect regulation in typically-developing children and adolescents? The field of affective neuroscience aims to answer this question using a number of techniques, including behavioral experiments, neuroimaging, genetics, and temperament.
From infancy, affect regulation begins as the interplay between interactions with caregivers and an infant’s temperament, the latter of which includes individual differences in (a) reactivity [the speed and intensity of emotional activation] and (b) self-regulation [the capacity to modify the intensity and duration of an emotion through behaviors, such as self-sucking or approaching/avoiding contact with caregivers] 44;45. Such early affect regulation can be observed and studied using constructs, such as, behavioral inhibition [the tendency of a child to become fearful and withdraw from novel situations] 46. With time, cognitive development and emotional experience enable the infant to exert greater affect self-regulation 47. Affect regulation is inherited to some degree, as demonstrated by identical twins performing more similarly than fraternal twins on parental reports of emotion regulation 48;49.
Adolescence is also a time of great change, including not only physical changes and growth but also the development of the many sub-processes of affect regulation. Paradoxically, adolescence involves both greater ability to self-regulate as well as greater tendency to engage in risk-taking behavior, including driving, unprotected sex, and experimentation with illegal substances 50. Recent longitudinal structural MRI studies have demonstrated that as children become young adults there is a loss of cortical gray matter in prefrontal and temporal cortices and increase in white matter volume 51;52. Functional MRI (fMRI) studies have shown that age-related changes in recruitment of the PFC during tasks of working memory, response inhibition, and verbal fluency 53–57. With all of this change and development, how does affect regulation account for the seeming inconsistency between adolescence as a time of cognitive development and a time of increased risk-taking? Recently, Casey has proposed a neurobiological model of adolescence to account for these inconsistencies. According to this model, during adolescence affect regulation represents a dynamic balance between (a) bottom-up processing of rewards by sub-cortical limbic regions, including the amygdala and accumbens area, and (b) top-down processing of cognitive control by the prefrontal cortex, with limbic structures maturing earlier than the PFC 58.
AFFECTS IN BIPOLAR DISORDER: KEY QUESTIONS
(A) IRRITABILITY
While irritability is one of the most common symptoms spurring parents to bring their children and adolescents for psychiatric treatment, the diagnosis and treatment of irritability remains a major challenge for clinicians and researchers alike. In part, this is due to the fact that irritability is a non-specific symptom in current psychiatric nosology. Irritability is an explicit DSM-IV-TR diagnostic criterion for a manic episode, generalized anxiety disorder, post-traumatic stress disorder, and, for children or adolescents, for a major depressive episode. Irritability is an associated symptom for pervasive developmental delay-spectrum disorders (autism, Asperger’s), attention deficit hyperactivity disorder (ADHD), and oppositional defiant disorder (ODD) 59.
Despite its inclusion in the above diagnoses, there is no widely accepted definition of irritability. Moreover, there is no uniform assessment measure of irritability, as standardized diagnostic interviews used both clinically and in research use different prompts to probe for irritability 60. For example, irritability is defined as: (a) “anger; crankiness; bad temper; short-tempered; resentment or annoyance, whether overtly expressed or not; easily lose temper; touchy or easily annoyed” (Child Schedule for Affective Disorders [KSADS]) 61, (b) “cranky; angry toward people you had no reason to; talk back; temper tantrum” (Diagnostic Interview Schedule for Children [DISC]) 62, and (c) “grumpy, crabby, talked back, sassy, wouldn’t do something your parents asked you to do” (Children’s Depression Rating Scale [CDRS]) 63. Thus, the challenge for all those involved is to determine whether the irritability is a manifestation of normal childhood or adolescence, or if it is a symptom of an underlying psychiatric disorder, including BD 64.
Moreover, there is no solitary biological cause of irritability, whether brain region, neurotransmitter, or gene. The brain region most frequently implicated is the orbitofrontal cortex (OFC), dating back to notable case reports such as Phineas Gage, whose personality was transformed from good-natured and hardworking to angry and labile after an iron tamping rod was blown through his skull and OFC, as shown by modern reconstruction of the incident 65. Imbalances in virtually every major neurotransmitter, including serotonin, glutamate, GABA, and catecholamines, also play a role 66. Moreover, recent studies have begun to explore the link between irritability and genotype related to these neurotransmitters 67;68.
