Abstract
BACKGROUND
Patatin like phospholipase domain containing 8 (PNPLA8) has been shown to play a significant role in various cancer entities. Previous studies have focused on its roles as an antioxidant and in lipid peroxidation. However, the role of PNPLA8 in colorectal cancer (CRC) progression is unclear.
AIM
To explore the prognostic effects of PNPLA8 expression in CRC.
METHODS
A retrospective cohort containing 751 consecutive CRC patients was enrolled. PNPLA8 expression in tumor samples was evaluated by immunohistochemistry staining and semi-quantitated with immunoreactive scores. CRC patients were divided into high and low PNPLA8 expression groups based on the cut-off values, which were calculated by X-tile software. The prognostic value of PNPLA8 was identified using univariate and multivariate Cox regression analysis. The overall survival (OS) rates of CRC patients in the study cohort were compared with Kaplan-Meier analysis and Log-rank test.
RESULTS
PNPLA8 expression was significantly associated with distant metastases in our cohort (P = 0.048). CRC patients with high PNPLA8 expression indicated poor OS (median OS = 35.3, P = 0.005). CRC patients with a higher PNPLA8 expression at either stage I and II or stage III and IV had statistically significant shorter OS. For patients with left-sided colon and rectal cancer, the survival curves of two PNPLA8-expression groups showed statistically significant differences. Multivariate analysis also confirmed that high PNPLA8 expression was an independent prognostic factor for overall survival (hazard ratio HR = 1.328, 95%CI: 1.016-1.734, P = 0.038).
CONCLUSION
PNPLA8 is a novel independent prognostic factor for CRC. These findings suggest that PNPLA8 is a potential target in clinical CRC management.
Keywords: Biomarker, Colorectal cancer, Expression level, Overall survival, Patatin like phospholipase domain containing 8, Prognosis
Core Tip: Patatin like phospholipase domain containing 8 (PNPLA8) has been shown to be associated with a variety of cancers, but its role in the progression of colorectal cancer (CRC) is unclear. In this study, 751 consecutive CRC patients were retrospectively analyzed. The results of this study indicate that PNPLA8 is a new independent prognostic factor for CRC. High PNPLA8 expression in CRC leads to impaired survival. These findings suggest that PNPLA8 is a potential target for clinical CRC therapy, providing important insights to help personalize therapy for CRC patients.
INTRODUCTION
Colorectal cancer (CRC) is among the deadliest tumors[1]. The only curative treatment for localized CRC is surgery, and patients with lymph node metastases are usually advised to undergo adjuvant chemotherapy[2]. The relatively low 5-year survival rate of about 56.9% is further affected by inadequate screening methods and increasing resistance to chemotherapy during the clinical course[3,4]. Currently, several reliable prognostic factors are widely used in clinical practice, such as molecular subtype, therapeutic response to previous adjuvant chemotherapy, time between adjuvant therapy and metastasis development (shorter is associated with poorer prognosis), comorbidities, and frailty[5,6]. Therefore, considering the heterogeneity of CRC, it is essential to develop new prognostic and therapeutic strategies. However, widely accepted new prognostic biomarkers are scarce.
Patatin like phospholipase domain-containing protein (PNPLA8), also termed Ca2+-independent phospholipase A2γ (iPLA2γ), is localized to the mitochondrial matrix, where it may manifest its unique activity to cleave phospholipid side-chains from both sn-1 and sn-2 positions, consequently releasing either saturated or unsaturated fatty acids, including oxidized fatty acids[7]. As a calcium-independent and membrane-bound phospholipase, PNPLA8 catalyzes the esterolytic cleavage of fatty acids from glycerophospholipids to yield free fatty acids and lysophospholipids, hence regulating membrane physical properties and the release of lipid second messengers and growth factors[8,9]. It is essential for maintaining efficient bioenergetic mitochondrial function by regulating mitochondrial membrane lipid metabolism and composition[9]. Mutations in the PNPLA8 gene have been linked to multiple diseases such as mitochondrial myopathy with lactic acidosis and mitochondrial myopathy[10]. Recently, it was found that the dysregulation of iPLA2γ can therefore be a critical factor in the development of many diseases[11,12], including metabolic diseases and multiple cancers, such as colitis and CRC[13]. However, the expression status of PNPLA8 in CRC and its relationship with clinicopathological features and prognosis are largely unknown.
In this study, to investigate the potential biomarker value of PNPLA8, 751 cases of tumor samples from a cohort of CRC patients were selected to analyze PNPLA8 protein expression by immunohistochemical staining. Additionally, concentrated analyses on the correlations between PNPLA8 expression and overall survival of CRC patients in this cohort were conducted to unveil the prognostic significance of PNPLA8 in CRC. Our results suggest that higher PNPLA8 expression is an independent predictor for poor prognosis in CRC patients, which could be potentially used to guide the clinical management of CRC patients.
