Abstract
Introduction: Patients with ischemic stroke (IS) and atrial fibrillation (AF) face a higher risk of recurrent vascular events. This study evaluates the impact of atherosclerotic vascular disease burden across different vascular territories on the risk of vascular events in patients with recent ischemic stroke and AF within 90 days. Patients and Methods: We included patients with IS and AF from the International RAF network in a prospective 90-day follow-up. Atherosclerotic vascular disease was identified by at least one of the following: Symptomatic ischemic heart disease, symptomatic peripheral artery disease, internal carotid stenosis ≥50%, or the presence of plaques in the aorta. The primary outcome was a composite of stroke, transient ischemic attack, systemic embolism, cerebral bleeding, and major extracranial bleeding within 90 days postacute stroke. Patients were categorized into 5 groups based on the number of affected atherosclerotic vascular territories, with those with no atherosclerotic vascular disease as the reference. Kaplan–Meier curves were generated and compared using the log-rank test to determine the predictive value of the number of diseased territories for the risk of events. Data analysis was performed with SPSS/PC Win Package 25.0. Results: Of the 2148 patients (mean age 77.59; 53.86% female), 744 (34.60%) had atherosclerosis. Multivariable analysis revealed that involvement of 3 (hazard ratio [HR] 2.80, 95% confidence interval [CI]: 1.20-6.53) or 4 (HR 6.81, 95% CI: 1.02-36.24) vascular territories was significantly associated with the risk of combined events. Conclusions: In patients with recent ischemic stroke and AF, atherosclerosis across multiple territories correlates with a higher risk of future vascular events.
Keywords: stroke, ischemic stroke, atherosclerosis, atrial fibrillation, ischemic heart disease, peripheral artery disease, internal carotid stenosis, aortic plaque
Introduction
Atrial fibrillation (AF) is the most common chronic cardiac arrhythmia and is a major cause of stroke and thromboembolic events. 1 AF-related ischemic strokes are associated with increased mortality, poorer functional outcomes, and increased recurrence rates 2 and survivors are left more disabled and are more likely to suffer a recurrence compared to other non-AF-related stroke patients. Epidemiological studies have identified several risk factors for ischemic stroke (IS), both nonmodifiable ones, such as sex and age, and modifiable ones, including hypertension, smoking, and diabetes mellitus. 3 These stroke risk factors have been used to formulate risk stratification scores to predict the risk of stroke in patients with AF. 1
AF frequently coexists with vascular diseases, such as coronary artery disease (CAD) and peripheral vascular disease (PAD). The CHA2DS2-VASc score, which includes vascular disease (V) as a criterion, is widely used to assess stroke risk in individuals with AF. 4 This score accounts for the presence of conditions like myocardial infarction (MI), aortic plaque, carotid stenosis, or PAD, with PAD notably raising the risk by 17%-22%—a fact particularly pronounced in Asian populations. 5
Analyses of the Swedish Atrial Fibrillation cohort study by Friberg et al 6 highlight the importance of clinically significant CAD as an independent risk factor for ischemic stroke in patients with AF, with an adjusted incidence rate ratio of 1.29 (95% CI 1.08-1.53). Additionally, the presence of complex aortic plaques, particularly in the descending aorta, has been identified as a significant predictor of ischemic stroke risk, indicating severe vascular disease. 7 It is crucial to recognize, however, that the CHA2DS2-VASc score, while comprehensive, does not account for the heightened risk that comes with the involvement of multiple vascular territories.
Moreover, vascular disease in more than one arterial territory is a marker of advanced diffused atherosclerosis and is associated with a worse prognosis than single-vessel disease. 8 Indeed, atherosclerotic disease may confer a poor outcome in patients with AF.9,10 Previous studies have reported that higher CHADS2 and CHA2DS2-VASc scores were proportionally associated with cerebral atherosclerotic vascular disease (AVD) burden in stroke patients with AF. 5 However, data regarding the impact of atherosclerotic burden as reflected by the involvement of multiple diseased vascular territories on vascular outcomes in stroke patients with AF are limited. In a recent paper, Lin et al, 5 reported that the presence of PAD or CAD in patients with AF does not contribute equally to the risk prediction and presentation of ischemic stroke and systemic thromboembolism and polyvascular disease, defined as the presence of atherosclerosis in more than one arterial bed; coronary arteries, peripheral arteries, and cerebrovascular arteries, should be considered at a higher risk compared to those with only one of these conditions.
This study aimed to assess the impact of AVD burden across affected vascular territories including coronary artery, carotid, aorta (ascending, arch, descending and abdominal, peripheral arteries) on the risk of vascular events among patients with recent stroke and AF within 90 days. Additionally, we explored the relationship between an increasing number of affected territories on the risk of vascular events.
