Abstract
We previously reported that infection of goats with caprine arthritis encephalitis virus (CAEV) tat− proviral DNA or virus results in persistent infection, since the animals seroconverted and direct virus isolation from cultures of blood-derived macrophages was positive. In this study we wanted to determine whether goats injected with CAEV tat− proviral DNA or virus were protected against challenge with the pathogenic homologous virus and to investigate whether CAEV tat− was still pathogenic. All animals injected with CAEV tat− became infected as indicated by seroconversion and virus isolation. Challenge at 8 or 9 months postinfection demonstrated protection in four of four animals injected with CAEV tat− but did not in three of three mock-inoculated challenged goats. Challenge virus was undetectable in the blood macrophages of protected animals during a period of 6 or 10 months postchallenge. In two of four protected animals, however, we were able to detect the challenge wild-type virus by reverse transcriptase PCR on RNA directly extracted from synovial membrane cells surrounding the inoculation site. This result suggests that protection was achieved without complete sterilizing immunity. Animals injected with CAEV tat− and mock challenged developed inflammatory lesions in the joints, although these lesions were not as severe as those in CAEV wild-type-injected goats. These results confirm the dispensable role of Tat in CAEV replication in vivo for the establishment of infection and pathogenesis and demonstrate in another lentivirus infection model the efficacy of live attenuated viruses to induce resistance to superinfection.
Like other lentiviral infections, caprine arthritis encephalitis virus (CAEV) infection is characterized by viral persistence in the face of an immune response and the onset of slow and progressive degenerative diseases (20). Synovitis, mastitis, pneumonia, and encephalitis characterize the course of disease in CAEV infection (29). These inflammatory diseases are the result of viral infection of cells of monocyte/macrophage lineage, which are the main target cells in vivo (8–10). Infection of macrophages is a common feature of lentiviral infections and plays a central role in the development of associated diseases. The CAEV infection model thus provides a system to analyze the pathogenesis of diseases associated with macrophage infection and to develop vaccine strategies against macrophage-tropic viruses. The use of an infectious molecular clone of CAEV (34, 37) allowed us to investigate the role of different genes in the induction of infection and pathogenesis. We previously demonstrated the essential role of the vif gene for efficient CAEV replication in vitro and in vivo (13, 15), whereas the tat gene was shown to be dispensable in both instances (14). Goats inoculated with CAEV vif− virus or proviral DNA were not protected against CAEV wild-type (wt) challenge, due to the reduced level of CAEV vif− replication (15, 16). Similar results were obtained in the simian immunodeficiency virus (SIV) macaque model, in which an inverse correlation between the degree of virus attenuation and the induction of protection was demonstrated (26, 42). In the macaque model, it is now clearly established that long-term protection against systemic challenge can be achieved by systemic immunization with live attenuated SIVmac239 Δnef or Δ3 (7, 42) or SIVmac32H C8 (31). This approach was also shown to be effective against mucosal challenge after either systemic SIVmac32H C8 (6) or mucosal simian/human immunodeficiency virus 89.6 (28) immunization. All these results were obtained with lymphocyte-tropic viruses. Since macrophage-tropic strains of human immunodeficiency virus type 1 (HIV-1) seem to be the transmitted viruses responsible for initial infection (36, 39, 44), they might be the initial targets to consider in vaccine strategies. Indeed, in the SIV model, immunization of macaques with attenuated macrophage-tropic SIV/17E-CI resulted in protective immunity against heterologous challenge (4).
In a previous study, we reported that proviral DNA of the CAEV Cork molecular clone with deleted tat sequences (CAEV tat−) produced persistent infection in goats, with an antibody response showing kinetics of appearance and a reactivity pattern against viral proteins that were similar to those in CAEV wt-infected goats (14). The present study was designed to evaluate the capacity of this live attenuated virus to induce resistance to superinfection and to investigate the pathogenic properties of CAEV tat− compared to those of CAEV wt. Protection against challenge with a high dose (>250 100% animal infectious dose [AID100]) of homologous CAEV wt was achieved in all goats inoculated with CAEV tat− without complete sterilizing immunity. A control animal inoculated with CAEV tat− and mock challenged developed mild inflammatory lesions in the joints, ruling out an essential role for the CAEV tat gene in virus-induced pathogenesis. Although further attenuation of CAEV tat− is required to obtain a nonpathogenic live attenuated vaccine strain, these experiments demonstrated the efficacy of this vaccine strategy in an additional lentiviral animal model.
MATERIALS AND METHODS
Viruses.
The infectious proviral DNA of the CAEV Cork strain was generated by ligation of the 9- and 0.5-kb HindIII CAEV fragments (34, 37). The tat-deleted mutant, containing a 153-bp in-frame deletion between positions 5677 and 5829, was previously described (14). Viral stocks were obtained by transfection of CAEV wt or tat− proviral DNA into primary goat synovial membrane cells or by infection of goat synovial membrane cells with transfection supernatants. These stocks contained 105 50% tissue culture infectious doses (TCID50) per ml. Preliminary in vivo virus titration with fivefold stock virus dilutions revealed that five of five goats that were given the last 2.5 × 10−2 dilution by the intratracheal route became persistently infected (data not shown). Therefore, the stock virus is estimated to contain >250 AID100 per ml.
Immunization and challenge protocols.
Goats were obtained from two separate CAEV-free flocks and were included in two protocols summarized in Table 1. In the first experiment, four goats were inoculated intra-articularly in the right carpus with 105 TCID50 of CAEV wt (goat 9317) or tat− viral stocks (goats 9319, 9324, and 9327). Mock control goat 9315 was inoculated with culture medium. The goats were challenged on day 228 postinjection (p.i.) by inoculation of >250 AID100 of homologous CAEV wt in the left carpus and necropsied at day 185 postchallenge (p.c.). In the second experiment, five goats were inoculated in the right carpus with 100 μg of proviral CAEV wt DNA (goats 306 and 307) or with either 100 μg (goat 311) or 350 μg (goats 303 and 312) of CAEV tat− proviral DNA mixed with the cationic lipid DOTAP (Boehringer-Mannheim) as previously described (14). Mock control goat 308 was injected with 100 μg of plasmid DNA. Naive goat C70 was included as a positive control for infection at the time of challenge. These goats were challenged on day 279 p.i. by inoculation in the left carpus with >250 AID100 of homologous CAEV wt and necropsied on day 310 p.c. Blood samples were regularly drawn to purify macrophages for virus isolation and reverse transcriptase (RT)-PCR analyses and to determine the serological status by enzyme-linked immunosorbent assay (ELISA).
TABLE 1.