MATERIALS AND METHODS
Patients and specimens
A total of 751 patients with CRC that were admitted to Zhongshan Hospital, Fudan University (Shanghai, China) between May 2008 and November 2012 were retrospectively enrolled in this study. The inclusion criteria were as follows: (1) Receiving primary radical resection; (2) pathologically confirmed colorectal adenocarcinoma; (3) no treatment before surgery; and (4) clinicopathological data available. CRC patients with radical resections of synchronous liver metastases were also included. CRC cancer stages were defined according to the International Union Against Cancer/American Joint Committee on Cancer TNM classification 8th edition. The diagnostic procedures were concluded before the current study was conducted. During the analysis, the observers were fully blinded to patient data. The median follow-up time of the patient cohort was 46.1 mo (IQR = 32.9-59.5).
This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University. Informed consent was acquired from all patients of the primary cohort for the acquisition of clinical and pathological information and the use of surgical specimens.
Immunohistochemistry
Formalin-fixed paraffin-embedded surgical specimens were used for tissue microarray (TMA) construction and subsequent immunohistochemistry study as described previously[14]. Standard procedures were used to determine PNPLA8 expression levels in CRC tumor samples. After being dried overnight at 37˚C and deparaffinized in xylene, the TMA slide was rehydrated through graded alcohol and then immersed in 3% hydrogen peroxide to block endogenous peroxidase activity. After that, the sections were pretreated in a microwave oven (14 min in sodium citrate buffer, pH 6) and then incubated with 10% normal goat serum for 30 min. Primary antibody (rabbit anti-human PNPLA8 polyclonal antibody, ab223726, Abcam; diluted 1:150) was applied overnight in a moist chamber at 4˚C. After the primary antibody was washed off with PBS, the secondary goat anti-rabbit antibody (ab6721, Abcam; diluted 1:10000) was applied. Reaction products were visualized by incubation with 3,3'-diaminobenzidine and counterstained with hematoxylin. Negative controls were treated identically, but with the primary antibody omitted. In addition, the paracancerous tissues were used as controls.
Evaluation of immunohistochemical staining
Two independent pathologists who were blinded to the clinical data evaluated the immunostaining and the results were averaged. In case of significant discrepancies, a final score was established by reassessment on a double-headed microscope and a third person was asked to re-score the results and choose the value with the closest score. The scores for PNPLA8 intensity were set as follows: ‘+++’ was 3; ‘++’ was 2; ‘+’ was 1; and ‘−’ was 0. The area scores for PNPLA8 expression were set as follows: ‘1’ (0%-25% positive cells among all tumor cells), ‘2’(25%-50% positive cells), ‘3’ (51%-75% positive cells), and ‘4’ (more than 75% positive cells). The final score for PNPLA8 expression was the intensity score multiplied by the area score, resulting in a final score ranging from 0 to 12. Boundaries were based on the results from X-Tile Software (Yale University, version 3.6.1). A final score of 0-8 was considered low PNPLA8 expression, while 9-12 was considered high PNPLA8 expression.
Statistical analysis
The statistical analysis was performed using SPSS 23.0 (IBM, Armonk, NY, United States). The association between clinicopathological features and PNPLA8 expression were accessed by Chi-square test or Fisher’s exact test as appropriate. Kaplan-Meier analysis and Log-rank test were performed to evaluate the relationship between PNPLA8 expression and overall survival (OS). Univariate Cox regression analysis was performed to identify the independent prognostic factors among clinicopathological features and other information. Those factors with P < 0.1 in univariate Cox regression analyses were included in the multivariate Cox regression analysis. A two-sided P < 0.05 was considered statistically significant. To obtain the best prognostic efficacy, the cut-off values of PNPLA8 score were calculated using X-Tile Software (Yale University, version 3.6.1) based on the OS data[15].
RESULTS
Clinicopathologic characteristics of the enrolled patients with CRC
The clinicopathologic characteristics of the enrolled CRC patients are listed in Table 1. Approximately half of the patients (53.7%) were over 60-years-old, and their ages ranged from 19 years to 90 years with a median age of 62 (SD, 12.3) years. The male to female ratio was 60.3:39.7. The patients with CEA value over 5 ng/mL accounted for 47.3% of total patients, while those with CA199 value more than 37 U/mL accounted for 19.2%. The tumor location was categorized as right-sided colon in 209 cases (27.8%), left-sided colon in 200 cases (26.6%), and rectum in 342 cases (45.6%). There were 323 cases (43%) with tumor size over 4.0 cm, while the majority of all the cases were non-mucinous in terms of primary histological type. For primary tumor differentiation, 497 cases (66.2%) had well/moderate differentiation, while 254 cases (33.8%) were poor/anaplastic in tumor differentiation. TNM Staging results showed that a small portion of patients (197 cases, 26.2%) were at active metastasis stage, whereas only 96 cases (12.8%) and 56 cases (7.5%) showed vascular invasion and nerve invasion, respectively.