(B) EUPHORIA
While irritability is a non-specific, yet impairing, symptom of BD, the elevated and expansive mood (euphoria) found during mania is a specific symptom of BD. From an affective neuroscience perspective, euphoric mania may represent a hyper-hedonic period of increased responsivity, or seeking out, of rewards. In BD adults, this position is supported from several lines of research. From a temperament perspective, two studies have found that those with history of mania, although in remission, are more likely to endorse items reflecting perfectionism and the need to achieve goals including “If I try hard enough, I should be able to excel at anything I attempt” 69;70. Moreover, a longitudinal study using the Behavioral Inhibition System Behavioral Activation System (BIS/BAS), a scale designed to measure individual differences in the avoidance of punishment (BIS) or approach towards reward (BAS) 71, has shown that BAS hypersensitivity (i.e., hypersensitivity to rewards) predicted shorter time to onset of manic and hypomanic episodes 72. Neuroimaging studies also support the potential alteration in reward processing in BD adults, with a recent study showing that whereas schizophrenic or control adults have activation of dopaminergic brain areas, including ventral tegmentum areas, to the expectation of monetary rewards and accumbens area to receipt vs. omission of rewards, manic BD adults did not 73. Moreover, genetic studies have demonstrated that carriers of the short (S) polymorphism of the serotonin transporter gene has been associated with an increased risk for anti-depressant associated mania in BD adults 74.
(C) DEPRESSION
Whether called “bipolar disorder” or “manic-depressive illness”, BD is not just about mania, as it involves depression, too. As is the case with irritable and euphoric mania, we know far more about depression in BD adults than in minors. In adults, only 25–33% of those with lifetime history of mania deny episodes of depression 75. Moreover, several large prospective studies have shown that BD adults often spend more time depressed than manic in approximately a 3:1 ratio 76. For example, Post et al. showed that of 258 BD adults followed prospectively for one year spent 33.2% of their time depressed while only 10.8% manic 77. Anger attacks were more common in depressed BD adults (62%) than those with unipolar depression (26%) 78.
With respect to pediatric patients, there are several important questions. For example, do BD youth spend the majority of their time depressed as has been shown in BD adults? If not, does this potentially reflect differences in affect regulation? Also, can treatments resolve the depression found in PBD, given the risk for medication induced mania, especially with serotonergic medications? At present, there is a great need to understand the neurobiology of depression in PBD subjects.
AFFECT REGULATION IN PEDIATRIC BIPOLAR DISORDER
Against this backdrop of the basics of affect regulation in normal development and in BD, what do we know about affect regulation in PBD? The short answer is that the past 8–10 years have seen great progress in PBD research. However, there is still a great deal to learn about the pathophysiology, phenomenology, and treatment of PBD, and this work is being conducted at a number of sites around the country and worldwide.
Returning to the abovementioned sub-processes of affect regulation, several studies have evaluated face processing in PBD. Several of these have used the Diagnostic Analysis of Non-Verbal Accuracy (DANVA), which requires participants to view and to categorize sets of child and adult faces displaying high- and low-intensity expressions of happiness, sadness, anger, or fear 79. These studies have shown that PBD youth were significantly more likely to miscategorize child images of happy, sad, and fear as angry compared to minors with either anxiety disorders or controls 80. A second study found that an expanded sample of PBD participants made more errors on child and adult faces than an expanded sample of controls, with secondary analyses suggesting that PBD participants were less sensitive to anger and happiness in children’s faces and less sensitive to anger and sadness in adults’ faces than controls 81. Functional neuroimaging studies have followed up on these behavioral studies. PBD youth had greater activation of the amygdala and striatum than controls when they were attending to emotional aspects of faces 82. These same PBD youth had greater neural activation in the striatum and ACC when successfully encoding happy faces into memory, and greater activation of the OFC when successfully encoding angry faces into memory 83.
Specifically in regard to cognitive flexibility, studies have shown that PBD participants are impaired versus typically-developing controls on reversal learning tasks. PBD youth make more errors, require more trials to achieve minimal competence, and require more time to complete the simple reversal stage of the intra-dimensional/extra-dimensional shift task (IDED), a computerized version of the Wisconsin card sorting task (CANTAB, Cambridge UK) 84. Four prior studies found similar results in BD adults with this task in both mania and euthymia, suggesting this may be a trait deficit in BD, rather than a mood-state dependent phenomenon 85–88. In a separate study, PBD participants made more errors than controls on a probabilistic response reversal task (PRR) that further manipulates reward and punishment by adding probabilistic trials (e.g., for stimulus pair dog/cat, during initial acquisition trials, dog is rewarded 80% and punished 20% while cat is rewarded 20% and punished 80%; subsequent reversal trials have dog rewarded 20% and punished 80%, while cat is now rewarded 80% and punished 20%) 89;90.
