ABSTRACT
Sporothrix brasiliensis is an emerging zoonotic fungal pathogen that can be difficult to treat. Antifungal susceptibility testing was performed on the mold phase of a convenience sample of 61 Sporothrix spp. isolates from human and cat sporotrichosis cases in Brazil using the Clinical and Laboratory Standards Institute standard M38. A bimodal distribution of azole susceptibility was observed with 50% (28/56) of S. brasiliensis isolates showing elevated itraconazole minimum inhibitory concentrations ≥16 µg/mL. Phylogenetic analysis found the in vitro resistant isolates were not clonal and were distributed across three different S. brasiliensis clades. Single nucleotide polymorphism (SNP) analysis was performed to identify potential mechanisms of in vitro resistance. Two of the 28 resistant isolates (MIC ≥16 mg/L) had a polymorphism in the cytochrome P450 gene, cyp51, corresponding to the well-known G448S substitution inducing azole resistance in Aspergillus fumigatus. SNPs corresponding to other known mechanisms of azole resistance were not identified in the remaining 26 in vitro resistant isolates.
KEYWORDS: sporotrichosis, antifungal agents, antifungal resistance
INTRODUCTION
A large sporotrichosis epidemic, driven by Sporothrix brasiliensis, is ongoing in Brazil with rising cat-to-cat and cat-to-human transmission in 24 of 25 Brazilian states (1). More recently, cat cases with sporotrichosis caused by S. brasiliensis have been reported in Argentina (2, 3), Paraguay, Chile (4), and Uruguay (5). S. brasiliensis is characterized by high virulence, increased zoonotic transmissibility, and high antifungal resistance and can have a wide spectrum of clinical presentations (6–8). Treatment of infected cats is essential for sporotrichosis transmission control (9). Diverse antifungal therapeutic strategies are available to treat infections caused by Sporothrix spp.; itraconazole is the first-choice antifungal drug with terbinafine and potassium iodide as alternatives and amphotericin B used in cases of severe infections (10). The high number of feral cats and treatment interruption due to the high cost of itraconazole are the main challenges to effective cat treatment in Brazil (11). In this context, reports of antifungal-resistant isolates recovered from cat cases have been reported (8, 12). Despite the number of cases, the molecular mechanisms of antifungal resistance among Sporothrix spp. are still poorly understood.
Several studies have reported azole minimum inhibitory concentration (MIC) values for S. brasiliensis (7, 8, 13, 14), along with a multicenter international study with proposed S. brasiliensis epidemiologic cutoff values (ECVs) to azoles (14). The generation of additional S. brasiliensis MIC values would facilitate the establishment of S. brasiliensis ECVs by the Clinical and Laboratory Standards Institute (CLSI). The possibility of identifying isolates with reduced susceptibility to antifungals, in the context of the zoonotic sporotrichosis epidemic that is ongoing in Brazil, is essential for treatment management and will facilitate the tracking of emergence and spread of azole resistance. Here, we determined the MICs for both azoles and echinocandins against Sporothrix spp. isolates recovered from cat and humans living in Brazil and the United States (15). All isolates were tested in the mold phase using the CLSI broth microdilution method. Phylogenetic analysis reported in a previous study (15) was correlated with susceptibility to itraconazole and was used to investigate single nucleotide polymorphisms (SNPs) in the target gene for the azoles, cyp51.
