ABSTRACT
Primaquine (PQ) is the main drug used to eliminate dormant liver stages and prevent relapses in Plasmodium vivax malaria. It also has an effect on the gametocytes of Plasmodium falciparum; however, it is unclear to what extent PQ affects P. vivax gametocytes. PQ metabolism involves multiple enzymes, including the highly polymorphic CYP2D6 and the cytochrome P450 reductase (CPR). Since genetic variability can impact drug metabolism, we conducted an evaluation of the effect of CYP2D6 and CPR variants on PQ gametocytocidal activity in 100 subjects with P. vivax malaria. To determine gametocyte density, we measured the levels of pvs25 transcripts in samples taken before treatment (D0) and 72 hours after treatment (D3). Generalized estimating equations (GEEs) were used to examine the effects of enzyme variants on gametocyte densities, adjusting for potential confounding factors. Linear regression models were adjusted to explore the predictors of PQ blood levels measured on D3. Individuals with the CPR mutation showed a smaller decrease in gametocyte transcript levels on D3 compared to those without the mutation (P = 0.02, by GEE). Consistent with this, higher PQ blood levels on D3 were associated with a lower reduction in pvs25 transcripts. Based on our findings, the CPR variant plays a role in the persistence of gametocyte density in P. vivax malaria. Conceptually, our work points to pharmacogenetics as a non-negligible factor to define potential host reservoirs with the propensity to contribute to transmission in the first days of CQ–PQ treatment, particularly in settings and seasons of high Anopheles human-biting rates.
KEYWORDS: malaria, Plasmodium vivax, gametocytes, primaquine, pharmacogenetics, cytochrome P450 reductase, CYP2D6
INTRODUCTION
Plasmodium vivax is the most prevalent malaria species in the Americas, responsible for approximately 89% of the 160,000 cases annually reported in Brazil in the last 4 years (1). The highest incidence rates occur in the Legal Amazon region, which corresponds to 59% of the Brazilian territory in the country’s northern region (2).
During the malaria parasite’s life cycle, the sexual blood stage gametocytes link the vertebrate host and the vector, making them prime targets for intervention strategies aiming at blocking transmission. The lifespan of gametocytes is reduced by the action of primaquine (PQ) in infections caused by P. falciparum (3–5). Similarly, the gametocyte clearance time is significantly shorter when PQ is added to chloroquine to treat patients with P. vivax malaria (6). In addition, children who received PQ treatment in Papua New Guinea had a lower chance of becoming positive for P. vivax gametocytes than those who did not receive PQ (7).
PQ is a tissue schizonticide that needs to be bio-transformed into an active metabolite, i.e., it represents a prodrug that needs to be metabolized to generate molecules that exert hypnozoiticidal and gametocytocidal activity (8, 9). Hypnozoites are latent stages of the parasite that remain dormant in hepatocytes, leading to recurrence of symptoms when active (10). The PQ metabolism is rapid after drug ingestion, reaching peak plasma levels within 2–3 hours and then rapidly declining with a terminal elimination half-life between 4 and 9 hours (11, 12).
The mechanism of action of PQ is still unclear, but the involvement of CYP2D6 in PQ metabolism is well-established (8, 9). Pharmacokinetic studies of PQ have demonstrated its metabolic dependence via the CYP2D6 pathway, indicating that the presence of polymorphisms in the gene can alter the rate of PQ metabolism (13–15). The CYP2D6 gene is highly polymorphic, with over 130 alleles associated with variable phenotypes ranging from complete dysfunction to ultra-rapid metabolism (16, 17). The decreased enzymatic function associated with diminished prodrug activation may lead to increased clinical recurrences of malaria as the hypnozoite clearance is compromised. The metabolism of PQ by CYP2D6 occurs in a joint action between the CYP system and its substrates, including the electrons provided by the cytochrome P450 reductase enzyme (CPR) (18, 19). Mutations present in or near flavin adenine dinucleotide/flavin mononucleotide (FAD/FMN) domains can result in severe effects on CPR activity, which may affect metabolic processes significantly dependent on CYPs (20, 21). The most common A503V CPR polymorphism, located near the FAD domain, defines the CPR*28 allele. It presents a moderate effect (40%–50%) on CYP2D6 activity, possibly contributing to genetic variability in drug and xenobiotic metabolism (22).
PQ is converted into hydroxylated metabolites (OH-PQm) with consequent hydrogen peroxide (H2O2) formation, which accumulates at sites of metabolic transformation, leading to antiparasitic action (23, 24). PQ activity against P. falciparum gametocytes is significantly enhanced by direct reduction of OH-PQm by the complex CPR (24). A few studies have addressed the direct impact of CYP2D6 enzyme activity on the gametocytocidal effect of PQ in malaria. A retrospective study on African populations treated with a single dose of PQ (0.1 to 0.75 mg/kg) combined with artemisinin-based combination therapy (ACT) showed longer P. falciparum gametocyte clearance after treatment of patients with impaired CYP2D6 activity (25). On the other hand, another clinical trial conducted in Tanzania demonstrated that single-dose PQ treatment was sufficient to reduce P. falciparum gametocyte levels regardless of the CYP2D6 status (26).
The association between the CPR/CYP2D6 complex and gametocyte clearance has been investigated only in P. falciparum infections (24). Here, we further explored the effects of polymorphisms in the CPR/CYP2D6 complex on gametocyte clearance of P. vivax by conducting a 3-day follow-up study of individuals with P. vivax malaria from the Brazilian Amazon region. We hypothesized that mutations in this enzymatic complex could diminish the effectiveness of PQ, resulting in a delay in gametocyte clearance.
RESULTS
One hundred adults with a median age of 33 years (IQR, 26–42) were enrolled (Table 1). Most of them were males who possibly became infected in gold mining areas in the north region of Brazil. They are found to frequently migrate due to mining activities. Almost 40% experienced at least one episode of P. vivax malaria recurrence in 6 months.
TABLE 1.
