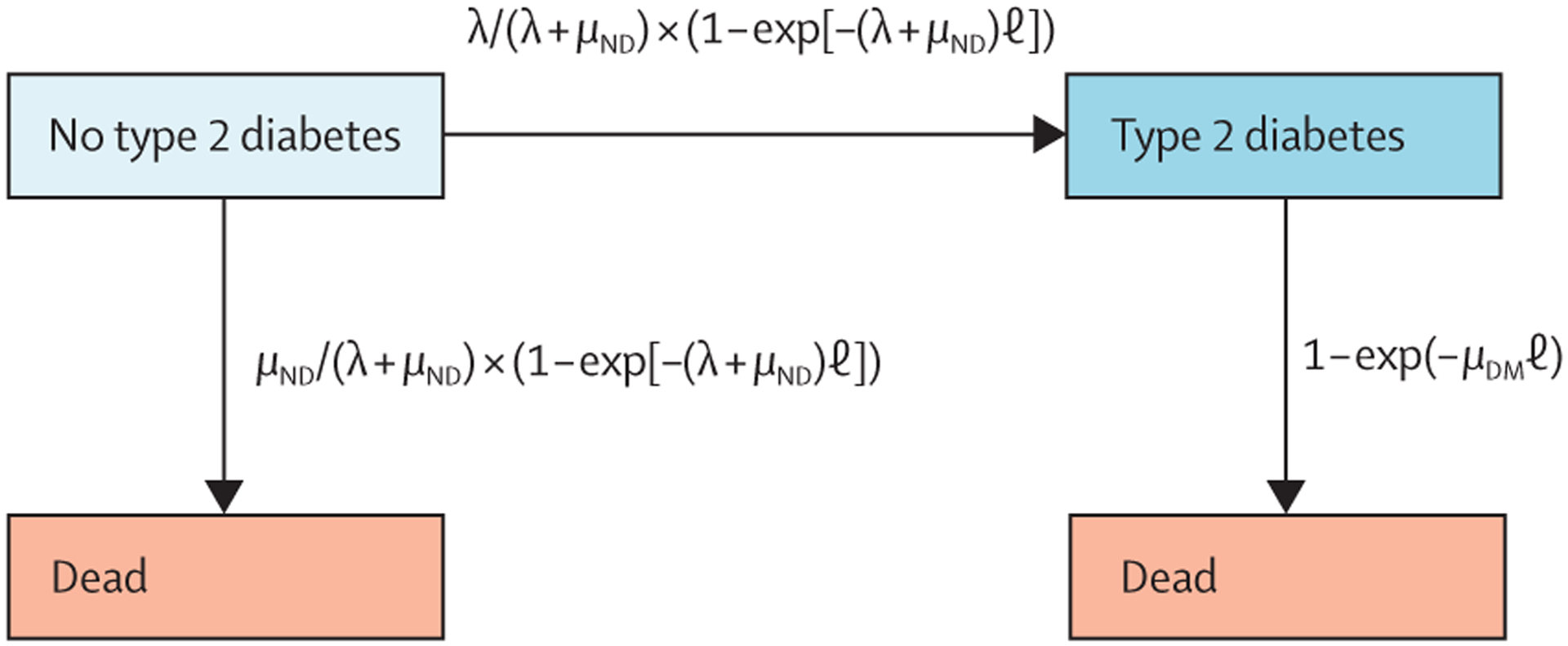
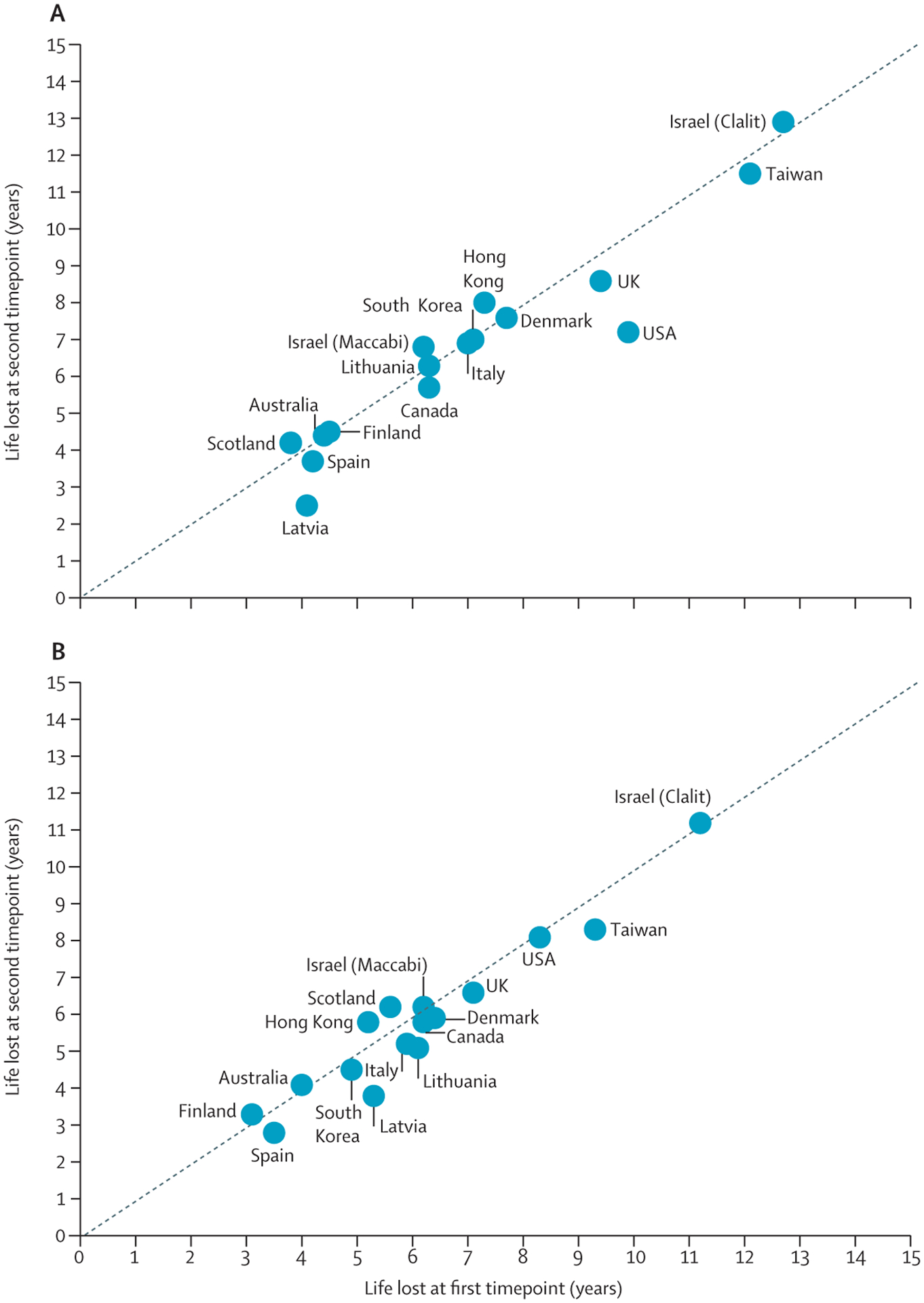
Dunya Tomic
Dunya Tomic
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Jedidiah I Morton
Jedidiah I Morton
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Lei Chen
Lei Chen
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Agus Salim
Agus Salim
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Edward W Gregg
Edward W Gregg
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Meda E Pavkov
Meda E Pavkov
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Martti Arffman
Martti Arffman
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Ran Balicer
Ran Balicer
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Marta Baviera
Marta Baviera
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Elise Boersma-van Dam
Elise Boersma-van Dam
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Ralph Brinks
Ralph Brinks
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Bendix Carstensen
Bendix Carstensen
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Juliana C N Chan
Juliana C N Chan
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Yiling J Cheng
Yiling J Cheng
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Sandrine Fosse-Edorh
Sandrine Fosse-Edorh
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Sonsoles Fuentes
Sonsoles Fuentes
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Hélène Gardiner
Hélène Gardiner
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Hanne L Gulseth
Hanne L Gulseth
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Romualdas Gurevicius
Romualdas Gurevicius
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Kyoung Hwa Ha
Kyoung Hwa Ha
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Annika Hoyer
Annika Hoyer
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
György Jermendy
György Jermendy
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Alexandra Kautzky-Willer
Alexandra Kautzky-Willer
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Ilmo Keskimäki
Ilmo Keskimäki
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Dae Jung Kim
Dae Jung Kim
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Zoltán Kiss
Zoltán Kiss
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Peter Klimek
Peter Klimek
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Maya Leventer-Roberts
Maya Leventer-Roberts
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Chun-Yi Lin
Chun-Yi Lin
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Paz Lopez-Doriga Ruiz
Paz Lopez-Doriga Ruiz
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Andrea O Y Luk
