Abstract
Background
Collarbone fracture is a common injury, particularly among athletes involved in contact sports and participating in endurance activities. Conventional treatment requires surgery and postoperative immobilization, resulting in an average return-to-sport timeframe of approximately 13 weeks. This case challenges the established treatment protocols, aiming to expedite recovery and enable a quicker resumption of high-intensity athletic activities.
Case presentation
A 24-year-old Caucasian athlete completed a Half-Ironman Triathlon (70.3) merely three weeks post-collarbone fracture. Utilizing Extracorporeal Magneto-Transduction Therapy (EMTT) alongside surgical intervention, the patient achieved accelerated healing and remarkable performance outcomes without encountering any adverse effects.
Conclusions
The integration of EMTT into the treatment paradigm for bone fractures alters the traditional understanding of recovery timelines and rehabilitation strategies. This case highlights the potential benefits of electromagnetic wave therapy in expediting the healing process and enabling athletes to resume high-level sports activities at an earlier stage.
Keywords: Return to sports, Accelerated healing, Electromagnetic field therapy, Fracture healing, Treatment guidelines, Collarbone fracture, Case report
Introduction
Clavicle fractures account for approximately 10% of all sport-related fractures [1, 2]. Additionally, they represent 2.6% of all fractures and 44% of fractures occurring in the shoulder region [3]. Allman classified clavicle fractures into three types based on the location of the fracture: Type 1 involves the middle third of the clavicle, Type 2 affects the distal end, and Type 3 affects the medial third [4]. Neer further subdivided lateral clavicle fractures into five subtypes based on the status of the coracoclavicular ligaments [5]. In our patient's case, he suffered from a Type 2a fracture, indicating that the ligaments located laterally were not affected. The optimal treatment protocol for clavicle fractures remains a topic of debate. Therefore, managing these fractures depends on the hospital´s and physicians’ preferences. Evidence proposes a higher risk of developing non-union with non-surgical treatment [6, 7]. Given the significant displacement of the medial fragment in our patient's case, we concluded that surgery may lead to a better outcome compared to conservative therapy. According to the evidence-based clinical practice guidelines from the AAOS (2022), we recommended immobilization with a sling for a few days after surgery. The surgical treatment for clavicular fractures is becoming increasingly common. In Finland, the incidence of surgical treatment significantly increased from 1.3 per 100,000 persons in 1987 to 10.8 per 100,000 persons in 2010 [8]. The average time off work is 49 days for operative treatment and 47 days for non-operative treatment [9]. The average time required to return to sports (including various surgical and non-surgical treatments) is approximately 13.7 weeks. Furthermore, only 81% of patients can return to their pre-injury level [10]. Considering both personal factors and the healthcare costs associated with a prolonged absence, there is an urgent need to investigate new rehabilitation protocols to expedite the return to daily life and sports activities. In our case, the patient used Extracorporeal Magneto-Transduction Therapy (EMTT) to recover faster.
According to some older reports, electrical stimulation has been used in medical treatment since 1841 [11]. One specific type of electromagnetic wave therapy is PEMF (pulsed electromagnetic field) therapy, which gained approval from the Food and Drug Administration (FDA) in 1979. PEMF devices have been used as a modality for treating various osteogenic disorders, including fracture healing [12–14] or reducing bone loss associated with osteoporosis [15, 16]. EMTT, on the other hand, is a newer device that falls within the category of electromagnetic treatment. It differs from PEMF in a few physical aspects. EMTT utilizes high-intensity PEMFs with a magnetic field strength of up to 150 mT. It operates at an effective transduction power exceeding 60 kT/s and has an oscillating frequency ranging between 100–300 kHz. These specifications enable EMTT to penetrate tissues up to 18 cm deep. In comparison, PEMF devices typically have lower power outputs, falling below 60 kT/s [17, 18].
Case presentation
We present the case of a 24-year-old Caucasian non-smoking male patient training for mid and long-distance triathlon competitions. While road cycling, he had an accidental fall and suffered a fracture in his left lateral clavicle (Fig. 1a). Based on the Neer classification, the fracture can be categorized as a Type IIa fracture, which is located medial to the coracoclavicular ligament. There is noticeable displacement of the medial fragment, but the conoid and trapezoid ligaments remained intact. The patient underwent surgery the following day, during which the dislocated bone fragments were stabilized using a clavicle plate system (VariAx, Fig. 1b).
