Abstract
A heterologous feline immunodeficiency virus (FIV) expression system permitted high-level expression of FIV proteins and efficient production of infectious FIV in human cells. These results identify the FIV U3 element as the sole restriction to the productive phase of replication in nonfeline cells. Heterologous FIV expression in a variety of human cell lines resulted in profuse syncytial lysis that was FIV env specific, CD4 independent, and restricted to cells that express CXCR4, the coreceptor for T-cell-line-adapted strains of human immunodeficiency virus. Stable expression of human CXCR4 in CXCR4-negative human and rodent cell lines resulted in extensive FIV Env-mediated, CXCR4-dependent cell fusion and infection. In feline cells, stable overexpression of human CXCR4 resulted in increased FIV infectivity and marked syncytium formation during FIV replication or after infection with FIV Env-expressing vectors. The use of CXCR4 is a fundamental feature of lentivirus biology independent of CD4 and a shared cellular link to infection and cytopathicity for distantly related lentiviruses that cause AIDS. Their conserved use implicates chemokine receptors as primordial lentivirus receptors.
The nonprimate lentiviruses include the ungulate lentiviruses and feline immunodeficiency virus (FIV). FIV was discovered in 1986 as a cause of acquired immune deficiency and neurological disease in domestic cats (Felis catus) (31). FIV and human immunodeficiency virus type 1 (HIV-1) are the only lentiviruses that cause selective loss of the CD4+ T-cell subset and AIDS in naturally infected host species. However, in contrast to the primate lentiviruses, FIV does not use CD4 for entry and displays broader cellular tropism in vivo, infecting large numbers of B cells and CD8+ T cells as well as CD4+ T cells and macrophages (9, 26). Uncoupling of selective CD4 depletion from the use of the CD4 molecule for entry is one of the most interesting features of the FIV model because it suggests that there exist basic lentivirus pathogenetic pathways that are CD4 molecule independent yet affect the CD4+ T-cell subset preferentially. Primary cell surface receptors have not been established for any of the nonprimate lentiviruses (13, 44).
Nucleotide sequence comparisons indicate that FIV is more closely related to the ungulate lentiviruses than to HIV or simian immunodeficiency virus (28). Phylogenetic and epidemiological data also suggest an ancient evolutionary divergence of FIV from ancestors of the primate lentiviruses (1, 8, 11, 29, 39). While serological cross-reactivity between structural proteins of FIV and ungulate lentiviruses has been observed, none is detectable between FIV and the primate lentiviruses (14, 27, 39). In addition, FIV encodes a dUTPase, a fifth pol-encoded enzymatic activity that is found only in the nonprimate lentiviruses (42). Although FIV infects 2 to 20% of domestic-cat populations as well as many free-roaming nondomestic members of the family Felidae worldwide, there is no evidence for FIV infection of nonfelids (29, 30). Neither human seroconversion nor any other detectable evidence of human infection or disease occurs, despite frequent exposure of humans to FIV by biting, the principal route of natural feline transmission (sexual transmission does not occur to any extent) (30).
At the human cellular level, restrictions to both viral production and infection by nonprimate lentiviruses are evident, although the mechanisms are not well understood (12, 22, 40). Reported obstacles to FIV expression in human cells have included poor Rev function (40) and poor long terminal repeat (LTR) transcriptional activity (22, 35). Because of these blocks, expression of the nonprimate lentivirus Rev-dependent structural proteins in nonhost animal cells has received little investigation. In the present study, we developed an expression system which revealed that human cells support high-level production of Rev-dependent FIV structural proteins and of infectious virus when the transcriptional inactivity of the FIV LTR is bypassed through promoter (U3 element) substitution. In addition, these chimeric constructs produced profuse syncytia in human as well as feline cells, a surprising finding that prompted further investigation of the molecular basis for this broad cytopathicity.
Expressing FIV directly in cells of a given species avoids the potential ambiguities of studies employing interspecies coculture, including the identity of the cells undergoing fusion and the potentially confounding role of xenotropic retroviruses. For example, feline cells contain multiple copies of an inducible, xenotropic, replication-competent type C endogenous retrovirus (RD114) that is related at the nucleotide sequence level to a primate retrovirus (baboon endogenous virus), replicates in human cells, and phenotypically mixes with FIV and other retroviruses (20, 38).