Experimental design
| Expt and goat no. | Priming witha:
|
Challengeb
|
|||
|---|---|---|---|---|---|
| Live virus
|
Proviral DNA
|
||||
| wt | tat− | wt | tat− | wt | |
| Expt 1 | |||||
| 9317 | + | + | |||
| 9319 | + | − | |||
| 9324 | + | + | |||
| 9327 | + | + | |||
| 9315 | + | ||||
| Expt 2 | |||||
| 306 | + | + | |||
| 307 | + | − | |||
| 303 | + | − | |||
| 311 | + | + | |||
| 312 | + | + | |||
| 308 | + | ||||
| C70 | + | ||||
Priming was carried out at day 0 by injection of 105 TCID50 of virus or 100 or 350 μg of proviral DNA into the right carpus.
Challenge occurred at day 228 p.i. (experiment 1) or at day 279 p.i. (experiment 2) by injection of >250 AID100 of CAEV wt into the left carpus.
Cells.
Primary goat macrophages were obtained from peripheral blood mononuclear cells purified on a Ficoll-Paque density gradient (Pharmacia) and maintained for 10 to 12 days in Teflon bags, as previously described (14). Cells were cultured in RPMI 1640 containing 10% sheep serum, 1% glutamine, penicillin and streptomycin, 10 mM HEPES, and 10−5 M β-mercaptoethanol. Macrophage culture supernatants were analyzed for RT activity as previously described (13). For RT-PCR analyses, blood-derived macrophages as well as necropsied tissues or lymph nodes were subjected to direct RNA extraction with the RNAB reagent (BioProbe) as described previously (13). A portion of the synovial membranes was frozen in liquid nitrogen for histological examination of tissue sections after hematoxylin and eosin staining.
RT-PCR analyses.
RT-PCR analyses and Southern blot hybridization of the amplified products were performed as previously described (13). According to the CAEV Cork sequence published previously (37), the sequences and positions of the primers used were as follows: for VIF5 (sense primer), 5′-GACACAACGGGATACACGCA-3′ (positions 5621 to 5640); for TAT (antisense primer), 5′-GATTATGTTCCCCACCCCGG-3′ (5953 to 5934); for TATH (hybridization oligonucleotide), 5′-CAAGGCGCCTGTGATTAGG-3′ (5891 to 5909); for ENV1 (antisense primer), 5′-CCCAGTTAAGCGCATGTATC-3′ (6047 to 6028); for POLS (sense primer), 5′-GATAGGATAGGAGTGCATTG-3′ (3721 to 3740); for POLH (hybridization oligonucleotide), 5′-TATTTCCGAAATATATTTGTC-3′ (3801 to 3781); and for POLA (antisense primer), 5′-TGAGTCTATGATTCCTCCT-3′ (4020 to 4002).
To detect CAEV tat-specific sequences, a seminested PCR was performed after the nonspecific reverse transcription step with the VIF5/ENV1 primer pair used in the initial PCR, followed by another reaction with the VIF5/TAT primers. As an internal control for RT-PCR, the caprine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified with the following primers: for CGAP1 (sense primer), 5′-GTTCCACTATGATTCCACCC-3′; for CGAP2 (hybridization oligonucleotide), 5′-CAGTCAAGGCAAGAGAATGGG-3′; and for CGAPR1 (antisense primer), 5′-TCCCTCCACGATGCCAAA-3′.
ELISA.
Antibody detection was performed by a whole-virus protein ELISA (Chekit CAEV/maedi-visna virus test; Hoechst Roussel Vet.) according to the manufacturer’s instructions. Results were expressed as the percentage of positive controls with the following formula: (mean tests − mean negative controls)/(mean positive controls − mean negative controls) × 100. Sera with percentage values below 30% were considered negative, sera with percentage values between 30 and 40% were considered doubtful, and sera with percentage values above 40% were considered positive.
Anti-Gag antibodies were screened by a Gag-glutathione S-transferase ELISA as previously described (43). Anti-transmembrane 3 (TM3) and anti-TM4 reactivities were analyzed with TM3 (amino acids 717 to 731) or TM4 (amino acids 749 to 762) peptide ELISAs, respectively, as described previously (1). A positive serum sample was defined as having a reactivity >25% of that of the positive control.
RESULTS
Inoculation of goats with CAEV tat−.
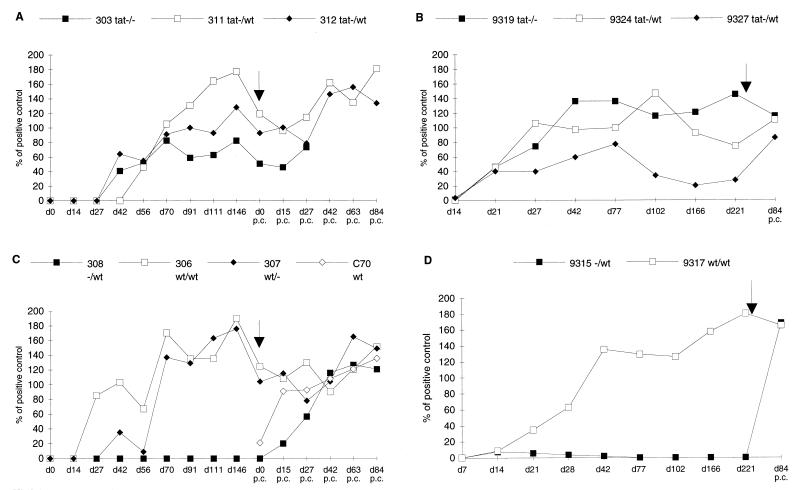
Goats 9319, 9324, and 9327 received one intra-articular injection of 105 TCID50 of CAEV tat− viral stock. Goats 303, 311, and 312 were inoculated once with 100 or 350 μg of CAEV tat− proviral DNA (Table 1). All six goats developed anti-CAEV antibodies detected by whole-virus protein ELISAs (Fig. 1), and no major difference was detected between animals inoculated with the CAEV tat− proviral DNA (Fig. 1A) and those inoculated with viral stock (Fig. 1B). Antibodies appeared between 3 to 8 weeks p.i. and persisted until the time of challenge. One goat in each group (goats 303 and 9327) exhibited a low level of antibody response, and the antibody response of goat 9327 dropped below the limit of positivity from day 102 p.i. Positive control goats inoculated with either CAEV wt proviral DNA (306 and 307) or viral stock (9317) seroconverted within 3 to 10 weeks (Fig. 1C and D, respectively). Compared to the three positive control animals, four of six goats inoculated with CAEV tat− developed similar levels of antibody response. These results confirmed that wt or tat− proviral DNA injection was as efficient as wt or tat− virus inoculation in inducing persistent infection (14, 15).
FIG. 1.