Fig. 1.
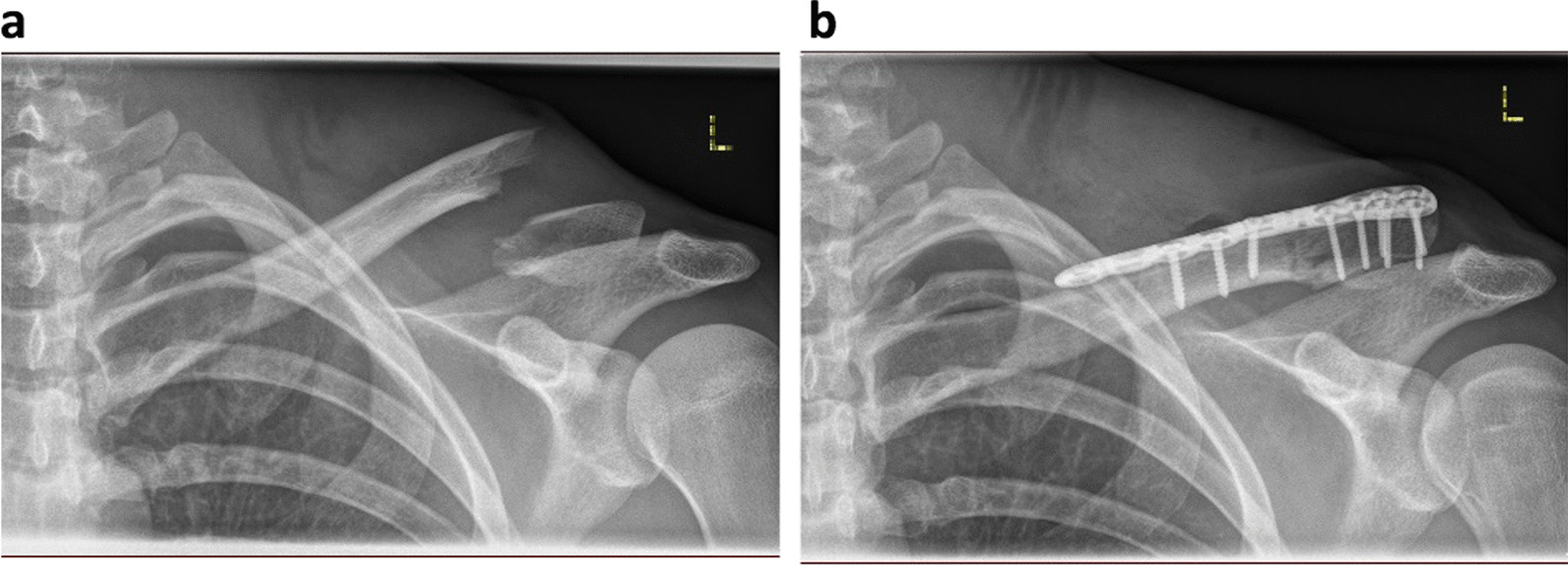
a Radiograph showing left clavicle fracture Typ IIa after the bike accident. b Post-surgery radiograph showing left clavicle plate system (VariAx) after plate osteosynthesis
One day after surgery, he was discharged. Since the patient's goal was to participate in the upcoming Half-Ironman (consisting of a 1.2 mile/1.9 km swim, a 56 mile/90 km bike ride, and a 13.1 mile/21.1 km run) in three weeks, we immediately initiated EMTT stimulation on the same day (Table 1). We opted for a relatively intense treatment protocol using the Magnetolith device (STORZ Medical), stimulating for 30 min at a frequency of 8 Hz and magnetic field strength of approximately 80 mT (14,400 impulses per session). The patient does not experience any additional pain or discomfort during the application of EMTT. For the first two weeks, we repeated this protocol every other day (Fig. 2).
Table 1.
