Abstract
BACKGROUND
The prevalence of metabolic-associated fatty liver disease (MAFLD) is a growing public health issue in people living with human immunodeficiency virus (PLWH). However, the pathophysiology of MAFLD is still unknown, and the role of genetic variables is only now becoming evident.
AIM
To evaluate the associations of gene-polymorphism-related MAFLD in PLWH.
METHODS
The study employed transient elastography with a controlled attenuation parameter ≥ 248 dB/m to identify MAFLD in patients from a Super Tertiary Hospital in central Thailand. Candidate single-nucleotide polymorphisms (SNPs) were genotyped using TaqMan® MGB probe 5' nuclease assays for seven MAFLD-related genes. Statistical analyses included SNP frequency analysis, Fisher's Exact and Chi-square tests, odds ratio calculations, and multivariable logistic regression.
RESULTS
The G-allele carriers of PNPLA3 (rs738409) exhibited a two-fold rise in MAFLD, increasing by 2.5 times in MAFLD with human immunodeficiency virus infection. The clinical features and genetic patterns imply that LEP rs7799039 A-allele carriers had a nine times (P = 0.001) more significant chance of developing aberrant triglyceride among PLWH.
CONCLUSION
The current study shows an association between PNPLA3 rs738409 and LEP rs7799039 with MAFLD in PLWH.
Keywords: PNPLA3, LEP, Metabolic-associated fatty liver disease, People living with HIV, Thai
Core Tip: The prevalence of metabolic-associated fatty liver disease (MAFLD) in people living with human immunodeficiency virus (PLWH) is increasing, becoming a public health concern. The current evidence suggests that aspartate transaminase, fasting plasma glucose, triglyceride, total cholesterol, low-density lipoprotein, and the genetic factors PNPLA3 rs738409 and LEP rs7799039 indicate genetic susceptibility for PLWH, leading to improvements in MAFLD.
INTRODUCTION
Metabolic-associated fatty liver disease (MAFLD) related to systemic insulin resistance is defined as an accumulation of fat in the hepatocytes of more than 5%, consisting of steatosis, non-alcoholic steatohepatitis, fibrosis, and cirrhosis[1,2]. Nowadays, the pathogenesis of MAFLD remains unclear. Furthermore, the prevalence of MAFLD in people living with human immunodeficiency virus (PLWH) was reported to be 40%-55%, based on different MAFLD phenotype-proven techniques in multiple ethnicities[3,4]. Additionally, liver disease is the second leading cause of death in PLWH[5]. Since 2005, several studies have suggested that MAFLD is common in human immunodeficiency virus (HIV) patients, and its prevalence appears to be increasing. Therefore, HIV infection remains a contributing factor of MAFLD that directly activates insulin resistance in adipose tissue and generates mitochondrial toxicity and reactive oxygen species in hepatocytes, which worsens the MAFLD prognosis[6].
Genetic differences in the risk of MAFLD or NASH progression in the general population are well described[7]. One of the strongest and most consistent associations with the presence and progression of MAFLD in certain populations is associated with the single-nucleotide polymorphism (SNP) on the PNPLA3 rs738409[8-12]. However, only limited studies exist regarding the role of rs738409 SNP on the PNPLA3 gene among people living with HIV. A previous report analyzed the association between PNPLA3 rs738409 polymorphism and the severity of liver disease, insulin resistance, and obesity in patients co-infected with HIV/hepatitis C virus, which was not associated with the duration of the HIV infection or antiretroviral therapy (ART), specific antiretroviral drugs, a history of opportunistic infection, the patient’s immune status, or the duration of the aminotransferase elevation[13,14]. Additionally, other studies indicate that genes involved in the lipid metabolism pathway, such as APOC3, APOB, APOA5, and LIPC, are associated with MAFLD[15-22]. Moreover, GHRL and LEP also exhibit an association with MAFLD pathogenesis[23-25]. In a previous study, it was shown that the APOC3 rs2854116 is associated with elevated serum levels of triglycerides, while this genotype did not affect the incidence of lipoatrophy after adjusting for gender and stavudine (d4T)-containing regimens in Thai people living with HIV[26]. Importantly, GHRL gene polymorphism was significantly correlated with insulin resistance, which is a hallmark of MAFLD and increased type 2 diabetes mellitus risk, particularly among Chinese people and in other populations[27-29]. However, the genome-wide associations replicated in people living with HIV and MAFLD remain inconclusive. A previous study showed both positive and negative associations between candidate SNP and MAFLD, making it difficult to determine the significance of these findings[7,30]. Hence, our study aimed to evaluate the association between several genes related to MAFLD in Thai people living with HIV.
MATERIALS AND METHODS
Study subjects
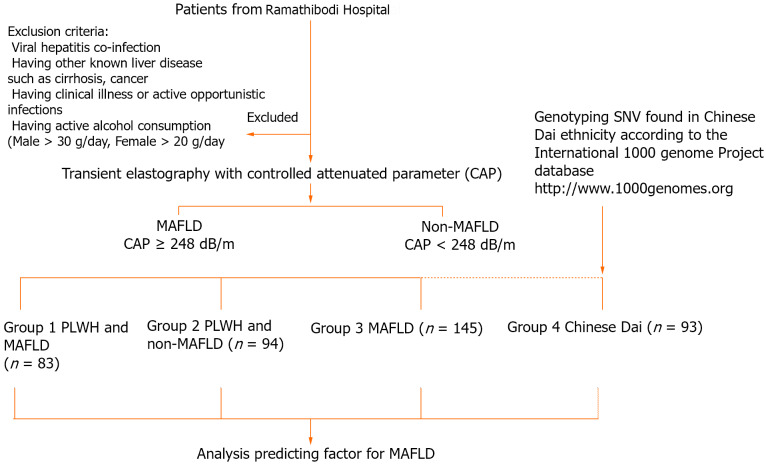
We enrolled patients from a Super Tertiary Hospital in central Thailand and classified them into 4 groups: 83 PLWH and MAFLD (Group 1); 94 people living with HIV and without MAFLD (Group 2); 145 with NAFLD without HIV infection (Group 3), and 93 Chinese Dai genotyping data from the 1000 Genome Project phase (http://www.1000genomes.org) used to represent the Thai ethnicity (Group 4). The presence of MAFLD was confirmed via transient elastography with a controlled attenuated parameter ≥ 248 dB/m, as prescribed by our colleague in a previous study[31]. The Infectious Disease Clinic enrolled PLWH who were on ART with full viral suppression and had no history of alcohol consumption in the trial. The key inclusion criteria were as follows: PLWH receiving ART with an undetectable HIV viral load for at least 6 months. Patients co-infected with hepatitis B or C virus, other known liver disorders such as cirrhosis or hepatocellular carcinoma, and critical liver disease were all excluded. The study protocol is shown in Figure 1.
Figure 1.
Protocol flowchart. MAFLD: Metabolic-associated fatty liver disease; PLWH: People living with HIV; SNV: Single nucleotide variant.