Methods
The data that supported the findings of this study are available from the corresponding author upon reasonable request. We merged the databases from 2 prospective observational studies: The Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation (RAF) study 11 conducted from January 2012 to March 2014 in 29 Stroke Units and the Early Recurrence and Major Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation Treated With Non-Vitamin K Oral Anticoagulants (RAF-NOAC) study, 12 carried out from April 2014 to June 2016 in 35 Stroke Units. These studies included regions in Europe, the US, and Asia.
The design of these studies has been previously published elsewhere.11,12
Briefly, these prospective observational multicenter studies included patients with acute ischemic stroke and AF. The RAF and RAF-NOAC aimed to evaluate the rates of early recurrence and major bleeding within 90 days to determine the best type and time for initiating anticoagulation treatment for secondary stroke prevention.11,12 The studies were approved by the local institutional review boards if required. Local investigators were responsible for filling in standardized forms with predefined variables using individual patient data from corresponding study databases. Completed forms were sent to the coordinating center in Perugia, Italy, where the pooled analysis was performed. The corresponding author and the co-authors had full access to the data and took responsibility for the integrity of the analysis.
We included patients with: (1) Acute IS with a systematic follow-up of at least 3 months or longer after the index event (patients with a fatal event before 3 months were also included in the analysis) (2) diagnosis of nonvalvular AF either known prior to the index event or detected after the event; (3) information on antithrombotic therapy (anticoagulation or antiplatelet) prior to and after index event available; (4) secondary stroke prevention with Direct oral anticoagulations or Vitamin K antagonist; and (5) information on the presence/absence of recurrent IS, systemic embolism, intracerebral hemorrhage, major extracranial bleeding and death. We excluded patients with (1) intracerebral hemorrhage that occurred before the index event; (2) mechanical heart valves; (3) rheumatic or severe mitral valve stenosis; (4) long-term secondary stroke prevention with antiplatelet only; or (5) missing information on antithrombotic treatment before and after the index event.
On admission, stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS). A noncontrast cerebral computed tomography (CT) or cerebral magnetic resonance (MR) scan was performed on admission to exclude intracranial hemorrhage. Revascularization treatments were given as per standard local protocol when appropriate. Standard Stroke Unit care, monitoring, and treatment were provided according to international acute ischemic stroke recommendations. Attending physicians decided the type of anticoagulants for secondary prevention and the day of initiating anticoagulant treatment. The RAF study included patients treated with either vitamin K antagonists or nonvitamin K antagonist oral anticoagulants (NOACs), while the RAF-NOAC study included only patients who received NOACs. AF was categorized as paroxysmal, persistent, and permanent.11,12
For the purpose of this study, AVD was defined as the presence of at least one of the following diseased vascular territories: (1) history of symptomatic ischemic heart disease (MI, history of angina or previous diagnosis of multiple lesions on thallium heart isotope scan, or evidence of coronary disease on coronary angiography); (2) history of symptomatic peripheral artery disease (intermittent claudication of presumed atherosclerotic origin, or ankle/arm systolic blood pressure ratio <0.85 in either leg at rest, or history of intermittent claudication with previous leg amputation, reconstructive surgery, or angioplasty); (3) presence of internal carotid stenosis ≥50% on duplex ultrasound examination and/or on CT angiography scan and (4) presence of plaques in the aorta (ascending aorta, arch of aorta, descending aorta, and abdominal aorta).
Risk Factors
Data on known stroke risk factors were collected: Age, gender, history of hypertension (blood pressure of >140/90 mmHg at least twice before stroke or already under treatment with antihypertensive drugs), history of diabetes mellitus (preprandial fasting glucose level >126 mg/dL on 2 examinations, or postprandial glucose level >200 mg/dL, or HbA1c > 6.5%, or under antidiabetic treatment), current cigarette smoking, past smoking (cessation less than 5 years prior), hyperlipidemia (total cholesterol >200 mg/dL or triglyceride >140 mg/dL or already under lipid-lowering therapy), alcohol abuse (>300 g per week), obesity (body mass index >30 kg/m2), or previous stroke/transient ischemic attack (TIA). White matter changes (leukoaraiosis defined on the first CT or MR) were categorized as ill-defined, and moderately hypodense (at CT) or hyperintense (on T2-weighted MR) areas of >5 mm according to published criteria). Leukoaraiosis in the deep white matter was dichotomized into absent versus present (independently if mild, moderate, or severe). 13
Other baseline variables obtained at admission included: fasting serum glucose, fasting serum cholesterol (total, HDL, and LDL), platelet count, international normalized ratios, activated partial thromboplastin time, systolic blood pressure, and diastolic blood pressure.
