Abstract
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), represents a substantial healthcare challenge. Provoked and unprovoked DVT cases carry distinct risks and treatment considerations. Recognizing the limitations of this classification, molecular markers may enhance diagnostic precision and guide anticoagulation therapy duration relying on patient history and risk factors. This preliminary, open-label, prospective cohort study was conducted including 15 patients (10 provoked DVT and 5 unprovoked DVT) and a control group of healthy plasmatic subjects. Plasma levels of 9 biomarkers were measured at diagnosis (baseline, day 0, and D0) and after 30 days (day 30-D30). Patient demographics, clinical data, and biomarker concentrations were analyzed. Serum concentrations of D-dimer, von Willebrand factor, C-reactive protein, and Anti-Xa were elevated in DVT groups at D0 compared to controls. No significant differences were observed between the provoked and unprovoked groups on the day of diagnosis and 30 days later. Over 30 days, the provoked group exhibited significant biomarker changes related to temporal assessment. No significant differences were noted in the biomarker profile between provoked and unprovoked DVT groups. This study is indicative of the concept of individualized thrombosis assessment and subsequent treatment for VTE. Larger cohorts are warranted to validate these findings and further define the most appropriate use of the molecular markers.
Keywords: venous thromboembolism, thrombosis biomarkers, provoked and unprovoked deep venous thrombosis, risk factors, thrombo-inflammation
Introduction
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), represents a substantial global burden in terms of morbidity and mortality. 1 Data from 2021 suggests the occurrence of nearly 10 million cases worldwide, with an annual incidence of 1 to 2 cases per 1000 population, rising exponentially with age for both men and women. 2
DVT varies between provoked and unprovoked instances. Provoked DVT results from a temporary environmental risk factor, such as surgery, trauma, immobilization, pregnancy, or puerperium. Unprovoked DVT occurs without any discernible provoking risk factors.3,4
Patients with provoked DVT exhibit a reduced risk of recurrence when compared to individuals with unprovoked DVT.5,6 Individuals with unprovoked PE exhibit elevated Wells scores and a more significant thrombotic burden when contrasted with provoked PE cases. 7 A first occurrence of unprovoked isolated DVT carries 2.1 times higher recurrent VTE episodes than a first episode of unprovoked PE. 8 This suggests that there may be variations in the natural progression and outcomes of provoked and unprovoked VTE. 6
The duration of treatment for VTE varies based on whether it is provoked or unprovoked. Guidelines have long advised a 3-month treatment period for VTE provoked by a reversible risk factor or a first unprovoked isolated distal DVT, given the low risk of recurrences.9,10 In contrast, patients with unprovoked proximal DVT or PE are recommended to undergo extended or indefinite-duration anticoagulation therapy due to the increased risk of recurrences.5,11 Despite the influence of this classification on clinical decisions, it still relies mainly on the precise collection of patient history and is susceptible to memory and information biases.
Nevertheless, it is known that recognizing molecular markers can aid in diagnosis, determining the duration of anticoagulant therapy, and forecasting the thrombus’ biological activity when caring for high-risk patients who may gain from extended anticoagulation.12,13
Markers such as D-dimer, 14 von Willebrand factor (vWF), 15 C-reactive protein (CRP), 16 thrombin generation (TG), 17 plasminogen activator inhibitor 1 (PAI-1), 18 thrombin-activatable fibrinolysis inhibitor (TAFI), 19 and neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR)20,21 have been linked to outcomes in the treatment of VTE and the risk of recurrences. Additional markers, such as the anti-factor Xa (Anti-Xa), while not yet established to provide reliable outcomes, are still employed in clinical practice as indicators for anticoagulation therapy.22,23
To the best of our understanding, no previous studies have assessed the distinct biomarker profile of provoked and unprovoked DVT cases. In this study, we introduce a preliminary prospective cohort of 20 patients to examine the serum levels of 9 biomarkers at diagnosis and after 30 days of follow-up in both provoked and unprovoked DVT patient groups to establish a comparative biomarker profile for these patients.
Methods
Study Design and Patient Enrolment
This was a preliminary, open-label, prospective cohort study comprising 2 follow-up arms: one for provoked DVT and another for unprovoked DVT, with a control group consisting of healthy individuals. Patient recruitment occurred between February and December 2022 at the Vascular Ultrasound Department of the Dante Pazzanese Cardiology Institute in São Paulo, Brazil.
A control group was included exclusively for plasmatic assessments, utilizing plasma from healthy volunteers produced by George King Biomedical, Inc. (Overland Park, KS, USA).
The laboratory assessments were conducted at the Hemostasis & Thrombosis Research Laboratories of Loyola University, Chicago, USA.
Hyphen Biomed provided the ELISA kit used in this study. Complete identification of the kit, including the manufacturer and reference number, was recorded to ensure traceability and transparency in the experimental process.
This study adhered to the principles outlined in the Helsinki Declaration and received approval from the Ethics Committee of the Dante Pazzanese Cardiology Institute under protocol number 40421120.2.0000.5462. Before participation, all patients received comprehensive information about the study's objectives and procedures, and signed informed consent was obtained from all participants.