MATERIALS AND METHODS
Cells, stable cell lines, and viruses.
Cell lines used were American Type Culture Collection lines propagated in Dulbecco minimal Eagle medium supplemented with glutamine, pyruvate, antibiotics, and 10% heat-inactivated fetal calf serum. Plasmid pZ.CXCR4 has the structure LTR-cxcr4-IRES-neo-LTR (where IRES is a poliovirus internal ribosome entry site) to permit selection for a bicistronic message encoding CXCR4 and neomycin phosphotransferase and was constructed by cloning the human CXCR4 cDNA (obtained from A. Gervaix) in place of the hygromycin resistance gene of retroviral vector pJZ308 (47). G418-stable lines were generated by transduction of the indicated cells with supernatant obtained from PA317 cells cotransfected with pZ.CXCR4 and a vesicular stomatitis virus glycoprotein G (VSV-G) expression plasmid, pHCMV-G, followed by selection and maintenance in medium containing G418 at 400 to 1,000 μg/ml. All lines were derived from at least 1,000 separate colonies. Infectious FIV was produced in 293T cells by calcium phosphate transfection of CT5 (10 μg/75-cm2 flask), harvesting at 24 to 48 h, centrifugation at 1,000 × g, and filtration through a 0.45-μm-pore-size filter. For production of FIV in Crandell feline kidney (CrFK) cells, chronic infection of CrFK cells was established by transfection with p34TF10 and passaging; supernatant was harvested, cleared, filtered (0.45-μm pore size), and stored at −80°C.
Chimeric FIV plasmid construction.
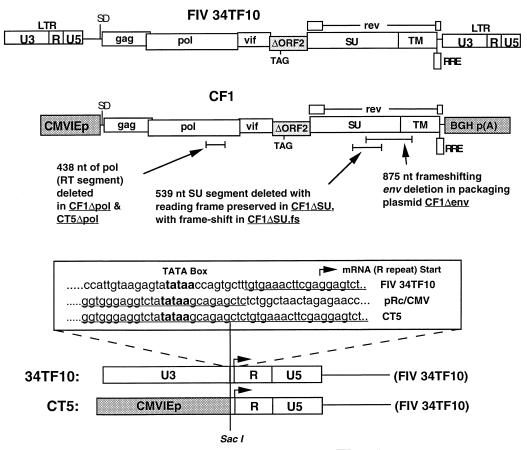
The numbering system used is based on that of Talbott et al. (39). CF1 was generated by blunt-end ligation of the SacI-EspI fragment of p34TF10 between the NotI and XbaI sites in the polylinker of the cytomegalovirus (CMV) expression plasmid pRc/CMV (Invitrogen). The 5′ junction is 97 nucleotides (nt) upstream of the major splice donor site. CF1 has been deleted of both LTRs except for the 89-nt portion of the 3′ U3 that overlaps rev and lacks the basis for reverse transcription and integration. CF1Δenv has an 875-nt deletion in env which spans the SU-TM junction and is also frameshifting (a SacII linker was inserted between the two PflMI sites of the subcloned env gene). CF1ΔSU and CF1ΔSU.fs are deleted of nt 1059 to 1596 of SU (encompassing the V3 and V4 hypervariable loops). To produce the fusion of the human CMV immediate-early promoter (hCMVIEp) to the FIV genome over the TATA box illustrated in Fig. 1, PCR was performed with a SacI-tailed sense PCR primer homologous to the nucleotides immediately downstream from the FIV TATA box (5′-ATATAGAGCTCTGTGAAACTTCGAGGAGTCTC-3′) in combination with an antisense PCR primer (5′-CCAATCTCGCCCCTGTCCATTCCCC-3′) homologous to the opposite strand of the FIV gag gene and 3′ to the leader sequence XhoI site. The 450-bp PCR product was first digested with XhoI (without SacI, to avoid cleaving the overlapping SacI site 3 nt downstream) and then subsequently digested with SacI to generate the 5′ cloning end. The 310-bp cleavage product was cloned into the SacI-XhoI backbone of pRc/CMV, generating plasmid CRF1. SalI-tailed PCR primers 5-TATATAGTCGACTAGGGACTGTTTACGAAC-3′ and 5′-ATATATAGTCGACGCGGCCGCTGCGAAGTTCTCG-3′ were then used to amplify the 3′ FIV LTR; after SalI digestion, the PCR product was