Data on antiplatelet, anticoagulants, or thrombolytic agents, prior to admission, at baseline, and during the follow-up period were recorded.
Outcome Evaluation
Patients were followed up prospectively by face-to-face or telephone interviews. Whenever an outcome event occurred, patients were requested to bring complete documentation of the event to the face-to-face appointment.
The primary outcome included the composite of recurrent ischemic cerebrovascular events (including stroke or TIA), symptomatic systemic embolism, intracerebral hemorrhage, and severe extracranial hemorrhage at 90 days. Furthermore, both ischemic and hemorrhagic outcomes were evaluated separately.
Stroke was defined as the sudden onset of a new focal neurological deficit of vascular origin in a site consistent with the territory of a major cerebral artery and categorized as either ischemic or hemorrhagic. TIA was defined as a transient episode of neurological dysfunction caused by focal brain ischemia without acute infarction. Systemic embolism was defined as an acute vascular occlusion of a limb or organ confirmed at imaging, surgery, or autopsy. Cerebral bleeding was considered symptomatic if associated with a decline in neurological status (an increase of ≥4 points in NIHSS score or leading to death). Major extracerebral bleeding was defined as a reduction in the hemoglobin level of at least 2 g/dL, a need of blood transfusion of at least 2 U, or symptomatic bleeding in a critical area or organ. 14
Statistical Analysis
The study patients were classified into 5 groups according to the number of AVD territories (0, 1, 2, 3, and 4). The group with no AVD was the reference group for comparison.
For patients with or without outcome events, differences in clinical characteristics and risk factors were evaluated using the χ2 test of proportions (with a 2-sided α level of 5%), by calculating odds ratio (OR) and 95% confidence intervals (CIs). Multivariable logistic regression analysis was performed to identify independent associations with outcome events, and the variables included in this analysis were the following: age, sex, hypertension, diabetes mellitus, hyperlipidemia, current alcohol abuse, current smoking habit, paroxysmal AF, history of stroke or TIA, therapy with oral anticoagulants, therapy with statins, presence of leukoaraiosis, and the presence of atherosclerosis. Furthermore, survival and empirical cumulative hazard functions were estimated via the Kaplan-Meier estimator for the 2 groups for outcome events. Patients were censored at the time of an outcome event or death.
Kaplan–Meier curves were compared, using the log-rank test to assess the predictive value of risk classes for the risk of outcome events. Data were analyzed using the SPSS/PC Win Package 25.0.
Results
Atherosclerotic Vascular Disease Versus None
Out of the total of 2156 patients, 2148 (mean age 77.59 years old; 53.86% female) were included in the final analysis. (sFigure 1 on the Supplemental Material) Among these, 744 (34.6%) had atherosclerosis. Baseline characteristics are summarized in Table 1. Patients with AVD were older and more likely to have higher baseline NIHSS-score at admission, diabetes mellitus, hypertension, previous stroke/TIA, congestive heart failure, current smoking, and leukoaraiosis. Patients without atherosclerosis were more likely to receive oral anticoagulant therapy after the index stroke. Regarding treatment, patients with AVD were more frequently pretreated with statins at admission (35.2%, P < .001) and with aspirin after the index event (16.1%, P < .001), while less frequently received oral anticoagulation therapy (76.6%, P < .001) and 441 (20.5%) patients were treated with acute revascularization (rt-PA or mechanical thrombectomy or combined). After the acute stroke, 344 patients with AVD (46.2%) were treated with NOACs; 126 (36.6%) with Rivaroxaban, 119 (34.5%) with Apixaban and 99 (28.7%) with Dabigatran. The results from the univariate analysis for variables associated with AVD are listed in Table 1. Male sex 407 (54.7%, P value <.001), diabetes 236 (31.7%, P < .001), hypertension 652 (87.6%, P < .0001), hyperlipidemia 342 (46.0%, P < .001) and history stroke/TIA 237 (31.5%, P < .001) were all significantly associated with atherosclerosis. On multivariate analysis, variables independently associated with AVD are listed in Table 2. The distribution of the affected vascular territories is listed in Table 3.
Table 1.