All data was handled confidentially and de-identified.
Patients were included following a positive diagnosis of lower- or upper-limb DVT, confirmed by a venous Doppler ultrasound of the limbs after clinical suspicion.
Patients who declined to sign the informed consent form, those already on therapeutic doses of anticoagulant medication for DVT for over 48 h before diagnosis, or individuals with contraindications to anticoagulation, including intracranial bleeding, active bleeding, active gastroduodenal ulcers, and blood disorders were excluded from the study.
Demographic and Clinical Data Collection
Upon enrolment, all patient's medical history, information on age, gender, race, weight, height, body mass index (BMI), preexisting medical conditions, and signs and symptoms of DVT were collected. Patients were also stratified according to their Wells score and quality of life (QoL) status as assessed by the EQ-5D-5L questionnaire.
In a 30-day follow-up visit, patients were reassessed regarding QoL status and interviewed about the use of concurrent medications and the occurrence of adverse events (AEs) and serious adverse events (SAEs) during treatment following the International Society on Thrombosis and Haemostasis (ISTH) definitions. 24
Serum Biomarker Collection
Serum biomarkers were collected on the day of diagnosis (D0) and after 30 days of treatment (D30).
The blood samples were adequately collected and stored for the assessment of D-dimer, von Willebrand factor, C-reactive protein, thrombin generation, anti-factor Xa; PAI-1, TAFI, NLR, and PLR.
All blood collections and laboratory analyses were conducted following the Hemostasis & Thrombosis Research Laboratories guidelines of Loyola University. The frozen samples were sent on dry ice and arrived at their destination within 24 to 48 h at the partner laboratory of this research.
The plasma levels of various biomarkers involved in this study were analyzed using commercially available sandwich enzyme-linked immunosorbent assays (ELISA). D-dimer, PAI-1 antigen, TAFI (TAFIa), von Willebrand factor (vWF), C-reactive protein (CRP), thrombin, and coagulation factor X were sourced from HYPHEN BioMed (Neuville dur Oise, France). The closed-kit protocol was employed for conducting the assays for all the studied biomarkers. The citrated plasma control was obtained from healthy volunteers without comorbidities, sourced from the commercial supplier George King Biomedical, Inc., based in Overland Park, Kansas, USA.
Group Selection
The patients were divided into “Provoked DVT” and “Unprovoked DVT” groups following the ISTH definitions 3 based on their clinical history.
Statistical Analysis
Demographic and clinical data, as well as relative frequencies and mean values, were presented.
The data for inflammatory biomarkers from the groups of provoked and unprovoked DVT and the plasmatic control group were presented with the median and interquartile interval values. The serum concentration of all biomarkers was compared between groups at D0 and D30. In-group concentration differences between the D0 and D30 were also assessed. All statistical analyses were conducted using the SPSS software, version 25.0. Due to the small sample sizes, nonparametric tests were employed for data analysis, with a significance level set at P < .05. Significant data were represented using boxplots.
Results
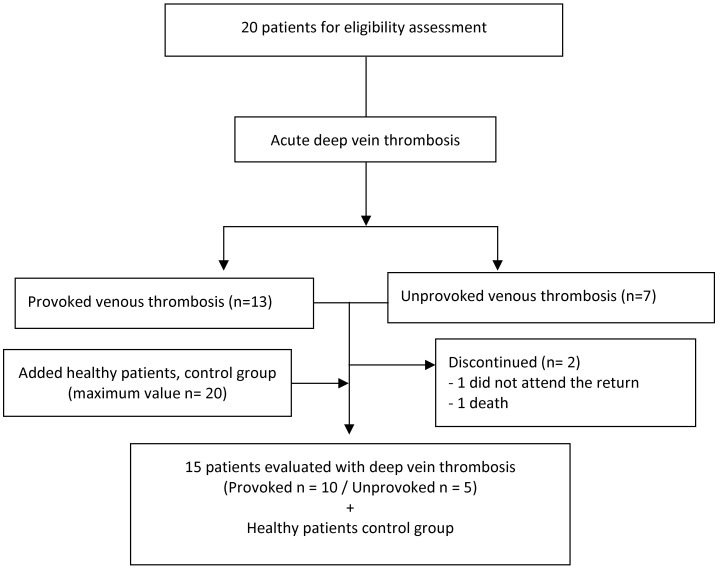
Fifteen patients with DVT were assessed, comprising 10 with provoked venous thrombosis and 5 with unprovoked venous thrombosis, as presented in the CONSORT diagram, as shown in Figure 1.
Figure 1.
Consort diagram.
Among patients with provoked venous thrombosis, the most common comorbidities were hypertension (60%) and diabetes mellitus (50%), while in the unprovoked group, hypertension (80%), coronary artery disease (40%), and cardiac insufficiency (40%) were prevalent. Previous surgeries in the provoked group were percutaneous coronary angioplasty followed by open pericardiotomy, reconstruction of the ascending aorta and resuspension of the aortic valve, angiography and debridement of the lower limb for peripheral obstructive arterial disease (PAD), varicose veins surgery with associated foam sclerotherapy, and myocardial revascularization for coronary disease. Patient demographic and clinical characteristics are detailed in Table 1.