Demographic and genetic characteristics of subjects enrolled in this study
| Characteristics | Total population |
|---|---|
| Age, median (IQR, years) | 33 (26–41.5) |
| Gender, n (%) | |
| Male | 80 (80) |
| Female | 20 (20) |
| Possible area of infection, n (%) | |
| Gold mining | 63 (63) |
| Others | 37 (37) |
| Number of malaria recurrence episodes, n (%) | |
| 0 | 61 (61) |
| 1 | 23 (23) |
| >2 | 16 (16) |
| CYP2D6 activity score, n (%)a | |
| <1.0 | 30 (30) |
| >1.0 | 67 (67) |
| CPR A503V, n (%)b | |
| CC | 65 (65) |
| CT | 28 (28) |
| TT | 7 (7) |
Three participants were not included in the analyses due to an indetermined phenotype.
1508C > T variant.
All subjects contributed with two blood samples before drug treatment (D0) and 72 hours (D3) after a standard CQ and PQ treatment regime. They were successfully genotyped for polymorphisms in CYP2D6 (97%) and CPR (100%). Among them, 30% had impaired enzyme activity for CYP2D6 as inferred from genotype data (AS ≤1.0) (Table 1; Table S1). There was no association between recurrence and CYP2D6 activity [27 out of 67 (40%) had one or more episodes of recurrence in the AS >1.0 group versus 10 out of 30 (33%) in the AS ≤1.0 group, P = 0.514 by the chi-squared test]. Also, the time to the first recurrence was not influenced by the CYP2D6 status (P = 0.423, Kaplan–Meier log-rank test). However, it is important to consider that most individuals exerted activities related to gold mining, where malaria transmission is high. Thus, one could not exclude the possibility of an infection due to a new mosquito bite.
We also evaluated the amino acid substitution A503V in CPR, which has been previously shown to reduce CYP2D6 activity. Thirty-five subjects carried the mutated allele in homo- (7%) or heterozygosity (28%). The CPR status was not associated with the number of recurrence episodes of P. vivax malaria (34% of carriers of the mutated allele in homo- or heterozygosity vs 42% without mutation experienced P. vivax recurrences; P = 0.525, by Fisher’s exact test).
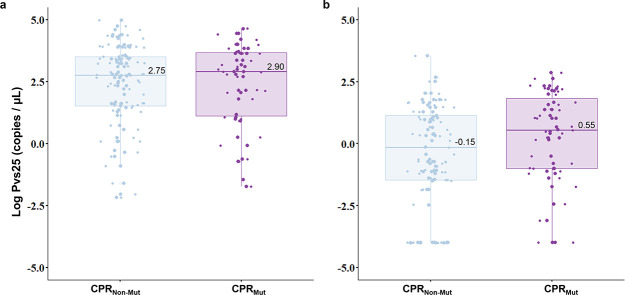
We next evaluated how PQ metabolism was associated with gametocyte density. The gametocyte positivity and density were assessed by quantifying pvs25 transcripts. Ninety-five percent of individuals presented with pvs25 transcripts at baseline (D0) and 77% on D3. The average levels of the pvs25 transcript were similar on D0 irrespective of the CPR status (Fig. 1A). By adjusting a longitudinal regression model GEE controlling for the effect of potentially confounding variables, we found that subjects with CPR mutations had gametocyte levels 11.5 times higher on average on D3 than individuals without mutation in CPR (95% CI 1.2–55.2, P = 0.013) (Fig. 1B; Table S2). The median number of gametocytes on D3 could be roughly estimated as 0.06 (IQR, 0.0–3.09) and 0.85 (IQR, 0.02–23.1) for CPR non-mutated and mutated subjects, respectively. The total parasitemia determined by the quantification of 18 s rRNA transcripts was also significantly associated with the levels of the pvs25 transcript over time. A similar trend regarding gametocyte densities on D3 was seen for CPR A503V genotypes (Fig. S1).
Fig 1.
Gametocyte density estimates according to the cytochrome P450 reductase (CPR) status. Gametocyte density is shown as the log of pvs25 transcript levels (A) at baseline and (B) 72 hours after the initiation of the treatment. According to the conversion of pvs25 transcript levels into numbers of gametocytes published by Koepfli and colleagues (27) (1 gametocyte = 4.17 pvs25 transcripts), the median number of gametocytes is 134.8 [(IQR, 7.35–825.6) for CPRNon-Mut and 191.5 (IQR, 2.82–1,217)] for CPRMut at baseline and 0.06 (IQR, 0.0–3.09) for CPRNon-Mut and 0.85 (IQR, 0.02–23.1) for CPRMut on D3. CPRNon-Mut, CPR non-mutated; CPRMut, CPR mutated (homo and heterozygous).
Primaquine blood level is associated with gametocyte densities
We evaluated whether the concentration of PQ in the whole blood was related to the CYP2D6 and CPR status, as well as to the gametocyte densities over time. Although the PQ concentration had high variability, it was associated with the predicted CYP2D6 phenotype (Fig. S2). PQ levels for carriers of the CPR mutated allele and impaired CYP2D6 activity showed a median of 197.5 ng/mL (IQR, 103–367) while individuals with normal activity of CYP2D6 presented a median of 167.5 ng/mL (IQR, 27–249). By fitting a linear regression model, we found evidence for carriers of CPR mutated allele showing higher dosage of PQ in the presence of impaired CYP2D6 activity when compared to individuals with normal activity of CYP2D6 (β = 66.6, 95% CI 8.5–125, P = 0.024) (Table S3). It is noteworthy that a lower reduction of pvs25 transcript levels between D3 and D0 was associated with a higher dosage of PQ on D3 (P = 0.010). Surprisingly, the dosage of PQ was lower for subjects with the CYP2D6 normal phenotype carrying the CPR mutated allele when compared with the subjects without mutation in both enzymes (β = −47.5, 95% CI −91.0–4.1, P = 0.036).
