Abstract
Background
Chronic Kidney Disease of unknown cause (CKDu) a disease of exclusion, and remains unexplained in various parts of the world, including India. Previous studies have reported mixed findings about the role of heavy metals or agrochemicals in CKDu. These studies compared CKDu with healthy controls but lacked subjects with CKD as controls. The purpose of this study was to test the hypothesis whether heavy metals, i.e. Arsenic (As), Cadmium (Cd), Lead (Pb), and Chromium (Cr) are associated with CKDu, in central India.
Methods
The study was conducted in a case-control manner at a tertiary care hospital. CKDu cases (n = 60) were compared with CKD (n = 62) and healthy subjects (n = 54). Blood and urine levels of As, Cd, Pb, and Cr were measured by Inductively Coupled Plasma- Optical Emission Spectrometry. Pesticide use, painkillers, smoking, and alcohol addiction were also evaluated. The median blood and urine metal levels were compared among the groups by the Kruskal-Wallis rank sum test.
Results
CKDu had significantly higher pesticide and surface water usage as a source of drinking water. Blood As levels (median, IQR) were significantly higher in CKDu 91.97 (1.3–132.7) µg/L compared to CKD 4.5 (0.0–58.8) µg/L and healthy subjects 39.01 (4.8–67.4) µg/L (p < 0.001) On multinominal regression age and sex adjusted blood As was independently associated with CKDu[ OR 1.013 (95%CI 1.003–1.024) P < .05].Blood and urinary Cd, Pb, and Cr were higher in CKD compared to CKDu (p > .05). Urinary Cd, Pb and Cr were undetectable in healthy subjects and were significantly higher in CKDu and CKD compared to healthy subjects (P = < 0.001). There was a significant correlation of Cd, Pb and Cr in blood and urine with each other in CKDu and CKD subjects as compared to healthy subjects. Surface water use also associated with CKDu [OR 3.178 (95%CI 1.029–9.818) p < .05).
Conclusion
The study showed an independent association of age and sex adjusted blood As with CKDu in this Indian cohort. Subjects with renal dysfunction (CKDu and CKD) were found to have significantly higher metal burden of Pb, Cd, As, and Cr as compared to healthy controls. CKDu subjects had significantly higher pesticide and surface water usage, which may be the source of differential As exposure in these subjects.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03564-4.
Keywords: Chronic kidney disease of unknown cause, CKDu, Heavy metals, Environmental toxins
Background
Chronic kidney disease of unknown cause (CKDu) has been reported in various parts of the world (i.e., Nicaragua, El Salvador, Sri Lanka), including India, as an endemic disease. The disease is a diagnosis of exclusion, made when a patient fulfils the Kidney Disease Improving Global Outcomes (KDIGO) CKD criteria but without the evidence of a recognized cause such as diabetes, hypertension, or glomerulonephritis [1]. No uniform and definite cause has yet been identified, though various environmental factors have been associated with and suggested to play a role in the pathogenesis. For example, heat stress, strenuous exercise, agrochemicals, and heavy metals have been held responsible for Mesoamerican nephropathy [2]. Mixed evidence has been reported for association with agrochemicals, heavy metals, and genetic variability for CKDu in Sri Lanka [3–5]. In India, one small study reported an association of heavy metals with CKD [6]. A study done on groundwater samples from the Uddanam region of Andhra Pradesh (India), which has a high prevalence of CKDu reported water as acidic (pH < 6.5) and to contain higher silica and lead in wet and dry seasons, respectively. Phthalates were also detected in the groundwater [7]. Previous studies have attempted to find a correlation between heavy metals and CKDu by comparing cases and endemic and nonendemic controls [4]. No study has yet tried to find the association of heavy metals comparing CKDu with CKD. Furthermore, previous studies have used urinary metal levels as a biomarker of metal exposure. There is an inherent issue of reverse causality i.e., reduced excretion of metals in urine with a reduction in glomerular filtration rate (GFR) [8]. Measurement of metals in blood has also been reported to be a promising biomarker of metal exposure [9, 10]. Some studies have employed urine to blood ratio for deciding whether urine or blood levels should be used for a particular metal. For metals with urine/blood ratio more than one blood metal levels, whereas for metals with urine/blood ratio less than one, urine metal levels were used in estimation analysis [11]. The purpose of this study was to test hypothesis whether heavy metals i.e. Arsenic (As), Cadmium (Cd), Lead (Pb) and Chromium (Cr) are associated with CKDu, in central India using blood and urine levels as biomarker of metal exposure.
Materials and methods
Study setting and population
Study was conducted in a tertiary care hospital setting in the Department of Nephrology in India in a case-control design between December 2019 to June 2022. Participants were enrolled between December 2019 -December 2021. The data collection was done simultaneously. The sample analysis was carried out between January to June-2022. The study was performed according to the guidelines of the Declaration of Helsinki. The study objective was to compare CKDu cases with CKD and healthy controls with regard to biomarkers of exposure of heavy metals [blood and urine levels of cadmium (Cd), lead (Pb), arsenic (As) and chromium (Cr)]. The study included adults aged 18–70 years with CKDu and two groups of the control population, one with CKD and another group of healthy controls without evidence of CKD.
The CKDu and CKD cases were inducted among the patients visiting the nephrology outpatient department and based on pre-defined criteria. At the same time, healthy controls were inducted among the healthy relatives accompanying the patients visiting other departments of the institute for treatment. Written informed consent was obtained from all the participants.
The case definition of CKDu was based on criteria proposed by the Indian Society of Nephrology for the diagnosis of CKDu [12]. The inclusion criteria included- eGFR < 60 mL/min/1.73m2 (CKD-EPI) [13] and albumin-to-creatinine ratio (ACR) > 30 mg/g for more than 3 months with:
Urine protein creatinine ratio(PCR) less than 2g/g.
No history of glomerulonephritis, pyelonephritis, renal calculi, polycystic kidneys or obstruction on renal ultrasound.
Not on treatment for diabetes and HbA1c less than 6.5%.
Blood pressure less than 140/90 if CKD stage 1 and 2; and less than 160/100 if CKD stage 3,4, and 5 and on a single drug for blood pressure control.
Case definition of CKD was based on: eGFR < 60 mL/min/1.73m2 (CKD-EPI) and albumin-to-creatinine ratio > 30 mg/g for more than 3 months. Patients were included in the CKD group only if PCR > 2g/g. Hypertension with BP > 140/90 in stages 1–2 and > 160/100 in stages 3–5 or on two or more drugs for BP control.
CKD staging was based on the KDIGO-2008 classification [1]. The same stages were applied to categorize the renal functions of subjects with CKDu.