Characteristics of the Patients With and Without Atherosclerosis.
| Atherosclerosis* N = 744 | No Atherosclerosis N = 1404 | P Value | |
|---|---|---|---|
| Age (mean) | 77.51 ± 9.04 | 75.68 ± 10.10 | <.001 |
| NIHSS at admission (mean) | 8.96 ± 6.78 | 8.10 ± v6.76 | .005 |
| Sex, male | 407 (54.7%) | 584 (41.6%) | <.0001 |
| Diabetes | 236 (31.7%) | 244 (17.4%) | <.0001 |
| Statins at admission | 262 (35.2%) | 322 (22.9%) | <.0001 |
| Hypertension | 652 (87.6%) | 1029 (73.3%) | <.0001 |
| Hyperlipidemia | 342 (46.0%) | 378 (26.9%) | <.0001 |
| Paroxysmal AF | 305 (41.0%) | 621 (44.2%) | .169 |
| History stroke/TIA | 237 (31.5%) | 328 (23.4%) | <.0001 |
| Current smoker | 79 (10.6%) | 124 (8.8%) | .188 |
| Alcoholism | 56 (7.5%) | 85 (6.0%) | .200 |
| Pacemaker | 70 (9.4%) | 80 (5.7%) | .002 |
| Congestive heart failure | 193 (25.9%) | 176 (12.5%) | <.0001 |
| Leukoaraiosis | 394 (52.9%) | 695 (49.5%) | .145 |
| Hemorrhagic transformation (24-72 h) | 81 (10.9%) | 157 (11.2%) | .885 |
| Oral anticoagulant therapy after index event | 570 (76.6%) | 1190 (84.7%) | <.0001 |
| DOAC therapy after index event | 344 (46.2%) | 869 (61.9%) | <.0001 |
| Aspirin after index event | 120 (16.1%) | 120 (8.5%) | <.0001 |
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; AF, atrial fibrillation; TIA, transient ischemic attack.
Table 2.
Multivariate Analysis on the Associations of Baseline Characteristics With Atherosclerosis.
| Atherosclerosis N = 744 | OR (95%CI) Lower Limit-Upper Limit | P Value | |
|---|---|---|---|
| Age (mean) | 77.51 ± 9.04 | 1.02 (1.01-1.03) | <.001 |
| Sex, male | 407 (54.7%) | 1.86 (1.52-2.29) | <.001 |
| NIHSS at admission (mean) | 8.96 ± 6.78 | 1.02 (1.01-1.03) | .001 |
| Diabetes | 236 (31.7%) | 1.83 (1.46-2.30) | <.001 |
| Hypertension | 652 (87.6%) | 1.95 (1.48-2.57) | <.001 |
| Hyperlipidemia | 342 (46.0%) | 1.84 (1.50-2.26) | <.001 |
| Paroxysmal AF | 305 (41.0%) | 1.15 (0.94-1.41) | .172 |
| History stroke/TIA | 237 (31.5%) | 1.20 (0.97-1.50) | .086 |
| Current smoker | 79 (10.6%) | 1.56 (1.10-2.21) | .013 |
| Alcoholism | 56 (7.5%) | 1.04 (0.70-1.55) | .820 |
| Pacemaker | 70 (9.4%) | 1.39 (0.95-2.01) | .082 |
| Congestive heart failure | 193 (25.9%) | 2.14 (1.66-2.75) | <.001 |
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; AF, atrial fibrillation; TIA, transient ischemic attack.
Table 3.
Characteristics of Atherosclerotic Territories.
| Atherosclerosis | Ischemic Heart Disease | Peripheral Artery Disease | Aorta Sclerosis | Carotid Stenosis |
|---|---|---|---|---|
| 1 Territory | 167 | 65 | 71 | 240 |
| 2 Territories | 84 | 71 | 57 | 87 |
| 3 Territories | 37 | 39 | 32 | 30 |
| 4 Territories | 6 | 6 | 6 | 6 |
Outcome Events
Over 90 days of follow-up, the combined outcome were recorded in 77 AVD patients (10.3%) and in 97 (6.9%) no-AVD patients (HR 1.22, 95% Cl: 0.87-1.92). Of the AVD patients, 33 (11.3%) had a history of symptomatic ischemic heart disease, 26 (14.7%) PAD, 26 (15.8%) aortic plaques, and 35 (9.7%) carotid stenosis. The Kaplan Meier Curve of the combined outcomes in patients with AVD and no AVD was overlapping for the 2 groups (sFigure 2 on the Supplemental Material).
Ischemic events during follow-up were recorded in 46 (6.2%) AVD patients and in 55 (3.9%) no-AVD patients (HR 1.24, 95%Cl: 0.80–1.92) (Table 4). Hemorrhagic events occurred in 31 (4.1%) AVD patients and in 42 events (3.0%) no-AVD patients (HR 1.22, 95%CI: 0.74–2.03), the increase in the atherosclerotic territories was not statistically significant (Table 4).
Table 4.