Different applied therapy modalities
| Week 1 | Week 2 | Week 3 | |
|---|---|---|---|
| EMTT Stimulation | 30 min, lvl 8, 8 Hz, every other day (− > 14.400 impulses) | 30 min, lvl 8, 8 Hz, every other day (− > 14.400 impulses) | |
| Physiotherapy | Active movements limited to pain-free range (< 90° in every axis) | Active movements within a pain-free range (> 90° if painless) | Shoulder muscle strengthening with resistance bands |
| Sling (Gilchrist) | During daily life and activities | During daily life, omitted for sport activities | No need for the sling anymore |
| Range of motion (passive) |
Flexion < 70° Abduction: < 60° |
Flexion: < 100° Abduction: < 120° |
Free range of motion |
| Range of motion (passive) |
Flexion < 70° Abduction: < 60° |
Flexion: < 100° Abduction: < 120° |
Free range of motion |
Physiotherapy exercises: 2–3 times a day for 15 min
min: minute; lvl: level; Hz: hertz
Fig. 2.
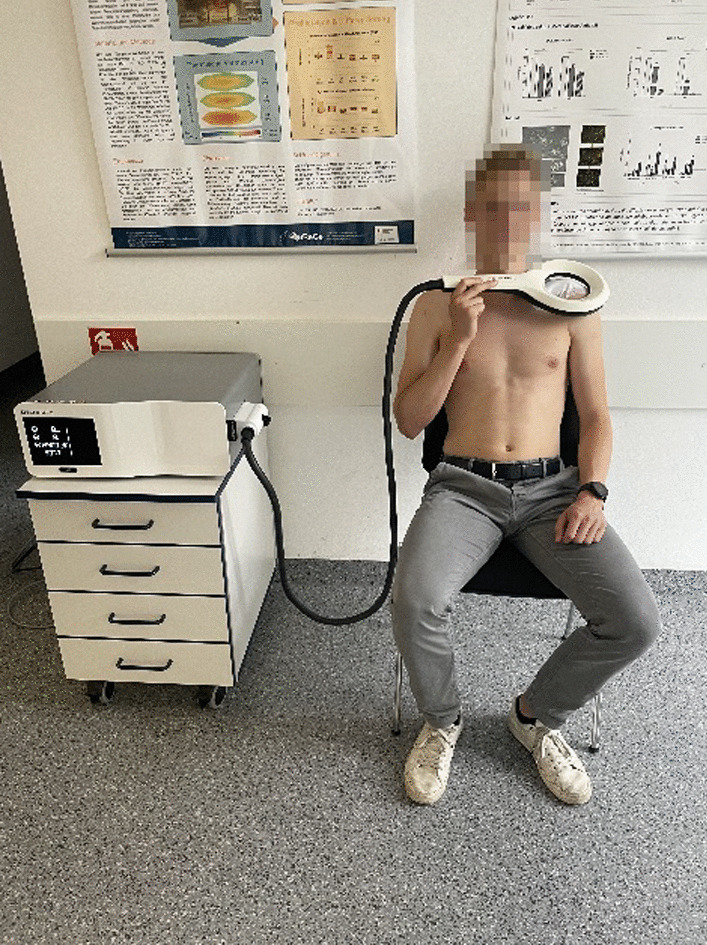
The patient applying extracorporeal magneto-transduction therapy (EMTT) 3 days post-surgery. Following protocol was used: Frequency 8 Hz, duration 30 min (14,400 impulses), and strength level 8 out of 8 (Device: STORZ Medical Magnetolith)
Additionally, we started with physiotherapeutic measures one day after the surgery (Table 1). In the first week, the patient's active and passive movements were limited to 90 degrees in flexion and abduction. Moreover, he resumed his training plan and began cycling on a home cycle trainer and running (Table 2), both while wearing a sling. The sling immobilization was maintained during the second week but omitted during the training sessions. Throughout the second week, the range of motion improved, allowing an elevation and abduction above 90° (Flexion: < 110°, Abduction: < 100°) under the guidance of the physiotherapist. Consequently, the athlete could perform bike training sessions on a TT-Bike (time trial) in conjunction with the TT-position (Fig. 3).
Table 2.
Triathlon specific training performed by the patient
| Week 1 | Week 2 | Week 3 | |
|---|---|---|---|
| Running | Running with a sling (5 to 10 km/session) |
Running at a moderate pace without a sling (10–20 km/session) |
Intervals (including race pace) |
| Cycling | Indoor cycling using a smart trainer with Zwift (ca. 60 min/session) | Indoor cycling using a smart trainer with Zwift in a time trial (TT) position (60 to 150 min/session) | Outdoor cycling on a time trial (TT) bike (> 100 km/session) |
| Swimming | 500 m, technique: Crawl |
Fig. 3.