Genotyping analysis
The genotyping of seven genes related to MAFLD was performed using an allele-specific TaqMan® MGB probe 5’ nuclease assay with a real-time polymerase chain reaction (PCR) ViiA7™ system (Applied Biosystems, Life Technologies). The allele-specific TaqMan® MGB probe 5’ nuclease chain reaction assay was performed with primers of PNPLA3 (rs738409); APOC3 (rs2854116); APOA5 (rs662799); APOB (rs10495712); LIPC (rs1800588); LEP (rs7799039); and GHRL (rs27647). Each 6 μL of the PCR mixture contained 2 μL of genomic DNA in a concentration of 5 ng/μL, 2.5 μL of the TaqMan® Genotyping Master mix, 0.25 μL of allele-specific TaqMan® MGB probe and a sequence-specific primer kit, and 1.25 μL of DNase-free water. The thermal cycler program started with 10 min at 95°C, followed by 50 cycles of 15 s at 92°C and 90 s at 60°C. The allelic discrimination plot was analyzed using ViiA7™ software (Applied Biosystems, Life Technologies). Allele 1 was labeled with VIC® dye fluorescence, and allele 2 was labeled with FAM® dye fluorescence.
Statistical analysis
The frequencies of all SNPs were checked for Hardy-Weinberg equilibrium using the R statistic, version 3.6.1, from the R Foundation for Statistical Computing. Fisher’s Exact and Chi-square tests were used to determine the statistical difference between the minor alleles between MAFLD patients and control patients using SPSS version 22.0 for Windows, SPSS Inc., Chicago, IL, United States. The association of the candidate genes’ polymorphisms with MAFLD was assessed by calculating the odds ratios and the corresponding 95% confidence intervals. Backward stepwise multivariable logistic regression analysis was used to assess whether one or more genetic factors predicted MAFLD. A P value of less than 0.05 was considered significant.
RESULTS
Characteristics of people living with HIV and with MAFLD (Group 1) and of non-MAFLD patients (Group 2) and MAFLD patients (Group 3)
When comparing the people living with HIV and with and without MAFLD, we enrolled a higher proportion of males with a higher BMI. The levels of fasting glucose, HbA1C, triglyceride, aspartate transaminase (AST), alanine transaminase (ALT), and gamma-glutamyl transferase were significantly higher in the MAFLD group compared to the non-MAFLD group in the metabolic profiles and liver function tests. When comparing the HIV treatment regimens, the proportion of non-nucleoside reverse transcriptase inhibitor, nucleoside reverse transcriptase inhibitor (NRTI), protease inhibitor (PI)-based, or alternative regimens did not differ between the two groups (P = 0.573), but comorbidities of dyslipidemia, hypertension, and diabetes mellitus were higher in people living with HIV and MAFLD (P = 0.002; P = 0.001 and P = 0.005, respectively) (Table 1).
Table 1.
Baseline characteristics of metabolic-associated fatty liver disease patients and controls
|
Characteristics
|
PLWH and MAFLD (n = 83)
|
PLWH and non-MAFLD (n = 94)
|
P value
|
MAFLD (n = 145)
|
P value
|
Chinese Dai (n = 93)
|
P value
|
| Age (yr) | 51.99 ± 7.65 | 49.55 ± 8.27 | 0.044a | 65.00 | < 0.001b | N/A | N/A |
| Gender | |||||||
| Male | 54 (65.10) | 48 (51.10%) | 0.060 | 68 (46.90%) | 0.008b | 44 (47.30%) | 0.950 |
| Female | 48 (34.90%) | 46 (48.90%) | 77 (53.10%) | 49 (52.70%) | |||
| BMI (kg/m2) | 25.42 | 21.79 | < 0.001a | 27.67 | < 0.001b | N/A | - |
| CD4 (cells/mm3) | 619.00 | 570.50 | 0.033a | N/A | - | N/A | - |
| %CD 4 | 26.10 ± 0.83 | 25.76 ± 0.81 | 0.853 | N/A | - | N/A | - |
| Hb (g/dL) | 14.36 1.62 | 13.84 1.90 | 0.015a | N/A | N/A | ||
| Platelets (-/mm3) | 259063 77332 | 261694 66966 | 0.744 | N/A | N/A | ||
| AP (U/L) | 85.00 | 86.00 | 0.821 | 72.00 | 0.001b | N/A | - |
| AST (U/L) | 33.00 | 28.00 | < 0.001a | 38.00 | 0.015b | N/A | - |
| ALT (U/L) | 38.00 | 25.00 | < 0.001a | 50.00 | 0.004b | N/A | - |
| GGT (U/L) | 47.00 | 35.50 | < 0.001a | 51.50 | 0.853 | N/A | - |
| Total protein (g/L) | 79.60 ± 0.56 | 78.28 ± 0.47 | 0.071 | 75.96 ± 0.42 | < 0.001b | N/A | - |
| Albumin (g/L) | 40.80 | 38.35 | < 0.001a | 40.65 | 0.255 | N/A | - |
| Total bilirubin (mg/dL) | 0.60 | 0.50 | 0.759 | 0.70 | 0.10 | N/A | - |
| Direct bilirubin (mg/dL) | 0.20 | 0.20 | 0.862 | 0.30 | 0.007b | N/A | - |
| HbA1C (mmol/L) | 5.68 | 5.38 | < 0.001a | 6.36 | < 0.001b | N/A | - |
| Fasting plasma Glucose (mg/dL) | 98 | 93 | 0.026 | 108 | < 0.001b | N/A | - |
| Triglyceride (mg/dL) | 169 | 109 | < 0.001a | 123 | < 0.0001b | N/A | - |
| Total Cholesterol (mg/dL) | 206.78 ± 5.57 | 199.10 ± 3.57 | 0.212 | 183 | 0.428 | N/A | - |
| HDL (mg/dL) | 44 | 49 | 0.004a | 49 | 0.10 | N/A | - |
| LDL (mg/dL) | 130.67 ± 4.27 | 122.46 ± 2.98 | 0.086 | 114.94 ± 2.83 | 0.001b | N/A | - |
| Drug-regimen, n (%) | |||||||
| NRTI+NNRTI | 62 (74.7) | 71 (75.5) | 0.573 | N/A | - | N/A | - |
| NRTI+PI | 19 (22.9) | 18 (19.1) | N/A | - | N/A | - | |
| Alternative | 2 (2.4) | 5 (5.3) | N/A | - | N/A | - | |
| Co-morbidities | |||||||
| Dyslipidemia | 30 (36.1) | 15 (16.0) | 0.002a | 82 (56.9) | 0.002b | N/A | - |
| Hypertension | 21 (25.3) | 6 (6.4) | 0.001a | 64 (44.1) | 0.003b | N/A | - |
| Diabetes mellitus | 16 (19.3) | 5 (5.3) | 0.005a | 63 (43.4) | < 0.001b | N/A | - |
P value < 0.05 compared between people living with human immunodeficiency virus (PLWH) and metabolic-associated fatty liver disease (MAFLD) vs PLWH and non-MAFLD.
P value < 0.05 compared between PLWH and MAFLD vs MAFLD.