Antibody response. Anti-CAEV antibodies to whole-virus proteins were measured by ELISA on different days (d) postinfection or p.c. Time of challenge is indicated by the arrows. (A) Goats inoculated with CAEV tat− proviral DNA and mock-challenged (303 tat−/−) or wt-challenged goats (311, 312 tat−/wt). (B) Goats inoculated with CAEV tat− and mock-challenged (9319 tat−/−) or wt-challenged (9324, 9327 tat−/wt) goats. (C) Mock-inoculated and wt-challenged goat (308 −/wt) or naive and wt-challenged (C70 wt) goat and CAEV wt proviral DNA-positive control animals mock challenged (307 wt/−) or wt challenged (306 wt/wt). (D) Mock-inoculated challenged control goat (9315 −/wt) and positive control goat challenged with CAEV wt (9317 wt/wt).
Virus production was monitored by measuring the RT activity of blood-derived macrophage culture supernatants. As shown in Table 2, virus isolation was infrequently positive for goats injected with CAEV wt or tat−. Blood-derived macrophages from two of three positive control goats (9317 and 306) allowed virus isolation in two of six and two of nine tests, respectively. The third positive control goat, 307, which exhibited the longest delay before seroconversion (day 70 p.i., Fig. 1C), remained negative for virus isolation in eight of eight assays. Among goats inoculated with CAEV tat−, virus isolation was positive in two of three goats injected with proviral DNA (311 and 312) and in only one of the three goats injected with the virus (9319). Goat 311, for which three of nine tests were positive, received one injection of 100 μg of DNA, whereas goats 303 and 312 were inoculated with 350 μg of DNA, suggesting that the inoculation dose did not affect the level of virus replication. Virus isolation remained negative for goats 303, 9327, and 9324, although they developed low to high antibody responses, respectively (Fig. 1A and B). These results probably reflect variation between animals in the ability to control viral replication, together with the difficulty of isolating virus from a small percentage of infected blood monocytes (9). No significant difference was observed in the frequency of isolation of viruses from animals injected with CAEV wt or tat− proviral DNA or virus-injected animals. RT-PCR analysis was performed on day 91 p.i. with RNA extracted from macrophage-produced viruses (Table 2, goats 306, 307, 303, 311, 312, and 308) and showed that the deletion introduced into the tat gene was still detectable in tat− virus particles (14), ruling out the possibility that a recombination event caused reversal to the wt phenotype, which would explain the lack of difference between goats injected with CAEV wt and those injected with tat−.
TABLE 2.
Virus isolation and RT-PCR detection in blood-derived macrophages
| Day | RT activity/RT-PCR detectiona in goat challenged as indicated
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expt 1
|
Expt 2
|
|||||||||||
| 9317 | 9319 | 9324 | 9327 | 9315 | 306 | 307 | 303 | 311 | 312 | 308 | C70 | |
| p.i.b | wt virus | tat− virus | tat− virus | tat− virus | Mock | wt DNA | wt DNA | tat− DNA | tat− DNA | tat− DNA | Mock | |
| 0 | − | − | − | − | − | − | ||||||
| 4 | − | − | − | − | − | − | − | − | − | − | − | |
| 14 | − | − | − | − | − | − | − | − | − | − | − | |
| 27 | + | + | − | − | − | − | − | − | − | − | − | |
| 42 | − | + | − | − | − | − | − | − | − | − | − | |
| 56 | + | + | + | − | ||||||||
| 70 | − | − | − | + | − | − | ||||||
| 77 | + | + | − | − | − | |||||||
| 91 | +/wt | −/− | −/− | +/tat− | −/− | −/− | ||||||
| 102 | − | − | − | − | − | |||||||
| 146 | − | − | − | − | − | − | ||||||
| p.c.c | wt | Mock | wt | wt | wt | wt | Mock | Mock | wt | wt | wt | wt |
| 15 | −/wt | −/− | +/tat− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/wt | −/− |
| 27 | −/− | −/− | −/− | −/− | +/wt | −/− | −/− | −/−d | −/− | −/− | +/wt | −/− |
| 42 | +/wt | −/− | −/− | −/− | +/wt | −/− | −/wt | −/− | −/− | −/wt | −/− | |
| 56 | −/wt | −/− | −/− | −/− | −/− | |||||||
| 63 | −/− | −/− | −/− | −/− | −/− | −/− | ||||||
| 84 | −/− | −/− | −/− | −/− | −/− | −/− | −/wt | −/− | −/− | −/wt | +/wt | |
| 118 | −/wt | −/− | −/− | −/− | +/wt | |||||||
| 124 | −/− | −/− | −/− | −/− | −/− | −/− | ||||||
| 152 | −/− | −/− | −/− | −/− | −/− | −/− | ||||||
Virus was isolated by RT activity measurement of 1 ml of macrophage culture supernatant at day 12, concentrated 100×/RT-PCR detection of RNA extracted from blood-derived macrophages cultured for 12 days with the VIF5/ENV1 primer pair. −, no fragment amplification; wt, detection of the 427-bp wt tat fragment; tat−, detection of the 274-bp tat-deleted fragment. Southern blots of the amplified fragments were hybridized with the 32P-labeled TATH oligonucleotide probe.
Right intracarpal inoculation of CAEV wt or tat− proviral DNA or virus.
Left intracarpal challenge with pathogenic CAEV.
Goat 303 died accidentally at day 30 p.c.
Antibody response analysis.
In the CAEV infection model, a correlation was previously described between the severity of disease in infected animals and the titers of anti-Env antibodies (23), especially the anti-TM antibodies (27). Four immunodominant epitopes were delineated in the CAEV gp38 TM, and antibody reactivity against two of them, TM3 and TM4, was shown to be associated with the presence of clinical arthritis (1). To compare the ability of CAEV tat− proviral DNA or virus infection to induce such antibody specificities with that of CAEV wt infection, we tested the goat sera by ELISA with peptides TM3 (amino acids 717 to 731) or TM4 (amino acids 749 to 762) as antigens. In parallel, an ELISA specific for the recombinant Gag fusion protein glutathione S-transferase-Gag was used to detect anti-Gag antibodies. Sera were tested at day 146 p.i. for the group injected with CAEV wt or tat− proviral DNA and at day 221 p.i. for the group injected with CAEV wt or tat− virus. We chose to analyze late sera, since it was reported that the kinetics of anti-TM antibody appearance were around 12 to 32 weeks p.i. compared to 3 to 4 weeks p.i. for anti-Gag antibodies (1). Results are summarized in Table 3 (left-hand side). Sera were considered positive for values >25% of the ELISA for the positive controls. All positive control goats, 306, 307, and 9317, developed anti-Gag and anti-TM3 antibodies, whereas only goat 306 also produced anti-TM4 antibodies. In the groups injected with CAEV tat−, goat 311 (tat− proviral DNA) and goat 9319 (tat− virus) developed all three antibody specificities. Goat 312 (tat− proviral DNA) was weakly positive against Gag and TM4. Goat 303 (tat− proviral DNA) and goats 9324 and 9327 (tat− virus) were considered unreactive in all three ELISAs. Western blot analysis against whole-virus proteins, however, demonstrated that day 146 p.i. sera from goats 303 and 312 were weakly reactive against mature Gag proteins p28, p18, and p14.5 (14). This discrepancy between ELISA and Western blot results has already been observed with sera from naturally infected goats (1). Noticeably, three of six animals inoculated with CAEV tat− developed anti-Gag and anti-TM antibodies after inoculation (goats 311, 312, and 9319) compared to three of three goats injected with CAEV wt, which developed both reactivities.