Table 1.
Clinicopathologic characteristics of the colorectal cancer patients in this study
|
Clinicopathologic parameters
|
n
|
Percentage (%)
|
| All patients | 751 | 100.0 |
| Age in yr | ||
| ≤ 60 | 348 | 46.3 |
| > 60 | 403 | 53.7 |
| Sex | ||
| Male | 453 | 60.3 |
| Female | 298 | 39.7 |
| CEA in ng/mL | ||
| ≤ 5 | 396 | 52.7 |
| > 5 | 355 | 47.3 |
| CA199 in U/mL | ||
| ≤ 37 | 607 | 80.8 |
| > 37 | 144 | 19.2 |
| Tumor location | ||
| Right-sided colon | 209 | 27.8 |
| Left-sided colon | 200 | 26.6 |
| Rectum | 342 | 45.6 |
| Tumor size in cm | ||
| ≤ 4.0 | 428 | 57.0 |
| > 4.0 | 323 | 43.0 |
| Primary histological type | ||
| Non-mucinous | 640 | 85.2 |
| Mucinous | 111 | 14.8 |
| Primary differentiation | ||
| Well/moderate | 497 | 66.2 |
| Poor/anaplastic | 254 | 33.8 |
| T stage | ||
| T1 | 24 | 3.2 |
| T2 | 132 | 17.6 |
| T3 | 354 | 47.1 |
| T4 | 241 | 32.1 |
| N stage | ||
| N0 | 410 | 54.6 |
| N1 | 231 | 30.8 |
| N2 | 110 | 14.6 |
| Vascular invasion | ||
| No | 655 | 87.2 |
| Yes | 96 | 12.8 |
| Nerve invasion | ||
| No | 695 | 92.5 |
| Yes | 56 | 7.5 |
| M stage | ||
| M0 | 554 | 73.8 |
| M1 | 197 | 26.2 |
CA199: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; M: Presence of metastasis; N: Extent of tumor spread to the lymph nodes; T: Extent of the tumor (the size of the tumor and any spread of tumor into nearby tissue).
Correlations between PNPLA8 expression and clinicopathological parameters
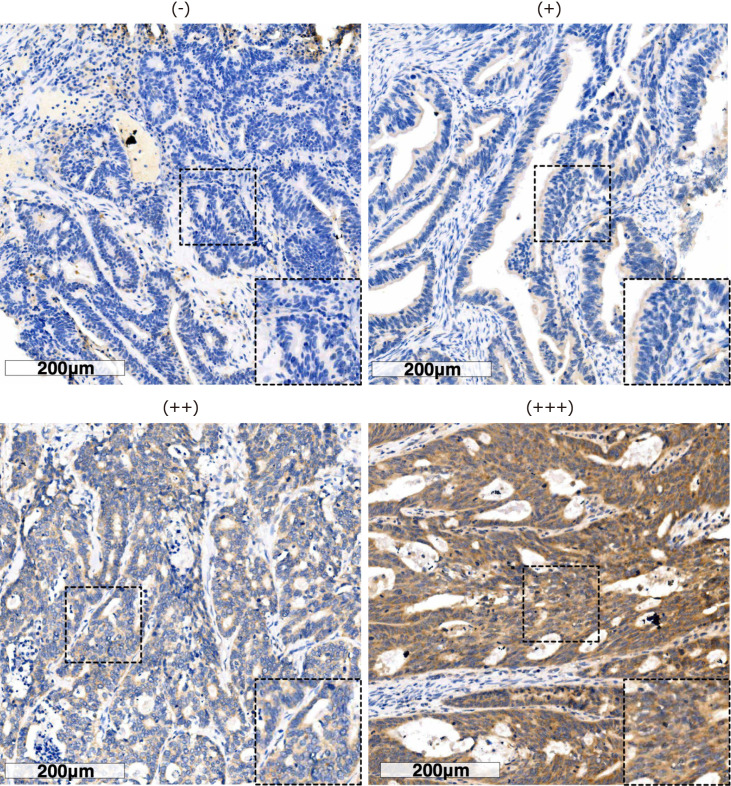
We next examined PNPLA8 expression in tumor samples using immunohistochemistry staining and scored each sample according to the staining intensity (Figure 1) and staining area. Out of 751 stained CRC specimens, 689 (91.7%) showed positive PNPLA8 expression. These 751 samples were categorized into a PNPLA8-low expression group and PNPLA8-high expression group, and the correlations between PNPLA8 expression and the clinicopathological parameters were analyzed (Table 2). A positive correlation was observed between high cytoplasmic PNPLA8 staining and M stage (P = 0.048). However, there were no significant correlations between PNPLA8 staining and other parameters (P > 0.05), including age, sex, CEA, CA199, tumor location, tumor size, primary histological type, primary differentiation, T stage, N stage, vascular invasion, and nerve invasion.
