Abstract
The Human Microbiome Project, Earth Microbiome Project, and next-generation sequencing have advanced novel genome association, host genetic linkages, and pathogen identification. The microbiome is the sum of the microbes, their genetic information, and their ecological niche. This study will describe how millions of bacteria in the gut affect the human body in health and disease. The gut microbiome changes in relation with age, with an increase in Bacteroidetes and Firmicutes. Host and environmental factors affecting the gut microbiome are diet, drugs, age, smoking, exercise, and host genetics. In addition, changes in the gut microbiome may affect the local gut immune system and systemic immune system. In this study, we discuss how the microbiome may affect the metabolism of healthy subjects or may affect the pathogenesis of metabolism-generating metabolic diseases. Due to the high number of publications on the argument, from a methodologically point of view, we decided to select the best papers published in referred journals in the last 3 years. Then we selected the previously published papers. The major goals of our study were to elucidate which microbiome and by which pathways are related to healthy and disease conditions.
Keywords: Gut microbiome, Dysbiosis, Pathobionts, Gut-brain axis, Heart-brain axis, Metabolic diseases, Omics techniques
Core Tip: Gut microbiome has relevant importance in healthy and diseased subjects. The production of several metabolites from the gut microbiome influences the immune system, brain, lung, heart, and metabolism. In the case of normal indigenous microbiota, metabolites produced have a benign action and contribute to the health. By contrast, the presence of pathobionts with their products may affect the different organs and produce diseases. The study of the gut microbiome is a difficult one and different omics technologies should be applied. The large quantity of studies highlights the relevance of the gut microbiome in health and disease.
INTRODUCTION
More than 150,000 papers with “Microbiome” in the title or abstract have been published since the term was introduced in 2001. Early-stage reports were cross-sectional studies of the microbiota at different body sites, associations with disease markers, and diseases themselves. More recently, three relevant projects, the Human Microbiome Project[1], the Earth Microbiome Project[2], and next-generating sequencing[3], have advanced novel genome associations, host genetic linkages, and pathogen identification. Current studies have principally focused on the functional or mechanistic aspects of differences in microbial composition.
In particular, a study from Gao et al[4] found that the more relevant studies cover six important aspects of microbiome research. They include best practices for analyzing microbiomes, the regulation of gut microbiomes in the human immune system, how microbiomes affect immune sensing, the role of microbiomes in the gut-brain interaction, the application of microbiomes in maternal and newborn health, and the study of nutri-microbiome epidemiology.
Due to the high number of publications on the argument, from a methodologically point of view, we decided to select the best papers published on referred journals in the last 3 years. Then, going in a backward way, we selected the previously published papers.
The aim of this study was to clarify the relationship between microbiome and healthy and disease conditions. In particular, we looked for which microbiome products and metabolites are involved in this relationship and which organs are strictly connected to microbiome normal or modified composition.
Before discussing the gut microbiome, and its function and its relationship with health and disease, exact definitions are needed for a correct understanding.
DEFINITIONS
Frequently, the terms microbiota and microbiome are mutually used, but they have a different significance. Table 1 shows the exact definitions needed to be understood.
Table 1.
Definitions related to gut microbiome and its action
|
Terminology
|
Significance
|
| Microbiota | All microorganisms living in the gut |
| Microbioma | Sum of microbes, their genetic information, and their ecological niche |
| Metagenome | Total genes providing information on genetic potential |
| Indigenous microbiota | Resident gut microbiota in the healthy subjects |
| Dysbiosis | Modification of the composition of gut microbiota causing diseases |
| Pathobionts | Gut microbiota causing diseases |
Microbiota are defined as the microorganisms (bacteria, archaea, viruses, fungi) that live in the human body in healthy conditions. All of these microorganisms make up the microbiome. The microbiome is the sum of microbes, their genetic information, and their ecological niche. The metagenome is the totality of genes from the genomics of a mixed microbial population that provides information about genetic potential. Indigenous microbiota are the resident microbiota resident in the healthy subjects. Dysbiosis is the change in indigenous microbiota composition causing disease. Pathobionts are the microbiota causing disease.
GUT MICROBIOME
The microbiome is spread across different organs and tissues of the human body, but the most important and best studied is the gut microbiome. A total of 1014 bacteria already represent the gut microbiome, and 1011 bacteria flow each day from the pharynx to the stomach. Changes in the gut microbiome are associated with diseases, but frequently, it is not known if this is a cause or an effect.
Under normal conditions, formation of the adult microbiome occurs over the first 3 years of life and is affected by life events such as weaning, starting solid food, and primarily cessation of breastfeeding. At birth, the most common bacteria are aerobic bacteria such as Enterococcus and Staphylococcus. Later, anaerobes prevail, with a prevalence of Firmicutes and Bacteroidetes[5]. Several studies have documented the distribution of normal gut flora in the different parts of the intestine in adults as shown in Table 2[6-8].
Table 2.
Distribution of normal gut flora in different parts of intestine
|
Intestine sections
|
Function
|
Normal flora
|
| Stomach | Acid production, pepsin, amylase, CFU < 103/mL | Lactobacillus; Streptococcus; Helicobacter pylori |
| Small intestine: Duodenum, jejunum | Pancreatic enzymes, bicarbonate ions, bile salts, CFU: 103-104/mL | Lactobacilli; Enterococci, Streptococci; Actinobacteria |
| Small intestine: Ileum | CFU: 103-109/mL | Enterococcus; Bacteroidetes; Lactobacillus; Clostridium; Corynebacteria |
| Large intestine: Cecum, colon | Mucus and bicarbonate; CFU: 1010-1012/mL | Bacteroidetes; Clostridium; Eubacterium; Ruminococcus; Streptococcus; Enterococcus; Lactobacillus; Fusobacteria |
CFU: Colony-forming unit.
FACTORS INFLUENCING THE GUT MICROBIOME
The Human Microbiome Project data suggest that the unperturbed microbiome is stable over short periods and that there is a degree of resilience of the microbiome due to several factors. In addition to age, several factors affect the composition of the gut microbiome, such as diet, host genetics, exercise, smoking, and drugs[9]. A vegetarian and rich fiber diet has a beneficial effect on the gut microbiome, favoring an increase in Firmicutes and Bacteroidetes[10,11]. Several genes associated with innate immunity influence the microbiome[12]. A recent study from Chen et al[13] documented the influence of host genetics on the gut microbiome. Exercise is associated with a beneficial effect on gut microbiome composition, as documented by Hughes et al[14] and Mailing et al[15]. It has also been documented that athletes have a reduced rate of inflammatory markers[16] and exercise has been proposed to reduce dysbiosis[17]. No smoker subjects showed an increase in the fecal microbiota of Firmicutes and Actinobacteria[18]. The same author in a different study found differences in the oral gut microbiota in smokers with respect to no smokers[19]. Several drugs have a powerful effect on microbiome composition. Antibiotics may damage the microbiome in two different ways. On the one hand, by destroying beneficial microbes, antibiotics may cause dysbiosis[20]. On the other hand, destroying beneficial microbes blocks the mechanism by which they inhibit pathogens[21]. These effects on the gut microbiome are related to the type of antibiotics and the treatment duration. Worse effects on the microbiome have been described with the use of clindamycin[22], clarithromycin, ciprofloxacin[23], and vancomycin[24]. All of these antibiotics cause a reduction in Bacteroides variety and Ruminococcus. Regarding non-antibiotic drugs, there is a complex bidirectional interaction between their use and the gut microbiome[25]. On the one hand, these commonly used drugs alter the gut microbiome composition and function; on the other hand, gut microbes can contribute to drug efficacy and safety by enzymatically transforming drug structure and altering drug bioavailability, bioactivity, or toxicity. Knowing these interactions enables interventions to modulate the gut microbiome and optimize treatment efficacy.
