Abstract
Introduction:
Bacteremia caused by Staphylococcus aureus is common, with cases caused by methicillin-resistant S. aureus (MRSA) especially formidable and often lethal. The mortality associated with MRSA bacteremia has not significantly decreased over the past couple of decades, and concerns about the efficacy and toxicity of standard therapy highlight the need for novel agents and therapeutic approaches to emerge.
Areas covered:
This review paper will explore clinical trials that investigate novel therapeutic approaches to treat S. aureus bacteremia, focusing on MRSA bacteremia. Specifically, this paper discusses monotherapy and combination therapy approaches, while also reviewing novel antimicrobials and adjunctive therapies that are only recently being established for therapeutic use.
Expert opinion:
The unfavorable safety profile of combination antimicrobial therapy in clinical trials has outweighed its benefits. Therefore, future investigation should focus on optimizing duration and de-escalation protocols. Antibody and bacteriophage lysin-based candidates have mostly been limited to safety trials, but progress with these agents is demonstrated through a lysin-based agent receiving a phase III trial. Antibiotics indicated for use in treating MRSA skin infections see continued investigation as treatments for MRSA bacteremia despite the difficulty of completing trials in this patient population. Overall, promising agents include dalbavancin, ceftobiprole, ceftaroline, and exebacase.
Keywords: Bacteremia, clinical trials, investigational agent, MRSA, Staphylococcus aureus
1. Introduction
Staphylococcus aureus is an important human pathogen that causes a wide variety of infections such as osteomyelitis, pneumonia, skin and soft tissue infections (SSTIs), endocarditis, and bacteremia. S. aureus is a leading cause of bacteremia in industrialized nations1, which is associated with significant mortality2. Although methicillin-sensitive S. aureus (MSSA) accounts for most cases of bacteremia3, 4, bacteremia caused by methicillin-resistant S. aureus (MRSA) is associated with worse clinical outcomes for patients5, 6. While the incidence of hospital-onset MRSA bacteremia decreased annually by 17.1% in hospitals in the United States from 2005 to 2012, the decline slowed between 2013 and 20167. The 30-day all-cause mortality of S. aureus bacteremia (SAB) is between 18% and 30%5,7,8, 9, and mortality due to MRSA bacteremia has not changed significantly over time10.
As antibiotic resistance continues to be a significant problem for therapeutic efficacy, there is a need to identify novel therapeutic agents and strategies to treat SAB. Therefore, this review will discuss novel therapeutic approaches in clinical trials, both completed and in progress, to discuss therapeutic compounds and approaches that may show promise as treatments for SAB, with a focus on MRSA bacteremia. This review will highlight the use of monotherapies as alternatives to standard of care (SOC), combination therapy, and novel antimicrobials and adjunctive therapies.
2. Treatment guidelines for SAB
The development of SAB is frequently associated with other primary infection sites, such as SSTIs, infective endocarditis, pneumonia, or infected indwelling medical devices. SAB can also lead to metastatic infections such as infective endocarditis, septic arthritis, and osteomyelitis11, underscoring the importance of resolving the infection in a timely manner. Current treatment guidelines for SAB are based on the antibiotic resistance of the isolate as well as the classification of the bacteremia as complicated or uncomplicated (Figure 1). Uncomplicated bacteremia is defined as a positive blood culture plus the following12: 1) exclusion of endocarditis; 2) no implanted prostheses; 3) blood cultures obtained after 2–4 days after the first set of blood cultures that do not contain MRSA; 4) defervescence within 72 hours of the beginning of therapy; 5) no evidence of metastatic infection. Complicated bacteremia is defined as a positive blood culture and failure to meet the criteria of uncomplicated bacteremia. Vancomycin and daptomycin are the standard therapies to treat MRSA bacteremia and are the only treatments that are approved by the US Food and Drug Administration (FDA) for this purpose.
Figure 1.
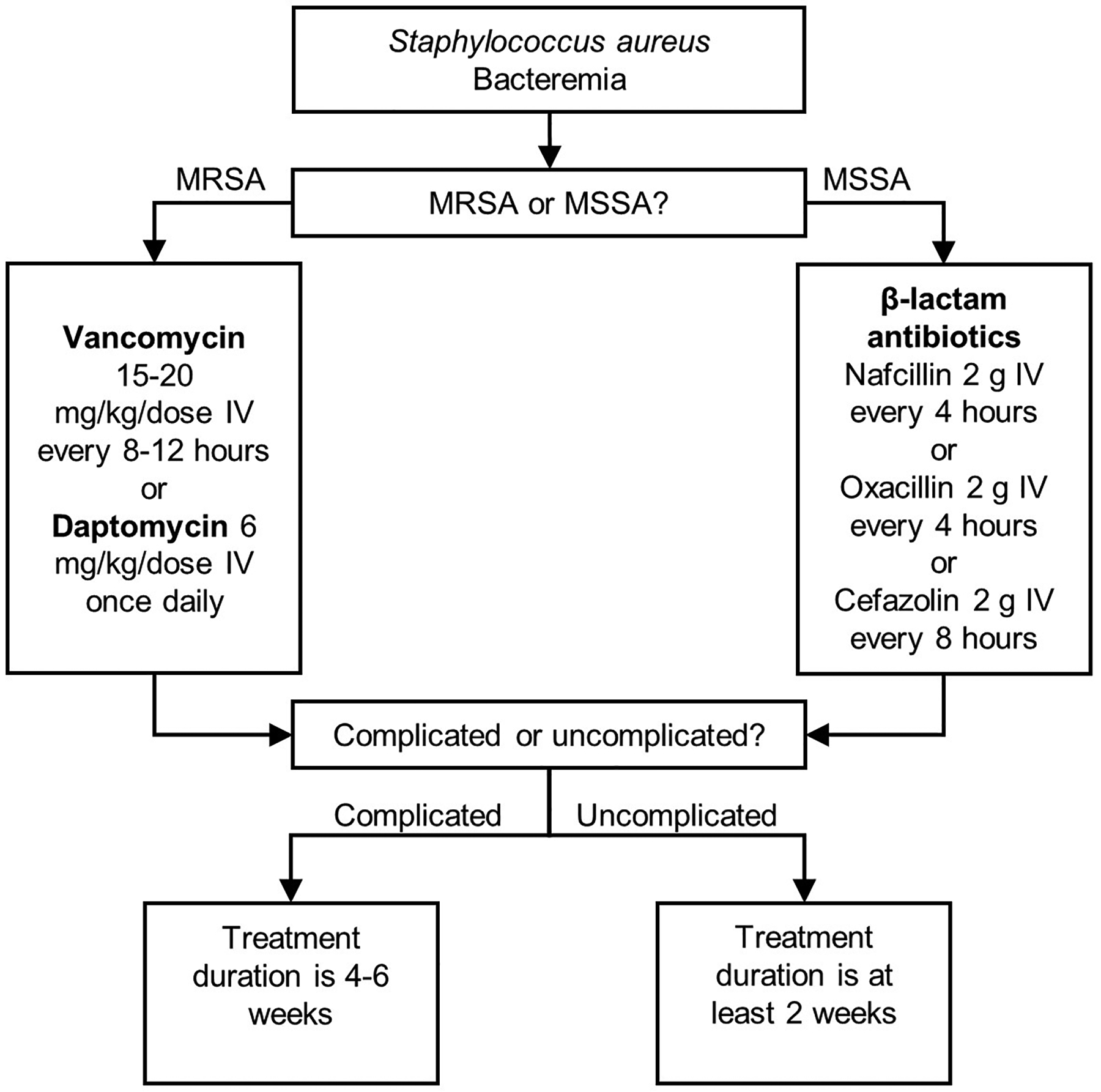
Treatment guidelines for bacteremia caused by Staphylococcus aureus.
3. Standard of care
3.1. Vancomycin
Vancomycin is a glycopeptide antibiotic that exerts antimicrobial activity by binding to d-alanyl d-alanine, which results in the inhibition of peptidoglycan polymerization by preventing the incorporation of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM) into the growing peptidoglycan layer13. The dosage and route of administration for vancomycin as a treatment for MRSA bacteremia are outlined in Figure 1. Trough levels of 15–20 mg/L should be maintained for the treatment of MRSA infections; however, such trough levels come with an increased risk of nephrotoxicity, and therefore must be monitored to assess patient risk14.Vancomycin is inferior to β-lactams for the treatment of patients with MSSA bacteremia and should not be administered in these cases, as its usage is associated with poor patient outcomes15. Vancomycin efficacy is also limited by its poor tissue penetration and slow bactericidal activity16. There is also the concern of a rise in bacterial resistance to vancomycin, mostly in the form of an ‘MIC (minimum inhibitory concentration) creep,’ which refers to an increase in the MIC of an antibiotic against a particular organism over time. While some studies have found an increase in the MIC of vancomycin against S. aureus clinical isolates in specific hospitals and regions over time17, 18, a meta-analysis found no such evidence that the phenomenon exists on a worldwide scale19. It is thought that differences in the laboratory methods used to determine MIC values20, 21 as well as differences in clonal dissemination22 may impact findings regarding the MIC creep. S. aureus isolates with some degree of vancomycin resistance are classified by their MICs in three groups23: 1) vancomycin-resistant S. aureus (VRSA), MIC ≥ 16 μg/mL 2) vancomycin-intermediate S. aureus (VISA), MIC between 4–8 μg/mL 3) heterogeneous VISA (hVISA), sub-population of cells (< 1 per 100,000 colony-forming units (CFU)) with an MIC between 4–8 μg/mL. Resistance mechanisms encountered in VISA strains include the thickening of the cell wall, which limits the localization of vancomycin to the division septum, as well as decreased autolysis24. High levels of vancomycin resistance seen in VRSA stains is conferred by the vanA operon, which is responsible for the production of d-alanyl d-lactate which has a poor affinity for vancomycin and other glycopeptides (teicoplanin, dalbavancin, telavancin)25, 26. The global prevalence of all three vancomycin-resistance groups has increased from pre-2010 to 2019, with VRSA increasing 2.0-fold (1.2% to 2.4%), VISA 3.6-fold (1.2% to 4.3%), and hVISA 1.3-fold (4.0% to 5.3%)27. There is no definitive link between the MIC of vancomycin and MRSA bacteremia patient outcomes. One meta-analysis found an association between a high vancomycin MIC value (≥ 1.5 μg/mL) and increased mortality28, while a meta-analysis and two retrospective cohort studies reported a correlation between increasing vancomycin MIC values and an increased risk of treatment failure28–30. However, other retrospective, single-center studies have found no relationship between vancomycin MIC and patient mortality31, 32. Nevertheless, the prevalence of VISA and hVISA has been increasing since 200633, and with vancomycin continuing to see significant use, it is plausible that isolates with decreased susceptibility to vancomycin may continue to emerge and threaten patient outcomes.
3.2. Daptomycin
Daptomycin is a lipopeptide antibiotic with a controversial mechanism of action, although it is generally accepted that daptomycin penetrates into the bacterial cell membrane, which disrupts membrane integrity and leads to cell death and membrane depolarization34. However, more recent studies have demonstrated that daptomycin’s mechanism of action might be more specific than previously thought, as it was shown that daptomycin can also bind to lipid II and disrupt cell wall biosynthesis35.While the dosage recommendation for daptomycin is 6 mg/kg/dose12, physicians often prescribe higher dosages in the range of 8–10 mg/kg/dose36, 37. Like vancomycin, the development of antibiotic resistance is also a concern with the use of daptomycin. Resistance mechanisms include the modification of membrane composition to decrease daptomycin binding. Mutations in mprF lead to increased conversion of phosphatidylglycerol (PG) to lysine-PG, which is positively charged and disrupts daptomycin binding, while overexpression of the dlt operon leads to an increase in biosynthesis of the machinery that attaches alanine to wall teichoic acid (WTA), which disrupts daptomycin binding by positively increasing the cell-surface charge34. In a clinical trial comparing the efficacy of daptomycin versus SOC to treat bacteremia and endocarditis caused by S. aureus, isolates retrieved during or after treatment from the daptomycin-treated group became less susceptible to daptomycin in 6 of 19 patients that experienced microbiological failure38. Daptomycin MIC values can also be correlated with those of vancomycin39, and up to 15% of hVISA isolates have a daptomycin-nonsusceptibility phenotype40. The use of daptomycin is inappropriate in patients with MRSA bacteremia secondary to pneumonia, as daptomycin is inactivated by the PG content of pulmonary surfactant41.