Table 1.
Demographic Characteristics and Comorbidities of Patients, Quantitative Variables Analyzed Using the Mann-Whitney Test, and Qualitative Variables Using Fisher's Exact Test or Chi-Square.
| Characteristics | Provoked | Unprovoked | P value |
|---|---|---|---|
| Age years | 62.2 (50.1; 73.7) | 69.8 (55.5; 79.0) | .462* |
| Weight (kg) | 68.0 (67.8; 87.3) | 86.0 (56.5; 106.0) | .354* |
| Height (cm) | 161.5 (155.8; 166.3) | 174.0 (162.5; 182.5) | .057* |
| Body mass index | 26.9 (24.6; 34.5) | 31.7 (19.2; 32.9) | .759* |
| Health perception | 50.0 (47.8; 80.0) | 50.0 (45.0; 71.0) | .750* |
| Wells score | 4.4 (2.8; 6.0) | 3.0 (1.5; 4.5) | .750* |
| Female sex - n (%) | 6 (60.0%) | 2 (40.0%) | .608** |
| Ethnicity | |||
| White | 4 (40%) | 2 (40.0%) | .861*** |
| Black | 1 (10.0%) | 1 (20.0%) | |
| Other | 5 (50.0%) | 2 (40.0%) | |
| Previous illnesses | |||
| Previous thrombosis | 3 (30.0%) | 1 (20.0%) | 1000** |
| Hypertension | 6 (60.0%) | 4 (80.0%) | .600** |
| Diabetes | 5 (50.0%) | 1 (20.0%) | .580** |
| Renal insufficiency | 0 (0.0%) | 1 (20.0%) | .333** |
| Coronary artery disease | 1 (10.0%) | 2 (40.0%) | .242** |
| Stroke | 1 (10.0%) | 1 (20.0%) | 1000** |
| Lung disease | 0 (0.0%) | 1 (20.0%) | .333** |
| Cardiac insufficiency | 3 (30.0%) | 2 (40.0%) | 1000** |
| Active smoking | 2 (20.0%) | 1 (20.0%) | 1000** |
| Heart transplant | 1 (10.0%) | 0 (0.0%) | 1000** |
| Others | 8 (80.0%) | 1 (20.0%) | .089** |
Quantitative variables – Median (Q1; Q3) – * Mann-Whitney test.
Qualitative variables – N and % – ** Fisher's exact test or *** chi-square.
The average Wells score in the provoked thrombosis group was 4.4 and 3.0 in the unprovoked group. Most patients with provoked and unprovoked DVT (60%) had distal vein involvement. In the provoked thrombosis group, the main veins affected were popliteal (60.0%) and peroneal (50.0%), and in the unprovoked group, the muscular veins (80.0%) and the popliteal vein (40.0%). When evaluating each group individually, the provoked group had more affected proximal veins than the unprovoked group, as depicted in Table 2 (veins affected by deep vein thrombosis according to the provoked and unprovoked groups, analysis using Fisher's exact test). Table 3 describes the main symptoms presented at the time of diagnosis, and analysis between the provoked and unprovoked groups with Fisher's exact test.
Table 2.
Veins Affected by Deep Vein Thrombosis (DVT) According to the Provoked and Unprovoked Groups, Analysis Using Fisher's Exact Test.
| Location of thrombosis | Provoked | Unprovoked | P value ** |
|---|---|---|---|
| N (%) | |||
| Proximal DVT | |||
| Iliac | 2 (20.0) | 0 (0.0) | 0.524 |
| Common femoral | 4 (40.0) | 1 (20.0) | 0.600 |
| Femoral | 4 (40.0) | 1 (20.0) | 0.600 |
| Deep femoral | 3 (30.0) | 0 (0.0) | 0.505 |
| Subclavian | 2 (20.0) | 0 (0.0) | 0.524 |
| Axillary | 2 (20.0) | 0 (0.0) | 0.524 |
| Brachial | 2 (20.0) | 0 (0.0) | 0.524 |
| Distal DVT | |||
| Popliteal | 6 (60.0) | 2 (40.0) | 0.608 |
| Tibial anterior | 1 (10.0) | 0 (0.0) | 1000 |
| Posterior tibial | 4 (40.0) | 1 (20.0) | 0.600 |
| Fibular | 5 (50.0) | 1 (20.0) | 0.580 |
| Muscles | 3 (30.0) | 4 (80.0) | 0.119 |
Fisher's exact test.
Table 3.
Main Symptoms Presented at the Time of Diagnosis, Analysis Between the Provoked and Unprovoked Groups with Fisher's Exact Test.
| Provoked | Unprovoked | P-value** | |
|---|---|---|---|
| Symptoms | N (%) | ||
| Spontaneous pain | 8 (80.0%) | 3 (60.0%) | 0.560 |
| Edema | 9 (90.0%) | 3 (60.0%) | 0.242 |
| Erythema | 5 (50.0%) | 1 (20.0%) | 0.580 |
| Cyanosis | 3 (30.0%) | 0 (0.0%) | 0.505 |
| Dilation of the superficial venous system | 6 (60.0%) | 1 (20.0%) | 0.282 |
| Temperature increase | 4 (40.0%) | 1 (20.0%) | 0.600 |
| Muscle filling | 8 (80.0%) | 2 (40.0%) | 0.251 |
| Pain on palpation | 8 (80.0%) | 4 (80.0%) | 1000 |
Fisher's exact test.
