Abstract
The goals of this review are to improve understanding of the aetiology of chronic muscle pain and identify new targets for treatments. Muscle pain is usually associated with trigger points in syndromes such as fibromyalgia and myofascial syndrome, and with small spots associated with spontaneous electrical activity that seems to emanate from fibers inside muscle spindles in EMG studies. These observations, added to the reports that large‐diameter primary afferents, such as those innervating muscle spindles, become hyperexcitable and develop spontaneous ectopic firing in conditions leading to neuropathic pain, suggest that changes in excitability of these afferents might make an important contribution to the development of pathological pain. Here, we review evidence that the muscle spindle afferents (MSAs) of the jaw‐closing muscles become hyperexcitable in a model of chronic orofacial myalgia. In these afferents, as in other large‐diameter primary afferents in dorsal root ganglia, firing emerges from fast membrane potential oscillations that are supported by a persistent sodium current (I NaP) mediated by Na+ channels containing the α‐subunit NaV1.6. The current flowing through NaV1.6 channels increases when the extracellular Ca2+ concentration decreases, and studies have shown that I NaP‐driven firing is increased by S100β, an astrocytic protein that chelates Ca2+ when released in the extracellular space. We review evidence of how astrocytes, which are known to be activated in pain conditions, might, through their regulation of extracellular Ca2+, contribute to the generation of ectopic firing in MSAs. To explain how ectopic firing in MSAs might cause pain, we review evidence supporting the hypothesis that cross‐talk between proprioceptive and nociceptive pathways might occur in the periphery, within the spindle capsule.
Keywords: astrocytes, chronic muscle pain, ectopic firing, muscle spindle afferent, trigeminal system
-
What is the topic of this review?
Firing in muscle spindle afferents (MSAs) is generated when their muscle is stretched, but sometimes generated elsewhere along the neuron (ectopic firing) in pathological pain. Appearance of ectopic firing coincides with appearance of pain. We examine mechanisms underlying emergence of ectopic firing in MSAs of the jaw‐closing muscles.
-
What advances does it highlight?
Firing in MSAs emerges from oscillations supported by a persistent sodium current, which varies with extracellular Ca2+, which is, in turn, regulated by astrocytes. We emphasize the potential role of astrocytes in appearance of ectopic firing. We proposed an explanation for how activity in MSAs crosses to nociceptors in the periphery.
1. INTRODUCTION
Muscle pain is a perplexing common condition that can, at times, arise from inflammation caused by minor injuries, strain, exercise, fatigue or stress, whereas at other times, pain can be completely absent in some muscle diseases associated with important inflammatory histopathological signs (Partanen, 2018). It is usually localized and resolves spontaneously or after simple treatments (Tantanatip & Chang, 2023). However, in 20%−30% of people, acute musculoskeletal pain develops into chronic pain that persists in the absence of inflammation and after tissue healing and can occasionally lead to devastating conditions, such as chronic widespread musculoskeletal pain, chronic fatigue syndrome, and fibromyalgia, which are major causes of disability (Henschke et al., 2008; Itz et al., 2013; Kasch et al., 2008). A classic example is the large proportion (40%−50%) of patients with acute whiplash‐associated disorders who develop a chronic pain condition (Carroll et al., 2008; Kamper et al., 2008). In the orofacial area, chronic musculoskeletal pain of sufficient intensity to impede speech and eating behaviors by affecting jaw muscle motor function and proprioception is reported in 22%−26% of the general population, with 7%−11% experiencing it persistently (Brattberg et al., 1989).
A typical feature of muscle pain syndromes is that pain is not spread uniformly across the muscle but is triggered at some specific points (Partanen, 2018). Trigger points are spots of hardening in the muscle tissue that are painful on compression (Simons et al., 1999). Typically, trigger points are a hallmark of myofascial pain syndrome (Money, 2017; Saxena et al., 2015), but they can also impact fibromyalgia by adding to the level of central sensitization that characterizes this condition (Ge, 2010). Even in EMG studies of healthy muscles, pain caused by the insertion of the EMG needle is elicited in small spots where spontaneous electric activity is encountered, but outside of these spots, there is no pain and no spontaneous activity (Lavelle et al., 2007; Partanen, 2018). Electrical stimulation near these spots is extremely painful even if no visible contraction can be detected, and because the same stimulation elsewhere in the muscle is painless, it has been proposed that pain spots occur when the needle touches an intramuscular nerve terminal (Partanen, 2018). Furthermore, these pain spots have been found often to coincide with increased resistance to the advancement of the needle, which has led to the suggestion that these terminals are located inside muscle spindle capsules (Partanen, 2018). These capsules are lamellated structures of connective tissue that enclose the intrafusal muscle fibers, around which coil the mechanosensitive sensory endings of large‐diameter primary afferents (PAs) of group Ia and II (Banks & Barker, 2004; Hulliger, 1984). In normal conditions, activity in these afferents conveys to the CNS information about muscle length and speed of stretch, whereas pain sensation is typically conveyed by small‐diameter PAs (groups III and IV), termed nociceptors (S. A. Armstrong & Herr, 2023).
Pain that persists in the absence of noxious stimuli and after tissue healing is considered pathological and often results from central or peripheral changes in excitability in nociceptors and their associated circuitry (Arendt‐Nielsen et al., 2011; Colloca et al., 2017; Finnerup et al., 2021; Voscopoulos & Lema, 2010). However, several lines of evidence suggest that changes in excitability of large‐diameter PAs might also contribute to appearance of pathological pain (Djouhri et al., 2012; Han et al., 2000; Khan et al., 2002; Y. I. Kim et al., 1998; C. N. Liu et al., 2000; Tal M et al., 2006; Tashima et al., 2018; Zhu & Henry, 2012). Here, we review this evidence and focus on the potential role of changes in excitability in muscle spindle afferents (MSAs) in chronic myalgia, using jaw‐closing muscles as an example. We examine the potential contribution of astrocytes, the most common type of glial cells, to these changes in excitability. Finally, we propose a model explaining how changes in excitability in proprioceptive pathways might lead to activation of nociceptive pathways translating into pain sensation.
2. EVIDENCE THAT LARGE‐DIAMETER PRIMARY AFFERENTS CONTRIBUTE TO PAIN SENSATION
Firing in PAs is usually generated in the periphery when their receptor in muscle, tendon or skin is activated by a stimulus. When generated elsewhere along the neuron, it is termed ectopic (LaMotte, 2007). In humans, after nerve injury, and in several animal neuropathic pain models, an increase in excitability of large‐diameter PAs leading to spontaneous ectopic firing coincides precisely with pain onset (Han et al., 2000; Khan et al., 2002; C. N. Liu et al., 2000; Tal M et al., 2006). In these conditions, vibration or light mechanical stimuli that activate only large‐diameter PAs can produce pain (Colloca et al., 2017; Zhu & Henry, 2012). More importantly, in mice in which transmission from large‐diameter PAs is left intact, but in which transmission specifically from nociceptors is prevented genetically (conditional VGluT2 knockouts), mechanical hypersensitivity that is normally observed after nerve injury develops and persists despite the fact that they have altered acute nociceptive responses and lose injury‐induced heat hyperalgesia (Scherrer et al., 2010).
Even in milder pain conditions not involving nerve injury, such as delayed onset muscle soreness, which results from excessive exercise or exercises with eccentric contractions and which is often used as a model of muscle pain in humans, there is evidence that specific stimulation of MSAs exacerbates the pain (Barlas et al., 2000; Weerakkody et al., 2001). To reproduce the pH changes observed in muscles after exercise or inflammation in humans or induced by increased levels of muscle lactate and pyruvate observed in chronic myalgia patients (Gerdle et al., 2008, 2010; Hood et al., 1988; Issberner et al., 1996; Reeh & Steen, 1996; Sjogaard et al., 2010), Sluka et al. (2001) developed an animal model using two injections of acidic saline spaced 2–5 days apart. Such injections made unilaterally in the gastrocnemius muscle produced bilateral hyperalgesia, which persisted for 4–5 weeks, even if they caused minimal damage and produced only a small reduction in the pH (to 6.0) that lasted for ∼10 min.
To obtain direct measures of MSA excitability in chronic myalgia conditions, Lund et al. (2010) took advantage of the trigeminal system innervating the orofacial region, where most PAs have their somata in the trigeminal ganglion, while those of MSAs and about half of the periodontal receptors, which are both large‐diameter PAs, are centrally located in the trigeminal mesencephalic nucleus (NVmes), making them easily accessible for identified recordings in brainstem slice preparations. They adapted Sluka's model to the jaw‐closing (masseter) muscles of rats and showed that two ipsilateral injections of acidic saline (20 μL, pH 4.0) caused a bilateral increase in mechanical sensitivity (allodynia) of the masseters that lasted for ∼5 weeks. This increase in sensitivity was accompanied by increased expression of the activity marker c‐Fos and several electrophysiological changes in NVmes cells.