3 weeks post-surgery, during a triathlon race in a time trial (TT) position (flexion: 90°, adduction: 30°, internal rotation: 45°)
At the end of the second week, the athlete could run 20 km without experiencing any pain. Given the patient's report of being almost pain-free during daily activities (Table 3), we advanced to a more competition-oriented protocol, incorporating strength training using resistance bands. Furthermore, the athlete included brief high-intensity sessions into his exercise regimen. To test his ability to swim in the upcoming Half-Ironman, he swam the crawl for 500 m while wearing a wetsuit. To avoid causing pain, he refrained from pulling and pushing forcefully during the arm crawl phases. The Half-Ironman was completed three weeks after surgery, even achieving a personal best time. A cautious approach was taken in the swimming discipline, resulting in a more restrained swim performance. A clinical assessment after the competition did not reveal any deterioration in terms of painless range of motion. However, to ensure the correct positioning of the screws and plate, an X-ray evaluation was conducted six weeks after surgery (Fig. 4a). The fixation plate and all screws were intact and not loosened. The fracture gap already exhibited slight bony consolidation, indicating ongoing fracture healing. The treatment can be successfully completed (clinically) at this stage. To confirm complete bone healing radiologically too, the patient received one final follow-up X-ray image ten weeks post-surgery (Fig. 4b).
- First week:
-
oElbow, wrist, hand exercises
-
oPendulum, Circular (Codman's Exercises)
-
oIsometric exercises
-
oAll movements were restricted to 90° (Flexion, Abduction)
-
o
- Second week:
-
oExercises from the first week
-
oArm lifting (Flexion) with support of the good arm (< 90°, end of the week: < 100°)
-
oWalk Up Exercise against the wall (Active)
-
oPassive movements supported by physiotherapist: Flexion: < 110°, Abduction: < 100°
-
o
- Third week:
-
oExercises from first and second week
-
oStrengthening exercises with resistance band (pain adopted)
-
oPassive movements supported by physiotherapist: Full range of motion (if painless)
-
o
Table 3.
Further assessment
| Week 1 | Week 2 | Week 3 | |
|---|---|---|---|
| Imaging | Pre- and post-surgery X-Ray | Post Race X-Ray | |
| Pain (VAS score) | 4–6 | 1–3 | 0–2 |
Pain management therapy was administered during the initial days following the surgery, involving the use of nonsteroidal anti-inflammatory drugs (NSAIDs) and ice application
Fig. 4.
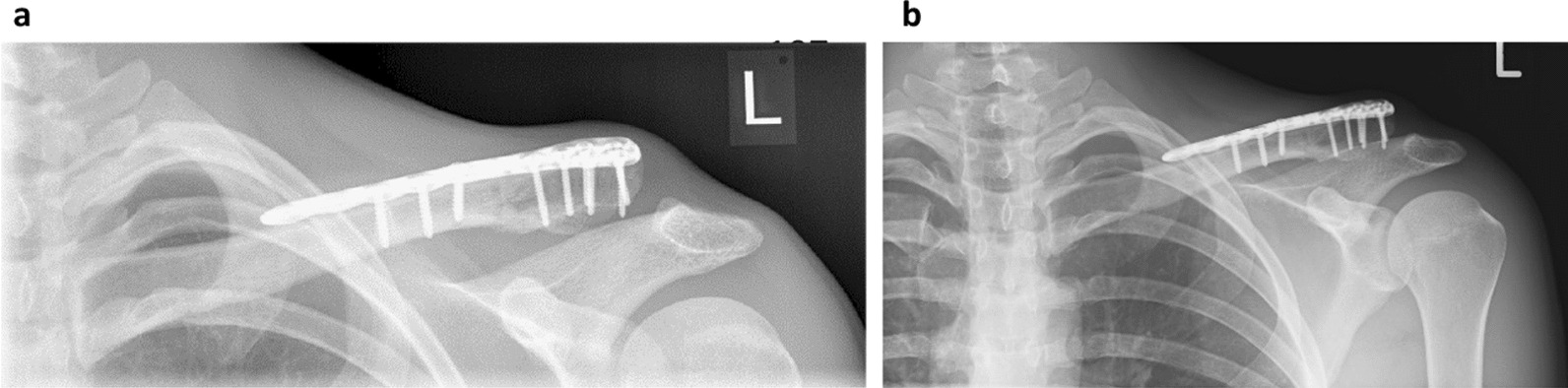
a Radiograph 6 weeks post-surgery. Condition after a lateral clavicle fracture treated with plate osteosynthesis and EMTT stimulation. The foreign material is intact, with no signs of loosening. The fracture gap appears partially healed. b Radiograph 10 weeks post-surgery showing complete bone healing following a clavicle fracture.
