Abstract
Introduction
In our previous work, we investigated the analgesic effects of ibuprofen gargle after mandibular third molar extractions. However, a subsequent detailed review of individual patient data revealed variations in postoperative pain reduction among patients. Consequently, the present study was designed to conduct post-hoc subanalyses that identified factors contributing to variation in the analgesic response to ibuprofen gargle after third molar extractions.
Materials and methods
This study involved thirty-five Japanese patients from a prior randomized, double-blind, placebo-controlled, crossover study, which focused on the analgesic effects of ibuprofen gargle after mandibular third molar extractions. Participants were categorized as responders (n = 13) and non-responders (n = 22) based on the within-subject difference (ibuprofen-placebo, IP) of visual analog scale (VAS) changes. Baseline characteristics were compared, along with variables, such as age, sex, the reason for extraction, extraction site, Pell Gregory (space and depth) classification, Winter’s classification, surgeon’s experience, and surgery time. Baseline characteristics predicting responder status were examined using multivariate logistic regression.
Results
In the univariate analysis, variables such as age, sex, and baseline VAS scores with p-values <0.2 were evaluated using a stepwise approach. This analysis identified age (per -10 years) with an odds ratio of 4.163 (95% confidence interval (CI): 1.170-31.952, p = 0.0233) and sex (female) with an odds ratio of 9.977 (95% CI: 1.336-208.256, p = 0.0213) as significant predictors of responder status.
Conclusions
In young and female patients, ibuprofen gargle decreased postoperative pain after mandibular third molar extractions.
Keywords: tooth extraction, postoperative pain, mouthwash, mandibular third molar, ibuprofen
Introduction
Extraction of the mandibular third molar is a common oral surgery procedure [1,2]. This procedure is invasive, particularly when bone removal and crown division is involved; it often leads to moderate-to-severe postoperative complications such as pain, swelling, and restricted mouth opening and swallowing, along with challenges in oral intake after surgery [2,3]. Post-extraction pain is a primary concern after dental extractions, often deterring patients from seeking treatment [1]. Nonsteroidal anti-inflammatory drugs (NSAIDs) and acetaminophen are standard analgesics for the management of post-extraction pain; NSAIDs, particularly acidic NSAIDs, are typically preferred for moderate pain, such as the pain associated with mandibular extractions [4]. For patients with gastrointestinal ulcers or aspirin-induced asthma, acetaminophen may serve as an alternative [4]. Various NSAIDs, including celecoxib [5,6], valdecoxib [7], ibuprofen [8], flurbiprofen [9], and opioid-containing medications such as oxycodone [10], have been closely evaluated to identify optimal analgesics for pain alleviation in mandibular third molar extractions. A systematic review by Weil et al. highlighted the safety and efficacy of oral paracetamol (acetaminophen) in postoperative pain management after embedded mandibular third molar extractions [11], whereas the Cochrane review by Bailey et al. highlighted the superiority of oral ibuprofen over oral paracetamol in this context [12].
When prescribing postoperative analgesics, clinicians must consider potential side effects [13]; opioids are primarily linked to respiratory depression, nausea, vomiting, and constipation, whereas NSAIDs are predominantly associated with gastrointestinal, renal, and hepatic complications, as well as platelet dysfunction [14]. Rindal et al. recently outlined a randomized controlled trial protocol to mitigate opioid-related issues, with a focus on interventions to reduce opioid prescriptions in favor of nonopioid alternatives [15]. Furthermore, topical analgesic application has emerged as a potentially safer approach to enhance postoperative pain relief [16]; studies have shown reduced side effects without decreases in analgesic efficacy [9,17,18].
Ibuprofen, introduced in the 1960s, is a potent inhibitor of prostaglandin synthesis that effectively mitigates fever, pain, and inflammation [19]. Despite its pharmacological activity against cyclooxygenase-1 and cyclooxygenase-2, systemic administration may cause adverse effects such as gastrointestinal and renal dysfunction. Nevertheless, numerous reviews and meta-analyses have confirmed ibuprofen’s efficacy and relatively low toxicity compared with other NSAIDs, for both adults and children [20-22]. We hypothesized that an oral ibuprofen gargle (0.6% or 1%) directly applied to the affected area could alleviate pain associated with oral mucositis [23]. Previous research indicated no significant safety concerns and suggested that some pain relief could be achieved in cases of chemotherapy- or chemoradiotherapy-induced oral mucositis [23].