According to the Belgium Flemish cohort[26] and the Twins United Kingdom cohort[27], the most common no antibiotic drugs associated with microbiome modification and dysbiosis are proton pump inhibitors (PPIs), statins, laxatives, metformin, and angiotensin-converting enzyme inhibitors. PPIs, by changing microbiome composition may favor the colonization of pathogens such as Clostridium difficile and Salmonella[28,29]. Metformin, used to treat type 2 diabetes, increases Escherichia coli and reduces Intestinibacter, favoring dysbiosis and causing gastrointestinal side effects[30,31]. Studies conducted in the United Kingdom, the Netherlands, and Belgium have documented modifications in microbiome composition after the prolonged assumption of laxatives, statins, and antidepressants[26,27,32].
FUNCTIONS OF THE MICROBIOME
Metabolic function
The gut microbiota has the capacity to metabolize dietary fibers not metabolized by digestive enzymes[33]. In this way, the microbiome provides additional energy by metabolizing large polysaccharides and alcohols. The MEROPS database showed that the microbiome produces proteases that are able to metabolize different substances in the large intestine[34,35]. Additionally, beneficial effects produced by the microbiome are related to the production of several vitamins[36,37]. A study by Afzaal et al[38] showed the principal metabolites produced by gut microbiota in normal conditions and their functions (Table 3)[39-49].
Table 3.
Metabolites produced by gut microbiota and their functions
|
Metabolites
|
Functions
|
Ref.
|
| Bile acid metabolites; including deoxycholic acid and lithocholic acid | Regulate bile acid, cholesterol, lipid, glucose, and activate host nuclear receptors and cell signaling pathways | Ramírez-Macías et al[39] |
| Short-chain fatty acids metabolites | Regulate food intake and insulin secretion, also aid in maintaining body weight | Psichas et al[40] |
| Branched-chain fatty acids including isobutyrate | Histone deacetylase inhibition, increased histone acetylation | Mischke et al[41] |
| Indole derivatives including indoxyl sulfate and IPA | IPA exhibits neuroprotective effects, acts as a powerful antioxidant and regulates intestinal barrier function | Hendrikx et al[42] |
| Lipopolysaccharide, peptidoglycan, lipoteicholic acid | Epigenetic regulation of genes in colorectal cancer, modulation of chromatine structure and transcriptional activity | Lightfoot et al[43] |
| Phenolic derivatives include 4-OH phenylacetic acid, urolithins, enterodiol and 9-prenylaringenin | Exhibit antimicrobial effect, maintain intestinal health and protect against oxidative stress | Larrosa et al[44] |
| Choline metabolites include choline, trimethylamine N-oxide, and betaine | Regulating lipid metabolism, and glucose synthesis contribute to the development of cardiovascular disease | Smallwood et al[45] |
| Polyamines include putrescine, spermidine and spermine | Sustaining the high proliferation rate of intestinal epithelial cells enhances intestinal barrier integrity and enhances the systematic adaptive immune system | Rooks et al[46] |
| Vitamins including thiamine (B1), riboflavin (B2), niacin (B3), pyridoxine (B6) panthotenic acid (B5), biotin (B7), folate (B11, B9), cobalamin (B12), and menaquinone (K2) | Help in red blood cell formation, DNA replication, and repair, work as an enzymatic co-factor, and enhance immune functioning | Nicholson et al[47] |
| Ethanol | Protein fermentation metabolism may be involved in NAFLD progression | Yao et al[48] |
| Hydrogen sulfide | Reduction/neutralization of reactive oxygen species | Afanas'ev et al[49] |
IPA: Indole-3-propionic acid; NAFLD: Non-alcoholic fatty liver disease.
Structural function
Under normal conditions, the microbiome contributes to maintaining the integrity of the gut epithelium. In this condition, cytokines present in the gut lumen do not pass through the gut epithelium. This function may be altered by pathogens such as E. coli and C. difficile. The dysbiosis produced by these bacteria facilitates the back diffusion of cytokines[50,51].
Protective function
The intestinal surface represents an important barrier, and the microbiome contributes to its stability[52]. The production of short-chain fatty acids (SCFAs) by the microbiome provides further energy for the epithelium and strengthens this barrier[53,54]. Table 4 shows examples of gut microbiome-derived metabolites and their beneficial effects on healthy conditions. In order of the metabolites, the pathway involved, the microbial agent responsible, and the health benefits produced, this information is shown in Table 4[55-70].
Table 4.
Examples of gut microbiota-derived metabolites and their beneficial effects on human health
|
Metabolite
|
Pathway
|
Microbial agent
|
Health benefits
|
| Butyrate | Carbohydrate metabolism | Clostridia; Faecalibacterium prausnitzii; Coprococcus catus; Anaerostipes hadrus | Increased intestinal barrier function; Modulate intestinal macrophage function; Suppression of colonic inflammation; Improvements in insulin sensitivity |
| Propionate | Carbohydrate metabolism | Blautia obeum; Coprococcus catus; Roseburia inulinivorans; Prevotella copri | Suppression of colonic inflammation; Decreased innate immune response to microbial stimulation; Protection from allergic airway inflammation; Improvements in insulin sensitivity and weight control in obese mice |
| Indole | Tryptophan metabolism | Lactobacillus; Bifinobacterium longum; Bacteroides fragilis | Maintenance of host-microbe homeostasis at mucosal surfaces via IL-22; Increased barrier function; Modulation of host metabolism |
| Indole-3-aldehyde | Tryptophan metabolism | Lactobacillus | Maintenance of mucosal homeostasis and intestinal barrier function via increased IL-22 production; Protection against intestinal inflammation in mouse models of colitis |
| Indole-3-propionate | Tryptophan metabolism | Clostridium sporogenes | Maintenance of intestinal barrier function and mucosal homeostasis; Increased production of antioxidant and neuroprotectant products |
| 10-hydroxy-cis-12-octadecoate | Linoleic acid derivative (lipid metabolism) | Lactobacillus | Maintenance of intestinal barrier function; Decreased inflammation; Increased intestinal IgA production |
IgA: Immunoglobulin A; IL-22: Interleukin 22.