4. Investigational agents for the treatment of MRSA bacteremia (Table 1)
Table 1.
Investigational agents for the treatment of staphylococcal bacteremia in clinical trials
| Clinicaltrials.gov Identifier | Intervention | Phase | Proposed Sample Size/Enrollment | Primary Endpoints | Status | Results |
|---|---|---|---|---|---|---|
| Monotherapy | ||||||
| NCT03138733 | Ceftobiprole vs. daptomycin (with or without Aztreonam) | III | 390 | Overall success at the post-treatment evaluation (around day 70) | Active | N/A |
| NCT04775953 | Dalbavancin vs. SOC | II | 200 | Resolution of clinical signs and symptoms, clinical failure, infectious complications | Recruiting | N/A |
| NCT00062647 | Telavancin vs. SOC for uncomplicated SAB | II | 60 (approximately 50% of patients had MRSA) | Presence or absence of clinical signs and symptoms associated with SAB, metastatic complications, positive culture at TOC evaluation | Completed | 7/8 patients (88%) given telavancin were cured of infection compared to 8/9 patients (89%) given SOC. |
| NCT02208063 | Telavancin vs. SOC | III | 121 | Alive at TOC, resolution of clinical signs and symptoms, no evidence of microbiological persistence or relapse, no new metastatic infections | Completed (terminated early) | 22/47 (47%) patients given telavancin were cured of infection compared to 27/52 (52%) given SOC. |
| NCT00427076 | Trimethoprim-sulfamethoxazole (TMP/SMX) vs. vancomycin for invasive MRSA infections | III | 252 (91 had bacteremia) | Improved patient condition or cure with or without antibiotic modifications, survival at 7 days, 30-day all-cause mortality | Completed | 51/135 (38%) patients given TMP/SMX had treatment failure compared to 32/117 (27%) given vancomycin. Patients with bacteremia given TMP/SMX had increased mortality (14/41, 34%) compared to patients given vancomycin (9/50, 18%). |
| Combination therapy | ||||||
| NCT02365493 | SOC with a β-lactam antibiotic vs. SOC for patients with MRSA bacteremia | III | 358 | All-cause mortality, persistent bacteremia at day 5 or later, microbiological relapse, microbiological treatment failure | Completed | 59/170 (35%) patients in the combination group reached a primary endpoint compared to 68/175 (39%) patients given SOC. Increased risk of acute-kidney injury in the combination therapy group (P < 0.001). |
| NCT02660346 | Daptomycin with ceftaroline vs. SOC for patients with MRSA bacteremia | IV | 40 | Time to bacteremia clearance | Completed | Significantly increased risk of mortality in the SOC group compared to the combination therapy group (P = 0.028). See section 4.2.2. |
| NCT01701219 | Ceftaroline fosamil in patients with SAB or MRSA bacteremia persisting 72 hours after initial treatment | IV | 56 | Time to bacteremia clearance, time to defervescence, clinical outcome, mortality, readmission | Completed | Results not posted. |
| NCT00871104 | Fosfomycin and imipenem vs. vancomycin for patients with bacteremia or infective endocarditis due to MRSA | IV | 50 | Negative blood culture at 7 days | Completed | 4/8 (50%) patients given combination therapy were cured of infection compared to 3/7 (43%) patients given vancomycin. |
| NCT01898338 | Fosfomycin and daptomycin vs. daptomycin for patients with MRSA bacteremia | III | 167 | Clinical and microbiological response at week 6 | Completed | 40/74 (54%) patients given combination therapy had treatment success at TOC compared to 34/81 (42%) patients given daptomycin. |
| Novel antimicrobials and adjunctive therapies | ||||||
| NCT00063089 | Altastaph (polyclonal immunoglobulin preparation) vs. placebo, both in addition to SOC | I/II | 40 (17 with MRSA) | Safety | Completed | Adverse events included fevers, rigors, and dyspnea. Patients given Altastaph had a significantly shorter median time to fever resolution (2 days versus 7 days, P = 0.09) and a shorter median duration of hospital stay (9 days versus 14 days, P = 0.03). |
| NCT00198302 | Tefibazumab (Aurexis®) (humanized monoclonal antibody) vs. placebo, both in addition to SOC | II | 60 (15 with MRSA) | Safety, pharmacokinetics, progression of uncomplicated SAB to complicated SAB, microbiological relapse, mortality | Completed | 2/30 (7%) patients given tefibazumab reached a primary endpoint compared to 4/30 (13%) patients given a placebo (P = 0.455). 12/30 (40%) patients given tefibazumab had at least one SAE, compared to 9/30 (30%) given a placebo. |
| NCT02357966 | 514G3 (human monoclonal antibody) vs. placebo, both in addition to SOC | I/II | 16 (Phase I). 52 (Phase II). 6 patients in phase I had MRSA. | Safety, tolerance, maximum tolerated dose (phase I). Duration of hospitalization, incidence of severe-adverse effects (phase II) | Completed | No adverse events attributed to the experimental treatment were reported in phase I. 56% relative risk reduction for the incidence of S. aureus related SAEs for patients given 514G3 compared to placebo (P = 0.23). Duration of hospitalization was decreased in patients given 51463 compared to placebo (8.6 ± 7 days for patients given 514G3, 12.7 ± 9 days for patients given a placebo, (P = 0.092) |
| NCT03162250 | DSTA4637S (antibody-antibiotic conjugate) vs placebo, both in addition to SOC | II | 27 | Incidence of adverse events | Completed | Full results not yet available. See section 4.3.4. |
| NCT03089697 | SAL-200 (anti-staphylococcal lysin) vs. placebo, both in addition to SOC | II | 25 | Incidence of adverse events | Completed (terminated early) | 10/12 (83%) patients given SAL-200 had a TEAE compared to 12/13 (92%) patients given a placebo. |
| NCT03163446 | Exebacase (CF-301) (anti-staphylococcal lysin) vs. placebo, both in addition to SOC for patients with SAB or infective endocarditis | II | 121 (37 with MRSA) | Incidence of adverse events, clinical outcome at day 14, pharmacokinetics | Completed | 50/71 (70%) patients given exebacase were clinical responders compared to 27/45 (60%) patients given a placebo (P = 0.31). 20/27 (74%) patients with MRSA given exebacase were clinical responders compared to 5/16 (31%) patients with MRSA given a placebo (P = 0.01). |
| NCT04160468 | Exebacase (CF-301) (antistaphylococcal lysin) vs. placebo, both in addition to SOC for patients with SAB or infective endocarditis | III | 348 | Clinical response at day 14, treatment-emergent adverse events through day 60 | Recruiting | N/A |
MRSA – methicillin-resistant Staphylococcus aureus; N/A – not applicable; SAB – Staphylococcus aureus bacteremia; SAEs – severe adverse events; SOC – standard of care; TEAE – treatment-emergent adverse event; TMP/SMX – trimethoprim/sulfamethoxazole; TOC – test-of-cure
4.1. Monotherapy
In an effort to identify antibiotics that may be viable alternatives to SOC for SAB, several antibiotics have been used off-label and compared to either vancomycin or daptomycin in clinical trials to assess evaluate their safety and efficacy for use as a monotherapy.
4.1.1. Ceftobiprole
Ceftobiprole is a fifth-generation cephalosporin antibiotic that was granted fast-track approval status by the FDA in 2003 to treat complicated skin and soft tissue infections (cSSTIs) and hospital-acquired bacterial pneumonia (HABP). Ceftobiprole been investigated as a treatment for cSSTIs in clinical trials42, 43, but full FDA approval for ceftobiprole for any indication has not yet been granted. While there is currently no data from randomized clinical trials pertaining to ceftobiprole’s efficacy and safety for use as a treatment for SAB, there is a phase III, double-blind, randomized clinical trial (NCT03138733) that will compare ceftobiprole to daptomycin (with or without aztreonam) in patients with SAB, with the primary endpoint being overall success rate44. Ceftobiprole shows promise in that it has bactericidal activity against both MSSA and MRSA45, 46, indicating it could see use as a general therapy for the treatment of SAB.
4.1.2. Ceftaroline
Like ceftobiprole, ceftaroline is a fifth-generation cephalosporin with bactericidal activity against MSSA and MRSA47. It is currently FDA-approved to treat ABSSSI and community-acquired bacterial pneumonia (CABP). Ceftaroline has shown promise in retrospective analyses and cohort studies – in a retrospective analysis of patients with SAB, 101 of 129 (78%) clinically evaluable patients had clinical success with ceftaroline, with 92.5% of SAB cases in the initial population caused by MRSA47. Ceftaroline has not yet been evaluated as a monotherapy for MRSA bacteremia in a randomized clinical trial, but there is evidence that it may useful, particularly as a salvage therapy. In a retrospective comparative study, 132 MRSA bacteremia patients were identified, and the outcomes of 30 patients given ceftaroline were compared to the matched control groups of 46 patients given daptomycin and 56 given vancomycin48. Out of the 30 patients given ceftaroline, 17 (57%) had failed initial treatment and therefore were switched to ceftaroline. Clinical outcomes were similar in all groups, including 30-day mortality, 42-day relapse, and 30-day readmission. In a review of the available studies investigating ceftaroline as a salvage therapy for MRSA bacteremia, it was found to be useful49. As ceftaroline usage has not been established in the treatment of MRSA bacteremia, its optimal dosage, particularly when used as a salvage monotherapy, has not yet been demonstrated in any clinical trial. Comparative studies will be needed to assess its standing as a monotherapy for MRSA bacteremia.
4.1.3. Dalbavancin
Dalbavancin is a semisynthetic lipoglycopeptide with activity against Gram-positive pathogens that is currently FDA-approved to treat acute bacterial skin and skin structure infections (ABSSSI). A phase II, randomized, controlled, open-label trial with results published in 2005 showed that dalbavancin may be a viable alternative to vancomycin in patients with catheter-related bloodstream infections caused by Gram-positive pathogens, as 20 of 23 (87%) patients given dalbavancin had treatment success (resolution of signs and symptoms and microbiological clearance of infection) compared to 14 of 28 (50%) patients given vancomycin50. However, it is important to note that only 14 patients had MRSA and that most infections in both treatment groups were caused by coagulase-negative staphylococci (CoNS). More recently, there is a phase II, multicenter, open-label, randomized clinical trial (NCT04775953) currently recruiting that will compare dalbavancin to SOC in patients with complicated bacteremia or right-sided endocarditis caused by S. aureus. For patients with MRSA, patients randomized to receive dalbavancin will receive a 1500 mg dose intravenously (IV) on day 1 and 1500 mg IV on day 8, while those randomized to receive SOC will receive either vancomycin or daptomycin. The primary outcome measure assesses the resolution of clinical signs of SAB, clinical failure, incidence of metastatic infections, and relapse of infection. It is anticipated that the study will be completed in August of 2023. Dalbavancin is a promising agent given its success in a clinical trial focused on treating Gram-positive bacteremia, while also having an acceptable safety profile. Dalbavancin is also notable for its long plasma-half life, and therefore can be administered once weekly, potentially in an outpatient setting when appropriate51. Future trials should hopefully help to clarify its usefulness against MRSA bacteremia. Large clinical trials with glycopeptides would also prove helpful in providing information about the potential for the development of resistance to emerge and affect treatment, as a dalbavancin non-susceptibility phenotype was observed after one patient was treated with both vancomycin and dalbavancin52 In vitro, the exposure of MRSA to dalbavancin led to a 64–128 fold increase in the MIC of dalbavancin and a 4–16 fold increase in the MIC of vancomycin53.