Considering the rapid absorption of locally administered drugs in post-extraction wounds due to the loss of keratinized mucosa [24], we hypothesized that an ibuprofen mouthwash could serve as an efficient drug delivery system for targeting post-extraction pain while minimizing systemic effects. In this context, we conducted a single-center, placebo-controlled, double-blind, randomized crossover study to evaluate the efficacy of ibuprofen mouthwash [25,26]. The study failed to demonstrate a statistically significant effect on post-extraction pain relief, despite not observing any major safety issues, including increases in postoperative bleeding [26].
On the basis of these findings, and in anticipation of the insurance coverage trials that will begin in Japan in June 2024, this clinical study was meticulously designed to evaluate the differential effects of ibuprofen gargle on postoperative pain relief after mandibular third molar extractions. The primary goal of the study was the identification of patient characteristics that could predict an effective analgesic response to ibuprofen gargle. Consequently, the study aims to establish a definitive relationship between its objectives and the hypothesis that specific baseline characteristics, including age, sex, and initial pain levels, significantly influence the efficacy of ibuprofen gargle as a postoperative analgesic treatment.
Materials and methods
Data source and study population
This study was conducted as a substudy of an exploratory clinical trial that evaluated the clinical effects of administering ibuprofen gargle to patients after mandibular third molar extractions. The design and main results of the exploratory clinical trial have been previously reported [26]. The primary objective of this substudy was to examine specific patient subgroups within the same cohort, particularly responders and non-responders, to determine whether baseline characteristics (e.g., age and sex) influenced the efficacy of the study treatment. The study protocol complied with the Declaration of Helsinki and was approved by the ethics committee of our hospital (C200024, date of approval: March 23, 2021). Written informed consent was obtained from all patients.
Briefly, the original study was an investigator-initiated, placebo-controlled, double-blind, randomized crossover, single-center clinical trial in which the study intervention comprised gargling 0.6% ibuprofen daily for five days [25]. Patients were enrolled after providing written informed consent to participate. The main exclusion criteria were peptic ulcers, a history of hypersensitivity to any component of the ibuprofen gargle, impaired cardiac function or clinically significant heart disease, and aspirin-induced asthma. A visual analog scale (VAS) was used to measure pain before and at five minutes and 15 minutes after gargling, daily for up to one week [25].
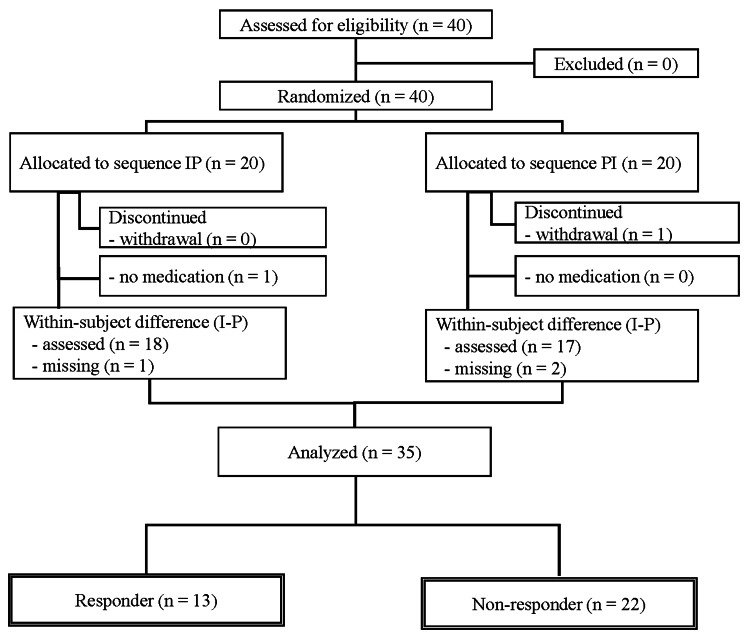
From 7 June 2021 to 26 May 2022, 40 patients were enrolled [26]. However, one patient withdrew informed consent, and four patients for whom VAS scores could not be obtained on postoperative day (POD) 1 and POD 2 were excluded [26]. Therefore, the study population in this substudy comprised 35 patients (Figure 1).
Figure 1. Patient flow chart for the assessment of patient characteristics associated with the efficacy of ibuprofen gargle treatment.