RESULTS
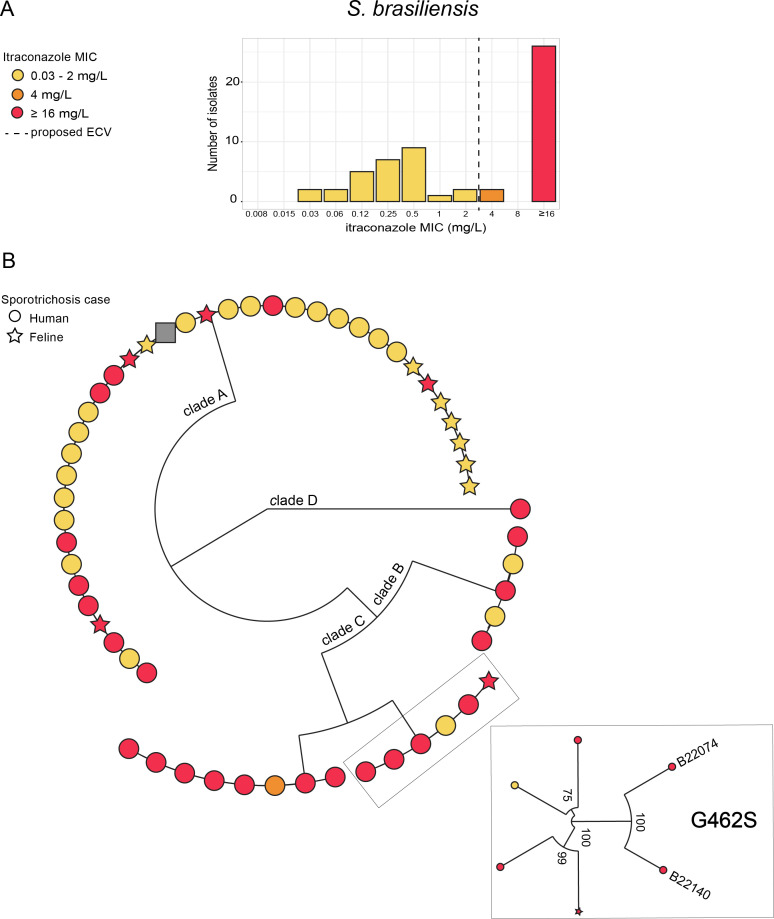
Antifungal susceptibility testing (AFST) was performed by broth microdilution as outlined in the CLSI reference standard M38 for filamentous fungi (16), for 56 S. brasiliensis, six S. schenckii, and one S. globosa isolates. As shown in Table 1, 50% (28/56) of the S. brasiliensis isolates had an MIC ranging from 0.03mg/L to 4 mg/L, and 50% had MICs of ≥16 mg/L to itraconazole (Fig. 1A). The same elevated MIC distribution was observed for the other azoles as follows: 32% (18/56) of isolates showed posaconazole MICs of ≥16 mg/L; 64% (36/56) of isolates showed isavuconazole MICs of >8 mg/L; and 39% (22/56) of isolates showed voriconazole MICs of ≥16 mg/L. Of the 28 S. brasiliensis with MIC ≥ 16 mg/L to itraconazole, 43% (12/28) also showed the highest MIC to posaconazole (>16 mg/L), 96% (27/28) to isavuconazole (≥16 mg/L), and 46% (13/28) to voriconazole (≥16 mg/L). Similarly, S. schenckii isolates showed a bimodal MIC distribution for itraconazole (Fig. S1), and elevated MIC values for isavuconazole and voriconazole (Table 1). For the echinocandins, the distribution of minimum effective concentration (MEC) values was low, ranging from ≤0.008mg/L to 0.03 mg/L for all isolates (Fig. S2).
TABLE 1.
Distribution of MIC values for azole activity against 56 clinical isolates of Sporothrix spp. according to speciesa
| Antifungal drug | Sporothrix spp. | 0.03 mg/L | 0.06 mg/L | 0.12 mg/L | 0.25 mg/L | 0.5 mg/L | 1 mg/L | 2 mg/L | 4 mg/L | 8 mg/L | ≥16 mg/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Itraconazole | S. brasiliensis | 2 | 2 | 5 | 7 | 8 | 1 | 2 | 1 | 28 | |
| S. schenckii | 1 | 1 | 4 | ||||||||
| Posaconazole | S. brasiliensis | 2 | 4 | 1 | 9 | 9 | 3 | 7 | 2 | 1 | 18 |
| S. schenckii | 2 | 2 | 1 | 1 | |||||||
| Isavuconazole | S. brasiliensis | 1 | 6 | 6 | 7 | 36 | |||||
| S. schenckii | 6 | ||||||||||
| Voriconazole | S. brasiliensis | 2 | 3 | 3 | 8 | 17 | 22 | ||||
| S. schenckii | 6 |
No isolates showed MIC of 0.004 mg/L, 0.008 mg/L, or 0.015 mg/L to the azoles represented in the table.
Fig 1.