Discussion
Numerous studies in the literature highlight the positive effects of PEMF therapy on various aspects of bone health, including chondrogenesis [19–21], cytoprotective effects against oxidative stress [22], anti-inflammatory effects [23–25], and bone metabolism. Studies conducted on osteoporosis-induced rats or mice (ovariectomized) have demonstrated improvements in bone mass, bone mineral density, and bone microarchitecture [15, 16]. Additionally, a wealth of research indicates accelerated fracture healing with PEMF therapy [12–14]. Potential mechanisms for these effects include increased osteoblast activity, enhanced osteoblast differentiation, inhibition of osteoclasts, and induction of angiogenesis [26–33].
The accelerated bone healing observed in our presented case aligns with current clinical research. A randomized, double-blind, sham-controlled pilot study involving 41 patients with radial fractures revealed significantly greater union extent and better functional outcomes in the group treated with electromagnetic stimulation compared to the control group [34]. Other clinical studies investigating the enhancement of fracture healing with PEMF have reported similar results [35–37]. However, there are critics and concerns regarding the effectiveness of PEMF treatment for fracture healing. One notable criticism is the inconsistency of results, which may be attributed to factors such as the low achievable impulse frequency of most PEMF devices, resulting in a lower biological impact. Practitioners have also expressed concerns about the lengthy treatment sessions required. In contrast, EMTT devices, producing a 40 × higher oscillation frequency, may provide noticeable clinical effects more rapidly. A follow-up study involving 1382 patients with delayed union and non-union fractures who received varying durations of PEMF stimulation showed a median healing time reduction of 35–60% [38]. Patients receiving ten hours of PEMF stimulation per day experienced healing 76 days earlier than those receiving only three hours per day. This study emphasizes the importance of more efficient electromagnetic wave therapy. Since EMTT is a relatively new form of electromagnetic wave therapy, the available evidence on its effectiveness is limited. An in vitro study investigating its influence on human bone marrow mesenchymal stem cells (MSCs) demonstrated increased VEGF concentration and a tendency toward higher expression of bone formation-specific genes (collagen I and alkaline phosphatase). These findings support the concept that electromagnetic waves can play a beneficial role in promoting bone fracture healing through mechanisms involving bone metabolism and angiogenesis [17]. A case report in the literature on EMTT describes a novel approach involving the combination of EMTT with high-energy focused extracorporeal shockwave therapy (ESWT) for treating a non-union of the metacarpal V bone [39]. The patient underwent a specific treatment protocol, including three ESWT sessions followed by EMTT stimulation. This dual therapy was administered once a week for a total of three weeks. Four weeks after the completion of the therapy, there was evidence of enhanced bony consolidation in the metacarpal bone. This case report highlights a potential advanced therapy concept for treating bone-specific disorders. Additional studies are necessary to determine the optimal treatment protocols for different types of bone disorders involving EMTT and ESWT. These studies would help establish the most effective parameters, frequencies, durations, and combinations of these therapies to maximize their therapeutic benefits in various bone-related conditions. A better understanding of the optimal protocol would aid in providing evidence-based guidelines for healthcare professionals and ensure the best possible patient outcomes.
Conclusion
Initiation of EMTT treatment soon after a bone fracture, regardless of whether the patient undergoes surgery or receives non-surgical treatment, could serve as an essential adjunct therapy to expedite recovery time and facilitate a return to sports activities, whether at an amateur or professional level.
Acknowledgements
Not applicable.
Author contributions
All three authors have made substantial contributions to the conception and implementation of the innovative therapy in this study. All authors read and approved the final manuscript.