Studies of attentional control and conflict monitoring have demonstrated the following. Using a Stroop color-naming task, PBD adolescents had increased activation in the left putamen and thalamus during incongruent (the word “BLUE” written in red ink) versus congruent (the word “BLUE” written in blue ink) trials 91. Using a stop-signal task that required participants to inhibit a pre-potent motor response, PBD participants had a reduced striatal “error signal” (thought to cause avoidance of similar mistakes on subsequent trials) during failed motor inhibition compared to controls 92. In a related study that used a change-signal task requiring participants to inhibit a prepotent response and substitute an alternative response, PBD participants had significantly more neural activation in the DLPFC and primary motor cortex than healthy controls during correctly performed change trials versus correctly performed go trials 93.
Several important studies have begun to address the controversy regarding irritability vs. euphoria in PBD. While there is no rating instrument that is sensitive and specific for irritability found in BD, a consensus has emerged that the development of such an instrument is vital. In this regard, the K-SADS Mania Rating Scale is designed to measure mania in children, and unlike other measures, has specific anchors for the irritability item 94. However, many assert that it is unlikely that a single item can capture the complexity of irritability in PBD 60;95. For example, more than 50% of consecutive adolescents admitted to an inpatient psychiatric unit were judged to have moderate to severe irritability using the K-SADS Mania Rating scale, but the consensus diagnoses given to each of these admitted adolescents were quite varied 96. Ongoing PBD studies from around the country report high rates of irritability in their samples, but differing rates of euphoria. The Course and Outcome of Bipolar Youth (COBY; 3 sites University of Pittsburgh, Brown University, UCLA) found that 84.5% of their BD type I youth (N=220) had irritability or anger and 91.8% had elevated or expansive mood during their most severe lifetime manic episode 97. The research group at Massachusetts General Hospital found that of N=86 PBD subjects, 94% irritable mood while only 51% had euphoria 98. Geller et al. at Washington University in St. Louis found that of N= PBD subjects, 97.9% had irritable mood while 89.3% had elated mood 99.
Leibenluft has proposed a classification system of PBD to facilitate research about irritability vs. elation in PBD 100. At the heart of this system a definition of irritability grounded in affect regulation that consists of “marked reactivity to negative emotional stimuli manifest verbally or behaviorally—i.e., temper tantrums out of proportion to the inciting event and/or child’s developmental stage occurring >3 times per week during the past 4 weeks”. Building upon this definition, “broad phenotype” youth (also known as “severe mood dysregulation” [SMD]) have functionally-impairing (1) irritability (as defined above), (2) baseline sad or angry mood, and (3) hyperarousal (≥3 of insomnia, agitation, distractibility, racing thoughts/flight of ideas, pressured speech, or intrusiveness) that are chronic and non-episodic (symptoms present for ≥12 months without ≥2 months symptom free). These SMD youth do not have euphoria, and they do not have distinct mood episodes. At the other extreme, “narrow phenotype BD” (NPBD) youth have (1) clearly defined episodes of euphoric mania accompanied by ≥3 DSM-IV-TR “B” symptoms of mania and (2) symptoms of euphoric mania must be present for most of the day, every day for ≥4 days for hypomania or ≥7 days for mania. Thus, NPBD and SMD groups are orthogonal to one another, to separate out issues of irritability vs. euphoria and chronic course vs. distinct episodes that have been controversial in PBD 101;102.