Data represent as mode, mean ± standard deviation or n (%), differences between groups were tested by Chi-square test or one-way ANOVA as appropriate. AP: Alkaline phosphatase; AST: Aspartate aminotransaminase; ALT: Alanine aminotransaminase; ALP: Alkaline phosphatase; Hb: Henoglobin; TB: Total bilirubin; MAFLD: Metabolic-associated fatty liver disease; N/A: Not available; PLWH: People living with human immunodeficiency virus; GGT: Gamma-glutamyl transferase; HbA1C: Hemoglobin A1C; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; NRTI: Nucleoside reverse transcriptase inhibitor; NNRTI: Non-nucleoside reverse transcriptase inhibitor; PI: Protease inhibitor.
Distribution of SNPs in people living with HIV and MAFLD (Group 1) or without MAFLD (Group 2), with MAFLD (Group 3), and Chinese Dai (Group 4)
All the SNP genotyping experiments were successful. Throughout the entire study, the genotype frequencies of each SNP did not deviate from Hardy-Weinberg equilibrium (P > 0.05). Table 2 shows the genotype distribution and minor allele frequency of the investigated SNPs in people living with HIV with or without MAFLD, MAFLD patients, and Chinese Dai. All potential SNP genotype distributions (PNPLA3 rs738409, APOC3 rs2854116, APOA5 rs662799, APOB rs10495712, LIPC rs1800588, LEP rs7799039, and GHRL rs27647) in people living with HIV with MAFLD were similar to those seen in patients living with HIV without MAFLD. In comparison to Chinese Dai, patients with MAFLD had a higher frequency of the PNPLA3 G-allele (P = 0.035). The frequencies of the other SNPs were not significant in persons living with HIV and those with or without MAFLD.
Table 2.
Genotype distributions and Minor allele frequency of candidates single-nucleotide polymorphisms
|
Polymorphism
|
PLWH and MAFLD (n = 83)
|
PLWH and non-MAFLD (n = 94)
|
P value
|
MAFLD (n = 145)
|
Chinese Dai (n = 94)
|
P value
|
| PNPLA3 rs738409 | ||||||
| MAF = G | 57 (34.33) | 56 (29.78) | 0.413 | 92 (31.73) | 43 (23.12) | 0.035a |
| APOC3 rs2854116 | ||||||
| MAF=T | 81 (47.09) | 87(46.28) | 0.691 | 128 (44.14) | 88 (47.31) | < 0.001b |
| LEP rs7799039 | ||||||
| MAF=G | 57 (33.14) | 57 (30.32) | 0.738 | 85 (29.31) | 48 (25.81) | 0.674 |
| GHRL rs27647 | ||||||
| MAF = C | 13 (7.56) | 25 (13.30) | 0.055 | 28 (9.66) | 18 (9.68) | 0.702 |
| LIPC rs1800588 | ||||||
| MAF = T | 70 (42.17) | 70 (37.33) | 0.421 | 101 (34.83) | 67 (36.02) | 0.872 |
| APOB rs10495712 | ||||||
| MAF = A | 15 (8.06) | 14 (7.45) | 0.853 | 22 (7.59) | 8 (4.30) | 0.339 |
| APOA5 rs662799 | ||||||
| MAF = G | 41 (22.04) | 51 (27.13) | 0.766 | 75 (25.86) | 52 (27.96) | 0.834 |
P value < 0.05 compared between people living with human immunodeficiency virus (PLWH) and metabolic-associated fatty liver disease (MAFLD) vs PLWH and non-MAFLD.
P value < 0.05 compared between PLWH and MAFLD vs MAFLD MAF minor allele frequency, Chinese Dai was represented as general population. Data represented as n (%), PLWH and MAFLD vs other groups, differences between groups were tested by Chi-square test. MAFLD: Metabolic-associated fatty liver disease; PLWH: People living with human immunodeficiency virus; MAF: Metabolic-associated fatty.
Association between PNPLA3 and other candidate SNPs with MAFLD
The data given in Tables 3 and 4 show the well-established PNPLA3 rs738409 gene, which is found on chromosome 22 and has a function related to lipid droplet formation in the hepatocytes; the G-carrier patients had an approximately two-fold higher risk of developing MAFLD when compared to MAFLD with Chinese Dai (P = 0.012). Importantly, people living with HIV and MAFLD exhibited a 2.5-fold increased risk (P = 0.002) when compared to Chinese Dai (Group 4). In addition, GHRL (Ghrelin) rs27647 is a promising susceptibility gene for insulin regulation; the C-allele carrier has exhibited a protective effect in MAFLD in people living with HIV. The odds of having MAFLD is 53% lower if the people living with HIV are C-carriers of GHRL rs27647 than if they are not C-carriers (P = 0.047). However, there was no statistically significant association with GHRL in the other groups. Furthermore, APOC3 rs2854116 C-allele carriers were also statistically significant in the MAFLD group, exhibiting a six-fold higher risk in the dominant model with Chinese Dai as the comparison (P < 0.001).
Table 3.
Genotype and allele frequencies of the single-nucleotide polymorphisms in the people living with human immunodeficiency virus and metabolic-associated fatty liver disease compared with people living with human immunodeficiency virus and non-metabolic-associated fatty liver disease group
| Gene | SNP | B allele |
Dominant model
|
Recessive model
|
||||||
|
PLWH and MAFLD vs PLWH and non-MAFLD
|
PLWH and MAFLD vs Chinese Dai
|
PLWH and MAFLD vs PLWH and non-MAFLD
|
PLWH and MAFLD vs Chinese Dai
|
|||||||
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
|||
| PNPLA3 | rs738409 | G | 1.476 (0.809-2.694) | 0.204 | 2.539 (1.382-4.665) | 0.002b | 0.94 (0.276-3.202) | 0.921 | 0.929 (0.273-3.166) | 0.907 |
| APOC3 | rs2854116 | C | 1.117 (0.543-2.297) | 0.764 | 1.203 (0.588-2.462) | 0.613 | 0.798 (0.411-1.550) | 0.506 | 0.828 (0.425-1.614) | 0.579 |
| LEP | rs7799039 | G | 0.704 (0.287-1.727) | 0.422 | 0.408 (0.146-1.142) | 0.080 | 0.886 (0.491-1.601) | 0.689 | 0.730 (0.403-1.322) | 0.298 |
| GHRL | rs27647 | G | 0.466 (0.217-1.001) | 0.047a | 0.704 (0.317-1.566) | 0.388 | 2.146 (1.832-2.514) | 0.469 | 2.134 (1.823-2.499) | 0.472 |
| LIPC | rs1800588 | T | 1.506 (0.806-2.815) | 0.198 | 1.676 (0.898-3.128) | 0.104 | 1.053 0.452-2.456) | 0.905 | 1.040 (0.446-2.427) | 0.928 |
| APOB | rs10495712 | A | 1.264 (0.557-2.871) | 0.575 | 2.156 (0.855-5.436) | 0.098 | 1.134 (0.070-18.422) | 1.000 | 2.134 (1.823-2.499) | 0.472 |
| APOA5 | rs662799 | G | 0.914 (0.505-1.654) | 0.766 | 0.960 (0.330-2.790) | 0.940 | 0.629 (0.177-2.231) | 0.470 | 0.734 (0.200-2.697) | 0.751 |
Data expressed as OR odds ratio, CI confidence interval.