In in vitro preparations from control animals, NVmes cells are usually silent, but if the cells are depolarized they display fast membrane potential oscillations, from which firing emerges. The rising phase of the oscillations depends on a persistent Na+ current (I NaP), while a low‐threshold, 4‐aminopyridine‐sensitive outward current, is probably responsible for the repolarizing phase (Pedroarena et al., 1999; Wu et al., 2001). Normally, the amplitude of the oscillations rises with increasing levels of membrane depolarization. A resurgent Na+ current (I res) activated during the oscillations sometimes leads to rhythmic bursting, but otherwise, it is difficult to maintain firing in these afferents because of a marked outward rectification with depolarization (Del Negro & Chandler, 1997; Verdier et al., 2004). However, in the muscle pain model, a greater proportion of cells displayed spontaneous or sustained firing upon depolarization in the acid‐treated groups versus control animals despite a surprising hyperpolarizing shift of the membrane potential which, counterintuitively, resulted in increased excitability. This is because the thresholds for firing, bursting, and fast membrane oscillations were also shifted to hyperpolarized potentials. The amplitude of the oscillations recorded from the acid‐treated neurons was significantly greater than the amplitude of those recorded from control animals at similar membrane potentials. These differences persisted for ≥35 days but returned to control values by 42 days after the injections, the time at which responses to mechanical stimuli also returned to control levels (Lund et al., 2010).
Large dorsal root ganglion (DRG) neurons have similar membrane potential oscillations, and in various neuropathic pain models involving injury or inflammation of nerves or of DRGs, the amplitude of these oscillations increases and leads to spontaneous somatic ectopic firing (Amir et al., 1999; Ke et al., 2012; C. N. Liu et al., 2000; Xie et al., 2012). Ectopic firing is believed to be a major contributor to chronic neuropathic pain (Devor & Seltzer, 1999), because onset of allodynia or hyperalgesia coincides with a sudden increase in the proportion of large DRG neurons expressing spontaneous firing and large membrane potential oscillations (C. N. Liu et al., 2000). In humans, injections of low doses of local anaesthetic into the DRG reduce phantom limb pain (Vaso et al., 2014). In animal models, blocking spontaneous activity by perfusing the damaged nerve with TTX prevents development of chronic pain, and perfusion of inflamed DRGs with riluzole, which blocks I NaP, also blocks spontaneous activity and bursting in Aαβ fibre afferents and reduces pain behavior for ≥3 weeks after application (Xie et al., 2012, 2005). Thus, there seems to be a clear link between changes in excitability of large‐diameter PAs and some forms of chronic pain.
3. CHANGES IN EXPRESSION OF ION CHANNELS UNDERLYING HYPEREXCITABILITY OF LARGE‐DIAMETER PRIMARY AFFERENTS
Many ion channels are differentially regulated in chronic pain states (reviewed by Alles & Smith, 2021; Bennett et al., 2019), but NaV1.3 and NaV1.6 are of particular interest for appearance of ectopic firing (X. Liu et al., 1999; Xie et al., 2015). NaV1.3 is an embryonic type of channel, whose expression is normally suppressed in adults. It is highly re‐expressed in all injured sensory neurons, but preferentially in medium‐ and large‐diameter DRG neurons (Abe et al., 2002; Black et al., 1999; Dib‐Hajj et al., 1996; C. H. Kim et al., 2001; Waxman et al., 1994). This overexpression of NaV1.3 occurs rapidly (within 24 hours of injury) and lasts as long as ectopic discharge and mechanical allodynia are observed (days to weeks) (H. S. Kim & Chung, 1992; C. N. Liu et al., 2000, X. Liu et al., 1999). In humans, NaV1.3 is also upregulated in biopsies from trigeminal neuralgia patients (Siqueira et al., 2009).
The second channel of interest, NaV1.6, has also been linked to several neuropathic pain states (L. Chen et al., 2020; Henry et al., 2007; Ren et al., 2012). In NVmes cells and in DRG cells, repetitive firing and excitatory persistent and resurgent currents have been shown to rely on I NaP and, in particular, on the NaV1.6 sodium channel isoform (Cummins et al., 2005; Xing et al., 2014). Spontaneously active bursting cells in inflamed DRGs express higher levels of NaV1.6 immunoreactivity, and local knockdown of NaV1.6 by injection of small interfering RNA into the DRG at the time of inflammation blocks mechanical pain behaviors and abnormal spontaneous activity in large myelinated PAs, with little effect on unmyelinated PAs in the radicular pain model, the spinal nerve ligation model and the chronic constriction of the sciatic nerve model (Xie et al., 2013, 2015). Furthermore, specific knockdown of NaV1.6 in small‐diameter PAs expressing NaV1.8 does not affect acute, inflammatory, or neuropathic pain behaviors, further supporting a role for large‐diameter PAs in chronic pain states (L. Chen et al., 2018). Expression of NaV1.6 is significantly increased in human skin biopsies taken from patients with complex regional pain syndrome and post‐herpetic neuralgia, and most interestingly, a recent study reported a gain‐of‐function mutation of this subunit in a patient with trigeminal neuralgia, adding to evidence from animal studies to support a role for this channel in pain (Dib‐Hajj et al., 1996; Sittl et al., 2012; Tanaka et al., 2016; Xie et al., 2013, 2015; Zhao et al., 2008).
In all cases, the kinetics and stability of NaV channels are regulated by Ca2+, which interacts directly with extracellular moieties and amino acid residues lining the pore of the channel, or indirectly, via its interaction with calmodulin, which modifies the gating behaviors of the channel, or calpain, which cleaves NaV channel α‐subunit NaV1.6 (C. M. Armstrong & Cota, 1990; C. Brocard et al., 2016; Santarelli et al., 2007; Sarhan et al., 2012). Decreases of the extracellular Ca2+ concentration ([Ca2+]o) are able to shift the activation threshold of I NaP and its half‐activation voltage (by ∼1 mV, every 0.1 mM) towards more hyperpolarized potentials (F. Brocard et al., 2013; Li & Hatton, 1996; Morquette et al., 2015; Su et al., 2001). Thus, even small variations in [Ca2+]o can have an important effect on the firing of a neuron by decreasing the threshold for I NaP activation and increasing its amplitude, perhaps explaining the increased excitability associated with the hyperpolarizing membrane potential shift observed in the muscle pain model above. Interestingly, Morquette et al. (2015) have shown, in a different trigeminal nucleus, that astrocytes, the most common type of glial cells, exert a powerful effect on I NaP by releasing a calcium‐binding protein, S100β, which modulates [Ca2+]o. On the basis of their findings, Ryczko et al. (2021) went on to show that photostimulation of astrocytes expressing channelrhodopsin (ChR2; a light‐sensitive cationic channel) in glial fibrillary acidic protein (GFAP)‐ChR2 mice causes reductions in [Ca2+]o that are sufficient to elicit NaV1.6‐driven firing in cortical layer 5 pyramidal neurons, which is prevented by infusion of an anti‐S100β antibody. Taken together, this evidence suggests that astrocytes can alter neuronal firing by releasing a protein, S100β, that potentiates I NaP by decreasing [Ca2+]o.
These observations raise the possibility that the hyperexcitability of NVmes neurons in the pain model results from an enhancement of I NaP caused by a decrease in [Ca2+]o following release of S100β from activated astrocytes (Figure 1). Unpublished (except in abstract form) results from Gaudel et al. (2022) show that local applications of S100β near NVmes neurons recorded in a rat brainstem slice preparation increase the amplitude of their oscillations and lead to firing (Figure 2a, left) in previously silent neurons, which is blocked with riluzole (Figure 2a, right), an I NaP blocker. S100β, in addition to BAPTA, a Ca2+ chelator that reproduces the effects of S100β on [Ca2+]o, also lowers the firing and oscillation thresholds (Figure 2b). Interestingly, astrocytic processes immunoreactive to S100β were also seen closely apposed onto sections of the axon initial segment of NVmes neurons enriched in NaV1.6, as shown in Figure 3a. Thus, experimentally lowering [Ca2+]o with local applications of BAPTA or S100β near the axon initial segment of NVmes neurons elicits I NaP‐dependent firing. This effect could be produced physiologically by release of S100β from astrocytic processes apposed to the axon initial segment.
FIGURE 1.
