Abstract
Managing diabetes during pregnancy is challenging, given the significant risk it poses for both maternal and foetal health outcomes. While traditional methods involve capillary self-monitoring of blood glucose level monitoring and periodic HbA1c tests, the advent of continuous glucose monitoring (CGM) systems has revolutionized the approach. These devices offer a safe and reliable means of tracking glucose levels in real-time, benefiting both women with diabetes during pregnancy and the healthcare providers. Moreover, CGM systems have shown a low rate of side effects and high feasibility when used in pregnancies complicated by diabetes, especially when paired with continuous subcutaneous insulin infusion pump as hybrid closed loop device. Such a combined approach has been demonstrated to improve overall blood sugar control, lessen the occurrence of preeclampsia and neonatal hypoglycaemia, and minimize the duration of neonatal intensive care unit stays. This paper aims to offer a comprehensive evaluation of CGM metrics specifically tailored for pregnancies impacted by type 1 diabetes mellitus.
Keywords: Type 1 diabetes mellitus, Continuous glucose monitoring, Pregnancy, Glycaemic control, Continuous glucose monitoring system
Core Tip: Intense glucose monitoring during pregnancy is crucial for the management of women with type 1 diabetes mellitus (T1DM) to ensure optimal maternal and foetal health outcomes. Continuous glucose monitoring (CGM) techniques are revolutionising diabetes care in patients with T1DM in recent years owing to its higher efficacy, relatively easier use for younger generation for testing compared to the cumbersome finger-prick capillary self-monitoring of blood glucose, and the options for integration CGM to continuous subcutaneous insulin infusion pump settings for simulating artificial pancreas. Understanding the CGM metrics is highly important for the correct management of these new technological advancements, which is the theme of this clinical update review.
INTRODUCTION
Sensors in healthcare, ranging from thermometers to wearable tech, have evolved significantly since the 19th century. Key developments include the introduction of electrocardiograms, implantable devices, and digital technology, leading to miniaturized, more accurate sensors[1]. The late 20th century saw advancements in glucose monitoring technology with wearable sensors revolutionizing the diabetes care[2]. The glucose sensor represents a pivotal advancement in biomedical engineering, integrating electrochemical principles to achieve real-time, non-invasive blood glucose monitoring. This innovation, crucial for diabetes management, emerged from extensive research into enzyme-based electrochemical sensors, harmonizing biocompatibility with analytical precision. Pioneered in the late 1990s of 20th century, these sensors utilized glucose oxidase to catalyse the oxidation of glucose, generating an electrical signal proportional to glucose concentration[3]. This breakthrough not only revolutionized diabetic care but also set the foundation for the development of wearable health monitoring technologies and personalized medicine[2].
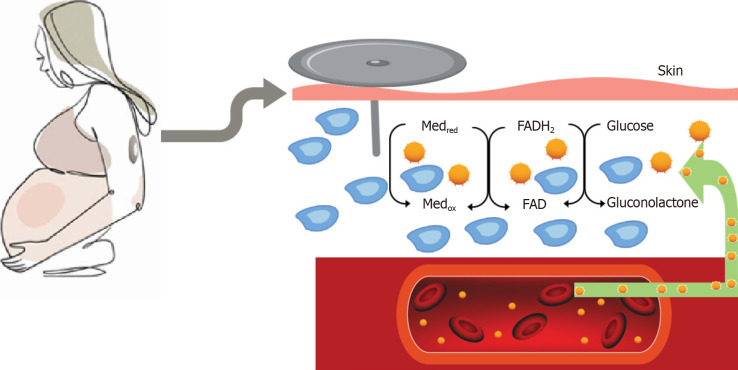
A tiny sensor that is commonly placed on the arm or abdomen for continuous glucose monitoring (CGM) measures blood sugar levels every five minutes, day and night, and transmits the results to an external device[3,4]. People with diabetes can more easily keep track of their blood sugar levels over time[5,6]. CGM provides up to 288 blood glucose readings daily, providing detailed information about changes in blood glucose levels over 24 h[7,8]. Figure 1 shows the basic operational mechanisms of CGM sensor. Monitoring blood glucose levels provides information and understanding about high blood glucose levels, low blood glucose levels, for titration of medication and insulin. On long term it has been shown to reduce the incidence of occurrence of microvascular and macrovascular complications in type 1 diabetes and type 2 diabetes[9-11]. CGM use in diabetes during pregnancy is challenging with rapid changes in the blood volume and fluid shift across body compartments, growing foetus in the abdomen, and different pregnancy specific glucose targets[12]. Pregnancy also alters insulin sensitivity and glucose tolerance in a dynamic state of continual metabolic adjustment[8,13]. With CGM becoming increasingly used in pregnant women with type 1 diabetes, it becomes very important to understand the various parameters, and how it reflects in the pregnancy outcome, which we have detailed in this review.
Figure 1.
Basic operational mechanism of continuous glucose monitoring sensor measuring interstitial glucose. Med: Artificial redox mediators such as Ferrocene or Ferricyanide, red-reduced form, ox-oxidized form; FAD: Flavin Adenine Dinucleotide; FADH2: Flavin Adenine Dinucleotide, reduced form.
CGM EFFECT ON PREGNANCY AND NEONATAL OUTCOMES
CGM plays a pivotal role in managing type 1 diabetes mellitus (T1DM) during pregnancy. The frequent and critical therapeutic decisions in T1DM pregnancies are primarily driven by glucose data, necessitating more rigorous monitoring than in non-pregnant individuals with T1DM[14,15]. Poor glycaemic control in pregnancy can have detrimental consequences not only for the mother but also for the developing foetus[16]. Therefore, CGM emerges as a protective tool for achieving favourable obstetric outcomes in pregnancies complicated by T1DM[17]. Studies have shown that pregnant women using CGM exhibit improved glycaemic control. For instance, Feig et al[18], reported a small yet significant difference in HbA1c levels (mean difference -0.19%; 95%CI -0.34 to -0.03) among pregnant women utilizing CGM compared to T1DM pregnancies managed by usual care. Additionally, these individuals spent a greater proportion of time in the target glucose range (68% with CGM vs 61% without) and less time in hyperglycaemia (27% with CGM vs 32% without)[18]. Scott et al[19], further emphasized that the primary efficacy of CGM was demonstrated by the increased duration pregnant users spent within the target glucose range.
Neonatal hypoglycaemia, a common complication in infants born to mothers with diabetes, can have long-lasting effects[20]. Stenninger et al[21], in their elegant study described that neonatal hypoglycaemia is usually a consequence of maternal hyperglycaemia especially during the labour. CONCEPTT trial, a landmark study, revealed that the use of CGM in pregnant women with T1DM was associated with better glycaemic control and reduced incidences of neonatal hypoglycaemia[18]. The study reported a lower rate of neonatal intensive care unit admissions and shorter hospital stays for newborns, highlighting the direct impact of maternal glycaemic control on neonatal health. Additionally, CGM use has been linked to a decrease in the incidence of large for gestational age (LGA) babies, a common complication associated with maternal hyperglycaemia[18]. Beyond the immediate neonatal outcomes, the implications of using CGM during pregnancy extend to long-term health benefits for the child. Maintaining optimal blood glucose levels through continuous monitoring can help to prevent complications that have lasting effects on the child's development and health[22]. Better maternal glucose control achieved through CGM has been correlated with lower risks of childhood obesity and metabolic disorders, which are often higher in children born to mothers with poorly controlled diabetes[23].