The was no difference in QoL status as assessed by the EQ-5D-5L questionnaire (health perception mean rank 8.25 vs 7.50, P = .750).
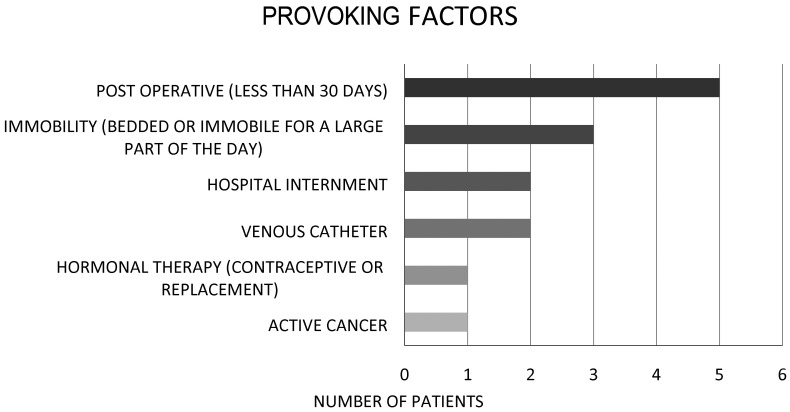
The frequency of DVT-provocating factors is listed in Figure 2.
Figure 2.
Factors that provoked deep vein thrombosis in the provoked group.
The analysis of the day of diagnosis between control groups of healthy patients and those with unprovoked and provoked venous thrombosis (using the Kruskal–Wallis test) and the comparison of unprovoked group versus control and provoked group versus control (using the Mann–Whitney test) are summarized in Table 4.
Table 4.
Analysis of the Day of Diagnosis Between Control Groups of Healthy Patients and Those With Unprovoked and Provoked Venous Thrombosis Using the Kruskal-Wallis Test. Analysis of Unprovoked Group Versus Control and Provoked Group Versus Control Using the Mann-Whitney Test.
| Biomarker | Control | Unprovoked | Provoked | Kruskal-Wallis (P value) | Mann-Whitney (P value) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median (Q1; Q3) | 95% CI | N | Median (Q1; Q3) | 95% CI | N | Median (Q1; Q3) | 95% CI | Control × unprovoked |
Control × provoked |
||
| Anti-Xa (%) | 19 | 32.5 (14.3; 34.6) | 38.0 (14.0; 61.0) | 5 | 45.0 (36.9; 62.3) | 49.0 (31.0; 66.0) | 10 | 40.2 (32.3; 51.9) | 43.0 (33.0; 53.0) | .016 | .015 | .043 |
| PCR (ng/mL) | 9 | 1138.3 (329.2; 5667.0) | 2776.0 (355.0; 5198.0) | 5 | 11 356.3 (3567.2; 13 580.2) | 9139.0 (2538.0; 15 723.0) | 10 | 14 629.7 (7500.8; 15 978.6) | 11 815.0 (7930.0; 15 700.0) | .003 | .002 | .028 |
| D-dimer (ng/mL) | 20 | 0.0 (0.0; 155.7) | 98.0 (7.0; 189.0) | 5 | 4210.1 (2025.2; 10 581.9) | 5885.0 (416.0; 1353.0) | 10 | 7922.3 (2095.6; 14 416.0) | 8397.0 (4179.0; 12 616.0) | <.001 | <.001 | <.001 |
| PAI-1 (ng/mL) | 9 | 24.1 (19.4; 27.8) | 24.0 (18.0; 31.0) | 5 | 48.4 (9.1; 55.6) | 36.0 (5.0; 66.0) | 10 | 44.1 (21.3; 56.7) | 41.0 (26.0; 56.0) | .295 | – | – |
| TAFI (%) | 9 | 108.7 (105.4; 115.9) | 110.0 (105.0; 115.0) | 5 | 109.3 (104.9; 116.4) | 110.0 (101.0; 120.0) | 10 | 114.2 (105.9; 122.4) | 114.0 (107.0; 121.0) | .567 | – | – |
| GT (%) | 19 | 158.0 (134.9; 196.7) | 165.0 (149.0; 181.0) | 5 | 73.5 (61.3; 167.8) | 106.0 (16.0; 197.0) | 10 | 137.5 (71.0; 170.8) | 125.0 (84.0; 166.0) | .072 | – | – |
| vWF (%) | 9 | 106.4 (91.1; 112.0) | 105.0 (91.0; 117.0) | 5 | 138.8 (120.4; 155.3) | 138.0 (114.0; 162.0) | 10 | 143.5 (137.4; 157.1) | 146.0 (137.0; 156.0) | .001 | < .001 | .014 |
Abbreviations: vWF, von Willebrand factor; PAI-1, plasminogen activator inhibitor 1; NLR, neutrophil-to-lymphocyte ratio; TAFI, thrombin-activatable fibrinolysis inhibitor; PCR, polymerase chain reaction; GT: group testing.