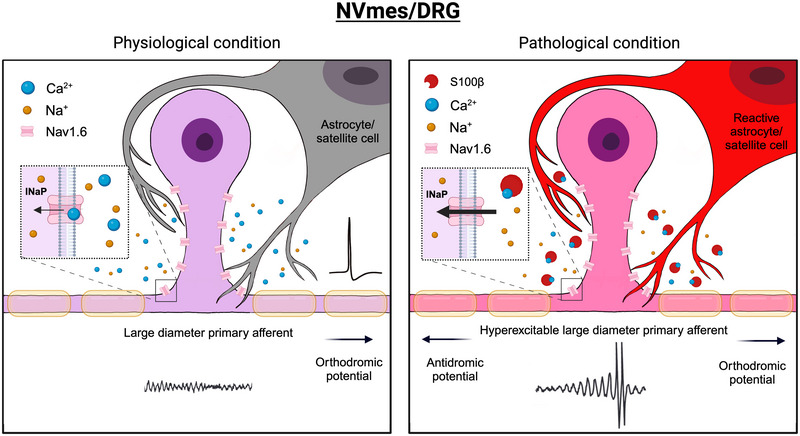
Schematic representation synthesizing the proposed mechanisms underlying the appearance of ectopic firing in large‐diameter PAs of NVmes and DRG neurons. Left: In physiological conditions, higher levels of extracellular Ca2+ limit activity in the cells by occluding the Na+ channels (NaV1.6) mediating I NaP that supports membrane potential oscillations from which firing normally emerges. Right: In pathological states, reactive astrocytes (perhaps activated initially by firing of large‐diameter PAs sensing acidosis) release S100β, which chelates extracellular Ca2+ and enhances I NaP, causing an increase in the amplitude of the fast membrane oscillations and emergence of ectopic firing. Abbreviations: DRG, dorsal root ganglion; I NaP, persistent sodium current; NVmes, trigeminal mesencephalic nucleus; PA, primary afferent.
FIGURE 2.
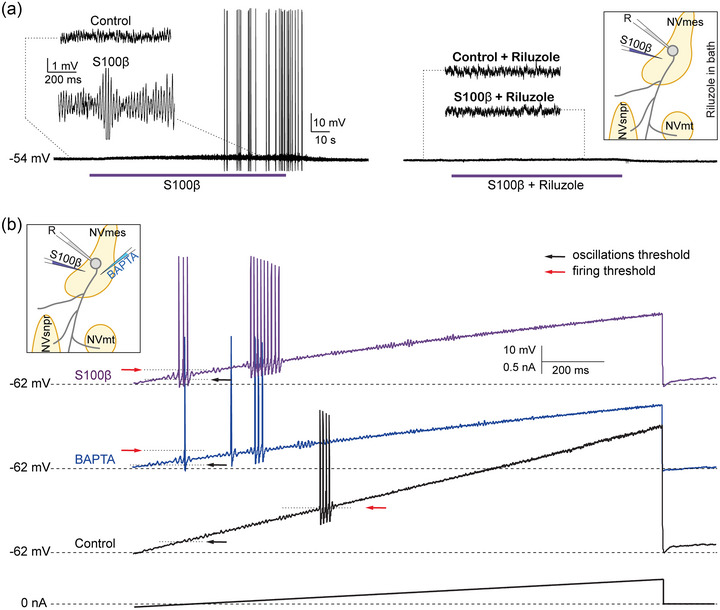
Effect of S100β and BAPTA on oscillations and firing properties of NVmes cells. (a) S100β locally applied on an NVmes cell increases the amplitude of the oscillations and induces firing (left), which is abolished by riluzole (right). (b) Both BAPTA and S100β decrease the threshold for firing (red arrows) and for oscillations (black arrows) in NVmes neurons subjected to a ramp protocol. Insets: Schematic representation of the experimental set‐up. Abbreviation: NVmes, trigeminal mesencephalic nucleus.
FIGURE 3.
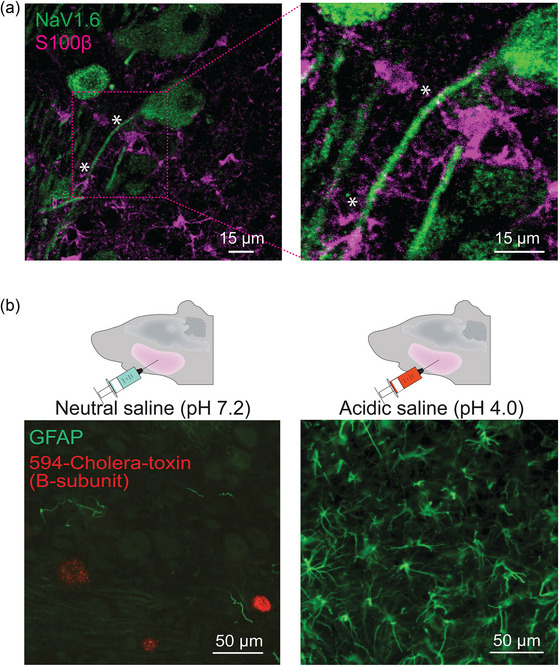
Proximity of astrocytic processes to NVmes axons and their activation in the acidic saline masseteric myalgia model. (a) S100β‐immunoreactive (pink) astrocytes have processes closely apposed (*) to NVmes somata and axons that are immunoreactive to NaV1.6 (green). (b) Expression of GFAP (green) in the NVmes area of rats from the control and pain groups at 7 days after the second neutral (left) or acidic (right) saline injection. Somata of NVmes neurons (red) are retrogradely labelled by intramuscular injections of fluorescent cholera toxin β‐subunit. Abbreviations: GFAP, glial fibrillary acidic protein; NVmes, trigeminal mesencephalic nucleus.
4. POTENTIAL ROLE FOR GLIAL CELLS IN PAIN AND IN MODULATION OF EXCITABILITY OF LARGE‐DIAMETER PRIMARY AFFERENTS
A large body of evidence indicates that glial cells, including satellite cells in DRGs and the trigeminal ganglion, and in microglia and astrocytes in the CNS, play important roles in chronic pain states (Chiang et al., 2011, 2012; Ohara et al., 2009; C. I. Svensson & Brodin, 2010). Several studies have shown that chronic pain is correlated with the appearance of reactive astrocytes, as indicated by increased expression of GFAP (Hansen & Malcangio, 2013; Zhuang et al., 2006). In models of orofacial inflammatory or neuropathic pain, both astroglia and microglia are activated very rapidly (within 20 minutes) in association with central sensitization in the subnucleus caudalis of the spinal trigeminal tract, which is considered functionally equivalent to the medullary dorsal horn (Chiang et al., 2011, 2012; Yeo et al., 2001). Similar increases of GFAP expression were observed in the vicinity of NVmes neurons of rats up to 9 days after injections of acidic saline into their masseters (Figure 3b; unpublished results from Gaudel et al., 2022). The mechanisms underlying this activation are unknown, but in two neuropathic pain models where microglia and satellite glial cells in the DRG are activated, perfusion of a Na+ channel blocker (TTX) into the DRG to reduce activity of spontaneously active sensory neurons also prevents glial activation, indicating that glial activation might result initially from an increase in activity of PAs (Xie et al., 2009). Reactive astrocytes, in contrast, release more gliotransmitters, raising the possibility that hyperexcitability of large, myelinated PAs results from increased release of S100β, as illustrated in Figure 1 (and see Agulhon et al., 2012). This is supported by the facts that S100β mRNA and protein are increased in the spinal cord after peripheral inflammation and nerve injury (Tanga et al., 2006) and that S100β knockout mice have decreased mechanical allodynia after nerve injury, whereas mice in which S100β is overexpressed have increased mechanical allodynia (Tanga et al., 2006).
5. HOW DO HYPEREXCITED LARGE‐DIAMETER PRIMARY AFFERENTS ACTIVATE NOCICEPTIVE PATHWAYS?
Several central mechanisms might lead to cross‐talk between large‐diameter PAs and nociceptive pathways. Lund & colleagues (2010) postulated that antidromic propagation of ectopic action potentials could cause glutamate release at peripheral endings and be a potential mechanism of activation of nociceptive fibers if they have endings nearby, within the spindle capsule (Figure 4, bottom left).
FIGURE 4.