4.1.4. Telavancin
Telavancin is another semisynthetic lipoglycopeptide that is FDA-approved to treat cSSTIs, HABP, and ventilator-associated bacterial pneumonia (VABP) caused by susceptible isolates of S. aureus when alternative treatments are not available. Telavancin was evaluated to treat MRSA bacteremia in a randomized phase II clinical trial (NCT00062647) in which a total of 60 patients with uncomplicated SAB were randomized to receive either telavancin (10 mg/kg/day IV every 24 hours up to 14 days) or SOC to treat either MRSA or MSSA depending on the methicillin-resistance status of the patient isolate. Approximately 50% of patients in both the telavancin-treated group and the SOC-group had MRSA (15 of 31 patients in the all-treated target population)54. At the test-of-cure (TOC) visit, seven of eight (88%) patients given telavancin were cured of infection compared to eight of nine (89%) patients given SOC. All patients with MRSA bacteremia were cured. Serious adverse events (SAEs) were more common in patients given telavancin, with 38% of telavancin-treated patients experiencing an SAE compared to 21% of patients receiving SOC. There was also an increase in serum creatinine levels in patients given telavancin, with 5 of 25 (20%) patients having serum creatinine ≥ 1.5 mg/dl and at least 50% greater than baseline compared to 2 of 25 (8%) patients given SOC. This study utilized a proof-of-concept design to demonstrate that telavancin could be used to treat SAB but the study population was restricted to patients with uncomplicated bacteremia, which is uncommon and therefore limited the target population55. Another trial that evaluated telavancin as a treatment for SAB is a phase III, randomized, open-label, noninferiority trial (NCT02208063) that was terminated due to a lack of statistical power56. In this trial, patients with SAB or infective endocarditis caused by S. aureus were randomized to receive either telavancin or SOC, with the primary endpoint being the number of participants that were cured at TOC. Out of 47 patients given telavancin, 22 (47%) were determined to be cured of infection compared to 27 of 52 (52%) patients given SOC. SAEs were more common in patients given telavancin, but there were no significant differences between groups in occurrence of acute kidney injury. Larger trials are needed with telavancin before conclusions can be made about its efficacy, as both trials have been too small to draw meaningful conclusions. Future trials with telavancin should also focus on including patients with complicated bacteremia in the study population.
4.1.5. Linezolid
Linezolid is a synthetic antibiotic belonging to the class of oxazolidinone antibiotics that inhibits protein synthesis by binding to both the 30S and 50S subunits of rRNA57. It is currently FDA approved for the treatment of HABP and cSSTIs caused by either MRSA or MSSA. The efficacy of linezolid as a treatment for catheter-related bloodstream infections was investigated in an open-label, multicenter trial in which patients with a suspected catheter-related infection were randomized to receive either linezolid 600 mg IV or vancomycin 1 g IV every 12 hours for 7–28 days58. Out of 363 patients that received linezolid, 49 (13%) had MRSA, compared to 40 of 363 (11%) patients that received vancomycin. Out of 24 patients with a MRSA bloodstream infection that received linezolid, 19 (79%) achieved clinical success at TOC compared to 16 of 21 (76%) patients with a MRSA bloodstream infection that received vancomycin (95% CI: −21.4 – 27.4). Patients with bacteremia caused by a Gram-negative pathogen were recommended to be given aztreonam or amikacin, although specific information about therapies given to these patients was not made available. An independent review of the treatments given to these patients by two academic physicians found that treatment was adequate in 22 of 59 (37%) patients given linezolid and 19 of 47 (40%) patients in the control group. In patients with Gram-negative bacteremia, negative blood cultures at baseline, or an infection with both Gram-positive and Gram-negative pathogens, a Kaplan-Meier survival curve analysis showed increased incidence of mortality in patients that received linezolid compared to the control group (patients with a negative culture at baseline: hazard ratio (HR) = 2.20, 95% CI: 1.07 – 4.50; patients with Gram-negative bacteremia: HR = 1.94, 95% CI: 0.78 – 4.81). This suggests that linezolid should not be given as an empirical therapy for the treatment of bacteremia and should only be given to patients with a microbiologically confirmed Gram-positive infection. The results of this study led to an FDA warning in 2007 advising against the use of linezolid in patients with bacteremia caused by a Gram-negative or an unknown organism59.
4.1.6. Trimethoprim-sulfamethoxazole
Trimethoprim-sulfamethoxazole (TMP/SMX) is a fixed antibiotic combination that is currently FDA-approved to treat otitis media in pediatric patients, urinary tract infections, and acute infective exacerbation of chronic bronchitis. It is also used off-label to treat skin infections caused by S. aureus. Sulfamethoxazole competes directly with 4-aminobenzoic acid to inhibit the synthesis of dihydrofolate, while trimethoprim inhibits dihydrofolate reductase, leading to an overall interruption in purine biosynthesis60. The potential of TMP/SMX to treat SAB was evaluated in a randomized clinical trial in 199261, which randomized intravenous drug users hospitalized with suspected SAB to receive vancomycin 1 g or TMP/SMX (320 mg:1600 mg) every 12 hours. Of 43 patients given TMP/SMX, 27 (63%) had bacteremia compared to 38 of 58 (66%) patients given vancomycin. 47% of all infections were caused by MRSA. TMP/SMX was inferior to vancomycin, as 37 of 43 (86%) patients given TMP/SMX were cured of infection compared to 57 of 58 (98%) patients given vancomycin (P = 0.014). In a more recent clinical trial, TMP/SMX was again compared to vancomycin to treat MRSA infections (NCT00427076)62. Of 252 patients in the trial, 91 (36%) had bacteremia. Treatment failure was observed in 51 of 135 (38%) patients given TMP/SMX compared to 32 of 117 (27%) given vancomycin, and TMP/SMX failed to meet the non-inferiority criterion (defined by a difference of less than 15% for treatment failure). For patients with bacteremia, mortality (30-day all-cause) was observed in 14 of 41 (34%) patients given TMP/SMX compared to 9 of 50 patients (18%) given vancomycin, which led to the authors of the study advising against the use of TMP/SMX to treat severe MRSA infections. Given that TMP/SMX has failed to be non-inferior to vancomycin in two large clinical trials, TMP/SMX is not a viable alternative to SOC for MRSA bacteremia.
4.2. Combination therapy
There has been an abundance of data in the form of in-vitro experiments, animal models, and observational studies to suggest that the use of combination antimicrobial therapy may benefit patients with SAB. Two of the largest clinical trials to date investigating novel therapeutic strategies to treat MRSA bacteremia have evaluated the efficacy and safety of combination therapy.
4.2.1. Rifampicin
Rifampicin is an antibiotic that specifically inhibits bacterial RNA polymerase with increased antimicrobial activity against Gram-positives, as it has difficulty penetrating through the outer membrane in Gram-negatives63. When used clinically, rifampicin is used in combination with other antibiotics to suppress the rapid emergence of resistance, which arises from mutations in the rpoB gene that encodes the β-subunit of RNA polymerase64. The use of rifampicin combination therapy has been established in patients with orthopedic device-related infections, which are particularly difficult to treat because of the recalcitrant nature of the colonizing biofilm65. Antibiotic combinations that contained rifampicin were more effective at reducing bacterial load than those without it in a mouse osteomyelitis model66, and rifampicin has also been implicated for use in combination therapy due to in-vitro evidence that it penetrates eukaryotic cells more effectively than glycopeptides and β-lactams67, indicating it may help to target intracellular bacteria that are shielded from antibiotics. In a rabbit endocarditis model, the use of rifampicin in combination with vancomycin led to decreased vegetation titers of MRSA compared to use of either antibiotic alone68.
In the largest clinical trial investigating a therapeutic strategy to treat SAB to date, 758 patients with SAB were randomized to receive rifampicin (600 mg or 900 mg per day) or a placebo in addition to SOC for 2 weeks69. The primary endpoints were the duration of time before treatment failure, recurrence, or death. Of the 758 patients, only 47 (6%) had MRSA bacteremia. Out of MRSA patients, 9 of 26 (35%) given rifampicin reached a primary endpoint by week 12 compared to 3 of 21 (14%) given a placebo. Overall, by week 12, 71 of 388 (18%) patients given rifampicin reached the primary endpoint compared to 62 of 370 (17%) patients given a placebo (P = 0.81). This trial showed no added benefit for the addition of rifampicin to standard antibiotic therapy, and instead there was evidence that the use of rifampicin in this manner may further complicate treatment, as 17% of patients given rifampicin had an antibiotic-modifying adverse event compared to 10% of patients given a placebo (P = 0.004).
4.2.2. β-lactam antibiotics
β-lactams, the most widely-used class of antibiotics70, exert antimicrobial activity by binding to penicillin-binding proteins (PBPs) and interrupting the transpeptidation process which results in the termination of peptidoglycan cross-linking71. Bacterial resistance to β-lactams is of serious concern, as numerous resistance mechanisms have been described, including drug efflux, production of β-lactamases, overproduction of PBPs, and the reduced affinity of β-lactams for PBPs72. This reduced affinity is a defining feature of MRSA, which contains the mecA gene that codes for a PBP, called PBP2A, that has reduced affinity for β-lactams73.
The “see-saw” effect refers to an observed increase in the susceptibility of MRSA to beta-lactams when susceptibility to glycopeptide and lipopeptide antibiotics such as vancomycin and daptomycin decrease74, suggesting that the addition of β-lactams to SOC may improve clinical outcomes for patients with MRSA bacteremia. In a multicenter, open-label, randomized clinical trial (NCT02365493), patients with MRSA bacteremia were randomized to receive either SOC or a β-lactam (flucloxacillin, 2 g IV every 6 hours; cloxacillin, 2 g IV every 6 hours) in addition to SOC for the first 7 days following randomization75. Cefazolin was used in patients with non-type 1 hypersensitivity allergies to penicillin (2 g every 8 hours) and in patients undergoing hemodialysis (2 g 3 times per week after hemodialysis). The primary endpoints of the study were mortality, persistent bacteremia at day 5, microbiological relapse, and microbiological treatment failure. Out of 170 patients, 59 (35%) in the combination therapy group reached a primary endpoint compared to 68 of 175 patients (39%) in the standard therapy group. Despite no difference between groups in the primary endpoint, persistence of bacteremia was observed in 19 of 166 (11%) patients in the combination therapy group compared to 35 of 172 (20%) patients at day 5 (P = 0.02). However, 34 of 145 (23%) patients given combination therapy had acute kidney injury compared to 9 of 145 (6%) patients given standard therapy (P < 0.001), which led to early termination of the trial. While the safety profile of combination therapy outweighed its clinical impact in this study, the study authors acknowledge that regional differences in vancomycin MICs may have impacted treatment response. This study was also overwhelmingly reliant on vancomycin with flucloxacillin or cloxacillin with only a few daptomycin and cefazolin treatments (349 out of 352 patients in the trial received vancomycin). Such reliance may have had an impact on the nephrotoxicity results, as cefazolin has been associated with a better safety profile than other anti-staphylococcal penicillins in patients with MSSA bacteremia76. Of the 27 patients who received cefazolin in addition to vancomycin, only one developed acute kidney injury.