ligated into the XhoI site of CRF1. The resulting plasmid, CRF(L), has the 5′-LTR fusion and a wild-type 3′ LTR. The major coding region of the FIV 34TF10 genome (the 8,845-nt BbeI-EspI fragment) was then inserted into the BbeI-EspI backbone of CRF(L), producing CT5, which encodes full-length, infectious FIV. The 438-ntpol deletion in CF1Δpol and CT5Δpol was constructed by deleting the NheI-Bsu36I fragment (nt 3122 to 3573). CF1Δenv was generated by a series of three-part ligations that deleted an 875-nt PflmI fragment of FIV env (nt 7322 to 8197) and inserted a SacII linker to produce a frameshift. The shorter, 539-nt deletion (nt 7321 to 7860) confined to SU (in CF1ΔSU and CF1ΔSU.fs) was produced by PCR amplification of a segment including env and the 3′ LTR (using SacI-tailed sense primer 5′-ATATACCGCGGTCTTGTACATCTGACTTGCCATCG-3′ and antisense primer 5′-ATATATAGTCGACCGGCCGTGCGAAGTTCTCGG-3′), digestion with SacII- and EagI, and insertion of the resulting fragment downstream of the SacII site in CF1 in several steps; blunted closure of the SacII site restored an open reading frame.
FIG. 1.
Diagram of chimeric FIV expression plasmids. CT5 contains the illustrated fusion of the hCVMIEp to the FIV genome at position −14 between the TATA box and the mRNA cap site. See Materials and Methods for details of construction. RRE, Rev response element.
Radioimmunoprecipitation and RT assays.
Radiolabeling with [35S]cysteine and [35S]methionine in cysteine- and methionine-free medium with 7.5% dialyzed fetal bovine serum was performed for 5 h after a 1-h preincubation in this medium without isotope. Cells were washed, detached with 5 mM EDTA in phosphate-buffered saline (PBS), and lysed in 1 ml of radioimmunoprecipitation buffer containing the protease inhibitors phenylmethylsulfonyl fluoride and leupeptin. Subsequent steps were performed at 0 to 4°C in the presence of freshly added protease inhibitors. Cell lysates were precleared with normal cat serum and protein A-Sepharose for 2 h and then incubated overnight with continuous mixing after addition of 10 μl of FIV-infected cat plasma. Antibody-protein complexes were precipitated with protein A-Sepharose. After repeated washing, samples were heated to 95°C for 5 min in sodium dodecyl sulfate (SDS) loading buffer and electrophoresed with prestained protein size markers (Bio-Rad) in SDS–10 or SDS–12.5% polyacrylamide gels. Reverse transcriptase (RT) assays were performed in triplicate with a 32P-based microtiter assay (46).
Transfection assays and transfection efficiency controls.
Comparisons of transfected cells were controlled by cotransfection of a GFP or LacZ reporter plasmid under the control of hCMVIEp as 10% of the input DNA; only experiments with transfection efficiencies varying <10% between compared cell lines are reported. Where syncytial destruction of the monolayer was extensive at 24 h for CF1, comparative transfection efficiencies were assayed in wells transfected in parallel and maintained in the presence of a 1:300 dilution of FIV-infected domestic-cat plasma to inhibit syncytium formation. Transfections were performed by calcium phosphate precipitation except for U87MG and U87MG.CXCR4 cells, which were electroporated (210 V).
FIA and titration of FIV and FIV-enveloped vectors.