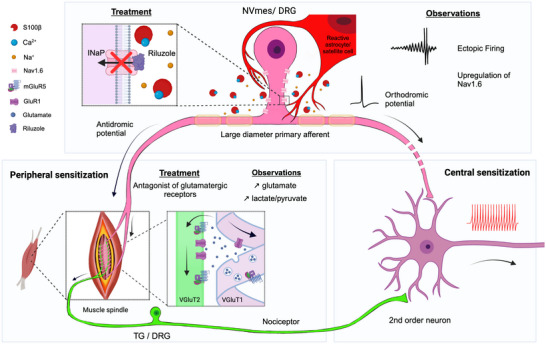
Schematic summary of mechanisms thought to underlie peripheral sensitization within spindle capsules, with indications of all locations where therapeutic interventions have been shown to have analgesic effects in humans. Top: Blockade of I NaP with riluzole (left) prevents ectopic firing in NVmes or the DRG (see Figure 1 for mechanisms underlying the appearance of the ectopic firing) and produces analgesia. Bottom left: Unless prevented, the ectopic firing travels antidromically to the peripheral endings of large‐diameter VGluT1‐positive PAs and induces glutamate release within the spindle capsule (using vesicular release), thereby activating nociceptors (which normally express VGluT2) through ionotropic and metabotropic glutamate receptors and contributing to the observed allodynia. Intramuscular injections of ionotropic and metabotropic glutamate receptor antagonists prevent activation of nociceptors and induction of allodynia. Bottom right: Chronic activation of nociceptors (having their soma in the TG or the DRG) and/or central endings of large‐diameter PAs leads to chronic activation of second‐order neurons and central sensitization. Abbreviations: DRG, dorsal root ganglion; I NaP, persistent sodium current; NVmes, trigeminal mesencephalic nucleus; PA, primary afferent; TG, trigeminal ganglion.
The possibility that antidromic discharges cause release of a chemical in the periphery was raised by Catton (1961) and Habgood (1950). Glutamate is the major neurotransmitter released by the central terminals of MSAs and thus, the primary candidate for release by their peripheral endings. Glutamate concentrations within the affected muscles increase in chronic myalgia, during delayed onset muscle soreness and following tissue injury or inflammation (deGroot et al., 2000; Lawand et al., 2000; Tegeder et al., 2002). Interstitial glutamate is also higher within the masseter muscles of patients suffering from myofascial temporomandibular disorders in comparison to healthy subjects (Castrillon et al., 2010). We suggest that this glutamate comes, in part, from MSA terminals. Bewick et al. (2005) showed that MSA endings release and recycle glutamate, which serves mainly to modulate their mechanical sensitivity. Injections of glutamate (or its agonists) into muscles, including the masseter, cause pain in humans (Cairns et al., 2003) and excite small‐diameter afferent fibers in animals (Bhave et al., 2001), presumably by activating glutamatergic receptors on their peripheral endings. Indeed, blocking of glutamate receptors decreases nocifensive behaviors in animal models (Dong et al., 2006; Ro & Capra, 2006; Ro et al., 2007; P. Svensson et al., 2003). Release of glutamate from MSA terminals could activate or sensitize muscle nociceptors if they are close to the release sites. Lund et al. (2010) showed that annulospiral MSA endings contain high levels of the glutamate transporter VGluT1, which is usually associated with glutamate release sites (see their figures 5–7). These endings intersected with fine fibers that expressed known nociceptor markers but did not express tyrosine hydroxylase, indicating that they are not sympathetic efferents. Both annulospiral MSA endings and fine fibers expressed metabotropic (mGluR5) glutamate receptors, and ionotropic receptors (GluR1) were also found in fine fibres innervating blood connective tissues. A recent study by Thompson et al. (2023) also confirmed the presence of mGluR5 glutamate receptors on fine‐caliber axons within the spindle capsules. However, the authors reported that only a GluK2 glutamate receptor subunit could be detected on spindle mechanosensory terminals. The homomeric GluK2 receptor formed by this subunit is an atypical metabotropic glutamate receptor coupled to phospholipase D (PLD‐mGluR), whose blockade or activation abolishes or greatly increases stretch‐evoked firing of MSAs, respectively. In line with studies showing that mGluR agonists and antagonists increase and decrease sensitivity, respectively, in an inflammatory pain model (Bhave et al., 2001) and that ionotropic receptor antagonists reduce glutamate‐induced masseter pain in humans (Cairns et al., 2006), Lund and collaborators found that mixtures of either ionotropic or metabotropic glutamate receptor antagonists given together with the second unilateral injections of acid saline prevented the induction of allodynia on both sides. The blockers were ineffective if given 2 days after the second injection (Figure 4; see figure 8 of Lund et al., 2010).
6. PERSPECTIVES AND CLINICAL IMPLICATIONS
Traditionally, research into the mechanisms driving chronic pain has focused predominantly on small‐diameter PAs and on mechanisms of either peripheral or central sensitization leading overall to increased activity in nociceptive pathways. However, strong evidence suggests that central sensitization is maintained dynamically by peripheral inputs because it subsides shortly after cessation of peripheral activity (reviewed by Devor, 2009; Gracely et al., 1992; Koltzenburg et al., 1994). This is supported by the observation that in syndromes of chronic widespread pain or fibromyalgia, attenuation of peripheral inputs alleviates or abolishes allodynia and hyperalgesia in patients (J. J. Chen et al., 2010).
Using the findings obtained from MSAs of the jaw‐closing muscles in a chronic myalgia model, in the present paper, we compile evidence supporting the proposal that initial activity in large‐diameter PAs, initiated or amplified by activation of astrocytes or satellite cells, leads to ectopic firing. Firing in these large‐diameter PAs depends on I NaP, which is enhanced by decreases of [Ca2+]o. A central hypothesis of the model proposed here is that astrocytes/satellite cells play a major role in the generation of ectopic firing by releasing S100β, a protein that chelates extracellular Ca2+. This ectopic firing can travel orthodromically and perhaps lead to increased firing in nociceptive pathways (central sensitization; Figure 4, bottom right), but here we propose that it also travels antidromically and leads to release of glutamate from large‐diameter PAs in the periphery. This released glutamate would then activate glutamatergic receptors carried by nearby free endings of nociceptors, thus initiating firing in nociceptive pathways (peripheral sensitization; Figure 4, bottom left).
Multiple animal models of pain have been designed to mimic distinct clinical diseases to evaluate the underlying mechanisms and potential treatments. The acid‐induced pain animal model, based on the observation of increased levels of lactate and pyruvate in exercised muscles of healthy subjects or muscles of patients with chronic muscle pain, has given inconsistent results in humans, with stronger algesic effects when using injections of a buffered acidic saline solution (pH 5.2) causing longer‐lasting pH changes, instead of an unbuffered solution (even with a lower pH of 3.3) (Castrillon et al., 2013; Law et al., 2008; Louca et al., 2013; Louca Jounger et al., 2019). Recent work from Chen's group and others (reviewed by Lee & Chen, 2023) has described a variety of proton‐sensing ion channels and receptors expressed in proprioceptors, enabling them to sense acidosis. However, it is possible that the inconsistent effects observed with injections in experimental conditions result from the fact that the proprioceptor terminals are isolated in the spindle capsule, whereas in pathological conditions acidification might be produced by muscular satellite cells located within the capsule, given that their presence has been reported in this location by Maynard and Cooper (1973).
Although it is uncertain whether the animal model used is relevant to human physiology, several observations in humans support the proposed theoretical model. First, as stated above, after nerve injury the onset of pain coincides in time with the appearance of ectopic firing in large‐diameter PAs (Campero et al., 1998). This ectopic firing could result from increased expression of some sodium channel subunits, such as NaV1.3 and NaV1.6, which are upregulated in biopsies from trigeminal neuralgia patients and in patients with complex regional pain syndrome and post‐herpetic neuralgia (Siqueira et al., 2009; Sittl et al., 2012; Tanaka et al., 2016; Zhao et al., 2008). NaV1.6, in particular, is responsible for I NaP, whose blockade with lignocaine or riluzole reduces phantom limb pain and tactile allodynia (Vaso et al., 2014; Xie et al., 2012, 2005).
Second, interstitial glutamate levels are higher in masseter muscles of patients suffering from temporomandibular disorders and in resting, low‐force exercise, or repetitive work in patients with chronic muscle pain, such as fibromyalgia, chronic shoulder pain, and chronic trapezius pain (Gerdle et al., 2010; Gerdle, Ghafouri et al., 2014; Gerdle, Larsson et al., 2014b; Larsson et al., 2008; Malatji et al., 2017; Sorensen et al., 2018). Moreover, glutamate injected into the human masseter muscle or temporomandibular joint causes pain and/or mechanical sensitivity, and intramuscular injection of glutamate receptor antagonists sometimes relieves the pain (reviewed by J. Liu et al., 2022). These observations support the model according to which ectopic firing in large‐diameter PAs resulting from enhanced I NaP leads to antidromic firing and release of glutamate within the muscle in the periphery causing pain, perhaps through activation of nociceptor terminals.
7. CONCLUSION AND RECOMMENDATIONS FOR FUTURE RESEARCH
The evidence reported here suggests that neuron–astrocyte interactions can cause ectopic spiking and that these interactions are enhanced in pathological pain conditions. Future work should examine how this abnormal firing can be prevented and/or how to prevent communications from MSA terminals to nociceptor terminals in the periphery. This should help to identify new drug targets, some of which are in the periphery, which is a clear advantage, from a therapeutic perspective, for the millions of people suffering from chronic musculoskeletal pain, which is by far the most common type of pain.