Concentrations of Anti-Xa, CRP, D-dimer, and vWF were significantly elevated in the thrombosis groups when compared to healthy patients. Significant differences were observed between the provoked and unprovoked groups compared to the control in 4 of the 7 tested biomarkers.
Serum concentration did not differ, at D0, between the provoked and unprovoked groups, as shown in Table 5.
Table 5.
Analysis Using the Mann-Whitney Test of Markers Between the Provoked and Unprovoked Groups on the Day of Diagnosis and 30 Days Later.
| Biomarker | Diagnosis (D0) | P value * | 30th | P value * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unprovoked (n = 5) | Provoked (n = 10) | Unprovoked (n = 5) | Provoked (n = 10) | |||||||
| Anti-Xa (%) | 45.0 (36.9; 62.3) | 49.0 (31.0; 66.0) | 40.2 (32.3; 51.9) | 43.0 (33.0; 53.0) | .270 | 57.9 (32.0; 79.0) | 56.0 (26.0; 86.0) | 57.6 (46.0; 76.0) | 59.0 (43.0; 54.0) | .759 |
| PCR (ng/mL) | 11 356.3 (3567.2; 13 580.2) | 9130.0 (2538.0; 15 723.0) | 14 629.7 (7500.8; 15 978.6) | 11 815.0 (7930.0; 15 700.0) | .221 | 6575.0 (4093.0; 10 499.0) | 7152.0 (2759.0; 11 545.0) | 4338.0 (930.0; 10 545.0) | 5061.0 (1521.0; 8600.0) | .270 |
| D-dimer (ng/mL) | 4210.1 (2025.2; 10 581.9) | 5885.0 (416.0; 11 353.0) | 7922.3 (2095.6; 14 416.0) | 8397.0 (4179.0; 12 616.0) | .462 | 2710.0 (546.0; 8410.0) | 4124.0 (−1645.0; 9894.0) | 789.9 (186.0; 2048.0) | 1473.0 (−161.0; 3108.0) | .178 |
| PAI-1 (ng/mL) | 48.4 (9.1; 55.6) | 36.0 (5.0; 66.0) | 44.1 (21.3; 56.7) | 41.0 (26.0; 56.0) | .668 | 23.7 (14.0; 73.0) | 40.0 (-14.0; 93.0) | 19.9 (14.0; 31.0) | 30.0 (10.0; 50.0) | .540 |
| TAFI (%) | 109.3 (104.9; 116.4) | 110.0 (101.0; 120.0) | 114.2 (105.9; 122.4) | 114.0 (107.0; 121.0) | .462 | 100.5 (90.0; 109.0) | 100.0 (86.0; 113.0) | 108.9 (101.0; 114.0) | 108.0 (102.0; 113.0) | .221 |
| GT (%) | 73.5 (61.3; 167.8) | 106.0 (16.0; 197.0) | 137.5 (71.0; 170.8) | 125.0 (84.0; 166.0) | .462 | 31.9 (24.0; 133.0) | 69.0 (-14.0; 152.0) | 74.8 (59.0; 145.0) | 98.0 (48.0; 147.0 | .391 |
| vWF (%) | 138.8 (120.4; 155.3) | 138.0 (114.0; 162.0) | 143.5 (137.4; 157.1) | 146.0 (137.0; 156.0) | .462 | 132.8 (117.0; 153.0) | 134.0 (112.0; 157.0) | 130.7 (113.0; 141.0) | 130.0 (111.0; 148.0) | .713 |
| Hemoglobin (g/dL) | 11.6 (10.5; 16.3) | 13.0 (9.0; 17.0) | 12.6 (10.8; 14.7) | 12.9 (11.3; 14.5) | .903 | 11.5 (10.0; 15.0) | 13.0 (9.0; 17.0) | 12.7 (11.0; 14.0) | 12.8 (11.2; 14.4) | .354 |
| Hematocrit (%) | 36.0 (33.8; 48.2) | 39.9 (30.3; 49.6) | 38.0 (33.9; 45.8) | 39.5 (34.5; 44.5) | .951 | 37.0 (33.0; 43.0) | 39.9 (30.3; 49.6) | 37.6 (35.0; 42.0) | 39.0 (34.1; 43.9) | .877 |
| Leukocytes ×10³/mm³) | 6.9 (4.0; 8.8) | 6.5 (3.4; 9.5) | 9.0 (8.2; 11.9) | 10.0 (7.1; 12.8) | .086 | 5.4 (4.0; 6.0) | 6.5 (3.4; 9.5) | 6.6 (5.0; 10.0) | 9.8 (6.2; 13.5) | .165 |
| Total lymphocytes ×10³/mm³) | 1.8 (1.2; 2.8) | 2.0 (0.8; 3.1) | 1.7 (1.3; 2.5) | 2.0 (1.2; 2.8) | .903 | 1.6 (1.0; 2.0) | 2.0 (0.8; 3.1) | 1.5 (1.0; 3.0) | 2.0 (1.0; 3.0) | .877 |
| Platelets (×10³/mm³) | 182.0 (131.5; 221.0) | 177.4 (99.3; 255.5) | 220.5 (155.0; 261.0) | 208.9 (150.5; 267.2) | .391 | 183.5 (145.0; 274.0) | 177.4 (99.3; 255.5) | 194.0 (183.0; 264.0) | 209.5 (139.8; 279.1) | .699 |
| NLR | 2.2 (1.3; 2.7) | 2.0 (1.1; 2.9) | 4.0 (2.0; 6.5) | 4.1 (2.4; 5.9) | .111 | 1.9 (1.0; 3.0) | 2.0 (1.1; 2.9) | 2.5 (2.0; 4.0) | 4.1 (1.9; 6.2) | .355 |