Discussed earlier as a monotherapy, ceftaroline has also been investigated for use in combination antimicrobial therapy. The use of ceftaroline in combination with daptomycin as salvage therapy for complicated MRSA bacteremia was demonstrated in a retrospective cohort study, as none of the 30 patients given ceftaroline experienced recurrence of bacteremia within 60 days compared to 9 of 30 patients given SOC (P < 0.01)77. Further evidence for the use of the daptomycin-ceftaroline combination as a salvage therapy was provided in a case series. In this study, bacteremia persisted for a median of 10 days on previous antibiotic treatment, with infection clearance achieved in a median of 2 days after the start of combination therapy in 23 out of 26 cases (20 were MRSA)78. Evidence supporting the efficacy of ceftaroline in combination with daptomycin in the form of data from randomized clinical trials is limited, with only one small phase IV trial with published data and another completed phase IV trial with data not yet available. In a phase IV, randomized, open-label trial (NCT02660346) with 40 participants, patients with MRSA bacteremia were randomized to receive either monotherapy (vancomycin or daptomycin) or 6 to 8 mg/kg/day of daptomycin and 600 mg of ceftaroline every 8 hours79. There was a significantly increased risk of mortality in the SOC group compared to patients in the combination therapy group (Kaplan-Meier 60-day survival analysis, P = 0.028). As a disproportionate number of deaths occurred in the group receiving monotherapy, the trial was halted early. Of note, two patients with stage IV lung cancer were randomized to the monotherapy group, suggesting random variability between the two groups could have impacted the study’s results. The other phase IV trial is a non-randomized, open-label trial evaluating the efficacy and safety of ceftaroline fosamil 600 mg IV given every 8 hours in patients with SAB that persists at least 72 hours after vancomycin and/or daptomycin treatment (NCT01701219). This study was completed in 2014, but the results have not yet been made available. The use of ceftaroline combination therapy is promising, especially with respect to its safety profile, but the only clinical trial with published results was underpowered to make conclusions about efficacy. Larger trials powered to show efficacy in comparison to SOC will prove useful. While we are awaiting full results on the study completed in 2014, this study is non-comparative in nature which limits conclusions about efficacy.
4.2.3. Fosfomycin
Fosfomycin is a phosphonic acid antibiotic first discovered in 1969 that is primarily used to treat lower urinary tract infections. Fosfomycin inhibits the MurA enzyme, which catalyzes the first committed step in peptidoglycan synthesis80. The efficacy and safety of fosfomycin in combination with imipenem (a carbapenem) was evaluated for use as a salvage therapy in patients with complicated bacteremia and endocarditis due to MRSA in a study conducted from 2001 to 201081. In total, 16 patients received primarily vancomycin or daptomycin (with some patients also receiving gentamicin, teicoplanin, or linezolid) as the initial therapy, with fosfomycin (2 g IV every 6 hours) in combination with imipenem (1 g IV every 6 hours) added as the salvage therapy. All patients had a negative blood culture 72 hours after the first dose, and 11 patients were cured and 5 had died at TOC. Adverse effects attributed to combination therapy were observed in five patients, and included leukopenia, fungal bloodstream infection, and liver cirrhosis. A follow-up study was completed in 2018 in the form of a randomized, open-label, multicenter clinical trial (NCT00871104)82. Among 15 patients with complicated bacteremia or infective endocarditis due to MRSA, 8 were randomized to receive fosfomycin and imipenem, and 7 to receive vancomycin. Four patients (50%) were cured of infection in the combination therapy group, while three patients (43%) that received vancomycin were cured. Hypernatremia was reported in one patient undergoing combination therapy.
In-vitro results and animal studies have shown synergistic activity between fosfomycin and daptomycin, specifically as a means to treat MRSA endocarditis in a rabbit model83. A recent, randomized, open-label trial sought to explore this relationship clinically (NCT01898338), in which patients with MRSA bacteremia were randomized to receive 10 mg/kg IV of daptomycin daily with or without 2 g IV of fosfomycin every 6 hours84. The primary endpoint was treatment success at TOC (6 weeks after the end of therapy), defined as the resolution of clinical manifestations and a negative blood culture. Out of 74 patients, 40 (54%) given combination therapy had treatment success, compared to 34 of 81 (42%) patients given monotherapy (P = 0.135). In the combination therapy group, 12 patients (16%) had complicated bacteremia at TOC compared to 26 (32%) in the SOC group. Adverse events were more common in the combination therapy group and included cardiac failure and electrolyte disorders, including hypokalemia and hypocalcemia. Notably, fosfomycin-related adverse events occurred after a median of 10 days post-treatment, while microbiological efficacy was achieved at 3 and 7 days, suggesting fosfomycin should be administered early during the first week of treatment to minimize the risk of side effects. Fosfomycin may have a role in aggressive treatment regimens, but the risk of side effects relative to monotherapy suggest treatment should be de-escalated to monotherapy when determined to be appropriate. Clinical trials investigating fosfomycin have also been limited in the enrollment of patients with complicated bacteremia. Future trials may look to increase the enrollment of patients with complicated SAB.
4.3. Novel antimicrobials and adjunctive therapies
Several novel antimicrobials, mostly in the form of anti-S. aureus antibodies and recombinant lysins, have been evaluated for their potential to treat SAB as adjunctive therapies to SOC.
4.3.1. Altastaph (S. aureus capsular polysaccharide immune globulin)
Altastaph (Nabi Biopharmaceuticals, Rockville, MD) is a S. aureus polyclonal immunoglobulin preparation that contains antibodies against capsular polysaccharide (CPS) types 5 and 8, which are produced by about 70% of S. aureus clinical isolates85. It should be noted however that CPS are not produced86 by the most abundant clonal lineage of MRSA in the United States, USA30087, which represents a drawback of therapeutics targeting capsular polysaccharides. Nonetheless, antibodies against CPS types 5 and 8 have been shown to have high opsonizing activity in vitro88 and to offer passive immunity against staphylococcal endocarditis and sepsis in several animal models88, 89. Previously, a phase II clinical trial evaluating the safety and ability of Altastaph to prevent nosocomial S. aureus infections in very low birth weight infants indicated that it was well-tolerated90. A randomized phase II clinical trial (NCT00063089) sought to determine the safety and pharmacokinetics of Altastaph in 40 patients with SAB and persistent fever, with patients receiving SOC plus either two infusions of Altastaph (200 mg/kg) 24 hours apart or placebo91. Out of the 40 patients, 17 had MRSA bacteremia. In the Altastaph group, high levels of antibodies (> 100 μg/mL) were maintained for four weeks post-treatment. Compared to the placebo group, patients receiving Altastaph had a significantly shorter median time to fever resolution (2 days versus 7 days, P = 0.09) and a shorter duration of hospital stay (9 days versus 14 days, P = 0.03). Among patients with MRSA bacteremia, the median time to resolution of bacteremia was 0 days in the Altastaph group compared to 2 days in the placebo group (P = 0.3), suggesting that Altastaph did not aid in the resolution of SAB. Adverse events determined to be drug and infusion related included fevers, rigors, dyspnea, and chest discomfort. The development of Altastaph was halted in 2005, after Nabi Biopharmaceuticals’ StaphVAX® vaccine, which is based on the same technology as Altastaph, failed to meet its primary endpoint in a phase III clinical trial92.
4.3.2. Tefibazumab (Aurexis®)
Tefibazumab (Aurexis®) is a humanized monoclonal antibody (immunoglobin G1 kappa) that binds to clumping factor A (ClfA), an MSCRAMM (microbial surface components recognizing adhesive matrix molecules) protein in S. aureus that promotes the binding of fibrin and fibrinogen to the bacterial cell surface. ClfA is found in nearly all S. aureus clinical isolates93 and is involved in staphylococcal colonization94–96. In a sepsis model, mice given a murine monoclonal antibody (MAb 12–9) that targets ClfA prior to bacterial challenge survived longer compared to the control group97, and in an infective endocarditis model, rabbits given tefibazumab prophylactically did not become bacteremic after bacterial challenge98. The safety and pharmacokinetics of tefibazumab in humans was evaluated in a randomized, double-blind, multi-center phase II trial (NCT00198302) that randomized 63 patients with SAB to receive either tefibazumab (20 mg/kg single infusion) or a placebo in addition to SOC99. At baseline, MRSA was identified in 37% of the blood culture isolates in the tefibazumab group and in 57% of the placebo group (n = 30 in each group). The biological activity of tefibazumab was defined by at least one of the following endpoints: 1) development of an SAB-related complication that was not present at baseline; 2) microbiologically confirmed relapse of SAB; 3) death. Out of 30 patients given tefibazumab, 2 (7%) reached an endpoint compared to 4 of 30 (13%) patients in the placebo group (P = 0.455). Only 2 of 28 (7%) patients with MRSA reached an endpoint. Adverse events were more common in the tefibazumab group, and a hypersensitivity reaction occurred in one patient given tefibazumab. Clinical development of tefibazumab was suspended in 2006, pending the outcome of licensing negotiations100.
4.3.3. 514G3 (monoclonal antibody)
514G3 (XBiotech, Austin, TX) is a human anti-SpA monoclonal antibody that binds to staphylococcal protein A (SpA), which promotes immune evasion by interfering with the development of humoral immunity101. Anti-SpA monoclonal antibodies have been shown to have protective effects in vivo, protecting mice against S. aureus sepsis, subsequent staphylococcal infections, and nasopharyngeal colonization102. Mice given 514G3 in addition to vancomycin had increased survival six days after bacterial challenge compared to vancomycin alone in a bacteremia model103. The safety of 514G3 was assessed in patients hospitalized with SAB in a Phase I-II clinical trial (NCT02357966), with subjects randomized to receive either a single dose of 514G3 (2, 10, or 40 mg/kg) or a placebo in addition to SOC104. In total, 16 subjects were enrolled in the phase I study, with 6 cases of MRSA (38%). SAEs were reported in 3 of 12 patients (25%) given 514G3 compared to 2 of 4 (50%) patients given a placebo, but the investigators determined these were not treatment-related. In the Phase II trial, patients with SAB were randomized to receive either a single dose of 514G3 at 40 mg/kg or a placebo, both in addition to SOC, with duration of hospitalization and incidence of SAEs the primary clinical endpoints105. While the length of hospital stay was decreased by 33% in the 514G3 group compared to the placebo group, the difference was not statistically significant. There was a 56% relative risk reduction for the incidence of S. aureus-related SAEs in patients receiving 514G3 compared to the placebo (4 of 36 (11%) versus 4 of 16 (25%), respectively), but no definition of S. aureus related SAEs was given and the results were not statistically significant (P = 0.23). 514G3 is the lone antibody-only candidate that is still seeing clinical investigation as an adjunctive treatment for MRSA bacteremia. Its phase II trial was designed to provide insight into both safety and efficacy, but the trial was too small to make conclusions regarding efficacy and lacked significant results. It should also be noted that no information was given on the prevalence of MRSA bacteremia or complicated bacteremia in the study population. Therefore, a larger trial – preferably with an emphasis on efficacy endpoints, should be completed to demonstrate its potential.
4.3.4. DSTA4637S (anti-S. aureus antibody-antibiotic conjugate)
Long-term colonization with S. aureus and associated clinical failures may be linked to the establishment of intracellular reservoirs inside host cells106, which shield the bacteria from antibiotics107. Aiming to target such reservoirs, DSTA4637S (Genentech, San Francisco, CA) is a Thiomab antibody-antibiotic that contains an engineered human immunoglobulin G1 (IgG1) anti-S. aureus monoclonal antibody linked to a novel rifamycin class bactericidal antibiotic (dmDNA31) via a protease-cleavable valine-citrulline (VC) linker108–110. The antibody targets the β-N-acetylglucosamine (β-GlcNAc) sugar modification of WTA. dmDNA31 only becomes active after cathepsins in the phagolysosome cleave the VC linker once bacteria bound to the antibody have been phagocytosed108. The in-vivo efficacy of this conjugate was demonstrated in a mouse model where mice pre-treated with the conjugate prior to MRSA challenge had a significantly lower CFU burden in the kidneys four days after infection compared to mice pre-treated with vancomycin108.