AUTHOR CONTRIBUTIONS
Dar'ya Sas contributed to writing and illustration of the paper. Fanny Gaudel and Dorly Verdier generated some of the data and figures presented. Arlette Kolta contributed to writing of the paper. All authors read and approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
CONFLICT OF INTEREST
None declared.
Sas, D. , Gaudel, F. , Verdier, D. , & Kolta, A. (2024). Hyperexcitability of muscle spindle afferents in jaw‐closing muscles in experimental myalgia: Evidence for large primary afferents involvement in chronic pain. Experimental Physiology, 109, 100–111. 10.1113/EP090769
Handling Editor: Ronan Berg
This review was presented at the ‘Mechanotransduction, Muscle Spindles and Proprioception’ symposium, which took place at Ludwig‐Maximilians‐Universität, Munich, 25–28 July 2022.
REFERENCES
- Abe, M. , Kurihara, T. , Han, W. , Shinomiya, K. , & Tanabe, T. (2002). Changes in expression of voltage‐dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Spine (Phila Pa 1976), 27(14), 1517–1524. discussion 1525. [DOI] [PubMed] [Google Scholar]
- Agulhon, C. , Sun, M. Y. , Murphy, T. , Myers, T. , Lauderdale, K. , & Fiacco, T. A. (2012). Calcium signaling and gliotransmission in normal vs. reactive astrocytes. Frontiers in Pharmacology, 3, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles, S. R. A. , & Smith, P. A. (2021). Peripheral voltage‐gated cation channels in neuropathic pain and their potential as therapeutic targets. Frontiers in Pain Research (Lausanne), 2, 750583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir, R. , Michaelis, M. , & Devor, M. (1999). Membrane potential oscillations in dorsal root ganglion neurons: Role in normal electrogenesis and neuropathic pain. The Journal of Neuroscience : the official journal of the Society for Neuroscience, 19(19), 8589–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt‐Nielsen, L. , Fernandez‐de‐Las‐Penas, C. , & Graven‐Nielsen, T. (2011). Basic aspects of musculoskeletal pain: From acute to chronic pain. Journal of Manual & Manipulative Therapy, 19(4), 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C. M. , & Cota, G. (1990). Modification of sodium channel gating by lanthanum. Some effects that cannot be explained by surface charge theory. Journal of General Physiology, 96(6), 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, S. A. , & Herr, M. J. (2023). Physiology, Nociception. In StatPearls. Treasure Island (FL). [PubMed] [Google Scholar]
- Banks, R. W. , & Barker, D. (2004). The muscle spindle. In Engel A. G., & Franzini‐Armstrong C. (Eds), Myology (pp. 489–509). McGraw‐Hill, Medical Publishing Division. [Google Scholar]
- Barlas, P. , Walsh, D. M. , Baxter, G. D. , & Allen, J. M. (2000). Delayed onset muscle soreness: Effect of an ischaemic block upon mechanical allodynia in humans. Pain, 87(2), 221–225. [DOI] [PubMed] [Google Scholar]
- Bennett, D. L. , Clark, A. J. , Huang, J. , Waxman, S. G. , & Dib‐Hajj, S. D. (2019). The role of voltage‐gated sodium channels in pain signaling. Physiological Reviews, 99(2), 1079–1151. [DOI] [PubMed] [Google Scholar]
- Bewick, G. S. , Reid, B. , Richardson, C. , & Banks, R. W. (2005). Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic‐like vesicles: Evidence from the rat muscle spindle primary sensory ending. The Journal of Physiology, 562(Pt 2), 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave, G. , Karim, F. , Carlton, S. M. , & Gereau, R. W. T. (2001). Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nature Neuroscience, 4(4), 417–423. [DOI] [PubMed] [Google Scholar]
- Black, J. A. , Cummins, T. R. , Plumpton, C. , Chen, Y. H. , Hormuzdiar, W. , Clare, J. J. , & Waxman, S. G. (1999). Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. Journal of Neurophysiology, 82(5), 2776–2785. [DOI] [PubMed] [Google Scholar]
- Brattberg, G. , Thorslund, M. , & Wikman, A. (1989). The prevalence of pain in a general population. The results of a postal survey in a county of Sweden. Pain, 37(2), 215–222. [DOI] [PubMed] [Google Scholar]
- Brocard, C. , Plantier, V. , Boulenguez, P. , Liabeuf, S. , Bouhadfane, M. , Viallat‐Lieutaud, A. , Vinay, L. , & Brocard, F. (2016). Cleavage of Na(+) channels by calpain increases persistent Na(+) current and promotes spasticity after spinal cord injury. Nature Medicine, 22(4), 404–411. [DOI] [PubMed] [Google Scholar]
- Brocard, F. , Shevtsova, N. A. , Bouhadfane, M. , Tazerart, S. , Heinemann, U. , Rybak, I. A. , & Vinay, L. (2013). Activity‐dependent changes in extracellular Ca2+ and K+ reveal pacemakers in the spinal locomotor‐related network. Neuron, 77(6), 1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns, B. E. , Svensson, P. , Wang, K. , Castrillon, E. , Hupfeld, S. , Sessle, B. J. , & Arendt‐Nielsen, L. (2006). Ketamine attenuates glutamate‐induced mechanical sensitization of the masseter muscle in human males. Experimental Brain Research, 169(4), 467–472. [DOI] [PubMed] [Google Scholar]
- Cairns, B. E. , Svensson, P. , Wang, K. , Hupfeld, S. , Graven‐Nielsen, T. , Sessle, B. J. , Berde, C. B. , & Arendt‐Nielsen, L. (2003). Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. Journal of Neurophysiology, 90(4), 2098–2105. [DOI] [PubMed] [Google Scholar]
- Campero, M. , Serra, J. , Marchettini, P. , & Ochoa, J. L. (1998). Ectopic impulse generation and autoexcitation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle & Nerve, 21(12), 1661–1667. [DOI] [PubMed] [Google Scholar]
- Carroll, L. J. , Holm, L. W. , Hogg‐Johnson, S. , Cote, P. , Cassidy, J. D. , Haldeman, S. , Nordin, M. , Hurwitz, E. L. , Carragee, E. J. , van der Velde, G. , Peloso, P. M. , Guzman, J. , & Bone, P. , Joint Decade—Task Force on Neck, and D. Its Associated . (2008). Course and prognostic factors for neck pain in whiplash‐associated disorders (WAD): Results of the Bone and Joint Decade 2000–2010 task force on neck pain and its associated disorders. Spine (Phila Pa 1976), 33(Suppl 1), S83–92. [DOI] [PubMed] [Google Scholar]
- Castrillon, E. E. , Cairns, B. , List, T. , Svensson, P. , & Ernberg, M. (2013). Acidic saline‐induced pain as a model for experimental masseter myalgia in healthy subjects. European Journal of Pain (London, England), 17(10), 1438–1446. [DOI] [PubMed] [Google Scholar]
- Castrillon, E. E. , Ernberg, M. , Cairns, B. E. , Wang, K. , Sessle, B. J. , Arendt‐Nielsen, L. , & Svensson, P. (2010). Interstitial glutamate concentration is elevated in the masseter muscle of myofascial temporomandibular disorder patients. Journal of Orofacial Pain, 24(4), 350–360. [PubMed] [Google Scholar]
- Catton, W. T. (1961). Threshold, recovery and fatigue of tactile receptors in frog skin. The Journal of Physiology, 158(2), 333–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. J. , Lue, J. H. , Lin, L. H. , Huang, C. T. , Chiang, R. P. , Chen, C. L. , & Tsai, Y. J. (2010). Effects of pre‐emptive drug treatment on astrocyte activation in the cuneate nucleus following rat median nerve injury. Pain, 148(1), 158–166. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Huang, J. , Benson, C. , Lankford, K. L. , Zhao, P. , Carrara, J. , Tan, A. M. , Kocsis, J. D. , Waxman, S. G. , & Dib‐Hajj, S. D. (2020). Sodium channel Nav1.6 in sensory neurons contributes to vincristine‐induced allodynia. Brain, 143(8), 2421–2436. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Huang, J. , Zhao, P. , Persson, A. K. , Dib‐Hajj, F. B. , Cheng, X. , Tan, A. , Waxman, S. G. , & Dib‐Hajj, S. D. (2018). Conditional knockout of Na(V)1.6 in adult mice ameliorates neuropathic pain. Scientific Reports, 8, 3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C. Y. , Dostrovsky, J. O. , Iwata, K. , & Sessle, B. J. (2011). Role of glia in orofacial pain. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17(3), 303–320. [DOI] [PubMed] [Google Scholar]
