Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Eulecanium giganteum (Hemiptera: Coccidae), the giant eulecanium scale, for the territory of the European Union, following the commodity risk assessment of Acer palmatum plants from China, in which E. giganteum came to attention as a pest of possible concern. The pest is only known to be present in Asia, where it has been reported from China, India, Iran, Japan and eastern Russia (Primorsky Krai). The pest has not been reported within the EU. It is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072. It is polyphagous, feeding on broad‐leaf trees and shrubs assigned to 41 genera in 22 plant families. Host plant species commonly found in the EU include apricot (Prunus armeniaca), elm (Ulmus spp.), grapevine (Vitis vinifera), maple (Acer spp.), oak (Quercus spp.), oriental plane (Platanus orientalis), pomegranate (Punica granatum), quince (Cydonia oblonga), silkworm mulberry (Morus alba), walnut (Juglans regia), and several ornamentals. Climatic conditions and availability of host plants in southern EU countries would most probably allow this species to successfully establish and spread. However, EU native natural enemies are anticipated to provide biological control and therefore reduce potential impacts. Phytosanitary measures are available to reduce the likelihood of entry and spread. E. giganteum satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest, other than the criterion on impact which is a key uncertainty.
Keywords: Coccidae, Giant eulecanium scale, Hemiptera, pest risk, plant health, plant pest, quarantine
1. INTRODUCTION
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high‐risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annex 1A, 1B, 1D and 1E (for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of High Risk Plants.
1.2. Interpretation of the Terms of Reference
Eulecanium giganteum is one of a number of pests relevant to Annex 1C of the terms of reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a potential Union quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform EU decision‐making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/ 2072. If a pest fulfils the criteria to be potentially listed as a Union quarantine pest, risk reduction options will be identified.
1.3. Additional information
This pest categorisation was initiated following the commodity risk assessment of Acer palmatum plants from China performed by EFSA (EFSA PLH Panel, 2022), in which E. giganteum was identified as a relevant non‐regulated EU pest of possible concern, which could potentially enter the EU on A. palmatum.
2. DATA AND METHODOLOGIES
2.1. Data
2.1.1. Literature search
A literature search on E. giganteum was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt to TRACES in May 2020.
GenBank was searched to determine whether it contained any nucleotide sequences for E. giganteum which could be used as reference material for molecular diagnosis. GenBank® (www.ncbi.nlm.nih.gov/genbank/) is a comprehensive publicly available database that as of August 2019 (release version 227) contained over 6.25 trillion base pairs from over 1.6 billion nucleotide sequences for 450,000 formally described species (Sayers et al., 2020).
2.2. Methodologies
The Panel performed the pest categorisation for E. giganteum, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013).
The criteria to be considered when categorising a pest as a potential Union quarantine pest (QP) is given in Regulation (EU) 2016/2031 Article 3 and Annex I, Section 1 of the Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met, the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
TABLE 1.
Pest categorisation criteria under evaluation, as derived from Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4 ) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways for entry and spread |
| Potential for consequences in the EU territory (Section 3.5 ) | Would the pests' introduction have an economic or environmental impact on the EU territory? |
|
Available measures (Section 3.6 ) |
Are there measures available to prevent pest entry, establishment, spread or impacts? |
| Conclusion of pest categorisation (Section 4 ) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. While the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. PEST CATEGORISATION
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
Is the identity of the pest clearly defined, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes, the identity of the pest is established and Eulecanium giganteum (Shinji) is the accepted name.
Eulecanium giganteum (Shinji, 1935) is an insect within the order Hemiptera, family Coccidae, and is commonly known as the giant eulecanium scale (García Morales et al., 2016; Kondo & Watson, 2022). It was originally described as Lecanium gigantea by Shinji (1935) from specimens collected in Morioka, Japan, on Magnolia kobus (northern Japanese magnolia). Later, Borchsenius (1955) redescribed and illustrated specimens of the same species collected in Primorsky Krai, Far East Region of Russia, on Quercus spp. as Eulecanium diminutum (García Morales et al., 2016). Wang (1980) changed the combination of genus and species to Eulecanium gigantea (Ben‐Dov, 1993; García Morales et al., 2016).
The EPPO code 1 (EPPO, 2019; Griessinger & Roy, 2015) for this species is: EULCGI (EPPO, online).
3.1.2. Biology of the pest
E. giganteum reproduces sexually and has one generation per year (García Morales et al., 2016; Kondo & Watson, 2022). Females have three development stages: egg, nymph (two instars) and adult, while males have two additional non‐feeding nymphal instars, the prepupa and pupa (Zhao & Xie, 2004). Most of the development stages are found on branches that are 1–3 years old. Females live about 20–34 days while males live only 1–2 days and die after mating (Wang, 2000; Xie, 1985; Yue et al., 2011). Fecundity is high, as an adult female can lay more than 6000 eggs on average during its life span (Kondo & Watson, 2022). The pest develops one annual generation in northern China (Gansu province) (Xie et al., 1995) Shanxi province (Kondo & Watson, 2022) and in Guanzhong region in Shaanxi province (Wang, 2000). It overwinters as a second‐instar nymph on twigs (Deng et al., 2016), and the sex ratio of overwintered female to male nymphs is 1:2 (Kondo & Watson, 2022). On average, each female can produce 677 nymphs that successfully reach the leaves (Kondo & Watson, 2022). Xie et al. (1995) have shown that in Taiyuan, Shanxi Province in China, the high level of urban air pollution (sulfur dioxide and lead) caused an increase in population densities of this species (García Morales et al., 2016). The reasons could be: (a) effect of pollutants improved nutrients for the host plant Styphnolobium japonicum, so scales became larger and more fertile, and (b) pollutants had negative effect on natural enemies of E. giganteum and the scale was no longer controlled (Xue et al., 1999) (Table 2).
TABLE 2.
Important features of the life history strategy of Eulecanium giganteum.
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Egg | In northern China, oviposition occurs from late April to early May. Eggs hatch in late May (Kondo & Watson, 2022) | The eggs take about 25 days to develop (Kondo & Watson, 2022) |
| Nymph | In spring, overwintering second‐instar nymphs complete development and new adults appear in May and start reproducing. The number of the hatched crawlers increases rapidly until June (Deng et al., 2016). From June to September, the crawlers feed on leaves, and then in September–October, the second‐instar nymphs move to the branches to overwinter (EFSA PLH Panel, 2022; Kondo & Watson, 2022) | The first‐instar nymphs are mobile (crawlers) while the second instars are sedentary (EFSA PLH Panel, 2022; Tao et al., 2002). The crawlers can be dispersed by the wind, insects or birds (EFSA PLH Panel, 2022; Zhao & Xie, 2004) |
| Prepupa‐Pupa (males) | Males have four development stages. Prepupa‐pupa stage takes places after the second‐instar male nymph (EFSA PLH Panel, 2022) | |
| Adult | Adults of both sexes emerge and mate from late April to early May (EFSA PLH Panel, 2022) |
3.1.3. Host range/species affected
E. giganteum is a polyphagous insect, feeding on plants assigned to more than 41 genera in 22 plant families (Appendix A provides a full list of hosts). E. giganteum has been recorded on broad‐leaf trees and shrubs such as apricot (Prunus armeniaca), elm (Ulmus spp.), grapevine (Vitis vinifera), maple (Acer spp.), oak (Quercus spp.), oriental plane (Platanus orientalis), pomegranate (Punica granatum), quince (Cydonia oblonga), silkworm mulberry (Morus alba), walnut (Juglans regia) and several ornamentals (García Morales et al., 2016; Suganthi et al., 2022).