| PLR | 89.1 (67.3; 128.4) | 96.1 (54.3; 137.8) | 107.8 (57.5; 167.0) | 122.6 (68.4; 176.7( | .713 | 123.1 (93.0; 176.0) | 96.1 (54.3; 137.8) | 128.1 (79.0; 222.0) | 122.9 (56.5; 189.3) | .758 |
| PT INR | 1.0 (1.0; 1.2) | 1.0 (0.8; 1.2) | 1.0 (0.9; 1.1) | 1.0 (0.9; 1.0) | .500 | 1.1 (1.0; 3.0) | 1.0 (0.8; 1.2) | 1.0 (1.0; 1.0) | 0.9 (0.9; 1.0) | .589 |
| APTT | 0.93 (0.8; 1.0) | 0.9 (0.8; 1.0) | 0.9 (0.8; 1.0) | 0.9 (0.7; 1.0) | .759 | 1.0 (1.0; 1.0) | 0.9 (0.8; 1.0) | 1.0 (1.0; 1.0) | 0.8 (0.7; 0.9) | .487 |
| Fibrinogen (mg/dL) | 369.9 (230.6; 430.9) | 339.0 (209.0; 468.0) | 374.4 (297.3; 516.5) | 395.0 (278.0; 511.0) | .505 | 315.9 (260; 361) | 339.0 (209.0; 468.0) | 339.4 (254.0; 388.0) | 395.0 (278.0; 511.0) | .610 |
Abbreviations: vWF, von Willebrand factor; PAI-1, plasminogen activator inhibitor 1; PLR, platelet-to-lymphocyte ratio; LR, neutrophil-to-lymphocyte ratio; TAFI, thrombin-activatable fibrinolysis inhibitor; PCR, polymerase chain reaction; GT: group testing; APTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normatized ratio.
Median (Q1; Q3), * Mann-Whitney test; 95% CI.
Discussion
The increase in Anti-Xa, CRP, D-dimer, and VWF concentrations in patients with thrombosis (provoked and unprovoked) compared to the control group of healthy patients reinforces the already-known interaction between inflammation and thrombosis. These inflammatory biomarkers aid in diagnosing thrombotic events and offer potential as predictive indicators of recurrence. 25
Comparative investigation of biomarker levels in patients with provoked and unprovoked venous thrombosis revealed compelling results that challenge traditional categorizations of VTE and argue for a more differentiated, patient-centered approach. In this study, 2 groups were examined: 10 individuals with provoked venous thrombosis and 5 with unprovoked thrombosis. Laboratory tests covering D-dimer, PAI-1, CRP, vWF, TAFI, thrombin, Anti Xa, blood count, and coagulogram were performed on the day of diagnosis and again 30 days later.
At baseline, serum concentrations of selected biomarkers showed no differences between the provoked and unprovoked groups on the day of diagnosis, challenging prevailing assumptions about distinctions in thrombotic mechanisms between these 2 groups.
However, when considering the temporal evolution of biomarkers over a 30-day interval, it was observed that the challenged group had a significant reduction in CRP, D-dimer, and VWF values, along with an increase in Anti-Xa. This dynamic change in biomarker levels suggests a distinct response to thrombosis in the challenged group over time. These evolutionary marker changes were not found in the unprovoked group, where biomarker levels remained relatively stable between the 2-time points.
When comparing the provoked and unprovoked groups, the most striking difference was found in the delta values (D0–D30), specifically in plasma vWF levels. Patients with provoked DVT exhibited a more pronounced drop in vWF levels 30 days after baseline compared to their unprovoked counterparts. This subtle distinction highlights the need for a more individualized assessment based on specific biomarkers’ temporal evolution, challenging VTE's simplistic dichotomization into provoked and unprovoked categories.