- Chiang, C. Y. , Sessle, B. J. , & Dostrovsky, J. O. (2012). Role of astrocytes in pain. Neurochemical Research, 37, 2419–2431. [DOI] [PubMed] [Google Scholar]
- Colloca, L. , Ludman, T. , Bouhassira, D. , Baron, R. , Dickenson, A. H. , Yarnitsky, D. , Freeman, R. , Truini, A. , Attal, N. , Finnerup, N. B. , Eccleston, C. , Kalso, E. , Bennett, D. L. , Dworkin, R. H. , & Raja, S. N. (2017). Neuropathic pain. Nature Reviews Disease Primers, 3, 17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins, T. R. , Dib‐Hajj, S. D. , Herzog, R. I. , & Waxman, S. G. (2005). Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. Febs Letters, 579(10), 2166–2170. [DOI] [PubMed] [Google Scholar]
- deGroot, J. , Zhou, S. , & Carlton, S. M. (2000). Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport, 11(3), 497–502. [DOI] [PubMed] [Google Scholar]
- Del Negro, C. A. , & Chandler, S. H. (1997). Physiological and theoretical analysis of K+ currents controlling discharge in neonatal rat mesencephalic trigeminal neurons. Journal of Neurophysiology, 77(2), 537–553. [DOI] [PubMed] [Google Scholar]
- Devor, M. (2009). Ectopic discharge in Abeta afferents as a source of neuropathic pain. Experimental Brain Research, 196(1), 115–128. [DOI] [PubMed] [Google Scholar]
- Devor, M. , & Seltzer, Z. (1999). Pathophysiology of damaged nerves in relation to chronic pain. In Wall P., & Melzack R. (Eds.), Texbook of pain (pp. 129–164). Churchill Livingstone. [Google Scholar]
- Dib‐Hajj, S. , Black, J. A. , Felts, P. , & Waxman, S. G. (1996). Down‐regulation of transcripts for Na channel alpha‐SNS in spinal sensory neurons following axotomy. Proceedings of the National Academy of Sciences of the United States of America, 93(25), 14950–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri, L. , Fang, X. , Koutsikou, S. , & Lawson, S. N. (2012). Partial nerve injury induces electrophysiological changes in conducting (uninjured) nociceptive and nonnociceptive DRG neurons: Possible relationships to aspects of peripheral neuropathic pain and paresthesias. Pain, 153(9), 1824–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X. D. , Mann, M. K. , Sessle, B. J. , Arendt‐Nielsen, L. , Svensson, P. , & Cairns, B. E. (2006). Sensitivity of rat temporalis muscle afferent fibers to peripheral N‐methyl‐D‐aspartate receptor activation. Neuroscience, 141(2), 939–945. [DOI] [PubMed] [Google Scholar]
- Finnerup, N. B. , Kuner, R. , & Jensen, T. S. (2021). Neuropathic pain: From mechanisms to treatment. Physiological Reviews, 101(1), 259–301. [DOI] [PubMed] [Google Scholar]
- Gaudel, F. , Giraud, J. , Couillard‐Laroque, M. , Verdier, D. , & Kolta, A. (2022). Decreases of extracellular calcium elicit sustained firing in axons of primary afferents through Nav1.6 channels. San Diego, CA: Society for Neuroscience. [Google Scholar]
- Ge, H. Y. (2010). Prevalence of myofascial trigger points in fibromyalgia: The overlap of two common problems. Current Pain and Headache Reports, 14(5), 339–345. [DOI] [PubMed] [Google Scholar]
- Gerdle, B. , Ghafouri, B. , Ernberg, M. , & Larsson, B. (2014). Chronic musculoskeletal pain: Review of mechanisms and biochemical biomarkers as assessed by the microdialysis technique. Journal of Pain Research, 7, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdle, B. , Larsson, B. , Forsberg, F. , Ghafouri, N. , Karlsson, L. , Stensson, N. , & Ghafouri, B. (2014). Chronic widespread pain: Increased glutamate and lactate concentrations in the trapezius muscle and plasma. Clinical Journal of Pain, 30, 409–420. [DOI] [PubMed] [Google Scholar]
- Gerdle, B. , Lemming, D. , Kristiansen, J. , Larsson, B. , Peolsson, M. , & Rosendal, L. (2008). Biochemical alterations in the trapezius muscle of patients with chronic whiplash associated disorders (WAD)–a microdialysis study. European Journal of Pain (London, England), 12, 82–93. [DOI] [PubMed] [Google Scholar]
- Gerdle, B. , Soderberg, K. , Puigvert, L. S. , Rosendal, L. , & Larsson, B. (2010). Increased interstitial concentrations of pyruvate and lactate in the trapezius muscle of patients with fibromyalgia: A microdialysis study. Journal of Rehabilitation Medicine: Official Journal of the UEMS European Board of Physical and Rehabilitation Medicine, 42, 679–687. [DOI] [PubMed] [Google Scholar]
- Gracely, R. H. , Lynch, S. A. , & Bennett, G. J. (1992). Painful neuropathy: Altered central processing maintained dynamically by peripheral input. Pain, 51, 175–194. [DOI] [PubMed] [Google Scholar]
- Habgood, J. S. (1950). Sensitization of sensory receptors in the frog's skin. The Journal of Physiology, 111, 195–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, H. C. , Lee, D. H. , & Chung, J. M. (2000). Characteristics of ectopic discharges in a rat neuropathic pain model. Pain, 84, 253–261. [DOI] [PubMed] [Google Scholar]
- Hansen, R. R. , & Malcangio, M. (2013). Astrocytes–multitaskers in chronic pain. European Journal of Pharmacology, 716, 120–128. [DOI] [PubMed] [Google Scholar]
- Henry, M. A. , Freking, A. R. , Johnson, L. R. , & Levinson, S. R. (2007). Sodium channel Nav1.6 accumulates at the site of infraorbital nerve injury. BMC Neuroscience [Electronic Resource], 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschke, N. , Maher, C. G. , Refshauge, K. M. , Herbert, R. D. , Cumming, R. G. , Bleasel, J. , York, J. , Das, A. , & McAuley, J. H. (2008). Prognosis in patients with recent onset low back pain in Australian primary care: Inception cohort study. Bmj, 337, a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, V. L. , Schubert, C. , Keller, U. , & Muller, S. (1988). Effect of systemic pH on pHi and lactic acid generation in exhaustive forearm exercise. The American Journal of Physiology, 255, F479–F485. [DOI] [PubMed] [Google Scholar]
- Hulliger, M. (1984). The mammalian muscle spindle and its central control. Reviews of Physiology, Biochemistry and Pharmacology, 101, 1–110. [DOI] [PubMed] [Google Scholar]
- Issberner, U. , Reeh, P. W. , & Steen, K. H. (1996). Pain due to tissue acidosis: A mechanism for inflammatory and ischemic myalgia? Neuroscience Letters, 208, 191–194. [DOI] [PubMed] [Google Scholar]
- Itz, C. J. , Geurts, J. W. , van Kleef, M. , & Nelemans, P. (2013). Clinical course of non‐specific low back pain: A systematic review of prospective cohort studies set in primary care. European Journal of Pain, 17, 5–15. [DOI] [PubMed] [Google Scholar]
- Kamper, S. J. , Rebbeck, T. J. , Maher, C. G. , McAuley, J. H. , & Sterling, M. (2008). Course and prognostic factors of whiplash: A systematic review and meta‐analysis. Pain, 138, 617–629. [DOI] [PubMed] [Google Scholar]
- Kasch, H. , Qerama, E. , Kongsted, A. , Bach, F. W. , Bendix, T. , & Jensen, T. S. (2008). Deep muscle pain, tender points and recovery in acute whiplash patients: A 1‐year follow‐up study. Pain, 140, 65–73. [DOI] [PubMed] [Google Scholar]
- Ke, C. B. , He, W. S. , Li, C. J. , Shi, D. , Gao, F. , & Tian, Y. K. (2012). Enhanced SCN7A/Nax expression contributes to bone cancer pain by increasing excitability of neurons in dorsal root ganglion. Neuroscience, 227, 80–89. [DOI] [PubMed] [Google Scholar]
- Khan, O. A. , Majumdar, S. , & Jones, N. S. (2002). Facial pain following sinonasal surgery or facial trauma. Clinical Otolaryngology & Allied Sciences, 27, 171–174. [DOI] [PubMed] [Google Scholar]
- Kim, C. H. , Oh, Y. , Chung, J. M. , & Chung, K. (2001). The changes in expression of three subtypes of TTX sensitive sodium channels in sensory neurons after spinal nerve ligation. Brain Research Molecular Brain Research, 95, 153–161. [DOI] [PubMed] [Google Scholar]
- Kim, H. S. , & Chung, M. J. (1992). An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain, 50, 355–363. [DOI] [PubMed] [Google Scholar]