Andrea O Y Luk
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Stefan Ma
Stefan Ma
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Manel Mata-Cases
Manel Mata-Cases
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Dídac Mauricio
Dídac Mauricio
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Stuart McGurnaghan
Stuart McGurnaghan
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Tomoaki Imamura
Tomoaki Imamura
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Sanjoy K Paul
Sanjoy K Paul
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Anna Peeters
Anna Peeters
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Santa Pildava
Santa Pildava
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Avi Porath
Avi Porath
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Cynthia Robitaille
Cynthia Robitaille
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Maria Carla Roncaglioni
Maria Carla Roncaglioni
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Takehiro Sugiyama
Takehiro Sugiyama
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Kang-Ling Wang
Kang-Ling Wang
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Sarah H Wild
Sarah H Wild
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Naama Yekutiel
Naama Yekutiel
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,
Jonathan E Shaw
Jonathan E Shaw
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,*,
Dianna J Magliano
Dianna J Magliano
1Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (D Tomic MBBS(Hons), J I Morton BSc(Hons), L Chen PhD, A Salim PhD, Prof J E Shaw MD, Prof D J Magliano PhD); School of Public Health and Preventive Medicine (D Tomic, J I Morton, Prof J E Shaw, Prof D J Magliano) and Centre for Medicine Use and Safety (J I Morton), Monash University, Melbourne, VIC, Australia; Melbourne School of Population and Global Health (A Salim), School of Mathematics and Statistics (A Salim), and Department of Medicine (Prof S K Paul PhD), University of Melbourne, Melbourne, VIC, Australia; Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Diseases Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, Y J Cheng PhD); Welfare State Research and Reform, Finnish Institute for Health and Welfare, Helsinki, Finland (M Arffman MSc, Prof I Keskimäki PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research, IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc); Institute for Biometrics and Epidemiology, German Diabetes Center, Duesseldorf, Germany (Prof R Brinks PhD); Institute for Medical Biometry and Epidemiology, University Witten/Herdecke, Witten, Germany (Prof R Brinks); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Herlev, Denmark (B Carstensen MSc[Stat]); Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Sciences, Hong Kong Institute of Diabetes and Obesity, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh PhD, S Fuentes MPH); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (H Gardiner MPH, C Robitaille MSc); Department for Chronic Diseases, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius PhD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (Prof K H Ha PhD, Prof D J Kim MD); Biostatistics and Medical Biometry, Medical School EWL, Bielefeld University, Bielefeld, Germany (Prof A Hoyer PhD); Third Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Department of Medicine III, Endocrinology and Metabolism (Prof A Kautzky-Willer MD) and Section for Science of Complex Systems (Prof P Klimek PhD), Medical University of Vienna, Vienna, Austria; Gender Institute, Gars am Kamp, Austria (Prof A Kautzky-Willer); Faculty of Social Sciences, Tampere University, Tampere, Finland (Prof I Keskimäki); Second Department of Medicine and Nephrological Center, University of Pécs, Pécs, Hungary (Z Kiss MD); Complexity Science Hub Vienna, Vienna, Austria (Prof P Klimek); Department of Pediatrics (M Leventer-Roberts) and Department of Environmental Medicine and Public Health (M Leventer-Roberts), Icahn School of Medicine at Mount Sinai, New York, NY, USA; General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (S Ma PhD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases MD, Prof D Mauricio MD); Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, Prof D Mauricio); Department of Endocrinology, Hospital de la Santa Creu I Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (Prof D Mauricio); Usher Institute, University of Edinburgh, Edinburgh, UK (S McGurnaghan PhD, Prof S H Wild PhD); Department of Public Health, Health Management and Policy, Nara Medical University, Nara, Japan (Prof T Imamura MD); Institute for Health Transformation, Deakin University, Melbourne, VIC, Australia (Prof A Peeters PhD); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg.Soc); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health Sciences, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Diabetes and Metabolism Information Center, Research Institute (T Sugiyama MD) and Institute for Global Health Policy Research, Bureau of International Health Cooperation (T Sugiyama), National Center for Global Health and Medicine, Tokyo, Japan; Department of Health Services Research, University of Tsukuba, Tsukuba, Japan (T Sugiyama)
1,*