Abstract
Background
To address the COVID-19 pandemic and future threats, VA leadership assembled research and clinical teams to coordinate a unified response, which included creating the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD).
Observations
VA SHIELD is a comprehensive specimen and data repository. It links specific types of biospecimens with data regarding genetics, exposure, and disease risk by connecting data sources and the collections of biospecimens across clinical and research environments. Researchers can test novel diagnostic platforms and therapeutics for new and existing diseases, allowing for an expedited, more robust, and informed response. The existing longitudinal disease risk-factor information, records of causal processes, and outcomes data present an unparalleled opportunity to optimize prevention, diagnosis, and treatment of many acute and chronic diseases.
Conclusions
VA SHIELD will expand to become an enterprise resource for investigators and public health officials. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement. VA SHIELD has the opportunity to transform the VA research enterprise by creating an entirely new biorepository.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary by research aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
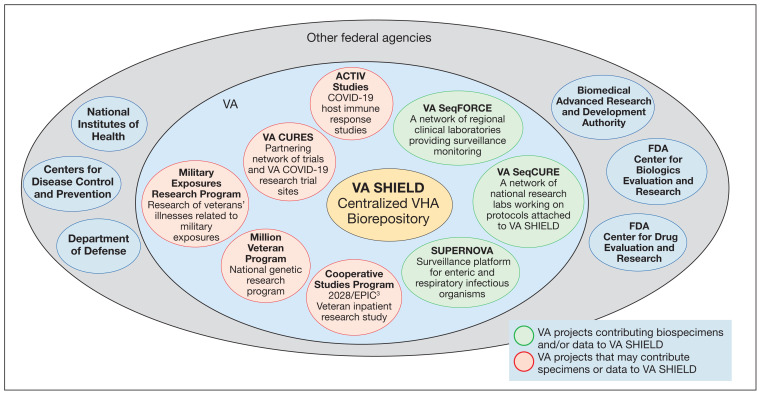
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
FIGURE.
VA SHIELD Intra- and Extramural Activities
Abbreviations: ACTIV, Accelerating COVID-19 Therapeutic Interventions and Vaccination; FDA, US Food and Drug Administration; SeqCURE, Sequencing Collaborations United for Research and Epidemiology; SeqFORCE, Sequencing for Research Clinical and Epidemiology; SHIELD, Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases; SUPERNOVA, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers; VA, US Department of Veterans Affairs.
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
From inception, VA SHIELD has discussed best practices for human research subject protection, how to ensure veterans’ privacy and protecting personal health information, and how to assess the benefit-risk ratio of veterans who participate in the biorepository. Ethical principles on access to and use of veteran data are embedded in human subject protection plans and patient consent. The PSRB is responsible for reviewing specimen use and data access requests in accordance with established programmatic and scientific goals. The PSRB balances limited sample availability by prioritizing requests to ensure utilization of biospecimens occurs in accordance with the guidelines, protocols, and strategic objectives of VA SHIELD.
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
CONCLUSIONS
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
Footnotes
Disclaimer: The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Ethics and consent: Not applicable.
Author disclosures: The authors report no actual or potential conflicts of interest or outside sources of funding with regard to this article.
References
- 1.Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380–386. doi: 10.1001/jamasurg.2020.6372. [DOI] [PubMed] [Google Scholar]
- 2.Zucker S, Crabbe JC, Cooper G, 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481–1486. doi: 10.1096/fj.04-1573lfe. [DOI] [PubMed] [Google Scholar]
- 3.Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi: 10.1093/ofid/ofac641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meites E, Bajema KL, Kambhampati A, et al. SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi: 10.3389/fpubh.2021.739076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajema KL, Dahl RM, Evener SL, et al. SUPERNOVA COVID-19 Surveillance Group. Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700–1705. doi: 10.15585/mmwr.mm7049a2externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44–S47. doi: 10.12788/fp.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274–e275. doi: 10.1016/S2468-2667(19)30081-7. [DOI] [PubMed] [Google Scholar]
- 8.Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451–40452. [Google Scholar]