MIC distribution to itraconazole and genomic diversity among S. brasiliensis isolates. (A) MIC distribution of S. brasiliensis isolates to itraconazole. (B) Maximum likelihood (ML) phylogenetic tree of the S. brasiliensis whole-genome sequecing (WGS) (15). Colors correspond to the MIC values to itraconazole. The shape represents the S. brasiliensis host (human or cat), with the diamond representing the S. brasiliensis strain 5110 reference genome (NCBI: txid1398154). The ML tree and MIC values were visualized together with metadata containing additional epidemiologic data (NCBI BioProject ID: PRJNA957313) for each sample using Microreact (http://microreact.org).
To better understand the relatedness of S. brasiliensis isolates with high and low MIC values to itraconazole, a maximum likelihood (ML) tree based on whole-genome sequencing (WGS) data (15) was visualized with susceptibility results for itraconazole using Microreact (http://microreact.org) (Fig. 1). In vitro itraconazole-resistant S. brasiliensis isolates were found in both animal and human cases. Fifty-two percent (23/44) human and 42% (5/12) of cat isolates had itraconazole MIC values of ≥16 mg/L. The ML tree showed that the tested isolates were distributed in four genetically distinct S. brasiliensis clades (A–D) supported by bootstrap values of 100% (Fig. 1A). There was no correlation between the S. brasiliensis MIC distribution to itraconazole and the phylogenetic clustering patterns in either human or animal cases. Isolates with high MIC were often highly genetically related to isolates with low MIC and were found within all three major clades on S. brasiliensis ML phylogeny. However, clade C had the highest number of isolates with high MIC (Fig. 1B).
SNP analysis was performed to identify potential mechanisms of resistance to itraconazole. SNP analysis of the S. brasiliensis cyp51 gene sequences revealed two isolates (B22074 and B22140) with a glycine to serine amino acid substitution in position 462 (G462S) of cyp51 and itraconazole MIC values of ≥16 mg/L (Fig. 1B; Table S1). The amino acid substitution in position 462 corresponds to the cyp51A G448S mutation in Aspergillus fumigatus that is known to be linked to azole resistance (17, 18). Cyp51 sequencing of the six S. shenckii isolates revealed one resistant isolate (B10282) with the amino acid substitution N48K in cyp51. (Table S1).
DISCUSSION
Cat-associated sporotrichosis is an emerging fungal disease of increasing public health concern. In this study, we determined MIC values for eight different antifungals among S. brasiliensis and S. schenckii isolates. We report a bimodal distribution of S. brasiliensis MICs to itraconazole, which is the first-choice drug for the treatment of sporotrichosis, and high MICs to most other azoles. Isolates resistant to itraconazole (MIC ≥16 µg/mL) in vitro originated from both human and animal cases and were distributed across three genetically distinct S. brasiliensis clades often clustering closely with susceptible isolates. This suggests multiple independent selection events for itraconazole resistance in vitro. Similar results were observed for S. schenckii. In addition, this was the first study to report S. brasiliensis isolates in vitro resistant to azoles containing a missense polymorphism in the cytochrome P450 gene (cyp51).
Currently, there are neither clinical breakpoints nor ECVs for Sporothrix spp. against any antifungal agent. An international multicenter study reporting antifungal susceptibility results of 306 S. brasiliensis isolates generated using CLSI M38-A2 broth microdilution found an itraconazole MIC distribution of ≤0.03 mg/L to ≥32 mg/L with a modal MIC of 1 mg/L (48% of isolates). As a result, the proposed ECV for itraconazole was 2 mg/L for both S. brasiliensis and S. schenckii (14, 19). According to this interpretation, 52% (29/56) of S. brasiliensis isolates included in our study would be considered “non-wild-type.” Other studies using S. brasiliensis isolates from Brazil have reported considerable variability in MIC range for itraconazole and posaconazole (0.12 mg/L to >8 mg/L) (7, 8, 13, 14, 19–21). Considering these previous studies, we found a considerable number of S. brasiliensis isolates showing high MIC values to itraconazole, which could indicate a potential increasing resistance to this drug. However, additional longitudinal studies tracking the antifungal susceptibility of clinical isolates are essential to better understand this point.