Outcomes of Patients With and Without Atherosclerosis.
| Atherosclerosis N = 744 |
No Atherosclerosis N = 1404 |
Odds Ratio (95%CI) | P Value | |
|---|---|---|---|---|
| Combined outcome | 77 (10.3%) | 97 (6.9%) | Unadjusted 1.56 (1.14-2.13) Adjusted 1.22 (0.87-1.92) |
.006 .2 |
| Ischemic outcomea | 46 (6.2%) | 55 (3.9%) | Unadjusted 1.62 (1.08-2.42) Adjusted 1.24 (0.80-1.92) |
.018 .3 |
| Hemorrhagic outcomeb | 31 (4.1%) | 42 (3.0%) | Unadjusted 1.41 (0.88-2.26) Adjusted 1.22 (0.74-2.03) |
.135 .4 |
Ischemic outcomes (ischemic stroke and systemic embolism including MI).
Hemorrhagic outcomes (intracranial hemorrhage and severe extracranial bleeding).
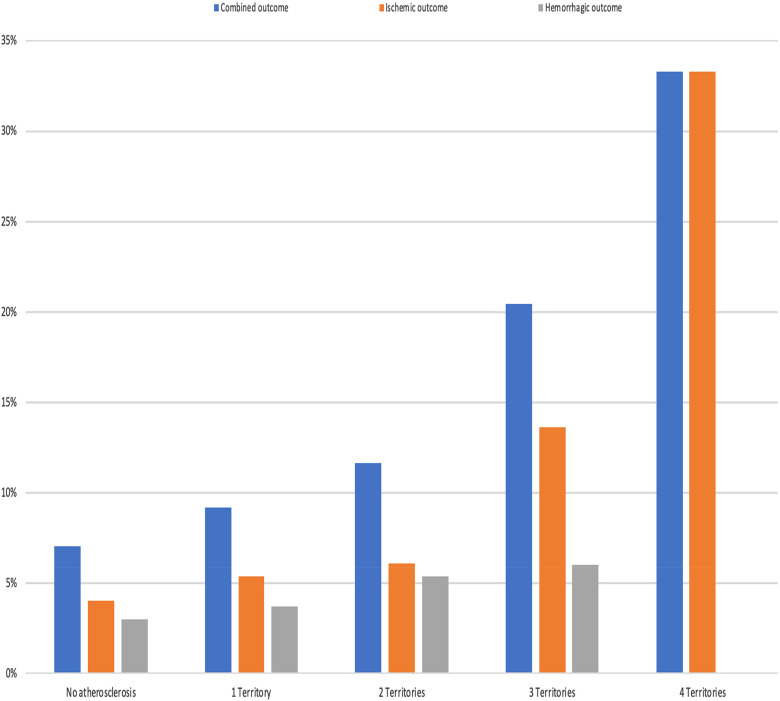
In the multivariable analysis, patients with AVD involving 3 or 4 vascular territories demonstrated a notably increased risk of combined vascular outcomes when compared to those with no-AVD. Specifically, for involvement of 3 territories, the hazard ratio (HR) was 2.80 (95% CI: 1.20–6.53), and for 4 territories, the HR was 6.81 (95% CI: 1.02–36.24) (Table 5 and Figure 1). Additionally, the risk for ischemic outcomes was significantly higher when 3 territories were involved (HR 2.88, 95% CI: 1.04–7.95), and even more pronounced with 4 territories (HR 7.76, 95% CI: 1.22–49.23).
Table 5.
Adjusted OR for Outcomes in Patients With Atherosclerosis According to the Number of Vascular Territories Involved.
| Adjusted OR (95%CI) | P value | |
|---|---|---|
|
Combined outcome No atherosclerosis Atherosclerosis
|
1 (Reference) 1.08 (0.74-1.58) 1.39 (0.77-2.49) 2.80 (1.20-6.52) 6.81 (1.02-36.24) |
.6 .2 .01 .04 |
|
Ischemic outcome No atherosclerosis Atherosclerosis
|
1 (Reference) 1.14 (0.70-1.85) 1.17 (0.54-2.52) 2.88 (1.04-7.95) 7.76 (1.22-49.23) |
.5 .6 .04 .03 |
|
Hemorrhagic outcome No atherosclerosis Atherosclerosis
|
1 (Reference) 1.08 (0.62-1.89) 1.72 (0.75-3.93) 2.16 (0.56-8.26) NA |
.7 .2 .2 |
Figure 1.
Outcomes in patients with atherosclerosis according to the number of atherosclerotic vascular territories involved.