Moreover, the psychological benefits for the mother, such as reduced anxiety over managing diabetes during pregnancy, can contribute to a healthier prenatal environment[24,25]. This aspect, although indirect, plays a significant role in the overall well-being of both the mother and the foetus[26]. It is important to note that while CGM offers significant benefits, its effectiveness is maximized when combined with comprehensive diabetes education and support, ensuring that pregnant women with T1DM can effectively interpret and act upon the data provided by these devices[27].
CLINICAL TARGETS FOR CGM MONITORING DATA IN PREGNANCY
CGM has revolutionized the management of diabetes in pregnancy by providing direct observation of glycaemic excursions, diurnal profiles, and the ability to detect patterns of hypoglycaemia and hyperglycaemia[12]. This real-time monitoring enables the implementation of appropriate treatment decisions and lifestyle changes on a day-to-day basis, enhancing overall diabetes management[15,28]. Despite its availability since the late 1990, the usage of CGM was initially limited due to the absence of clear, established targets for its application in clinical care. To address this gap, the Advanced Technologies & Treatments for Diabetes (ATTD) Congress in February 2019 convened an international panel comprising physicians, researchers, and individuals with diabetes proficient in CGM technologies[27]. This global panel, which included diabetics, medical professionals, and research experts in CGM, aimed to formulate standards to aid clinicians, researchers, and individuals with diabetes in the utilization, understanding, and reporting of CGM data in both routine clinical care and research settings. The consensus reached by this panel, known as the 2019 International Consensus on CGM metrics, has since become the foundation for current clinical care standards[27]. The panel's recommendations were inclusive and generalizable, thanks to the involvement of individuals with diabetes. This consensus statement standardized CGM metrics in pregnancy, establishing targets such as time in range (TIR), time above range (TAR), and time below range (TBR). Additional metrics included the glucose management indicator (GMI), mean glucose, and glycaemic variability. These metrics provide a comprehensive framework for assessing and managing glycaemic control in pregnancy[29].
Moreover, the consensus also highlighted the importance of other CGM-derived data, such as the number of days the device is worn, device capture rate and ambulatory glucose profile (AGP)[27]. AGP provides an average time plot of glucose with percentile confidence intervals, offers an overarching view of glucose control over weeks, enabling a more nuanced understanding of the patient's glycaemic profile[30]. CGM metrics and targets by the ATTD consensus has significantly enhanced the utility of CGM in pregnancy[26]. These standardized metrics have become instrumental in guiding clinicians and researchers in optimizing diabetes care for pregnant women, ensuring that treatment and monitoring strategies are both effective and tailored to individual needs.
CORRELATION OF EACH PARAMETER TO PREGNANCY AND NEONATAL OUTCOMES
In the realm of managing type 1 diabetes during pregnancy, CGM provides critical data through various parameters. Understanding the correlation between these parameters and pregnancy and neonatal outcomes is essential. Each CGM metric offers unique insights into the glycaemic environment of the mother, which in turn can have significant implications for both maternal and foetal health. This section delves into how specific CGM metrics are correlated with pregnancy and neonatal outcomes, providing a comprehensive understanding of their impact and importance.
Number of days CGM worn
The duration of CGM wear in pregnant women with type 1 diabetes critically influences maternal and neonatal outcomes[29]. Consistent use of CGM in pregnancy leads to more effective glucose control, which is pivotal in reducing risks associated with T1DM. Studies have repeatedly highlighted that longer periods of CGM use correlate with better glycaemic control. Hughes et al[31], showed that, women who consistently used CGM for more than four days per week throughout their pregnancy demonstrated significant improvements in maintaining glucose levels within the target range. Consistent CGM monitoring, characterized by at least 96 h of data including nocturnal readings, is essential for effective diabetes management during pregnancy[32]. This continuous monitoring is crucial for detecting and addressing periods of hyperglycaemia and hypoglycaemia, especially common in the first trimester. The use of real-time CGM for 6 days at crucial stages of pregnancy (weeks 8, 12, 21, and 33) provided important insights into glucose trends, aiding in timely therapeutic interventions[33]. However, intermittent or short-term CGM use was found to be less effective in significantly reducing maternal hyperglycaemia by the third trimester, suggesting the need for prolonged and continuous CGM application[34].
The number of days CGM is worn also has direct implications not only for maternal health but also for neonatal outcomes. Prolonged CGM usage has been associated with lower risks of neonatal complications such as LGA infants and preterm births[18]. These findings underline the importance of longer and consistent use of CGM in managing glucose levels effectively throughout pregnancy, thereby supporting healthier pregnancy outcomes for both the mother and the child.
Percentage of time CGM is operational
While there is a lack of specific data regarding the operational time of CGM in pregnant women with type 1 diabetes, insights can be drawn from studies conducted on adult individuals with type 1 and type 2 diabetes[35]. These studies suggest that for comprehensive glucose monitoring and accurate derivation of CGM metrics, the device should be operational for at least 70% of the time[36]. Translated into practical terms, this would mean that the sensor should be active for a minimum of 16 h and 48 min in a day or 10 d within a 14-d period. The significance of maintaining this level of operational consistency becomes more pronounced in the context of pregnancy. Incomplete data capture can result in a loss of critical information regarding glucose levels. This gap in data is particularly concerning during pregnancy, as it could lead to poor judgment in treatment decisions. For expectant mothers with type 1 diabetes, this might mean missed opportunities for timely interventions or adjustments in their diabetes management plan, potentially impacting both maternal and foetal health. Furthermore, the importance of consistent CGM use in pregnancy is underscored by the dynamic nature of glucose levels during this period[37].
Mean glucose
The metric of mean glucose in CGM systems often receives less attention compared to other CGM metrics, yet it holds significant clinical relevance, especially in the management of type 1 diabetes during pregnancy. Mean glucose levels, as recorded by CGM, play a pivotal role in the calculation of the GMI, a parameter we will explore in detail later[38]. Notably, any substantial fluctuation in mean glucose levels is reflected in the GMI and HbA1c values, which are crucial for assessing glycaemic control[39]. In pregnancies complicated by type 1 diabetes, defining an exact target for mean glucose can be challenging. However, for practical purposes, it is generally advised that the mean glucose should align with the target blood glucose range[27]. A strong correlation exists between HbA1c, a marker of hyperglycaemia, and mean glucose levels, although this correlation is less pronounced with hypoglycaemia[38]. Clinical studies have shed light on the implications of mean glucose levels during pregnancy. For instance, a significant difference was observed in mean overnight glucose levels in pregnancies resulting in LGA status. Pregnancies with LGA babies exhibited considerably higher mean overnight glucose levels [6.0 ± 1.0 mmol/L (108.0 ± 18 mg/dL)] compared to those without LGA [5.5 ± 0.8 mmol/L (99.0 ± 14.4 mg/dL)][40]. In the same study, 162 women with Gestational Diabetes Mellitus (GDM) reported higher mean glucose levels in pregnancies with LGA status [6.2 vs 5.8 mmol/L (111.6 vs 104.4 mg/dL)][40]. The potential of CGM in managing mean glucose levels during pregnancy is further highlighted by the work of Petrovski et al[4], which revealed that CGM users in their first trimester had significantly lower mean blood glucose levels compared to those using self-monitoring blood glucose (SMBG) (6.92 ± 2.1 mmol/L vs 7.42 ± 3.4 mmol/L). This evidence underscores the effectiveness of CGM in providing tight glucose control, which is particularly crucial during pregnancy in managing type 1 diabetes.