Studies probing differences in affect regulation using these constructs are in their early stages. Thus far, epidemiological samples of children have shown that SMD may have a prevalence of 3.3% among those 9–19 years, although when these same youth are followed into adulthood, they were significantly more likely to be diagnosed with unipolar depression than those without SMD 103. Several lines of ongoing research are probing the brain/behavior interactions mediating affect regulation in NPBD vs. SMD youth, including the processing of emotional faces and also processing of rewards/punishments. With respect to face processing, both NPBD and SMD youth required greater emotion intensity to correctly identify facial images (including those of happiness, surprise, fear, sadness, anger, and disgust) compared to controls 104. Moreover, NPBD and SMD youth made more errors in the identification of facial emotions than from those with anxiety and/or depression, those with ADHD and/or conduct disorder, and controls 105. With respect to rewards and punishments and to cognitive flexibility, while both NPBD and SMD youth performed worse than controls on the compound reversal stage of the intra-dimensional/extra-dimensional set shifting task (IDED), NPBD youth were specifically impaired on the simple reversal stage vs. both SMD and controls 106. The same study also demonstrated that NPBD youth performed worse than both SMD and controls on a task requiring subjects to inhibit a prepotent motor response and substitute a new response (i.e., press “1” if you see an “X”, press “2” if you see an “O”, and press “3” if you see a blue square after either an “X” or “O” are presented). In contrast, there was no difference between NPBD and SMD youth in decision-making task requiring subjects to select from differentially rewarded and punished stimuli 107. In an attempt to study differential response to frustration elicited via rigged feedback, a recent electrophysiology study found that, while both NPBD and SMD youth reported more arousal than controls, NPBD subjects had lower P3 event-related potential (brain wave) amplitude than either, reflecting impaired executive attention, whereas SMD youth had lower N1 event-related potential amplitude than either NPBD or controls during both frustration and non-frustration events, suggesting impaired initial orientation 108. In a separate study, there was no difference between NPBD, SMD, and controls in magnitude of startle response during reward, punishment, or neutral conditions 109.
THE FUTURE
In summary, the past 8–10 years have shown great progress in what we know about affect regulation, including the brain/behavior interactions that differentiate PBD youth from those typically-developing youth without psychopathology. Affective neuroscience techniques, such as behavioral tasks, neuroimaging, and electrophysiology and event-related potentials, have advanced what is known about affect regulation in PBD. In particular, these studies have shown that PBD involves alterations in several sub-processes involved with affect regulation, including emotional face processing, cognitive flexibility, and response to reward and to punishment. These deficits are mediated by alterations in several brain regions, including a distributed neural circuit encompassing the PFC, striatum, and amygdala. Ongoing and future studies will examine the diagnostic specificity of such impairments versus other psychiatric disorders. They will also probe the relationship between such processes and underlying neural, genetic, and developmental mechanisms and mediators. Lastly, they will evaluate the potential for treatments, including medications and/or computerized tasks, to improve affect regulation in children and adolescents with BD. More and more, the future will likely involve the integration of affective neuroscience into clinical practice.
Figure 1.
Brain Regions Implicated in Affect Regulation
Table 1.
Affect Regulation in Pediatric Bipolar Disorder
| Function/Task | Study (author, year) | Major Finding: |
|---|---|---|
| Face/Emotion Processing | McClure EB et al. 2003 | PBD youth more likely to mis-categorize happy, sad, fearful faces compared to anxious and control youth |
| McClure EB et al. 2005 | PBD youth less sensitive (does this mean more errors?) to angry/happy child faces and angry/sad adult faces than controls | |
| Rich BA et al. 2006 | Greater amygdala/striatum activation when processing emotional faces in PBD youth than controls | |
| Dickstein DP et al. 2007 | Greater ACC/striatum activation when encoding happy faces and greater OFC activation when encoding angry faces in PBD youth compared to controls | |
| Rich BA et al. 2008 | NPBD and SMD youth required higher intensity of emotion to correctly identify emotional faces | |
| Guyer AE et al. 2007 | NPBD and SMD youth made more errors identifying emotions compared to anxious, depressed, ADHD, CD, and control youth | |
| Cognitive Flexibility - Reversal Learning | Dickstein DP et al. 2004 | PBD youth made more errors and required more trials/time on reversals compared to controls |
| Gorrindo T et al. 2005 | PBD youth made more errors on probabilistic trials compared to controls | |