Discussion
Our analysis shows that patients with AVD involving 3 or 4 territories are at a significantly increased risk for combined vascular and ischemic events. Conversely, the degree of atherosclerotic involvement does not appear to correlate with bleeding outcomes. This indicates that the increased risk in the combined patient group is primarily attributable to ischemic rather than hemorrhagic events. Our findings are consistent with another study from Park et al, 15 who reported that concomitant AVD among stroke patients with AF was associated with a higher risk of major adverse vascular events MACE and all-cause death in a dose-dependent manner. The study observed a high presence of extracranial stenosis. However, the data reported by Park et al were limited to Korean Atrial Fibrillation Evaluation Registry; the higher prevalence of intracranial stenosis may limit the generalization to other ethnic stroke populations. 16 In our study, patients in the AVD group were older and more likely to have risk factors that are closely connected to atherosclerosis and increased stroke risk. 17 In our study, more than a third of the patients with AF had atherosclerotic disease, which is higher than the subanalysis of SPAF-III (Stroke Prevention in Atrial Fibrillation) trial, where the presence of complex atherosclerotic plaque was reported in 25% of patients with AF independent of sex and serum cholesterol levels. 17 However, the SPAF-III population was almost 10 years younger and less well-screened for vascular disease compared to our study population with different definition of atherosclerosis. In many observational studies, AF and AVD share common risk factors such as hypertension, obesity, and age, which is in-line with our study. 18 Our analysis of CHA2DS2-VASc score distribution among different vascular territories revealed that patients with increased atherosclerotic territory involvement tended to have higher CHA2DS2-VASc scores (data not shown). This supports the validity of the CHA2DS2-VASc score as a comprehensive indicator of vascular disease burden. The presence of atherosclerotic disease may play a significant role in the residual stroke risk of AF patients who are already on anticoagulation. In the RENO study, about 30% of the patients with cerebrovascular events had a stroke due to causes other than cardio-embolism. 19 Indeed, ischemic stroke in patients with AF is not exclusively cardio-embolic.20–22 For this reason, in patients with the breakthrough stroke while on oral anticoagulant therapy, the first step should be to diagnose the aetiology of the new event, which may require a reassessment of the original treatment strategy. The stroke etiologist in patients with AF while on oral anticoagulant are heterogeneous and may include nonAF-related competing stroke mechanisms (such as large artery and small vessel diseases); therefore, the AVD may still play a significant role.12,20,21 As reported in our study, the higher the burden of atherosclerosis with more vascular territories involved, the higher the risk of stroke events. Therefore, a thorough diagnostic workup should be performed on AF patients to define the risk burden. However, add-on treatment with aspirin does not reduce the recurrence risk but increases only the hemorrhagic risk. 23 A possible strategy in these patients would be an optimal control of vascular risk factors, which is part of the current holistic or integrated care approach to AF management recommended in guidelines. 24 Adherence to such an approach has been associated with improved clinical outcomes, including a lower relative risk of death, stroke, bleeding, and hospitalization.23,24 In our study, patients with AVD were taking less oral anticoagulant therapy and more statins on admission, consistent with a prior study. 25 Despite these patients having higher cardiovascular risk, they tended to be undertreated and to have more severe strokes at admission. These patients deserve evidence-based treatment with NOACs as recommended by the guidelines. 26
Our study has several limitations. Firstly, as a post hoc analysis of observational, nonrandomized studies, there is potential for selection bias despite the use of adjusted statistical models to control for confounders. Secondly, the relatively low number of outcome events may reduce the statistical power of our analyses. Outcomes were not centrally adjudicated but instead assessed by local investigators, which could affect the consistency of the diagnosis of AVD. Thirdly, the methodological approaches to defining vascular territories and outcome events carry certain limitations, as previously discussed in the RAF, RAF-NOAC, RENO, and RENO-EXTEND studies by our international group.11,12,19,27
Conclusions
AVD serves as a prognostic marker in individuals with recent stroke and AF, particularly when 3 to 4 territories are affected. The degree of vascular territory involvement directly correlates with an increased risk of combined and ischemic events.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296241240746 for The Impact of Atherosclerotic Burden on Vascular Outcomes in Patients with Stroke and Atrial Fibrillation: The ATHENA study by Andrea Galeazzo Rigutini and in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The Author thanks ARS UMBRIA for its unconditioned support.