The main objective of this study was to obtain preliminary information on serum levels of relevant biomarkers in provoked and unprovoked DVT patients to gain insight into the need to adjust VTE assessment and treatment in provoked and unprovoked DVT. The terms “provoked” and “unprovoked” have been criticized as this 2-fold designation. Many patients may exist within a spectrum of risk factor severity despite being included in one group or another. 26 Patients with unprovoked VTE have a high risk of recurrence. However, the concept that all patients with provoked VTE are considered generally with a low risk of VTE recurrence might be misleading. Patients with VTE provoked by persistent minor factors, such as heart failure, immobility, and frequent flyers with extended travel, have a cumulative risk of 15% over 5 years compared with 3% over 5 years in patients with a VTE provoked by major risk factor, such as orthopedic surgery. 27
The results of our study also highlight the importance of considering temporal changes in biomarker levels, particularly in the challenged group, where statistically significant changes were evident within 30 days.
The values found in the provoked group are higher than in the unprovoked group, which may be related to a greater thrombotic/inflammatory burden as this group of patients had a more significant number of proximal veins affected than the unprovoked group. In general, provoked thrombosis may result in a thrombotic load that is more predictable and related to the specific triggering event. In contrast, unprovoked thrombosis may be more unpredictable because it occurs without an apparent precipitating event. 28
The findings of this study resonate with the 2019 European Society of Cardiology (ESC) guideline that suggests that doctors abandon the dichotomization of VTE, preferring a more individualized assessment based on the patient's risk factors. This approach aligns with our results, highlighting the need for a personalized approach to anticoagulant treatment in patients with thrombosis, regardless of whether the thrombosis is provoked or unprovoked. 29
The current study has limitations, mainly the small sample size in each group, making it a pilot project. It is also important to notice that previous comorbidities such as hypertension and coronary artery disease were more prevalent in the unprovoked group, which may lead to biomarker bias. Additionally, because this was designed and approved as a preliminary study, there was an Institutional Review Board restriction that limited the number of healthy volunteers recruited, meaning that the control groups ought to be considered as reference only. Although the results provide valuable information, the generalizability of the results may be limited. Future research with larger cohorts is needed to validate and expand the implications of this pilot study.
Despite its limitations, this study presents, to the best of our knowledge, preliminary evidence into the biomarker profile of patients presenting with “provoked” and “unprovoked” DVT, and may present the basis for further research remarking on the importance of considering individual risk factors rather than a simple dichotomized classification, possibly altering therapeutic orientation as well as other clinical aspects such as electronic record forms, patient information material and others.
Conclusion
No significant differences in biomarker levels between provoked and unprovoked DVT groups were observed. This study is indicative of the concept of individualized thrombosis assessment and subsequent treatment for VTE. Larger cohorts are warranted to validate these findings and further define the most appropriate use of the molecular markers.
Acknowledgments
This study was partially supported by the Cardiovascular Research Institute of Loyola University, Chicago, USA, and by Science Valley Research Institute, Santo André, São Paulo, Brazil.
Footnotes
Authors’ Note: This article has not been presented anywhere. This article has not been published.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Srdjan Nikolovski https://orcid.org/0000-0002-3142-2199
Jawed Fareed https://orcid.org/0000-0003-3465-2499
Eduardo Ramacciotti https://orcid.org/0000-0002-5735-1333
References
- 1.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;2016:18(8):891-975. [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: Guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480-1483. DOI: 10.1111/jth.13336 [DOI] [PubMed] [Google Scholar]
- 3.Kovacs MJ, Kahn SR, Wells PS, et al. Patients with a first symptomatic unprovoked deep vein thrombosis are at higher risk of recurrent venous thromboembolism than patients with a first unprovoked pulmonary embolism. J Thromb Haemost. 2010;8(9):1926-1932. DOI: 10.1111/j.1538-7836.2010.03958.x [DOI] [PubMed] [Google Scholar]
- 4.Stoeva N, Kirova G, Staneva M, Lekova D, Penev A, Bakalova R. Recognition of unprovoked (idiopathic) pulmonary embolism-prospective observational study. Respir Med. 2018;135:57-61. DOI: 10.1016/j.rmed.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 5.Anghel L, Sascau R, Radu R, Statescu C. From classical laboratory parameters to novel biomarkers for the diagnosis of venous thrombosis. Int J Mol Sci. 2020;21(6). DOI: 10.3390/ijms21061920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman LR. In search of venous thromboembolism: the first 2913 years. AJR Am J Roentgenol. 2013;201(4):W576-W581. DOI: 10.2214/AJR.13.10604 [DOI] [PubMed] [Google Scholar]