GMI
The transition from the estimated A1C (eA1C) to the GMI marks a significant advancement in diabetes management, particularly in the context of pregnancy. While eA1C served as an earlier method to estimate average glucose levels, it was replaced by GMI due to its derivation from a larger, more representative dataset[38]. This new dataset provided a more accurate correlation with laboratory-measured HbA1c values, enhancing the precision of glucose monitoring. GMI is calculated using mean glucose levels obtained from CGM systems, offering a more direct and immediate assessment of an individual’s glucose control[41]. This method diverges from the HbA1c approach, which depends on the glycation of haemoglobin over longer periods. GMI's ability to provide real-time analysis makes it particularly valuable during pregnancy, where rapid fluctuations in glucose levels can occur due to physiological changes[38].
The significance of GMI in pregnancy is underscored by studies that demonstrate its correlation with TIR and its ability to reflect glycaemic control more accurately than HbA1c alone[39]. For example, Bergenstal et al[38], observed that GMI, calculated using CGM data, showed a strong correlation with TIR, especially in the second and third trimesters of pregnancy. Shah et al[39], further supported this by highlighting a notable negative association between TIR with GMI providing a clearer picture of glucose management. One significant benefit of GMI is its reduced susceptibility to the physiological changes that occur during pregnancy[41]. Unlike HbA1c levels, which can be influenced by the accelerated turnover of haemoglobin in pregnancy, GMI remains a more stable and reliable indicator of glucose control[38,41]. This stability is crucial in managing the dynamic glycaemic environment of pregnancy, where rapid changes in glucose levels can significantly impact maternal and foetal health. Moreover, GMI is derived from sensor-based average glucose readings, representing a cost-effective solution, particularly in resource-limited settings. This aspect of GMI is especially important considering the financial constraints and accessibility issues that can limit the use of extensive laboratory testing in some regions. The ability to estimate GMI directly from CGM data eliminates the need for frequent laboratory visits and blood draws, thereby reducing the overall cost and burden on healthcare systems and patients.
Another critical aspect of GMI in pregnancy management is the emphasis on trends rather than single-point measurements. The trend in GMI values provides a more comprehensive picture of glucose control over time, allowing for more nuanced and effective management strategies[41]. This is particularly relevant in pregnancy, where continuous monitoring and adjustments are vital to ensure both maternal and foetal well-being. The ability to track GMI trends enables healthcare providers to make more informed decisions, potentially leading to better outcomes by promptly addressing any adverse glycaemic patterns. In summary, GMI's resilience to physiological changes in pregnancy, its cost-effectiveness in glucose monitoring, and its focus on trends rather than isolated values, make it an indispensable tool in the management of type 1 diabetes during pregnancy.
Glycaemic variability
Glycaemic variability (GV) is an essential aspect of managing type 1 diabetes during pregnancy, characterized by the degree of fluctuation in blood glucose levels. CGM provides an invaluable tool for detailed analysis of these fluctuations, which are crucial for the health and well-being of both the mother and the developing foetus. GV is traditionally assessed using two primary metrics: Glucose standard deviation (SD) and coefficient of variation (CV)[42]. SD measures the extent of blood glucose fluctuations around the mean glucose level, with a high SD indicating larger swings[43]. These fluctuations are particularly significant in pregnancy due to potential impacts on foetal development. Kovatchev et al[43], have highlighted the importance of SD in CGM, emphasizing its strong correlation with mean glucose and HbA1c levels. On the other hand, CV offers a dimensionless measure of glucose variability relative to the mean glucose level. Its independence from mean glucose or HbA1c renders CV a unique and valuable tool in assessing glycaemic stability[44].
The clinical implications of GV during pregnancy are profound. GV has been identified as a potential risk factor for pregnancy complications, such as large for gestational age (LGA) infants[37]. Studies have shown that women with GDM exhibit higher GV, as indicated by increased SD and mean amplitude of glycaemic excursion (MAGE) values[28]. Quah et al[45] and Shindo et al[46], revealed that participants with GDM had significantly higher SD and MAGE values in both the first and second trimesters compared to those without GDM. Further research underscores the impact of GV on maternal and foetal health. Rodbard et al[47], found that women with GDM using CGM experienced less glucose variability and better glycaemic control compared to those not using CGM. This finding is supported by Dalfrà et al[42], who identified a relationship between macrosomia and maternal glycaemic variability in diabetic pregnancies. Additional studies by Feig et al[18] and Wei et al[48] have demonstrated that CGM users exhibit significantly lower glucose standard deviation and MAGE compared to SMBG users, indicating the efficacy of CGM in managing GV. The distinction in GV between type 1 and type 2 diabetes, as highlighted by El-Laboudi et al[49], points to the variability in glucose profiles and the need for tailored management strategies in pregnancy. Their study reported significantly higher CV in type 1 diabetic patients compared to those with type 2 diabetes[49]. This difference underscores the complexity and individualized nature of glucose management in type 1 diabetes pregnancy. Current research suggests that a CV value below 36% indicates a stable glucose profile, while values of 36% or higher suggest higher variability and an unstable profile[27].
Despite the evident association between GV and pregnancy outcomes, some studies have presented nuanced findings. For example, Dalfrà et al[42] in 2011 showed that women using CGM experienced reduced glycaemic variability, as indicated by lower SD and MAGE. However, a retrospective cohort study by Mulla et al[50] did not find trimester-specific relationships between GV and birth weight in women with type 1 diabetes, suggesting the multifaceted nature of GV's impact on pregnancy outcomes. In summary, GV, as assessed through CGM, plays a pivotal role in the management of type 1 diabetes during pregnancy. The metrics of SD and CV provide essential insights into glucose fluctuations, which are critical for both maternal and foetal health. The nuanced and variable impact of GV on pregnancy outcomes underscores the need for individualized monitoring and management strategies[42]. As research continues to evolve, the role of CGM in understanding and managing GV in pregnancy remains a vital component of diabetes care.