Inclusion criteria for healthy controls included: Absence of CKD as evidenced by eGFR more than 90 ml/min/1.73m2, ACR < 30mg/g and lack of anatomical renal disease, obstruction or stone on renal ultrasound, no history of diabetes, HbA1C less than 6.5 and BP less than 140/90.
Biases were kept a minimum by adhering to the case definition described above, and study exposures are mainly objectively assessed with very less dependency on recall i.e. for pesticide or painkiller use. The urine metal levels were adjusted for urine dilution by estimating metals per gram of creatinine in urine.
Sample size
Assuming a difference of moderate effect size (0.25), between three groups (CKDu cases, CKD Controls, Normal Controls) with a confidence level of 95% and power of 80%, the calculated sample size was 159. The final sample size estimated, including a 10% non-response rate, was 180 (60 per group).
Specimen collection and analysis
For the analysis of heavy metals, venous blood (2 ml) was collected in trace element free Trace Element K2-EDTA Vacutainer (Cat# BD 368381). Whole blood was stored at -40 °C until analysis. Ten millilitres (10 ml) of first-morning urine was collected in 50 ml polypropylene tubes. Urine was stored at -40 °C in aliquots until analysis. Serum and urine creatinine was measured using a modified kinetic Jaffe’s method using a Random Access Fully Automated Chemistry Analyzer (Beckman Coulter). Urinary protein and urine albumin were estimated using a colorimetric and immune-turbidimetric methods, respectively, using a Random Access Fully Automated Chemistry Analyzer (Beckman Coulter). HbA1c was analyzed by ion-exchange high pressure liquid chromatography method using a D10 Haemoglobin testing system (BioRad Laboratories). eGFR was calculated from serum creatinine and CKD -EPI equation (Ref). A kidney ultrasound was performed in standard B Mode grey scale in 3.5–5 MHz, the longitudinal length was measured along with the width and thickness of the kidney, renal stones, and any other anatomical abnormality.
Estimation of heavy metals in blood and urine
Levels of Cd, Pb, Cr and As were measured in whole blood and urine. Urinary spot sample results of metal analysis were adjusted for dilution by urine creatinine. Metal analysis was carried out at NIREH, Bhopal (India).
Levels of various heavy metals, viz. Cd, Pb, Cr, As in the collected blood and urine samples were analyzed through inductively coupled plasma optical emission spectroscopy (iCAP® 7400 Duo ICP-OES, ThermoFisher Scientific® Pvt. Ltd). Blood and urine samples were acid-digested in a microwave oven prior to metal detection on ICP-OES. For blood digestion, 1 mL of whole blood sample was mixed with 6 ml of a freshly prepared mixture of concentrated trace metal grade nitric acid (HNO3) and hydrogen peroxide (H2O2) in a ratio 2:1 (v/v) in high-purity polytetrafluoroethylene (PTFE-TFM) vessels. For urine digestion, 5 mL of urine sample was mixed with 6 ml of a freshly prepared mixture of HNO3 and H2O2 in a ratio of 2:1. After gentle mixing of these reactants with blood, the PTFE-TFM vessels were arranged in the rotor (24HVT80, Anton PAAR) and digestion was carried out in the Anton Paar, multi microwave PRO Reaction System at 200 C for 15 min. Digested samples were cooled to 40°C and diluted to 30 ml with distilled water. Blank was prepared for each cycle of digestion using distilled water, nitric acid, and hydrogen peroxide mixture. All the chemicals were trace-element free.
Before the analysis of metal ions in processed blood and urine samples, calibration standards for each element were prepared from multi-element stock solutions (1000 mg L − 1) in triple distilled water. Detection of Cd, Pb, and Cr was performed using a standard sample introduction setup, whereas for As, the hydride generation sample introduction system was utilized. Online hydride generation for As was achieved with an Enhanced Vapor System sample introduction kit using 0.5% m/v sodium tetrahydroborate (NaBH4) stabilized in 0.5% m/v NaOH and 50% v/v HCl solution. Emission data acquisition was performed using the Qtegra ISDS Software at interference-free wavelengths.
Statistical analysis
Statistical analyses were performed with R version 4.2 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS 26 version. The distribution of data in groups was evaluated with Shapiro-Wilk, kurtosis, skewness, and histograms. Skewed data for three groups was compared with the Kruskal-Wallis test. Subgroup analysis in three groups was performed with pairwise comparisons by Dunn test. Parameters with homogeneous distribution were compared with the chi-square test. Data are presented as %, for categorical variables or as median (Q1-Q3) for continuous variables.
Detection rates for blood and urinary metal levels were calculated. For urine metal levels, all statistical analyses were performed with creatinine-adjusted metal concentrations.
Urine to blood ratio was calculated for all metal levels. Spearman correlation coefficient was used to find the association between blood and urine metal levels of individual metals as well as for the association between different metals both in blood and urine. Correlation of blood and urine As with GFR was also performed.
We performed multinominal regression analysis for significantly different metal level in CKDu cases with respect to CKD and healthy controls. We included age and gender (confounding factors) in the model to see the y independence of association and effect estimate of the factor associated with CKDu. Regression model matrices and goodness-of-fit were also determined by the pseudo R2 coefficient and Hosmer-Lemeshow goodness-of-fit test.
For all analyses, we have considered a p-value less than 0.05 as statistically significant.
Results
A total of 568 patients who visited Nephrology OPD during the study period were screened for inclusion in the study. Out of these, 66 CKDu and 70 CKD cases were found eligible to enroll in the study. Eight patients withdrew consent in the CKD group, whereas four patients in the CKDu group had uncontrolled blood pressure with a single drug, and two withdrew consent. Finally, 60 CKDu and 62 CKD cases were included in the study for outcome analysis. We have approached 120 relatives of patients attending other OPDs and screened them for eligibility criteria of the healthy control group. Out of these, 60 were eligible, and 54 provided consent for participation in the study.
Demography and lab parameters
The CKD and CKDu subjects were similar in demographics for age and sex. However, healthy subjects were younger (Table 1). There was no significant difference between CKDu and CKD with reference to stage V (32 vs. 44, P-0.107).There were 05 diabetic kidney disease 04 CKD due to secondary glomerular disease patients (3-lupus nephritis, 1-FSGS), 12 hypertension-associated renal disease, 01 ADPKD, 36 Chronic glomerulonephritis patients, and 04 Chronic pyelonephritis patients in the CKD group. Use of smoking, Alcohol, and painkillers was similar across the three groups (Table 1). A significant difference was found between the three study groups with respect to the source of drinking water (ground or surface water). A significantly higher number of CKDu subjects used surface water as a source of drinking water (Table 1 and Table-S1 and Fig-S1) and a higher number of CKDu subjects reported pesticide usage. As shown in Table 1, blood pressures were significantly higher in CKD subjects compared to CKDu and healthy subjects and reflect the inclusion criteria with appropriate patient inclusion in three groups. Both ACR and PCR were also significantly different between CKD and CKDu. The eGFR was calculated based on the CKD-EPI formula and was not significantly different between the CKD and CKDu subjects, however, CKD subjects had lower median eGFR compared to CKDu subjects. The healthy subjects had significantly higher eGFR compared to both groups. HbA1c, were similar across the three groups (Table 1).