I: ibuprofen, P: placebo.
Patient characteristics associated with treatment efficacy
In the original study, patients were randomized at a 1:1 ratio into two groups: ibuprofen-placebo (IP) and placebo-ibuprofen (PI). On POD 1, ibuprofen gargle administration was initiated in the IP group, whereas the PI group began using a placebo gargle. On POD 2, the IP group switched to a placebo gargle, and the PI group switched to an ibuprofen gargle. On PODs 3-5, both groups were prescribed an ibuprofen gargle. Approximately 10 mL of gargle solution was dispensed into a measuring cup and retained in the mouth in contact with the affected area for at least 30 seconds (preferably one minute), then discarded [25].
The primary endpoint was the change in pain intensity, as measured by the VAS, within each patient before and five minutes after the first use of either the ibuprofen or placebo gargle on PODs 1 and 2 (ΔVAS5_ibuprofen−ΔVAS5_placebo). This change is denoted as ΔVAS5, where 'Δ' represents the difference and '5' indicates the time in minutes after administration. Specifically, ΔVAS5_ibuprofen and ΔVAS5_placebo refer to changes in VAS scores after the use of ibuprofen and placebo gargles, respectively.
The equation ΔVAS5_ibuprofen−ΔVAS5_placebo calculates the difference in pain score changes between the ibuprofen and placebo treatments. A negative value indicates that the ibuprofen gargle led to a greater reduction in pain compared with the placebo gargle. For example, in our study, the within-patient changes in VAS5 for the IP and PI groups were 1.25 ± 12.0 and −5.26 ± 8.93 mm, respectively, demonstrating how pain levels changed after each treatment. The overall treatment effect of the ibuprofen gargle was calculated as −2.01 ± 10.62 mm (p = 0.246), suggesting that, on average, the ibuprofen gargle reduced pain slightly more than the placebo; however, this difference was not statistically significant. The treatment effect was determined by calculating the mean of within-patient changes in VAS scores for both the IP and PI groups, providing a measure of the ibuprofen gargle's mean effectiveness in pain reduction across the study population. Instead of performing the evaluation solely after five minutes, clinical acceptability was defined as the persistence of the effect after 15 minutes. A subanalysis of VAS changes was performed with a response of 10 mm or greater in the group of patients. Therefore, the responders defined within-patient difference (I-P) at five or 15 minutes as −10 or less in this study. Correlations of within-patient differences (I-P) at five and 15 minutes were also examined. After excluding patients with no VAS data for both POD 1 and 2, the characteristics of responders and non-responders were compared in this substudy.
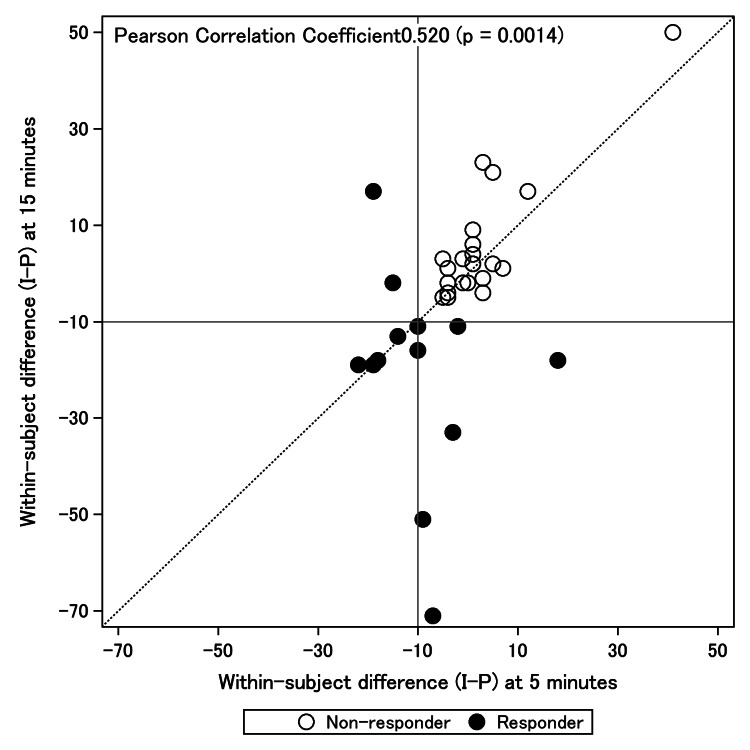
As shown in Figure 2, the correlation of within-subject difference (I-P) at five and 15 minutes had a Pearson correlation coefficient of 0.520 (p = 0.0014).