Figure 1.
Immunohistochemical staining of patatin like phospholipase domain containing 8 protein in colorectal cancer specimens. Representative immunohistochemistry images show the staining intensities of patatin like phospholipase domain containing 8 protein, which were scored as 0 ("-"), 1 ("+"), 2 ("++"), and 3 ("+++"). Scale bar: 200 µm.
Table 2.
Correlations between patatin like phospholipase domain containing 8 expression and clinicopathological parameters of colorectal cancer patients in this study
|
Variables
|
PNPLA8 expression
|
P value
|
|
|
Low (%)
|
High (%)
|
||
| All patients | 331 | 420 | |
| Age in yr | 0.811 | ||
| ≤ 60 | 155 (46.8) | 193 (46.0) | |
| > 60 | 176 (53.2) | 227 (54.0) | |
| Sex | 0.375 | ||
| Male | 199 (60.1) | 239 (56.9) | |
| Female | 132 (39.9) | 181 (43.1) | |
| CEA in ng/mL | 0.821 | ||
| ≤ 5 | 173 (52.3) | 223 (53.1) | |
| > 5 | 158 (47.7) | 197 (46.9) | |
| CA199 in U/mL | 0.900 | ||
| ≤ 37 | 267 (80.7) | 340 (81.0) | |
| > 37 | 64 (19.3) | 80 (19.0) | |
| Tumor location | 0.434 | ||
| Right-sided colon | 97 (29.3) | 112 (26.7) | |
| Left-sided colon | 92 (27.8) | 108 (25.7) | |
| Rectum | 142 (42.9) | 200 (47.6) | |
| Tumor size in cm | 0.589 | ||
| ≤ 4.0 | 185 (55.9) | 243 (57.9) | |
| > 4.0 | 146 (44.1) | 177 (42.1) | |
| Primary histological type | 0.848 | ||
| Non-mucinous | 283 (85.5) | 357 (85.0) | |
| Mucinous | 48 (14.5) | 63 (15.0) | |
| Primary differentiation | 0.160 | ||
| Well/moderate | 210 (63.4) | 287 (68.3) | |
| Poor/anaplastic | 121 (36.6) | 133 (31.7) | |
| T stage | 0.618 | ||
| T1/T2 | 66 (19.9) | 90 (21.4) | |
| T3/T4 | 265 (80.1) | 330 (78.6) | |
| N stage | 0.684 | ||
| N0 | 180 (54.4) | 230 (54.8) | |
| N1 | 106 (32.0) | 125 (29.8) | |
| N2 | 45 (13.6) | 65 (15.4) | |
| Vascular invasion | 0.710 | ||
| No | 287 (86.7) | 368 (87.6) | |
| Yes | 44 (13.3) | 52 (12.4) | |
| Nerve invasion | 0.638 | ||
| No | 308 (93.1) | 387 (92.1) | |
| Yes | 23 (6.9) | 33 (7.9) | |
| M stage | 0.048 | ||
| M0 | 256 (77.3) | 298 (71.0) | |
| M1 | 75 (22.7) | 122 (29.0) | |
CA199: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; PNPLA8: Patatin like phospholipase domain containing 8; M: Presence of metastasis; N: Extent of tumor spread to the lymph nodes; T: Extent of the tumor (the size of the tumor and any spread of tumor into nearby tissue).
High PNPLA8 expression is associated with poor overall survival of CRC patients
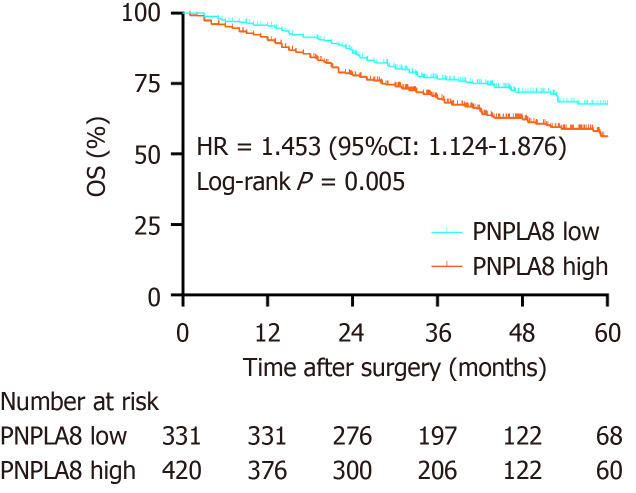
To further substantiate the importance of high PNPLA8 expression in CRC progression, we compared the OS of CRC patients in our study cohort with differential PNPLA8 expression levels. The median follow-up OS of the CRC patients was 46.1 mo (IQR = 36.9-60.9). We found that PNPLA8 expression was statistically significantly associated with a shorter OS (HR 1.445; 43.1 mo for PNPLA8-low group vs 35.4 mo for PNPLA8-high group; P = 0.005) (Figure 2). Therefore, higher PNPLA8 expression could predict poor overall survival in CRC patients, suggesting that PNPLA8 is a prognostic factor of CRC.