Relationship between the gut microbiome and immune system
The gut microbiome has important effects on the local and general immune systems. The local gut microbiome drives the maturation of gut-associated lymphoid tissue (GALT)[71] and maintains barrier function by mucus production and antimicrobial peptide production. In addition, GALT has specific influences on inflammatory vs no inflammatory cell phenotypes. One-sixth of the cells of the gut epithelium are represented by T lymphocytes. In addition, B lymphocytes, dendritic cells, and plasma cells are present in lymphoid tissue, principally in the colon mucosa or in Peyer’s patches[72,73].
Indigenous microbiota or pathobionts may differentiate T helper (Th) cells into Th1, Th2, Th17, and regulatory T (Treg) cells via the production of microbiota metabolites. Filamentous bacteria induce the growth of Th17 and Th1; Clostridia stimulate Tregs and the anti-inflammatory cytokine interleukin 10 (IL-10). B. fragilis stimulates IL-10 and Tregs. By contrast, excessive stimulation of Th1 and Th2 cells due to the condition of dysbiosis may cause excessive production of proinflammatory cytokines[74]. Intestinal colonization has an important role in the development of tolerance[75]. This fact is relevant in the prevention of immune-mediated diseases. Indeed, the loss of immune tolerance may cause the development of allergic diseases or autoimmune diseases. Dysbiosis caused by E. coli or C. difficile is associated with eczema or atopic dermatitis. The production of SCFAs has a double effect. They constitute the main energy substrate for enterocytes and stimulate the maturation and correct function of Tregs[76]. Several axes have been established between the gut microbiome and different organs. In the case of dysbiosis they can generate diseases. The gut-brain axis may generate stress, anxiety, depression, schizophrenia, cognitive decline, and autism. The gut-brain endocrine axis generates regulatory, metabolic, behavioral, and hormonal disorders. The gut-heart axis generates cardiovascular diseases, atherosclerosis, thrombotic events, and hypertension. The gut-lung axis generates chronic obstructive pulmonary disease. The gut-liver axis generates liver inflammation, hepatocellular carcinoma, and non-alcoholic fatty liver. The gut-pancreas axis generates diabetes and pancreas cell inflammation. The gut-bone axis generates bone demineralization and osteoporosis. The gut-muscle axis generates muscle impairment, fragility, and sarcopenia. The gut-skin axis generates acne, psoriasis, atopic dermatitis, wrinkles, and aging. The gut-reproductive axis generates infertility, ovarian dysfunction, ovarian cancer, and postmenopausal osteoporosis. The gut-kidney axis generates chronic kidney disease, acute kidney injury, inflammation, nephrolithiasis, and nephropathy. The gut-bladder axis generates urinary tract infection and an overactive painful bladder. Some of these axes will be discussed below.
Gut microbiome and the brain
Recently, significant studies have documented the existence of the so-called “gut-brain axis.” This is a bidirectional communication system between the gut and brain. On the one hand, the brain may control gastrointestinal functions such as peristalsis, mucus production, and the gut immune system[77]. On the other hand, the gut microbiome releases SCFAs and other metabolites influencing brain function, whereas several neurotransmitters are involved in the bidirectional communication between the host and the microbiota[78].
The gut microbiome may affect the brain directly by the gut nervous system, sending signals to the brain or indirectly by the production of intestinal hormones or transforming diet components into substances such as SCFAs, neurotransmitters such as serotonins, gamma amino butyric acid (GABA), tryptophan, and vitamins that influence the blood-brain barrier (BBB) and cerebral functions[79].
In healthy conditions, bacteria such as Lactobacillus, Bifidobacterium, Enterococcus, and Streptococcus are among the principal producers of neurotransmitters[80,81]. In addition, SCFAs affect the BBB, and several other immune pathways affect behavior, memory, and locomotion[82,83].
It should also be highlighted that the Mediterranean diet rich in vegetables and fibers stimulates the activity and growth of beneficial bacteria for the brain[84]. More than 20% of patients with gut dysbiosis are affected by sleep disorders and depression[85,86].
Similarly, recent studies have documented that microbiota composition differs significantly between healthy controls and patients affected by neurovegetative disorders such as multiple sclerosis (MS), Alzheimer’s disease (AD), and Parkinson’s disease (PD)[87]. In MS, a higher abundance of Firmicutes and the absence of Fusobacteria are frequently found[88]. The stool microbial profile of AD patients has a decreased number of Firmicutes and Actinobacteria. Firmicutes, such as Ruminococcoceae and Turicibacteriaceae, are less abundant in these patients[89]. PD patients have a lower production of SCFAs and fewer gut bacteria, such as Lechnospiraceae and Faecalibacterium prausnitzii producing these substances[90].
In conclusion, several recent studies have documented that a different composition of the gut microbiome may contribute to the development of neurodegenerative disorders causing chronic inflammation of neuronal cells and loss of the BBB.
MICROBIOME IN HEALTH AND DISEASE
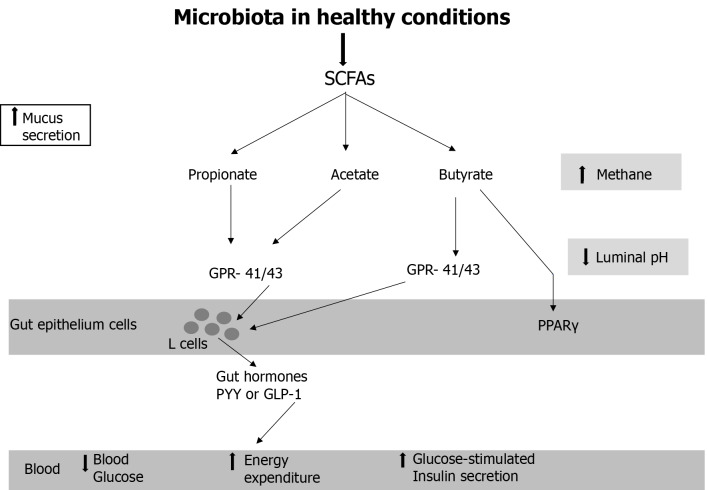
Table 4 shows the gut microbiome in healthy conditions. A metabolically healthy microbiota (mainly achieved by a high fiber, low animal fat, and low protein diet and other aforementioned environmental factors) is shown in Figure 1. Microbial production of SCFAs provides an energy source for colonocytes and causes a decrease in luminal pH. The SCFAs acetate, butyrate, and propionate can bind to the G protein-coupled receptor 41 (GPR41) and GPR43, which are expressed on enteroendocrine L cells, and subsequently induce the secretion of glucagon-like peptide 1 and peptide YY, which contribute to increased energy expenditure, reduced food intake, and improved glucose metabolism and insulin secretion[91]. Butyrate is an activator of peroxisome proliferator-activated receptor gamma and a stimulator of β-oxidation and oxygen consumption in the gut, which maintains an anaerobic environment in the gut lumen[92].