DISCUSSION
Several factors can affect the potential of an infected individual to transmit malaria parasites to mosquitoes, such as gametocyte maturity, the ratio of female to male gametocytes, as well as the human host immunity levels (5). Here, we addressed how impairment of PQ metabolism might affect gametocyte clearance in P. vivax infection, which can contribute to maintaining human infection reservoirs. This is an area that still needs to be explored in appropriate depth, particularly in P. vivax malaria.
We identified a direct association between CPR status and gametocyte density at 72 hours after PQ–CQ treatment initiation by analyzing polymorphisms in main enzymes related to PQ metabolism. Carriers of polymorphism in CPR had a lower reduction in gametocyte density on day 3 compared to non-mutated individuals. This effect was confirmed after considering the initial total parasitemia, which has already been described to influence gametocyte densities (24, 28, 29). These findings agree with those of Camarda and colleagues (24), who observed that P. falciparum gametocytocidal activity in vitro was greater when in the presence of the active CPR enzyme. CPR has a direct role in the redox cycling of OH-PQm produced by the complex CPR/CYP2D6. The OH-PQm undergo spontaneous oxidation to quinoneimines, leading to the production of H2O2. Quinoneimines in turn can be reduced back to hydroxylated forms by CPR, resulting in the accumulation of H2O2, significantly enhancing PQ gametocytocidal activity (24). On the other hand, the A503V variant, despite its highly conservative change and its location near the protein’s surface, away from the FAD-binding site, exhibits reduced catalytic activity (30). Thus, our results provide further evidence for a role of the CPR variant in interindividual differences in response to PQ treatment.
Besides the direct role of CPR in generating H2O2 and parasite killing, this enzyme is a CYP2D6 redox partner (8, 24). In vitro experiments support that hydroxylated PQ metabolites produced through the CPR/CYP2D6 complex exert per se modest activity against gametocyte stages, confirming that PQ biotransformation is required for gametocyte killing activity (24). Consistent with that, individuals with impaired CP2D6 activity were associated with prolonged P. falciparum gametocyte carriage after treatment (31). Here, we did not find evidence of an association between predicted CYP2D6 enzymatic activity and gametocyte clearance. In the meantime, it is important to stress that only a few individuals were classified as poor (gPM, 2%) or intermediate (gIM, 3%) metabolizers, which restricted us from assessing the effect of these more extreme phenotypes on gametocyte clearance. Here, most subjects with impaired CYP2D6 were classified as normal–slow metabolizers (gNM-S), a phenotype group that shows enzymatic activity between gIM and normal–fast metabolizers (gNM-F) (16). For now, we cannot exclude the possibility that PQ metabolites generated by gNM-S metabolizers through the CPR/CYP2D6 pathway are converted into H2O2 via CPR-mediated redox cycling exerting effective gametocytocidal activity. Another possible explanation for this lack of association is that CPR is not the exclusive electron donor for CYP2D6 (32, 33). Other pathways may play a role in the process and compensate for alterations in CPR, allowing CYP2D6 to sustain its function during gametocyte clearance.
Our findings were corroborated by the analysis of the PQ concentration in the blood with clear evidence for lower reduction in pvs25 transcripts among individuals with higher concentrations of PQ on day 3 of drug administration. Consistent with these results, carriers of the CPR mutated allele showed a higher dosage of PQ in the presence of impaired CYP2D6 activity compared with the normal CYP2D6 activity. A clear difference in PQ blood levels was also observed for poor metabolizers showing higher concentrations of the drug and reduced gametocyte clearance on D3. Our study has a limitation because we could not follow a standard approach related to the time of blood collection after drug intake, which may have contributed to the high variability observed in drug blood levels among subjects. Despite that, our results corroborate with those of previous studies showing higher plasma drug levels in gPM/gIM metabolizers and that monitoring blood levels of PQ during treatment can provide a reliable assessment of the parasite exposure to the drug (34, 35). This finding prompts us to inquire whether antimalarial therapy optimization could enhance patient outcomes. However, optimizing drug dosing necessitates a thorough understanding of the drug’s pharmacokinetic and pharmacodynamic properties, along with insights into its metabolizing enzymes. Additionally, achieving optimization should ideally maximize cost–benefit (36). Hence, it becomes imperative to evaluate these conditions in more extensive studies.
This is the first study to evaluate how the CPR/CYP2D6 genetic variability affects the clearance of P. vivax gametocytes, highlighting the importance of understanding how pharmacogenetic factors influence the risk of malaria transmission. Specifically, we found that during standard CQ-PQ treatment, the median number of gametocytes on D3 was significantly higher among CPR mutated allele carriers. Of importance, the median of 0.9 gametocytes/µL found among these patients is in the range of the recently determined lowest infective P. vivax gametocyte density: 0.2 to 5 gametocytes/µL, with a median of 0.8 gametocytes/µL (37). This contrasts with the significantly lower median of 0.06 gametocytes/µL reported for the CPR wild-type carriers, a value markedly below the documented lower limit for effective infectivity. In operational terms, this suggests that CPR 503V carriers are likely to be still contributing to transmission 72 hours after treatment initiation, while the wild-type–harboring subjects are expected in most cases not to. This fits previous reports of P. vivax infectivity studies which showed that, although at much lower rates, asymptomatic individuals with low parasitemia were still able to infect mosquitoes from the Brazilian Amazon (38). Conceptually, our work points to pharmacogenetics as a non-negligible factor to define potential host reservoirs with the propensity to contribute to transmission during CQ–PQ treatment for 3 days, particularly in settings and seasons of high Anopheles human-biting rates.