Table 1.
Demography and lab parameters
| Population Characteristics | CKD, N = 62 | CKDu, N = 60 | Healthy, N = 54 | P-value | P-value for Pair-wise comparisons by Dunn’s test | ||
|---|---|---|---|---|---|---|---|
| CKD vs. CKDu | CKD vs. Healthy | CKDu vs. Healthy | |||||
| Age (Years) | 43.0 (32.0, 52.0) | 44.0 (36.0, 50.0) | 31.0 (27.0, 39.8) | < 0.001* | 0.7 | ||
| Male | 29 (46.8) | 26 (43.3) | 33 (61.1) | 0.136* | |||
| Female | 33 (53.2) | 34 (56.7) | 21 (38.9) | ||||
| SBP (mmHg) | 142.0 (138.0, 154.0) | 138.0 (124.0, 144.0) | 125.0 (116.8, 132.0) | < 0.001** | < 0.001 | < 0.001 | < 0.001 |
| DBP (mmHg) | 89.5 (83.0, 97.8) | 89.0 (80.0, 94.0) | 86.0 (73.2, 89.0) | < 0.001** | 0.11 | < 0.001 | 0.015 |
| Smoking (yes) | 5 (8.2%) | 2 (3.4%) | 4 (7.4%) | 0.4# | 0.5 | 0.7 | 0.3 |
| Alcohol use (yes) | 4 (6.6%) | 7 (12%) | 3 (5.6%) | 0.4# | 0.3 | 0.8 | 0.2 |
| Painkillers use(yes) | 1 (1.6%) | 6 (10%) | 1 (1.9%) | 0.08# | 0.028 | > 0.9 | 0.037 |
| Pesticide use(yes) | 7 (12%) | 12 (20%) | 1 (1.9%) | 0.01# | 0.15 | 0.1 | 0.002 |
| Source drinking water (Surface water) | 15(24) | 25(42) | 10(18) | 0.016* | 0.04 | 0.458 | 0.007 |
| ACR1st (mcg/mg) | 1,259.4 (487.0, 3,253.6) | 321.9 (56.4, 611.6) | 13.7 (6.8, 17.4) | < 0.001** | < 0.001 | < 0.001 | 0.1 |
| ACR2nd (mcg/mg) | 988.1 (370.5, 2,314.0) | 315.8 (98.9, 584.0) | 15.0 (8.8, 19.2) | < 0.001** | < 0.001 | < 0.001 | 0.024 |
| PCR (gm/gm) | 3.1 (2.0, 5.8) | 1.1 (0.4, 1.8) | 0.040 (0.020, 0.075) | < 0.001** | 0.00 | 0.00 | 0.00 |
| HbA1C (pct) | 5.4 (5.1, 5.7) | 5.4 (5.1, 5.8) | 5.8 (5.2, 6.0) | 0.12** | > 0.9 | 0.4 | 0.12 |
| Sr.Creatinine (mg/dL) | 6.1 (3.5, 10.7) | 3.8 (1.9, 7.2) | 0.8 (0.7, 0.9) | < 0.001** | 0.002 | < 0.001 | < 0.001 |
| eGFR (ml/min/1.73m2) | 9.0 (6.0, 17.0) | 14.5 (7.0, 34.2) | 109 (95.0,122.0) | < 0.001** | 0.08 | 0.000 | 0.000 |
Median (IQR); n (%) p-values for three group comparison without any superscript was obtained by Kruskal-Wallis rank sum test ** for three group comparison; *Pearson’s Chi-squared test; #Fisher Freeman Halton test CKD-chronic kidney disease, CKDu Chronic kidney disease of unknown cause, SBP Systolic blood pressure, DBP Diastolic blood pressure, ACR_1st Albumin creatinine ratio on 1st visit, ACR_2nd Albumin creatinine ratio on 2nd visit, Sr Serum, eGFR Estimated glomerular filtration rate, PCR Protein creatinine ratio
Analytical results
The urinary and blood levels of As, Cd, Pb, and Cr (Table 2) were measured in ppb (micrograms per litre), and median with interquartile ranges were reported. Urinary metal levels were also measured in ppb (micrograms per liter) and then adjusted for urinary dilution by urine creatinine value and were finally expressed as micrograms/grams of urine creatinine (Table 2).
Table 2.