- Kim, Y. I. , Na, H. S. , Kim, S. H. , Han, H. C. , Yoon, Y. W. , Sung, B. , Nam, H. J. , Shin, S. L. , & Hong, S. K. (1998). Cell type‐specific changes of the membrane properties of peripherally‐axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience, 86, 301–309. [DOI] [PubMed] [Google Scholar]
- Koltzenburg, M. , Torebjork, H. E. , & Wahren, L. K. (1994). Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain, 117(Pt 3), 579–591. [DOI] [PubMed] [Google Scholar]
- LaMotte, R. (2007). Ectopia, spontaneous. In Schmidt R., & Willis W. (Eds.), Encyclopedia of pain (pp. 677–681). Springer. [Google Scholar]
- Larsson, B. , Rosendal, L. , Kristiansen, J. , Sjogaard, G. , Sogaard, K. , Ghafouri, B. , Abdiu, A. , Kjaer, M. , & Gerdle, B. (2008). Responses of algesic and metabolic substances to 8 h of repetitive manual work in myalgic human trapezius muscle. Pain, 140, 479–490. [DOI] [PubMed] [Google Scholar]
- Lavelle, E. D. , Lavelle, W. , & Smith, H. S. (2007). Myofascial trigger points. Medical Clinics of North America, 91, 229–239. [DOI] [PubMed] [Google Scholar]
- Law, L. A. F. , Sluka, K. A. , McMullen, T. , Lee, J. , Arendt‐Nielsen, L. , & Graven‐Nielsen, T. (2008). Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain, 140, 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawand, N. B. , McNearney, T. , & Westlund, K. N. (2000). Amino acid release into the knee joint: Key role in nociception and inflammation. Pain, 86, 69–74. [DOI] [PubMed] [Google Scholar]
- Lee, C. H. , & Chen, C. C. (2023). Role of proprioceptors in chronic musculoskeletal pain. Experimental Physiology, 109(1), 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , & Hatton, G. I. (1996). Oscillatory bursting of phasically firing rat supraoptic neurones in low‐Ca2+ medium: Na+ influx, cytosolic Ca2+ and gap junctions. The Journal of Physiology, 496(Pt 2), 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. N. , Wall, P. D. , Ben‐Dor, E. , Michaelis, M. , Amir, R. , & Devor, M. (2000). Tactile allodynia in the absence of C‐fiber activation: Altered firing properties of DRG neurons following spinal nerve injury. Pain, 85(3), 503–521. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Jia, S. , Huang, F. , He, H. , & Fan, W. (2022). Peripheral role of glutamate in orofacial pain. Frontiers in Neuroscience, 16, 929136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Chung, K. , & Chung, J. M. (1999). Ectopic discharges and adrenergic sensitivity of sensory neurons after spinal nerve injury. Brain Research, 849(1–2), 244–247. [DOI] [PubMed] [Google Scholar]
- Louca, S. , Ernberg, M. , & Christidis, N. (2013). Influence of intramuscular granisetron on experimentally induced muscle pain by acidic saline. Journal of Oral Rehabilitation, 40(6), 403–412. [DOI] [PubMed] [Google Scholar]
- Louca Jounger, S. , Eriksson, N. , Lindskog, H. , Oscarsson, A. , Simonsson, V. , Ernberg, M. , & Christidis, N. (2019). Repeated buffered acidic saline infusion in the human masseter muscle as a putative experimental pain model. Scientific Reports, 9, 15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, J. P. , Sadeghi, S. , Athanassiadis, T. , Caram Salas, N. , Auclair, F. , Thivierge, B. , Arsenault, I. , Rompre, P. , Westberg, K. G. , & Kolta, A. (2010). Assessment of the potential role of muscle spindle mechanoreceptor afferents in chronic muscle pain in the rat masseter muscle. PLoS ONE, 5(6), e11131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatji, B. G. , Meyer, H. , Mason, S. , Engelke, U. F. H. , Wevers, R. A. , van Reenen, M. , & Reinecke, C. J. (2017). A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurology, 17(1), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard, J. A. , & Cooper, R. R. (1973). Two unusual satellite cell‐intrafusal muscle fiber relationships. Zeitschrift fur Anatomie und Entwicklungsgeschichte, 140(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Money, S. (2017). Pathophysiology of trigger points in myofascial pain syndrome. Journal of Pain & Palliative Care Pharmacotherapy, 31(2), 158–159. [DOI] [PubMed] [Google Scholar]
- Morquette, P. , Verdier, D. , Kadala, A. , Fethiere, J. , Philippe, A. G. , Robitaille, R. , & Kolta, A. (2015). An astrocyte‐dependent mechanism for neuronal rhythmogenesis. Nature Neuroscience, 18(6), 844–854. [DOI] [PubMed] [Google Scholar]
- Ohara, P. T. , Vit, J. P. , Bhargava, A. , Romero, M. , Sundberg, C. , Charles, A. C. , & Jasmin, L. (2009). Gliopathic pain: When satellite glial cells go bad. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 15(5), 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen, J. V. (2018). Muscle pain and muscle spindles. In Anatomy, posture, prevalence, pain, treatment and interventions of musculoskeletal disorders. Intechopen, 10.5772/intechopen.72223 [DOI] [Google Scholar]
- Pedroarena, C. M. , Pose, I. E. , Yamuy, J. , Chase, M. H. , & Morales, F. R. (1999). Oscillatory membrane potential activity in the soma of a primary afferent neuron. Journal of Neurophysiology, 82(3), 1465–1476. [DOI] [PubMed] [Google Scholar]
- Reeh, P. W. , & Steen, K. H. (1996). Tissue acidosis in nociception and pain. Progress in Brain Research, 113, 143–151. [DOI] [PubMed] [Google Scholar]
- Ren, Y. S. , Qian, N. S. , Tang, Y. , Liao, Y. H. , Yang, Y. L. , Dou, K. F. , & Toi, M. (2012). Sodium channel Nav1.6 is up‐regulated in the dorsal root ganglia in a mouse model of type 2 diabetes. Brain Research Bulletin, 87(2–3), 244–249. [DOI] [PubMed] [Google Scholar]
- Ro, J. Y. , & Capra, N. F. (2006). Assessing mechanical sensitivity of masseter muscle in lightly anesthetized rats: A model for craniofacial muscle hyperalgesia. Neuroscience Research, 56(1), 119–123. [DOI] [PubMed] [Google Scholar]
- Ro, J. Y. , Capra, N. F. , Lee, J. S. , Masri, R. , & Chun, Y. H. (2007). Hypertonic saline‐induced muscle nociception and c‐fos activation are partially mediated by peripheral NMDA receptors. European Journal of Pain (London, England), 11(4), 398–405. [DOI] [PubMed] [Google Scholar]
- Ryczko, D. , Hanini‐Daoud, M. , Condamine, S. , Breant, B. J. B. , Fougere, M. , Araya, R. , & Kolta, A. (2021). S100beta‐mediated astroglial control of firing and input processing in layer 5 pyramidal neurons of the mouse visual cortex. The Journal of Physiology, 599(2), 677–707. [DOI] [PubMed] [Google Scholar]
- Santarelli, V. P. , Eastwood, A. L. , Dougherty, D. A. , Ahern, C. A. , & Horn, R. (2007). Calcium block of single sodium channels: Role of a pore‐lining aromatic residue. Biophysical Journal, 93(7), 2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan, M. F. , Tung, C. C. , Van Petegem, F. , & Ahern, C. A. (2012). Crystallographic basis for calcium regulation of sodium channels. Proceedings of the National Academy of Sciences of the United States of America, 109(9), 3558–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, A. , Chansoria, M. , Tomar, G. , & Kumar, A. (2015). Myofascial pain syndrome: An overview. Journal of Pain & Palliative Care Pharmacotherapy, 29(1), 16–21. [DOI] [PubMed] [Google Scholar]
- Scherrer, G. , Low, S. A. , Wang, X. , Zhang, J. , Yamanaka, H. , Urban, R. , Solorzano, C. , Harper, B. , Hnasko, T. S. , Edwards, R. H. , & Basbaum, A. I. (2010). VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury‐induced heat hypersensitivity. Proceedings of the National Academy of Sciences of the United States of America, 107(51), 22296–22301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, D. G. , Travelll, J. G. , & Simons, L. S. (1999). Myofascial pain and dysfunction, the trigger point manual. Williams & Wilkins. [Google Scholar]
- Siqueira, S. R. , Alves, B. , Malpartida, H. M. , Teixeira, M. J. , & Siqueira, J. T. (2009). Abnormal expression of voltage‐gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience, 164(2), 573–577. [DOI] [PubMed] [Google Scholar]