MATERIALS AND METHODS
Study site and subjects
To evaluate gametocyte clearance during treatment with PQ–CQ of P. vivax malaria, 100 patients were enrolled between October 2019 and March 2020. Eligible subjects were patients with symptomatic P. vivax infection of either sex, aged >12 years, attending the government-run malaria clinic Policlínica Cosme e Silva in Boa Vista city. Boa Vista is the capital of Roraima State, in the North Region of Brazil, with about 437,000 inhabitants. According to the Epidemiological Surveillance System for Malaria (SIVEP-Malaria), around 30,000 cases were recorded in 2020, most of them autochthonous cases caused by P. vivax (80%). Although Boa Vista is considered a low-risk area for malaria transmission, many cases from other regions have been recorded in recent years (39). In addition, the region and neighboring countries’ surroundings have mining areas in which working conditions are considered a significant problem for disease control. Since 2017, Roraima has experienced intense migration of the Venezuelan and Guyanese population, which has affected malaria control measures and contributed to the spread of the disease in the state (39).
The enrolled participants were diagnosed with a mono-infection by P. vivax through microscopic examination of Giemsa-stained thick blood smears evaluated by well-trained microscopists following the malaria diagnosis guidelines of the Brazilian Ministry of Health. Subsequently, a non-ribosomal qPCR assay was conducted for the purpose of molecular diagnosis, targeting Pvr47/Pfr364 as described in a previous study (40). The prescribed treatment for the subjects followed the guidelines of the Ministry of Health, which consisted of a combination of CQ and PQ. The total dosage for CQ was 25 mg/kg administered orally over 3 consecutive days, while PQ was given over 7 days with a total dosage of 3.5 mg/kg (41).
We obtained the number of P. vivax malaria episodes for each participant from the Epidemiological Surveillance System for Malaria (SIVEP-Malaria). Recurrence of P. vivax was defined as a new episode that was microscopically diagnosed according to the case report, and it occurred within an interval ranging from 29 to 180 days after the initial episode. In Brazil, the first recurrence commonly occurs within this interval (42). We considered overall recurrence rates due to the inability to distinguish reliably relapse from reinfection or recrudescence by clinical assessment or parasite genotyping (43, 44).
Blood sample collection and DNA and RNA extraction
In this exploratory study, the number of individuals enrolled was limited by logistic reasons. Blood samples were collected from participants at two time points: on admission (0 hours) before treatment and on the third day (72 hours) after starting antimalarials. Blood samples collected within 72 hours were obtained through active search, where the study team visited the participant’s home to collect the blood sample. This timeframe was selected based on published data indicating that the median time for P. vivax gametocyte clearance is approximately 3 days (45, 46). From each patient, 5 mL peripheral blood was drawn during the survey and stored at 4°C in a tube containing the anticoagulant EDTA. The QIAamp DNA Mini Kit (Qiagen) was used following the manufacturer’s instructions for extracting DNA from 200 µL of whole blood, resulting in 50 µL of DNA.
Two 200-µL aliquots of whole blood were collected with 1 mL of RNAprotect (Qiagen) added to each sample for RNA extraction. The samples were then stored at −20°C until transportation to the René Rachou Institute laboratory in Belo Horizonte, where they were kept at −80°C until extraction. RNA extraction was performed using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s instructions, with both blood aliquots processed together. Pellets from both samples were combined to proceed with the extraction. To remove any trace of DNA contamination during RNA extraction, DNA digestion was performed using the commercial Turbo DNA-free Kit (Invitrogen) according to the manufacturer’s protocol.
CYP2D6 polymorphism genotyping and copy number analysis
Eight single-nucleotide polymorphisms (SNPs) (C1584G, C100T, C1023T, G1846A, C2580T, G2988A, G3183A, and G4180C) and one deletion (2615_2617delAAG) in the CYP2D6 gene and the copy number of the gene were assayed by qPCR. We used specific hydrolysis probes for each polymorphism (TaqMan SNP genotyping assays; Thermo Fisher Scientific). All amplification reactions were performed according to the protocol described by Silvino and colleagues (15). Amplification and fluorescence detection were carried out using the ViiA 7 real-time PCR system (Thermo Fisher Scientific). The results were analyzed by QuantStudio real-time PCR software (Thermo Fisher Scientific) and the Thermo Fisher cloud platform (Thermo Fisher Scientific).
The copy number of the CYP2D6 gene was determined by quantitative PCR (qPCR) using the Hs00010001_cn assay (Thermo Fisher Scientific) for gene deletion and amplification detection. All amplification reactions were performed according to the protocol described by Silvino and colleagues (15). Amplification and fluorescence detection were carried out in the ViiA 7 real-time PCR system (Thermo Fisher Scientific), and the number of copies was estimated in CopyCaller v.2.0 software. Only samples with confidence values greater than 95% and absolute z-scores of <1.75 were considered in our analysis.
Prediction of the CYP2D6 phenotype based on the AS model
The inferred haplotypes were compared to the CYP2D6 haplotypes derived from the Pharmacogene Variation Consortium (PharmVar) database for allele designation. Haplotypes that did not match known CYP2D6 alleles were designated undetermined. For each allelic variant, the value of CYP2D6 metabolic activity relative to that of the fully functional CYP2D6*1 reference allele was assigned. A value of 1 was assigned to the fully functional reference CYP2D6*1 allele, and a value of 0 (zero) was assigned to nonfunctional alleles. Reduced activity alleles received a value of 0.5 to reflect the perceived level of activity reduction. Alleles carrying gene duplications or multiplications received double the value compared to that assigned to an allele with a single gene copy. The sums of these values permitted us to classify the subjects as poor metabolizers [gPM (the prefix “g” indicates that the CYP2D6 phenotype was predicted from genotype data); AS of 0], intermediate metabolizers (gIM; AS of 0.25–0.5), normal–slow metabolizers (gNM-S; AS of 1), normal–fast metabolizers (gNM-F; AS of 1.5 and AS of 2), and ultrarapid metabolizers (gUM; AS of >2).