P value < 0.05 compared between people living with human immunodeficiency virus (PLWH) and metabolic-associated fatty liver disease (MAFLD) vs PLWH and non-MAFLD.
P value < 0.05 compared between PLWH and MAFLD vs Chinese Dai was represented as general population, B-allele expressed risk allele. MAFLD: Metabolic-associated fatty liver disease; PLWH: People living with human immunodeficiency virus; SNP: Single-nucleotide polymorphism.
Table 4.
Genotype and allele frequencies of the single-nucleotide polymorphisms in the metabolic-associated fatty liver disease compared with Chinese Dai
| Gene | SNP | B allele |
Dominate model
|
Recessive model
|
||
|
OR (95%CI)
|
P value
|
OR (95%CI)
|
P value
|
|||
| PNPLA3 | rs738409 | G | 1.970 (1.160-3.345) | 0.012a | 1.074 (0.377-3.061) | 0.894 |
| APOC3 | rs2854116 | C | 6.109 (2.490-14.986) | < 0.001a | 0.485 (0.259-0.907) | 0.022b |
| LEP | rs7799039 | G | 0.840 (0.300-2.355) | 0.740 | 0.790 (0.469-1.332) | 0.376 |
| GHRL | rs27647 | G | 0.953 (0.491-1.850) | 0.888 | 1.646 (1.486-1.823) | 1.000 |
| LIPC | rs1800588 | T | 0.889 (0.525-1.504) | 0.661 | 1.042 (0.494-2.199) | 0.914 |
| APOB | rs10495712 | A | 1.799 (0.762-4.251) | 0.176 | 1.642 (1.486-1.823) | 1.000 |
| APOA5 | rs662799 | G | 0.854 (0.507-1.438) | 0.552 | 0.960 (0.330-2.790) | 0.940 |
P value < 0.05 compared between metabolic-associated fatty liver disease (MAFLD) vs Chinese Dai in dominant model.
P value < 0.05 compared between MAFLD vs Chinese Dai in recessive model, B-allele expressed risk allele. Data expressed as n (%). MAFLD: Metabolic-associated fatty liver disease; PLWH: People living with human immunodeficiency virus; SNP: Single-nucleotide polymorphism; OR: Odds ratio.
Association between candidate SNPs and the lipid profile, liver function, and glucose metabolisms
We performed a subgroup analysis of people living with HIV in terms of their metabolic profiles and compared the genotypes of candidate genes. As shown in Table 5, the mean or median values of the lipid profile [triglyceride, total cholesterol, LDL]; liver function (AST, ALT); and glucose metabolisms (HbA1C and fasting plasma glucose) were higher in the people living with HIV and MAFLD than in the control group (people living with HIV and non-MAFLD). The association between the genotypes of the APOA5 rs662799 SNP and serum lipid parameters in the control group is presented in Table 5 and Supplementary Table 1. Serum total cholesterol levels in control patients differed between the AA and AG/GG genotypes (P < 0.05). The APOA5 rs662799 G allele carriers had a lower proportion of total cholesterol levels in the normal range (< 200 mg/dL) than the A allele non-carriers and indicated the protective effect of APOA5 rs662799 in an abnormal range of total cholesterol (> 200 mg/dL); these results showed statistical significance (P = 0.045). Furthermore, LEP rs7799039 AG and AA carriers exhibited a significant nine-fold higher risk in an abnormal range of triglyceride (> 150 mg/dL) when compared with non-carriers (P = 0.001). Unfortunately, none of the individual SNPs were associated with LDL. Moreover, in men, APOC3 rs2854116 TT alleles also showed a protective effect on the high-density lipoprotein (HDL) profile (Table 5 and Supplementary Table 1). Furthermore, AST is known to be a reliable surrogate marker for outcome measures in MAFLD. Table 6 shows that PNPLA3 rs738409 G-carrier patients have an approximately 2.5 times higher chance of AST abnormality (> 34 U/L) when compared with non-carriers (statistically significant at P = 0.010).
Table 5.
Association between genetic polymorphism and Lipid profile
| Genetic polymorphisms |
Lipid parameters
|
|||||||
|
Triglyceride (mg/dL), (n = 177)
|
Total cholesterol (mg/dL), (n = 177)
|
LDL-cholesterol (mg/dL), (n = 177)
|
HDL-cholesterol (mg/dL), (n = 66)
|
HDL-cholesterol (mg/dL), (n = 111)
|
||||
|
|
< 150
|
≥ 150
|
< 200
|
≥ 200
|
< 130
|
≥ 130
|
Men ≥ 40 mg/dL, women ≥ 50 mg/dL
|
Men < 40 mg/dL, women < 50 mg/dL
|
| PNPLA3 rs738409 CC vs CG+GG | ||||||||
| OR (95%CI) | 0.699 (0.383-1.277) | 1.053 (0.580-1.912) | 1.088 (0.597-1.981) | 0.9967 (0.538 -1.846) | ||||
| P value | 0.243 | 0.865 | 0.784 | 0.992 | ||||
| APOC3 rs2854116 TT vs CT+CC | ||||||||
| OR (95%CI) | 0.796 (0.387-1.635) | 1.611 (0.780-3.328) | 1.173 (0.568-2.423) | 0.4696 (0.253-0.873) | ||||
| P value | 0.534 | 0.195 | 0.666 | 0.017a | ||||
| APOA5 rs662799 AA vs AG+GG | ||||||||
| OR (95%CI) | 1.021 (0.562-1.855) | 0.543 (0.299-0.989) | 0.595 (0.326-1.084) | 0.739 (0.374-1.461) | ||||
| P value | 0.946 | 0.045a | 0.089 | 0.385 | ||||
| APOB rs10495712 (GG vs AG+AA) | ||||||||
| OR (95%CI) | 0.749 (0.322-1.743) | 0.719 (0.315-1.639) | 0.807 (0.351-1.855) | 0.816 (0.343-1.938) | ||||
| P value | 0.501 | 0.431 | 0.613 | 0.645 | ||||
| LIPC rs1800588 CC vs CT+TT | ||||||||
| OR (95%CI) | 0.870 (0.467-1.621) | 0.732 (0.393-1.363) | 0.607 (0.326-1.132) | 0.911 (0.482-1.722) | ||||
| P value | 0.661 | 0.325 | 0.115 | 0.774 | ||||
| LEP rs7799039 GG vs AG+AA | ||||||||
| OR (95%CI) | 9.316 (2.064-40.428) | 1.623 (0.655-4.017) | 1.518 (0.602-3.825) | 1.317 (0.507-3.419) | ||||
| P value | 0.001a | 0.292 | 0.374 | 0.572 | ||||
| GHRL rs27647 (AA vs AG+GG) | ||||||||
| OR (95%CI) | 0.570 (0.265-1.224) | 0.997 (0.483-2.058) | 0.905 (0.436-1.878) | 0.889 (0.417-1.895) | ||||
| P value | 0.147 | 0.993 | 0.788 | 0.761 | ||||
P < 0.05 compare between normal level vs abnormal level. OR: Odds ratio; HDL: High-density lipoprotein; LDL: Low-density lipoprotein.