Figure 1.
Microbiota in healthy conditions. GLP-1: Glucagon-like peptide 1; GPCR: G protein-coupled receptor; PPARγ: Peroxisome proliferator-activated receptor gamma; PYY: Peptide YY; SCFA: Short chain fatty acid.
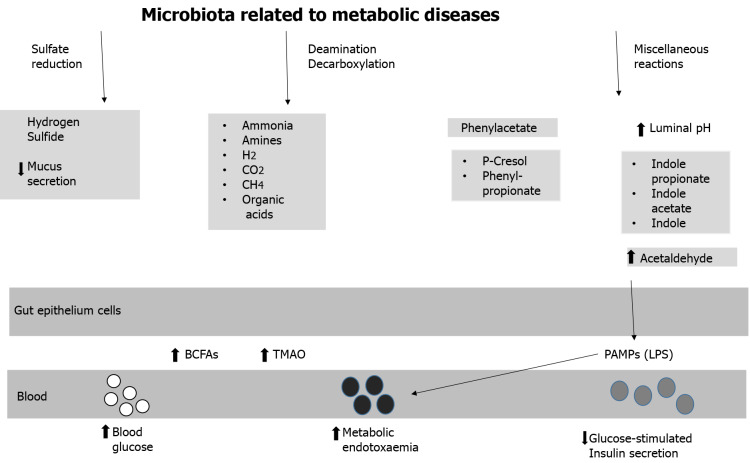
Pathobionts often responsible for dysbiosis are shown in Table 5. Figure 2 shows the metabolic pathways induced by gut dysbiosis. A dysbiotic microbiota is often associated with a prolonged colonic transit time, resulting in a shift in colonic metabolism leading to increased microbial proteolysis. Even though the preferred substrate for bacterial fermentation is fermentable dietary fibers, bacteria will not switch to protein metabolism until fermentable polysaccharides are depleted. As a result of increased protein fermentation, branched-chain fatty acids (2-methylbutirate, isobutyrate, and isovalerate), trimethylamine, organic acids, gases, and trace amounts of phenols, amines, indoles, and ammonia are produced, causing an increase in luminal pH[93]. Together, such changes in the microbial environment and metabolites cause leakage of pathogen-associated molecular patterns, including lipopolysaccharides (LPS), which increase in the blood and trigger systemic low-grade inflammation and insulin resistance[94]. It should be noted, however, that some indole derivatives, such as 3-indolepropionic acid, produced by the fermentation of dietary fibers have been shown to improve glucose metabolism[95].
Table 5.
Some examples of potentially harmful gut microbiota bacterial species
|
Bacteria
|
Associated physiologic changes
|
Associated diseases states
|
| Bacteroides | Activate CD4+ T cells | Increased with animal-based diet; Increased in obesity |
| Bilophila | Promote pro-inflammatory immunity | Increased in colitis; Decreased in autism |
| Clostridium | Promote generation Th17 cells | Increased after smoke exposure; Increased in autism and Rett syndrome; Positive correlation with plasma insulin and weight gain; Increased in type 2 diabetes; Clostridium perfrigens increased in old age |
| Escherichia coli | TLR activation | Increased in inflammatory bowel disease; Increased in type 2 diabetes |
| Neisseria | Sugar fermentation | Only two species are pathogenic: Neisseria meningitides and Neisseria gonorrhoeae |
Th17: T helper 17: TLR: Toll-like receptor.
Figure 2.
Microbiota related to metabolic diseases. BCFA: Branched-chain fatty acid; LPS: Lipopolysaccharides; PAMP: Pathogen-associated molecular pattern; TMAO: Trimethilamine oxide.
DISEASES ASSOCIATED OR RELATED TO DYSBIOSIS
Some diseases associated with gut microbiota abnormalities are shown in Table 6. Neurological diseases associated with gut dysbiosis have already been discussed. Other diseases associated with gut dysbiosis are allergic diseases, inflammatory bowel syndromes or diseases, and metabolic diseases.
Table 6.
Diseases associated with gut microbiota abnormalities
|
Disease
|
Features
|
| Irritable bowel syndrome | An abundance of Firmicutes and a decrease of Bacteroidetes |
| Type I diabetes | In genetically predisposed individuals, autoimmune against pancreatic β-cells. Deficient development or alteration of the microbiota may contribute to dysfunctional immunity with the devastation of autoimmune β-cells and increased leakiness of the intestinal barrier. Variability of microbiomes reduced |
| Asthma | Outbreaks of Chlamidophila pneumonia during bronchitis and pneumonia development affect the airway microbiome. Gut microbiota is influenced by the introduction of microbiota to the environment, particularly in early life, which helps immune function growth and the development of defending against allergic sensitization |
| Food-borne pathogens and food poisoning | Opportunistic pathogens (Campylobacter, Salmonella, Escherichia coli, Shigella) disturb the microbiome’s balance leading to dysbiosis |
| Malnutrition | Decrease or missing species that either process food categories efficiently or produce vitamins may reduce the absorption of nutrients. An overabundance of Enetrobacteriaceae can lead to epithelial damage, diarrhea, and limited absorption of nutrients |
| Depression | In physiologic system, Bifidobacterium infantis, generally found in infants’ gastrointestinal tract and administered probiotic drugs, can have antidepressant effects |
| Anxiety | Oral administration of Campylobacter jejuni subclinical doses in murine models induced anxiety like behavior without stimulating immunity |
Allergic diseases: Gut microbiota dysbiosis is reportedly associated with allergic diseases such as eczema and asthma[96]. Lower levels of gut S. aureus and Clostridium and higher levels of Bifidobacterium are present in children affected by allergic symptoms[97]. The cause has been ascribed to LPS that cause a reduced immune system response[98]. The relationship of gut dysbiosis and asthma or other lung syndromes justifies the term “gut-lung axis”[99].
Irritable and inflammatory bowel syndromes: Irritable bowel syndrome (IBS) is a condition affecting 10% to 20% of adults, and children may also be affected. Bennet et al[100] described alterations in the gut microbiome of patients affected by IBS. Firmicutes are increased with a reduction in Ruminococcus and B. fragilis. The result is an excessive increase in SCFAs with consequent increase in serotonin that alters intestinal motility. Inflammatory bowel disease (IBD) is a more severe condition. The most significant types of IBD are ulcerative colitis and Crohn’s disease[101]. Several types of gut dysbiosis have been found in patients affected by IBD. Bacteroidetes and Firmicutes are decreased[102]. Recently Zhu et al[103] found an increase in Proteobacteria and E. coli. One of the consequences of such dysbiosis is a reduction in mucus production that allows gut flora to pass more easily across the intestinal barrier, thus enhancing the inflammatory process[104].