Heavy metal levels comparison in different groups
| Exposures (ppb) | CKD, N = 62 | CKDu, N = 60 | Healthy, N = 54 | P value | P-vale for Pair-wise comparisons by Dunn’s test | ||
|---|---|---|---|---|---|---|---|
| CKD vs. CKDu | CKD vs. Healthy | CKDu vs. Healthy | |||||
| As DL (mcg/Lt) | 0.191 ppb | ||||||
| U/B ratio | 0.13 (0.00–0.80) | 0.73 (0.00–32.37) | 2.2 (0.86–70.1) | ||||
| Blood_As (mcg/Lt)a | 4.5 (0.0, 58.8) | 91.97 (1.3, 132.7) | 39.01 (4.8, 67.4) | < 0.001* | 0.001 | 0.006 | 0.005 |
| % above DL | 64.2 | 78.3 | 81.2 | < 0.001# | |||
| Urine As (mcg/gm)a | 17.1 (7.7, 29.9) | 11.1 (2.8, 22.1) | 97.4 (52.4, 152.5) | < 0.001* | 0.4 | < 0.001 | < 0.001 |
| % above DL | 94 | 83.4 | 100 | < 0.001# | |||
| Cd DL (mcg/Lt) | 0.0 | ||||||
| U/B ratio | 0.09 (0.04-.17) | 0.10 (0.06–0.21) | 0.03 (0.01–0.14) | ||||
| Blood_Cd (mcg/Lt)a | 10.9 (3.4, 14.0) | 9.8 (0.7, 15.2) | 2.7 (1.6, 3.8) | < 0.001* | 0.5 | < 0.001 | < 0.001 |
| % above DL | 91.9 | 91.6 | 100 | < 0.001# | |||
| Urine_Cd (mcg/gm)a | 1.0 (0.3, 1.7) | 0.5 (0.0, 1.5) | 0.0 (0.0, 0.1) | < 0.001* | 0.063 | < 0.001 | < 0.001 |
| % above DL | 80.5 | 62.8 | 24.1 | < 0.001# | |||
| Lead DL (mcg/Lt) | 0.822 | ||||||
| U/B ratio | 2.13 (0.38–5.2) | 1.6 (0.49–3.47) | 0.06 (0.04–0.27) | ||||
| Blood_Pb (mcg/Lt)a | 54.9 (7.1, 77.6) | 27.1 (4.0, 66.3) | 10.9 (0.0, 30.0) | < 0.001* | 0.14 | < 0.001 | 0.006 |
| % above DL | 80 | 85 | 74.1 | 0.510# | |||
| Urine_Pb (mcg/gm)a | 70.5 (17.6, 162.2) | 29.0 (1.1, 142.0) | 0.0 (0.0, 1.1) | < 0.001* | 0.5 | < 0.001 | < 0.001 |
| % above DL | 80.6 | 76.7 | 29.6 | < 0.001# | |||
| Chromium DL (mcg/Lt) | 3.156 | ||||||
| U/B ratio | 0.13 (0.04–0.18) | 0.13 (0.07–0.22) | 0.03 (0.01–0.05) | ||||
| Blood Cr (mcg/Lt)a | 477.2 (253.5, 597.4) | 455.0 (156.4, 629.2) | 221.4 (177.5, 268.6) | < 0.001* | 0.4 | < 0.001 | < 0.001 |
| % above DL | 100 | 86.7 | 100 | < 0.001# | |||
| Urine Cr (mcg/gm)a | 59.2 (21.7, 82.8) | 34.7 (6.3, 82.0) | 0.0 (0.0, 0.0) | < 0.001* | 0.5 | < 0.001 | < 0.001 |
| % above DL | 85.5 | 86.7 | 14.8 | < 0.001# | |||
aMedian (IQR); n (%) p-values for three group comparison without any superscript was obtained by Kruskal-Wallis rank sum test * for three group comparison. #Pearson’s Chi-squared test. DL Detection limit, U/B Ratio-urine blood ratio. All values blood metals in ppb (parts per billion or micrograms/liter). All values of urinary metals in microgram/gm). CKD Chronic kidney disease, CKDu Chronic kidney disease of unknown cause, As Arsenic, Cd Cadmium, Pb Lead, Cr Chromium
Detection limits
The lowest detectable concentrations of various heavy metals analyzed on ICP OES with a signal-to-noise ratio of 1 were as follows: As (193.759 nm) - 0.191 ppb; Cd (214.438 nm) - 0 ppb; Pb (220.353 nm) - 0.822 ppb; Cr (283.563 nm) - 3.156 ppb (Table 2, Figs-S2-S5).
Detection percentage
The number of subjects with blood and urine metal levels above the respective detection limits in each study group is reported in Table 2.
Urine to blood ratio
A urine/blood ratio for each metal in all study groups was calculated for patients with metal levels above the detection limit. The distribution of urine/blood ratios for all metals is presented in Table 2. Ratios were different between healthy and subjects with deranged kidney functions i.e. low GFR (CKD and CKDu). Median urine/blood Ratio for As was > 1 in healthy subjects and < 1 in CKD and CKDu, reflecting higher urinary levels compared to blood in healthy and reverse in CKD and CKDu subjects. For Pb, it was < 1 in healthy subjects and > 1 in subjects with CKD and CKDu, reflecting higher blood levels compared to urine in healthy and reverse in CKD and CKDu subjects. For Cd and Cr the ratio were < 1 across all three groups suggesting higher urine levels compared to blood levels.
Correlation
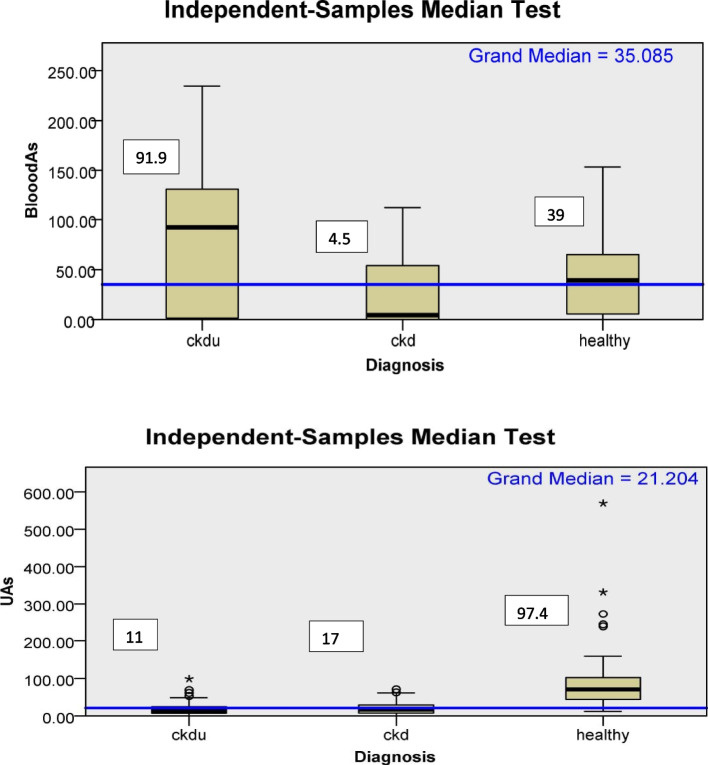
A spearman correlation (ρ) was also performed to see the association between each urine and blood metal and among the metals with each other as well. In CKDu, UAs were negatively associated with BAs (ρ-0.260, p-0.11) and in CKD positively (0.138, p-0.37). There was a positive association between urine and blood levels of As,Pb, and Cr and negative association of urine and blood Cd in CKD. In CKDu, a positive association was found in blood and urine Cd,Pb and Cr. In addition, there was a strong correlation of blood Cd, Pb, and Cr (p < 0.01) [ρ = 0.68 (BCd and BPb), 0.88 (BCd and BCr), 0.71 (BPb and BCr) in CKDu and [ρ = 0.55 (BCd and BPb), 0.82 (BCd and BCr), 0.65 (BPb and BCr) in CKD. The Urine Cd, Pb, and Cr also had strong correlations [ρ = 0.33 (UCd and UPb), and 0.48(UPb and UCr)] in CKD and [ρ = 0.19(UCd and UPb), 0.67 (UCd and UCr), and 0.69 (UPb and UCr)] in CKDu < 0.05 (Table-S2-S4 and Fig-S6). Association of Blood and urine As with GFR was also evaluated, and BAs were found to be negatively associated with GFR (ρ = -0.097, p = 0.56), whereas UAs were positively associated (ρ = 0.14, p = 0.25) with GFR (Table-S5). Metal levels: Blood As: was significantly higher in CKDu (n = 37) subjects compared to CKD (n = 41) and healthy (n = 53) subjects (Table 2). On the other hand, the urinary As (UAs) was significantly low in CKD (n = 50) and CKDu (n = 48) subjects compared to healthy subjects (n = 38) and was non significantly higher in CKD subjects compared to CKDu subjects (Fig. 1, Table 2).The blood and urine As values were below detection limits in 21.6%, 35.7%, and 18.8% and in 6%, 16.6%, and 0% of subjects in CKDu, CKD, and healthy groups, respectively.