Figure 2. Correlation of within-subject difference (I-P) at five minutes with within-subject difference (I-P) at 15 minutes.
I: ibuprofen, P: placebo.
Statistical analysis of endpoints
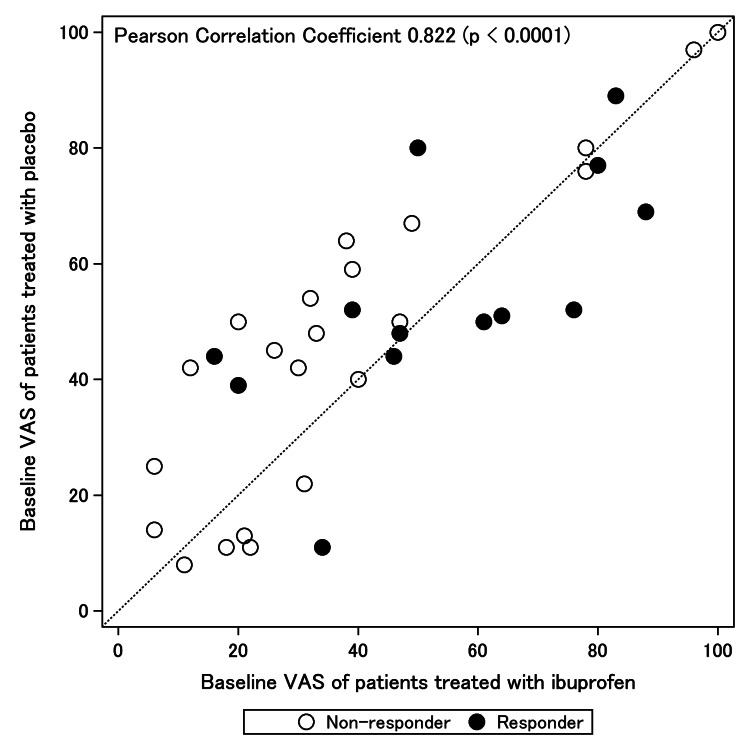
The results are primarily presented as descriptive statistics because of the limited sample size. Continuous variables are expressed as means ± standard deviations (SDs), whereas categorical variables are represented as numbers (%). Baseline characteristics and variables were assessed using Welch’s t-test and Fisher’s exact test. Multivariate logistic regression analysis was performed with ‘responder’ as the dependent variable. This analysis utilized a stepwise approach, selecting variables with a p-value of <0.2 from the univariate analysis of baseline characteristics as candidate explanatory variables. Akaike’s information criterion (AIC) was used to guide the variable selection process. The stepwise approach determined the final model, which provided the odds ratios and 95% confidence intervals (CIs) for the variables. Because baseline VAS scores were highly correlated with both ibuprofen and placebo use (Pearson correlation coefficient of 0.822), as shown in Figure 3, we used the mean of both VAS scores. Data were analyzed using R version 4.2.1 software (R Core Team, Vienna, Austria).
Figure 3. Correlation of the baseline visual analog scale (VAS) of ibuprofen treatment with the baseline VAS of the placebo.
VAS: visual analog scale, I: ibuprofen, P: placebo.
Results
Patient characteristics associated with the treatment efficacy
Of the 35 patients who received ibuprofen gargle, 13 were responders and 22 were non-responders. The characteristics of responders and non-responders are shown in Table 1.
Table 1. Comparison of baseline characteristics between responders and non-responders to ibuprofen gargle treatment.