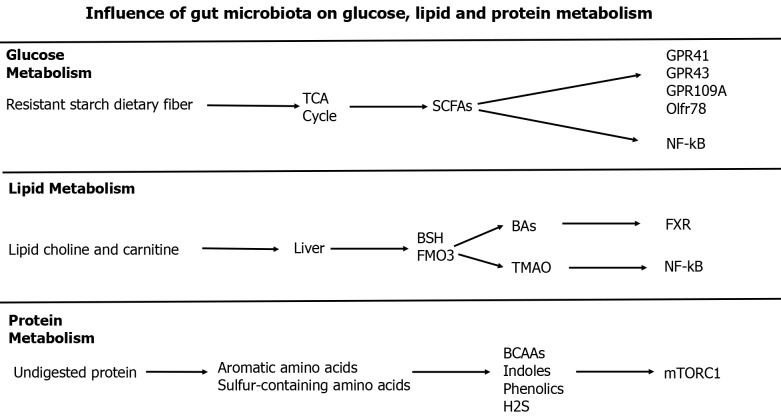
Gut dysbiosis and metabolic diseases: The gut microbiota participates in material metabolism to produce metabolites. The gut microbiota can affect the metabolism of glucose, lipids, and proteins by generating a series of metabolites and activating downstream signaling pathways as shown in Figure 3.
Figure 3.
Influence of gut microbiota on glucose, lipid, and protein metabolism. BCAAs: Branched-chain amino acids; BSH: Bile salt hydrolase; FMO3: Flavin monooxigenase 3; FXR: Farnesoid X receptor; GPR: G protein-coupled receptor; Olftr: Olfactory receptor 78; mTORC1: Mammalian target of rapamycin complex 1; NF-kB: Nuclear factor kappa B; SCFAs: Short-chain fatty acids; TGR5: Takeda G protein-coupled receptor 5; TMAO: Trimethylamine N-oxide.
Obesity: In addition to genetic and behavioral factors, the gut microbiome has an important role in the genesis of obesity[105]. Obese patient gut flora have higher levels of Firmicutes and lower levels of Bacteroidetes[106]. Other bacteria found in the gut of obese patients are Bacteroides, Ruminococcus, and Staphylococcus[107]. These bacteria cause the increased degradation of β-glucuronide and aromatic amino acids, higher generation of organic acids and H2, and higher biosynthesis of phenylalanine, tyrosine, and tryptophan[108]. In this condition, chronic inflammation is generated by the production of IL-1, tumor necrosis factor alpha, monocyte chemoattractant protein-1, and IL-6. In addition, LPS are produced that bind to the cluster of differentiation 14 receptor on the surface of immune cells to produce further inflammatory factors[109,110].
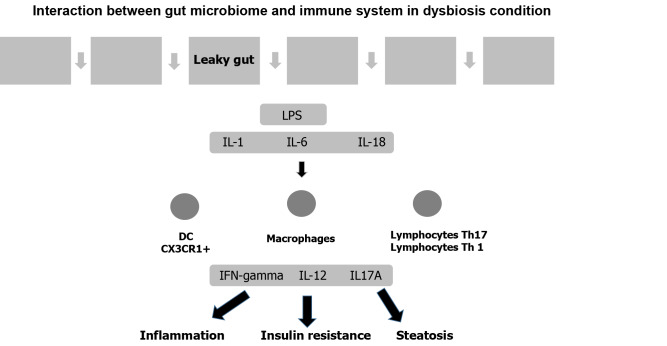
Type 2 diabetes mellitus: Several studies have documented the influence of the gut microbiome on the pathophysiology of type 2 diabetes mellitus. In this condition, pathogenic flora prevail over protective flora. Gurung et al[111] analyzed 42 studies and found that the protective bacteria were Bifidobacterium and Bacteroides as well as Rosaburia and Faecalibacterium. By contrast, Ruminococcus, Fusobacterium, and Blautia are pathobionts that, through the production of LPS, increase the permeability of the intestinal epithelium. In this condition, inflammatory molecules are produced, which increases insulin resistance (Figure 4)[112]. By contrast, protective flora produce IL-10 and other anti-inflammatory cytokines. In addition, protective flora exert their effect through the production of SCFAs and butyrate[113]. SCFAs also act as substrates for lipogenesis and gluconeogenesis in the liver.
Figure 4.
Interaction between gut microbiome and immune system in dysbiosis condition. DC: Dendritic cell; IFN-γ: Interferon gamma; IL-1: Interleukin 1; IL-6: Interleukin 6; IL-18: Interleukin 18; LPS: Lipopolysaccharides.
Heart disease: Recent studies have also allowed identified a heart-gut axis. As in similar conditions, the effects of this axis are bivalent. Indeed, on the one hand, heart failure induces an increase in permeability of the gut barrier (the so-called leaky gut) with consequent passage of gut microbiota and its products in the circulation inducing inflammation. On the other hand, several products of the gut microbiota entering the blood may induce hypertension, atherosclerosis, and heart failure. The gut flora that is able to induce cardiovascular diseases is composed as follows[108]. There is an increase in Enterobacteriaceae as E. coli and Klebsiella, and a decrease in Roseburia and F. prausnizii[114]. Several metabolic pathways are involved in the gut-heart axis: trimethylamine (TMA) and trimethylamine n-oxide (TMAO), SCFAs, and bile acids[115]. Dietary sources including choline and l-carnitine provide substrates for microbiota-mediated production of trimethylamine. TMA after entering the portal circulation is converted by the hepatic host flavin-containing monooxygenase to TMAO. TMAO can promote atherogenesis and heart failure development. Adverse cardiac remodeling is also associated with elevated TMAO levels[116-118]. A beneficial effect is exerted by SCFAs. SCFAs improve intestinal barrier function by promoting mucous production. In addition, they improve vascular tone through G protein-coupled receptor signaling. Finally, SCFAs activate histone acetyltransferase and inhibit histone deacetylase, thereby inhibiting inflammation and modulating immune cell activation[119].
Bile acids: Bile acids may be modified by the microbiome. They are produced by the liver, and a small portion are metabolized by colon microbiota with the production of secondary bile acids such as deoxycholate, lithocholate, and ursodeoxycholate. These secondary bile acids are powerful agonists of the farnesoid nuclear receptor (FXR), which modulates metabolism and inflammation. Therefore, bile acids inhibit the anti-inflammatory activity of FXR. At the cardiovascular level, bile acids favor atherosclerotic disease, cardiac hypertrophy and hypertension[120].
All of these studies are important, but often they are conflicting or have some drawbacks. Some examples are as follows.