Genomic analysis of isolates in our study did not identify an association between MIC values to itraconazole and phylogenetic structure; isolates with MICs to itraconazole above 16 mg/L clustered with isolates with MICs as low as 0.03 µg/mL. These results suggest ongoing selective pressure for reduced susceptibility to itraconazole and recent emergence of this phenotype. However, previous studies using a less discriminatory method of strain typing, multilocus sequence typing, found that isolates with high itraconazole MIC values could be genetically differentiated from susceptible isolates (22). Furthermore, no association between azole resistance and previous drug exposure has been reported by others (7, 8, 23). Although our phylogenetic and gene sequencing results suggest that in vitro resistance arose on multiple independent occasions, more studies associating clinical and genomic data are needed.
Several mechanisms have been associated with antifungal resistance including chromosome rearrangement, differential gene expression, and nucleotide substitutions in the target promoter genes. There has been little effort to characterize the genetic mechanisms of antifungal resistance in the genus Sporothrix (24–26). A recent study explored molecular mechanisms involved in antifungal drug resistance in four S. brasiliensis strains in Brazil and provided a working hypothesis for linking S. brasiliensis resistance profile to chromosomal variation (26). Multiple substitutions in the cyp51 gene are associated with resistance to azoles in A. fumigatus (27, 28). In addition, an in silico study has recently suggested that intrinsic resistance to ketoconazole in S. schenckii complex could be explained by the fixed substitutions in the cyp51 gene (25). We found two isolates of S. brasiliensis with high MIC values to itraconazole that had the cyp51 amino acid substitution G462S. This substitution directly corresponds to the G448S mutation in A. fumigatus known to be linked to azole resistance (17, 18). Additional work is necessary to better understand the mechanisms of azole resistance in S. brasiliensis, but we demonstrate that more than one mechanism is involved.
There are several limitations to this study. Only the cyp51 gene, rather than the entire genome, was explored for the possibility of polymorphisms linked to in vitro itraconazole resistance, in which other possible resistance mechanisms may have been missed. In the future, genetic mechanisms of resistance can be investigated by comparing genomes of isolates with different AFST profiles; however, larger collections of resistant and susceptible are needed for the genome-wide association studies. Another limitation is that the number of isolates from Brazil was relatively small and constituted a convenience sample with a disproportionate distribution of the three different Sporothrix species, which may not accurately reflect the distribution of susceptibility pattern for the different species or within a species. Finally, clinical information for the sporotrichosis cases was not available, and the in vitro AFST results may not correspond to the clinical response to the antifungal treatment. Our study did not evaluate the MIC distribution to terbinafine, an allylamine widely used to treat sporotrichosis in combination with itraconazole, or when itraconazole or KI is not tolerated or cannot be used. Other studies evaluating the S. brasiliensis MIC distribution to terbinafine have shown low MIC values, ranging from ≤0.01 mg/L to 1 mg/L (14).
Altogether, our results suggest that more than one mechanism is involved in in vitro itraconazole resistance in S. brasiliensis. Further studies linking population structure with S. brasiliensis strains, antifungal susceptibility data, and clinical outcome of sporotrichosis would enhance understanding of the spread and possible emergence of antifungal resistance in the zoonotic sporotrichosis context.
MATERIALS AND METHODS
Isolates
Species identification and genomic epidemiology were previously reported (15). Among the 63 Sporothrix spp. isolates included in this study, 61 were from Brazil and 2 were from the United States. Isolates from Brazil were received by three different laboratories: Microbiology Section of Grupo Fleury (São Paulo, Brazil), Parasitology and Mycology Center of Adolfo Lutz Institute (São Paulo, Brazil), and the Central Public Health Laboratory of Mato Grosso do Sul (Campo Grande, Brazil). These isolates were from clinical samples received by the three reference laboratories for diagnosis purposes and were kept as part of the isolate collection bank for each laboratory. Clinical isolates were obtained from human (N = 48) and cat (N = 13) cases, and those with collection site information (N = 40) were from five different Brazilian states: São Paulo (N = 24), Rio de Janeiro (N = 2), Mato Grosso do Sul (9), Rio Grande do Norte (N = 3), Pernambuco (N = 1), and Bahia (N = 1). All Sporothrix spp. isolates collected from 2013 to 2022 by the three different laboratories in Brazil were included in this study. The two Sporothrix spp. isolates from the United States were obtained from human (N = 2) cases received by the U.S. Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch (MDB) laboratory (Atlanta, United States) for diagnosis purposes and were randomly chosen to be included in the study. Details of phylogenetic analysis, epidemiologic, and demographic information of cat and human cases associated with the isolates were previously described (15) and can be found at NCBI BioProject ID: PRJNA957313.