VAS: visual analog scale, I: ibuprofen, P: placebo.
| Characteristic | All (n = 35) | Responders (n = 13)a | Non-responders (n = 22)a | p-value | |
| Age (years) | 29.7 ± 8.8 | 25.2 ± 2.6 | 32.4 ± 10.2 | 0.0044b | |
| Sex | |||||
| Male | 12 (34.3%) | 1 (12%) | 11 (50. 0%) | 0.0132c | |
| Female | 23 (65.7%) | 12 (88%) | 11 (50.0%) | ||
| Reason for extraction | 0.2591c | ||||
| Pericoronitis | 25 (71.4%) | 11 (84.6%) | 14 (63.6%) | ||
| Other | 10 (28.6%) | 2 (15.4%) | 8 (36.4%) | ||
| Pell Gregory classification (distal_space) | 0.2591c | ||||
| Class I | 3 (8.6%) | 0 (0.0%) | 3 (13.6%) | ||
| Class II | 32 (91.4%) | 13 (100.0%) | 19 (86.0%) | ||
| Pell Gregory classification (depth) | 0.7260c | ||||
| Class A | 23 (65.7%) | 8 (61.5%) | 15 (68.2%) | ||
| Class B | 12 (34.3%) | 5 (38.5%) | 7 (31.8%) | ||
| Winter’s classification | 0.4930c | ||||
| Horizontal | 13 (37.1%) | 6 (46.2%) | 7 (31.8%) | ||
| Vertical | 13 (37.1%) | 3 (23.1%) | 10 (45.5%) | ||
| Mesioangular | 6 (17.1%) | 2 (15.4%) | 4 (18.2%) | ||
| Distoangular | 3 (8.6%) | 2 (15.4%) | 1 (4.5%) | ||
| Surgeon experience (years) | 6.1 ± 1.6 | 6.0 ± 1.8 | 6.1 ± 1.5 | 0.8780b | |
| Surgery time (min) | 28.4 ± 10.2 | 29.2 ± 12.7 | 28.0 ± 8.7 | 0.7666b | |
| Baseline VAS (mean of I and P) | 46.6 ± 24.7 | 54.2 ± 20.0 | 42.1 ± 26.5 | 0.1353b | |
| Treatment sequence | 0.3053c | ||||
| PI | 17 (48.6%) | 8 (61.5%) | 9 (40.9%) | ||
| IP | 18 (51.4%) | 5 (38.5%) | 13 (59.1%) | ||
| Within-subject difference (I-P) (VAS5) | −2.1 ± 11.5 | −10.0 ± 10.5 | 2.5 ± 9.6 | 0.0017b | |
| Within-subject difference (I-P) (VAS15) | −4.2 ± 20.6 | −20.4 ± 21.7 | 5.4 ± 12.7 | 0.0011b | |
| aMean ± SD; n (%) | |||||
| bWelch’s t-test | |||||
| cFisher’s exact test | |||||
Univariate analysis of responder status revealed statistically significant differences in age and sex (age: p = 0.0044, sex: p = 0.0132). The means and SDs of baseline VAS scores were 56.4 ± 23.4 for women (n = 23) and 27.8 ± 14.6 for men (n = 12).
A stepwise analysis was conducted using variables such as age, sex, and baseline VAS score (mean of I and P), each of which had a p-value of <0.2 in the univariate analysis shown in Table 1. We initially fitted a logistic regression model with age, sex, and baseline VAS score as explanatory variables; however, baseline VAS score did not predict responder status (Table 2).
Table 2. Results of multivariate logistic regression analysis.
AIC: Akaike’s information criterion; VAS: visual analog scale; I: ibuprofen; P: placebo.
| Estimation of odds ratios (n = 35, AIC = 41.525) | |||||
| Reference | Unit | Odds ratio | 95% Confidence interval | p-value | |
| Sex (female) | Male | 11.43 | 1.153-287.199 | 0.0365 | |
| Age | −10 years | 4.143 | 1.178-31.490 | 0.0226 | |
| Baseline VAS (mean of I and P) | 10 mm | 0.956 | 0.641-1.403 | 0.8151 | |
The AIC served as the criterion for model selection. Notable findings included an odds ratio of 4.163 (95% CI: 1.170-31.952, p = 0.0233) for age (per -10 years) and an odds ratio of 9.977 (95% CI: 1.336-208.256, p = 0.0213) for sex (female), as detailed in Table 3.
Table 3. Results of multivariate logistic regression analysis (final model).