| Dickstein DP et al. 2007 | NPBD youth performed worse than SMD and controls on reversal trials, with SMD performing worse than controls | |
| Attentional Control and Conflict Monitoring | Blumberg HP et al. 2003 | Greater activation in left putamen and thalamus during incongruent trials compared to congruent trials of the Stroop Task |
| Leibenluft E et al. 2007 | Reduced striatal activation during failed motor inhibition in PBD versus controls on the Stop-signal task | |
| Nelson EE et al. 2007 | Greater activation in DLPFC and primary motor cortex when correctly inhibiting a pre-potent motor response and executing an alternative response in PBD youth versus controls | |
| Response to reward and punishment | Rau G et al. 2008 | No difference in performance on reward decision-making task between NPBD and SMD |
| Electrophysiology/ERP response to reward and frustration. | Rich BA et al. 2007 | NPBD and SMD showed more arousal compared to controls during frustration and non-frustration events |
| Rich BA et al. 2005 | No differences in magnitude of arousal found between NPBD, SMD, and controls during reward, punishment, or neutral conditions. |
Footnotes
Disclosures: Dr. Dickstein is supported by Bradley Hospital and NIMH K22 MH 74945 and a NARSAD Young Investigator Award. Dr. Hunt is supported by Bradley Hospital and NIMH R01 MH59691
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kessler RC, Akiskal HS, Ames M, Birnbaum H, Greenberg PE, Hirschfeld RM, Wang PS. Considering the costs of bipolar depression. Behav Healthc. 2007;27:45–47. [PubMed] [Google Scholar]
- 2.Blader JC, Carlson GA. Increased Rates of Bipolar Disorder Diagnoses Among U.S. Child, Adolescent, and Adult Inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno C, Laje G, Blanco C, Jiang H, Schmidt AB, Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 4.Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Dev Psychopathol. 2005;17:827–850. doi: 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickstein DP, Leibenluft E. Emotion regulation in children and adolescents: boundaries between normalcy and bipolar disorder. Dev Psychopathol. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- 6.Bauer MS, Mitchner L. What is a “mood stabilizer”? An evidence-based response. Am J Psychiatry. 2004;161:3–18. doi: 10.1176/appi.ajp.161.1.3. [DOI] [PubMed] [Google Scholar]
- 7.Keck PE, Jr, McElroy SL, Richtand N, Tohen M. What makes a drug a primary mood stabilizer? Mol Psychiatry. 2002;7(Suppl 1):S8–14. doi: 10.1038/sj.mp.4001013. [DOI] [PubMed] [Google Scholar]
- 8.Nathanson DL. Shame and Pride. New York: W.W. Norton & Company, Inc; 1992. [Google Scholar]
- 9.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RA. Emotion regulation: a theme in search of definition. Monogr Soc Res Child Dev. 1994;59:25–52. [PubMed] [Google Scholar]
- 11.Fox NA. Definitions and concepts of emotion regulation. Introduction. Monogr Soc Res Child Dev. 1994;59:3–6. [PubMed] [Google Scholar]
- 12.Campos JJ, Frankel CB, Camras L. On the nature of emotion regulation. Child Dev. 2004;75:377–394. doi: 10.1111/j.1467-8624.2004.00681.x. [DOI] [PubMed] [Google Scholar]
- 13.Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier I, Behrmann M, Tarr MJ. Can face recognition really be dissociated from object recognition? J Cogn Neurosci. 1999;11:349–370. doi: 10.1162/089892999563472. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 16.Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann C, Mueller T, Federspiel A, Hubl D, Schroth G, Huber O, Strik W, Dierks T. Dissociation between overt and unconscious face processing in fusiform face area. Neuroimage. 2004;21:75–83. doi: 10.1016/j.neuroimage.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Bukach CM, Gauthier I, Tarr MJ. Beyond faces and modularity: the power of an expertise framework. Trends Cogn Sci. 2006;10:159–166. doi: 10.1016/j.tics.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;16:7678–7687. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- 21.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Morris AP, McGlone F, Abbott DF, Mattingley JB. Amygdala responses to fearful and happy facial expressions under conditions of binocular suppression. J Neurosci. 2004;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SC, Morgan M, Young AW, Gray JA. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. Neuroimage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 25.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 26.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amiez C, Joseph JP, Procyk E. Anterior cingulate error-related activity is modulated by predicted reward. Eur J Neurosci. 2005;21:3447–3452. doi: 10.1111/j.1460-9568.2005.04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellebaum C, Koch B, Schwarz M, Daum I. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain. 2008;131:829–841. doi: 10.1093/brain/awn011. [DOI] [PubMed] [Google Scholar]