Figure 2.
Kaplan-Meier analyses of overall survival rates in 751 colorectal cancer patients with a low or high patatin like phospholipase domain containing 8 protein expression. The colorectal cancer (CRC) patients with a high patatin like phospholipase domain containing 8 (PNPLA8) expression (n = 420) had an overall survival (OS) rate (P = 0.005) than those with a low PNPLA8 expression (n = 331) as determined using the Kaplan-Meier method.
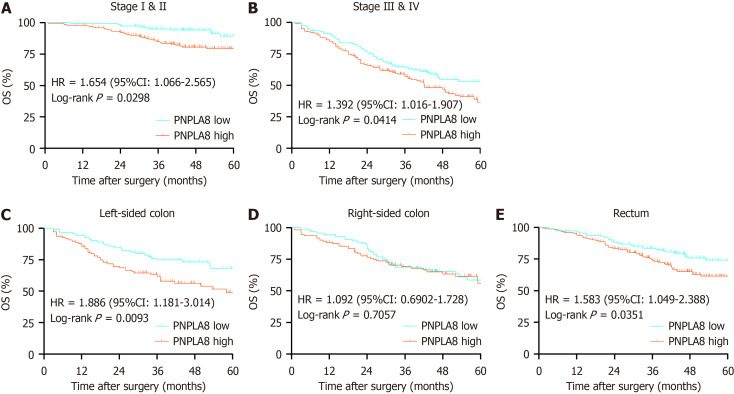
We then conducted further stratified analysis according to the TNM stage of CRC patients. For CRC patients at Stage I and II, PNPLA8 expression was a significant prognostic factor (HR 2.578, P < 0.01; Figure 3A). For CRC patients at Stage III, OS did not show statistical differences among patients with different PNPLA8 expression levels (HR 1.061, P = 0.083; Figure 3B). For CRC patients at Stage IV, patients with a higher PNPLA8 expression also had statistically significantly shorter OS (HR 1.476, P = 0.036; Figure 3C). In stratified analysis according to tumor location, PNPLA8 expression was not a significant prognostic factor for patients with right-sided colon cancer (Figure 3E) (P = 0.7057). However, for patients with left-sided colon (Figure 3D) and rectal cancer (Figure 3F), the survival curves of the two PNPLA8-expression groups showed statistically significant differences (HR 1.886, P = 0.009 for left-sided colon cancer; HR 1.583, P = 0.035 for rectal cancer).
Figure 3.
Patatin like phospholipase domain containing 8 protein expression according to TNM stage and tumor location. A: Patients at stage I and stage II [n = 153 for patatin like phospholipase domain containing 8 (PNPLA8) low group; n = 200 for PNPLA8 high group]; B: Patients at stage III and stage IV (n = 178 for PNPLA8 low group; n = 220 for PNPLA8 high group); C: Patients with left-sided colon cancer (n = 92 for PNPLA8 low group; n = 108 for PNPLA8 high group); D: Patients with right-sided colon cancer (n = 97 for PNPLA8 low group; n = 112 for PNPLA8 high group); E: Patients with rectal cancer (n = 142 for PNPLA8 low group; n = 200 for PNPLA8 high group). The hazard ratio (HR), 95% confidence interval (95%CI), and log-rank P values are indicated in each panel. OS: Overall survival.
PNPLA8 and several clinicopathological parameters are independent prognostic factors of CRC
Using univariate analysis, we found that CRC patients with PNPLA8-high expression showed significant differences compared to PNPLA8-low expression in terms of multiple parameters, including CEA (P < 0.001), CA199 (P < 0.001), primary differentiation (P = 0.02), T stage (P < 0.001), N stage (P < 0.001), M stage (P < 0.001), vascular invasion (P < 0.001), and nerve invasion (P < 0.001) (Table 3). Therefore, multivariate analysis was performed using the Cox proportional hazards model for all of the significant variables examined in the univariate analysis. We found that PNPLA8 expression was a statistically significant independent prognostic factor (HR 1.328, P = 0.038). In addition, CA199 (HR 1.548, P = 0.004), N stage (HR 1.701, P < 0.001), M stage (HR 4.862, P < 0.001) and vascular invasion (HR 1.512, P = 0.017) (Table 3) were also found to be independent factors.
Table 3.