In humans, DSTA4637S was first used in a phase I randomized, placebo-controlled, single-ascending dose study (NCT02596399) that assessed its safety, tolerability, pharmacokinetics, and immunogenicity in healthy volunteers111. Treatment-emergent adverse events (TEAEs) were considered mild and included nasal discharge discoloration, headache, and nausea. The plasma pharmacokinetics of DSTA4637S were approximately dose-proportional with a mean half-life ranging from 4.3 to 6.1 days, and no anti-drug antibodies were detected post-baseline. The second phase I trial (NCT03162250) randomized 27 patients with SAB to receive DSTA4637S at a low, intermediate, or high dosage (infusion within 24 hours of randomization on day 1 and then every 7 days up to 6 doses) or a placebo in addition to SOC. The full results of the trial have not yet been made available but a comparison of the pharmacokinetics of DSTA4637S between healthy volunteers and SAB patients has been published. Peak serum concentrations of DSTA4637S were reduced 26.7% to 51.3% in SAB patients compared to healthy volunteers, potentially due to factors such as disease status, target-mediated clearance and/or non-specific uptake of the antibody-antibiotic conjugant112. As clinical trials with DSTA4637S have been focused on characterizing its safety and pharmacokinetics, a larger trial focused on efficacy is necessary to demonstrate its potential as a novel therapeutic agent for SAB. It would also be beneficial if such a trial can investigate if reduced serum concentrations of DSTA4637S has a meaningful effect on treatment efficacy.
4.3.5. SAL200 (N-Rephasin™)
Endolysins are bacteriophage-derived hydrolytic enzymes that target the cell wall and show promise as potential therapeutic agents for infections caused by Gram-positive pathogens. For the treatment of SAB, SAL200 (N-Rephasin™) is an endolysin-based candidate that utilizes SAL-1, a recombinant bacteriophage endolysin derived from SAP-1113, 114, a Staphylococcus-specific bacteriophage. Antibacterial activity of SAL200 and synergism with antibiotics against both MRSA and MSSA has been demonstrated both in vitro and in a mouse model115. Preclinical evaluations of the safety of SAL200 in dogs and rats revealed only mild, transient adverse effects after injection116. In a placebo-controlled single-dosing and dose-escalating phase I clinical trial (NCT01855048), which was also the first-in-human phase I study to intravenously administer a bacteriophage endolysin-based drug to human patients, the safety profile, pharmacokinetics, and pharmacodynamics of SAL200 were evaluated in 36 healthy male volunteers117. SAL200 was well tolerated, with no SAEs and only mild and transient adverse events. Another phase I, multiple-ascending dose trial (NCT03446053) was recently completed118. Among patients given SAL200, adverse events included chills, pyrexia, and headache, but no SAEs were reported. To evaluate the safety and efficacy of SAL200 in patients with SAB, a phase II placebo-controlled trial (NCT03089697)119 was completed, which randomized patients to receive either a placebo or SAL200 in addition to SOC. Incidence of TEAEs were similar in both the placebo and experimental groups. Efficacy endpoints, which included the percentage of patients in each group with treatment failure by day 14, percentage of patients who died of SAB within 14 days of diagnosis, and percentage of patients with a negative first blood culture, were also similar in both groups. This trial was terminated by the study’s sponsor, Intron Biotechnology, prior to completion for strategic reasons. There is currently no data to suggest that SAL-200 is effective when used as an adjunctive therapy, although this is because its clinical investigation has focused on characterizing its safety and pharmacokinetics. These studies suggest that it is well-tolerated, but future studies will need to be designed to make conclusions about its efficacy.
4.3.6. Exebacase (CF-301)
Another bacteriophage lysin-based candidate for the treatment of SAB is exebacase (CF-301). In vitro, exebacase has rapid bacteriolytic activity against MRSA and MSSA, anti-biofilm activity, synergy with SOC antibiotics and human blood components (albumin and lysozyme), and a lower resistance profile relative to other antibiotics120–123. Notably, exebacase is bacteriolytic against 120 MRSA isolates and has a lower molar MIC against MRSA compared to vancomycin and daptomycin120. The safety of exebacase was first evaluated in a phase I, dose-escalating trial with 20 healthy subjects (NCT02439359), with no reported adverse effects124. Since then, a phase II, randomized, double blind trial has been completed, primarily evaluating the safety, efficacy, and pharmacokinetics of exebacase in patients with SAB (NCT03163446)125.
In this trial, 121 patients with SAB/endocarditis were randomized to receive either a single dose of exebacase (0.25 mg/kg) or a placebo in addition to SOC. Out of 121 patients, 116 had a confirmed S. aureus bloodstream infection (BSI), with a total of 37 (32%) BSI patients with MRSA. After 14 days, 50 of 71 (70%) patients given exebacase were clinical responders compared to 27 of 45 patients (60%) given antibiotics-alone (P = 0.31). Out of 27 MRSA patients given exebacase, 20 (74%) were clinical responders compared to 5 of 16 (31%) MRSA patients given antibiotics-alone (P = 0.01). Among patients with complicated MRSA bacteremia, 13 of 17 (76%) patients were clinical responders compared to 3 of 13 (23%) patients given antibiotics-alone (90% CI: 21.0 – 85.8). There was no statistically significant difference in clinical responder rates between the exebacase-treated group and the antibiotics-alone group in patients with MSSA and the overall cohort. This is potentially due to differences in the in-vivo antibacterial activity of the antibiotics used to treat MSSA compared to MRSA, as well as differences in the distribution of subgroup comorbidities such as left-sided endocarditis (15% of patients given exebacase had left-sided endocarditis compared to 7% of patients given antibiotics-alone). The incidence of TEAEs were similar in the exebacase-treated and antibiotics-alone groups and no hypersensitivity reactions to exebacase were reported, despite 21% of patients in the exebacase-treated group having anti-drug antibodies at baseline. Although the sample size of MRSA patients is small, this study is unique among clinical trials for adjunctive therapies for MRSA bacteremia because it employed a superiority-design that provides proof-of-concept for the use of exebacase as an adjunctive therapy. The efficacy and safety of exebacase for SAB treatment is currently undergoing further evaluation in a phase III, randomized, placebo-controlled study with an estimated enrollment of 348 patients (NCT04160468).
5. Conclusion
With only two FDA-approved antibiotics for the treatment of MRSA bacteremia, there is an urgent need to identify new treatments as mortality remains high and antibiotic resistance continues to increase the risk of treatment failure. While there remains a lack of high-quality evidence supporting the use of specific alternatives to vancomycin or daptomycin, clinical trials have revealed promising agents and therapeutic approaches while providing valuable information that will inform clinical management of MRSA bacteremia and lead to improved patient outcomes.
6. Expert Opinion
Vancomycin has remained the primary treatment for MRSA bacteremia for decades, with daptomycin emerging as another treatment option after it was shown to be non-inferior to vancomycin in 200638. There is an urgent need to identify novel therapeutic agents and approaches to treat MRSA bacteremia as the mortality rates associated with MRSA bacteremia have remained stable since the 1990s5, in addition to concerns over both vancomycin’s efficacy and toxicity and the emergence of bacterial non-susceptibility to daptomycin. In the pursuit of novel agents and treatments, investigations have focused primarily on three types of approaches: alternative antibiotics to SOC that can be used as monotherapies, combination antimicrobial therapies, and novel antimicrobials that can be used as adjunctive therapies to SOC.
Several different classes of antibiotics have been evaluated for use as a monotherapy to treat MRSA bacteremia, including cephalosporins, lipoglycopeptides, and oxazolidinones. Ceftobiprole and ceftaroline, both cephalosporins, are promising in that they have bactericidal activity against both MRSA and MSSA. This feature gives them the potential to act as a general treatment for SAB, regardless of the methicillin-resistance status of the isolate, but their efficacy as treatments for MRSA bacteremia have not yet been established through randomized clinical trials. There is an active clinical trial that will compare ceftobiprole to daptomycin as a treatment for SAB that seeks to establish its efficacy. Similarly, there is limited data substantiating the usefulness of dalbavancin and telavancin. Dalbavancin had success in treating bacteremia caused by Gram-positive bacteria in a clinical trial, but only a small subset of the study population had MRSA bacteremia. Telavancin did not perform better than SOC in the two clinical trials previously discussed, although it should be noted that one was a proof-of-concept study while another was terminated early. Although not discussed extensively in this review, oritavancin is another lipoglycopeptide that is FDA-approved to treat ABSSSI that has seen some success as a treatment for Gram-positive bacteremia126, but there is a lack of high quality data in patients with SAB. Notably, oritavancin is a candidate for outpatient parenteral antimicrobial therapy (OPAT) due to its extremely long half-life of 2 weeks127. There is a phase IV proof-of-concept trial (NCT03761953) that will evaluate the safety and efficacy of oritavancin as a treatment for bacteremia and endocarditis caused by S. aureus in opioid users128. This trial may pave the way for a larger, randomized trial with oritavancin in the future.
Linezolid was shown to be non-inferior to vancomycin in patients with Gram-positive infections but concerns about increased mortality in patients without Gram-positive infections make microbiological confirmation of the isolate crucial when considering the use of linezolid. Tedizolid is another oxazolidinone antibiotic, but it has not yet been evaluated as a treatment for MRSA bacteremia in clinical trials. Tedizolid may be a good candidate for future trials as it can be administered once daily, in addition to having a more favorable safety profile and shorter therapy duration than linezolid as a treatment for ABSSSI129.
Trimethoprim-sulfamethoxazole was shown to be inferior to vancomycin as a treatment for MRSA bacteremia in two clinical trials discussed in this review, arguing against its use as a frontline therapy for MRSA bacteremia. Apart from dalbavancin, ceftaroline, and ceftobiprole, which are currently under investigation, there is no evidence to suggest that any of these monotherapy agents should be considered a frontline alternative to SOC; instead, it seems that their usefulness may lie as salvage therapies when patients do not respond to initial treatment. Future clinical trials investigating the efficacy of these potential salvage therapies may clarify which agents show the most promise.
A combination of in-vitro, animal model, and observational cohort studies have suggested that combination antimicrobial therapy may benefit patients with MRSA bacteremia. In the first large-scale, clinical evaluation of the usefulness of combination therapy, there was no added benefit of the addition of rifampicin to SOC as combination therapy lead to an increase in antibiotic-modifying adverse events. Since then, investigation has largely focused on evaluating the efficacy and safety of the addition of β-lactams to SOC, driven by the observation of the aforementioned “see-saw” effect. This effect is proposed to arise after the disruption of PrsA, a lipoprotein that acts as a post-translocational chaperone, leads to impaired post-translational maturation of PBP2A and a subsequent increase in susceptibility to β-lactams130. The clinical efficacy of β-lactams in addition to SOC was explored in the CAMERA-2 trial, which showed no benefit for this type of combination therapy. Despite bacteremia persistence decreasing in patients given combination therapy, the increased risk of acute kidney injury cannot be understated. However, it is important to note that 99% of patients in the trial were given vancomycin – therefore, increased usage of daptomycin may have allowed for a comparison of the safety profiles for both antibiotics when used in conjunction with β-lactams. Future clinical trials may consider exploring this topic. Additionally, future trials may emphasize using β-lactam antibiotics that have more favorable safety profiles, such as cefazolin or ceftaroline, compared to those primarily used in the CAMERA-2 trial. These approaches should help elucidate which combinations are most effective while keeping patient safety in mind. Ceftaroline in combination with daptomycin has been investigated in a clinical trial but the small sample size limited conclusions about its efficacy. Nonetheless, it was well-tolerated and warrants future investigation in a larger trial. The combination of daptomycin and fosfomycin has also shown promise as more patients given the combination therapy compared to SOC reached treatment success at six weeks post-treatment. However, the comparison was not statistically significant, and these results were accompanied by an increased incidence of adverse events in the combination therapy group. Fosfomycin is currently not available for intravenous use in the United States, and larger trials should reveal more about fosfomycin’s safety profile and its potential as a treatment for MRSA bacteremia.