3.1.4. Intraspecific diversity
Chinese literature between 1989 and 2016 considered what is now recognised as E. giganteum and E. kuwanai Kanda, 1934 as the same species with wide intraspecific diversity. Shi and Lü (1989) determined that E. giganteum and E. kuwanai were two different ecological types of the same species, with the different phenotypes resulting from varying population densities. This was because both species are morphologically similar, sympatrically distributed in China, share many of the same hosts, and often appear together on the same plants, and even on the same twigs. Deng et al. (2016), however, demonstrated that they were indeed distinct species using molecular techniques.
There are no reports for intraspecific diversity since E. giganteum has been separated from E. kuwanai.
3.1.5. Detection and identification of the pest
Are detection and identification methods available for the pest?
Yes, visual detection is possible, and morphological and molecular identification methods are available.
Detection
Visual examination of plants is an effective way for the detection of E. giganteum due to the large size of adult female scales (Kondo & Watson, 2022). Accumulation of honeydew, sooty mould and honeydew‐seeking ants are general signs of phloem‐feeding insect infestations; they can be used to pinpoint the areas where plants may be inspected for the presence of soft scales (Camacho & Chong, 2015; Deng et al., 2016). Sticky bands around branches can be used to detect crawlers (Bethke & Wilen, 2010).
Symptoms
According to Wang et al. (2012), EFSA PLH Panel (2022) and Kondo and Watson (2022), the main symptoms of E. giganteum infestation are:
honeydew egested by the scales;
black sooty mould growing on the honeydew;
partial necrosis and wilting of twigs and leaves, and;
yellowing, defoliation, reduced plant growth, dieback of the branches or of the entire plant caused by heavy infestations.
These symptoms are similar to those caused by many other phloem‐feeding insects and should not be considered as diagnostic.
Identification
The identification of E. giganteum requires microscopic examination of slide‐mounted adults and verification of the presence of key morphological characteristics. Detailed morphological descriptions, illustrations and keys of adult E. giganteum can be found in Danzig (1980), and Zhao and Xie (2004), while egg and nymphal stages are described by Xie (1985).
Molecular identification based on the nucleotide sequence of e.g. the mitochondrial cytochrome c oxidase subunit I (COI) gene can be used for species identification (Deng et al., 2016). GenBank contains gene nucleotide sequences for E. giganteum (https://www.ncbi.nlm.nih.gov/nuccore/?term=Eulecanium+giganteum).
Description
Young adult females are almost hemispherical, reddish brown to purple‐brown with dark irregular lines. The dorsum is covered by thin grey‐white powdery wax. At maturity, the body is nearly 19 mm long, 18 mm wide and 14 mm high, making it the largest species in the genus. In mature adult females, there is no visible wax, the scale is dark and often with reddish‐brown patches on the dorsum (Kondo & Watson, 2022). Males are winged and have robust legs (Zhao & Xie, 2004).
In addition to its large size, E. giganteum can be differentiated from other species in the genus by the following combination of characteristics: (a) marginal setae of one type, conical, present in a single row; (b) the stigmatic spines are not differentiated from the marginal setae; (c) the dorsal tubercles are absent; (d) small dorsal tubular ducts are present; and (e) anal ring with eight setae (Kondo & Watson, 2022).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
E. giganteum is an Asiatic species first described in Morioka, Japan (García Morales et al., 2016). Its present known distribution includes most of northern China, India, Iran, Japan and eastern Russia (Primorsky Krai) (García Morales et al., 2016; Deng et al., 2016; Kondo & Watson, 2022; Suganthi et al., 2022; see Figure 1).
FIGURE 1.
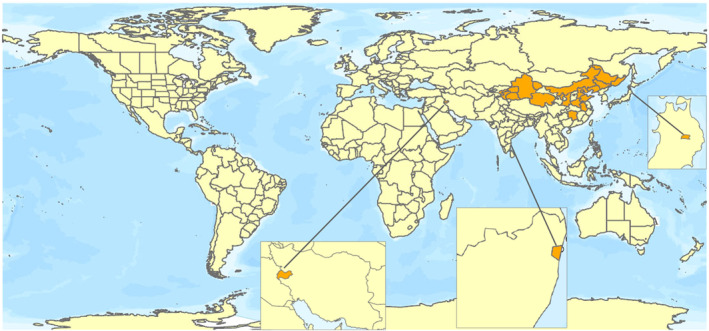
Global distribution of Eulecanium giganteum (data source: Deng et al., 2016; García Morales et al., 2016; Kondo & Watson, 2022; Suganthi et al., 2022). The polygons with highlighted orange colour indicate the administrative areas where E. giganteum is present.
3.2.2. Pest distribution in the EU
Is the pest present in the EU territory? If present, is the pest in a limited part of the EU or is it scarce, irregular, isolated or present infrequently? If so, the pest is considered to be not widely distributed.
No. E. giganteum has not been recorded in the EU territory.
3.3. Regulatory status
3.3.1. Commission implementing regulation 2019/2072
E. giganteum is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031, or in any emergency plant health legislation.
3.3.2. Hosts or species affected that are prohibited from entering the union from third countries
According to the Commission Implementing Regulation (EU) 2019/2072, Annex VI, introduction of several E. giganteum hosts in the Union from certain third countries is prohibited (Table 3).
TABLE 3.
List of plants, plant products and other objects that are Eulecanium giganteum hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI.
| List of plants, plant products and other objects whose introduction into the union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN code | Third country, group of third countries or specific area of third country | |
| 2. | Plants of [...] Quercus L., with leaves, other than fruit and seeds |
ex 0602 10 90 ex 0602 20 20 ex 0602 20 80 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 99 ex 0604 20 90 ex 1404 90 00 |
Third countries other than Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District [Tsentralny federalny okrug], Northwestern Federal District [Severo‐ Zapadny federalny okrug], Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District [Severo‐Kavkazsky federalny okrug] and Volga Federal District [Privolzhsky federalny okrug]), San Marino, Serbia, Switzerland, Türkiye, Ukraine and the United Kingdom |
| 5. | Isolated bark of Quercus L., other than Quercus suber L. |
ex 1404 90 00 ex 4401 40 90 |
Mexico |
| 8. | Plants for planting of [...] Cydonia Mill., [...] Prunus L., [...] and Rosa L., other than dormant plants free from leaves, flowers and fruits |
ex 0602 10 90 ex 0602 20 20 ex 0602 20 80 ex 0602 40 00 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 47 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐ Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Türkiye, Ukraine and the United Kingdom |
| 9. | Plants for planting of Cydonia Mill., [...] Prunus L. and [...] and their hybrids, and [...] other than seeds |
ex 0602 10 90 ex 0602 20 20 ex 0602 90 30 ex 0602 90 41 ex 0602 90 45 ex 0602 90 46 ex 0602 90 48 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 |
Third countries other than Albania, Algeria, Andorra, Armenia, Australia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canada, Canary Islands, Egypt, Faeroe Islands, Georgia, Iceland, Israel, Jordan, Lebanon, Libya, Liechtenstein, Moldova, Monaco, Montenegro, Morocco, New Zealand, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (Severo‐Zapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐ Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug)), San Marino, Serbia, Switzerland, Syria, Tunisia, Türkiye, Ukraine, the United Kingdom and United States other than Hawaii |
| 10. | Plants of Vitis L., other than fruits |
0602 10 10 0602 20 10 ex 0604 20 90 ex 1404 90 00 |
Third countries other than Switzerland |
Plants for planting of Acer L., Corylus L., Fraxinus L., Juglans L., Quercus L., Robinia L., Rosa L., Prunus L., Salix L. and Ulmus L., which are hosts of E. giganteum (Appendix A), are considered high‐risk plants for the EU and their import is prohibited pending risk assessment (EU 2018/2019).