AIC: Akaike’s information criterion; VAS: visual analog scale; I: ibuprofen; P: placebo.
| Estimation of odds ratios (n = 35, AIC = 39.580) | |||||
| Reference | Unit | Odds ratio | 95% Confidence interval | p-value | |
| Sex (female) | Male | 9.977 | 1.366-208.256 | 0.0213 | |
| Age | −10 years | 4.163 | 1.170-31.952 | 0.0233 | |
Discussion
To our knowledge, this study represents a pioneering effort to explore to examine the correlations of patient characteristics with ibuprofen gargle efficacy in improving pain after mandibular third molar extractions. Regarding the efficacy endpoint in our study, responders were female and younger than non-responders. The influence of the higher baseline VAS score for women was minimal, despite the large number of women who were responders. Consistent with existing literature, our findings suggest that women exhibit lower pain thresholds, and baseline pain levels in women appear to be higher. Additionally, systematic reviews have found that women are more likely to respond to pain treatment [27]. Our data support the notion that the efficacy of NSAIDs in postoperative pain management varies according to age and sex; the results of some studies have suggested that naproxen alone could manage acute postoperative pain more effectively in women than in men [28]. The results of the present study suggest that women are more likely to benefit from the short-term analgesic effects of ibuprofen gargle after mandibular third molar extraction. Generally, local NSAID therapies do not differ in efficacy according to age, but there are differences in analgesic efficacy among NSAIDs [29]. The results highlight rapid recovery and better quality of life in patients aged <21 years compared with older patients [30]. Thus, age-related variation in the effect of ibuprofen-containing gargles may have been influenced by differences in recovery after third molar extraction.
In addition to our current findings regarding gargle treatment that future research will explore, we are currently planning to conduct a clinical trial (https://jrct.niph.go.jp/latest-detail/jRCTs051230162) in which cyclooxygenase-2 paste will be directly applied to the extraction socket for local pain control; such an approach may be required for non-responder patients, such as men.
Although this study represents a substantial exploratory advance of the differential effects of ibuprofen gargle on postoperative pain relief after mandibular third molar extractions, it also had limitations that influence its implications for future research. The methodology used in this study provides a robust framework for assessing the immediate analgesic effects of ibuprofen gargle, offering valuable insights into patient-specific responses according to baseline characteristics. However, the findings were derived from a relatively small cohort, and the statistical analysis was primarily descriptive. Although this limitation may impact the external validity, the results of this study establish a foundation for future meta-analyses and highlight areas that warrant further investigation. Moreover, the study's assessment of pain at only two-time points (five minutes and 15 minutes post-treatment) may not have fully captured the dynamic nature of postoperative pain management. The absence of well-established objective markers for evaluating the analgesic effects of ibuprofen gargles underscores the need for continued research in this area. By addressing these issues, we aim to provide a comprehensive perspective regarding our study's contributions to the field and to provide clear directions for future research endeavors.
Conclusions
Based on data from a well-conducted clinical trial, this study showed that patient characteristics are associated with ibuprofen gargle efficacy in improving pain among patients who have undergone mandibular third molar extractions. Our results suggest that patients who are female and young tend to show greater improvement in pain control. Further studies are required to evaluate the relationship between the analgesic effect and baseline patient characteristics.
Acknowledgments
We extend special thanks to all staff involved in this study. This work was supported by Toru Hibi from Pharma Seeds Create, Limited Liability Co. (4-12-10 Tsutsujigaoka-Minami, Sanda, Hyogo 669-1347, Japan), who instructed us in the production of ibuprofen gargle used in the study. Additionally, we thank Helen Jeays, BDSc, and Ryan Chastain-Gross, PhD, from Edanz (https://jp.edanz.com/ac) for editing drafts of this manuscript.
The authors have declared that no competing interests exist.
Funding Statement
This work was supported by the Japan Society for the Promotion of Science, KAKENHI, Grant-in-Aid for Young Scientists (Grant No. 19K16413). The grant funder for this study played no role in the study design; collection, management, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication. The funding agency can be contacted at https://www.jsps.go.jp/english/index.html.