The Firmicutes/Bacteroidetes ratio is cited in several studies as an important marker of health or disease; however, several of these studies have relevant bias due to methodological procedures or discrepancies in enrolling subjects for the study.
Modification in gut microbiota seems to cause irritable bowel disease, but the microbiota responsible and their metabolites are poorly understood. The same is true for the interrelation between not well defined microbiota and brain diseases such as PD and autism. Again, methodological problems and presence of confounders may be the cause.
More importantly, these problems concern the treatment. Indeed, meta-analyses on the use of prebiotics and probiotics have given contradictory results and to date, the majority of authors retain that large-scale randomized controlled studies are needed to evaluate the efficacy of these treatments.
TREATMENT AND PERSPECTIVES
Dysbiosis treatment is based on the use of prebiotics or probiotics. In addition, fecal microbiota transplantation has been proposed. Prebiotics are dietary products that may change the composition and functions of microbiota by enhancing the presence of indigenous microbiota or by favoring the growth of specific bacteria. Probiotics are live beneficial bacteria. Among these favorable effects to re-equilibrate, gut microbiota dysbiosis has been documented by Akkermansia muciniphila, F. prausnizii, B. uniformis, predator bacteria, and phage therapy[8]. Fecal microbiota transplantation is the process of transplantation of fecal microorganisms from healthy people to re-equilibrate gut microbiota dysbiosis. In the future we can imagine the use of sequestrate or binding resins to eliminate harmful products or to sequestrate microbial metabolites. Other approach is the use of 3, 3-dimethyl-1-butanol to reduce TMAO or TMA-lyase inhibitors. Overall, several problems remain to be resolved.
There is substantial heterogeneity in the microbiota in both normal conditions and dysbiosis. In general, there is not a single bacterium but a collection of bacteria. This collection will likely not be best defined by the individual bacteria but rather by their metabolic capacities. This refers to the enzymes that are expressed, functioning and determining downstream metabolism.
CONCLUSION
The human gut possesses millions of microorganisms that is called microbiota. Microbiota in normal conditions exerts beneficial effects over the whole body and is connected with many organs forming different axis. Several factors may modify the microbiota composition and favor the presence of dangerous microorganisms, better known as pathobionts. This condition is called dysbiosis and is linked with different diseases, such as neurological diseases, metabolic diseases, and circulatory diseases. An understanding of microbiota in both the healthy subject and sick patient and understanding of the biological pathway connecting the gut microbiota with different organs are essential to finding therapeutic measures. The use of probiotics, prebiotics, phages, and feces transplantation represent to date the therapeutic measures more frequently adopted. However, several trials are ongoing looking for new, more efficient therapeutic strategies.
Future directions, among others could be as follows: The development of multiomics techniques such as metagenomics, metabolomics, and metatranscriptomes should be further developed to answer the critical questions of which microbiota are involved in health and disease. Indeed, in addition to metagenomics, the microbiome should also be analyzed by metabolomics studies in order to find its metabolic organization.
In addition, studies on genome-wide association will be used to correlate different genotypes with diseases phenotypes, and studies of metabolome wide association will correlate metabolic phenotypes with disease phenotypes.
The key findings of our study were as follows: (1) In normal conditions, the gut microbiome exerts a beneficial effect on the organism. (2) This is related to the production of several metabolites. (3) Several axis connect the microbiome with several organs. (4) Gut microbiome modifications with the appearance of pathobionts are at the basis of several diseases in different organs.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 23, 2023
First decision: December 6, 2023
Article in press: January 27, 2024
Specialty type: Medicine, general and internal
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fu M, China; Meng Y, China; Qureshi W, India S-Editor: Liu JH L-Editor: Filipodia P-Editor: Guo X
Contributor Information
Maurizio Salvadori, Department of Renal Transplantation, Careggi University Hospital, Florence 50139, Tuscany, Italy. maurizio.salvadori1@gmail.com.
Giuseppina Rosso, Division of Nephrology, San Giovanni di Dio Hospital, Florence 50143, Toscana, Italy.
References
- 1.Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature. 2019;569:641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R Earth Microbiome Project Consortium. A communal catalogue reveals Earth's multiscale microbial diversity. Nature. 2017;551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthijs G, Souche E, Alders M, Corveleyn A, Eck S, Feenstra I, Race V, Sistermans E, Sturm M, Weiss M, Yntema H, Bakker E, Scheffer H, Bauer P EuroGentest; European Society of Human Genetics. Guidelines for diagnostic next-generation sequencing. Eur J Hum Genet. 2016;24:2–5. doi: 10.1038/ejhg.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Li D, Liu YX. Microbiome research outlook: past, present, and future. Protein Cell. 2023;14:709–712. doi: 10.1093/procel/pwad031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M, Eggersdorfer M. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, Nelson KE, White O, Methé BA, Huttenhower C. The Human Microbiome Project: a community resource for the healthy human microbiome. PLoS Biol. 2012;10:e1001377. doi: 10.1371/journal.pbio.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIH HMP Working Group. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. The NIH Human Microbiome Project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. doi: 10.1007/s10482-020-01474-7. [DOI] [PubMed] [Google Scholar]
- 10.Ray K. Gut microbiota: Filling up on fibre for a healthy gut. Nat Rev Gastroenterol Hepatol. 2018;15:67. doi: 10.1038/nrgastro.2018.2. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359:1151–1156. doi: 10.1126/science.aao5774. [DOI] [PubMed] [Google Scholar]
- 12.Iebba V, Totino V, Gagliardi A, Santangelo F, Cacciotti F, Trancassini M, Mancini C, Cicerone C, Corazziari E, Pantanella F, Schippa S. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39:1–12. [PubMed] [Google Scholar]
- 13.Chen C, Huang X, Fang S, Yang H, He M, Zhao Y, Huang L. Contribution of Host Genetics to the Variation of Microbial Composition of Cecum Lumen and Feces in Pigs. Front Microbiol. 2018;9:2626. doi: 10.3389/fmicb.2018.02626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes RL. A Review of the Role of the Gut Microbiome in Personalized Sports Nutrition. Front Nutr. 2019;6:191. doi: 10.3389/fnut.2019.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- 16.Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB, Wood-Martin R, Kerins DM, Quigley E, Ross RP, O'Toole PW, Molloy MG, Falvey E, Shanahan F, Cotter PD. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913–1920. doi: 10.1136/gutjnl-2013-306541. [DOI] [PubMed] [Google Scholar]