TABLE 3.
Antibody specificitiesa after inoculation with CAEV tat− or wt and pathogenic challenge
| Goat no. | Type of inoculation | % of ELISA for positive control p.i.
|
Type of challenge | % of ELISA for positive control p.c.
|
Severity of lesionsb | Protection | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-Gag | Anti-TM3 | Anti-TM4 | Anti-Gag | Anti-TM3 | Anti-TM4 | |||||
| 308 | Mock | 0 | 0 | 0 | wt | 27 | 530 | 254 | +++ | − |
| 9315 | Mock | 0 | 0 | 0 | wt | 63 | 86 | 0 | +++ | − |
| 306 | wt DNA | 66 | 418 | 39 | wt | 108 | 524 | 254 | +++ | − |
| 307 | wt DNA | 78 | 67 | 0 | Mock | 37 | 446 | 4 | +++ | − |
| 9317 | wt virus | 82 | 28 | 0 | wt | 88 | 20 | 0 | +++ | − |
| 303 | tat− DNA | 11 | 13 | 2 | Mock | ND | ND | ND | ND | |
| 311 | tat− DNA | 91 | 42 | 43 | wt | 123 | 336 | 80 | ++ | + |
| 312 | tat− DNA | 25 | 18 | 26 | wt | 54 | 493 | 13 | ++ | + |
| 9319 | tat− virus | 31 | 398 | 70 | Mock | 19 | 314 | 47 | + | |
| 9324 | tat− virus | 21 | 24 | 0 | wt | 39 | 119 | 0 | ++ | + |
| 9327 | tat− virus | 6 | 0 | 0 | wt | 25 | 0 | 0 | ++ | + |
Antibody reactivities were measured by ELISAs specific for a glutathione S-transferase-Gag fusion protein, the TM3 peptide (amino acids 717 to 731), and the TM4 peptide (amino acids 749 to 762). Sera were diluted 1:100, 1:60, and 1:20, respectively, and values higher than 25% were considered positive. Samples analyzed were taken at day 146 p.i. and day 152 p.c. for goats 308, 306, 307, 311, and 312 and at day 221 p.i. and day 84 p.c. for goats 9315, 9317, 9319, 9324, and 9327.
Histopathological lesions observed in sections of synovial membranes were graded low (+), mild (++), or severe (+++). ND, not determined.
Challenge of goats inoculated with CAEV tat−.
Challenge was performed with a high dose of homologous CAEV wt (>250 AID100) injected in the joint, which is one of the main targets of infection. Goats inoculated with CAEV tat− or wt virus were challenged on day 228 p.i., whereas goats injected with CAEV tat− or wt proviral DNA were challenged on day 279 p.i. A naive animal, goat C70, was included at that time as an additional positive control for infection (Table 1). Two of three goats inoculated with CAEV wt, 306 and 9317, were challenged with the homologous CAEV wt, whereas goat 307 was mock challenged. Two goats inoculated with CAEV tat−, 303 (tat− proviral DNA) and 9319 (tat− virus), were mock challenged. Most additional results were obtained with goat 9319, since goat 303 died accidentally at day 30 p.c. The four other animals inoculated with CAEV tat−, 311 and 312 (tat− proviral DNA) and 9324 and 9327 (tat− virus), were challenged with CAEV wt. All control animals became infected as shown by the seroconversion curves (goats 308 and C70, Fig. 1C and goat 9315, Fig. 1D). The antibody response level for goat 9319 remained high (Fig. 1B), suggesting continuous stimulation by viral antigens. An increase in antibody response was observed in the four wt-challenged animals inoculated with CAEV tat−, goats 311 and 312 (Fig. 1A) and goats 9324 and 9327 (Fig. 1B), as measured on day 84 p.c. Isolation of virus was possible from blood-derived macrophages of positive control goats (Table 2). Of the samples from the two animals challenged and inoculated with CAEV wt, only those from goat 9317 allowed positive virus isolation by RT activity measurement and RT-PCR on RNA from blood-derived macrophages (Table 2). Positive control mock-challenged goat 307 developed a persistent low-level infection as revealed by the late occasional RT-PCR virus detection on blood-derived macrophages (Table 2). Virus isolation was negative for all animals immunized with CAEV tat−, regardless of whether they were mock challenged or challenged with CAEV wt (Table 2). In only one case (goat 9324 on day 15 p.c.) was the immunizing tat− virus detected in blood-derived macrophages by RT-PCR with the VIF5/TAT primer pair (Table 2). This result suggests a transient reactivation of the CAEV tat− by the wt challenge virus. These observations indicate that prior immunization with the attenuated CAEV tat− induced protection or superinfection resistance against homologous pathogenic challenge at the peripheral blood level.
Protein-specific ELISAs revealed that the two positive control animals tested, 308 and 9315, developed both anti-Gag and anti-TM3 antibodies, but only goat 308 produced anti-TM4 antibodies (Table 3, right-hand side). An increase of anti-Gag, anti-TM3, and anti-TM4 antibodies was observed in sera from goat 306, whereas goat 9317 demonstrated no change in antibody specificity. The late development of anti-TM3 antibodies was detected in goat 307 (Table 3, right-hand side). Whereas a slight decrease was observed in the anti-Gag, anti-TM3, and anti-TM4 reactivities of goat 9319, all these antibody specificities were strengthened in the four wt-challenged animals that were inoculated with CAEV tat−, without the appearance of new reactivities (Table 3, right-hand side), suggesting that the humoral response was stimulated by the wt challenge virus. RT-PCR analyses performed on RNA extracted from different tissues of positive control goats allowed the detection of challenge virus (Table 4). Virus was also detected in the synovial membranes and lymph nodes of goat 9317 as well as in the lymphoid tissues of goat 306 (Table 4). The infected status of goat 307 was confirmed by RT-PCR analyses on synovial membranes, mammary secretions, and lymphoid tissues (Table 4). Most of the necropsied tissues of goat 9319 were RT-PCR positive with the POLS/POLA primer pair (Table 4), suggesting a continuous replication of CAEV tat− in target tissues. RT-PCR analyses with the POLS/POLA primer pair on different tissues of wt-challenged goats inoculated with CAEV tat− gave positive reactions in the synovial membranes and mammary secretions of goats 311 and 312 and in the lymph nodes and bone marrow of goats 9324 and 9327 (Table 4). Since this reaction did not allow us to distinguish between the tat− immunizing virus and the wt challenge virus, further RT-PCR analyses were performed with the VIF5/TAT primer pair to amplify the tat gene.