Footnotes
Authors Note: The whole list of Authors and their affiliations are listed on the supplemental file. This article is called as the “ATHENA” study.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Paciaroni received honoraria as a member of the speaker bureau of Aspen, Sanofi-Aventis, Boehringer Ingelheim, Bayer, BMS, Daiichi Sankyo, and Pfizer. Caso received honoraria as a member of the speaker bureau of Boehringer Ingelheim, Bayer, and Daiichi Sankyo (all fees were paid to Associazione Ricerca Stroke, Umbria). She received honoraria as consultant or advisory board member of Boehringer Ingelheim, Bayer, Daiichi Sankyo, and Pfizer. Ntaios received research funding from Pfizer. He received honoraria from Pfizer, Boehringer Ingelheim, and Bayer. He received consultant honoraria from Pfizer, Boehringer Ingelheim, and Bayer. Tsivgoulis has received funding for travel or speaker's honoraria from Bayer, Pfizer, and Boehringer Ingelheim. He has served on scientific advisory boards for Bayer, Boehringer Ingelheim, and Daiichi Sankyo. Putaala has received personal fees from Boehringer Ingelheim, Bayer, and Portola. He has also received grants and personal fees from BMS-Pfizer and Abbott/St Jude Medical. Del Sette has received honoraria for speaking from Bayer and Boehringer Ingelheim. Zedde received speaking and consulting fees from Daiichi Sankyo, Amicus Therapeutics, Sanofi Genzyme, Abbott, and Takeda. Rota has received speaker fees from Bayer and Novartis. Stretz has received departmental funding from Massachusetts General Hospital/Boston Scientific for his site's participation in the Neuro Afib study. Ornello has received nonfinancial support from Novartis, Allergan, and Teva. Ageno has received grants and personal fees from Bayer, personal fees from Boehringer Ingelheim, BMS/Pfizer, Portola, Jansen, Aspen, Sanofi, and Daiichi Sankyo. Sacco has received personal fees as speaker or advisor from Abbott, Allergan, Astra Zeneca, Eli Lilly, Lundbeck, Novartis, NovoNordisk, Teva and research grants from Allergan, Novartis, and Uriach. Giannopoulos has received funding for travel from Bayer and speaker's honoraria from Pfizer. Cappellari has received consulting fees from Boehringer Ingelheim, Pfizer—BMS, and Daiichi Sankyo. Dawson reports honoraria as a member of the speaker bureau of Boehringer Ingelheim, Bayer, BMS, Daiichi Sankyo, Medtronic, and Pfizer. He has also received research funding from Pfizer. Toni has received personal fees from Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, and Pfizer. Agnelli received honoraria as a member of the speaker bureau of Boehringer Ingelheim and Bayer. Becattini received honoraria as a member of the speaker bureau of Bristol Meyer Squibb, Daiichi Sankyo, and Bayer. Michel received Research Grant by Swiss National Science Foundation and Swiss Heart Foundation; he received speaker fees by Bayer, Boehringer Ingelheim, Covidien, St. Jude Medical; he received honoraria as advisory relationship by Pierre-Fabre, Bayer, Bristol Meyer Squibb, Amgen, and Boehringer Ingelheim. Tatlisumak received honoraria as consultant or advisory relationship by Lundbeck and Boehringer Ingelheim. Lip Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Anthos. No fees are received personally. GYHL is a National Institute for Health and Care Research (NIHR) Senior Investigator and co-principal investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 899871. The other authors report no conflicts.
Ethical Statements: The studies were approved by the local Institutional Review Boards if required.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Informed consents were provided for the consecutive data collection based on current laws.
ORCID iD: Andrea Galeazzo Rigutini https://orcid.org/0009-0003-7119-1254
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Lip GYH, Gue Y, Zhang J, et al. Stroke prevention in atrial fibrillation. Trends Cardiovasc Med. 2022;32(8):501-510. 20211005. DOI: 10.1016/j.tcm.2021.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. 2016;388(10046):806-817. DOI: 10.1016/S0140-6736(16)31257-0 [DOI] [PubMed] [Google Scholar]
- 3.Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472-495. DOI: 10.1161/CIRCRESAHA.116.308398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest. 2010;137(2):263-272. 20090917. DOI: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 5.Lin YS, Wu VC, Chen YL, et al. Thromboembolic events in atrial fibrillation: Different level of risk and pattern between peripheral artery disease and coronary artery disease. Arch Cardiovasc Dis. 2021;114(3):176-186. 20210129. DOI: 10.1016/j.acvd.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish atrial fibrillation cohort study. Eur Heart J. 2012;33(12):1500-1510. 20120113. DOI: 10.1093/eurheartj/ehr488 [DOI] [PubMed] [Google Scholar]