Cox regression analyses for overall survival of colorectal cancer patients in this study
|
Variates
|
Overall survival
|
|||
|
Univariate analysis
|
Multivariate analysis
|
|||
|
HR (95%CI)
|
P value
|
HR (95%CI)
|
P value
|
|
| PNPLA8 | 0.004 | 0.038 | ||
| Low | 1 (reference) | 1 (reference) | ||
| High | 1.472 (1.132-1.914) | 1.328 (1.016-1.734) | ||
| Age in yr | 0.561 | |||
| ≤ 60 | 1 (reference) | |||
| > 60 | 1.079 (0.835-1.394) | |||
| Sex | 0.087 | 0.903 | ||
| Male | 1 (reference) | 1 (reference) | ||
| Female | 0.792(0.606-1.304) | 0.903 (0.687-1.187) | ||
| CEA in ng/mL | < 0.001 | 0.811 | ||
| ≤ 5 | 1 (reference) | 1 (reference) | ||
| > 5 | 2.126(1.636-2.763) | 0.964 (0.715-1.300) | ||
| CA199 in U/mL | < 0.001 | 0.004 | ||
| ≤ 37 | 1 (reference) | 1 (reference) | ||
| > 37 | 2.870 (2.191-3.759) | 1.548 (1.150-2.083) | ||
| Tumor location | 0.050 | 0.895 | ||
| Right-sided colon | 1 (reference) | 1 (reference) | ||
| Left-sided colon | 0.952 (0.687-1.320) | 0.933 (0.667-1.305) | ||
| Rectum | 0.706 (0.518-0.962) | 0.934 (0.680-1.284) | ||
| Tumor size in cm | 0.512 | |||
| ≤ 4.0 | 1 (reference) | |||
| > 4.0 | 1.090 (0.843-1.409) | |||
| Primary histological type | 0.371 | |||
| Non-mucinous | 1 (reference) | |||
| Mucinous | 0.854 (0.603-1.208) | |||
| Primary differentiation | 0.002 | 0.162 | ||
| Well/moderate | 1 (reference) | 1 (reference) | ||
| Poor/anaplastic | 1.507 (1.162-1.954) | 1.210 (0.926-1.581) | ||
| T stage | < 0.001 | 0.163 | ||
| T1/T2 | 1 (reference) | 1 (reference) | ||
| T3/T4 | 3.058 (1.934-4.834) | 1.415 (0.869-2.306) | ||
| N stage | < 0.001 | < 0.001 | ||
| N0 | 1 (reference) | 1 (reference) | ||
| N1/N2 | 2.948 (2.852-3.859) | 1.701 (1.272-2.274) | ||
| M stage | < 0.001 | < 0.001 | ||
| M0 | 1 (reference) | 1 (reference) | ||
| M1 | 7.193 (5.520-9.372) | 4.862 (3.608-6.551) | ||
| Vascular invasion | < 0.001 | 0.017 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 2.265 (1.649-3.111) | 1.512 (1.078-2.121) | ||
| Nerve invasion | < 0.001 | 0.218 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 2.901 (1.986-4.236) | 1.291 (0.860-1.939) | ||
CA199: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; HR: Hazard ratio; M: Presence of metastasis; N: Extent of tumor spread to the lymph nodes; PNPLA8: Patatin like phospholipase domain containing 8; T: Extent of the tumor (the size of the tumor and any spread of tumor into nearby tissue).
DISCUSSION
In this study, a large patient cohort was evaluated for the prognostic value of PNPLA8 in CRC. Patients with a higher PNPLA8 expression had a significantly impaired OS. Moreover, PNPLA8 expression was identified as a new independent prognostic factor for OS of CRC patients.
PNPLA8 is part of a diverse family of phospholipase A2 enzymes (PLA2s) that hydrolyze the sn-2 substituent from membrane phospholipids to release a free fatty acid and a lysolipid[16,11]. These enzymes are ubiquitously expressed, and in contrast to secretory PLA2s and cytosolic PLA2s, do not require Ca2+ for either translocation or activity. Some of the first descriptions of iPLA2 activity were in the mid- to late-1980s with the identification of a plasmalogen-selective iPLA2 in PNPLA8, when PNPLA8 was found to function as a phospholipase and was better characterized[17]. During the past few years, knockout and transgenic mice for manipulated PNPLA8 expression have been established[18], and studies using these gene-manipulated mice have provided models with which to elucidate the physiological and pathophysiological roles of PNPLA8. Recently, the mechanisms by which phospholipase A2 enzymes mediate lipid reprogramming and glycerophospholipid remodeling in cancer cells have been elucidated[19,20]. As the upstream regulators of the arachidonic acid cascade, PLA2s are generally highly expressed and activated in various cancers[19,20]. Therefore, they are potential pharmacological targets and biomarkers in cancer.