TABLE 4.
Virus detection by RT-PCR analysis on necropsy tissues
| Expt and animal no. | Priming | Type of challenge | Necropsy tissuea
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SM
|
MS | RMLN
|
PSLN
|
BM | ||||||
| Left | Right | Left | Right | Left | Right | |||||
| Expt 1 | ||||||||||
| 9317 | wt virus | wt | + | + | + | NT | + | NT | + | NT |
| 9319 | tat− virus | Mock | + | + | + | + | + | − | − | + |
| 9324 | tat− virus | wt | + | − | − | − | − | + | − | + |
| 9327 | tat− virus | wt | + | + | − | − | + | − | + | + |
| 9315 | Mock | wt | + | + | + | + | NT | + | NT | + |
| Expt 2 | ||||||||||
| 306 | wt DNA | wt | + | + | NT | − | + | − | + | − |
| 307 | wt DNA | Mock | + | − | + | − | − | − | − | − |
| 311 | tat− DNA | wt | − | + | + | − | − | − | − | − |
| 312 | tat− DNA | wt | + | + | + | − | − | − | − | − |
| 308 | Mock | wt | + | − | NT | − | − | − | − | − |
| C70b | wt | + | + | + | NT | NT | NT | NT | + | |
RT-PCR analysis on RNA directly extracted from tissues taken at sacrifice was performed with the POLS/POLA primer pair. Southern blots of the amplified fragments were hybridized with the 32P-labeled POLH oligonucleotide probe. +, detection of the 299-bp pol fragment; −, no detectable amplified products; NT, not tested. Tissue samples analyzed were from left or right synovial membranes (SM), mammary secretions (MS), left or right retromammary lymph node (RMLN), left or right prescapular lymph node (PSLN), and bone marrow (BM).
Goat C70 received no priming and was included at the time of challenge.
Viral RNA detection in necropsied tissues and histopathology.
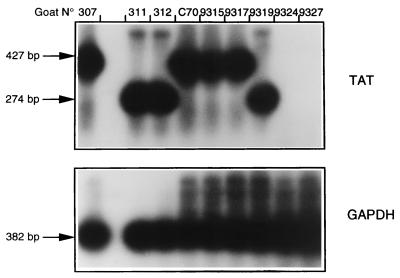
As CAEV wt challenge virus was not detected at the peripheral level, we next examined whether sequestration of the challenge virus occurred in protected wt-challenged goats immunized with tat−. Tissue samples were taken at necropsy and analyzed by RT-PCR for the presence of deleted or wt tat viral RNA. Figure 2 shows results obtained from cells from mammary secretions of female goats. wt tat RNA could be amplified from negative control challenged goats 9315 and C70 as well as from positive control mock-challenged goat 307 or wt-challenged goat 9317. Only a deleted tat product was amplified from mock-challenged goat 9319 or wt-challenged animals 311 and 312 which had been inoculated with CAEV tat−. An RT-PCR specific for tat was negative for samples from wt-challenged goats 9324 and 9327, which had been injected with CAEV tat−. No wt challenge virus was detected in fluids secreted by animals immunized with tat−, confirming the results obtained with blood-derived macrophages.
FIG. 2.
Challenge virus detection by RT-PCR on RNA extracted from cells in mammary secretions. Amplification was performed with the VIF5/ENV1 primer pair, and a Southern blot of the amplified products was hybridized with the 32P-labeled TATH oligonucleotide probe. The CGAP1/CGAP2 primer pair was used to amplify the caprine housekeeping gene GAPDH as an internal control. Molecular size markers are indicated at the left.
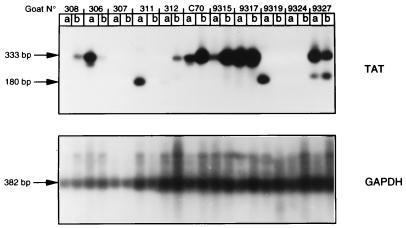
Figure 3 shows results obtained with RNA extracted from synovial membranes surrounding the inoculation sites. A wt tat amplification product could be observed in all negative control challenged animals, in both the right and the left joints of goats 9315 and C70 but in goat 308 in only the joint inoculated with CAEV wt. Among positive control animals, mock-challenged goat 307 was negative for the tat-specific RT-PCR in both joints, whereas the pol-specific primer pair allowed viral RNA detection in the left joint (Table 4). For the two other positive control challenged animals, 306 and 9317, amplification of wt tat was positive in synovial membranes from both joints. tat-deleted amplification products could be observed in three of four wt-challenged goats inoculated with CAEV tat−, in goats 311 and 312 in the joint injected with CAEV tat−, and in both joints of goat 9327, although in this last case the tat− amplified product exhibited a slightly different size. Both wt tat and tat− amplification products were detected in the wt-injected joint of goat 312 and in both synovial membranes of goat 9327. Samples from goat 9324 were negative for tat-specific RT-PCR analysis, whereas parallel amplification performed with pol-specific primers allowed viral RNA detection (Table 4). This discrepancy, also observed for goat 307, could be due to the lower efficiency of the VIF5/TAT primer pair compared to that of POLA/POLS and/or to a lower viral load in these infected animals. We were able to detect the wt challenge virus at the inoculation site in two of four wt-challenged animals that had been immunized with CAEV tat−; this suggests that control of the wt virus replication occurred in these animals without complete sterilizing immunity.
FIG. 3.
Challenge virus detection by RT-PCR on RNA extracted from right (a) or left (b) synovial membranes. Amplification was performed by seminested PCR, first with the VIF5/ENV1 primer pair and then with primers VIF5/TAT, resulting in the production of a 333-bp fragment for the wt tat gene and a 180-bp fragment for the tat− gene. Amplification of the GADPH control gene and Southern blot hybridization were as described in the legend for Fig. 3.