- 30.Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 32.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto N, Hanakawa T, Maki S, Graybiel AM, Kimura M. Role of [corrected] nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. J Neurophysiol. 1999;82:978–998. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- 34.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 35.Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol. 2001;29:499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- 36.Blair RJ. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J Neurol Neurosurg Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 38.Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Kosson DS, Chen G, Towbin KE, Leibenluft E, Pine DS, Blair JR. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- 40.Fuster JM. Prefrontal neurons in networks of executive memory. Brain Res Bull. 2000;52:331–336. doi: 10.1016/s0361-9230(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 41.Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Dev Psychobiol. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- 42.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 43.MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends Cogn Sci. 2000;4:383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- 44.Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J Pers Soc Psychol. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- 45.Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Dev. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- 46.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 47.Calkins SD. Origins and outcomes of individual differences in emotion regulation. Monogr Soc Res Child Dev. 1994;59:53–72. [PubMed] [Google Scholar]
- 48.Goldsmith HH, Buss KA, Lemery KS. Toddler and childhood temperament: expanded content, stronger genetic evidence, new evidence for the importance of environment. Dev Psychol. 1997;33:891–905. doi: 10.1037//0012-1649.33.6.891. [DOI] [PubMed] [Google Scholar]
- 49.Van Hulle CA, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on socio-emotional behavior in toddlers: an initial twin study of the infant-toddler social and emotional assessment. J Child Psychol Psychiatry. 2007;48:1014–1024. doi: 10.1111/j.1469-7610.2007.01787.x. [DOI] [PubMed] [Google Scholar]
- 50.Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Shanklin S, Lim C, Grunbaum JA, Wechsler H. Youth risk behavior surveillance--United States, 2005. MMWR Surveill Summ. 2006;55:1–108. [PubMed] [Google Scholar]
- 51.Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 54.Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- 55.McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS. A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 57.Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th Edition Text Revision (DSM-IV-TR) Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- 60.Leibenluft E, Blair RJ, Charney DS, Pine DS. Irritability in pediatric mania and other childhood psychopathology. Ann N Y Acad Sci. 2003;1008:201–218. doi: 10.1196/annals.1301.022. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 62.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Poznanski E, Freeman LNMH. Children’s Depression Rating Scale-Revised. Psychopharmacol Bull. 1985;21(4):979–984. [Google Scholar]
- 64.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 65.Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 66.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165:429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonda X, Rihmer Z, Zsombok T, Bagdy G, Akiskal KK, Akiskal HS. The 5HTTLPR polymorphism of the serotonin transporter gene is associated with affective temperaments as measured by TEMPS-A. J Affect Disord. 2006;91:125–131. doi: 10.1016/j.jad.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 68.Kang JI, Namkoong K, Kim SJ. The association of 5-HTTLPR and DRD4 VNTR polymorphisms with affective temperamental traits in healthy volunteers. J Affect Disord. 2008;109:157–163. doi: 10.1016/j.jad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Scott J, Stanton B, Garland A, Ferrier IN. Cognitive vulnerability in patients with bipolar disorder. Psychol Med. 2000;30:467–472. doi: 10.1017/s0033291799008879. [DOI] [PubMed] [Google Scholar]
- 70.Lam D, Wright K, Smith N. Dysfunctional assumptions in bipolar disorder. J Affect Disord. 2004;79:193–199. doi: 10.1016/S0165-0327(02)00462-7. [DOI] [PubMed] [Google Scholar]
- 71.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 72.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–322. doi: 10.1111/j.1399-5618.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 73.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferreira AD, Neves FS, da Rocha FF, Silva GS, Romano-Silva MA, Miranda DM, De Marco L, Correa H. The role of 5-HTTLPR polymorphism in antidepressant-associated mania in bipolar disorder. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Kessler RC, Rubinow DR, Holmes C, Abelson JM, Zhao S. The epidemiology of DSM-III-R bipolar I disorder in a general population survey. Psychol Med. 1997;27:1079–1089. doi: 10.1017/s0033291797005333. [DOI] [PubMed] [Google Scholar]
- 76.Thase ME. STEP-BD and bipolar depression: what have we learned? Curr Psychiatry Rep. 2007;9:497–503. doi: 10.1007/s11920-007-0068-9. [DOI] [PubMed] [Google Scholar]