- 7.Barnes DM, Wakefield TW, Rectenwald JE. Novel biomarkers associated with deep venous thrombosis: a comprehensive review. Biomark Insights. 2008;3(3):93-100. https://www.ncbi.nlm.nih.gov/pubmed/19578498https://doi.org/10.1177/117727190800300004 [PMC free article] [PubMed] [Google Scholar]
- 8.Ramacciotti E, Blackburn S, Hawley AE, et al. Evaluation of soluble P-selectin as a marker for the diagnosis of deep venous thrombosis. Clin Appl Thromb Hemost. 2011;17(4):425-431. DOI: 10.1177/1076029611405032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downing LJ, Wakefield TW, Strieter RM, et al. Anti-P-selectin antibody decreases inflammation and thrombus formation in venous thrombosis. J Vasc Surg. 1997;25(5):816-827; discussion 828. DOI: 10.1016/s0741-5214(97)70211-8. [DOI] [PubMed] [Google Scholar]
- 10.Aujesky D, Roy PM, Guy M, Cornuz J, Sanchez O, Perrier A. Prognostic value of D-dimer in patients with pulmonary embolism. Thromb Haemost. 2006;96(4):478-482. https://www.ncbi.nlm.nih.gov/pubmed/17003925 doi: 10.1160/TH06-07-0416 [DOI] [PubMed] [Google Scholar]
- 11.Kalodiki E, Fareed J, Syed D, Geroulakos G, Hoppensteadt D, Lattimer CR. Blood sampled directly from varicose veins reveals activation of inflammatory processes. J Vasc Surg Venous Lymphat Disord. 2015;3(1):119. DOI: 10.1016/j.jvsv.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 12.Lattimer CR, Kalodiki E, Geroulakos G, Hoppensteadt D, Fareed J. Endogenous pro-thrombotic biomarkers from the arm and leg may not have the same value. Phlebology. 2016;31(4):275-282. DOI: 10.1177/0268355515589678 [DOI] [PubMed] [Google Scholar]
- 13.Poredos P, Spirkoska A, Rucigaj T, Fareed J, Jezovnik MK. Do blood constituents in varicose veins differ from the systemic blood constituents? Eur J Vasc Endovasc Surg. 2015;50(2):250-256. DOI: 10.1016/j.ejvs.2015.04.031 [DOI] [PubMed] [Google Scholar]
- 14.Hou H, Ge Z, Ying P, et al. Biomarkers of deep venous thrombosis. J Thromb Thrombolysis. 2012;34(3):335-346. DOI: 10.1007/s11239-012-0721-y [DOI] [PubMed] [Google Scholar]
- 15.Delluc A, Le Mao R, Tromeur C, et al. Incidence of upper-extremity deep vein thrombosis in western France: a community-based study. Haematologica. 2019;104(1):e29-e31. DOI: 10.3324/haematol.2018.194951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170(19):1710-1716. DOI: 10.1001/archinternmed.2010.367 [DOI] [PubMed] [Google Scholar]
- 17.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2):e419S-e496S. DOI: 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484-3488. DOI: 10.1182/blood-2002-01-0108 [DOI] [PubMed] [Google Scholar]
- 19.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ. 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(6):761-768. DOI: 10.1001/archinte.160.6.761 [DOI] [PubMed] [Google Scholar]
- 20.Coleman DM, Wakefield TW. Biomarkers for the diagnosis of deep vein thrombosis. Expert Opin Med Diagn. 2012;6(4):253-257. DOI: 10.1517/17530059.2012.692674 [DOI] [PubMed] [Google Scholar]
- 21.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70(19):2411-2420. DOI: 10.1016/j.jacc.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 22.Dix C, Zeller J, Stevens H, et al. C-reactive protein, immunothrombosis and venous thromboembolism. Front Immunol. 2022;13(1002652). DOI: 10.3389/fimmu.2022.1002652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korte WC, Wuillemin WA, Caliezi C, Riesen WF. CRP Measured by a high sensitivity assay correlates with clinical probability (wells score) testing for deep venous thrombosis. Thromb Haemost. 2004;91(4):841-842. https://www.ncbi.nlm.nih.gov/pubmed/15045158 doi: 10.1055/s-0037-1614281 [DOI] [PubMed] [Google Scholar]
- 24.Bozic M, Blinc A, Stegnar M. D-dimer, other markers of haemostasis activation and soluble adhesion molecules in patients with different clinical probabilities of deep vein thrombosis. Thromb Res. 2002;108(2-3):107-114. DOI: 10.1016/s0049-3848(03)00007-0 [DOI] [PubMed] [Google Scholar]
- 25.Nagler M, Van Kuijk SMJ, Ten Cate H, Prins MH, Ten Cate-Hoek AJ. Predicting recurrent venous thromboembolism in patients with deep-vein thrombosis: development and internal validation of a potential new prediction model (continu-8). Front Cardiovasc Med. 2021;8(8):655226. DOI: 10.3389/fcvm.2021.655226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becattini C, Cimini LA. Provoked vs minimally provoked vs unprovoked VTE: Does it matter? Hematology Am Soc Hematol Educ Program 2023;2023(1):600-605. DOI: 10.1182/hematology.2023000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albertsen IE, Piazza G, Goldhaber SZ. Let's stop dichotomizing venous thromboembolism as provoked or unprovoked. Circulation. 2018;138(23):2591-2593. DOI: 10.1161/CIRCULATIONAHA.118.036548 [DOI] [PubMed] [Google Scholar]
- 28.Ageno W, Farjat A, Haas S, et al. Provoked versus unprovoked venous thromboembolism: findings from GARFIELD-VTE. Res Pract Thromb Haemost. 2021;5(2):326-341. DOI: 10.1002/rth2.12482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstantinides SV, Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2019;40(42):3453-3455. DOI: 10.1093/eurheartj/ehz726 [DOI] [PubMed] [Google Scholar]