Author Contributions
Concept and design: Yasumasa Kakei, Takumi Hasegawa, Masaya Akashi, Takeshi Ioroi, Keiko Miyakoda, Takahiro Ito, Toshiyasu Sakane, Ikuko Yano
Acquisition, analysis, or interpretation of data: Yasumasa Kakei, Takeshi Ioroi, Takahiro Ito, Tatsuya Shirai, Masahiko Kashin
Drafting of the manuscript: Yasumasa Kakei, Takeshi Ioroi, Keiko Miyakoda
Critical review of the manuscript for important intellectual content: Takumi Hasegawa, Masaya Akashi, Keiko Miyakoda, Takahiro Ito, Tatsuya Shirai, Masahiko Kashin, Toshiyasu Sakane, Ikuko Yano
Supervision: Masaya Akashi
Human Ethics
Consent was obtained or waived by all participants in this study. The Clinical Research Ethics Committee of Kobe University issued approval (protocol code: C200024, and date of approval: March 23, 2021)
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Do antimicrobial photodynamic therapy and low-level laser therapy minimize postoperative pain and edema after molar extraction? Fraga RS, Antunes LA, Fialho WL, Valente MI, Gomes CC, Fontes KB, Antunes LS. J Oral Maxillofac Surg. 2020;78:2155–2110. doi: 10.1016/j.joms.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Effect of submucosal dexamethasone injections in the prevention of postoperative pain, trismus, and oedema associated with mandibular third molar surgery: a systematic review and meta-analysis. O'Hare PE, Wilson BJ, Loga MG, Ariyawardana A. Int J Oral Maxillofac Surg. 2019;48:1456–1469. doi: 10.1016/j.ijom.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 3.A systematic review and meta-analysis evaluating antibiotic prophylaxis in dental implants and extraction procedures. Singh Gill A, Morrissey H, Rahman A. Medicina (Kaunas) 2018;54:95. doi: 10.3390/medicina54060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The pharmacological management of dental pain. Pergolizzi JV, Magnusson P, LeQuang JA, Gharibo C, Varrassi G. Expert Opin Pharmacother. 2020;21:591–601. doi: 10.1080/14656566.2020.1718651. [DOI] [PubMed] [Google Scholar]
- 5.Comparison of low pre-emptive oral doses of celecoxib versus acetaminophen for postoperative pain management after third molar surgery: a randomized controlled study. Xie L, Yang RT, Lv K, Zhou HH, Li Z. J Oral Maxillofac Surg. 2020;78:75–76. doi: 10.1016/j.joms.2019.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Evaluation of the efficacy of celecoxib and ibuprofen on postoperative pain, swelling, and mouth opening after surgical removal of impacted third molars: a randomized, controlled clinical trial. Isola G, Matarese M, Ramaglia L, Cicciù M, Matarese G. Int J Oral Maxillofac Surg. 2019;48:1348–1354. doi: 10.1016/j.ijom.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 7.The selective and non-selective cyclooxygenase inhibitors valdecoxib and piroxicam induce the same postoperative analgesia and control of trismus and swelling after lower third molar removal. Benetello V, Sakamoto FC, Giglio FP, et al. Braz J Med Biol Res. 2007;40:1133–1140. doi: 10.1590/s0100-879x2006005000123. [DOI] [PubMed] [Google Scholar]
- 8.Does single-dose preemptive intravenous ibuprofen reduce postoperative pain after third molar surgery? a prospective, randomized, double-blind clinical study. Demirbas AE, Karakaya M, Bilge S, Canpolat DG, Kütük N, Alkan A. J Oral Maxillofac Surg. 2019;77:1990–1997. doi: 10.1016/j.joms.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 9.The effect of zinc lozenge on pain, oedema, and trismus after impacted third molar surgery: a prospective randomized, double-blinded, placebo-controlled study. Rajanna T, Hemalatha BR. J Dent Specialities. 2021;9:13–18. [Google Scholar]
- 10.Managing acute pain and opioid risks following wisdom teeth extraction: an illustrative case. Pruskowski J, Childers J, Moore PA, et al. MedEdPORTAL. 2019;15:10855. doi: 10.15766/mep_2374-8265.10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paracetamol for pain relief after surgical removal of lower wisdom teeth. Weil K, Hooper L, Afzal Z, Esposito M, Worthington HV, van Wijk AJ, Coulthard P. Cochrane Database Syst Rev. 2007;2007:0. doi: 10.1002/14651858.CD004487.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth. Bailey E, Worthington HV, van Wijk A, Yates JM, Coulthard P, Afzal Z. Cochrane Database Syst Rev. 2013;2013:0. doi: 10.1002/14651858.CD004624.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opioid receptors. Stein C. Annu Rev Med. 2016;67:433–451. doi: 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- 14.NSAIDs in the treatment of postoperative pain. Gupta A, Bah M. Curr Pain Headache Rep. 2016;20:62. doi: 10.1007/s11916-016-0591-7. [DOI] [PubMed] [Google Scholar]