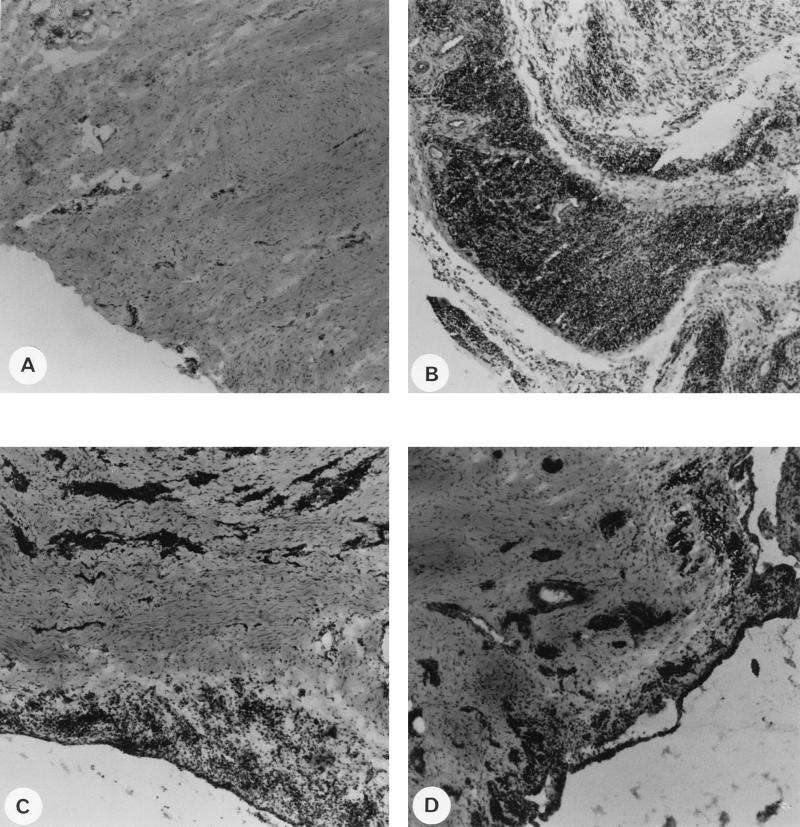
The pathogenic properties of the tat− attenuated virus were then analyzed. A portion of the necropsied synovial membranes of the challenged animals was frozen, and tissue sections were examined for the presence of inflammatory lesions. Different tissue sections from each animal were examined independently by two or three examiners, and representative pictures for each group are shown in Fig. 4. Compared to the synovial membrane section of a naive animal (Fig. 4A), a tissue section from the wt-challenged goat inoculated with CAEV wt (goat 9317) (Fig. 4B) showed the characteristics of severe virus-induced inflammatory lesions with the high lymphocytic and monocytic infiltration that we and others have already described with similar experimental infections (14, 38). CAEV tat− injection resulted in lesions that were less severe than those resulting from the wt virus, as observed on a tissue section from the mock-challenged goat injected with CAEV tat− virus (goat 9319) (Fig. 4C). Thickening of the synovial membrane as well as lymphocytic infiltration of the connective tissue was observed. Mild lesions were also observed in tissue sections of wt-challenged goat 9327, which was injected with CAEV tat− (Fig. 4D); these lesions were less severe than those in the positive control goat (Fig. 4B), indicating that wt-challenged animals immunized with CAEV tat− resisted the pathogenicity of the challenge virus, since lesions observed in these animals were most probably due to replication of the immunizing virus itself. Most of the animals developed either only anti-TM3 antibodies (9315, 307, 312, and 9324) or only anti-TM3 and anti-TM4 antibodies (308, 306, 311, and 9319) and lesions that ranged from low to severe (Table 3). Goats 9317 and 9327 developed severe and mild lesions, respectively, in the absence of either of these antibody reactivities (Table 3). In agreement with results previously obtained with a larger panel of sera (1), most sera in this study (80%) reacted with TM3 peptide and Gag and correlated with an arthritic condition.
FIG. 4.
Histopathology of synovial membranes from a naive goat (A), from positive control goat 9317, which was injected with CAEV wt (B), from mock-challenged goat 9319, which was inoculated with CAEV tat− (C), and from wt-challenged goat 9327, which was inoculated with CAEV tat− (D). Synovial membrane samples were taken at necropsy, and frozen sections were stained with hematoxylin and eosin. Magnification, ×10.
DISCUSSION
The infectivity of retroviral DNA has been proved in several models, including SIV, bovine leukemia virus, CAEV, and feline immunodeficiency virus (14, 15, 24, 35, 41). This method prevents variation in infection efficacy due to the presence of various proportions of defective viruses in individual viral stocks and allows the evaluation of the infectious and pathogenic properties of genetically modified retroviral genomes. In this study, we extended our previous results (14) and demonstrated the reliability and efficiency of this infection procedure compared to those of infection with viral stocks obtained in vitro by transfection of the same infectious CAEV molecular clone, either wt or tat−. Overall, time to seroconversion and virus isolation were not significantly different between animals inoculated with CAEV tat− or wt proviral DNA and goats injected with CAEV tat− or wt virus. A more detailed analysis of the antibody response, however, revealed that infection with CAEV tat− was less efficient than injection with CAEV wt in inducing a high-level antibody response and stimulating the production of anti-Gag and anti-TM reactivities. The differences detected in the antibody response in animals injected with CAEV tat− compared to that in goats inoculated with CAEV wt could be due to an attenuated replication of CAEV tat− in vivo. We observed that goats 303 and 9327, with the lowest antibody response, had no detectable anti-Gag or anti-TM antibodies and were negative for virus isolation. Goats 312 and 9324, with a high antibody response, reacted weakly against Gag and TM epitopes before challenge and remained negative for virus isolation. Finally, goats 311 and 9319, with the highest antibody level, reacted strongly against the Gag and TM epitopes, and virus could be isolated from blood-derived macrophage cultures. Taking into account the variation between the animals’ ability to control infection, these differences were not observed in the three positive control goats. In the CAEV infection model, there seems to be a correlation between the intensity of the immune response and the level of virus replication (15, 16); this correlation was also observed in the case of SIVmac32H C8 infection (6) but not in the SIVmac239 Δnef (7, 22) or equine infectious anemia virus ΔDU models (25).
The results demonstrate that infection with attenuated CAEV tat− via intracarpal inoculation can protect goats from homologous pathogenic challenge in the contralateral joint. Protection was defined as the absence of challenge virus detection in peripheral blood-derived macrophages during the postchallenge period—185 days for the group injected with CAEV tat− proviral DNA and 310 days for the group inoculated with CAEV tat−. No anamnestic antibody response was detected; however, an increase in anti-CAEV response was observed in goats 311 and 312, suggesting limited exposure to CAEV antigens. As in some vaccination studies with attenuated SIV (6, 28, 31), we found no correlation between the level of antibody response and protection. Protection was achieved without complete sterilizing immunity, since RT-PCR analyses of different tissues obtained at necropsy allowed the detection of the challenge wt virus in the inoculated joints of two of four protected animals. In one of these two goats, 9327, CAEV wt RNA was present in both carpal joints, showing that the challenge virus may have diffused through the body before it was brought under control. The local immune response, either antibody or cell-mediated, may be responsible for this control mechanism, since the synovial fluid and synovium of infected goats are rich in plasmocytes, activated CD4+ and CD8+ lymphocytes, and macrophages (12, 21, 40).