Antifungal susceptibility testing
Fifty-six S. brasiliensis, six S. schenckii, and one S. globosa isolates were used in this study. AFST was performed by broth microdilution as outlined in the CLSI reference standard M38 for filamentous fungi (16). Isolates Aspergillus fumigatus ATCC MYA-3626 and A. fumigatus MYA-3627 were included as quality controls. Fluconazole, voriconazole, itraconazole, isavuconazole, posaconazole, anidulafungin, caspofungin, and micafungin were tested. The MICs were determined visually after 48–72 hours of incubation at 35°C. For isavuconazole, itraconazole, posaconazole, and voriconazole, the MIC endpoint was the lowest concentration that produced complete inhibition of growth and for echinocandins, it was the lowest concentration producing a visual change in the appearance of the growth (MEC). Although there are no breakpoints for Sporothrix and itraconazole, an MIC value of ≥16 µg/mL was presumed to be resistant. Data visualizations were created with ggplot2 and ggpubr R packages.
Single-nucleotide polymorphism and phylogenetic analysis
SNPs were identified from WGS data using MycoSNP v 1.4 (https://github.com/CDCgov/mycosnp-nf/) as described by Bagal et al. (29) . The MycoSNP pipeline generated the ML tree and VCFs from the filtered SNPs calling file. ML tree was visualized together with metadata containing additional epidemiologic data for each sample using Microreact (http://microreact.org). Using the VCFs and SnpEff (v 5.1) (30), we searched for SNPs in the cyp51 gene orthologue (SPBR_08369 for S. brasiliensis and SPSK_09044 for S. schenckii) (31) in all analyzed genomes. All nonsynonymous polymorphisms in the respective cyp51 genes present only in resistant isolates were retrieved. Protein cytochrome P450, family 51 (Sterol 14-demethylase) orthologues in S. brasiliensis, S. schenckii, and A. fumigatus were aligned using Geneious Prime v 2021.0.3 software to find the corresponding amino acid positions for each species.
ACKNOWLEDGMENTS
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control (CDC).
Contributor Information
Amanda Ribeiro dos Santos, Email: tbu6@cdc.gov.
Damian J. Krysan, The University of Iowa, Iowa City, Iowa, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.01620-23.
This file contains supplementary analysis done for Sporothrix schenckii isolates, as well as complete results of MIC of each isolate included in this study.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Rodrigues AM, Gonçalves SS, de Carvalho JA, Borba-Santos LP, Rozental S, Camargo Z de. 2022. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi (Basel) 8:776. doi: 10.3390/jof8080776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Córdoba S, Isla G, Szusz W, Vivot W, Hevia A, Davel G, Canteros CE. 2018. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses 61:441–448. doi: 10.1111/myc.12760 [DOI] [PubMed] [Google Scholar]
- 3. Etchecopaz AN, Lanza N, Toscanini MA, Devoto TB, Pola SJ, Daneri GL, Iovannitti CA, Cuestas ML. 2020. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J Mycol Med 30:100908. doi: 10.1016/j.mycmed.2019.100908 [DOI] [PubMed] [Google Scholar]
- 4. Thomson P, González C, Blank O, Ramírez V, Río CD, Santibáñez S, Pena P. 2023. Sporotrichosis outbreak due to Sporothrix Brasiliensis in domestic cats at Magallanes, Chile: A one-health-approach study. J Fungi (Basel) 9:226. doi: 10.3390/jof9020226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cognialli RCR, Cáceres DH, Bastos F de AGD, Cavassin FB, Lustosa BPR, Vicente VA, Breda GL, Santos-Weiss I, Queiroz-Telles F. 2023. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011–2022. Emerg Infect Dis 29:1330–1339. doi: 10.3201/eid2907.230155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orofino-Costa R, Macedo PM de, Rodrigues AM, Bernardes-Engemann AR. 2017. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol 92:606–620. doi: 10.1590/abd1806-4841.2017279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almeida-Paes R, Oliveira MME, Freitas AFS, Valle ACF, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. 2017. Refractory Sporotrichosis due to Sporothrix Brasiliensis in humans appears to be unrelated to in vivo resistance. Med Mycol Open Access 55:507–217. [DOI] [PubMed] [Google Scholar]