TAR (> 10.0 mmol/L)
In the management of type 1 diabetes during pregnancy, CGM offers critical insights into glucose control, particularly in assessing TAR. TAR, an indicator of hyperglycaemia, is categorized into two distinct levels: Level 1 (mild hyperglycaemia, > 180 mg/dL to 250 mg/dL or 10.1–13.9 mmol/L) and Level 2 (significant hyperglycaemia, > 250 mg/dL or > 13.9 mmol/L)[38]. However, for pregnant individuals with type 1 diabetes, the threshold for TAR is more stringent, defined by sensor glucose values exceeding 140 mg/dL (> 7.8 mmol/L)[27]. This adjustment acknowledges the critical need for tighter glycaemic control to mitigate risks associated with maternal and foetal hyperglycaemia. Clinical guidelines recommend minimizing TAR, aiming for it to constitute no more than 25% of the time, equivalent to less than 6 h per day[27]. This target is imperative given the heightened risk of ketosis and diabetic ketoacidosis in pregnancy, conditions exacerbated by the physiological state of accelerated starvation inherent to this period[51].
Murphy et al[34], demonstrated the effectiveness of CGM in managing TAR, with CGM users showing a significantly lower percentage of time above range (27%) compared to SMBG users (32%). This reduction in TAR is of paramount importance in the context of pregnancy, where sustained hyperglycaemia can have detrimental effects on both maternal and foetal health. Further elucidating the impact of TAR on neonatal outcomes, research by Yamamoto et al[26], provided further insights into the neonatal impacts of TAR. Their study found that in cases of neonatal hypoglycaemia, maternal plasma glucose in the second trimester spent significantly less time within normal ranges (46% ± 14% vs 53% ± 15%) and more time above the optimal range (50% ± 16% vs 42% ± 17%) compared to infants without hypoglycaemia[26]. Similar trends were observed in the third trimester, with the percentage of time in range at 60% ± 16% vs 66 ± 14%, and time above range at 35% ± 16% vs 29% ± 14% for the respective groups[26]. Additionally, Scott et al[19] reported notable differences in glucose management with CGM use. Patients using CGM spent a greater proportion of time within the glucose goal range (67.6% ± 12.6% vs 61.3% ± 15.5%) and significantly less time above the target range (27.9% ± 13.4% vs 33.1% ± 15.0%) compared to SMBG users[19]. These findings underscore the superiority of CGM in achieving and maintaining optimal glucose levels during pregnancy.
Meticulous management of TAR is a crucial aspect of diabetes care in pregnancy as it is a critical component of optimal glycaemic control, with CGM emerging as an indispensable tool in this endeavour. The ability of CGM to accurately track and reduce TAR enhances the management strategies for diabetes in pregnancy, thereby playing a crucial role in promoting favourable maternal and neonatal outcomes. The continued investigation and application of CGM in this domain underscore its significance as a cornerstone in the management of diabetes during pregnancy.
TIR: (3.9–10.0 mmol/L)
TIR is increasingly recognized as a pivotal marker in managing type 1 diabetes during pregnancy. It offers comprehensive insights into the glucose profile by indicating the duration blood glucose levels stay within the target range of 63–140 mg/dL (3.5–7.8 mmol/L)[27]. This range is notably lower than in non-pregnant individuals, reflecting the physiological adaptations where glucose levels are generally lower in pregnancy[52]. Achieving a TIR of more than 70% of the time, equivalent to over 16 h and 48 minutes daily, is highly recommended[27]. This emphasis on maintaining a higher TIR underscores the importance of minimizing time spent in hyperglycaemic or hypoglycaemic states.
The relationship between TIR and pregnancy outcomes has been substantiated through various studies. For instance, Murphy et al[53], noted that every 5% increase in TIR is associated with improved neonatal outcomes. This finding highlights the direct impact of glycaemic control on foetal health, emphasizing the need for meticulous monitoring and management of blood glucose levels during pregnancy. The CONCEPTT study provides critical evidence on the effectiveness of CGM in improving TIR among pregnant women with type 1 diabetes[18]. This randomized trial included 215 pregnant women and 110 women planning pregnancy, comparing SMBG with CGM use. Remarkably, the TIR was significantly higher in the CGM group (68% with CGM vs 61% with SMBG), translating to an approximate difference of 1.5 h per day[18]. This study underscores the superiority of CGM over traditional SMBG in achieving optimal glucose control. The CONCEPTT study further revealed that women who had previously used CGM experienced a marked improvement in TIR during the first trimester, from 40% (10 h per day) in the early postpartum period to 55% (13.2 h per day) by the end of the first trimester[32]. Although the increase in TIR during the second trimester was minimal, a 5-percentage point gain in the third trimester elevated the TIR to 60% (14.4 h per day)[18,32]. These longitudinal improvements highlight the benefits of early and continued CGM use throughout pregnancy.
The focus on TIR in pregnancy management is not just about numerical targets; it embodies a broader strategy to ensure the health and well-being of both the mother and the foetus. Higher TIR correlates with reduced risks of pregnancy-related complications, such as preterm birth, preeclampsia, and neonatal hypoglycaemia[54]. Additionally, maintaining glucose levels within this targeted range can alleviate the psychological burden on expectant mothers, reducing anxiety and stress associated with diabetes management during this critical period[55,56]. The data from various studies, including the influential CONCEPTT trial, provide compelling evidence of the benefits of maintaining a high TIR. The focus on achieving and sustaining a TIR above 70% not only enhances maternal and foetal health outcomes but also sets a new standard in the approach to diabetes care during pregnancy.
TBR
TBR is a critical metric in CGM, particularly for pregnant women with type 1 diabetes, as it indicates periods of hypoglycaemia. TBR is categorized into two levels: Level 1 (mild hypoglycaemia, between 54 and 63 mg/dL or 3.0–3.5 mmol/L) and level 2 (significant hypoglycaemia, less than 54 mg/dL or < 3.0 mmol/L)[27]. These thresholds are lower than those for non-pregnant individuals, reflecting the physiological changes in pregnancy[52]. This adaptation was acknowledged in large clinical trials such as the Swedish and CONCEPTT trials[18,26]. Moreover, the occurrence of hypoglycaemia or decreasing insulin requirement, especially in the third trimester, has been strongly linked to uteroplacental insufficiency, making it crucial to monitor these levels for critical decision-making, such as considering early induction of labour[57].
Kristensen et al[58] observed a significant rise in the percentage of time spent below the threshold of 3.5 mmol/L, starting at 6 wk and peaking at 12-16 wk of gestation. This period coincides with an increased risk of severe hypoglycaemia in mothers. These findings suggest that minimizing the time that blood glucose levels fall below 3.5 mmol/L to less than 4% (less than 1 h per day) is particularly challenging in early pregnancy due to the limiting factor of maternal hypoglycaemia in achieving stringent glycaemic goals. The CONCEPTT study further highlights the dynamics of TBR during pregnancy[18,32]. Although severe hypoglycaemia events were too infrequent for detailed correlation analysis with CGM time below range criteria, a notable trend was observed. Between 12 and 34 wk of pregnancy, the amount of time spent below 3.5 mmol/L decreased by half for both insulin pump and multiple daily injection users (from 6% to 3% and from 8% to 4%, respectively)[18]. This decrease indicates an evolving glycaemic profile as pregnancy progresses, emphasizing the need for continuous monitoring and adjustment of diabetes management strategies.