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes , E. giganteum could enter the EU territory. Possible pathways of entry are plants for planting (except seeds, bulbs, and tubers), fruits and cut flowers, and isolated bark.
Comment on plants for planting as a pathway.
Plants for planting are the main pathway for E. giganteum to enter the EU (Table 4).
TABLE 4.
Potential pathways for Eulecanium giganteum into the EU.
| Pathways (e.g. host/intended use/source) | Life stage | Relevant mitigations (e.g. prohibitions [Annex VI], special requirements [Annex VII] or phytosanitary certificates [Annex XI] within Implementing Regulation 2019/2072) |
|---|---|---|
| Plants for planting | All life stages |
Plants for planting that are hosts of E. giganteum and are prohibited to import from third countries (Regulation 2019/2072, Annex VI), are listed in Table 3 Plants for planting from third countries require a phytosanitary certificate (Regulation 2019/2072, Annex XI, Part A) Some hosts are considered high‐risk plants (EU 2018/2019) for the EU and their import is prohibited subject to risk assessment |
| Fruits and cut flowers | All life stages | Fruits and cut flowers from third countries require a phytosanitary certificate to be imported into the EU (2019/2072, Annex XI, Part A). However, no requirements are specified for E. giganteum |
| Host isolated bark | Eggs | Annex VI prohibitions apply to the bark of some hosts i.e. Quercus sp., Table 3, point 5, but for countries where E. giganteum is not known to occur |
Plants for planting, fruits and cut flowers are the main potential pathways for entry of E. giganteum (Table 4).
Annual imports of E. giganteum hosts from countries where the pest is known to occur are provided in Appendix C.
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As of November 2023, there were no records of interception of E. giganteum in the Europhyt and TRACES databases.
3.4.2. Establishment
Is the pest able to become established in the EU territory?
Yes, the climate in EU countries is suitable and there are many available hosts that can support establishment.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker, 2002; Baker et al., 2000). Availability of hosts is considered in Section 3.4.2.1. Climatic factors are considered in Section 3.4.2.2.
3.4.2.1. EU distribution of main host plants
Many genera of E. giganteum host plants are present or are grown widely across the EU. Among others, Acer, Cydonia, Ficus, Juglans, Morus, Prunus, Quercus, Ulmus, Vitis and some ornamental plants. The main hosts of the scale insect cultivated in the EU between 2017 and 2022 are shown in Table 5.
TABLE 5.
Crop area of Eulecanium giganteum key hosts in the EU in 1000 ha (Eurostat accessed on 30 November 2023).
| Crop | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|
| Apricots | 72.23 | 72.57 | 73.22 | 76.13 | 73.48 | 74.90 |
| Berries (excluding strawberries)* | 146.27 | 150.42 | 154.44 | 154.27 | 157.07 | – |
| Figs | 24.63 | 24.99 | 25.59 | 27.63 | 25.79 | 26.42 |
| Walnuts | 74.15 | 80.60 | 87.62 | 99.21 | 97.00 | 104.74 |
| Grapes | 3133.32 | 3135.50 | 3155.20 | 3146.24 | 3120.22 | 3132.12 |
Only a proportion of these berries are host species, specifically Morus alba (mulberries).
3.4.2.2. Climatic conditions affecting establishment
E. giganteum occurs in continental, temperate and dry areas in Asia. The biology of this pest is little studied and no temperature thresholds for development have been reported. Consequently, there is some uncertainty regarding the climatic requirements of the insect. Figure 2 shows the world distribution of Köppen–Geiger climate types (Kottek et al., 2006) that occur in the EU, and which occur in countries where E. giganteum has been reported. In Russia, it was found to occur in the most southerly tip of the Primorsky Krai (Far East) Territory which experiences a humid continental climate (Dfb). Winters are cold and dry with daily mean temperatures between December and March below zero, and the average low temperature in January −13.9°C (EFSA PLH Panel, 2021). Based on locations where E. giganteum is reported in literature such as Anhui, Henan and Hunan in China (Cfa climate); Honshu in Japan (Cfa climate); and Kermanshah in Iran (Csa climate), southern EU countries may provide suitable climatic conditions for establishment. Distribution of the pest in its native range might be broader. The comparison of the current distribution of the pest with the suitability of the environment in the EU indicates that Southern Scandinavia and Southern Europe are climatically suitable but not central Europe. However, the Panel considers that climates between these areas would also enable survival of the pest (a map including Cfb is included in Appendix D).
FIGURE 2.
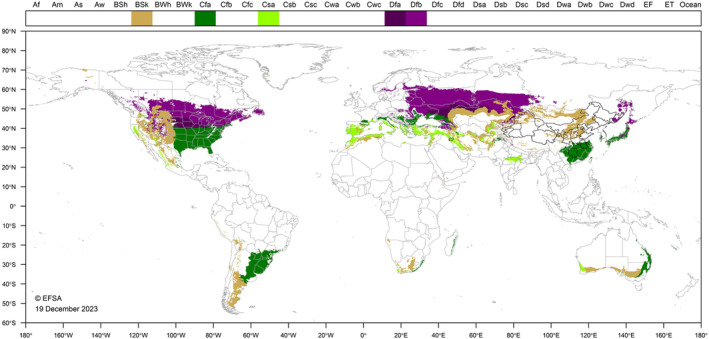
World distribution of Köppen–Geiger climate types that occur in the EU and which occur in countries where Eulecanium giganteum has been reported.
3.4.3. Spread
Describe how the pest would be able to spread within the EU territory following establishment?
Natural spread by first instar nymphs crawling or being carried by wind, or by hitchhiking on other animals, humans or machinery, will occur locally and relatively slowly. All stages may be moved over long distances in trade of infested plant materials, specifically plants for planting, fruits, and cut flowers.
Comment on plants for planting as a mechanism of spread.
Plants for planting provide a main spread mechanism for E. giganteum.
First‐instar nymphs (crawlers) may be carried to neighbouring plants by their own movement, wind or by hitchhiking on clothing, equipment or animals (EFSA PLH Panel, 2020).
Plants for planting, scion and rough wood are the main pathways of spread of E. giganteum, especially over long distances (EFSA PLH Panel, 2022).
3.5. Impacts
Would the pests' introduction have an economic or environmental impact on the EU territory?