- 7.Zabalgoitia M, Halperin JL, Pearce LA, et al. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke prevention in atrial fibrillation III investigators. J Am Coll Cardiol. 1998;31(7):1622-1626. DOI: 10.1016/s0735-1097(98)00146-6 [DOI] [PubMed] [Google Scholar]
- 8.Tsivgoulis G, Bogiatzi C, Heliopoulos I, et al. Low ankle-brachial index predicts early risk of recurrent stroke in patients with acute cerebral ischemia. Atherosclerosis. 2012;220(2):407-412. 20111116. DOI: 10.1016/j.atherosclerosis.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Nicolajsen CW, Nielsen PB, Jensen M, et al. Stroke and myocardial infarction in patients with abdominal aortic aneurysm and new-onset atrial fibrillation. Thromb Haemost. 2023;123(05):555-564. 20230110. DOI: 10.1055/a-2009-8954. [DOI] [PubMed] [Google Scholar]
- 10.Steensig K, Olesen KKW, Thim T, et al. Should the presence or extent of coronary artery disease be quantified in the CHA2DS2-VASc score in atrial fibrillation? A report from the western Denmark heart registry. Thromb Haemost 2018;118(12):2162-2170. 20181112. DOI: 10.1055/s-0038-1675401. [DOI] [PubMed] [Google Scholar]
- 11.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: Effect of anticoagulation and its timing: The RAF study. Stroke 2015;46(8):2175-2182. 20150630. DOI: 10.1161/STROKEAHA.115.008891. [DOI] [PubMed] [Google Scholar]
- 12.Paciaroni M, Agnelli G, Falocci N, et al. Early recurrence and Major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) study. J Am Heart Assoc. 2017;6(12):20171129. DOI: 10.1161/JAHA.117.007034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32(6):1318-1322. DOI: 10.1161/01.str.32.6.1318 [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3(4):692-694. DOI: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Chung JW, Bang OY, et al. Atherosclerotic burden and vascular risk in stroke patients with atrial fibrillation. Stroke 2021;52(5):1662-1672. 20210402. DOI: 10.1161/STROKEAHA.120.032232. [DOI] [PubMed] [Google Scholar]
- 16.Hoshino T, Sissani L, Labreuche J, et al. Prevalence of systemic atherosclerosis burdens and overlapping stroke etiologies and their associations with long-term vascular prognosis in stroke with intracranial atherosclerotic disease. JAMA Neurol. 2018;75(2):203-211. DOI: 10.1001/jamaneurol.2017.3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators, Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke prevention in atrial fibrillation III study. The SPAF III writing committee for the stroke prevention in atrial fibrillation investigators. JAMA 1998;279(16):1273-1277. [PubMed] [Google Scholar]
- 18.Batta A, Hatwal J, Batta A, et al. Atrial fibrillation and coronary artery disease: An integrative review focusing on therapeutic implications of this relationship. World J Cardiol. 2023;15(5):229-243. DOI: 10.4330/wjc.v15.i5.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paciaroni M, Agnelli G, Caso V, et al. Causes and risk factors of cerebral ischemic events in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants for stroke prevention. Stroke 2019;50(8):2168-2174. 20190625. DOI: 10.1161/STROKEAHA.119.025350. [DOI] [PubMed] [Google Scholar]
- 20.Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol 2020;87(5):677-687. 20200212. DOI: 10.1002/ana.25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polymeris AA, Meinel TR, Oehler H, et al. Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. J Neurol Neurosurg Psychiatry 2022;93(6):588-598. 20220408. DOI: 10.1136/jnnp-2021-328391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: The IAC study. J Neurol Neurosurg Psychiatry 2021;92(10):1062-1067. 20210426. DOI: 10.1136/jnnp-2021-326166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raf and Investigators* R-E. Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy. Eur Stroke J 2023;8(3):722-730. 20230717. DOI: 10.1177/23969873231183211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romiti GF, Pastori D, Rivera-Caravaca JM, et al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: Impact on clinical outcomes-A systematic review and meta-analysis of 285,000 patients. Thromb Haemost. 2022;122(03):406-414. 20210621. DOI: 10.1055/a-1515-9630. [DOI] [PubMed] [Google Scholar]
- 25.Aivo J, Ruuskanen JO, Tornio A, et al. Lack of statin therapy and outcomes after ischemic stroke: A population-based study. Stroke 2023;54(3):781-790. 20230207. DOI: 10.1161/STROKEAHA.122.040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373-498. [DOI] [PubMed] [Google Scholar]
- 27.Paciaroni M, Caso V, Agnelli G, et al. Recurrent ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: The RENO-EXTEND study. Stroke 2022;53(8):2620-2627. 20220511. DOI: 10.1161/STROKEAHA.121.038239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296241240746 for The Impact of Atherosclerotic Burden on Vascular Outcomes in Patients with Stroke and Atrial Fibrillation: The ATHENA study by Andrea Galeazzo Rigutini and in Clinical and Applied Thrombosis/Hemostasis