Our findings are in line with previous reports that PNPLA8 expression is increased in CRC[21,12]. The ability of PNPLA8 to promote cell proliferation becomes prominent in the context of tumorigenesis. Several in vitro studies revealed higher expression of PNPLA8 in stimulated immortal cell lines and that chemical inhibition or siRNA-mediated PNPLA8 knockdown reduces proliferation and promotes apoptosis[22,23]. Subsequent studies targeting specific cancers suggest that PNPLA8 promotes cancer cell growth via signal transduction pathways involving epidermal growth factor receptors, mitogen-activated protein kinases, E3 ubiquitin-protein ligase Mdm2, tumor suppressor protein p53, and cell cycle regulator p21[24,25]. Therefore, there is increasing support for a role of PNPLA8 and PNPLA8-associated phospholipase A in promoting cancer cell proliferation and metastasis, which plausibly provides the molecular mechanisms underlying our finding of PNPLA8 as a novel prognostic factor for CRC.
However, a couple of limitations of this study must be noted. First, our study is a retrospective one. To further validate our conclusion, a prospective study with data from multiple centers is necessary, especially for patients with left-sided colon and rectal cancer as well as Stage I and II patients. Second, the scores for PNPLA8 protein intensity and area were not determined in a fully automated way, resulting in potential artificial errors due to possible human bias. Third, in-depth in vitro and in vivo experiments are urgently needed to unveil the mechanisms of PNPLA8 in CRC.
CONCLUSION
In summary, PNPLA8 was identified as a new independent prognostic factor for CRC. CRC with high PNPLA8 expression conferred survival impairment. Our findings provide critical insights into aiding the individualized treatment of CRC patients.
ARTICLE HIGHLIGHTS
Research background
The role of patatin like phospholipase domain containing 8 (PNPLA8) in colorectal cancer (CRC) progression is unclear.
Research motivation
The research motivation is to explore the prognostic effects of PNPLA8 expression in CRC.
Research objectives
To evaluate the prognostic value of PNPLA8 expression level for CRC patient survival and its correlation with clinicopathological features of CRC patients.
Research methods
PNPLA8 expression in tumor samples was evaluated by immunohistochemistry staining and semi-quantitated with immunoreactive scores.
Research results
CRC patients with high PNPLA8 expression indicated poor overall survival (OS) (median OS = 35.3, P = 0.005). The multivariate analysis also confirmed that high PNPLA8 expression was a significantly independent prognostic factor for overall survival (hazard ratio HR = 1.328, 95%CI: 1.016-1.734, P = 0.038).
Research conclusions
High PNPLA8 expression level is associated with poorer survival outcomes in CRC patients, indicating its prognostic value for predicting patient outcomes.
Research perspectives
Further studies are needed to validate the prognostic value of PNPLA8 in multicenter cohorts of CRC patients. The mechanism of PNPLA8 in CRC development and progression remains to be fully investigated to help to identify potential therapeutic targets and develop new treatment strategies.
Footnotes
Institutional review board statement: This study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital, Fudan University.
Informed consent statement: Informed consent was acquired from all patients of the primary cohort for the acquisition of clinical and pathological information and the use of surgical specimens.
Conflict-of-interest statement: The authors report having no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 22, 2023
First decision: November 21, 2023
Article in press: January 22, 2024
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Basson MD, United States; Hamaya Y, Japan; Osera S, Japan S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Xu ZH
Contributor Information
Peng-Yang Zhou, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China.
De-Xiang Zhu, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China.
Yi-Jiao Chen, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China.
Qing-Yang Feng, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China.
Yi-Hao Mao, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China.
Ao-Bo Zhuang, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China.
Jian-Min Xu, Department of General Surgery, Zhongshan Hospital Fudan University, Shanghai 200032, China. xujmin@aliyun.com.
Data sharing statement
No additional data are available.