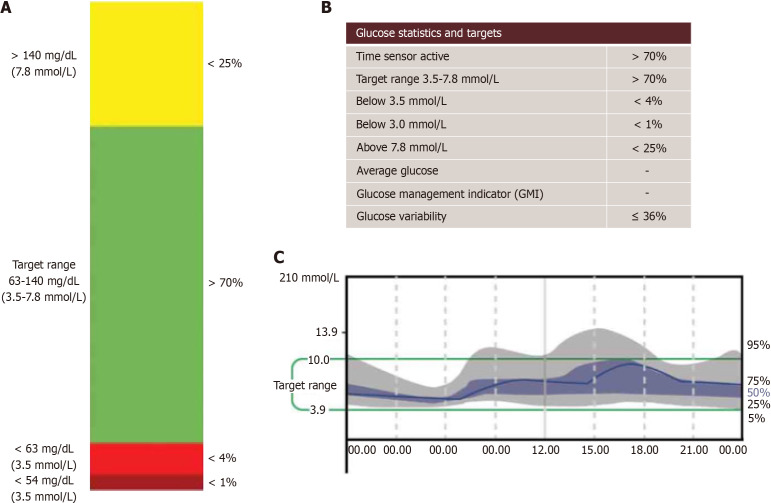
The imperative to maintain TBR values below critical thresholds (< 4%; <1 h below 63 mg/dL or value < 3.5 mmol/L, and < 1% or < 15 min below 54 mg/dL or < 3.0 mmol/L) is paramount in pregnancy[27]. Effective management of TBR is essential not only for maternal health but also for foetal well-being, as fluctuations in maternal glucose levels can have direct implications for foetal development[42]. In managing Type 1 diabetes during pregnancy, TBR as assessed through CGM plays a vital role in navigating the risks of hypoglycaemia. Continuous and vigilant monitoring of TBR, especially in the context of the changing glycaemic landscape of pregnancy, is crucial for achieving optimal maternal and neonatal outcomes. Figure 2 shows the graphical CGM metrics during pregnancy.
Figure 2.
Shows the graphical continuous glucose monitoring metrics during pregnancy. A and B: Recommended metrics for continuous glucose monitoring during pregnancy; C: Illustrates a typical ambulatory glucose profile (AGP) model. When AGP is generated on certain devices, it defaults to standard time-in-range settings for non-pregnant individuals. Therefore, careful interpretation is necessary, or settings should be adjusted to reflect pregnancy-specific parameters prior to generating the AGP.
PITFALLS OF USING CGM IN PREGNANCY
CGM has become a vital tool in managing type 1 diabetes during pregnancy, yet it presents several pitfalls that necessitate careful consideration. One of the primary concerns lies in the realm of accuracy. CGM sensors, which measure glucose in the interstitial fluid, can sometimes lag behind actual blood glucose levels. This delay is particularly problematic given the rapid glucose fluctuations typical in pregnancy[8]. Klonoff et al[59], highlighted that the accuracy of CGM systems, especially in extreme glucose ranges, could vary, potentially leading to mismanagement of hyperglycaemia or hypoglycaemia. Furthermore, technical challenges such as sensor adhesion issues, exacerbated by physiological changes during pregnancy, can lead to gaps in monitoring[60]. The necessity for regular calibration in previous generation sensor posed additional hurdles, which has now been mostly resolved with factory calibrated sensors.
User-related issues and psychological impacts constitute another set of challenges. The phenomenon of alarm fatigue, where users become desensitized to frequent alerts, can lead to critical glucose changes being overlooked[61]. A survey by Polsky et al[12], highlighted that approximately 30% of CGM users experienced alarm fatigue, risking overlooked hypoglycaemic or hyperglycaemic events. Additionally, the constant stream of data and the need for continual decision-making can heighten anxiety and stress in pregnant women, potentially impacting their overall health. Economic and accessibility constraints also play a crucial role. The financial burden of CGM, not universally covered by insurance plans, can limit its accessibility. Cost and insurance limitations are significant barriers to wider CGM adoption, impacting its feasibility for many pregnant women with type 1 diabetes[62,63].
Clinical management challenges and the risk of over-treatment are further pitfalls in using CGM during pregnancy[64]. The interpretation of CGM data requires expertise and a nuanced understanding of diabetes management, a challenge for both patients and healthcare providers. The changing physiological landscape of pregnancy necessitates frequent adjustments in CGM settings, a complex task that can lead to either over-treatment or under-treatment. For instance, the CONCEPTT trial, highlighted the intricacies of managing insulin dosages based on CGM data, underscoring the need for specialized knowledge and continuous monitoring[18]. Additionally, indirect impacts on foetal health due to misinterpretation of CGM data or technical issues can have lasting consequences, emphasizing the need for accurate and reliable use of this technology. Hence, effective use of CGM requires a comprehensive understanding of these pitfalls, continuous education, and support for healthcare providers and patients alike[64]. Addressing these challenges is crucial to harness the full potential of CGM and ensure optimal maternal and foetal health outcomes in pregnancies complicated by type 1 diabetes. More data is necessary regarding how twin or multiple pregnancies affect utility of CGM metrics in pregnancy as there is inadequate evidence available currently.
PRACTICAL APPROACH TO OPTIMIZING CGM USE IN PREGNANCY
The first and foremost step in effectively using CGM during pregnancy is to actively engage the patient in the process. Open dialogues where the patient’s opinions and observations are valued play a crucial role. This collaborative approach not only empowers the patient but also provides valuable insights into individualized management. Always check the sensor site and the injection or pump site. This step is crucial to ensure proper device functioning and to rule out any technical issues contributing to glycaemic variations. When opening the results data view, ensure that the cut-offs specific to pregnancy are set, as the default range can differ. Here are the steps that we commonly advocate for a complete assessment of the CGM data.
Data review and analysis
Data availability: Begin by confirming the adequacy of available data. For current CGM users, it’s ideal to have at least 70% of data over a two-week period. In cases of significant hypoglycaemia or hyperglycaemia, a shorter period may suffice for analysis.
Pattern identification using AGP: Utilize the AGP to discern overarching patterns within the two-week data. Engage the patient in identifying factors contributing to these trends. This process is not just diagnostic but educational, helping patients understand the interplay between insulin, diet, lifestyle, and glucose levels.
Prioritizing glycaemic patterns
Time in range assessment: Assess the TIR to quantify the average duration the patient spends within the target glucose levels each day. This metric is crucial in evaluating the effectiveness of current management strategies.
Identifying problematic patterns: Focus on identifying problematic glycaemic patterns in order of priority: Firstly, episodes of hypoglycaemia; secondly, periods of hyperglycaemia; and thirdly, instances of wide glycaemic variability. Review the overall glucose profile to pinpoint specific times of day when these patterns occur.
Daily graph review: Delve into daily graphs to verify if these patterns are isolated incidents or part of a recurring trend. This step is crucial in understanding the consistency and triggers of glycaemic fluctuations.