CPR polymorphism genotyping
The genotyping of A503V (rs1057868), which changes GCC to GTC at codon 1508 in the gene that encodes CPR, was assayed by qPCR. We used a specific hydrolysis probe for the target region (Assay ID C___8890131_30; Thermo Fisher Scientific). All amplification reactions were performed in 384-well plates in a total volume of 5 µL in the presence of 2.5 µL 2 × TaqMan universal PCR master mix (Thermo Fisher Scientific), 0.25 µL TaqMan SNP genotyping assay reagent (Thermo Fisher Scientific), 1.25 µL ultrapure water (free of nucleases), and 1 µL target DNA (≅ 10 ng/ µL). A negative control was prepared containing no template DNA, and heterozygous and homozygous samples were used as positive controls. The thermocycling conditions were initial denaturation at 95°C for 10 min, followed by 50 cycles of 15 sec at 92°C and 90 sec at 60°C, and one cycle of 30 sec at 60°C. Amplification and fluorescence detection were carried out using the ViiA 7 real-time PCR system (Thermo Fisher Scientific). The results were analyzed by QuantStudio real-time PCR software (Thermo Fisher Scientific) and the Thermo Fisher cloud platform (Thermo Fisher Scientific).
Quantification of P. vivax gametocytes by qPCR
We obtained complementary DNA (cDNA) through reverse transcription (RT) that was performed using the SuperScript IV Reverse Transcriptase (Invitrogen, Life Technologies) together with random primers according to the manufacturer’s protocol, except for the volume used of SuperScript IV (0.5 µL was used in this study). All reactions were performed in a Veriti 96-well Thermal Cycler (Thermo Fisher Scientific).
The quantification of gametocytes was performed using plasmids containing fragments of interest. For that, we used pGEM-T Easy Vector (Promega) and linearized the plasmids with ApaI enzyme (Promega). The primer sequences used to amplify the 18 s rRNA and pvs25 targets were previously described by Wampfler and colleagues (47). pvs25 qPCR experiments were performed using the following protocol: 5 µL of GoTaq qPCR Master Mix (Promega), 200 nM of primers, 3.6 µL of ultrapure water (free of nucleases), and 1 µl of cDNA (≅ 200 ng). The quantification of the 18 s rRNA gene was performed using 5 µL of GoTaq qPCR Master Mix (Promega), 900 nM of each primer, 2.2 µL of ultrapure water (free of nucleases), and 1 µL of diluted cDNA (≅ 2 ng). As this gene is present in greater abundance, the cDNA samples were diluted 100 x for the quantification of this target. The cycling parameters used for the quantification of gametocytes are the following: 2 min at 95°C and 40 cycles of 15 sec at 95°C and 1 min at 60°C, followed by a melt curve analysis. All amplification and fluorescence detection procedures were performed on the ViiA7 Real-Time PCR System (Applied Biosystems). Quantification results were analyzed in QuantStudio Real Time PCR Software v1.3.7. All samples were analyzed in triplicate in 384-well plates. Replicates with standard deviation >0.3 were omitted from the analysis.
The limit of detection (LOD) and the limit of quantification (LOQ) for the pvs25 assay were estimated from five-fold serial dilutions of cDNA ranging from 4 ng/μL to 0.007 ng/μL with five replicates for each dilution point, following the methodology proposed by Forootan and colleagues (48). The pvs25 assay was performed with an efficiency of 87% (R2 = 0.993). The LOD for pvs25 was equal to 0.045 copies/uL and the LOQ was equal to 0.174 copies/μL.
Measurement of primaquine levels
The measurement of primaquine levels was conducted on blood samples collected at 72 hours during the treatment. Primaquine was measured in a reverse-phase HPLC system with UV detection (Flexar, Perkin Elmer, Shelton, MA US), after a previous step of separation of the filter paper following by liquid–liquid extraction with methyl-ter-butyl-ether in alkaline media (49). The column was an RP-18 (X terra 4.6 × 150 mm, i.d. 5 µm) at 25°C, and the mobile phase was methanol and formic acid (1:3) eluted at a flow rate of 1.0 mL/min and monitored at 263 nm. The limit of detection was 15 ng/mL, and the limit of quantification was 25 ng/mL. The assay was linear from 25 to 2,000 ng/mL and the intra- and inter-assay mean coefficients of variation were 12.5% and 15.5%, respectively. The mean recovery was 82% for the parent compound. Chloroquine, desethylchloroquine, mefloquine, carboxy-mefloquine, or acetaminophen did not interfere in the detection of the analyte.
Statistical analysis
Proportions are given with 95% confidence intervals and compared with χ2 test or Fisher’s exact test. Comparison of continuous variables was performed with the Mann–Whitney test or Kruskal–Wallis test, with Dunn’s post hoc test, as appropriate. The association between two variables was estimated using Spearman’s rank correlation test. The time to the first P. vivax malaria recurrence for the CYP2D6 activity level groups was estimated with Kaplan–Meier survival analysis and compared using the log-rank test. All tests were two-sided, and a P-value less than 0.05 was considered statistically significant. We used the generalized estimating equations (GEEs) approach to examine the effects of CYP2D6/CPR mutation on gametocyte densities within 72 hours of treatment. We included the relevant covariates, such as age (as a measure of exposure to malaria), 18 s rRNA transcript levels, CYP2D6 predicted activity, and CPR status. Linear regression modeling (LRM) was performed to explain the relationship between PQ blood levels and the covariates: gametocyte densities, 18 s rRNA transcript levels, CYP2D6 predicted activity, and CPR status. To ensure the validity of our analysis, we assessed the assumptions of the LRM. We examined residual plots and Q-Q plots to evaluate the linearity, independence, homoscedasticity, and normality assumptions for model residuals. Appropriate adjustments or transformations were applied to meet these assumptions in case of any violations. Covariates were selected for inclusion in the GEE and LRM models if they were associated with the outcome at the 15% level of significance in exploratory unadjusted analysis. The models GEE and LRM were fitted using, respectively, the geeglm (geepack) and lm functions in R. Statistical analysis was performed using STATA v.14 software, GraphPad Prism version 8.0.2 (GraphPad Software, San Diego, California/USA), and the R v.4.1.1 package.