Clinical trials investigating novel antibodies and bacteriophage endolysins as adjunctive therapeutics for MRSA bacteremia have been mostly limited to small trials that primarily focus on safety evaluations, with SAL200 and exebacase being the most notable candidates in the clinical evaluation stage. These are the only bacteriophage lysin-based candidates that currently show the most promise for therapeutic use; candidates in the pre-clinical stage include LysGH15, P128, and ClyF, which have had success as treatments in MRSA bacteremia mouse models, but have no timetable on future development131–133. Encouragingly, SAL200 and exebacase have been well-tolerated, with generally mild adverse events and only a few instances of hypersensitivity reactions. Although anti-drug antibodies have been reported, specifically with SAL200 and exebacase, it remains to be seen how they affect treatment. Nevertheless, it is important to monitor the development of anti-drug antibodies to assess their potential to play a role in hypersensitivity reactions or treatment failure. The most promising agent in this class is exebacase, which is the first of its class to receive a phase III trial. Not only was it shown to be effective as an adjunctive therapy when added to SOC, but it also has an acceptable safety profile and no hypersensitivity reactions were reported in a phase II trial despite the presence of anti-drug antibodies. Only 8 of 43 patients with MRSA in the trial were given daptomycin; thus, the efficacy of exebacase was mostly established in combination with vancomycin, but the ongoing phase III trial may further establish efficacy with daptomycin. Exebacase was granted Breakthrough Therapy designation by the FDA following the results of the phase II trial134, and it is anticipated that the phase III trial will be completed in late 2022. In order for antibody and bacteriophage lysin-based candidates to reach their full potential, future trials will need to focus on demonstrating their efficacy. In this regard, SAL200, 514G3, and DSTA4637S should hopefully see larger trials with efficacy endpoints in the future.
The approval of novel agents to treat MRSA bacteremia significantly lags behind the approval of agents for use in treating ABSSSI or cSSTIs caused by MRSA. While there are only two drugs indicated for use as treatments for MRSA bacteremia, several drugs discussed in this review have already been approved for use in treating either ABSSSI or cSSTIs. Contributing to this is the relative difficulty of completing SAB trials compared to trials for ABSSSI/cSSTIs. Compared to these trials, bacteremia trial design for marketing approval lacks guidance from the FDA, which makes these trials difficult to standardize, in addition to being very expensive to run55. Bacteremia trials are also challenging because of the inherent difficulty in treating MRSA bacteremia; patients must be given an appropriate empirical therapy, and investigators must distinguish between uncomplicated and complicated bacteremia. The resistance profile of the patient isolate must also be considered in order to compare the efficacy of the investigational agent to SOC for either MRSA or MSSA bacteremia. Such difficulties present challenges to patient enrollment and make it difficult to conduct carefully controlled studies with largely homogeneous patient populations. In summary, investigators have learned through clinical trials that the drawbacks associated with combination therapy argue against widespread usage, but investigation focused on optimizing treatment duration and best practices for de-escalation to monotherapy should provide insight into maximizing patient safety during aggressive treatment regimens135. Only a few antibody and lysin-based candidates are seeing active development as adjunctive treatments for bacteremia, with their safety being established first and foremost – but exebacase’s upcoming phase III trial demonstrates that progress has been made towards conducting larger trials with these agents that are designed to show efficacy. While several antibiotics investigated as monotherapies have already been studied for treating cSSTIs and ABSSSIs caused by MRSA, they will need to be studied in patients with MRSA bacteremia before conclusions are made. To this end, clinical trials with ceftobiprole and dalbavancin should hopefully be completed within the next five years. Based on this timeline, the near future will reveal more about the standing of novel monotherapies than the other approaches discussed in this review.
While vancomycin and daptomycin will continue to remain the first-line treatments for MRSA bacteremia, clinical trials have identified other agents that may prove to be alternative treatment options in the future such as dalbavancin, ceftobiprole, ceftaroline, and exebacase. Despite the fact that previous clinical trials have failed to support strong alternatives to standard therapy, the future of therapeutics for MRSA bacteremia looks bright, as several ongoing and planned clinical trials indicate the continued motivation of the pharmaceutical industry in this field.
Article highlights.
The mortality associated with MRSA bacteremia has remained high and steady for decades, and there are concerns about the toxicity of standard therapy and the potential for antibiotic resistance to affect treatment efficacy.
In large trials investigating the efficacy of combination therapy, no benefit has been shown for vancomycin plus rifampicin or β-lactam antibiotics. Moreover, safety profiles favor monotherapy over combination therapy.
Ceftobiprole and ceftaroline are unusual in that they are β-lactam antibiotics that are used to treat MRSA infections. Both are currently under investigation as treatments for MRSA bacteremia and show acceptable safety profiles.
Trials that are focused on investigating novel antimicrobials and adjunctive therapies, including antibody and bacteriophage lysin-based agents, have mostly been limited to small studies that seek to establish their safety and pharmacokinetics.
Exebacase, a bacteriophage lysin-based candidate, is a standout among its class with efficacy as an adjunctive therapy to vancomycin for MRSA bacteremia shown in a phase II trial. Exebacase is undergoing further investigation in an upcoming phase III trial.
There are no clear alternatives to vancomycin or daptomycin for treating MRSA bacteremia, but several agents currently under investigation demonstrate a continued effort to improving patient outcomes.
Abbreviations:
- ABSSSI
acute bacterial skin and skin structure infections
- β-GlcNAc
β-N-acetylglucosamine
- BSI
bloodstream infection
- CABP
community-acquired bacterial pneumonia
- CoNS
coagulase-negative staphylococci
- CPS
capsular polysaccharide
- cSSTIs
complicated skin and soft tissue infections
- FDA
U.S. Food and Drug Administration
- HABP
hospital-acquired bacterial pneumonia
- HR
hazard ratio
- hVISA
heterogeneous vancomycin-intermediate Staphylococcus aureus
- IV
intravenous
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- MSCRAMM
microbial surface components recognizing adhesive matrix molecules
- MSSA
methicillin-sensitive Staphylococcus aureus
- NAG
N-acetylglucosamine
- NAM
N-acetylmuramic acid
- PBP
penicillin-binding protein
- PG
phosphatidylglycerol
- SAB
Staphylococcus aureus bacteremia
- SAE
serious adverse event
- SOC
standard of care
- SSTIs
skin and soft tissue infections
- TEAE
treatment-emergent adverse event
- TMP/SMX
trimethoprim-sulfamethoxazole
- TOC
test-of-cure
- VC
valine-citrulline
- VABP
ventilator-associated bacterial pneumonia
- VISA
vancomycin-intermediate Staphylococcus aureus
- VRSA
vancomycin-resistant Staphylococcus aureus
- WTA
wall teichoic acid
References
- 1.Laupland KB. Incidence of bloodstream infection: a review of population-based studies. Clin Microbiol Infect 2013. Jun;19(6):492–500. [DOI] [PubMed] [Google Scholar]
- 2.Bearman GM, Wenzel RP. Bacteremias: a leading cause of death. Arch Med Res 2005. Nov-Dec;36(6):646–59. [DOI] [PubMed] [Google Scholar]
- 3.David MZ, Daum RS, Bayer AS, Chambers HF, Fowler VG Jr., Miller LG, et al. Staphylococcus aureus bacteremia at 5 US academic medical centers, 2008–2011: significant geographic variation in community-onset infections. Clin Infect Dis 2014. Sep 15;59(6):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr., Hellmich M, Hopkins S, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 2014. Mar;68(3):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 2012. Apr;25(2):362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Y. C. Comparison of Mortality Associated with Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Meta-analysis. Clin Infect Dis 2003;36(1):53–59. [DOI] [PubMed] [Google Scholar]
- 7.Kourtis AP, Hatfield K, Baggs J, Mu Y, See I, Epson E, et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep 2019. Mar 8;68(9):214–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassetti M, Trecarichi EM, Mesini A, Spanu T, Giacobbe DR, Rossi M, et al. Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2012. Sep;18(9):862–9. [DOI] [PubMed] [Google Scholar]
- 9.Anantha RV, Jegatheswaran J, Pepe DL, Priestap F, Delport J, Haeryfar SM, et al. Risk factors for mortality among patients with Staphylococcus aureus bacteremia: a single-centre retrospective cohort study. CMAJ Open 2014. Oct;2(4):E352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Austin ED, Sullivan SS, Macesic N, Mehta M, Miko BA, Nematollahi S, et al. Reduced Mortality of Staphylococcus aureus Bacteremia in a Retrospective Cohort Study of 2139 Patients: 2007–2015. Clin Infect Dis 2020. Apr 10;70(8):1666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horino T, Hori S. Metastatic infection during Staphylococcus aureus bacteremia. Journal of Infection and Chemotherapy 2020;26(2):162–69. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011. Feb 1;52(3):e18–55. [DOI] [PubMed] [Google Scholar]; *: This reference reviews clinical practice guidelines for MRSA infections, including MRSA bacteremia.