Collaborative solution development
Patient reflection and solution discussion: Encourage patients to reflect on potential causes for observed glycaemic issues and engage in a discussion to brainstorm potential solutions.
Action plan formulation: Develop a collaborative action plan with the patient. Ensure that they fully understand and are equipped with the necessary skills to implement the plan effectively.
Action plan documentation: Provide the patient with a copy of the action plan. Given the complexity and volume of information, this step is vital to ensure they have a reference to rely on.
The practical application of CGM in the management of type 1 diabetes during pregnancy requires a meticulous and patient-centred approach. By engaging patients in the process, thoroughly analysing CGM data, prioritizing glycaemic patterns, and collaboratively developing action plans, healthcare providers can enhance the efficacy of CGM. This approach not only improves glycaemic control but also empowers patients with the knowledge and skills necessary for successful diabetes management during this crucial phase of their lives.
CONCLUSION
CGM stands as a transformative tool in the management of type 1 diabetes during pregnancy. CGM's real-time glucose monitoring capability offers unparalleled benefits in optimizing glycaemic control, a crucial factor for ensuring the health and well-being of both the mother and the foetus. The implementation of CGM in pregnancy has demonstrated significant improvements in key metrics such as TIR, TAR, and TBR. These metrics provide a nuanced view of the patient's glycaemic profile, allowing for more precise adjustments in diabetes management strategies. The ability of CGM to identify patterns of glycaemic variability and to facilitate early interventions in cases of hypo- or hyperglycaemia is instrumental in mitigating risks associated with diabetes in pregnancy. However, the utilization of CGM is not without its challenges. Accuracy concerns, technical limitations, and the need for proper patient education and engagement are critical considerations. The importance of a patient-centred approach in CGM use cannot be overstated. By involving patients in the decision-making process, addressing their concerns, and ensuring they understand and can respond to their CGM data, healthcare providers can enhance the effectiveness of this technology. Furthermore, the economic and accessibility aspects of CGM use, along with the need for healthcare providers to stay updated with evolving technology, are areas that require ongoing attention and resources. Despite these challenges, the benefits of CGM in the context of pregnancy are clear and impactful. In conclusion, CGM represents a significant advancement in the management of Type 1 diabetes during pregnancy. Its comprehensive monitoring capability, coupled with a patient-centred approach, paves the way for more effective, personalized diabetes care, ultimately leading to improved maternal and neonatal outcomes.
Footnotes
Conflict-of-interest statement: The authors have nothing to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 29, 2023
First decision: December 12, 2023
Article in press: January 16, 2024
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Belosludtseva NV, Russia; Yu H, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao YQ
Contributor Information
Mohammad Sadiq Jeeyavudeen, Metabolic Unit, Western General Hospital, Edinburgh EH4 2XU, United Kingdom.
Mairi Crosby, Department of Endocrinology and Metabolism, University Hospitals of Edinburgh, Edinburgh EH16 4SA, United Kingdom.
Joseph M Pappachan, Department of Endocrinology and Metabolism, Lancashire Teaching Hospitals NHS Trust, Preston PR2 9HT, United Kingdom; Faculty of Science, Manchester Metropolitan University, Manchester M15 6BH, United Kingdom; Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PL, United Kingdom. drpappachan@yahoo.co.in.
References
- 1.Wilson CB. Sensors 2010. BMJ. 1999;319:1288. doi: 10.1136/bmj.319.7220.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes Metab Syndr. 2018;12:181–187. doi: 10.1016/j.dsx.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Didyuk O, Econom N, Guardia A, Livingston K, Klueh U. Continuous Glucose Monitoring Devices: Past, Present, and Future Focus on the History and Evolution of Technological Innovation. J Diabetes Sci Technol. 2021;15:676–683. doi: 10.1177/1932296819899394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrovski G, Dimitrovski C, Bogoev M, Milenkovic T, Ahmeti I, Bitovska I. Is there a difference in pregnancy and glycemic outcome in patients with type 1 diabetes on insulin pump with constant or intermittent glucose monitoring? A pilot study. Diabetes Technol Ther. 2011;13:1109–1113. doi: 10.1089/dia.2011.0081. [DOI] [PubMed] [Google Scholar]
- 5.Funtanilla VD, Candidate P, Caliendo T, Hilas O. Continuous Glucose Monitoring: A Review of Available Systems. P T. 2019;44:550–553. [PMC free article] [PubMed] [Google Scholar]
- 6.Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations : A joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia. 2017;60:2319–2328. doi: 10.1007/s00125-017-4463-4. [DOI] [PubMed] [Google Scholar]
- 7.Patton SR, Clements MA. Continuous Glucose Monitoring Versus Self-monitoring of Blood Glucose in Children with Type 1 Diabetes- Are there Pros and Cons for Both? US Endocrinol. 2012;8:27–29. [PMC free article] [PubMed] [Google Scholar]
- 8.Rodbard D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol Ther. 2016;18 Suppl 2:S3–S13. doi: 10.1089/dia.2015.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nusinovici S, Sabanayagam C, Lee KE, Zhang L, Cheung CY, Tai ES, Tan GSW, Cheng CY, Klein BEK, Wong TY. Retinal microvascular signs and risk of diabetic kidney disease in asian and white populations. Sci Rep. 2021;11:4898. doi: 10.1038/s41598-021-84464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez M, Santamarina J, Pavesi A, Musso C, Umpierrez GE. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/bmjdrc-2020-002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj R, Mishra R, Jha N, Joshi V, Correa R, Kern PA. Time in range, as measured by continuous glucose monitor, as a predictor of microvascular complications in type 2 diabetes: a systematic review. BMJ Open Diabetes Res Care. 2022;10 doi: 10.1136/bmjdrc-2021-002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polsky S, Garcetti R. CGM, Pregnancy, and Remote Monitoring. Diabetes Technol Ther. 2017;19:S49–S59. doi: 10.1089/dia.2017.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Patterson A, Gich I, Amini SB, Catalano PM, de Leiva A, Corcoy R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia. 2010;53:446–451. doi: 10.1007/s00125-009-1633-z. [DOI] [PubMed] [Google Scholar]