This is a key uncertainty. Evidence from Asia indicates E. giganteum is a pest. However, the closely related species E. excrescens, also a pest in Asia, has been established in England since the 1990s but is not an economically important pest due in part to natural enemies providing control.
E. giganteum feeds on the phloem and egests sugary honeydew, which serves as a medium for the growth of sooty moulds. The mould reduces photosynthesis and gas exchange, causing a loss of vigour and yield (Kondo & Watson, 2022). Infestations of E. giganteum may completely cover the lower surfaces of the foliage, forming a dense mat of waxy secretions. Fruits from infested plants and infested ornamental plants are unmarketable (Kondo & Watson, 2022).
In China, E. giganteum is reported to cause serious damage to garden trees (EFSA PLH Panel, 2022). The production of jujube (Ziziphus jujuba) in Xinjiang, China has been reported to be severely threatened by E. giganteum (Deng et al., 2016, Li and Xu, 2013). Xie (1985) reported that in Lanzhou, China, E. giganteum causes serious damage between April and May in black locust (Robinia pseudoacacia), and Japanese pagoda trees (S. japonicum) while in the Kunming area, China, it mainly damages trident maple (Acer buergerianum), and triangle maple (Celtis tetrandra) (Tao et al., 2002). In Taiyuan, China, the population of E. giganteum on its host S. japonicum was reported to be positively correlated with air pollutants (Kondo & Watson, 2022; Xie et al., 1995).
The closely related Eulecanium excrescens (Ferris) is native to Asia, highly polyphagous, and is recorded feeding on many deciduous orchard and ornamental trees. In China, it is reported to be a pest of apple, pear and peach trees, although in the UK and USA, where it has been introduced (Malumphy, 2005), it does not cause economic damage. In the UK, this is due to high levels of parasitism as native parasitoids were rapidly recruited to attack the new resource (CP Malumphy, DEFRA, oral communication at the working group meeting on 9 February, 2024). Several E. giganteum parasitoid species are reported in the EU (Noyes, 2019). In addition, there are predators of E. giganteum that also occur in the EU (Kondo & Watson, 2022). Therefore, should E. giganteum be introduced into the EU, we would expect these natural enemies to accept this new host and provide biological control.
3.6. Available measures and their limitations
Are there measures available to prevent pest entry, establishment, spread or impacts such that the risk becomes mitigated?
Yes. Although the existing phytosanitary measures identified in Section 3.3.2 do not specifically target E. giganteum, they mitigate the likelihood of its entry into, establishment, and spread within the EU (see also Section 3.6.1).
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Additional potential risk reduction options and supporting measures are shown in Sections 3.6.1.1 and 3.6.1.2.
3.6.1.1. Additional potential risk reduction options
Potential additional control measures are listed in Table 6.
TABLE 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance.
| Control measure/risk reduction option (Blue underline = Zenodo doc, Blue = WIP) ) | RRO summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Require pest freedom | Pest‐free place of production (e.g. place of production and its immediate vicinity is free from pest over an appropriate time period, e.g. since the beginning of the last complete cycle of vegetation, or past 2 or 3 cycles). Pest‐free production site | Entry/Establishment/Spread |
| Growing plants in isolation | Place of production is insect proof originate in a place of production with complete physical isolation | Entry (reduce infestation)/Establishment/Spread |
| Managed growing conditions | Used to mitigate likelihood of infestation at origin. Plants collected directly from natural habitats, have been grown, held and trained for at least two consecutive years prior to dispatch in officially registered nurseries, which are subject to an officially supervised control regime | Entry (reduce infestation)/Establishment/Spread |
| Roguing and pruning | Roguing is defined as the removal of infested plants and/or uninfested host plants in a delimited area, whereas pruning is defined as the removal of infested plant parts only without affecting the viability of the plant | Entry/Spread/Impact |
| Biological control and behavioural manipulation |
Zhang and Huang (2001) reported Oriencyrtus liaoi sp. nov. (Hymenoptera: Encyrtidae) as a parasitoid of E. giganteum on willow (Salix spp.) in Zhongwei, China. In northern China, the parasitoid wasp Encyrtus eulecaniumiae sp. nov. (Hymenoptera: Encyrtidae) was reported on E. giganteum (Wang et al., 2016). In Iran (Ghazanchi, Kermanshah) larvae of Dicrodiplosis manihoti (Diptera: Cecidomyiidae) were observed feeding on egg masses and crawlers of E. giganteum on Canadian phlox (Phlox divaricate) (Jalilvand et al., 2013). In Xinjiang, Eunotus aequalivena (Hymenoptera: Pteromalidae) was reported to be a highly parasitic species to E. giganteum (Zhang et al., 2016). Tao et al. (2002) reported that, in Kunming, there are six natural enemies of E. giganteum such as Blastothrix sericea, Metaphycus pulvinariae, Cocophagus hawaiiensis, Microterys ericeri and Cocophagus sp. The first two parasitoid wasps have about 88% parasitism rate under natural conditions (Tao et al., 2002). Some of the parasitoid species that have been recorded to parasitise on E. giganteum in its distribution range, such as Blastothrix sericea, Metaphycus pulvinariae and Cocophagus spp., are also recorded in the EU territory |
Impact |
| Chemical treatments on crops including reproductive material | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments. The effectiveness of insecticide applications against soft scales may be reduced by the waxy coating of the adult. The efficacy of insecticides was tested on different nymphal stages of E. giganteum. Only the control of nymphs at the end of first instar and the beginning of second instar was effective, with mortality rate over 94% (Xie, 1985) | Entry/Establishment/Spread/Impact |
| Cleaning and disinfection of facilities, tools and machinery | The physical and chemical cleaning and disinfection of facilities, tools, machinery, facilities and other accessories (e.g. boxes, pots, hand tools) | Entry/Spread |
| Heat and cold treatments | Controlled temperature treatments aimed to kill or inactivate pests without causing any unacceptable prejudice to the treated material itself. Treatments relevant for this risk mitigation measure are: autoclaving; steam; hot water; hot air; cold treatment | Entry/Spread |
| Controlled atmosphere | Treatment of plants by storage in a modified atmosphere (including modified humidity, O2, CO2, temperature, pressure) | Entry/Spread (via commodity) |
3.6.1.2. Additional supporting measures
Potential additional supporting measures are listed in Table 7.
TABLE 7.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance.
| Supporting measure (Blue underline = Zenodo doc, Blue = WIP) | Summary | Risk element targeted (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping |
ISPM 5 (FAO, 2023) defines inspection as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques |
Entry/Establishment/Spread/Impact |
| Laboratory testing | Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests | Entry/Establishment/Spread |
| Sampling |
According to ISPM 31 (FAO, 2008), it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing For inspection, testing and/or surveillance purposes, the sample may be taken according to a statistically based or a non‐statistical sampling methodology |
Entry/Establishment |
| Phytosanitary certificate and plant passport |
According to ISPM 5 (FAO, 2023), a phytosanitary certificate and a plant passport are official paper documents or their official electronic equivalents, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements: a) export certificate (import) b) plant passport (EU internal trade) |
Entry/Establishment/Spread |
| Certified and approved premises | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by the NPPO in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries | Entry/Spread |
| Certification of reproductive material (voluntary/official) | Plants come from within an approved propagation scheme and are certified pest free (level of infestation) following testing; used to mitigate against pests that are included in a certification scheme | Entry/Spread |
| Delimitation of Buffer zones | ISPM 5 (FAO, 2023) defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’. The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest‐free production place (PFPP), site (PFPS) or area (PFA) | Spread |
| Surveillance | Surveillance to guarantee that plants and produce originate from a pest‐free area could be an option | Establishment/Spread |
3.6.1.3. Biological or technical factors limiting the effectiveness of measures
E. giganteum is polyphagous, making the inspections of all consignments containing hosts from countries where the pest occurs difficult.