- 15.De-implementing opioid use and implementing optimal pain management following dental extractions (DIODE): protocol for a cluster randomized trial. Rindal DB, Asche SE, Gryczynski J, et al. JMIR Res Protoc. 2021;10:0. doi: 10.2196/24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Effect of diclofenac mouthwash on postoperative pain after periodontal surgery. Mishra A, Amalakara J, Avula H, Reddy K. J Clin Diagn Res. 2017;11:0. doi: 10.7860/JCDR/2017/22165.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Efficacy of chlorhexidine, dexpanthenol, allantoin and chitosan gel in comparison with bicarbonate oral rinse in controlling post-interventional inflammation, pain and cicatrization in subjects undergoing dental surgery. Lopez-Lopez J, Jan-Pallí E, lez-Navarro BG, Jané-Salas E, Estrugo-Devesa A, Milani M. Curr Med Res Opin. 2015;31:2179–2183. doi: 10.1185/03007995.2015.1108909. [DOI] [PubMed] [Google Scholar]
- 18.Submucosal diclofenac for acute postoperative pain in third molar surgery: a randomized, controlled clinical trial. Gorecki P, Rainsford KD, Taneja P, et al. J Dent Res. 2018;97:381–387. doi: 10.1177/0022034517744207. [DOI] [PubMed] [Google Scholar]
- 19.Ibuprofen: pharmacology, efficacy and safety. Rainsford KD. Inflammopharmacology. 2009;17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- 20.Working towards an appropriate use of ibuprofen in children: an evidence-based appraisal. de Martino M, Chiarugi A, Boner A, Montini G, de' Angelis GL. Drugs. 2017;77:1295–1311. doi: 10.1007/s40265-017-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibuprofen in the treatment of children's inflammatory pain: a clinical and pharmacological overview. Barbagallo M, Sacerdote P. Minerva Pediatr. 2019;71:82–99. doi: 10.23736/S0026-4946.18.05453-1. [DOI] [PubMed] [Google Scholar]
- 22.Ibuprofen safety at the golden anniversary: are all NSAIDs the same? A narrative review. Varrassi G, Pergolizzi JV, Dowling P, Paladini A. Adv Ther. 2020;37:61–82. doi: 10.1007/s12325-019-01144-9. [DOI] [PubMed] [Google Scholar]
- 23.Ibuprofen gargle for chemo- or chemoradiotherapy-induced oral mucositis: a feasibility study. Ioroi T, Kiyota N, Imamura Y, et al. J Pharm Health Care Sci. 2020;6:12. doi: 10.1186/s40780-020-00168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Advances in oral transmucosal drug delivery. Patel VF, Liu F, Brown MB. J Control Release. 2011;153:106–116. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Efficacy of ibuprofen gargle for postoperative pain after mandibular third molar extraction: protocol for a phase II, placebo-controlled, double-blind, randomized crossover trial. Kakei Y, Ioroi T, Ito T, Okazaki Y, Hasegawa T, Yano I, Akashi M. JMIR Res Protoc. 2022;11:0. doi: 10.2196/35533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efficacy and safety of ibuprofen gargle for postoperative pain after mandibular third molar extraction: a phase II, placebo-controlled, double-blind, randomized crossover trial. Ioroi T, Kakei Y, Ito T, et al. Clin Exp Dent Res. 2023;9:1058–1068. doi: 10.1002/cre2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sex and gender differences in pain: past, present, and future. Keogh E. Pain. 2022;163:108–116. doi: 10.1097/j.pain.0000000000002738. [DOI] [PubMed] [Google Scholar]
- 28.Efficacy of naproxen with or without esomeprazole for pain and inflammation in patients after bilateral third molar extractions: a double blinded crossover study. Weckwerth GM, Simoneti LF, Zupelari-Gonçalves P, et al. Med Oral Patol Oral Cir Bucal. 2017;22:0–31. doi: 10.4317/medoral.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An evidence-based update on nonsteroidal anti-inflammatory drugs. Ong CK, Lirk P, Tan CH, Seymour RA. Clin Med Res. 2007;5:19–34. doi: 10.3121/cmr.2007.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quality of life after extraction of mandibular wisdom teeth: a systematic review. Hallab L, Azzouzi A, Chami B. Ann Med Surg (Lond) 2022;81:104387. doi: 10.1016/j.amsu.2022.104387. [DOI] [PMC free article] [PubMed] [Google Scholar]