- 14.Newman C, Ero A, Dunne FP. Glycaemic control and novel technology management strategies in pregestational diabetes mellitus. Front Endocrinol (Lausanne) 2022;13:1109825. doi: 10.3389/fendo.2022.1109825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lason I, Cyganek K, Witek P, Matejko B, Malecki MT, Skupien J. Continuous glucose monitoring and insulin pump therapy in pregnant women with type 1 diabetes mellitus. Ginekol Pol. 2021;92:675–681. doi: 10.5603/GP.a2021.0029. [DOI] [PubMed] [Google Scholar]
- 16.Buhary BM, Almohareb O, Aljohani N, Alzahrani SH, Elkaissi S, Sherbeeni S, Almaghamsi A, Almalki M. Glycemic control and pregnancy outcomes in patients with diabetes in pregnancy: A retrospective study. Indian J Endocrinol Metab. 2016;20:481–490. doi: 10.4103/2230-8210.183478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohan V, Joshi S, Mithal A, Kesavadev J, Unnikrishnan AG, Saboo B, Kumar P, Chawla M, Bhograj A, Kovil R. Expert Consensus Recommendations on Time in Range for Monitoring Glucose Levels in People with Diabetes: An Indian Perspective. Diabetes Ther. 2023;14:237–249. doi: 10.1007/s13300-022-01355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, Asztalos E, Barrett JFR, Sanchez JJ, de Leiva A, Hod M, Jovanovic L, Keely E, McManus R, Hutton EK, Meek CL, Stewart ZA, Wysocki T, O'Brien R, Ruedy K, Kollman C, Tomlinson G, Murphy HR CONCEPTT Collaborative Group. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390:2347–2359. doi: 10.1016/S0140-6736(17)32400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott EM, Feig DS, Murphy HR, Law GR CONCEPTT Collaborative Group. Continuous Glucose Monitoring in Pregnancy: Importance of Analyzing Temporal Profiles to Understand Clinical Outcomes. Diabetes Care. 2020;43:1178–1184. doi: 10.2337/dc19-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitanchez D, Yzydorczyk C, Simeoni U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J Diabetes. 2015;6:734–743. doi: 10.4239/wjd.v6.i5.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenninger E, Lindqvist A, Aman J, Ostlund I, Schvarcz E. Continuous Subcutaneous Glucose Monitoring System in diabetic mothers during labour and postnatal glucose adaptation of their infants. Diabet Med. 2008;25:450–454. doi: 10.1111/j.1464-5491.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 22.Griffith RJ, Harding JE, McKinlay CJD, Wouldes TA, Harris DL, Alsweiler JM CHYLD Study Team. Maternal glycemic control in diabetic pregnancies and neurodevelopmental outcomes in preschool aged children. A prospective cohort study. Early Hum Dev. 2019;130:101–108. doi: 10.1016/j.earlhumdev.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landon MB, Mele L, Varner MW, Casey BM, Reddy UM, Wapner RJ, Rouse DJ, Tita ATN, Thorp JM, Chien EK, Saade G, Grobman W, Blackwell SC, VanDorsten JP Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. The relationship of maternal glycemia to childhood obesity and metabolic dysfunction(‡) J Matern Fetal Neonatal Med. 2020;33:33–41. doi: 10.1080/14767058.2018.1484094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieß C, Heimann Y, Schleußner E, Groten T, Weschenfelder F. Disease Perception and Mental Health in Pregnancies with Gestational Diabetes-PsychDiab Pilot Study. J Clin Med. 2023;12 doi: 10.3390/jcm12103358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall M, Oakey H, Penno MAS, McGorm K, Anderson AJ, Ashwood P, Colman PG, Craig ME, Davis EA, Harris M, Harrison LC, Haynes A, Morbey C, Sinnott RO, Soldatos G, Vuillermin PJ, Wentworth JM, Thomson RL, Couper JJ ENDIA Study Group. Mental Health During Late Pregnancy and Postpartum in Mothers With and Without Type 1 Diabetes: The ENDIA Study. Diabetes Care. 2022 doi: 10.2337/dc21-2335. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto JM, Murphy HR. Benefits of Real-Time Continuous Glucose Monitoring in Pregnancy. Diabetes Technol Ther. 2021;23:S8–S14. doi: 10.1089/dia.2020.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ 3rd, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Nørgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer SA, Phillip M. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrar D, Tuffnell DJ, West J, West HM. Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes. Cochrane Database Syst Rev. 2016;2016:CD005542. doi: 10.1002/14651858.CD005542.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang X, Fu Y, Lu S, Shuai M, Miao Z, Gou W, Shen L, Liang Y, Xu F, Tian Y, Wang J, Zhang K, Xiao C, Jiang Z, Shi MQ, Wu YY, Wang XH, Hu WS, Zheng JS. Continuous glucose monitoring-derived glycemic metrics and adverse pregnancy outcomes among women with gestational diabetes: a prospective cohort study. Lancet Reg Health West Pac. 2023;39:100823. doi: 10.1016/j.lanwpc.2023.100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baretić M, Lekšić G, Ivanišević M. Ambulatory Glucose Profile Changes During Pregnancy in Women With Type 1 Diabetes Using Intermittently Scanned Continuous Glucose Monitoring Empowered by Personalized Education. Diabetes Spectr. 2023;36:373–378. doi: 10.2337/ds22-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes RC, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014;37:2953–2959. doi: 10.2337/dc14-1312. [DOI] [PubMed] [Google Scholar]
- 32.Farrar D, Campbell MD. Does continuous glucose monitoring during pregnancy improve glycaemic and health outcomes in women with type 1 diabetes?-what the CONCEPTT trial adds. Ann Transl Med. 2018;6:188. doi: 10.21037/atm.2018.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013;36:1877–1883. doi: 10.2337/dc12-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy HR, Rayman G, Duffield K, Lewis KS, Kelly S, Johal B, Fowler D, Temple RC. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30:2785–2791. doi: 10.2337/dc07-0500. [DOI] [PubMed] [Google Scholar]
- 35.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA(1c) Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care. 2017;40:994–999. doi: 10.2337/dc17-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing D, Kollman C, Beck RW, Tamborlane WV, Laffel L, Buckingham BA, Wilson DM, Weinzimer S, Fiallo-Scharer R, Ruedy KJ Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther. 2011;13:351–358. doi: 10.1089/dia.2010.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leksic G, Baretić M, Gudelj L, Radic M, Milicic I, Ivanišević M, Jurisic-Erzen D. Glycemic Variability in Type 1 Diabetes Mellitus Pregnancies-Novel Parameters in Predicting Large-for-Gestational-Age Neonates: A Prospective Cohort Study. Biomedicines. 2022;10 doi: 10.3390/biomedicines10092175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, Brown AS, Heinemann L, Aleppo G, Ryan DB, Riddlesworth TD, Cefalu WT. Glucose Management Indicator (GMI): A New Term for Estimating A1C From Continuous Glucose Monitoring. Diabetes Care. 2018;41:2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah VN, Snell-Bergeon JK, Demmitt JK, Joshee P, Garcetti R, Pyle L, Polsky S. Relationship Between Time-in-Range, HbA1c, and the Glucose Management Indicator in Pregnancies Complicated by Type 1 Diabetes. Diabetes Technol Ther. 2021;23:783–790. doi: 10.1089/dia.2021.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Law GR, Ellison GT, Secher AL, Damm P, Mathiesen ER, Temple R, Murphy HR, Scott EM. Analysis of Continuous Glucose Monitoring in Pregnant Women With Diabetes: Distinct Temporal Patterns of Glucose Associated With Large-for-Gestational-Age Infants. Diabetes Care. 2015;38:1319–1325. doi: 10.2337/dc15-0070. [DOI] [PubMed] [Google Scholar]