Egg masses may be difficult to detect on large trees.
Limited effectiveness of insecticides due to the presence of protective cover over the scales.
Limited biological data on developmental threshold temperatures.
3.7. Uncertainty
Noting that the related species E. excrescens is reported as a pest of apple, pear and peach trees in China, but following its establishment in USA and UK (Malumphy, 2005), has failed to cause any economic and environmental impacts, there is a key uncertainty as to whether E. giganteum will cause economic or environmental impact if it were to establish in the EU.
4. CONCLUSIONS
Eulecanium giganteum satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest, other than the criterion on impact which is a key uncertainty (Table 8).
TABLE 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column).
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1 ) | The identity of E. giganteum is established. Taxonomic keys based on morphology of adults exist. There are also molecular techniques for species identification | None |
| Absence/presence of the pest in the EU (Section 3.2 ) | No, E. giganteum is not known to occur in the EU | None |
| Pest potential for entry, establishment and spread in the EU (Section 3.4 ) | E. giganteum is able to enter, become established and spread within the EU territory especially in the southern EU MS. The main pathways are plants for planting, cut flowers, and fruits | None |
| Potential for consequences in the EU (Section 3.5 ) | The introduction of the pest could cause yield and quality losses on several crops and reduce the value of ornamental plants | There is uncertainty whether E. giganteum will cause economic or environmental impact if it were to establish in the EU |
| Available measures (Section 3.6 ) | There are measures available to prevent entry, establishment and spread of E. giganteum in the EU. Risk reduction options include inspections, chemical and physical treatments on consignments of fresh plant material from infested countries and the production of plants for import in the EU in pest free areas. Natural biological control could prevent impact | None |
| Conclusion (Section 4 ) | E. giganteum satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest, other than the criterion on impact which is a key uncertainty | There is uncertainty whether E. giganteum will cause economic or environmental impact if it were to establish in the EU |
| Aspects of assessment to focus on/scenarios to address in future if appropriate: | ||
ABBREVIATIONS
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
GLOSSARY
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2023).
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2023).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2023).
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2023).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2023).
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Hitchhiker
An organism sheltering or transported accidentally via inanimate pathways including with machinery, shipping containers and vehicles; such organisms are also known as contaminating pests or stowaways (Toy & Newfield, 2010).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2023).
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2023).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2023).
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2023).
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2023).
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2023‐00324
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen, and Lucia Zappalà.
MAP DISCLAIMER
The designations employed and the presentation of material on any maps included in this scientific output do not imply the expression of any opinion whatsoever on the part of the European Food Safety Authority concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries.
APPENDIX A. Eulecanium giganteum host plants/species affected
A.1.
Source: García Morales et al. (2016) (ScaleNet, online), and literature.
| Plant family | Host name | Common name | ReferenceA |
|---|---|---|---|
| Anacardiaceae | Spondias pinnata | Andaman mombin, Indian hog plum, Indian mombin, wild mango | García Morales et al. (2016) |
| Asteraceae | Taraxacum mongolicum | Dandelion | García Morales et al. (2016) |
| Betulaceae | Corylus heterophylla | Japanese hazel, Siberian filbert, Siberian hazel | García Morales et al. (2016) |
| Betulaceae | Corylus sieboldiana | Japanese filbert | García Morales et al. (2016) |
| Cannabaceae | Celtis tetrandra | Nilgiri elm | García Morales et al. (2016) |
| Elaeagnaceae | Elaeagnus angustifolia | Oleaster, Russian olive, Trebizond date, wild olive | García Morales et al. (2016) |
| Fabaceae | Albizia julibrissin | Persian acacia, pink siris, silk tree, varay cotton | García Morales et al. (2016) |
| Fabaceae | Amorpha fruticosa | Bastard indigo, desert false indigo, false indigo, indigo bush | García Morales et al. (2016) |
| Fabaceae | Caragana sinica | Chinese Peashrub | García Morales et al. (2016) |
| Fabaceae | Gleditsia sinensis | Chinese honey locust | García Morales et al. (2016) |
| Fabaceae | Glycyrrhiza uralensis | Chinese liquorice | García Morales et al. (2016) |
| Fabaceae | Halimodendron halodendron | Russian salt tree, Siberian salt tree | García Morales et al. (2016) |
| Fabaceae | Maackia amurensis | Amur maackia | García Morales et al. (2016) |
| Fabaceae | Robinia hispida | Bristly locust, moss locust | García Morales et al. (2016) |
| Fabaceae | Robinia pseudoacacia | Black locust, false acacia, locust, locust tree, robinia | García Morales et al. (2016) |
| Fabaceae | Styphnolobium japonicum * | Japanese pagoda tree, pagoda tree, Сhinese scholar tree | Deng et al. (2016), García Morales et al. (2016) |
| Fabaceae | Wisteria sinensis | Chinese wisteria, purple wisteria | Deng et al. (2016), García Morales et al. (2016) |
| Fagaceae | Quercus acutissima | Japanese chestnut oak, sawtooth oak | García Morales et al. (2016) |
| Fagaceae | Quercus mongolica | Mongolian oak | García Morales et al. (2016) |
| Juglandaceae | Juglans mandshurica | Manchurian walnut | García Morales et al. (2016) |
| Juglandaceae | Juglans regia | Common walnut, Persian walnut, walnut | Deng et al. (2016), García Morales et al. (2016) |
| Lythraceae | Lagerstroemia indica | Cannonball, carrion tree, crepe myrtle, Indian crape myrtle, June rose, lilac of the south | García Morales et al. (2016) |
| Lythraceae | Punica granatum | Carthaginian apple, pomegranate | García Morales et al. (2016) |
| Magnoliaceae | Magnolia denudata | Magnolia yulan, yulan | García Morales et al. (2016) |
| Magnoliaceae | Magnolia kobus | Northern Japanese magnolia | García Morales et al. (2016) |
| Malvaceae | Hibiscus rosa‐sinensis | China rose, Chinese hibiscus, Chinese rose, Hawaiian hibiscus, rose mallow, rose of China, shoe‐black plant, shoe‐flower | Suganthi et al. (2022) |
| Moraceae | Broussonetia papyrifera | Common paper mulberry, paper mulberry, tapa‐cloth tree | Deng et al. (2016), García Morales et al. (2016) |