- 8. Nakasu CCT, Waller SB, Ripoll MK, Ferreira MRA, Conceição FR, Gomes ADR, Osório L da G, de Faria RO, Cleff MB. 2021. Feline sporotrichosis: a case series of itraconazole-resistant Sporothrix brasiliensis infection. Braz J Microbiol 52:163–171. doi: 10.1007/s42770-020-00290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gremião IDF, Martins da Silva da Rocha E, Montenegro H, Carneiro AJB, Xavier MO, de Farias MR, Monti F, Mansho W, de Macedo Assunção Pereira RH, Pereira SA, Lopes-Bezerra LM. 2021. Guideline for the management of feline sporotrichosis caused by Sporothrix brasiliensis and literature revision. Braz J Microbiol 52:107–124. doi: 10.1007/s42770-020-00365-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kauffman CA, Bustamante B, Chapman SW, Pappas PG, Infectious Diseases Society of America . 2007. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the infectious diseases society of America clinical infectious diseases. Clin Infect Dis 45:1255–1265. doi: 10.1086/522765 [DOI] [PubMed] [Google Scholar]
- 11. Gremião IDF, Miranda LHM, Reis EG, Rodrigues AM, Pereira SA. 2017. Zoonotic epidemic of sporotrichosis: cat to human transmission. PLoS Pathog 13:e1006077. doi: 10.1371/journal.ppat.1006077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fischman Gompertz O, Rodrigues AM, Fernandes GF, Bentubo HDL, de Camargo ZP, Petri V. 2016. Atypical clinical presentation of sporotrichosis caused by Sporothrix globosa resistant to itraconazole. Am J Trop Med Hyg 94:1218–1222. doi: 10.4269/ajtmh.15-0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanchotene KO, Brandolt TM, Klafke GB, Poester VR, Xavier MO. 2017. In vitro susceptibility of Sporothrix brasiliensis: Comparison of yeast and Mycelial phases. Med Mycol Open Access 55:869–876. doi: 10.1093/mmy/myw143 [DOI] [PubMed] [Google Scholar]
- 14. Espinel-Ingroff A, Abreu DPB, Almeida-Paes R, Brilhante RSN, Chakrabarti A, Chowdhary A, Hagen F, Córdoba S, Gonzalez GM, Govender NP, et al. 2017. Multicenter, international study of MIC/MEC distributions for definition of epidemiological cutoff values for Sporothrix species identified by molecular methods. Antimicrob Agents Chemother 61:e01057-17. doi: 10.1128/AAC.01057-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santos AR, Misas E, Min B, Ngoc L, Bagal UR, Parnell LA, Sexton DJ, Lockhart SR, Melhem MSC, Takahashi JPF, Oliboni GM, Bonfieti LX, Capellano P, Sampaio JLM, Araujo LS, Alves-Filho HL, Venturini J, Chiller TM, Litvintseva AP, Chow NA. 2024. Emergence of Zoonotic Sporotrichosis in Brazil: A Genomic epidemiology study in press. [DOI] [PMC free article] [PubMed]
- 16. CaLSI . 2017. CLSI M38 reference method for broth dilution antifungal susceptibility testing of Filamentous fungi, p 62 [Google Scholar]
- 17. Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failur. Emerg Infect Dis 15:1068–1076. doi: 10.3201/eid1507.090043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krishnan S, Alangaden G, Chandrasekar PH. 2003. Cytochrome P450 14-alpha-sterol demethylase mutation dependent triazole cross-resistance in Aspergillus fumigatus Conference on Antimicrobial Agents and Chemotherapy- Chicago, IL, USA, p 14–17 [Google Scholar]