Table 6.
Association between genetic polymorphism and metabolic traits
| Genetic polymorphisms |
Metabolic traits
|
|||||||
|
FPG (mg/dL), n = 175
|
HbA1C (mmol/L), n = 159
|
AST (U/L), n = 175
|
ALT (U/L), n = 177
|
|||||
|
|
< 100
|
≥ 100
|
< 6.5
|
≥ 6.5
|
< 34
|
≥ 34
|
< 40
|
≥ 40
|
| PNPLA3 rs738409 (CC vs CG+GG) | ||||||||
| OR (95%CI) | 0.354 (0.063-1.984) | 1.055 (0.437-2.543) | 2.568 (1.243-5.305) | 1.679 (0.713-3.953) | ||||
| P value | 0.219 | 0.906 | 0.010a | 0.232 | ||||
| APOC3 rs2854116 (TT vs CT+CC) | ||||||||
| OR (95%CI) | 0.956 (0.923-0.991) | 1.553 (0.495-4.897) | 0.735 (0.335-1.614) | 0.630 (0.253-1.570) | ||||
| P value | 0.342 | 0.448 | 0.442 | 0.318 | ||||
| APOA5 rs662799 (AA + AG+GG) | ||||||||
| OR (95%CI) | 1.167 (0.229-5.946) | 1.213 (0.509-2.893) | 0.823 (0.421-1.611) | 0.730 (0.320-1.664) | ||||
| P value | 1.000 | 0.663 | 0.570 | 0.453 | ||||
| APOB rs10495712 (GG vs AG+AA) | ||||||||
| OR (95%CI) | 1.100 (0.123-9.802) | 1.150 (0.356-3.720) | 2.063 (0.879-4.840) | 1.255 (0.431-3.651) | ||||
| P value | 0.923 | 0.815 | 0.092 | 0.774 | ||||
| LIPC rs1800588 (CC vs CT+TT) | ||||||||
| OR (95%CI) | 0.947 (0.907-0.989) | 1.709 (0.636-4.592) | 1.476 (0.719-3.027) | 2.208 (0.844-5.776) | ||||
| P value | 0.093 | 0.284 | 0.287 | 0.100 | ||||
| LEP rs7799039 (GG vs AG+AA) | ||||||||
| OR (95%CI) | 0.268 (0.046-1.561) | 1.008 (0.272-3.746) | 1.329 (0.462-3.827) | 2.016 (0.444-9.155) | ||||
| P value | 0.166 | 1.000 | 0.579 | 0.535 | ||||
| GHRL rs27647 (AA vs AG+GG) | ||||||||
| OR (95%CI) | 1.045 (1.009-1.083) | 0.700 (0.222-2.206) | 0.676 (0.285-1.605) | 0.795 (0.280-2.256) | ||||
| P value | 0.345 | 0.541 | 0.373 | 0.666 | ||||
P value < 0.05. AST: Aspartate aminotransaminase; ALT Alanine aminotransaminase; OR: Odds ratio; FPG: Fasting plasma glucose; HbA1C: Hemoglobin A1C.
Association between the genetic profile, clinical factors, and MAFLD
A stepwise multiple logistic regression was performed to investigate the relationship between the genetics profiles, clinical factors, and MAFLD. Sixteen variables, including gender, AST, ALT, total cholesterol, triglycerides, HDL, LDL, fasting plasma glucose, HbA1C, and the genetic profiles of PNPLA3 rs738409, APOC3 rs2854116, APOA5 rs662799, APOB rs10495712, LIPC rs1800588, LEP rs7799039, and GHRL rs27647 were entered into the original equation. The results showed that seven variables, namely, AST, total cholesterol triglycerides, LDL, fasting plasma glucose, APOB rs10495712, and APOA5 rs662799, were significantly associated with MAFLD (Table 7).
Table 7.
Logistic regression analysis of factors associated with metabolic-associated fatty liver disease (people living with human immunodeficiency virus and metabolic-associated fatty liver disease vs people living with human immunodeficiency virus and non-metabolic-associated fatty liver disease)
| Factor | Exp(B) | 95%CI | P value |
| AST | 4.615 | 1.081-19.709 | 0.039a |
| Fasting Plasma glucose | 21.5 | 5.327-86.767 | < 0.001a |
| Triglyceride | 6.747 | 1.747-26.047 | 0.006a |
| Total cholesterol | 0.125 | 0.019-0.819 | 0.030a |
| LDL | 12.97 | 1.983-84.827 | 0.007a |
| APOB rs10495712 | 4.195 | 1.304-18.532 | 0.019a |
| APOA5 rs662799 | 0.012 | 0.002-0.770 | < 0.001a |
| LEP rs7799039 | 0.321 | 0.070-1.469 | 0.143a |
P value < 0.2. AST: Aspartate aminotransaminase; LDL: Low-density lipoprotein.
DISCUSSION
Risk factors for MAFLD in people living with HIV (PLWH) include the normal factors seen in the general population, such as components of metabolic syndrome (obesity, diabetes, hypertension, dyslipidemia, a sedentary lifestyle, and excessive dietary intake)[1]. Hepatic steatosis and mitochondrial oxidative stress are pivotal to MAFLD pathogenesis. In PLWH with MAFLD, HIV-specific factors such as lipodystrophy, ART, and HIV infection itself are strongly linked to the development of MAFLD[31,32]. However, our study did not find NRTI-based and PI-based regimens to be predictive factors for MAFLD.
The strongest and most consistent associations with the presence and progression of MAFLD in the studied populations are related to the SNP on the PNPLA3 rs738409, which was discovered by the first GWAS in 2003[8]. Our study demonstrated the significance of PNPLA3 rs738409 in MAFLD when compared to the general population, indicating the impact of genetic factors. Moreover, we evaluated the effect of both HIV infection and genetic factors by conducting a comparison between people living with HIV and Chinese Dai, finding that it increased the chance of the development of MAFLD between 2 and 2.5 times when compared to the genetic factor alone. Moreover, our results agree with previous studies that demonstrated the significant association with PNPLA3 rs738409 and biopsy-proven fibrosis or steatosis among HIV/hepatitis C virus or HBV co-infected patients, HIV-mono infection, and the group with no viral infection[33-35].
Insulin resistance has been characterized as the crucial pathophysiological factor in MAFLD. The advanced reports found that insulin resistance is associated with the reduction of circulating ghrelin level[21,36,37]. Interestingly, our study has shown that the G/A genotype and G/G genotype of GHRL rs27647 were associated with a 53% decreased risk of MAFLD in people living with HIV when compared with non-MAFLD patients. Moreover, a previous study observed higher levels of ghrelin in patients with hypertriglyceridemia, as well as a positive correlation between ghrelin and triglyceride levels in patients with hypertriglyceridemia[38,39]. Unfortunately, our study failed to detect the association between SNP and triglyceride levels in people living with HIV.