| Moraceae | Ficus carica | Common fig, fig | García Morales et al. (2016) |
| Moraceae | Morus alba | Silkworm mulberry, white mulberry | García Morales et al. (2016) |
| Oleaceae | Fraxinus bungeana | Bunge ash | García Morales et al. (2016) |
| Oleaceae | Fraxinus chinensis | Chinese ash | García Morales et al. (2016) |
| Oleaceae | Ligustrum quihoui | Waxyleaf privet | García Morales et al. (2016) |
| Platanaceae | Platanus orientalis | Chenar tree, oriental plane | García Morales et al. (2016) |
| Poaceae | Stipa splendens | Chee grass | García Morales et al. (2016) |
| Rhamnaceae | Ziziphus jujuba | Chinese date, Chinese jujube, common jujube, Indian jujube, Indian plum, jujube tree | Deng et al. (2016), García Morales et al. (2016), Zhu et al. (2014) |
| Rosaceae | Cydonia oblonga | Quince | García Morales et al. (2016) |
| Rosaceae | Prunus armeniaca ** | Apricot | Deng et al. (2016), García Morales et al. (2016) |
| Rosaceae | Prunus cerasifera var. divaricata | Jalilvand et al. (2013) | |
| Rosaceae | Rosa | Rose | Deng et al. (2016), García Morales et al. (2016) |
| Rosaceae | Sorbaria kirilowii | Chinese sorbaria | García Morales et al. (2016) |
| Rutaceae | Zanthoxylum bungeanum | Chinese pepper tree, Chinese prickly ash, flat‐spine prickly ash | García Morales et al. (2016) |
| Salicaceae | Populus tomentosa | Chinese white poplar | Deng et al. (2016), García Morales et al. (2016) |
| Salicaceae | Salix | García Morales et al. (2016) | |
| Salicaceae | Salix babylonica | Chinese willow, mourning willow, Peking willow, weeping willow | Deng et al. (2016), García Morales et al. (2016) |
| Sapindaceae | Acer buergerianum | Trident maple | García Morales et al. (2016) |
| Sapindaceae | Acer elegantulum | Elegant maple | Deng et al. (2016), García Morales et al. (2016) |
| Sapindaceae | Acer negundo | Ash‐leaf maple, ash‐leaved maple, box elder, Manitoba maple | García Morales et al. (2016) |
| Sapindaceae | Acer oliverianum ssp. formosanum | Oliver's maple | García Morales et al. (2016) |
| Sapindaceae | Acer pictum | Yellow‐paint maple | García Morales et al. (2016) |
| Sapindaceae | Koelreuteria paniculata | Chinese varnish tree, golden rain, pride of India | Deng et al. (2016), García Morales et al. (2016) |
| Sapindaceae | Xanthoceras sorbifolium *** | Chinese flowering chestnut, goldenhorn, shiny‐leaved yellowhorn, yellowhorn | García Morales et al. (2016) |
| Ulmaceae | Ulmus | García Morales et al. (2016) | |
| Ulmaceae | Ulmus macrocarpa | Large‐fruited elm | Zhang et al. (2016), García Morales et al. (2016) |
| Ulmaceae | Ulmus pumila | Dwarf Asiatic elm, Siberian elm | Deng et al. (2016), García Morales et al. (2016) |
| Vitaceae | Vitis vinifera | Common grapevine | García Morales et al. (2016) |
Reported as Sophora japonica.
Reported as Armeniaca vulgaris.
Reported as Xanthoceras sorbifolia.
APPENDIX B. Distribution of Eulecanium giganteum
B.1.
Distribution records based on García Morales et al. (ScaleNet, online) and literature (Deng et al., 2016; Jalilvand et al., 2013; Suganthi et al., 2022; Zhu et al., 2014).
| Region | Country | Sub‐national (e.g. state) | Status |
|---|---|---|---|
| Asia | China | Anhui | Present, no details |
| Beijing | Present, no details | ||
| Lanzhou, Gansu | Present, no details | ||
| Hebei | Present, no details | ||
| Henan | Present, no details | ||
| Hunan | Present, no details | ||
| Liaoning | Present, no details | ||
| Nei Mongol | Present, no details | ||
| Ningxia | Present, no details | ||
| Qinghai | Present, no details | ||
| Shaanxi | Present, no details | ||
| Shandong | Present, no details | ||
| Taiyuan, Shanxi | Present, no details | ||
| Hami, Xinjiang | Present, no details | ||
| Bazhou, Xinjiang | Present, no details | ||
| Aksu, Xinjiang | Present, no details | ||
| Kashgar, Xinjiang | Present, no details | ||
| Khotan, Xinjiang | Present, no details | ||
| Heilongjiang | Present, no details | ||
| Jilin | Present, no details | ||
| India | Thyagaraja Nagar, Chennai | Present, no details | |
| Iran | Kermanshah | Present, no details | |
| Japan | Honshu | Present, no details | |
| Morioka* | Present, no details | ||
| Russia (non‐European) | Primorsky Krai* | Present, no details |
Deng et al. (2016) declare that in the last decades, the pest had not been reported to be present in Morioka, Japan and Primorsky Krai, non‐European Russia. However, this reference is almost 10 years old and there is no more recent information.
APPENDIX C. Import data
C.1.
TABLE C .1 Edible fruit or nut trees, shrubs and bushes, whether or not grafted) imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 404.63 | 642.61 | 305.32 | 31.80 | 1.68 |
| India | 0.22 | 0.03 | |||
| Iran, Islamic Republic of | 8.17 | ||||
| Japan | 0.95 | 41.26 | 0.55 | 0.40 | 139.38 |
| Russian Federation (Russia) | 2.08 | 189.39 | 1777.42 | 112.21 |
TABLE C .2 Conifer and evergreen outdoor trees, shrubs and bushes, incl. their roots (excl. with bare roots, cuttings, slips, young plants and fruit, nut and forest trees) imported in 100 kg into the EU (27) from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 68.30 | 14.50 | 36.58 | ||
| Japan | 735.11 | 705.20 | 437.92 | 303.18 | 202.38 |
| Russian Federation (Russia) | 295.54 |
TABLE C .3 Grapes, fresh or dried imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 07 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 87,690.22 | 191,986.55 | 156,789.04 | 80,255.76 | 23,689.94 |
| India | 741,303.06 | 970,130.19 | 767,803.65 | 852,065.11 | 896,702.51 |
| Iran, Islamic Republic of | 101,488.05 | 165,329.68 | 201,689.92 | 298,066.29 | 114,871.64 |
| Japan | 1.52 | 1.19 | 21.09 | 34.49 | 4.99 |
| Russian Federation (Russia) | 1.00 | 0.71 | 16.90 | 7.85 | 25.88 |
TABLE C .4 Roses, whether or not grafted imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 2510.23 | 623.75 | 3.01 | 623.10 | 0.00 |
| India | 17.18 | 17.67 | 17.80 | 24.68 | 0.05 |
| Japan | 0.01 | 0.15 | 0.85 | 0.02 | |
| Russian Federation (Russia) | 2.50 | 7.25 |
TABLE C .5 Fresh or dried figs imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 340.30 | 192.97 | 55.21 | 141.58 | 250.59 |
| India | 15.49 | 20.64 | 8.03 | 1.63 | 0.14 |
| Iran, Islamic Republic of | 780.01 | 540.56 | 1055.88 | 718.26 | 542.18 |
| Japan | 0.00 | 0.03 | |||
| Russian Federation (Russia) | 0.01 | 0.00 |
TABLE C .6 Fresh quinces imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 178.68 | 0.01 | |||
| India | |||||
| Iran, Islamic Republic of | 21.75 | ||||
| Japan | |||||
| Russian Federation (Russia) |
TABLE C .7 Apricots, cherries, peaches incl. nectarines, plums and sloes, fresh imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 0.90 | 3.24 | 0.14 | 19.79 | |
| India | 0.45 | 0.00 | 3.76 | 0.81 | |
| Iran, Islamic Republic of | 42.15 | 29.18 | 589.22 | 381.90 | 123.82 |
| Japan | 1.00 | 2.82 | 37.40 | 4.50 | |
| Russian Federation (Russia) | 218.50 | 693.16 |
TABLE C .8 Fresh blackberries, mulberries and loganberries imported in 100 kg into the EU from regions where Eulecanium giganteum is known to occur (Source: Eurostat accessed on 7 November 2023).