- 41.Ling P, Yang D, Wang C, Zheng X, Luo S, Yang X, Deng H, Xu W, Yan J, Weng J. A pregnancy-specific Glucose management indicator derived from continuous glucose monitoring in pregnant women with type 1 diabetes. Diabetes Metab Res Rev. 2023;39:e3689. doi: 10.1002/dmrr.3689. [DOI] [PubMed] [Google Scholar]
- 42.Dalfrà MG, Sartore G, Di Cianni G, Mello G, Lencioni C, Ottanelli S, Sposato J, Valgimigli F, Scuffi C, Scalese M, Lapolla A. Glucose variability in diabetic pregnancy. Diabetes Technol Ther. 2011;13:853–859. doi: 10.1089/dia.2010.0145. [DOI] [PubMed] [Google Scholar]
- 43.Kovatchev B, Cobelli C. Glucose Variability: Timing, Risk Analysis, and Relationship to Hypoglycemia in Diabetes. Diabetes Care. 2016;39:502–510. doi: 10.2337/dc15-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McLean A, Barr E, Tabuai G, Murphy HR, Maple-Brown L. Continuous Glucose Monitoring Metrics in High-Risk Pregnant Women with Type 2 Diabetes. Diabetes Technol Ther. 2023;25:836–844. doi: 10.1089/dia.2023.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quah PL, Tan LK, Lek N, Thain S, Tan KH. Glycemic Variability in Early Pregnancy May Predict a Subsequent Diagnosis of Gestational Diabetes. Diabetes Metab Syndr Obes. 2022;15:4065–4074. doi: 10.2147/DMSO.S379616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shindo R, Aoki S, Kasai J, Saigusa Y, Nakanishi S, Miyagi E. Impact of introducing the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria on pregnancy outcomes in Japan. Endocr J. 2020;67:15–20. doi: 10.1507/endocrj.EJ19-0279. [DOI] [PubMed] [Google Scholar]
- 47.Rodbard D. Continuous Glucose Monitoring: A Review of Recent Studies Demonstrating Improved Glycemic Outcomes. Diabetes Technol Ther. 2017;19:S25–S37. doi: 10.1089/dia.2017.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei Q, Sun Z, Yang Y, Yu H, Ding H, Wang S. Effect of a CGMS and SMBG on Maternal and Neonatal Outcomes in Gestational Diabetes Mellitus: a Randomized Controlled Trial. Sci Rep. 2016;6:19920. doi: 10.1038/srep19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Laboudi AH, Godsland IF, Johnston DG, Oliver NS. Measures of Glycemic Variability in Type 1 Diabetes and the Effect of Real-Time Continuous Glucose Monitoring. Diabetes Technol Ther. 2016;18:806–812. doi: 10.1089/dia.2016.0146. [DOI] [PubMed] [Google Scholar]
- 50.Mulla BM, Noor N, James-Todd T, Isganaitis E, Takoudes TC, Curran A, Warren CE, O'Brien KE, Brown FM. Continuous Glucose Monitoring, Glycemic Variability, and Excessive Fetal Growth in Pregnancies Complicated by Type 1 Diabetes. Diabetes Technol Ther. 2018;20:413–419. doi: 10.1089/dia.2017.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanner HL, Dekker Nitert M, Callaway LK, Barrett HL. Ketones in Pregnancy: Why Is It Considered Necessary to Avoid Them and What Is the Evidence Behind Their Perceived Risk? Diabetes Care. 2021;44:280–289. [Google Scholar]
- 52.Angueira AR, Ludvik AE, Reddy TE, Wicksteed B, Lowe WL Jr, Layden BT. New insights into gestational glucose metabolism: lessons learned from 21st century approaches. Diabetes. 2015;64:327–334. doi: 10.2337/db14-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy HR. Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia. 2019;62:1123–1128. doi: 10.1007/s00125-019-4904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voormolen DN, DeVries JH, Sanson RME, Heringa MP, de Valk HW, Kok M, van Loon AJ, Hoogenberg K, Bekedam DJ, Brouwer TCB, Porath M, Erdtsieck RJ, NijBijvank B, Kip H, van der Heijden OWH, Elving LD, Hermsen BB, Potter van Loon BJ, Rijnders RJP, Jansen HJ, Langenveld J, Akerboom BMC, Kiewiet RM, Naaktgeboren CA, Mol BWJ, Franx A, Evers IM. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): A multicentre randomized controlled trial. Diabetes Obes Metab. 2018;20:1894–1902. doi: 10.1111/dom.13310. [DOI] [PubMed] [Google Scholar]
- 55.Runge AS, Kennedy L, Brown AS, Dove AE, Levine BJ, Koontz SP, Iyengar VS, Odeh SA, Close KL, Hirsch IB, Wood R. Does Time-in-Range Matter? Perspectives From People With Diabetes on the Success of Current Therapies and the Drivers of Improved Outcomes. Clin Diabetes. 2018;36:112–119. doi: 10.2337/cd17-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gavin JR, Bailey CJ. Real-World Studies Support Use of Continuous Glucose Monitoring in Type 1 and Type 2 Diabetes Independently of Treatment Regimen. Diabetes Technol Ther. 2021;23:S19–S27. doi: 10.1089/dia.2021.0211. [DOI] [PubMed] [Google Scholar]
- 57.Padmanabhan S, Lee VW, Mclean M, Athayde N, Lanzarone V, Khoshnow Q, Peek MJ, Cheung NW. The Association of Falling Insulin Requirements With Maternal Biomarkers and Placental Dysfunction: A Prospective Study of Women With Preexisting Diabetes in Pregnancy. Diabetes Care. 2017;40:1323–1330. doi: 10.2337/dc17-0391. [DOI] [PubMed] [Google Scholar]
- 58.Kristensen K, Ögge LE, Sengpiel V, Kjölhede K, Dotevall A, Elfvin A, Knop FK, Wiberg N, Katsarou A, Shaat N, Kristensen L, Berntorp K. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62:1143–1153. doi: 10.1007/s00125-019-4850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: A review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178–192. doi: 10.1016/j.diabres.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving Skin Integrity with Chronic Device Use in Diabetes. Diabetes Technol Ther. 2018;20:S254–S264. doi: 10.1089/dia.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kern-Goldberger AR, Hamm RF, Raghuraman N, Srinivas SK. Reducing Alarm Fatigue in Maternal Monitoring on Labor and Delivery: A Commentary on Deimplementation in Obstetrics. Am J Perinatol. 2023;40:1378–1382. doi: 10.1055/a-1785-9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanenbaum ML, Commissariat PV. Barriers and Facilitators to Diabetes Device Adoption for People with Type 1 Diabetes. Curr Diab Rep. 2022;22:291–299. doi: 10.1007/s11892-022-01469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Datye KA, Tilden DR, Parmar AM, Goethals ER, Jaser SS. Advances, Challenges, and Cost Associated with Continuous Glucose Monitor Use in Adolescents and Young Adults with Type 1 Diabetes. Curr Diab Rep. 2021;21:22. doi: 10.1007/s11892-021-01389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li A, Brackenridge A. The role of continuous glucose monitoring in pregnancy. Obstet Med. 2022;15:6–10. doi: 10.1177/1753495X211014716. [DOI] [PMC free article] [PubMed] [Google Scholar]