- 77.Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck PE, Jr, McElroy SL, Luckenbaugh DA, Pollio C, Kupka R, Nolen WA. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- 78.Perlis RH, Smoller JW, Fava M, Rosenbaum JF, Nierenberg AA, Sachs GS. The prevalence and clinical correlates of anger attacks during depressive episodes in bipolar disorder. J Affect Disord. 2004;79:291–295. doi: 10.1016/S0165-0327(02)00451-2. [DOI] [PubMed] [Google Scholar]
- 79.Nowicki S, Duke M. Individual differences in the nonverbal communication of affect: The Diagnostic Analysis of Nonverbal Accuracy Scale. Journal of Nonverbal Behavior. 1994;18:9–35. [Google Scholar]
- 80.McClure EB, Pope K, Hoberman AJ, Pine DS, Leibenluft E. Facial expression recognition in adolescents with mood and anxiety disorders. Am J Psychiatry. 2003;160:1172–1174. doi: 10.1176/appi.ajp.160.6.1172. [DOI] [PubMed] [Google Scholar]
- 81.McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- 82.Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Fromm SJ, Pine DS, Leibenluft E. Limbic Hyperactivation During Processing of Neutral Facial Expressions in Children with Bipolar Disorder. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0603246103. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disord. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dickstein DP, Treland JE, Snow J, McClure EB, Mehta MS, Towbin KE, Pine DS, Leibenluft E. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 85.Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med. 2000;30:1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- 86.Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biol Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- 87.Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158:1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- 88.Clark L, Iversen SD, Goodwin GM. Sustained attention deficit in bipolar disorder. Br J Psychiatry. 2002;180:313–319. doi: 10.1192/bjp.180.4.313. [DOI] [PubMed] [Google Scholar]
- 89.Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 90.Budhani S, Blair RJ. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J Child Psychol Psychiatry. 2005;46:972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 91.Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 92.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 93.Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, Rich BA, Brotman MA, Pine DS, Leibenluft E. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord. 2007;9:810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 94.Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 95.Carlson GA, Meyer SE. Phenomenology and diagnosis of bipolar disorder in children, adolescents, and adults: complexities and developmental issues. Dev Psychopathol. 2006;18:939–969. doi: 10.1017/S0954579406060470. [DOI] [PubMed] [Google Scholar]
- 96.Hunt JI, Dyl J, Armstrong L, Litvin E, Sheeran T, Spirito A. Frequency of manic symptoms and bipolar disorder in psychiatrically hospitalized adolescents using the K-SADS Mania Rating Scale. J Child Adolesc Psychopharmacol. 2005;15:918–930. doi: 10.1089/cap.2005.15.918. [DOI] [PubMed] [Google Scholar]
- 97.Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 98.Wozniak J, Biederman J, Kwon A, Mick E, Faraone S, Orlovsky K, Schnare L, Cargol C, van Grondelle A. How Cardinal are Cardinal Symptoms in Pediatric Bipolar Disorder? An Examination of Clinical Correlates. Biol Psychiatry. 2005;58:583–588. doi: 10.1016/j.biopsych.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 99.Geller B, Zimerman B, Williams M, DelBello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 100.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 101.Biederman J, Klein RG, Pine DS, Klein DF. Resolved: mania is mistaken for ADHD in prepubertal children. J Am Acad Child Adolesc Psychiatry. 1998;37:1091–1096. doi: 10.1097/00004583-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 102.Biederman J, Faraone SV, Wozniak J, Mick E, Kwon A, Aleardi M. Further evidence of unique developmental phenotypic correlates of pediatric bipolar disorder: findings from a large sample of clinically referred preadolescent children assessed over the last 7 years. J Affect Disord. 2004;82(Suppl 1):S45–S58. doi: 10.1016/j.jad.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 103.Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, Egger HL, Angold A, Pine DS, Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 104.Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Dev Psychopathol. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Pine DS, Ernst M, Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 106.Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, Rich BA, Pine DS, Leibenluft E. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 107.Rau G, Blair KS, Berghorst L, Knopf L, Skup M, Luckenbaugh DA, Pine DS, Blair RJ, Leibenluft E. Processing of differentially valued rewards and punishments in youths with bipolar disorder or severe mood dysregulation. J Child Adolesc Psychopharmacol. 2008;18:185–196. doi: 10.1089/cap.2007.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 109.Rich BA, Bhangoo RK, Vinton DT, Berghorst LH, Dickstein DP, Grillon C, Davidson RJ, Leibenluft E. Using affect-modulated startle to study phenotypes of pediatric bipolar disorder. Bipolar Disord. 2005;7:536–545. doi: 10.1111/j.1399-5618.2005.00265.x. [DOI] [PubMed] [Google Scholar]