ACKNOWLEDGMENTS
The authors thank all the patients who participated in this study and Policlínica Cosme e Silva (RR) teams. We thank Agatha Cristina Pereira for the support in the fieldwork. We thank Dr. Viviane Santos from FIOCRUZ-MG and the Program for Technological Development in Tools for Health-PDTISFIOCRUZ for use of the Real-Time PCR Facility (RPT09D) at René Rachou Institute. We thank Eduardo Silva and Renata Freitas for the statistical consulting at the René Rachou Institute. Y.E.A.R.S. thanks CNPq for scholarship support. Y.E.A.R.S., M.C.S.B.P., and L.F.F.G. thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – for scholarship support (Finance Code 001) and the Programa de Pós-Graduação em Ciências da Saúde do Instituto René Rachou. T.N.S. and C.F.A.B. are CNPq Research Productivity fellows.
This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Swedish Research Council Grant (Grant ref. 200075/2022–5), Bill & Melinda Gates Foundation- Grand Challenges Brazil, and Programa PrInt-Fiocruz-CAPES. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conceptualization: T.N.S. formal analysis: Y.E.A.R.S., T.N.S, M.C.S.B.P, and J.L.F.V. Funding acquisition: T.N.S and A.M.S. Investigation: T.N.S., C.F.A.B., and J. P. G. Resources: J.L, A.M.S., and C.F.A.B. Methodology: Y.E.A.R.S., M.C.S.B.P., J.L.F.V., and L.F.F.G. Writing - original draft: Y.E.A.R.S. and T.N.S. Writing - review & editing: T.N.S., C.F.A.B., A.M.S., and J. P. G.
Contributor Information
Tais Nobrega de Sousa, Email: tais.sousa@fiocruz.br.
Audrey Odom John, The Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, USA.
ETHICS APPROVAL
This study’s ethical and methodological aspects were approved by the Ethics Committee of Research Involving Human Subjects of Institute René Rachou/Fiocruz (report no. 2.803.756 and no. 2.243.058). All participants signed a written informed consent form, and the next of kin, caretakers, or guardians signed on behalf of minors/children enrolled in the study.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01204-23.
Supplemental tables and figures.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. WHO . 2021. World malaria report. Swiss: Geneva [Google Scholar]
- 2. Wolfarth-Couto B, Silva R da, Filizola N. 2019. Variability in malaria cases and the association with rainfall and rivers water levels in Amazonas State, Brazil. Cad Saude Publica 35:e00020218. doi: 10.1590/0102-311X00020218 [DOI] [PubMed] [Google Scholar]
- 3. Rieckmann KH, McNamara JV, Frischer H, Stockert TA, Carson PE, Powell RD. 1968. Gametocytocidal and Sporontocidal effects of Primaquine and of Sulfadiazine with Pyrimethamine in a chloroquine-resistant strain of Plasmodium Falciparum. Bulletin of the World Health Organization doi:4876731:625–632. [PMC free article] [PubMed] [Google Scholar]
- 4. Shekalaghe S, Drakeley C, Gosling R, Ndaro A, van Meegeren M, Enevold A, Alifrangis M, Mosha F, Sauerwein R, Bousema T. 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2:e1023. doi: 10.1371/journal.pone.0001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arroyo-Arroyo M, Arango E, Carmona-Fonseca J, Aristizabal B, Yanow S, Maestre A. 2017. Efficacy of different primaquine regimens to control Plasmodium falciparum gametocytemia in Colombia. Am J Trop Med Hyg 97:712–718. doi: 10.4269/ajtmh.16-0974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pukrittayakamee S, Imwong M, Singhasivanon P, Stepniewska K, Day NJ, White NJ. 2008. Effects of different antimalarial drugs on gametocyte carriage in P. vivax malaria. Am J Trop Med Hyg 79:378–384. [PubMed] [Google Scholar]
- 7. Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CSN, Hofmann NE, Kinboro B, Waltmann A, Brewster J, Lorry L, Tarongka N, Samol L, Silkey M, Bassat Q, Siba PM, Schofield L, Felger I, Mueller I. 2015. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med 12:e1001891. doi: 10.1371/journal.pmed.1001891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pybus BS, Sousa JC, Jin X, Ferguson JA, Christian RE, Barnhart R, Vuong C, Sciotti RJ, Reichard GA, Kozar MP, Walker LA, Ohrt C, Melendez V. 2012. CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine. Malar J 11:259. doi: 10.1186/1475-2875-11-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, Caridha D, Zeng Q, Reichard GA, Ockenhouse C, Bennett J, Walker LA, Ohrt C, Melendez V. 2013. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J 12:212. doi: 10.1186/1475-2875-12-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, Alonso PL, del Portillo HA. 2009. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9:555–566. doi: 10.1016/S1473-3099(09)70177-X [DOI] [PubMed] [Google Scholar]
- 11. Mihaly GW, Ward SA, Edwards G, Nicholl DD, Orme ML, Breckenridge AM. 1985. Pharmacokinetics of primaquine in man. I. Studies of the absolute bioavailability and effects of dose size. Br J Clin Pharmacol 19:745–750. doi: 10.1111/j.1365-2125.1985.tb02709.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhatia SC, Saraph YS, Revankar SN, Doshi KJ, Bharucha ED, Desai ND, Vaidya AB, Subrahmanyam D, Gupta KC, Satoskar RS. 1986. Pharmacokinetics of primaquine in patients with P. vivax malaria. Eur J Clin Pharmacol 31:205–210. doi: 10.1007/BF00606660 [DOI] [PubMed] [Google Scholar]
- 13. Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, Deye G, Melendez V, Ockenhouse CF. 2013. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 369:1381–1382. doi: 10.1056/NEJMc1301936 [DOI] [PubMed] [Google Scholar]