The APOC3 gene plays a crucial role in the circulation and clearance of very-low-density lipoprotein, HDL, and chylomicron remnants[40,41]. The polymorphism in the promotor region of the APOC3 rs2854116 (-455T>C) gene has been extensively studied and has been found to be related with insulin resistance at the transcriptional level. Consequently, the overexpression of APOC3, which functions to inhibit lipoprotein lipase and the cellular uptake of triglyceride-rich lipoprotein particles, may result in hypertriglyceridemia, as has been confirmed by in vivo and clinical studies[23,24,42-44]. In this study, we showed that APOC3 rs2854116 C-allele carrier patients have a six-fold higher risk of developing MAFLD in a dominant model. Our findings are also consistent with previous reports that the APOC3 rs2854116 genetic variant leads to increased plasma concentrations of apolipoprotein C3, resulting in hepatic insulin resistance and MAFLD in multiethnic populations[23,45,46].
Our results show a similar trend to those of a previous report, which demonstrated a positive correlation between AST levels and the accumulation of intrahepatic triglyceride[47]. Interestingly, our results indicate a robust association in LEP rs7799039 with the lipid profile, especially with triglyceride levels. According to a subgroup analysis of patients infected with HIV, a patient who is a carrier of the A-allele (AG and AA) has a nine-times-higher risk of exhibiting abnormal triglyceride levels (> 150 mg/dL). Further information suggests that LEP rs7799039, located on chromosome 7, encodes 167 amino acid peptide variants with a molecular weight of 16 ku, which may subsequently affect the biological functions of LEP[48]. In recent years, LEP has been found to regulate the energy balance in coordination with the regulation of the glucose and lipid metabolisms. Thus, it plays a vital role in the development of MAFLD. This finding aligns with that of previous reports that evaluated the association between LEP rs7799039 and diabetes mellitus, metabolic syndrome, MAFLD, and cardiovascular disease[49,50]. Our findings should be interpreted while bearing in mind several potential limitations. First, the small sample size of each group may have limited the study’s ability to detect a significant relationship. Second, the patients included in this study were exclusively Thai, so our findings may not apply to patients of other ethnic origins. Further, long-term studies are still needed to confirm these findings in other ethnicities. Although the results of the available research are satisfactory, they have not been proven in randomized control trials. Further studies of genetic predispositions for MAFLD with the absence or presence with MAFLD will certainly provide a better understanding of the molecular mechanisms of MAFLD.
CONCLUSION
The prevalence of MAFLD in people living with HIV is increasing, representing a public health concern. The existing evidence suggests that AST, fasting plasma glucose, triglyceride, total cholesterol, LDL, and the genetic factors PNPLA3 rs738409 and LEP rs7799039 indicate genetic susceptibility for PLWH, leading to improvements in the treatment of MAFLD.
ARTICLE HIGHLIGHTS
Research background
Metabolic-associated fatty liver disease (MAFLD), which is characterized by hepatocyte fat accumulation, poses substantial health risks; it affects a significant number of people globally, especially those living with obesity, diabetes, dyslipidemia, hypertension, and metabolic syndrome. Despite its prevalence, the precise mechanisms underlying MAFLD, which involve factors including viral hepatitis, human immunodeficiency virus (HIV), antiretroviral treatment, and genetics, remain unclear.
Research motivation
MAFLD is prevalent among individuals with HIV, with rates ranging from 40% to 55%; it is influenced by both antiretroviral medications and specific genetic variants. Notably, the PNPLA3 rs738409 variant, a genetic factor, plays a significant role in the development of MAFLD.
Research objectives
The present investigation sought to assess the correlation between gene polymorphisms and MAFLD in individuals living with HIV.
Research methods
We employed transient elastography and set a threshold for the controlled attenuated parameter at ≥ 248 dB/m for the identification of MAFLD. All participants underwent genotyping for candidate single-nucleotide polymorphisms.
Research results
Individuals carrying the G-allele of PNPLA3 (rs738409) demonstrated a two-fold increased risk of developing MAFLD; this risk rose to 2.5 times in cases of MAFLD with HIV infection. The clinical characteristics and genetic profiles suggested that carriers of the A-allele of LEP rs7799039 had a nine-fold higher likelihood of developing abnormal triglyceride levels among individuals living with HIV.
Research conclusions
The present research reveals a connection between PNPLA3 rs738409 and LEP rs7799039 and MAFLD in individuals with HIV.
Research perspectives
Genetic factors play a crucial role in the pathophysiology of MAFLD. In upcoming research, targeting the PNPLA3 gene in clinical trials may emerge as a promising direction for precision medicine in the treatment of MAFLD.
ACKNOWLEDGEMENTS
We appreciate the study patients' participation and the support of the attending staffs and all staffs in the Divisions of Personalized medicine and Personalized Medicine, research nurses in the Divisions of Infectious Diseases and Gastroenterology and Hepatology, and the Department of Radiology.
Footnotes
Institutional review board statement: The study was approved by the ethics committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University (Bangkok, Thailand) (COA. MURA2019/645). All study procedures were conducted in accordance with the 1964 Helsinki Declaration.
Informed consent statement: In this investigation, genomic material was isolated from residual specimens of the study subjects, demonstrating minimal risk to patients and participant consent was not necessary.
Conflict-of-interest statement: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
STROBE statement: The authors have read the STROBE Statement – checklist of items, and the manuscript was prepared and revised according to the STROBE Statement – checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 1, 2023
First decision: December 5, 2023
Article in press: February 8, 2024
Specialty type: Genetics and heredity
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mendez-Sanchez N, Mexico S-Editor: Liu JH L-Editor: A P-Editor: Cai YX
Contributor Information
Kanuengnit Choochuay, Program in Translational Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand; School of Pharmacy, Walailak University, Nakhon Si Thammarat 80161, Thailand; Laboratory for Pharmacogenomics, Division of Pharmacogenomics and Personalized Medicine, Somdech Phra Debaratana Medical Center, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Punna Kunhapan, Department of Medical Sciences, Ministry of Public Health, Nonthaburi 11000, Thailand.
Apichaya Puangpetch, Laboratory for Pharmacogenomics, Division of Pharmacogenomics and Personalized Medicine, Somdech Phra Debaratana Medical Center, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Sissades Tongsima, National Biobank of Thailand, National Center for Genetic Engineering and Biotechnology, Pathum Thani 12120, Thailand.
Pornpen Srisawasdi, Division of Clinical Chemistry, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Abhasnee Sobhonslidsuk, Division of Gastroenterology and Hepatology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand.
Somnuek Sungkanuparph, Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan 10540, Thailand.
Mohitosh Biswas, Department of Pharmacy, University of Rajshahi, Rajshahi 6205, Bangladesh.
Chonlaphat Sukasem, Laboratory for Pharmacogenomics, Division of Pharmacogenomics and Personalized Medicine, Somdech Phra Debaratana Medical Center, Department of Pathology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok 10400, Thailand; Pharmacogenomics Clinic, Bumrungrad Genomic Medicine Institute, Bumrungrad International Hospital, Bangkok 10110, Thailand; Research and Development Laboratory, Bumrungrad International Hospital, Bangkok 10110, Thailand; MRC Centre for Drug Safety Science, Department of Pharmacology and Therapeutics, Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 3GL, United Kingdom; Faculty of Pharmaceutical Sciences, Burapha University, Chonburi 20131, Thailand. chonlaphat.suk@mahidol.ac.th.