Abbreviations
- EMTT
Extracorporeal magneto-transduction therapy
- ESWT
Extracorporeal shockwave therapy
- AAOS
American Academy of Orthopaedic Surgeons
- PEMF
Pulsed electromagnetic field
- FDA
Food and Drug Administration
- TT (-Bike/-Position)
Time trial
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- MSCs
Mesenchymal stem cells
- VEGF
Vascular endothelial growth factor
Author contribution
All authors contributed to the case data and design. Data collection was performed by LG and RB. The first draft of the manuscript was written by LG and supervised by AS and RB. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The study was supported by STORZ Medical by providing the treatment device within the study time. The sponsor did not have influence on handling of subjects, data collection, data analysis or preparation of the manuscript. Open Access funding enabled and or
ganized by Projekt DEAL.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aitken SA, Watson BS, Wood AM, Court-Brown CM. Sports-related fractures in South East Scotland: an analysis of 990 fractures. J Orthop Surg (Hong Kong) 2014;22(3):313–317. doi: 10.1177/230949901402200309. [DOI] [PubMed] [Google Scholar]
- 2.Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39(12):1365–1372. doi: 10.1016/j.injury.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Postacchini F, Gumina S, De Santis P, Albo F. Epidemiology of clavicle fractures. J Shoulder Elbow Surg. 2002;11(5):452–456. doi: 10.1067/mse.2002.126613. [DOI] [PubMed] [Google Scholar]
- 4.Allman FL. Fractures and ligamentous injuries of the clavicle and its articulation. J Bone Jt Surg Am. 1967;49(4):774–784. doi: 10.2106/00004623-196749040-00024. [DOI] [PubMed] [Google Scholar]
- 5.Stenson J, Baker W. Classifications in brief: the modified neer classification for distal-third clavicle fractures. Clin Orthop Relat Res. 2021;479(1):205–209. doi: 10.1097/CORR.0000000000001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh JH, Kim SH, Lee JH, Shin SH, Gong HS. Treatment of distal clavicle fracture: a systematic review of treatment modalities in 425 fractures. Arch Orthop Trauma Surg. 2011;131(4):525–533. doi: 10.1007/s00402-010-1196-y. [DOI] [PubMed] [Google Scholar]
- 7.Uittenbogaard SJ, van Es LJM, den Haan C, van Deurzen DFP, van den Bekerom MPJ. Outcomes, union rate, and complications after operative and nonoperative treatments of neer type II distal clavicle fractures: a systematic review and meta-analysis of 2284 patients. Am J Sports Med. 2023;51(2):534–544. doi: 10.1177/03635465211053336. [DOI] [PubMed] [Google Scholar]
- 8.Huttunen TT, Kannus P, Lepola V, Pihlajamäki H, Mattila VM. Surgical treatment of clavicular fractures in Finland—a register based study between 1987 and 2010. Injury. 2013;44(12):1899–1903. doi: 10.1016/j.injury.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Kask G, Raittio L, Mattila VM, Launonen AP. Cost-effectiveness of operative versus non-operative treatment for clavicle fracture: a systematic literature review. Curr Rev Musculoskelet Med. 2020;13(4):391–399. doi: 10.1007/s12178-020-09640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robertson GAJ, Wood AM. Return to sport following clavicle fractures: a systematic review. Br Med Bull. 2016;119(1):111–128. doi: 10.1093/bmb/ldw029. [DOI] [PubMed] [Google Scholar]
- 11.Hannouche D, Petite H, Sedel L. Current trends in the enhancement of fracture healing. J Bone Jt Surg Br. 2001;83(2):157–164. doi: 10.1302/0301-620x.83b2.12106. [DOI] [PubMed] [Google Scholar]
- 12.Del Buono A, Zampogna B, Osti L, Fontanarosa A, Garofalo R, Papalia R. Pulsed electromagnetic fields after intramedullary nailing of tibial fractures: a case control study. Int Orthop. 2021;45(11):2945–2950. doi: 10.1007/s00264-021-05125-y. [DOI] [PubMed] [Google Scholar]