References
- 1.Bao S, Song H, Tan M, Wohltmann M, Ladenson JH, Turk J. Group VIB Phospholipase A(2) promotes proliferation of INS-1 insulinoma cells and attenuates lipid peroxidation and apoptosis induced by inflammatory cytokines and oxidant agents. Oxid Med Cell Longev. 2012;2012:989372. doi: 10.1155/2012/989372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard H, Taha AY, Cheon Y, Kim HW, Turk J, Rapoport SI. iPLA2β knockout mouse, a genetic model for progressive human motor disorders, develops age-related neuropathology. Neurochem Res. 2014;39:1522–1532. doi: 10.1007/s11064-014-1342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Papillon J, Aoudjit L, Li H, Cybulsky AV, Takano T. Role of calcium-independent phospholipase A2 in complement-mediated glomerular epithelial cell injury. Am J Physiol Renal Physiol. 2008;294:F469–F479. doi: 10.1152/ajprenal.00372.2007. [DOI] [PubMed] [Google Scholar]
- 6.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 7.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara S, Yoda E, Sasaki Y, Nakatani Y, Kuwata H. Calcium-independent phospholipase A(2)γ (iPLA(2)γ) and its roles in cellular functions and diseases. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:861–868. doi: 10.1016/j.bbalip.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Hooks SB, Cummings BS. Role of Ca2+-independent phospholipase A2 in cell growth and signaling. Biochem Pharmacol. 2008;76:1059–1067. doi: 10.1016/j.bcp.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jabůrek M, Průchová P, Holendová B, Galkin A, Ježek P. Antioxidant Synergy of Mitochondrial Phospholipase PNPLA8/iPLA2γ with Fatty Acid-Conducting SLC25 Gene Family Transporters. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji M, Ren L, Lv Y, Lao X, Feng Q, Tang W, Zhuang A, Liu T, Zheng P, Xu J. Small Nuclear Ribonucleoprotein Polypeptide N Accelerates Malignant Progression and Poor Prognosis in Colorectal Cancer Transcriptionally Regulated by E2F8. Front Oncol. 2020;10:561287. doi: 10.3389/fonc.2020.561287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SA, Ilies MA. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int J Mol Sci. 2023;24 doi: 10.3390/ijms24021353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, Chen H, Dai M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255–268. doi: 10.1016/j.canlet.2021.09.034. [DOI] [PubMed] [Google Scholar]
- 14.Liu GY, Moon SH, Jenkins CM, Li M, Sims HF, Guan S, Gross RW. The phospholipase iPLA(2)γ is a major mediator releasing oxidized aliphatic chains from cardiolipin, integrating mitochondrial bioenergetics and signaling. J Biol Chem. 2017;292:10672–10684. doi: 10.1074/jbc.M117.783068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon SH, Jenkins CM, Liu X, Guan S, Mancuso DJ, Gross RW. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J Biol Chem. 2012;287:14880–14895. doi: 10.1074/jbc.M111.336776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami M, Masuda S, Ueda-Semmyo K, Yoda E, Kuwata H, Takanezawa Y, Aoki J, Arai H, Sumimoto H, Ishikawa Y, Ishii T, Nakatani Y, Kudo I. Group VIB Ca2+-independent phospholipase A2gamma promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipases A2. J Biol Chem. 2005;280:14028–14041. doi: 10.1074/jbc.M413766200. [DOI] [PubMed] [Google Scholar]
- 17.Murase R, Taketomi Y, Miki Y, Nishito Y, Saito M, Fukami K, Yamamoto K, Murakami M. Group III phospholipase A(2) promotes colitis and colorectal cancer. Sci Rep. 2017;7:12261. doi: 10.1038/s41598-017-12434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nappi A, Nasti G, Romano C, Berretta M, Ottaiano A. Metastatic Colorectal Cancer: Prognostic and Predictive Factors. Curr Med Chem. 2020;27:2779–2791. doi: 10.2174/0929867326666190620110732. [DOI] [PubMed] [Google Scholar]
- 19.Peng Z, Chang Y, Fan J, Ji W, Su C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021;497:165–177. doi: 10.1016/j.canlet.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Ramanadham S, Ali T, Ashley JW, Bone RN, Hancock WD, Lei X. Calcium-independent phospholipases A2 and their roles in biological processes and diseases. J Lipid Res. 2015;56:1643–1668. doi: 10.1194/jlr.R058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders CJ, Moon SH, Liu X, Thiffault I, Coffman K, LePichon JB, Taboada E, Smith LD, Farrow EG, Miller N, Gibson M, Patterson M, Kingsmore SF, Gross RW. Loss of function variants in human PNPLA8 encoding calcium-independent phospholipase A2 γ recapitulate the mitochondriopathy of the homologous null mouse. Hum Mutat. 2015;36:301–306. doi: 10.1002/humu.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott KF, Sajinovic M, Hein J, Nixdorf S, Galettis P, Liauw W, de Souza P, Dong Q, Graham GG, Russell PJ. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie. 2010;92:601–610. doi: 10.1016/j.biochi.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 24.Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585:72–76. doi: 10.1016/s1388-1981(02)00326-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhu D, Xia J, Gu Y, Lin J, Ding K, Zhou B, Liang F, Liu T, Qin C, Wei Y, Ren L, Zhong Y, Wang J, Yan Z, Cheng J, Chen J, Chang W, Zhan S, Ding Y, Huo H, Liu F, Sun J, Qin X, Xu J. Preoperative Hepatic and Regional Arterial Chemotherapy in Patients Who Underwent Curative Colorectal Cancer Resection: A Prospective, Multi-center, Randomized Controlled Trial. Ann Surg. 2021;273:1066–1075. doi: 10.1097/SLA.0000000000004558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.