Fig. 1.
Box plot for distribution of blood and urine arsenic according to diagnosis categories. Median; microgram/Lt (blood); microgram/gm(urine); UAs- Urine arsenic;CKDu-Chronic kidney disease of unknown cause; CKD-Chronic kidney disease
Cadmium
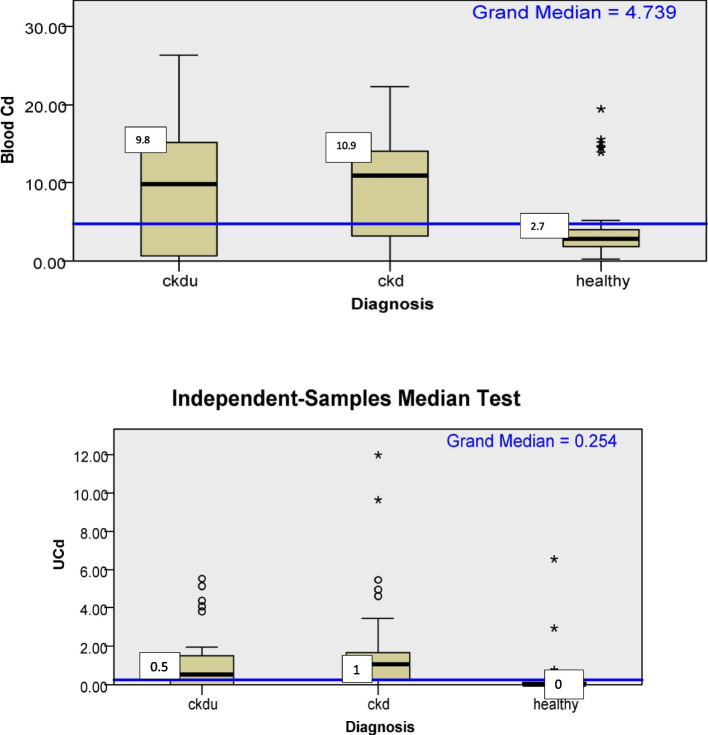
Blood Cd also was significantly higher in CKD and CKDu subjects compared to healthy subjects. Urinary Cd (UCd) levels were significantly higher in CKD and CKDu subjects compared to healthy subjects,. There was a weak association of (p = 0.06) UCd with CKD subjects compared to CKDu subjects.UCd was higher in CKD subjects compared to CKDu (Fig. 2, Table 2). The blood and urine Cd values were below detection limits in 8.3%, 8.1%, and 0% and 37.2%, 19.3%, and 75.9% of subjects in CKDu, CKD, and healthy groups, respectively.
Fig. 2.
Box plot for distribution of blood and urine cadmium according to diagnosis categories. Median, microgram/Lt (blood); micrograms/gm (urine); UCd- Urine cadmium;CKDu-Chronic kidney disease of unknown cause; CKD-Chronic kidney disease
Lead
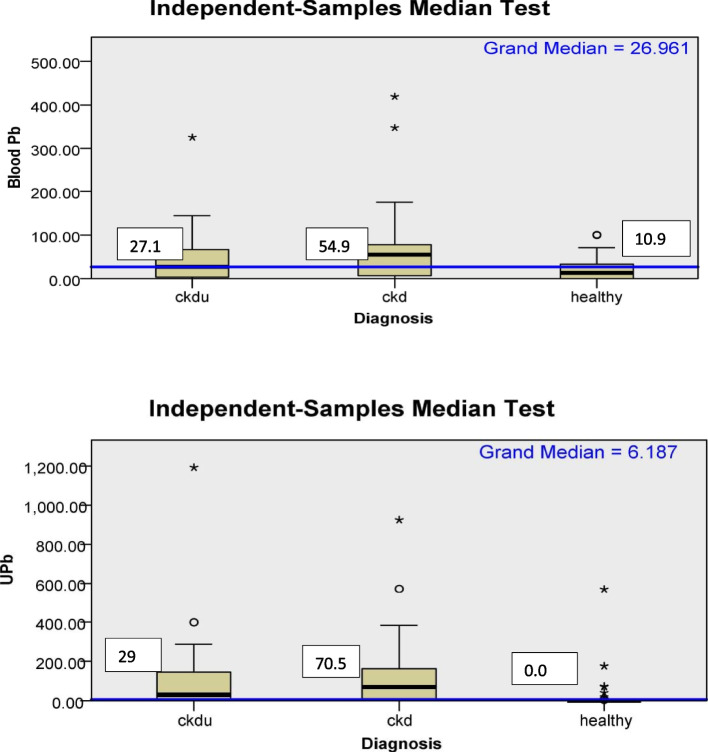
Pb levels in the blood of CKD and CKDu as well as in urine of CKD and CKDu subjects were significantly higher compared to healthy subjects. The Pb levels were higher in CKD subjects compared to CKDu subjects, but it was not statistically significant (Fig. 3, Table 2). The blood and urine Pb values were below detection limits in 15%, 20%, and 25.9% and 23.3%, 19.4% and 70.4% of subjects in CKDu, CKD, and healthy groups, respectively.
Fig. 3.