- 13.The classic: fundamental aspects of fracture treatment by Iwao Yasuda, reprinted from J Kyoto Med Soc. 1953; 4:395–406. Clin Orthop Relat Res. 1977;124: 5–8. [PubMed]
- 14.Tsai M-T, Li W-J, Tuan RS, Chang WH. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J Orthopaed Res. 2009;27(9):1169–1174. doi: 10.1002/jor.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei T, Liang Z, Li F, Tang C, Xie K, Wang P, Dong X, Shan S, Jiang M, Xu Q, Luo E, Shen G. Pulsed electromagnetic fields (PEMF) attenuate changes in vertebral bone mass, architecture and strength in ovariectomized mice. Bone. 2018;108:10–19. doi: 10.1016/j.bone.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Liao Y, Zeng Y, Xie H, Fu C, Li N. Effect of intervention initiation timing of pulsed electromagnetic field on ovariectomy-induced osteoporosis in rats. Bioelectromagnetics. 2017;38(6):456–465. doi: 10.1002/bem.22059. [DOI] [PubMed] [Google Scholar]
- 17.Gerdesmeyer L, Zielhardt P, Klüter T, Gollwitzer H, Gerdesmeyer L, Hausdorf J, Ringeisen M, Knobloch K, Saxena A, Krath A. Stimulation of human bone marrow mesenchymal stem cells by electromagnetic transduction therapy—EMTT. Electromagn Biol Med. 2022;41(3):304–314. doi: 10.1080/15368378.2022.2079672. [DOI] [PubMed] [Google Scholar]
- 18.Knobloch K. Knochenstimulation 4.0—Kombination aus EMTT und ESWT bei Humeruspseudarthrose: Ein Fallbericht. Unfallchirurg. 2022;125(4):323–326. doi: 10.1007/s00113-021-01025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzsimmons RJ, Gordon SL, Kronberg J, Ganey T, Pilla AA. A pulsing electric field (PEF) increases human chondrocyte proliferation through a transduction pathway involving nitric oxide signaling. J Orthopaed Res. 2008;26(6):854–859. doi: 10.1002/jor.20590. [DOI] [PubMed] [Google Scholar]
- 20.Littman J, Aaron RK. Stimulation of chondrogenesis in a developmental model of endochondral bone formation by pulsed electromagnetic fields. Int J Mol Sci. 2023;24(4):3275. doi: 10.3390/ijms24043275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parate D, Franco-Obregón A, Fröhlich J, Beyer C, Abbas AA, Kamarul T, Hui JHP, Yang Z. Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci Rep. 2017;7(1):9421. doi: 10.1038/s41598-017-09892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gökçek-Saraç Ç, Şimşek T, Karakurt S. Cytoprotective effects of low-frequency pulsed electromagnetic field against oxidative stress in glioblastoma cells. Gen Physiol Biophys. 2023;42(1):97–106. doi: 10.4149/gpb_2022056. [DOI] [PubMed] [Google Scholar]
- 23.Ross CL, Ang DC, Almeida-Porada G. Targeting mesenchymal stromal cells/pericytes (MSCs) with pulsed electromagnetic field (PEMF) has the potential to treat rheumatoid arthritis. Front Immunol. 2019;10:266. doi: 10.3389/fimmu.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross CL, Zhou Y, McCall CE, Soker S, Criswell TL. The use of pulsed electromagnetic field to modulate inflammation and improve tissue regeneration: a review. Bioelectricity. 2019;1(4):247–259. doi: 10.1089/bioe.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincenzi F, Targa M, Corciulo C, Gessi S, Merighi S, Setti S, Cadossi R, Goldring MB, Borea PA, Varani K. Pulsed electromagnetic fields increased the anti-inflammatory effect of A2A and A3 adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS ONE. 2013;8(5):e65561. doi: 10.1371/journal.pone.0065561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Zhang Y, Chen J, Zheng S, Huang H, Dong X. Effects of pulsed electromagnetic fields on the expression of NFATc1 and CAII in mouse osteoclast-like cells. Aging Clin Exp Res. 2015;27(1):13–19. doi: 10.1007/s40520-014-0239-6. [DOI] [PubMed] [Google Scholar]