In a previous study, we reported that immunization with the highly attenuated CAEV vif− failed to protect goats against homologous pathogenic challenge (15)—even when the goats were challenged after a long period of immunization (about 8 months p.i.)—whereas the slightly attenuated CAEV tat− immunization was protective. The fact that these two experimental groups were inoculated and challenged with the same protocol allows us to determine the impact of the degree of attenuation of the vaccine viruses on their efficiency to induce protection. In another lentiviral model, these results confirm the inverse correlation established with live attenuated SIVs between the level of vaccine strain replication and the induction of protection (26, 42). In addition to the replication efficiency of the vaccine strain, an important parameter of efficient protection is duration of the vaccination. In the macaque model, protection was obtained with live attenuated SIVs, and results demonstrate a clear trend toward increased protection with the time of vaccination (4, 7, 17, 31, 42). Most effective vaccination assays against lentiviral infection have failed to clearly demonstrate whether protection was immunity mediated or based on superinfection resistance due to interference. Further analyses are necessary to evaluate the maturation of the immune response, described in the SIV and equine infectious anemia virus models (4, 5, 11), as well as to investigate the induction of a cellular immune response to CAEV tat− immunization.
Examination of synovial membrane sections from a mock-challenged goat inoculated with CAEV tat− revealed mild histopathological changes compared to the severe inflammatory lesions observed in the joints of goats infected with CAEV wt. Since the CAEV tat gene is not strictly required to establish persistent infection and the onset of clinical signs, the function of Tat is still unknown. Several studies reported the correlation between the level of anti-Env (anti-SU and anti-TM) antibodies and the development of arthritic lesions in the CAEV infection model (our results and references 1, 23, and 27), together with the high level of virus expression in tissue macrophages (45) and the massive infiltration of the arthritic synovium by B lymphocytes, plasmocytes, and activated CD4+ and CD8+ lymphocytes (2, 12, 21, 40). Recent reports suggested the role of differential activation of CAEV-reactive T-helper subsets in virus expression control and disease outcome and associated the dominance of T-helper 2-like cells with arthritis (3, 33, 40). Our results suggest a role for CAEV Tat in the increase of the viral replication level, independently of its weak transactivation of the viral long terminal repeat (14, 18). As Tat of visna virus was reported to regulate the expression of cellular genes involved in activation pathways (30), one hypothesis is that Tat of small ruminant lentiviruses activates the infected macrophages, resulting in increased viral expression and thereby augmenting the reactivities of antibodies and activated lymphocytes to viral antigens and infected cells in the synovial tissue (2, 12, 21, 40). Nontranscriptional function of HIV-1 Tat in virion infectivity and immune activation of HIV-1-infected cells by Tat were recently described (19, 32) and may be a general feature of lentiviral Tat proteins.
ACKNOWLEDGMENTS
We thank J. M. Guibert, M. Vignoni (CNEVA, Sophia-Antipolis, France), E. Pardo, and P. Bolland (ENV, Lyon, France) for their excellent technical assistance and Sophie Dufour (IVV, Bern, Switzerland) for her precious help. We thank our colleagues at INSERM U372 for their support and M. Guyader and K. E. Willett for critical reading of the manuscript.
A. Harmache was the recipient of a doctoral fellowship from the French Agency against AIDS (ANRS). G. Bertoni was supported by grant 31-41859.94 from the Swiss National Science Foundation. This work was supported by INSERM and ANRS.
REFERENCES
- 1.Bertoni G, Zahno M-L, Zanoni R, Vogt H-R, Peterhans E, Ruff G, Cheevers W P, Sonigo P, Pancino G. Antibody reactivity to the immunodominant epitopes of the caprine arthritis encephalitis virus gp38 transmembrane protein associates with the development of arthritis. J Virol. 1994;68:7139–7147. doi: 10.1128/jvi.68.11.7139-7147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheevers W P, Knowles D P, McGuire T C, Cunningham D R, Adams D S, Gorham J R. Chronic disease in goats orally infected with two isolates of caprine arthritis encephalitis virus. Lab Investig. 1988;58:510–517. [PubMed] [Google Scholar]
- 3.Cheevers W P, Beyer J C, Knowles D P. Type 1 and type 2 cytokine gene expression by viral gp135 surface protein-activated T lymphocytes in caprine arthritis encephalitis lentivirus infection. J Virol. 1997;71:6259–6263. doi: 10.1128/jvi.71.8.6259-6263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements J E, Montelaro R C, Zink M C, Amedee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole K S, Rowles J L, Jagerski B A, Murphey-Corb M, Unangst T, Clements J E, Robinson J, Wyand M S, Desrosiers R C, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cranage M P, Whatmore A M, Sharpe S A, Cook N, Polyanskaya N, Leech S, Smith J D, Rud E W, Dennis M J, Hall G A. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology. 1997;229:143–154. doi: 10.1006/viro.1996.8419. [DOI] [PubMed] [Google Scholar]
- 7.Daniel M D, Kirchoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 8.Gendelman H E, Narayan O, Molineaux S, Clements J E, Ghotbi Z. Slow persistent replication of lentiviruses: role of macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci USA. 1985;82:7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gendelman H E, Narayan O, Kennedy-Stoskopf S, Kennedy P G E, Ghotbi Z, Clements J E, Stanley J, Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986;58:67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorrell M D, Brandon M R, Sheffer D, Adams R J, Narayan O. Ovine lentivirus is macrophagetropic and does not replicate productively in T lymphocytes. J Virol. 1992;66:2679–2688. doi: 10.1128/jvi.66.5.2679-2688.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harkiss G D, Watt N J, King T J, Williams J, Hopkins J. Retroviral arthritis: phenotypic analysis of cells in the synovial fluid of sheep with inflammatory synovitis associated with visna virus infection. Clin Immunol Immunopathol. 1991;60:106–117. doi: 10.1016/0090-1229(91)90116-r. [DOI] [PubMed] [Google Scholar]
- 13.Harmache A, Bouyac M, Audoly G, Hiéblot C, Peveri P, Vigne R, Suzan M. The vif gene is essential for replication of caprine arthritis encephalitis virus in goat synovial membrane cells and affects the late steps of the virus replication cycle. J Virol. 1995;69:3247–3257. doi: 10.1128/jvi.69.6.3247-3257.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmache A, Vitu C, Russo P, Bouyac M, Hieblot C, Peveri P, Vigne R, Suzan M. The caprine arthritis encephalitis virus tat gene is dispensable for efficient viral replication in vitro and in vivo. J Virol. 1995;69:5445–5454. doi: 10.1128/jvi.69.9.5445-5454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harmache A, Russo P, Guiguen F, Vitu C, Vignoni M, Bouyac M, Hiéblot C, Pépin M, Vigne R, Suzan M. Requirement of caprine arthritis encephalitis virus vif gene for in vivo replication. Virology. 1996;224:246–255. doi: 10.1006/viro.1996.0526. [DOI] [PubMed] [Google Scholar]