| Country | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| China | 53.91 | 20.20 | 0.00 | 88.39 | |
| Iran, Islamic Republic of | 0.80 | 0.06 | |||
| Russian Federation (Russia) | 4.43 |
APPENDIX D. World distribution of Köppen–Geiger climate types that occur in the EU and which occur in countries where E. giganteum has been reported (including Cfb climate type)
D.1.
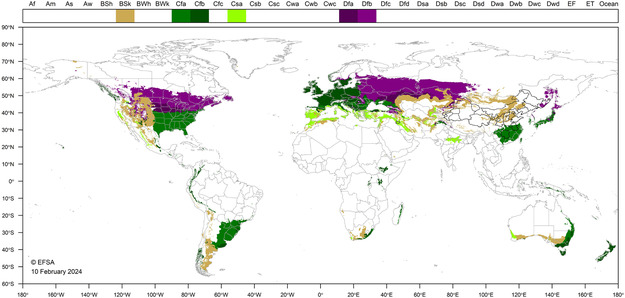
EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , Zappalà, L. … MacLeod, A. (2024). Pest categorisation of Eulecanium giganteum . EFSA Journal, 22 (4), e8666. 10.2903/j.efsa.2024.8666
Adopted: 22 February 2024
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger & Roy, 2015; EPPO, 2019).
REFERENCES
- Baker, R. H. A. (2002). Predicting the limits to the potential distribution of alien crop pests. In Hallman G. J. & Schwalbe C. P. (Eds.), Invasive arthropods in agriculture: Problems and solutions (pp. 207–241). Science Publishers Inc. [Google Scholar]
- Baker, R. H. A. , Sansford, C. E. , Jarvis, C. H. , Cannon, R. J. C. , MacLeod, A. , & Walters, K. F. A. (2000). The role of climatic mapping in predicting the potential geographical distribution of non‐indigenous pests under current and future climates. Agriculture, Ecosystems & Environment, 82(1‐3), 57–71. [Google Scholar]
- Ben‐Dov, Y. (1993). A systematic catalogue of the soft scale insects of the world (Homoptera: Coccoidea: Coccidae) (p. 536). Sandhill Crane Press Gainesville. [Google Scholar]
- Bethke, J. A. , & Wilen, C. A. (2010). UC IPM pest management guidelines: floriculture and ornamental nurseries, UCANR Publication 3392. https://ipm.ucanr.edu/agriculture/floriculture‐and‐ornamental‐nurseries/soft‐scales/
- Camacho, E. R. , & Chong, J. H. (2015). General biology and current management approaches of soft scale pests (Hemiptera: Coccidae). Journal of Integrated Pest Management, 6, 1–22. 10.1093/jipm/pmv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzig, E. M. (1980). Coccoids of the Far East USSR (Homoptera, Coccinea) with phylogenetic analysis of scale insects of the world. Nauka, Leningrad. 367 pp. [in Russian, translated into English and published in 1986 by Amerind publishing Co., New Delhi, India. 450 pp.].
- Deng, J. , Li, H.‐B. , Wang, X.‐B. , Yu, F. , Zhang, Y.‐Z. , & Wu, S.‐A. (2016). Molecular identification of two morphologically similar Eulecanium species: E. Giganteum and E. Kuwanai (Hemiptera: Coccidae). The Canadian Entomologist, 148(1), 1–7. 10.4039/tce.2015.42 [DOI] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Baptista, P. , Chatzivassiliou, E. , Di Serio, F. , Jaques Miret, J. A. , Justesen, A. F. , MacLeod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Stefani, E. , Thulke, H.‐H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , … Gonthier, P. (2022). Scientific opinion on the commodity risk assessment of Acer palmatum plants grafted on Acer davidii from China. EFSA Journal, 20(5), 7298. 10.2903/j.efsa.2022.7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Dehnen‐Schmutz, K. , Di Serio, F. , Gonthier, P. , Jacques, M. A. , Jaques Miret, J. A. , Justesen, A. F. , Mac Leod, A. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Reignault, P. L. , Thulke, H.‐H. , Van der Werf, W. , Civera, A. V. , Yuen, J. , Zappalà, L. , … Potting, R. (2020). Scientific opinion on the commodity risk assessment of Jasminum polyanthum plants from Israel. EFSA Journal, 18(8), 6928. 10.2903/j.efsa.2020.6225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C. , Di Serio, F. , Gonthier, P. , Jaques Miret, J. A. , Justesen, A. F. , Magnusson, C. S. , Milonas, P. , Navas‐Cortes, J. A. , Parnell, S. , Potting, R. , Reignault, P. L. , Thulke, H. H. , Van der Werf, W. , Vicent Civera, A. , Yuen, J. , Zappalà, L. , Grégoire, J.‐C. , Malumphy, C. , … MacLeod, A. (2021). Scientific opinion on the pest categorisation of Crisicoccus pini . EFSA Journal, 19(11), 6928. 10.2903/j.efsa.2021.6928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , Jeger, M. , Bragard, C. , Caffier, D. , Candresse, T. , Chatzivassiliou, E. , Dehnen‐Schmutz, K. , Gregoire, J.‐C. , Jaques Miret, J. A. , MacLeod, A. , Navajas Navarro, M. , Niere, B. , Parnell, S. , Potting, R. , Rafoss, T. , Rossi, V. , Urek, G. , Van Bruggen, A. , Van Der Werf, W. , … Gilioli, G. (2018). Guidance on quantitative pest risk assessment. EFSA Journal, 16(8), 5350. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy, A. , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Benfenati, E. , Chaudhry, Q. M. , Craig, P. , … Younes, M. (2017). Scientific opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal, 15(8), 4971. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) . (2019). EPPO codes. https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO (European and Mediterranean Plant Protection Organization) . (online). EPPO Global Database. https://gd.eppo.int [Accessed on: 20 December 2023].