- 27.He W-F, Qin R, Gao Y-H, Zhou J, Wei J-J, Liu J, Hou X-F, Ma H-P, Xian CJ, Li X-Y, Chen K-M. The interdependent relationship between the nitric oxide signaling pathway and primary cilia in pulse electromagnetic field-stimulated osteoblastic differentiation. FASEB J. 2022;36(6):e22376. doi: 10.1096/fj.202101577RR. [DOI] [PubMed] [Google Scholar]
- 28.He Z, Selvamurugan N, Warshaw J, Partridge NC. Pulsed electromagnetic fields inhibit human osteoclast formation and gene expression via osteoblasts. Bone. 2018;106:194–203. doi: 10.1016/j.bone.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Jing D, Cai J, Wu Y, Shen G, Li F, Xu Q, Xie K, Tang C, Liu J, Guo W, Wu X, Jiang M, Luo E. Pulsed electromagnetic fields partially preserve bone mass, microarchitecture, and strength by promoting bone formation in hindlimb-suspended rats. J Bone Miner Res. 2014;29(10):2250–2261. doi: 10.1002/jbmr.2260. [DOI] [PubMed] [Google Scholar]
- 30.Selvamurugan N, He Z, Rifkin D, Dabovic B, Partridge NC. Pulsed electromagnetic field regulates microRNA 21 expression to activate TGF-β signaling in human bone marrow stromal cells to enhance osteoblast differentiation. Stem Cells Int. 2017;2017:2450327. doi: 10.1155/2017/2450327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S, Gan J, Simon B, Hopper RA, Levine JP, Gurtner GC. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004;18(11):1231–1233. doi: 10.1096/fj.03-0847fje. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Liu J, Yang Y, Zhai M, Shao X, Yan Z, Zhang X, Wu Y, Cao L, Sui B, Luo E, Jing D. Differential intensity-dependent effects of pulsed electromagnetic fields on RANKL-induced osteoclast formation, apoptosis, and bone resorbing ability in RAW264.7 cells. Bioelectromagnetics. 2017;38(8):602–612. doi: 10.1002/bem.22070. [DOI] [PubMed] [Google Scholar]
- 33.Yan J-L, Zhou J, Ma H-P, Ma X-N, Gao Y-H, Shi W-G, Fang Q-Q, Ren Q, Xian CJ, Chen K-M. Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia. Mol Cell Endocrinol. 2015;404:132–140. doi: 10.1016/j.mce.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Factor S, Druckmann I, Atlan F, Rosenblatt Y, Tordjman D, Krespi R, Kazum E, Pritsch T, Eisenberg G. The effects of novel pulsed electromagnetic field therapy device on acute distal radius fractures: a prospective, double-blind, sham-controlled, randomized pilot study. J Clin Med. 2023;12(5):1866. doi: 10.3390/jcm12051866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Handoll HHG, Madhok R, Howe TE. Rehabilitation for distal radial fractures in adults. Cochrane Database Syst Rev. 2006;3:03324. doi: 10.1002/14651858.CD003324.pub2. [DOI] [PubMed] [Google Scholar]
- 36.Lazović M, Kocić M, Dimitrijević L, Stanković I, Spalević M, Cirić T. Pulsed electromagnetic field during cast immobilization in postmenopausal women with Colles’ fracture. Srp Arh Celok Lek. 2012;140(9–10):619–624. doi: 10.2298/SARH1210619L. [DOI] [PubMed] [Google Scholar]
- 37.Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J Bone Jt Surg Am. 2008;90(11):2322–2330. doi: 10.2106/JBJS.H.00111. [DOI] [PubMed] [Google Scholar]
- 38.Murray HB, Pethica BA. A follow-up study of the in-practice results of pulsed electromagnetic field therapy in the management of nonunion fractures. Orthop Res Rev. 2016;8:67–72. doi: 10.2147/ORR.S113756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knobloch K. Extracorporeal magnetotransduction therapy (EMTT) and high-energetic focused extracorporeal shockwave therapy (ESWT) as bone stimulation therapy for metacarpal non-union—a case report. Handchirurgie, Mikrochirurgie, Plastische Chirurgie: Organ Der Deutschsprachigen Arbeitsgemeinschaft Fur Handchirurgie: Organ Der Deutschsprachigen Arbeitsgemeinschaft Fur Mikrochirurgie Der Peripheren Nerven Und Gefasse: Organ Der V. 2021;53(1):82–86. doi: 10.1055/a-1344-8126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.