Box plot for distribution of blood and urine lead according to diagnosis categories. microgram/Lt (blood); microgram/gm(urine); UPb- Urine lead;CKDu-Chronic kidney disease of unknown cause; CKD-Chronic kidney disease
Chromium
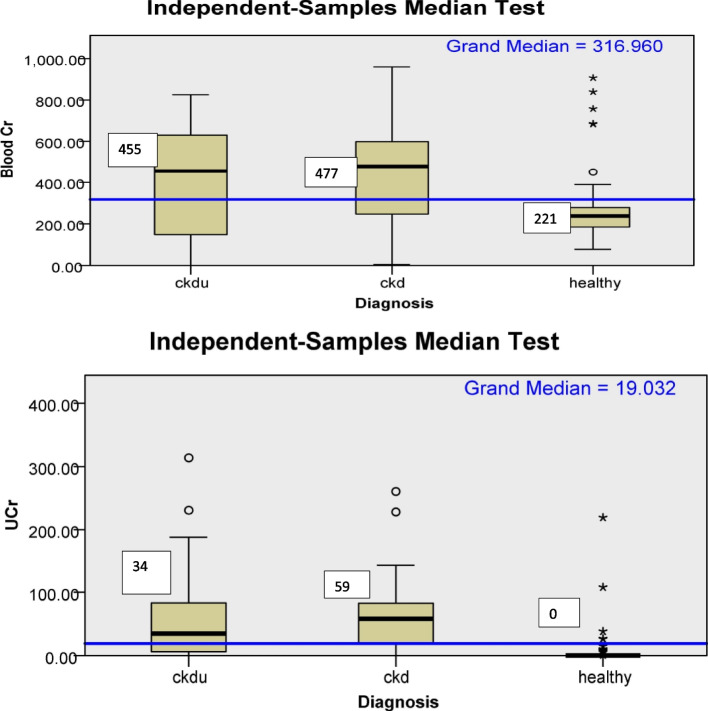
As shown in Table 2 and Fig. 4, urinary and blood Cr was significantly higher in CKD, and CKDu patients than healthy subjects. The blood and urine Cr values were below detection limits in 13%, 0%, and 0% and 13.3%, 14.5% and 85.2% of subjects in CKDu, CKD, and healthy groups, respectively.
Fig. 4.
Box plot for distribution of blood and urine chromium according to diagnosis categories. Median; microgram/Lt(blood); microgram/gm(urine); UCr-urine chromium, CKDu-Chronic kidney disease of unknown cause; CKD-Chronic kidney disease
Multinominal regression
Though age, gender, were not significantly different between CKDu and CKD, on univariate analysis, we included these In the multinominal regression analysis between CKDu and CKD in reference to healthy subjects in addition to factors found significantly different (p < 0.01 on univariate analysis) i.e. blood As and source of drinking water.After the final model, gender had no association with CKDu. Blood As, surface water as drinking water source and age were independently associated with CKDu. Age was associated independently with CKD also (Table 3).
Table 3.
Multinominal regression
| Diagnosis | Predictors | Odds ratio | CI | P value |
|---|---|---|---|---|
| CKDu | Age | 1.104 | 1.055–1.156 | 0.000 |
| Gender | 0.964 | 0.351–2.652 | 0.944 | |
| Blood As | 1.013 | 1.003–1.024 | 0.014 | |
| Surface water use | 3.178 | 1.029–9.818 | 0.045 | |
| CKD | Age | 1.053 | 1.013–1.094 | 0.010 |
| Gender | 0.208 | |||
| Blood As | 0.988 | 0.977–1.000 | 0.041 | |
| Surface water use | 1.320 | 1.320–0.456 | 0.609 | |
| Goodness of fit | Chi-square 0.256 | |||
| Pseudo R2 | Cox snell-0.340 | |||
Discussion
To the best of our knowledge, this is the first study wherein an attempt has been made to analyze the association of heavy metals with CKDu in central India, using blood and urine levels as biomarkers of metal exposure. In addition, CKD and healthy subjects have been used as control groups.
The current study showed that blood and urine creatinine-adjusted urinary levels of heavy metals Cd, Pb and Cr were significantly higher in patients with CKD and CKDu as compared to healthy subjects. The urinary levels of the above metals were undetectable in healthy subjects. The study also showed a weak association of (p = < 0.06) higher urinary Cd in CKD subjects compared to CKDu subjects of this Indian cohort.
The study also showed that Blood As was significantly higher in CKDu subjects compared to CKD and healthy subjects. On multinominalregression, blood As was independently (p < 0.05) associated with CKDu after age adjustment.
In our study, median GFR was rather high in CKDu subjects [14.5 (7.0, 34.2)] compared to GFR in CKD subjects [9.0 (6.0, 17.0)ml/min/1.73m2)] and it was non significantly different between the two groups. On correlation analysis, there was a negative correlation between Blood As and GFR and a positive correlation of urine As with GFR. Based on this, the higher blood As in CKDu with higher GFR appears to be truly elevated.
Previously a study from Sri Lanka has also reported an association of CKDu with chronic As toxicity. In that study, 48% of CKDu patients and 17.4% of the control subjects fulfilled the criteria to be diagnosed with chronic arsenical toxicity(CAT), indicating the potential link between CAT and CKDu and suggesting agrochemicals could be the possible source [14]. Later, it was reported that glyphosate was the most widely used pesticide in Sri Lanka, which contains an average of 1.9 mg/kg arsenic. Findings suggest that agrochemicals, especially phosphate fertilizers, are a major source of inorganic arsenic in CKDu endemic areas [15]. However, another study from Sri Lanka did not find any difference in UAs levels in patients of CKDu in endemic areas and controls from endemic and nonendemic areas [4].
Some other studies have reported associations of As with CKD. A study from Taiwan found total UAs to be associated with a four-fold risk of CKD [6]. Another study reported an association of MMAV (mono methyl arsenate pentavalent) and DMAV (dimethyl arsenate pentavalent) in urine with prevalence of CKD [16]. However, in both studies, the type of CKD was not reported.
The higher blood As in CKDu compared to CKD may be associated with exposures in our study; a significantly higher number of subjects in CKDu group reported use of pesticides, surface water as a source of drinking water in CKDu subjects.On regression analysis also, surface water was independently associated with CKDu.
A study from north India reported increased levels of OCPs, namely α-HCH, aldrin, and β-endosulfan, in CKDu patients as compared to healthy control and CKD patients of known etiology [17] and it is also known that arsenic is an important component of pesticides [18]. The contamination of surface water with various pollutants i.e. pesticides, is common [19]. Arsenic is a known nephrotoxin, and one of the case reports where kidney histopathology was evaluated reported As causes tubulointerstitial disease (TID) [20]. The difference in methylation processes of As has also been found responsible for various diseases associated with As i.e. for example, high proportions of urinary MMAs (%U-MMAs) have been associated with a higher risk of cancers and skin lesions [21]. In contrast, high %U-DMAs has been associated with diabetes risk [22]. We have measured only iAs in our study. Whether methylation resulting in various metabolite species has different associations with CKDu or CKD should be explored further. We recently found a significant association of single nucleotide polymorphism in a gene coding for sodium-dependent dicarboxylate transporter (SLC13A3) with the susceptibility to CKDu [23].