- 14. Baird JK, Louisa M, Noviyanti R, Ekawati L, Elyazar I, Subekti D, Chand K, Gayatri A, Soebianto S, Crenna-Darusallam C, Djoko D, Hasto BD, Meriyenes D, Wesche D, Nelwan EJ, Sutanto I, Sudoyo H, Setiabudy R, Instiaty . 2018. Association of impaired cytochrome P450 2D6 activity genotype and phenotype with therapeutic efficacy of primaquine treatment for latent Plasmodium vivax malaria. JAMA Netw Open 1:e181449. doi: 10.1001/jamanetworkopen.2018.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silvino ACR, Kano FS, Costa MA, Fontes CJF, Soares IS, de Brito CFA, Carvalho LH, Sousa TN. 2020. Novel insights into Plasmodium vivax therapeutic failure: CYP2D6 activity and time of exposure to malaria modulate the risk of recurrence. Antimicrob Agents Chemother 64:e02056-19. doi: 10.1128/AAC.02056-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. 2008. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83:234–242. doi: 10.1038/sj.clpt.6100406 [DOI] [PubMed] [Google Scholar]
- 17. Gaedigk A, Dinh JC, Jeong H, Prasad B, Leeder JS. 2018. Ten years’ experience with the CYP2D6 activity score: a perspective on future investigations to improve clinical predictions for precision therapeutics. J Pers Med 8:15. doi: 10.3390/jpm8020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang SL, Han JF, He XY, Wang XR, Hong JY. 2007. Genetic variation of human cytochrome P450 reductase as a potential biomarker for mitomycin C-induced cytotoxicity. Drug Metab Dispos 35:176–179. doi: 10.1124/dmd.106.011056 [DOI] [PubMed] [Google Scholar]
- 19. Gong L, Zhang CM, Lv JF, Zhou HH, Fan L. 2017. Polymorphisms in cytochrome P450 oxidoreductase and its effect on drug metabolism and efficacy. Pharmacogenet Genomics 27:337–346. doi: 10.1097/FPC.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 20. Strohmaier SJ, Huang W, Baek JM, Hunter DJB, Gillam EMJ. 2019. Rational evolution of the cofactor-binding site of cytochrome P450 reductase yields variants with increased activity towards specific cytochrome P450 enzymes. FEBS J 286:4473–4493. doi: 10.1111/febs.14982 [DOI] [PubMed] [Google Scholar]
- 21. Esteves F, Campelo D, Gomes BC, Urban P, Bozonnet S, Lautier T, Rueff J, Truan G, Kranendonk M. 2020. The role of the FMN-domain of human cytochrome P450 oxidoreductase in its promiscuous interactions with structurally diverse redox partners. Front Pharmacol 11:299. doi: 10.3389/fphar.2020.00299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandee D, Morrissey K, Agrawal V, Tam HK, Kramer MA, Tracy TS, Giacomini KM, Miller WL. 2010. Effects of genetic variants of human P450 oxidoreductase on catalysis by CYP2D6 in vitro. Pharmacogenet Genomics 20:677–686. doi: 10.1097/FPC.0b013e32833f4f9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vásquez-Vivar J, Augusto O. 1992. Hydroxylated metabolites of the Antimalarial drug Primaquine. oxidation and redox Cycling. J Biol Chem 267:6848–6854. doi: 10.1016/S0021-9258(19)50504-X [DOI] [PubMed] [Google Scholar]
- 24. Camarda G, Jirawatcharadech P, Priestley RS, Saif A, March S, Wong MHL, Leung S, Miller AB, Baker DA, Alano P, Paine MJI, Bhatia SN, O’Neill PM, Ward SA, Biagini GA. 2019. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat Commun 10:3226. doi: 10.1038/s41467-019-11239-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pett H, Bradley J, Okebe J, Dicko A, Tiono AB, Gonçalves BP, Stone W, Chen I, Lanke K, Neuvonen M, Mustaniemi A-L, Eziefula AC, Gosling R, D’Alessandro U, Drakeley C, Niemi M, Bousema T. 2019. CYP2D6 polymorphisms and the safety and gametocytocidal activity of single-dose primaquine for Plasmodium falciparum. Antimicrob Agents Chemother 63:e00538-19. doi: 10.1128/AAC.00538-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mwaiswelo RO, Ngasala B, Msolo D, Kweka E, Mmbando BP, Mårtensson A. 2022. A single low dose of primaquine is safe and sufficient to reduce transmission of Plasmodium falciparum gametocytes regardless of cytochrome P450 2D6 enzyme activity in Bagamoyo district, Tanzania. Malar J 21:84. doi: 10.1186/s12936-022-04100-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, Betuela I, Nuitragool W, Barry AE, Siba P, Felger I, Mueller I. 2015. Blood-stage parasitaemia and age determine Plasmodium falciparum and P. vivax gametocytaemia in Papua New Guinea. PLoS One 10:e0126747. doi: 10.1371/journal.pone.0126747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wampfler R, Hofmann NE, Karl S, Betuela I, Kinboro B, Lorry L, Silkey M, Robinson LJ, Mueller I, Felger I. 2017. Effects of liver-stage clearance by primaquine on gametocyte carriage of Plasmodium vivax and P. falciparum. PLoS Negl Trop Dis 11:e0005753. doi: 10.1371/journal.pntd.0005753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tadesse FG, van den Hoogen L, Lanke K, Schildkraut J, Tetteh K, Aseffa A, Mamo H, Sauerwein R, Felger I, Drakeley C, Gadissa E, Bousema T. 2017. The shape of the iceberg: quantification of submicroscopic Plasmodium falciparum and Plasmodium vivax parasitaemia and gametocytaemia in five low endemic settings in Ethiopia. Malar J 16:99. doi: 10.1186/s12936-017-1749-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang N, Agrawal V, Giacomini KM, Miller WL. 2008. Genetics of P450 oxidoreductase: sequence variation in 842 individuals of four ethnicities and activities of 15 missense mutations. Proc Natl Acad Sci U S A 105:1733–1738. doi: 10.1073/pnas.0711621105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pett H, Bradley J, Okebe J, Dicko A, Tiono AB, Gonçalves BP, Stone W, Chen I, Lanke K, Neuvonen M, Mustaniemi A-L, Eziefula AC, Gosling R, D’Alessandro U, Drakeley C, Niemi M, Bousema T. 2019. CYP2D6 polymorphisms and the safety and gametocytocidal activity of single-dose primaquine for Plasmodium falciparum. Antimicrob Agents Chemother 63:e00538-19. doi: 10.1128/AAC.00538-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller WL. 2021. Steroidogenic electron-transfer factors and their diseases. Ann Pediatr Endocrinol Metab 26:138–148. doi: 10.6065/apem.2142154.