- 16.Harmache A, Russo P, Vitu C, Guiguen F, Mornex J F, Pépin M, Vigne R, Suzan M. Replication in goats in vivo of caprine arthritis encephalitis virus deleted in vif or tat genes: possible use of these deletion mutants as live vaccines. AIDS Res Hum Retroviruses. 1996;12:409–411. doi: 10.1089/aid.1996.12.409. [DOI] [PubMed] [Google Scholar]
- 17.Heeney, J. L., L. Holtermann, P. TenHaaft, R. Dubbes, W. Koornstra, V. Teeuwsen, P. Bourquin, S. Norley, and H. Niphuis. 1994. Vaccine protection and reduced virus load from heterologous macaque-propagated SIV challenge. AIDS Res. Hum. Retroviruses 10(Suppl. 2):S117–S121. [PubMed]
- 18.Hess J L, Pyper J M, Clements J E. Nucleotide sequence and transcriptional activity of the caprine arthritis encephalitis virus long terminal repeat. J Virol. 1986;60:385–393. doi: 10.1128/jvi.60.2.385-393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang L, Joshi A, Willey R, Orenstein J, Jeang K-T. Human immunodeficiency viruses regulated by alternative trans-activators: genetic evidence for a novel non-transcriptional function of Tat in virion infectivity. EMBO J. 1994;13:2886–2896. doi: 10.1002/j.1460-2075.1994.tb06583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joag S V, Stephens E B, Narayan O. Lentiviruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1977–1996. [Google Scholar]
- 21.Kennedy-Stoskopf S, Zink C, Narayan O. Pathogenesis of ovine lentivirus-induced arthritis: phenotypic evaluation of T lymphocytes in synovial fluid, synovium and peripheral circulation. Clin Immunol Immunopathol. 1989;52:323–330. doi: 10.1016/0090-1229(89)90183-9. [DOI] [PubMed] [Google Scholar]
- 22.Kestler H, III, Ringler D J, Mori K, Panicali D L, Segal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 23.Knowles D, Jr, Cheevers W, McGuire T, Stem T, Gorham P. Severity of arthritis is predicted by antibody response to gp135 in chronic infection with caprine arthritis encephalitis virus. J Virol. 1990;64:2396–2398. doi: 10.1128/jvi.64.5.2396-2398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letvin N, Lord C I, King N W, Wyand M S, Myrick K V, Haseltine W A. Risks of handling HIV. Nature (London) 1991;349:573. doi: 10.1038/349573a0. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein D L, Rushlow K E, Cook R F, Raabe M L, Swardson C J, Kociba G J, Issel C J, Montelaro R C. Replication in vitro and in vivo of an equine infectious anemia virus mutant deficient in dUTPase activity. J Virol. 1995;69:2881–2888. doi: 10.1128/jvi.69.5.2881-2888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K A, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire T C, Knowles D P, Jr, Davis W C, Brassfield A L, Stem T A, Cheevers W P. Transmembrane protein oligomers of caprine arthritis encephalitis virus are immunodominant in goats with progressive arthritis. J Virol. 1992;66:3247–3250. doi: 10.1128/jvi.66.5.3247-3250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller C J, McChesney M B, Lü X, Dailey P J, Chutkowski C, Lu D, Brosio P, Roberts B, Lu Y. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J Virol. 1997;71:1911–1921. doi: 10.1128/jvi.71.3.1911-1921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayan O, Cork L C. Lentiviral diseases of sheep and goats: chronic pneumonia, leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985;7:89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- 30.Neuveut C, Vigne R, Clements J E, Sire J. The visna transcriptional activator Tat: effects on the viral LTR and on cellular genes. Virology. 1993;197:236–244. doi: 10.1006/viro.1993.1584. [DOI] [PubMed] [Google Scholar]
- 31.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a Nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 32.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T, Pahwa S, Verdin E. Immune hyperactivation of HIV-1 infected T cells mediated by Tat and the CD28 pathway. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 33.Perry L L, Wilkerson M J, Hullinger G A, Cheevers W P. Depressed CD4+ T lymphocyte response and enhanced antibody response to viral antigen in chronic lentivirus-induced arthritis. J Infect Dis. 1995;171:328–334. doi: 10.1093/infdis/171.2.328. [DOI] [PubMed] [Google Scholar]
- 34.Pyper J M, Clements J E, Strandberg J D, Cork L C, Griffin D E. Sequence homology between cloned caprine arthritis encephalitis virus and visna virus, two neurotropic lentiviruses. J Virol. 1986;58:665–670. doi: 10.1128/jvi.58.2.665-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigby M A, Hosie M J, Willett B J, Mackay N, McDonald M, Cannon C, Dunsford T, Jarrett O, Neil J C. Comparative efficiency of feline immunodeficiency virus infection by DNA inoculation. AIDS Res Hum Retroviruses. 1997;13:405–412. doi: 10.1089/aid.1997.13.405. [DOI] [PubMed] [Google Scholar]
- 36.Roos M T L, Lange J M A, de Goede R E Y, Coutinho R A, Schellekens P T A, Miedema F, Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- 37.Saltarelli M, Quérat G, Konings D A, Vigne R, Clements J E. Nucleotide sequence and transcriptional analysis of molecular clones of CAEV which generate infectious virus. Virology. 1990;179:347–364. doi: 10.1016/0042-6822(90)90303-9. [DOI] [PubMed] [Google Scholar]
- 37a.Suzan, M., et al. Unpublished results.
- 38.Turelli P, Guiguen F, Mornex J-F, Vigne R, Quérat G. dUTPase-minus caprine arthritis-encephalitis virus is attenuated for pathogenesis and accumulates G-to-A substitutions. J Virol. 1997;71:4522–4530. doi: 10.1128/jvi.71.6.4522-4530.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van’t Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrecht-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral and vertical transmission. J Clin Investig. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkerson M J, Davis W C, Baszler T V, Cheevers W P. Immunopathology of chronic lentivirus-induced arthritis. Am J Pathol. 1995;146:1433–1443. [PMC free article] [PubMed] [Google Scholar]
- 41.Willems L, Portetelle D, Kerkhofs P, Chen G, Burny A, Mammerickx M, Kettman R. In vivo transfection of bovine leukemia virus into sheep. Virology. 1992;189:775–777. doi: 10.1016/0042-6822(92)90604-n. [DOI] [PubMed] [Google Scholar]
- 42.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zanoni R G, Nauta I M, Pauli U, Peterhans E. Expression in Escherichia coli and sequencing of the coding region for the capsid protein of Dutch maedi-visna strain ZZV 1050: application of recombinant protein in enzyme-linked immunosorbent assay for the detection of caprine and ovine lentiviruses. J Clin Microbiol. 1991;29:1290–1294. doi: 10.1128/jcm.29.7.1290-1294.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 45.Zink M C, Yager J A, Myers J D. Pathogenesis of caprine arthritis encephalitis virus—cellular localization of viral transcripts in tissues of infected goats. Am J Physiol. 1990;136:843–854. [PMC free article] [PubMed] [Google Scholar]