Data sharing statement
No additional data are available.
References
- 1.Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What's in a name? Renaming 'NAFLD' to 'MAFLD'. Liver Int. 2020;40:1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 2.Verna EC. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with HIV. Lancet Gastroenterol Hepatol. 2017;2:211–223. doi: 10.1016/S2468-1253(16)30120-0. [DOI] [PubMed] [Google Scholar]
- 3.Non-alcoholic Fatty Liver Disease Study Group. Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, Cortez-Pinto H, Grieco A, Machado MV, Miele L, Targher G. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis. 2015;47:997–1006. doi: 10.1016/j.dld.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 5.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, Kowalska JD, de Wit S, Law M, el Sadr W, Kirk O, Friis-Moller N, Monforte Ad, Phillips AN, Sabin CA, Lundgren JD D:A:D Study Group. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 6.Coronel-Castillo CE, Qi X, Contreras-Carmona J, Ramírez-Pérez OL, Méndez-Sánchez N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in HIV infection: a metabolic approach of an infectious disease. Expert Rev Gastroenterol Hepatol. 2019;13:531–540. doi: 10.1080/17474124.2019.1599284. [DOI] [PubMed] [Google Scholar]
- 7.Soti S, Corey KE, Lake JE, Erlandson KM. NAFLD and HIV: Do Sex, Race, and Ethnicity Explain HIV-Related Risk? Curr HIV/AIDS Rep. 2018;15:212–222. doi: 10.1007/s11904-018-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Nakamura T, Teranishi H, Mizusawa S, Ueno T, Chayama K, Nakajima A, Nakao K, Sekine A, Hotta K. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum Genet. 2013;132:783–792. doi: 10.1007/s00439-013-1294-3. [DOI] [PubMed] [Google Scholar]
- 10.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, Eiriksdottir G, Garcia ME, Launer LJ, Nalls MA, Clark JM, Mitchell BD, Shuldiner AR, Butler JL, Tomas M, Hoffmann U, Hwang SJ, Massaro JM, O'Donnell CJ, Sahani DV, Salomaa V, Schadt EE, Schwartz SM, Siscovick DS NASH CRN; GIANT Consortium; MAGIC Investigators, Voight BF, Carr JJ, Feitosa MF, Harris TB, Fox CS, Smith AV, Kao WH, Hirschhorn JN, Borecki IB; GOLD Consortium. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Namjou B, Lingren T, Huang Y, Parameswaran S, Cobb BL, Stanaway IB, Connolly JJ, Mentch FD, Benoit B, Niu X, Wei WQ, Carroll RJ, Pacheco JA, Harley ITW, Divanovic S, Carrell DS, Larson EB, Carey DJ, Verma S, Ritchie MD, Gharavi AG, Murphy S, Williams MS, Crosslin DR, Jarvik GP, Kullo IJ, Hakonarson H, Li R eMERGE Network, Xanthakos SA, Harley JB. GWAS and enrichment analyses of non-alcoholic fatty liver disease identify new trait-associated genes and pathways across eMERGE Network. BMC Med. 2019;17:135. doi: 10.1186/s12916-019-1364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Sousa MA, Berenguer J, García-Álvarez M, Gutierrez-Rivas M, Aldámiz-Echevarria T, Tejerina F, Diez C, Vázquez-Morón S, Resino S. Impact of patatin-like phospholipase domain-containing 3 gene polymorphism (rs738409) on severity of liver disease in HIV/hepatitis C virus-coinfected patients. AIDS. 2016;30:465–470. doi: 10.1097/QAD.0000000000000908. [DOI] [PubMed] [Google Scholar]
- 14.Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. 2020;42:101092. doi: 10.1016/j.molmet.2020.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puppala J, Siddapuram SP, Akka J, Munshi A. Genetics of nonalcoholic Fatty liver disease: an overview. J Genet Genomics. 2013;40:15–22. doi: 10.1016/j.jgg.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Taliento AE, Dallio M, Federico A, Prati D, Valenti L. Novel Insights into the Genetic Landscape of Nonalcoholic Fatty Liver Disease. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16152755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15:21–35. doi: 10.1007/s12072-020-10121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Farha M, Ghosh A, Al-Khairi I, Madiraju SRM, Abubaker J, Prentki M. The multi-faces of Angptl8 in health and disease: Novel functions beyond lipoprotein lipase modulation. Prog Lipid Res. 2020;80:101067. doi: 10.1016/j.plipres.2020.101067. [DOI] [PubMed] [Google Scholar]
- 19.Posadas-Sánchez R, Ocampo-Arcos WA, López-Uribe ÁR, Posadas-Romero C, Villarreal-Molina T, León EÁ, Pérez-Hernández N, Rodríguez-Pérez JM, Cardoso-Saldaña G, Medina-Urrutia A, Vargas-Alarcón G. Hepatic lipase (LIPC) C-514T gene polymorphism is associated with cardiometabolic parameters and cardiovascular risk factors but not with fatty liver in Mexican population. Exp Mol Pathol. 2015;98:93–98. doi: 10.1016/j.yexmp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Li XL, Sui JQ, Lu LL, Zhang NN, Xu X, Dong QY, Xin YN, Xuan SY. Gene polymorphisms associated with non-alcoholic fatty liver disease and coronary artery disease: a concise review. Lipids Health Dis. 2016;15:53. doi: 10.1186/s12944-016-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin B, Huang Y, Zhang M, Wang J, Wu Y. Association between apolipoprotein C3 Sst I, T-455C, C-482T and C1100T polymorphisms and risk of coronary heart disease. BMJ Open. 2014;4:e004156. doi: 10.1136/bmjopen-2013-004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagi S, Bianconi M, Sammarco MA, Artuso R, Giglio S, de Martino M. New thoughts on pediatric genetic obesity: pathogenesis, clinical characteristics and treatment approach. InAdiposity: omics and molecular understanding 2017; 22: 1320-1678. InTechOpen, London. [Google Scholar]
- 24.Cappiello V, Ronchi C, Morpurgo PS, Epaminonda P, Arosio M, Beck-Peccoz P, Spada A. Circulating ghrelin levels in basal conditions and during glucose tolerance test in acromegalic patients. Eur J Endocrinol. 2002;147:189–194. doi: 10.1530/eje.0.1470189. [DOI] [PubMed] [Google Scholar]
- 25.Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Dore F, Fonda M, Ciocchi B, Cattin L, Guarnieri G. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3935–3940. doi: 10.1210/jc.2006-2527. [DOI] [PubMed] [Google Scholar]