- FAO (Food and Agriculture Organization of the United Nations) . (2008). ISPM (international standards for Phytosanitary measures) No 31. Methodologies for sampling of consignments. FAO, Rome, 19 pp. https://www.ippc.int/static/media/files/publication/en/2016/11/ISPM_31_2008_Sampling_of_consignments_EN.pdf
- FAO (Food and Agriculture Organization of the United Nations) . (2013). ISPM (international standards for Phytosanitary measures) No 11. Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014‐04‐30_201405121523‐494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations) . (2023). ISPM (international standards for Phytosanitary measures) No 5. Glossary of phytosanitary terms. FAO, Rome, 40 pp. https://assets.ippc.int/static/media/files/publication/en/2023/07/ISPM_05_2023_En_Glossary_PostCPM‐17_2023‐07‐12_Fixed.pdf
- García Morales, M. , Denno, B. D. , Miller, D. R. , Miller, G. L. , Ben‐Dov, Y. , & Hardy, N. B. (2016). ScaleNet: A literature‐based model of scale insect biology and systematics. Database: The Journal of Biological Databases and Curation, 2016, 1–5. 10.1093/database/bav118 [Accessed on: 20 December 2023]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessinger, D. , & Roy, A.‐S. (2015). EPPO codes: a brief description. https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Jalilvand, K. , Shirazi, M. , Vahedi, H. , Fallahzadeh, M. , Naghadeh, N. , & Samih, M. (2013). A preliminary study on natural enemies of coccoidea (Hemiptera, Sternorrhyncha) in Kermanshah Province, Western Iran. Acta Phytopathologica et Entomologica Hungarica, 48(2), 299–308. 10.1556/aphyt.48.2013.2.11 [DOI] [Google Scholar]
- Kondo, T. , & Watson, G. W. (Eds.). (2022). Encyclopedia of scale insect pests (p. 608). CABI International. [Google Scholar]
- Kottek, M. , Grieser, J. , Beck, C. , Rudolf, B. , & Rubel, F. (2006). World map of the Köppen_Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. 10.1127/0941-2948/2006/0130 [DOI] [Google Scholar]
- Li, J. , & Xu, Q. (2013). Eulecanium gigantea occurrence and control measures. Shaanxi Forest Science Technology, 2, 81–83. [Google Scholar]
- Malumphy, C. P. (2005). Eulecanium excrescens (Ferris) (Hemiptera: Coccidae), an Asian pest of woody ornamentals and fruit trees, new to Britain. British Journal of Entomology and Natural History, 18, 45–49. https://www.biodiversitylibrary.org/partpdf/263854 [Google Scholar]
- Malumphy, C. P. (2024). Personal communication at the working group meeting. 9 February, 2024.
- Noyes, J. S. (2019). Universal Chalcidoidea database. World Wide Web Electronic Publication. https://www.nhm.ac.uk/chalcidoids
- Sayers, E. W. , Cavanaugh, M. , Clark, K. , Ostell, J. , Pruitt, K. D. , & Karsch‐Mizrachi, I. (2020). Genbank. Nucleic Acids Research, 48(Database issue), D84–D86. 10.1093/nar/gkz956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. L. , & Lü, J. P. (1989). Studys on morphology and biology of Eulecanium kuwanai (Kanda). Journal of Shandong Agricultural University, 1, 12–19. [Google Scholar]
- Suganthi, M. , Logeshwaran, R. , Abirami, G. , Rupa Shree, B. , Anandaraj, P. , & Senthilkumar, P. (2022). Molecular identification of scale insect (Eulecanium giganteum) in Hibiscus rosa‐sinensis . Journal of Experimental Biology and Agricultural Sciences, 10(4), 797–804. 10.18006/2022.10(4).797.804 [DOI] [Google Scholar]
- Tao, M. , Guohua, C. , & Benli, H. (2002). Research of parasitoid of E. Giganteum (Shinji) in Kunming. Journal of Yunnan Agricultural University, 17(3), 225–227 [In Chinese]. [Google Scholar]
- Toy, S. J. , & Newfield, M. J. (2010). The accidental introduction of invasive animals as hitchhikers through inanimate pathways: A New Zealand perspective. Revue Scientifique et Technique (International Office of Epizootics), 29(1), 123–133. [DOI] [PubMed] [Google Scholar]
- Wang, T. C. (1980). Handbook for the determination of the common coccoids. Chinese Academy of Sciences, p. 252. [Google Scholar]
- Wang, H. (2000). A Study on Eulecanium Gigantea . Acta Agriculturae Boreali‐Occidentalis Sinica, 9, 83–86. [Google Scholar]
- Wang, Y. , Zhou, Q. S. , Qiao, H. J. , Yu, F. , Ai‐B, Z. , Wang, X. B. , Zhu, C. D. , & Zhang, Y. Z. (2016). Formal nomenclature and description of cryptic species of the Encyrtus sasakii complex (hymenoptera: Encyrtidae). Scientific Reports, 6, 34372. 10.1038/srep34372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. I. , Ai‐H, L. , Zhang, J. W. , Zhao, B. J. , Yue, Z. Y. , Zhang, X. P. , & Tang, L. (2012). Coincidence level in spatial distribution of Eulecanium gigantea and Blastothrix sericae[J]. Journal of Zhejiang A&F University, 29(5), 799–802. 10.11833/j.issn.2095-0756.2012.05.026 [DOI] [Google Scholar]
- Xie, X. X. (1985). A preliminary study on Eulecanium gigantea . Scientia Silvae Sinicae (Linye Kexue), 21, 44–52. [In Chinese]. [Google Scholar]
- Xie, Y. P. , Liu, X. , Li, J. , & Tang, M. (1995). The effect of urban air pollution on populations of Eulecanium gigantea (Shinji) (Coccidae) in Taiyuan City, China. Israel Journal of Entomology, 29, 165–168. [Google Scholar]
- Xue, J. , Xie, Y. , Liu, H. , Liu, J. , & Li, Y. (1999). The effect of air pollution on Sophora japonica (Leguminosae) and Eulecanium giganteum (Shinji) (Hemiptera: Coccoidea: Coccidae) in urban areas in China. Entomologica‐Bari, 33, 383–388. [Google Scholar]
- Yue, C. , Zhang, J. , & Zhang, X. (2011). Damage Degularity and control techniques of Eulecanium giganteum around Tarim Basin in Xinjiang. Procedia Engineering, 18, 133–138. 10.1016/j.proeng.2011.11.021 [DOI] [Google Scholar]
- Zhang, P. , Xiao, H. , Li, Q. , Ma, J. , & Xue, G. (2016). Eunotus aequalivena, an important parasitoid of Eulecanium gigantea . Chinese Journal of Biological Control, 32(1), 125–128. 10.16409/j.cnki.2095-039x.2016.01.019 [DOI] [Google Scholar]
- Zhang, Y. Z. , & Huang, D. W. (2001). Two new Encyrtid parasites (hymenoptera: Chalcidoidea) from China. Oriental Insects, 35(1), 311–319. 10.1080/00305316.2001.10417310 [DOI] [Google Scholar]
- Zhao, X. , & Xie, Y. (2004). Morphological characteristics of the different developmental stages of the male scale insect, Eulecanium giganteum . Entomological Knowledge, 41, 60–64. [Google Scholar]
- Zhu, X. S. , Wang, D. Y. , & Yu, J. N. (2014). Genetic variation of Eulecanium giganteum (Shinji) geographical populations and its correlations with ecological factors. Chinese Journal of Ecology, 33, 1267–1273. [Google Scholar]