- Sittl, R. , Lampert, A. , Huth, T. , Schuy, E. T. , Link, A. S. , Fleckenstein, J. , Alzheimer, C. , Grafe, P. , & Carr, R. W. (2012). Anticancer drug oxaliplatin induces acute cooling‐aggravated neuropathy via sodium channel subtype Na(V)1.6‐resurgent and persistent current. Proceedings of the National Academy of Sciences of the United States of America, 109(17), 6704–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogaard, G. , Rosendal, L. , Kristiansen, J. , Blangsted, A. K. , Skotte, J. , Larsson, B. , Gerdle, B. , Saltin, B. , & Sogaard, K. (2010). Muscle oxygenation and glycolysis in females with trapezius myalgia during stress and repetitive work using microdialysis and NIRS. European Journal of Applied Physiology, 108(4), 657–669. [DOI] [PubMed] [Google Scholar]
- Sluka, K. A. , Kalra, A. , & Moore, S. A. (2001). Unilateral intramuscular injections of acidic saline produce a bilateral, long‐lasting hyperalgesia. Muscle & Nerve, 24(1), 37–46. [DOI] [PubMed] [Google Scholar]
- Sorensen, L. B. , Gazerani, P. , Wahlen, K. , Ghafouri, N. , Gerdle, B. , & Ghafouri, B. (2018). Investigation of biomarkers alterations after an acute tissue trauma in human trapezius muscle, using microdialysis. Scientific Reports, 8(1), 3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, H. , Alroy, G. , Kirson, E. D. , & Yaari, Y. (2001). Extracellular calcium modulates persistent sodium current‐dependent burst‐firing in hippocampal pyramidal neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(12), 4173–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson, C. I. , & Brodin, E. (2010). Spinal astrocytes in pain processing: Non‐neuronal cells as therapeutic targets. Molecular Interventions, 10(1), 25–38. [DOI] [PubMed] [Google Scholar]
- Svensson, P. , Cairns, B. E. , Wang, K. , Hu, J. W. , Graven‐Nielsen, T. , Arendt‐Nielsen, L. , & Sessle, B. J. (2003). Glutamate‐evoked pain and mechanical allodynia in the human masseter muscle. Pain, 101(3), 221–227. [DOI] [PubMed] [Google Scholar]
- Tal, M. , Kim, J. , Back, S. K. , Na, H. S. , & Devor, M. (2006). Onset of ectopic Wring in the Chung model of neuropathic pain coincides with the onset of tactile allodynia. In Flor H., Kalso E., & Dostrovsky J. O. (Eds.), Proceedings of the 11th World Congress on pain (pp. 119–130). IASP Press: Seattle. [Google Scholar]
- Tanaka, B. S. , Zhao, P. , Dib‐Hajj, F. B. , Morisset, V. , Tate, S. , Waxman, S. G. , & Dib‐Hajj, S. D. (2016). A gain‐of‐function mutation in Nav1.6 in a case of trigeminal neuralgia. Molecular Medicine, 22, 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga, F. Y. , Raghavendra, V. , Nutile‐McMenemy, N. , Marks, A. , & Deleo, J. A. (2006). Role of astrocytic S100beta in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience, 140(3), 1003–1010. [DOI] [PubMed] [Google Scholar]
- Tantanatip, A. , & Chang, K. V. (2023). Myofascial pain syndrome. In StatPearls. [PubMed]
- Tashima, R. , Koga, K. , Sekine, M. , Kanehisa, K. , Kohro, Y. , Tominaga, K. , Matsushita, K. , Tozaki‐Saitoh, H. , Fukazawa, Y. , Inoue, K. , Yawo, H. , Furue, H. , & Tsuda, M. (2018). Optogenetic activation of non‐nociceptive abeta fibers induces neuropathic pain‐like sensory and emotional behaviors after nerve injury in rats. eNeuro, 5(1), ENEURO.0450‐17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder, L. , Zimmermann, J. , Meller, S. T. , & Geisslinger, G. (2002). Release of algesic substances in human experimental muscle pain. Inflammation Research, 51(8), 393–402. [DOI] [PubMed] [Google Scholar]
- Thompson, K. J. , Watson, S. , Zanato, C. , Dall'Angelo, S. , De Nooij, J. C. , Pace‐Bonello, B. , Shenton, F. C. , Sanger, H. E. , Heinz, B. A. , Broad, L. M. , Grosjean, N. , McQuillian, J. R. , Dubini, M. , Pyner, S. , Greig, I. , Zanda, M. , Bleakman, D. , Banks, R. W. , & Bewick, G. S. (2023). The atypical ‘hippocampal’ glutamate receptor coupled to phospholipase D that controls stretch‐sensitivity in primary mechanosensory nerve endings is homomeric purely metabotropic GluK2. Experimental Physiology, 109(1), 81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaso, A. , Adahan, H. M. , Gjika, A. , Zahaj, S. , Zhurda, T. , Vyshka, G. , & Devor, M. (2014). Peripheral nervous system origin of phantom limb pain. Pain, 155(7), 1384–1391. [DOI] [PubMed] [Google Scholar]
- Verdier, D. , Lund, J. P. , & Kolta, A. (2004). Synaptic inputs to trigeminal primary afferent neurons cause firing and modulate intrinsic oscillatory activity. Journal of Neurophysiology, 92(4), 2444–2455. [DOI] [PubMed] [Google Scholar]
- Voscopoulos, C. , & Lema, M. (2010). When does acute pain become chronic? British Journal of Anaesthesia, 105, Suppl 1, i69–85. [DOI] [PubMed] [Google Scholar]
- Waxman, S. G. , Kocsis, J. D. , & Black, J. A. (1994). Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. Journal of Neurophysiology, 72(1), 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody, N. S. , Whitehead, N. P. , Canny, B. J. , Gregory, J. E. , & Proske, U. (2001). Large‐fiber mechanoreceptors contribute to muscle soreness after eccentric exercise. The Journal of Pain: Official Journal of the American Pain Society, 2(4), 209–219. [DOI] [PubMed] [Google Scholar]
- Wu, N. , Hsiao, C. F. , & Chandler, S. H. (2001). Membrane resonance and subthreshold membrane oscillations in mesencephalic V neurons: Participants in burst generation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(11), 3729–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Strong, J. A. , Kim, D. , Shahrestani, S. , & Zhang, J. M. (2012). Bursting activity in myelinated sensory neurons plays a key role in pain behavior induced by localized inflammation of the rat sensory ganglion. Neuroscience, 206, 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Strong, J. A. , Meij, J. T. , Zhang, J. M. , & Yu, L. (2005). Neuropathic pain: Early spontaneous afferent activity is the trigger. Pain, 116(3), 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Strong, J. A. , Ye, L. , Mao, J. X. , & Zhang, J. M. (2013). Knockdown of sodium channel NaV1.6 blocks mechanical pain and abnormal bursting activity of afferent neurons in inflamed sensory ganglia. Pain, 154(8), 1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Strong, J. A. , & Zhang, J. M. (2009). Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience, 160(4), 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, W. , Strong, J. A. , & Zhang, J. M. (2015). Local knockdown of the NaV1.6 sodium channel reduces pain behaviors, sensory neuron excitability, and sympathetic sprouting in rat models of neuropathic pain. Neuroscience, 291, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, J. L. , Hu, S. J. , & Yang, J. (2014). Electrophysiological Features of Neurons in the Mesencephalic Trigeminal Nuclei. Neuro‐Signals, 22(2), 79–91. [DOI] [PubMed] [Google Scholar]
- Yeo, J. F. , Liu, H. P. , & Leong, S. K. (2001). Sustained microglial immunoreactivity in the caudal spinal trigeminal nucleus after formalin injection. Journal of Dental Research, 80(6), 1524–1529. [DOI] [PubMed] [Google Scholar]
- Zhao, P. , Barr, T. P. , Hou, Q. , Dib‐Hajj, S. D. , Black, J. A. , Albrecht, P. J. , Petersen, K. , Eisenberg, E. , Wymer, J. P. , Rice, F. L. , & Waxman, S. G. (2008). Voltage‐gated sodium channel expression in rat and human epidermal keratinocytes: Evidence for a role in pain. Pain, 139(1), 90–105. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. F. , & Henry, J. L. (2012). Excitability of Abeta sensory neurons is altered in an animal model of peripheral neuropathy. BMC Neuroscience [Electronic Resource], 13, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, Z. Y. , Wen, Y. R. , Zhang, D. R. , Borsello, T. , Bonny, C. , Strichartz, G. R. , Decosterd, I. , & Ji, R. R. (2006). A peptide c‐Jun N‐terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(13), 3551–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]