- 17.Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, Ahmadi-Vand Z, Marsden KR, Gibson DL. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biedermann L, Zeitz J, Mwinyi J, Sutter-Minder E, Rehman A, Ott SJ, Steurer-Stey C, Frei A, Frei P, Scharl M, Loessner MJ, Vavricka SR, Fried M, Schreiber S, Schuppler M, Rogler G. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS One. 2013;8:e59260. doi: 10.1371/journal.pone.0059260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biedermann L, Brülisauer K, Zeitz J, Frei P, Scharl M, Vavricka SR, Fried M, Loessner MJ, Rogler G, Schuppler M. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014;20:1496–1501. doi: 10.1097/MIB.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 20.Klingensmith NJ, Coopersmith CM. The Gut as the Motor of Multiple Organ Dysfunction in Critical Illness. Crit Care Clin. 2016;32:203–212. doi: 10.1016/j.ccc.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramnani P, Chitarrari R, Tuohy K, Grant J, Hotchkiss S, Philp K, Campbell R, Gill C, Rowland I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe. 2012;18:1–6. doi: 10.1016/j.anaerobe.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Reading) 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 23.Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5:e9836. doi: 10.1371/journal.pone.0009836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaac S, Scher JU, Djukovic A, Jiménez N, Littman DR, Abramson SB, Pamer EG, Ubeda C. Short- and long-term effects of oral vancomycin on the human intestinal microbiota. J Antimicrob Chemother. 2017;72:128–136. doi: 10.1093/jac/dkw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D'hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 27.Jackson MA, Verdi S, Maxan ME, Shin CM, Zierer J, Bowyer RCE, Martin T, Williams FMK, Menni C, Bell JT, Spector TD, Steves CJ. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9:2655. doi: 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Pedersen HK, Arumugam M, Kristiansen K, Voigt AY, Vestergaard H, Hercog R, Costea PI, Kultima JR, Li J, Jørgensen T, Levenez F, Dore J MetaHIT consortium, Nielsen HB, Brunak S, Raes J, Hansen T, Wang J, Ehrlich SD, Bork P, Pedersen O. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 32.Imhann F, Vich Vila A, Bonder MJ, Lopez Manosalva AG, Koonen DPY, Fu J, Wijmenga C, Zhernakova A, Weersma RK. The influence of proton pump inhibitors and other commonly used medication on the gut microbiota. Gut Microbes. 2017;8:351–358. doi: 10.1080/19490976.2017.1284732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 34.Rawlings ND, Barrett AJ, Thomas PD, Huang X, Bateman A, Finn RD. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018;46:D624–D632. doi: 10.1093/nar/gkx1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portune KJ, Benítez-Páez A, Del Pulgar EM, Cerrudo V, Sanz Y. Gut microbiota, diet, and obesity-related disorders-The good, the bad, and the future challenges. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201600252. [DOI] [PubMed] [Google Scholar]
- 36.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J MetaHIT Consortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, Hassoun A, Pateiro M, Lorenzo JM, Rusu AV, Aadil RM. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 2022;13:999001. doi: 10.3389/fmicb.2022.999001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramírez-Macías I, Orenes-Piñero E, Camelo-Castillo A, Rivera-Caravaca JM, López-García C, Marín F. Novel insights in the relationship of gut microbiota and coronary artery diseases. Crit Rev Food Sci Nutr. 2022;62:3738–3750. doi: 10.1080/10408398.2020.1868397. [DOI] [PubMed] [Google Scholar]
- 40.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–429. doi: 10.1038/ijo.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mischke M, Plösch T. The Gut Microbiota and their Metabolites: Potential Implications for the Host Epigenome. Adv Exp Med Biol. 2016;902:33–44. doi: 10.1007/978-3-319-31248-4_3. [DOI] [PubMed] [Google Scholar]
- 42.Hendrikx T, Schnabl B. Indoles: metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J Intern Med. 2019;286:32–40. doi: 10.1111/joim.12892. [DOI] [PubMed] [Google Scholar]
- 43.Lightfoot YL, Yang T, Sahay B, Mohamadzadeh M. Targeting aberrant colon cancer-specific DNA methylation with lipoteichoic acid-deficient Lactobacillus acidophilus. Gut Microbes. 2013;4:84–88. doi: 10.4161/gmic.22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Smallwood T, Allayee H, Bennett BJ. Choline metabolites: gene by diet interactions. Curr Opin Lipidol. 2016;27:33–39. doi: 10.1097/MOL.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 48.Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43:181–196. doi: 10.1111/apt.13456. [DOI] [PubMed] [Google Scholar]
- 49.Afanas'ev I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. 2014;5:52–62. doi: 10.14336/AD.2014.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, Tamura A, Tsukita S, Fujiyoshi Y. Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science. 2015;347:775–778. doi: 10.1126/science.1261833. [DOI] [PubMed] [Google Scholar]
- 51.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 52.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 53.Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT, Garcia-Ródenas CL, Wells JM. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr. 2017;117:93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH, Bhutani T, Liao W. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Nguyen V, Taylor CT, Colgan SP. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simeoli R, Mattace Raso G, Pirozzi C, Lama A, Santoro A, Russo R, Montero-Melendez T, Berni Canani R, Calignano A, Perretti M, Meli R. An orally administered butyrate-releasing derivative reduces neutrophil recruitment and inflammation in dextran sulphate sodium-induced murine colitis. Br J Pharmacol. 2017;174:1484–1496. doi: 10.1111/bph.13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan S, Jena G. Sodium butyrate reduces insulin-resistance, fat accumulation and dyslipidemia in type-2 diabetic rat: A comparative study with metformin. Chem Biol Interact. 2016;254:124–134. doi: 10.1016/j.cbi.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Tong LC, Wang Y, Wang ZB, Liu WY, Sun S, Li L, Su DF, Zhang LC. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front Pharmacol. 2016;7:253. doi: 10.3389/fphar.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciarlo E, Heinonen T, Herderschee J, Fenwick C, Mombelli M, Le Roy D, Roger T. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci Rep. 2016;6:37944. doi: 10.1038/srep37944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 62.den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, Bakker BM. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes. 2015;64:2398–2408. doi: 10.2337/db14-1213. [DOI] [PubMed] [Google Scholar]
- 63.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9:1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang IK, Yoo KY, Li H, Park OK, Lee CH, Choi JH, Jeong YG, Lee YL, Kim YM, Kwon YG, Won MH. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J Neurosci Res. 2009;87:2126–2137. doi: 10.1002/jnr.22030. [DOI] [PubMed] [Google Scholar]
- 69.Miyamoto J, Mizukure T, Park SB, Kishino S, Kimura I, Hirano K, Bergamo P, Rossi M, Suzuki T, Arita M, Ogawa J, Tanabe S. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J Biol Chem. 2015;290:2902–2918. doi: 10.1074/jbc.M114.610733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaikiri H, Miyamoto J, Kawakami T, Park SB, Kitamura N, Kishino S, Yonejima Y, Hisa K, Watanabe J, Ogita T, Ogawa J, Tanabe S, Suzuki T. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/nga mice. Int J Food Sci Nutr. 2017;68:941–951. doi: 10.1080/09637486.2017.1318116. [DOI] [PubMed] [Google Scholar]