In the current study, the UAs results suggest that As levels of 97 µg/gm of creatinine in healthy subjects were not associated with decreased GFR or proteinuria. Similar results were reported by a study from China where researchers found a lower confidence limit on the benchmark dose (LBMD) of 102 and 0.88 µg/gm creatinine for As and Cd, respectively, in order to prevent renal damage in the general population co-exposed to arsenic and cadmium [24]. The UAs in healthy subjects in our study were nearly similar to the LBMD reference and, not surprisingly, not to be associated with CKD or proteinuria.
Some studies have reported lead to be associated with CKDu. An Indian study reported high levels of lead and silicon concentrations in Indian groundwater in the endemic Uddanam area [7]. Jaysuman et al. also reported higher levels of Pb (26.5 µg/gm) in the urine of patients with Sri Lankan agricultural nephropathy compared to endemic and nonendemic control [25].
In the current study, although the median level of blood Pb was almost double in CKD patients compared to CKDu, the result was not statistically significant.
Our study showed that Cd was significantly associated with renal disease. Blood Cd and urine Cd (UCd) levels were significantly higher in patients with renal disease (CKD and CKDu) as compared to healthy subjects. The findings of UCd also showed a weak association (p-0.06) of Cd with CKD compared to CKDu among patients with renal diseases. There are some concerns that UCd may not be truly reflective of metal burden in patients with advanced CKD [26], because initially, in the course of Cd toxicity with early tubular damage, the normal reabsorption of cadmium-metallothionein decreases, and the UCd concentration increases. However, in the long run, cadmium-induced kidney damage gives rise to low Cd concentrations in both the kidney and urine, while the tubular damage remains [27]. The U/B ratio of < 1 for Cd in our study supports the above findings.
The mean eGFR in our CKD cohort was lower compared to CKDu; despite this, higher UCd values in patients with CKD compared to CKDu in our study indicate a potential association of Cd with CKD.
Studies have reported variable association of Cd with CKDu when compared to healthy subjects. Nanayakkara et al. [28] did not find an association of UCd with CKDu in stages 1–4 compared to healthy controls. Whereas another Sri Lankan [4] study found significantly high UCd in patients with CKDu against the endemic and nonendemic controls. We also observed significantly higher UCd in CKDu vs. healthy controls.
In the current study, urinary Cr (UCr) was not detected in healthy subjects, whereas it was significantly higher in patients with CKD and CKDu as compared to healthy subjects. UCr levels were higher in CKD compared to CKDu. Epidemiologically, Cr exposure has been reported to be associated with kidney damage in occupational populations [26]. Recently, a study from Taiwan reported that a significant and independent association between Cr exposure and decreased renal function in the general population, and co-exposure to Cr with Pb and Cd is potentially associated with an additional decline in the GFR in Taiwanese adults [27]. A study from Bangladesh reported outcomes similar to our study; however, the study included only CKD (n = 30) patients and compared them with healthy subjects (n = 20). In that study, compared to the controls, CKD patients exhibited significantly higher levels of Pb, Cd, and Cr levels in their urine samples. This signifies a potential association between heavy metal co-exposure and CKD [29]. In the current study a significant correlation between blood Cd, Pb, and Cr and urine Cd, Pb, and Cr were found in CKDu and CKD subjects compared to healthy subjects. The levels of UCd, UPb, and UCr in CKD and CKDu patients were significantly higher compared to healthy controls; The possibility of the combined effect of Cd, Pb, and Cr in the causation of renal diseases could be evaluated further in future studies. As CKDu is an endemic disease, the results of our study suggest an association of arsenic with CKDu in the Indian population, and so the generalizability of the result should be used with caution.
Strengths and limitations
This is the first study which has included two controls (CKD and healthy) and compared metal levels in patients with CKDu. In addition, the comparison of metals in both blood and urine is another advantage, as falling GFR levels and urine levels of several metals do not reflect true metal burden in patients. Inclusion of CKDu patients, as per the suggested definition by the Indian society of Nephrology, is another strength of our study.
The small sample size of our study may be a limitation of our study though it was calculated scientifically. The study involved Indian patients and controls only so the generalization of the results should be with caution. Healthy controls were of younger age is also a limitation of the study.
Also the study included patients from central India, comparatively a larger area and does not points out endemicity.
Conclusion
The study finds an association of environmental toxins with CKDu and CKD. The age and sex-adjusted As were observed to have an independent association with CKDu. A weak association of Cd with CKD was also observed in this Indian cohort. Subjects with renal dysfunction (CKDu and CKD) were observed to have a significantly higher metal burden of Pb, Cd, As, and Cr as compared to healthy controls. CKDu patients may have higher exposure to As via pesticides, surface water usage, or both.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- CKDu
Chronic Kidney Disease of unknown cause
- CKD
Chronic Kidney Disease
- As
Arsenic
- Cd
Cadmium
- Pb
Lead
- Cr
Chromium
- KDIGO
Kidney Disease Improving Global Outcomes
- GFR
Glomerular filteration rate
- IHEC
Institutional Human Ethics committee
- HNO3
Concentrated trace metal grade nitric acid
- H2O2
Hydrogen peroxide
- PTFE-TFM
High-purity polytetrafluoroethylene
- UAs
Urinary As
- UCd
Urinary Cd
- UPb
Urine Pb
- UCr
Urinary Cr
- iAs
Inorganic As
- TID
Tubulo-interstitial disease
- MMA V
Pentavalent monomethylarsonic acid
- DMA V
Pentavalent dimethylarsinic acid
- MAs III
Methylarsenous acid
- SLC13A3
Sodium-dependent dicarboxylate transporter
- LBMD
Limit on the benchmark dose
- AsB
Arsenobetaine
- U-MMAs
Urinary MMAs
- U-DMAs
Urinary DMAs
- BLL
Blood lead levels
- ESKD
End-stage kidney disease
Authors’ contributions
MA and AK were equally involved in conceptualising the study, winning the grant, analyzing the results, monitoring the progress of study. MA prepared the manuscript. AK and SKG did the editing. RA supervised analysis of metal levels, sample collection done by MMN, RK. Metal analysis done by AA. Data entry and file preparation for results done by MMN, AA, SKV and STR.
Funding
The Study was funded by Indian council of Medical Research (ICMR),New Delhi, India. Sanction no.:5/4/7-14/2019-NCD-II.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Human Ethics Committee (IHEC) approval No- EF0125. The study was performed according to the guidelines of the Declaration of Helsinki. All participants gave informed consent for participation before enrolment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mahendra Atlani and Ashok Kumar contributed equally to this work.