- 26.Likanonsakul S, Rattanatham T, Feangvad S, Uttayamakul S, Prasithsirikul W, Srisopha S, Nitiyanontakij R, Tengtrakulcharoen P, Tarkowski M, Riva A, Nakayama EE, Shioda T. Polymorphisms in Fas gene is associated with HIV-related lipoatrophy in Thai patients. AIDS Res Hum Retroviruses. 2013;29:142–150. doi: 10.1089/aid.2012.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhuang L, Li M, Yu C, Li C, Zhao M, Lu M, Zheng T, Zhang R, Zhao W, Bao Y, Xiang K, Jia W, Wang N, Liu L. The Leu72Met polymorphism of the GHRL gene prevents the development of diabetic nephropathy in Chinese patients with type 2 diabetes mellitus. Mol Cell Biochem. 2014;387:19–25. doi: 10.1007/s11010-013-1865-6. [DOI] [PubMed] [Google Scholar]
- 28.Li YY, Lu XZ, Yang XX, Wang H, Geng HY, Gong G, Zhan YY, Kim HJ, Yang ZJ. GHRL Gene Leu72Met Polymorphism and Type 2 Diabetes Mellitus: A Meta-Analysis Involving 8,194 Participants. Front Endocrinol (Lausanne) 2019;10:559. doi: 10.3389/fendo.2019.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R, Tian S, Cai R, Sun J, Shen Y, Wang S. Ethnicity-Specific Association Between Ghrelin Leu72Met Polymorphism and Type 2 Diabetes Mellitus Susceptibility: An Updated Meta-Analysis. Front Genet. 2018;9:541. doi: 10.3389/fgene.2018.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, Lemoine M. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS. 2017;31:1621–1632. doi: 10.1097/QAD.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 31.Jongraksak T, Sobhonslidsuk A, Jatchavala J, Warodomwichit D, Kaewduang P, Sungkanuparph S. Prevalence and predicting factors of metabolic-associated fatty liver disease diagnosed by transient elastography with controlled attenuation parameters in HIV-positive people. Int J STD AIDS. 2021;32:266–275. doi: 10.1177/0956462420960997. [DOI] [PubMed] [Google Scholar]
- 32.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 33.Romeo S, Sentinelli F, Cambuli VM, Incani M, Congiu T, Matta V, Pilia S, Huang-Doran I, Cossu E, Loche S, Baroni MG. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol. 2010;53:335–338. doi: 10.1016/j.jhep.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Zou Y, Zhong L, Hu C, Sheng G. Association between the alanine aminotransferase/aspartate aminotransferase ratio and new-onset non-alcoholic fatty liver disease in a nonobese Chinese population: a population-based longitudinal study. Lipids Health Dis. 2020;19:245. doi: 10.1186/s12944-020-01419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Amador N, Nolasco-Hipolito C, Rojas-Jimeno MD, Carvajal-Zarrabal O. Liver enzymes in patients diagnosed with non-alcoholic fatty liver disease (NAFLD) in Veracruz: a comparative analysis with the literature. Clinical Investigation. 2017;7:25–32. [Google Scholar]
- 36.Zhang X, Zhai L, Rong C, Qin X, Li S. Association of Ghrelin Gene Polymorphisms and Serum Ghrelin Levels with the Risk of Hepatitis B Virus-Related Liver Diseases in a Chinese Population. PLoS One. 2015;10:e0143069. doi: 10.1371/journal.pone.0143069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estep M, Abawi M, Jarrar M, Wang L, Stepanova M, Elariny H, Moazez A, Goodman Z, Chandhoke V, Baranova A, Younossi ZM. Association of obestatin, ghrelin, and inflammatory cytokines in obese patients with non-alcoholic fatty liver disease. Obes Surg. 2011;21:1750–1757. doi: 10.1007/s11695-011-0475-1. [DOI] [PubMed] [Google Scholar]
- 38.Falasca K, Manigrasso MR, Racciatti D, Zingariello P, Dalessandro M, Ucciferri C, Mancino P, Marinopiccoli M, Petrarca C, Conti P, Pizzigallo E, Guagnano MT, Vecchiet J. Associations between hypertriglyceridemia and serum ghrelin, adiponectin, and IL-18 Levels in HIV-infected patients. Ann Clin Lab Sci. 2006;36:59–66. [PubMed] [Google Scholar]
- 39.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 40.Groenendijk M, Cantor RM, de Bruin TW, Dallinga-Thie GM. The apoAI-CIII-AIV gene cluster. Atherosclerosis. 2001;157:1–11. doi: 10.1016/s0021-9150(01)00539-1. [DOI] [PubMed] [Google Scholar]
- 41.Jong MC, Hofker MH, Havekes LM. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler Thromb Vasc Biol. 1999;19:472–484. doi: 10.1161/01.atv.19.3.472. [DOI] [PubMed] [Google Scholar]
- 42.Young K, Bjerregaard P. Health Transitions in Arctic Populations. University of Toronto Press. 2008 [Google Scholar]
- 43.Li MR, Zhang SH, Chao K, Liao XH, Yao JY, Chen MH, Zhong BH. Apolipoprotein C3 (-455T>C) polymorphism confers susceptibility to nonalcoholic fatty liver disease in the Southern Han Chinese population. World J Gastroenterol. 2014;20:14010–14017. doi: 10.3748/wjg.v20.i38.14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu H, Sun J, Sun L, Shu X, Xu Y, Xie D. Polymorphism of human leptin receptor gene is associated with type 2 diabetic patients complicated with non-alcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2009;24:228–232. doi: 10.1111/j.1440-1746.2008.05544.x. [DOI] [PubMed] [Google Scholar]
- 45.Marzuillo P, Miraglia del Giudice E, Santoro N. Pediatric fatty liver disease: role of ethnicity and genetics. World J Gastroenterol. 2014;20:7347–7355. doi: 10.3748/wjg.v20.i23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Ye C, Fei S. Association between APOC3 polymorphisms and non-alcoholic fatty liver disease risk: a meta-analysis. Afr Health Sci. 2020;20:1800–1808. doi: 10.4314/ahs.v20i4.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Z, Han CK, Pan LL, Zhang HJ, Ma ZM, Huang ZF, Chen S, Zhuang XJ, Li ZB, Li XY, Li XJ, Yang SY. Serum alanine aminotransferase independently correlates with intrahepatic triglyceride contents in obese subjects. Dig Dis Sci. 2014;59:2470–2476. doi: 10.1007/s10620-014-3214-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Wang C, Han W, Geng C, Chen D, Wu B, Zhang J, Jiang P. Association of leptin and leptin receptor polymorphisms with coronary artery disease in a North Chinese Han population. Rev Soc Bras Med Trop. 2020;53:e20190388. doi: 10.1590/0037-8682-0388-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swellam M, Hamdy N. Association of nonalcoholic fatty liver disease with a single nucleotide polymorphism on the gene encoding leptin receptor. IUBMB Life. 2012;64:180–186. doi: 10.1002/iub.597. [DOI] [PubMed] [Google Scholar]
- 50.Zain SM, Mohamed Z, Mahadeva S, Cheah PL, Rampal S, Chin KF, Mahfudz AS, Basu RC, Tan HL, Mohamed R. Impact of leptin receptor gene variants on risk of non-alcoholic fatty liver disease and its interaction with adiponutrin gene. J Gastroenterol Hepatol. 2013;28:873–879. doi: 10.1111/jgh.12104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.