- 19. Almeida-Paes R, Brito-Santos F, Figueiredo-Carvalho MHG, Machado ACS, Oliveira MME, Pereira SA, Gutierrez-Galhardo MC, Zancopé-Oliveira RM. 2017. Minimal inhibitory concentration distributions and epidemiological cutoff values of five antifungal agents against Sporothrix brasiliensis. Mem Inst Oswaldo Cruz 112:376–381. doi: 10.1590/0074-02760160527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galhardo MCG, De Oliveira RMZ, Valle A, Paes RDA, Silvatavares PME, Monzon A, Mellado E, Rodriguez-Tudela JL, Cuenca-Estrella M. 2008. Molecular epidemiology and antifungal susceptibility patterns of Sporothrix schenckii isolates from a cat-transmitted epidemic of sporotrichosis in Rio de Janeiro. Med Mycol 46:141–151. doi: 10.1080/13693780701742399 [DOI] [PubMed] [Google Scholar]
- 21. Gonçalves SS, da Cruz Bahiense Rocha I, Rediguieri BC, de Carvalho JA, Maifrede SB, Kruschewsky WLL, Falqueto A, Rodrigues AM. 2023. Human and feline sporotrichosis in a reference center of southeastern Brazil: genetic differentiation, diversity, and antifungal susceptibility of Sporothrix species. J Fungi (Basel) 9:831. doi: 10.3390/jof9080831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodrigues AM, de Hoog GS, de Cássia Pires D, Brihante RSN, Sidrim JJ da C, Gadelha MF, Colombo AL, de Camargo ZP. 2014. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect Dis 14:219. doi: 10.1186/1471-2334-14-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernardes-Engemann AR, Tomki GF, Rabello V de S, Almeida-Silva F, Freitas DFS, Gutierrez-Galhardo MC, Almeida-Paes R, Zancopé-Oliveira RM. 2022. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol 12:893501. doi: 10.3389/fcimb.2022.893501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waller SB, Dalla Lana DF, Quatrin PM, Ferreira MRA, Fuentefria AM, Mezzari A. 2021. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol 52:73–80. doi: 10.1007/s42770-020-00307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matowane RG, Wieteska L, Bamal HD, Kgosiemang IKR, Van Wyk M, Manume NA, Abdalla SMH, Mashele SS, Gront D, Syed K. 2018. In silico analysis of cytochrome P450 monooxygenases in chronic granulomatous infectious fungus Sporothrix schenckii: special focus on CYP51. Biochim Biophys Acta Proteins Proteom 1866:166–177. doi: 10.1016/j.bbapap.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 26. Teixeira MM, Almeida-Paes R, Bernardes-Engemann AR, Nicola AM, de Macedo PM, Valle ACF, Gutierrez-Galhardo MC, Freitas DFS, Barker BM, Matute DR, Stajich JE, Zancopé-Oliveira RM. 2022. Single nucleotide polymorphisms and chromosomal copy number variation may impact the Sporothrix brasiliensis antifungal susceptibility and sporotrichosis clinical outcomes. Fungal Genet Biol 163:103743. doi: 10.1016/j.fgb.2022.103743 [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Li L, Lv Q, Yan L, Wang Y, Jiang Y. 2019. The fungal CYP51S: their functions, structures, related drug resistance, and inhibitors. Front Microbiol 10:691. doi: 10.3389/fmicb.2019.00691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med Mycol 49 Suppl 1:S90–5. doi: 10.3109/13693786.2010.508469 [DOI] [PubMed] [Google Scholar]
- 29. Bagal UR, Phan J, Welsh RM, Misas E, Wagner D, Gade L, Litvintseva AP, Cuomo CA, Chow NA. 2022. Mycosnp: A portable Workflow for performing whole-genome sequencing analysis of Candida auris. Methods Mol Biol 2517:215–228. doi: 10.1007/978-1-0716-2417-3_17 [DOI] [PubMed] [Google Scholar]
- 30. Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, Land SJ, Lu X, Ruden DM. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 6:80–92. doi: 10.4161/fly.19695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teixeira MM, de Almeida LGP, Kubitschek-Barreira P, Alves FL, Kioshima ES, Abadio AKR, Fernandes L, Derengowski LS, Ferreira KS, Souza RC, et al. 2014. Comparative genomics of the major fungal agents of human and animal sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genomics 15:943. doi: 10.1186/1471-2164-15-943 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains supplementary analysis done for Sporothrix schenckii isolates, as well as complete results of MIC of each isolate included in this study.