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bart AG, Scott EE. 2017. Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes. J Biol Chem 292:20818–20833. doi: 10.1074/jbc.RA117.000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mello A, Vieira M, Sena L de, Paixão T da, Pinto ACG, Grisólia D de A, Silva MT, Vieira JLF. 2018. Levels of primaquine and carboxyprimaquine in patients with malaria vivax from the Brazilian Amazon basin. Rev Inst Med Trop Sao Paulo 60:e66. doi: 10.1590/S1678-9946201860066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spring MD, Sousa JC, Li Q, Darko CA, Morrison MN, Marcsisin SR, Mills KT, Potter BM, Paolino KM, Twomey PS, Moon JE, Tosh DM, Cicatelli SB, Froude JW, Pybus BS, Oliver TG, McCarthy WF, Waters NC, Smith PL, Reichard GA, Bennett JW. 2019. Determination of cytochrome P450 isoenzyme 2D6 (CYP2D6) genotypes and pharmacogenomic impact on primaquine metabolism in an active-duty US military population. J Infect Dis 220:1761–1770. doi: 10.1093/infdis/jiz386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White NJ. 2013. Pharmacokinetic and pharmacodynamic considerations in antimalarial dose optimization. Antimicrob Agents Chemother 57:5792–5807. doi: 10.1128/AAC.00287-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Surit T, Sripoorote P, Kumpitak C, Suansomjit C, Maneechai N, Cui L, Sattabongkot J, Roobsoong W, Nguitragool W. 2023. Transmission efficiency of Plasmodium vivax at low parasitaemia. Malar J 22:22. doi: 10.1186/s12936-022-04435-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martins-Campos KM, Kuehn A, Almeida A, Duarte APM, Sampaio VS, Rodriguez ÍC, da Silva SGM, Ríos-Velásquez CM, Lima JBP, Pimenta PFP, Bassat Q, Müller I, Lacerda M, Monteiro WM, Barbosa Guerra M das G. 2018. Infection of anopheles aquasalis from symptomatic and asymptomatic Plasmodium vivax infections in Manaus, western Brazilian Amazon. Parasit Vectors 11:288. doi: 10.1186/s13071-018-2749-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Louzada J, de Almeida NCV, de Araujo JLP, Silva J, Carvalho TM, Escalante AA, Oliveira-Ferreira J. 2020. The impact of imported malaria by gold miners in roraima: characterizing the spatial dynamics of autochthonous and imported malaria in an urban region of boa vista. Mem Inst Oswaldo Cruz 115:e200043. doi: 10.1590/0074-02760200043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amaral LC, Robortella DR, Guimarães LFF, Limongi JE, Fontes CJF, Pereira DB, de Brito CFA, Kano FS, de Sousa TN, Carvalho LH. 2019. Ribosomal and non-ribosomal PCR targets for the detection of low-density and mixed malaria infections. Malar J 18:154. doi: 10.1186/s12936-019-2781-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ministério da Saúde . Guia de tratamento da malária no Brasil, 2nd edn. Brasília, 2021. www.bvsms.saude.gov.br. [Google Scholar]
- 42. Daher A, Silva J, Stevens A, Marchesini P, Fontes CJ, Ter Kuile FO, Lalloo DG. 2019. Evaluation of Plasmodium vivax malaria recurrence in Brazil. Malar J 18:18. doi: 10.1186/s12936-019-2644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Araujo FCF, de Rezende AM, Fontes CJF, Carvalho LH, Alves de Brito CF. 2012. Multiple-clone activation of hypnozoites is the leading cause of relapse in Plasmodium vivax infection. PLoS One 7:e49871. doi: 10.1371/journal.pone.0049871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, Guthmann J-P, Nosten F, Carlton J, Looareesuwan S, Nair S, Sudimack D, Day NPJ, Anderson TJC, White NJ. 2007. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. Infect Dis Soc Am 195:927–933. doi: 10.1086/512241 [DOI] [PubMed] [Google Scholar]
- 45. Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, Poespoprodjo JR, Singhasivanon P, Anstey NM, White NJ, Tjitra E, Nosten F, Price RN. 2013. Gametocyte dynamics and the role of drugs in reducing the transmission potential of Plasmodium vivax. J Infect Dis 208:801–812. doi: 10.1093/infdis/jit261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beyene HB, Beyene MB, Ebstie YA, Desalegn Z. 2016. Efficacy of chloroquine for the treatment of vivax malaria in northwest Ethiopia. PLoS One 11:e0161483. doi: 10.1371/journal.pone.0161483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck H-P, Mueller I, Felger I. 2013. Strategies for detection of Plasmodium species gametocytes. PLoS One 8:e76316. doi: 10.1371/journal.pone.0076316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forootan A, Sjöback R, Björkman J, Sjögreen B, Linz L, Kubista M. 2017. Methods to determine limit of detection and limit of quantification in quantitative real-time PCR (qPCR). Biomol Detect Quantif 12:1–6. doi: 10.1016/j.bdq.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Na-Bangchang K, Guirou EA, Cheomung A, Karbwang J. 2014. Determination of primaquine in whole blood and finger - pricked capillary blood dried on filter paper using HPLC and LCMS / MS. Chromatographia 77:561–569. doi: 10.1007/s10337-014-2639-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental tables and figures.