- 71.Mörbe UM, Jørgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, Agace WW. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021;14:793–802. doi: 10.1038/s41385-021-00389-4. [DOI] [PubMed] [Google Scholar]
- 72.Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17:323–333. [PubMed] [Google Scholar]
- 73.Rescigno M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. 2014;16:1004–1013. doi: 10.1111/cmi.12301. [DOI] [PubMed] [Google Scholar]
- 74.Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473–493. doi: 10.1007/s00018-018-2943-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker WA. The importance of appropriate initial bacterial colonization of the intestine in newborn, child, and adult health. Pediatr Res. 2017;82:387–395. doi: 10.1038/pr.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohajeri MH, La Fata G, Steinert RE, Weber P. Relationship between the gut microbiome and brain function. Nutr Rev. 2018;76:481–496. doi: 10.1093/nutrit/nuy009. [DOI] [PubMed] [Google Scholar]
- 79.Rosario D, Boren J, Uhlen M, Proctor G, Aarsland D, Mardinoglu A, Shoaie S. Systems Biology Approaches to Understand the Host-Microbiome Interactions in Neurodegenerative Diseases. Front Neurosci. 2020;14:716. doi: 10.3389/fnins.2020.00716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Matsunami RK, Lugo M, Major A, Mori-Akiyama Y, Hollister EB, Dann SM, Shi XZ, Engler DA, Savidge T, Versalovic J. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ribeiro M, Brigas HC, Temido-Ferreira M, Pousinha PA, Regen T, Santa C, Coelho JE, Marques-Morgado I, Valente CA, Omenetti S, Stockinger B, Waisman A, Manadas B, Lopes LV, Silva-Santos B, Ribot JC. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aay5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singhal G, Jaehne EJ, Corrigan F, Baune BT. Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front Cell Neurosci. 2014;8:97. doi: 10.3389/fncel.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Redondo-Useros N, Nova E, González-Zancada N, Díaz LE, Gómez-Martínez S, Marcos A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients. 2020;12 doi: 10.3390/nu12061776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ, Parikh E, Lopez JV, Tartar JL. Gut microbiome diversity is associated with sleep physiology in humans. PLoS One. 2019;14:e0222394. doi: 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Limbana T, Khan F, Eskander N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus. 2020;12:e9966. doi: 10.7759/cureus.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cryan JF, O'Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 88.Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, Morita H, Hattori M, Yamamura T. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10:e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Gut microbiome alterations in Alzheimer's disease. Sci Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 91.Holz GG 4th, Kühtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Byndloss MX, Olsan EE, Rivera-Chávez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Bäumler AJ. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57:523–535. doi: 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- 94.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 95.de Mello VD, Paananen J, Lindström J, Lankinen MA, Shi L, Kuusisto J, Pihlajamäki J, Auriola S, Lehtonen M, Rolandsson O, Bergdahl IA, Nordin E, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Landberg R, Eriksson JG, Tuomilehto J, Hanhineva K, Uusitupa M. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017;7:46337. doi: 10.1038/srep46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alashkar Alhamwe B, López JF, Zhernov Y, von Strandmann EP, Karaulov A, Kolahian S, Geßner R, Renz H. Impact of local human microbiota on the allergic diseases: Organ-organ interaction. Pediatr Allergy Immunol. 2023;34:e13976. doi: 10.1111/pai.13976. [DOI] [PubMed] [Google Scholar]
- 97.O'Connor GT, Lynch SV, Bloomberg GR, Kattan M, Wood RA, Gergen PJ, Jaffee KF, Calatroni A, Bacharier LB, Beigelman A, Sandel MT, Johnson CC, Faruqi A, Santee C, Fujimura KE, Fadrosh D, Boushey H, Visness CM, Gern JE. Early-life home environment and risk of asthma among inner-city children. J Allergy Clin Immunol. 2018;141:1468–1475. doi: 10.1016/j.jaci.2017.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gehring U, Bolte G, Borte M, Bischof W, Fahlbusch B, Wichmann HE, Heinrich J LISA study group. Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood. Exposure to endotoxin decreases the risk of atopic eczema in infancy: a cohort study. J Allergy Clin Immunol. 2001;108:847–854. doi: 10.1067/mai.2001.119026. [DOI] [PubMed] [Google Scholar]
- 99.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos AM, Kunøe A, Fink NR, Chawes BL, Bønnelykke K, Brejnrod AD, Mortensen MS, Al-Soud WA, Sørensen SJ, Bisgaard H. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9:141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bennet SM, Ohman L, Simren M. Gut microbiota as potential orchestrators of irritable bowel syndrome. Gut Liver. 2015;9:318–331. doi: 10.5009/gnl14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schirmer M, Franzosa EA, Lloyd-Price J, McIver LJ, Schwager R, Poon TW, Ananthakrishnan AN, Andrews E, Barron G, Lake K, Prasad M, Sauk J, Stevens B, Wilson RG, Braun J, Denson LA, Kugathasan S, McGovern DPB, Vlamakis H, Xavier RJ, Huttenhower C. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nat Microbiol. 2018;3:337–346. doi: 10.1038/s41564-017-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lane ER, Zisman TL, Suskind DL. The microbiota in inflammatory bowel disease: current and therapeutic insights. J Inflamm Res. 2017;10:63–73. doi: 10.2147/JIR.S116088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu W, Winter MG, Byndloss MX, Spiga L, Duerkop BA, Hughes ER, Büttner L, de Lima Romão E, Behrendt CL, Lopez CA, Sifuentes-Dominguez L, Huff-Hardy K, Wilson RP, Gillis CC, Tükel Ç, Koh AY, Burstein E, Hooper LV, Bäumler AJ, Winter SE. Precision editing of the gut microbiota ameliorates colitis. Nature. 2018;553:208–211. doi: 10.1038/nature25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parekh PJ, Balart LA, Johnson DA. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin Transl Gastroenterol. 2015;6:e91. doi: 10.1038/ctg.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villanueva-Millán MJ, Pérez-Matute P, Oteo JA. Gut microbiota: a key player in health and disease. A review focused on obesity. J Physiol Biochem. 2015;71:509–525. doi: 10.1007/s13105-015-0390-3. [DOI] [PubMed] [Google Scholar]
- 106.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 108.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 109.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 110.Vetrani C, Di Nisio A, Paschou SA, Barrea L, Muscogiuri G, Graziadio C, Savastano S, Colao A On Behalf Of The Obesity Programs Of Nutrition Education Research And Assessment Opera Group. From Gut Microbiota through Low-Grade Inflammation to Obesity: Key Players and Potential Targets. Nutrients. 2022;14 doi: 10.3390/nu14102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Wang S, Zhang Q, He C, Fu C, Wei Q. The role of the gut microbiota in health and cardiovascular diseases. Mol Biomed. 2022;3:30. doi: 10.1186/s43556-022-00091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe. 2018;23:41–53.e4. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137–154. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 120.Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]