Contributor Information
Mahendra Atlani, Email: mkatlani9@gmail.com, Email: mahendra.nephro@aiimsbhopal.edu.in.
Ashok Kumar, Email: ashok.biochemistry@aiimsbhopal.edu.in.
References
- 1.Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011). 2013;3(1):19–62. 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed]
- 2.Gifford FJ, Gifford RM, Eddleston M, Dhaun N. Endemic nephropathy around the world. Kidney Int Rep. 2017;2(2):282–292. doi: 10.1016/j.ekir.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanayakkara S, Senevirathna S, Parahitiyawa NB, et al. Whole-exome sequencing reveals genetic variants associated with chronic kidney disease characterized by tubulointerstitial damages in North Central Region, Sri Lanka. Environ Health Prev Med. 2015;20(5):354–359. doi: 10.1007/s12199-015-0475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayatilake N, Mendis S, Maheepala P, Mehta FR. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14(1):180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayasumana C, Gunatilake S, Senanayake P. Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health. 2014;11(2):2125–2147. doi: 10.3390/ijerph110202125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bawaskar HS, HimmatraoBawaskar P, Himmatrao BP. Chronic renal failure associated with heavy metal contamination of drinking water: a clinical report from a small village in Maharashtra. Clin Toxicol. 2010;48(7):768–768. doi: 10.3109/15563650.2010.497763. [DOI] [PubMed] [Google Scholar]
- 7.Lal K, Sehgal M, Gupta V, et al. Assessment of groundwater quality of CKDu affected Uddanam region in Srikakulam district and across Andhra Pradesh, India. Groundw Sustain Dev. 2020;11:100432. doi: 10.1016/j.gsd.2020.100432. [DOI] [Google Scholar]
- 8.Zhang X, Cornelis R, de Kimpe J, et al. Accumulation of arsenic species in serum of patients with chronic renal disease. Clin Chem. 1996;42(8):1231–1237. doi: 10.1093/clinchem/42.8.1231. [DOI] [PubMed] [Google Scholar]
- 9.Bommarito PA, Beck R, Douillet C, et al. Evaluation of plasma arsenicals as potential biomarkers of exposure to inorganic arsenic. J Expo Sci Environ Epidemiol. 2019;29(5):718–729. doi: 10.1038/s41370-019-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Environmental Health Criteria 165. Joint sponsorship of The United Nations Environment Programme, The International Labour Organization, and The World Health Organization. 1995. https://apps.who.int/iris/bitstream/handle/10665/37241/9241571659-eng.pdf?sequence=1. Accessed 10 Nov 2022.
- 11.Ashrap P, Watkins DJ, Mukherjee B, et al. Predictors of urinary and blood Metal(loid) concentrations among pregnant women in Northern Puerto Rico. Environ Res. 2020;183:109178. doi: 10.1016/j.envres.2020.109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anupama Y, Sankarasubbaiyan S, Taduri G. Chronic kidney disease of unknown etiology: case definition for India – a perspective. Indian J Nephrol. 2019 doi: 10.4103/ijn.IJN_327_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasumana MACS, Paranagama PA, Amarasinghe KMRC, et al. Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J Nat Sci Res. 2013;3(1). www.iiste.org ISSN 2224-3186 (Paper) ISSN 2225-0921 (Online).
- 15.Jayasumana C, Fonseka S, Fernando A, et al. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. Springerplus. 2015;4:90. doi: 10.1186/s40064-015-0868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng LY, Umans JG, Yeh F, et al. The association of urine arsenic with prevalent and incident chronic kidney disease. Epidemiology. 2015;26(4):601–612. doi: 10.1097/EDE.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh R, Siddarth M, Singh N, et al. Organochlorine pesticide level in patients with chronic kidney disease of unknown etiology and its association with renal function. Environ Health Prev Med. 2017;22(1):49. doi: 10.1186/s12199-017-0660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Ye F, Wang A, et al. Chronic arsenic poisoning probably caused by arsenic-based pesticides: findings from an investigation study of a household. Int J Environ Res Public Health. 2016;13(1):133. doi: 10.3390/ijerph13010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal A, Pandey R, Sharma B. Water pollution with special reference to pesticide contamination in India. J Water Resource Prot. 2010;2(5):432–48. 10.4236/jwarp.2010.25050.
- 20.Prasad GVR, Rossi NF. Arsenic intoxication associated with tubulointerstitial nephritis. Am J Kidney Dis. 1995;26(2):373–376. doi: 10.1016/0272-6386(95)90660-6. [DOI] [PubMed] [Google Scholar]
- 21.Kuo CC, Moon KA, Wang SL, Silbergeld E, Navas-Acien A. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: a systematic review of the epidemiological evidence. Environ Health Perspect. 2017;125(8):087001. doi: 10.1289/EHP577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.del Razo LM, García-Vargas GG, Valenzuela OL, et al. Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: a cross-sectional study in the Zimapán and Lagunera regions in Mexico. Environ Health. 2011;10(1):73. doi: 10.1186/1476-069X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumari R, Tiwari S, Atlani M, et al. Association of single nucleotide polymorphisms in KCNA10 and SLC13A3 genes with the susceptibility to chronic kidney disease of unknown etiology in central Indian patients [published online ahead of print, 2023 Jan 25]. Biochem Genet. 2023. 10.1007/s10528-023-10335-7. [DOI] [PubMed]
- 24.Hong F, Jin T, Zhang A. Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals. 2004;17(5):573–580. doi: 10.1023/B:BIOM.0000045741.22924.d. [DOI] [PubMed] [Google Scholar]
- 25.Jayasumana C, Gunatilake S, Siribaddana S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015;16(1):103. doi: 10.1186/s12882-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilbur S, Abadin H, Fay M, et al. Toxicological profile for chromium. Atlanta: Agency for Toxic Substances and Disease Registry (US); 2012. [PubMed] [Google Scholar]
- 27.Tsai TL, Kuo CC, Pan WH, et al. The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int. 2017;92(3):710–720. doi: 10.1016/j.kint.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Nanayakkara S, Senevirathna STMLD, Karunaratne U, et al. evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: a cross-sectional study. Environ Health Prev Med. 2012;17(2):109–117. doi: 10.1007/s12199-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhury TR, Zaman SKZ, Chowdhury TI, Begum BA, Islam MDA, Rahman MDM. Status of metals in serum and urine samples of chronic kidney disease patients in a rural area of Bangladesh: an observational study. Heliyon. 2021;7(11):e08382. doi: 10.1016/j.heliyon.2021.e08382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.