- 13.Patel S, Preuss CV, Bernice F. Vancomycin. StatPearls. Treasure Island (FL) 2021. [Google Scholar]
- 14.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013. Feb;57(2):734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Kim KH, Kim HB, Kim NJ, Kim EC, Oh MD, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2008. Jan;52(1):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 2006;42:S35–S39. [DOI] [PubMed] [Google Scholar]
- 17.Aljohani S, Layqah L, Masuadi E, Al Alwan B, Baharoon W, Gramish J, et al. Occurrence of vancomycin MIC creep in methicillin resistant isolates in Saudi Arabia. J Infect Public Health 2020. Oct;13(10):1576–79. [DOI] [PubMed] [Google Scholar]
- 18.Kehrmann J, Kaase M, Szabados F, Gatermann SG, Buer J, Rath PM, et al. Vancomycin MIC creep in MRSA blood culture isolates from Germany: a regional problem? Eur J Clin Microbiol Infect Dis 2011. May;30(5):677–83. [DOI] [PubMed] [Google Scholar]
- 19.Diaz R, Afreixo V, Ramalheira E, Rodrigues C, Gago B. Evaluation of vancomycin MIC creep in methicillin-resistant Staphylococcus aureus infections-a systematic review and meta-analysis. Clin Microbiol Infect 2018. Feb;24(2):97–104. [DOI] [PubMed] [Google Scholar]
- 20.Prakash V, Lewis JS II, Jorgensen JH. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob Agents Chemother 2008. Dec;52(12):4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards B, Milne K, Lawes T, Cook I, Robb A, Gould IM. Is vancomycin MIC “creep” method dependent? Analysis of methicillin-resistant Staphylococcus aureus susceptibility trends in blood isolates from North East Scotland from 2006 to 2010. J Clin Microbiol 2012. Feb;50(2):318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sader HS, Fey PD, Limaye AP, Madinger N, Pankey G, Rahal J, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009. Oct;53(10):4127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover FC, Moellering RC Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007. May 1;44(9):1208–15. [DOI] [PubMed] [Google Scholar]
- 24.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev 2010. Jan;23(1):99–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuinness WA, Malachowa N, DeLeo FR. Vancomycin Resistance in Staphylococcus aureus. Yale J Biol Med 2017. Jun;90(2):269–81. [PMC free article] [PubMed] [Google Scholar]
- 26.Butler MS, Hansford KA, Blaskovich MA, Halai R, Cooper MA. Glycopeptide antibiotics: back to the future. J Antibiot (Tokyo) 2014. Sep;67(9):631–44. [DOI] [PubMed] [Google Scholar]
- 27.Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep 2020. Jul 29;10(1):12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis 2012. Mar;54(6):755–71. [DOI] [PubMed] [Google Scholar]
- 29.Lodise TP, Graves J, Evans A, Graffunder E, Helmecke M, Lomaestro BM, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008. Sep;52(9):3315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr., Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004. Jun;42(6):2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adani S, Bhowmick T, Weinstein MP, Narayanan N. Impact of Vancomycin MIC on Clinical Outcomes of Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia Treated with Vancomycin at an Institution with Suppressed MIC Reporting. Antimicrob Agents Chemother 2018. Apr;62(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh YC, Yeh KM, Lin TY, Chiu SK, Yang YS, Wang YC, et al. Impact of vancomycin MIC creep on patients with methicillin-resistant Staphylococcus aureus bacteremia. J Microbiol Immunol Infect 2012. Jun;45(3):214–20. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Sun X, Chang W, Dai Y, Ma X. Systematic Review and Meta-Analysis of the Epidemiology of Vancomycin-Intermediate and Heterogeneous Vancomycin-Intermediate Staphylococcus aureus Isolates. PLoS One 2015;10(8):e0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller WR, Bayer AS, Arias CA. Mechanism of Action and Resistance to Daptomycin in Staphylococcus aureus and Enterococci. Cold Spring Harb Perspect Med 2016. Nov 1;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grein F, Muller A, Scherer KM, Liu X, Ludwig KC, Klockner A, et al. Ca(2+)-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat Commun 2020. Mar 19;11(1):1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Figueroa DA, Mangini E, Amodio-Groton M, Vardianos B, Melchert A, Fana C, et al. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin Infect Dis 2009. Jul 15;49(2):177–80. [DOI] [PubMed] [Google Scholar]
- 37.Falcone M, Russo A, Venditti M, Novelli A, Pai MP. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2013. Dec;57(11):1568–76. [DOI] [PubMed] [Google Scholar]
- 38.Fowler VG Jr., Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006. Aug 17;355(7):653–65. [DOI] [PubMed] [Google Scholar]
- 39.Patel N, Lubanski P, Ferro S, Bonafede M, Harrington S, Evans A, et al. Correlation between vancomycin MIC values and those of other agents against gram-positive bacteria among patients with bloodstream infections caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2009. Dec;53(12):5141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev 2013. Oct;26(4):759–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis 2005. Jun 15;191(12):2149–52. [DOI] [PubMed] [Google Scholar]
- 42.Martinez Perez-Crespo PM, Lopez Cortes LE. Ceftobiprole: a clinical view. Rev Esp Quimioter 2021. Sep;34 Suppl 1:32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noel GJ, Strauss RS, Amsler K, Heep M, Pypstra R, Solomkin JS. Results of a double-blind, randomized trial of ceftobiprole treatment of complicated skin and skin structure infections caused by gram-positive bacteria. Antimicrob Agents Chemother 2008. Jan;52(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamed K, Engelhardt M, Jones ME, Saulay M, Holland TL, Seifert H, et al. Ceftobiprole versus daptomycin in Staphylococcus aureus bacteremia: a novel protocol for a double-blind, Phase III trial. Future Microbiology 2020;15(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morosini MI, Diez-Aguilar M, Canton R. Mechanisms of action and antimicrobial activity of ceftobiprole. Rev Esp Quimioter 2019. Sep;32 Suppl 3:3–10. [PMC free article] [PubMed] [Google Scholar]
- 46.Liapikou A, Cilloniz C, Torres A. Ceftobiprole for the treatment of pneumonia: a European perspective. Drug Des Devel Ther 2015;9:4565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casapao AM, Davis SL, Barr VO, Klinker KP, Goff DA, Barber KE, et al. Large retrospective evaluation of the effectiveness and safety of ceftaroline fosamil therapy. Antimicrob Agents Chemother 2014. May;58(5):2541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arshad S, Huang V, Hartman P, Perri MB, Moreno D, Zervos MJ. Ceftaroline fosamil monotherapy for methicillin-resistant Staphylococcus aureus bacteremia: a comparative clinical outcomes study. Int J Infect Dis 2017. Apr;57:27–31. [DOI] [PubMed] [Google Scholar]
- 49.Burnett YJ, Echevarria K, Traugott KA. Ceftaroline as Salvage Monotherapy for Persistent MRSA Bacteremia. Ann Pharmacother 2016. Dec;50(12):1051–59. [DOI] [PubMed] [Google Scholar]
- 50.Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005. Feb 1;40(3):374–80. [DOI] [PubMed] [Google Scholar]
- 51.Billeter M, Zervos MJ, Chen AY, Dalovisio JR, Kurukularatne C. Dalbavancin: a novel once-weekly lipoglycopeptide antibiotic. Clin Infect Dis 2008. Feb 15;46(4):577–83. [DOI] [PubMed] [Google Scholar]
- 52.Werth BJ, Jain R, Hahn A, Cummings L, Weaver T, Waalkes A, et al. Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen. Clin Microbiol Infect 2018. Apr;24(4):429 e1–29 e5. [DOI] [PubMed] [Google Scholar]
- 53.Werth BJ, Ashford NK, Penewit K, Waalkes A, Holmes EA, Ross DH, et al. Dalbavancin exposure in vitro selects for dalbavancin-non-susceptible and vancomycin-intermediate strains of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 2021. Jun;27(6):910 e1–10 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stryjewski ME, Lentnek A, O’Riordan W, Pullman J, Tambyah PA, Miró JM, et al. A randomized Phase 2 trial of telavancin versus standard therapy in patients with uncomplicated Staphylococcus aureus bacteremia: the ASSURE study. BMC Infectious Diseases 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holland TL, Chambers HF, Boucher HW, Corey GR, Coleman R, Castaneda-Ruiz B, et al. Considerations for Clinical Trials of Staphylococcus aureus Bloodstream Infection in Adults. Clin Infect Dis 2019. Feb 15;68(5):865–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; **: This paper is notable for discussing the challenges that are associated with conducting clinical trials in patients with SAB.
- 56.A Phase 3 Telavancin Staphylococcus Aureus (S. Aureus) Bacteremia Trial 2020. [cited; Available from: https://clinicaltrials.gov/ct2/show/results/NCT02208063
- 57.Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther 2018;12:1759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: noninferiority of linezolid in a phase 3 study. Clin Infect Dis 2009. Jan 15;48(2):203–12. [DOI] [PubMed] [Google Scholar]
- 59.Senior K FDA issue linezolid warning. The Lancet Infectious Diseases 2007;7(5):310. [Google Scholar]
- 60.Kemnic TR, Coleman M. Trimethoprim Sulfamethoxazole. StatPearls. Treasure Island (FL) 2021. [PubMed] [Google Scholar]
- 61.Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med 1992. Sep 1;117(5):390–8. [DOI] [PubMed] [Google Scholar]
- 62.Paul M, Bishara J, Yahav D, Goldberg E, Neuberger A, Ghanem-Zoubi N, et al. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: randomised controlled trial. BMJ 2015. May 14;350:h2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wehrli W Rifampin: mechanisms of action and resistance. Rev Infect Dis 1983. Jul–Aug;5 Suppl 3:S407–11. [DOI] [PubMed] [Google Scholar]
- 64.Forrest GN, Tamura K. Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev 2010. Jan;23(1):14–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmerli W, Sendi P. Role of Rifampin against Staphylococcal Biofilm Infections In Vitro, in Animal Models, and in Orthopedic-Device-Related Infections. Antimicrob Agents Chemother 2019. Feb;63(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jorgensen NP, Skovdal SM, Meyer RL, Dagnaes-Hansen F, Fuursted K, Petersen E. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog Dis 2016. Jun;74(4):ftw019. [DOI] [PubMed] [Google Scholar]
- 67.Darouiche RO, Hamill RJ. Antibiotic penetration of and bactericidal activity within endothelial cells. Antimicrob Agents Chemother 1994. May;38(5):1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bayer AS, Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis 1985. Jan;151(1):157–65. [DOI] [PubMed] [Google Scholar]
- 69.Thwaites GE, Scarborough M, Szubert A, Nsutebu E, Tilley R, Greig J, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. The Lancet 2018;391(10121):668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]; **: This is the largest clinical trial for SAB to date; it investigated the efficacy of adjunctive rifampicin for treating SAB.
- 70.Bush K, Bradford PA. beta-Lactams and beta-Lactamase Inhibitors: An Overview. Cold Spring Harb Perspect Med 2016. Aug 1;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pandey N, Cascella M. Beta Lactam Antibiotics. StatPearls. Treasure Island (FL) 2021. [PubMed] [Google Scholar]
- 72.Tang SS, Apisarnthanarak A, Hsu LY. Mechanisms of beta-lactam antimicrobial resistance and epidemiology of major community- and healthcare-associated multidrug-resistant bacteria. Adv Drug Deliv Rev 2014. Nov 30;78:3–13. [DOI] [PubMed] [Google Scholar]
- 73.Beck WD, Berger-Bachi B, Kayser FH. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol 1986. Feb;165(2):373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barber KE, Ireland CE, Bukavyn N, Rybak MJ. Observation of “seesaw effect” with vancomycin, teicoplanin, daptomycin and ceftaroline in 150 unique MRSA strains. Infect Dis Ther 2014. Jun;3(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tong SYC, Lye DC, Yahav D, Sud A, Robinson JO, Nelson J, et al. Effect of Vancomycin or Daptomycin With vs Without an Antistaphylococcal beta-Lactam on Mortality, Bacteremia, Relapse, or Treatment Failure in Patients With MRSA Bacteremia: A Randomized Clinical Trial. JAMA 2020. Feb 11;323(6):527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eljaaly K, Alshehri S, Erstad BL. Systematic Review and Meta-analysis of the Safety of Antistaphylococcal Penicillins Compared to Cefazolin. Antimicrob Agents Chemother 2018. Apr;62(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson TM, Molina KC, Miller MA, Kiser TH, Huang M, Mueller SW. Combination ceftaroline and daptomycin salvage therapy for complicated methicillin-resistant Staphylococcus aureus bacteraemia compared with standard of care. Int J Antimicrob Agents 2021. Apr;57(4):106310. [DOI] [PubMed] [Google Scholar]
- 78.Sakoulas G, Moise PA, Casapao AM, Nonejuie P, Olson J, Okumura CY, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014. Oct 1;36(10):1317–33. [DOI] [PubMed] [Google Scholar]
- 79.Geriak M, Haddad F, Rizvi K, Rose W, Kullar R, LaPlante K, et al. Clinical Data on Daptomycin plus Ceftaroline versus Standard of Care Monotherapy in the Treatment of Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother 2019. May;63(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silver LL. Fosfomycin: Mechanism and Resistance. Cold Spring Harb Perspect Med 2017. Feb 1;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.del Rio A, Gasch O, Moreno A, Pena C, Cuquet J, Soy D, et al. Efficacy and safety of fosfomycin plus imipenem as rescue therapy for complicated bacteremia and endocarditis due to methicillin-resistant Staphylococcus aureus: a multicenter clinical trial. Clin Infect Dis 2014. Oct 15;59(8):1105–12. [DOI] [PubMed] [Google Scholar]
- 82.Pericas JM, Moreno A, Almela M, Garcia-de-la-Maria C, Marco F, Munoz P, et al. Efficacy and safety of fosfomycin plus imipenem versus vancomycin for complicated bacteraemia and endocarditis due to methicillin-resistant Staphylococcus aureus: a randomized clinical trial. Clin Microbiol Infect 2018. Jun;24(6):673–76. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-de-la-Maria C, Gasch O, Garcia-Gonzalez J, Soy D, Shaw E, Ambrosioni J, et al. The Combination of Daptomycin and Fosfomycin Has Synergistic, Potent, and Rapid Bactericidal Activity against Methicillin-Resistant Staphylococcus aureus in a Rabbit Model of Experimental Endocarditis. Antimicrob Agents Chemother 2018. Jun;62(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pujol M, Miro JM, Shaw E, Aguado JM, San-Juan R, Puig-Asensio M, et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin Infect Dis 2021. May 4;72(9):1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arbeit RD, Karakawa WW, Vann WF, Robbins JB. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagnostic Microbiology and Infectious Disease 1984. 1984/04/01/;2(2):85–91. [DOI] [PubMed] [Google Scholar]
- 86.Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, et al. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. mBio 2015. Apr 7;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, et al. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect Control Hosp Epidemiol 2014. Mar;35(3):285–92. [DOI] [PubMed] [Google Scholar]
- 88.Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun 1997. Oct;65(10):4146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun 1996. May;64(5):1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benjamin DK, Schelonka R, White R, Holley HP, Bifano E, Cummings J, et al. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. J Perinatol 2006. May;26(5):290–5. [DOI] [PubMed] [Google Scholar]
- 91.Rupp ME, Holley HP Jr., Lutz J, Dicpinigaitis PV, Woods CW, Levine DP, et al. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2007. Dec;51(12):4249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nabi Biopharmaceuticals Announces Results of StaphVAX® Confirmatory Phase III Clinical Trial. 2005. [cited; Available from: https://www.sec.gov/Archives/edgar/data/72444/000119312505214434/dex99.htm
- 93.Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, et al. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun 2002. Sep;70(9):4987–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colque-Navarro P, Palma M, Soderquist B, Flock JI, Mollby R. Antibody responses in patients with staphylococcal septicemia against two Staphylococcus aureus fibrinogen binding proteins: clumping factor and an extracellular fibrinogen binding protein. Clin Diagn Lab Immunol 2000. Jan;7(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siboo IR, Cheung AL, Bayer AS, Sullam PM. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect Immun 2001. May;69(5):3120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolz C, Goerke C, Landmann R, Zimmerli W, Fluckiger U. Transcription of clumping factor A in attached and unattached Staphylococcus aureus in vitro and during device-related infection. Infect Immun 2002. Jun;70(6):2758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, et al. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun 2003. Dec;71(12):6864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Patti JM. A humanized monoclonal antibody targeting Staphylococcus aureus. Vaccine 2004. Dec 6;22 Suppl 1:S39–43. [DOI] [PubMed] [Google Scholar]
- 99.Weems JJ Jr., Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, et al. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006. Aug;50(8):2751–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.John JF. Drug evaluation: tefibazumab--a monoclonal antibody against staphylococcal infection. Current opinion in molecular therapeutics 2006. 2006/10//;8(5):455–60. [PubMed] [Google Scholar]
- 101.Falugi F, Kim HK, Missiakas DM, Schneewind O. Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio 2013. Aug 27;4(5):e00575–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun Y, Emolo C, Holtfreter S, Wiles S, Kreiswirth B, Missiakas D, et al. Staphylococcal Protein A Contributes to Persistent Colonization of Mice with Staphylococcus aureus. J Bacteriol 2018. May 1;200(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varshney AK, Kuzmicheva GA, Lin J, Sunley KM, Bowling RA Jr., Kwan TY, et al. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS One 2018;13(1):e0190537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huynh T, Stecher M, McKinnon J, Jung N, Rupp ME. Safety and Tolerability of 514G3, a True Human Anti-Protein A Monoclonal Antibody for the Treatment of S. aureus Bacteremia. Open Forum Infectious Diseases 2016;3(suppl_1). [Google Scholar]
- 105.XBiotech Announces Top-Line Results for 514G3 Antibody Therapy in Serious Staphylococcus aureus Infections. 2017. [cited; Available from: https://www.globenewswire.com/news-release/2017/04/03/953500/0/en/XBiotech-Announces-Top-Line-Results-for-514G3-Antibody-Therapy-in-Serious-Staphylococcus-aureus-Infections.html
- 106.Garzoni C, Kelley WL. Return of the Trojan horse: intracellular phenotype switching and immune evasion by Staphylococcus aureus. EMBO Mol Med 2011. Mar;3(3):115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peyrusson F, Tulkens PM, Van Bambeke F. Cellular Pharmacokinetics and Intracellular Activity of Gepotidacin against Staphylococcus aureus Isolates with Different Resistance Phenotypes in Models of Cultured Phagocytic Cells. Antimicrob Agents Chemother 2018. Apr;62(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nature 2015. 2015/11/01;527(7578):323–28. [DOI] [PubMed] [Google Scholar]
- 109.Staben LR, Koenig SG, Lehar SM, Vandlen R, Zhang D, Chuh J, et al. Targeted drug delivery through the traceless release of tertiary and heteroaryl amines from antibody-drug conjugates. Nat Chem 2016. Dec;8(12):1112–19. [DOI] [PubMed] [Google Scholar]
- 110.Zhou C, Lehar S, Gutierrez J, Rosenberger CM, Ljumanovic N, Dinoso J, et al. Pharmacokinetics and pharmacodynamics of DSTA4637A: A novel THIOMAB antibody antibiotic conjugate against Staphylococcus aureus in mice. MAbs 2016. Nov/Dec;8(8):1612–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peck M, Rothenberg Michael E, Deng R, Lewin-Koh N, She G, Kamath Amrita V, et al. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrobial Agents and Chemotherapy 2019;63(6):e02588–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rymut SM, Deng R, Owen R, Saad O, Berhanu A, Lim J, et al. 1305. Comparison of Pharmacokinetics of DSTA4637S, a novel THIOMAB(TM) Antibody-Antibiotic Conjugate, in Patients with Staphylococcus aureus Bacteremia Receiving Standard-of-Care Antibiotics with Pharmacokinetics in Healthy Volunteers. Open Forum Infectious Diseases 2020;7(Suppl 1):S666–S67. [Google Scholar]
- 113.Jun SY, Jung GM, Son JS, Yoon SJ, Choi YJ, Kang SH. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob Agents Chemother 2011. Apr;55(4):1764–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jun SY, Jung GM, Yoon SJ, Oh MD, Choi YJ, Lee WJ, et al. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 2013. Feb;41(2):156–61. [DOI] [PubMed] [Google Scholar]
- 115.Kim N-H, Park Wan B, Cho Jeong E, Choi Yoon J, Choi Su J, Jun Soo Y, et al. Effects of Phage Endolysin SAL200 Combined with Antibiotics on Staphylococcus aureus Infection. Antimicrobial Agents and Chemotherapy 2018;62(10):e00731–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jun SY, Jung GM, Yoon SJ, Choi YJ, Koh WS, Moon KS, et al. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother 2014;58(4):2084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jun SY, Jang IJ, Yoon S, Jang K, Yu KS, Cho JY, et al. Pharmacokinetics and Tolerance of the Phage Endolysin-Based Candidate Drug SAL200 after a Single Intravenous Administration among Healthy Volunteers. Antimicrob Agents Chemother 2017. Jun;61(6). [DOI] [PMC free article] [PubMed] [Google Scholar]; *: This study is notable for being the first-in-human phase I study to intravenously administer a bacteriophage endolysin-based drug to human patients.
- 118.A Study to Evaluate the Safety, PK, PD, Immunogenicity of N-Rephasin® SAL200 in Healthy Male Volunteers. 2021. [cited; Available from: https://clinicaltrials.gov/ct2/show/results/NCT03446053
- 119.Phase IIa Clinical Study of N-Rephasin® SAL200. 2021. [cited; Available from: https://clinicaltrials.gov/ct2/show/results/NCT03089697
- 120.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 2014. May 1;209(9):1469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob Agents Chemother 2017. Jul;61(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, et al. The Antistaphylococcal Lysin, CF-301, Activates Key Host Factors in Human Blood To Potentiate Methicillin-Resistant Staphylococcus aureus Bacteriolysis. Antimicrob Agents Chemother 2019. Apr;63(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Watson A, Sauve K, Cassino C, Schuch R. Exebacase Demonstrates In Vitro Synergy with a Broad Range of Antibiotics against both Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus. Antimicrob Agents Chemother 2020. Jan 27;64(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Exebacase Pipeline. 2021. [cited; Available from: https://www.contrafect.com/pipeline/exebacase
- 125.Fowler VG Jr., Das AF, Lipka-Diamond J, Schuch R, Pomerantz R, Jauregui-Peredo L, et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J Clin Invest 2020. Jul 1;130(7):3750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; **: This study demonstrated the efficacy of a lysin-based agent when used as an adjunctive treatment for SAB in a phase II trial.
- 126.Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-World Experience with Oritavancin Therapy in Invasive Gram-Positive Infections. Infect Dis Ther 2017. Jun;6(2):277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tice A Oritavancin: a new opportunity for outpatient therapy of serious infections. Clin Infect Dis 2012. Apr;54 Suppl 3:S239–43. [DOI] [PubMed] [Google Scholar]
- 128.Oritavancin for Staphylococcus Aureus Infections in Opioid Users. 2018. [cited; Available from: https://clinicaltrials.gov/ct2/show/NCT03761953
- 129.Hall RG 2nd, Smith WJ, Putnam WC, Pass SE. An evaluation of tedizolid for the treatment of MRSA infections. Expert Opin Pharmacother 2018. Sep;19(13):1489–94. [DOI] [PubMed] [Google Scholar]
- 130.Renzoni A, Kelley WL, Rosato RR, Martinez MP, Roch M, Fatouraei M, et al. Molecular Bases Determining Daptomycin Resistance-Mediated Resensitization to beta-Lactams (Seesaw Effect) in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother 2017. Jan;61(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gu J, Xu W, Lei L, Huang J, Feng X, Sun C, et al. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol 2011. Jan;49(1):111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Channabasappa S, Chikkamadaiah R, Durgaiah M, Kumar S, Ramesh K, Sreekanthan A, et al. Efficacy of chimeric ectolysin P128 in drug-resistant Staphylococcus aureus bacteraemia in mice. J Antimicrob Chemother 2018. Dec 1;73(12):3398–404. [DOI] [PubMed] [Google Scholar]
- 133.Yang H, Zhang H, Wang J, Yu J, Wei H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci Rep 2017. Jan 9;7:40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.ContraFect Announces U.S. FDA Grants Breakthrough Therapy Designation to Exebacase for the Treatment of Methicillin-Resistant Staphylococcus aureus (MRSA) Bacteremia, Including Right-Sided Endocarditis 2020. [cited; Available from: https://www.globenewswire.com/en/news-release/2020/02/24/1989110/0/en/ContraFect-Announces-U-S-FDA-Grants-Breakthrough-Therapy-Designation-to-Exebacase-for-the-Treatment-of-Methicillin-Resistant-Staphylococcus-aureus-MRSA-Bacteremia-Including-Right-S.html
- 135.Rose W, Fantl M, Geriak M, Nizet V, Sakoulas G. Current Paradigms of Combination Therapy in Methicillin-Resistant Staphylococcus aureus (MRSA) Bacteremia: Does it Work, Which Combination, and For Which Patients? Clin Infect Dis 2021. Dec 16;73(12):2353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