For titration of replication-competent FIV, each line was seeded into 48-well plates at 104 cells per well and infected in sextuplicate the next day with serial fourfold dilutions. For FIA, the focal infectivity assay (FIA), foci were scored by immunoperoxidase staining 42 h later, employing FIV Petaluma cat serum and a secondary horseradish peroxidase-conjugated goat antibody to feline immunoglobulin G (ICN Pharmaceuticals) as described by Remington et al. (34). With this protocol, no background staining was seen in any cell line. For endpoint dilution, cells were infected in 48-well plates in the same manner but allowed to proliferate for 4 weeks, with trypsinization and splitting at 4- to 5-day intervals; positive wells were scored by RT production, and titers were calculated by the method of Spearman (36). To generate FIV-enveloped vectors, CT5Δpol and CF1Δenv were cotransfected into 293T cells. Serial fivefold dilutions of filtered supernatant collected at 48 h posttransfection were used to infect 24-well plates seeded 24 h earlier with 3 × 104 cells of each cell line. Foci were scored by FIA 48 h after infection.
Production and titration of pseudotyped CT5Δpol vector.
To produce VSV-G-pseudotyped CT5Δpol, 293T cells were cotransfected with CF1Δenv, CT5Δpol, and the VSV-G expression plasmid pHCMV-G (10). At 48 to 96 h after transfection, supernatants were cleared by centrifugation, filtered (0.45-μm pore size), aliquoted, and stored at −80°C. Serial fourfold dilutions were used to infect 24-well plates seeded 24 h earlier with 3 × 104 cells of each cell line. Foci were scored by FIA 48 h after infection. Control experiments showed that no background staining occurred in uninfected cells, in cells infected with heat-treated (56°C, 30 min) vector, or in cells exposed to supernatant generated with only CT5Δpol and pHCMV-G (leaving the gag-pol expression plasmid out).
Human-feline cell coculture.
Cell lines were each plated (3 × 105 cells) in six-well plates. The next day, 105 3201-FIV cells (ATCC CRL 10909, maintained in 10% RPMI) were added, in a 1:1 mixture of 10% RPMI and 10% Dulbecco minimal Eagle medium, per well.
Analysis of CXCR4 mRNA expression.
RNA was prepared by direct addition of TRIZOL reagent (Gibco BRL) to 106 cells in accordance with the manufacturer’s instructions. RNA was dissolved in 50 μl of RNA transcription buffer, treated with 20 U of RNase-free DNase (Boehringer Mannheim) for 30 min at 37°C, and then heated to 70°C for 10 min. For reverse transcription-PCR, 10 μl of each DNase-treated RNA sample was reverse transcribed at 48°C for 45 min in a 50-μl reaction volume containing 1× Promega Access AMV/Tfl buffer, 1 mM MgSO4, 200 μM each deoxynucleoside triphosphate 50 pmol of each primer, 5 U of avian myeloblastosis virus RT, and 5 U of Tfl polymerase and then subjected to 40 cycles of 94°C for 30 s, 55°C for 45 s, and 68°C for 1 min followed by a final 7-min extension at 72°C. Primers used for amplification of human CXCR4 were 5′-GAAGCTGTTGGCTGAAAAGG-3′ and 5′-GATCCCAATGTAGTAAGGCAGC-3′. Primers used for amplification of human CXCR4 were 5′-GATAACTACACCGAAGATGACTTG-3′ and 5′-AAGATGAAATCAGGAATAGTCAAC-3′. Primers used for amplification of ribosomal protein L32 were 5′-ATGCCCAACATTGGTTATGG-3′ and 5′-ATTTGTTGCACATCAGCAGC-3′. To confirm the adequacy of the DNase treatment, samples were also subjected to PCR without addition of avian myeloblastosis virus RT. For analysis, 10 μl of each product was run on a 6% polyacrylamide gel and stained with SYBR Green dye (Molecular Probes, Eugene, Oreg.). Gels were imaged with a Speedlite Platinum system (LightTools Research, San Diego, Calif.), and bands were quantitated with ImageQuant software (Molecular Dynamics). Expression of CXCR4 RNA was plotted relative to L32 mRNA expression, subtracting the local background from each window and correcting for relative band sizes (332 bp for human CXCR4, 497 bp for feline CXCR4, and 120 bp for L32).
Flow cytometric analysis of cell surface CXCR4 expression.
Cells (106) were detached with 4 mM EDTA in PBS, incubated with 20 mg of anti-CXCR4 monoclonal antibody (no. 36195X, R-phycoerythrin-conjugated 12G5 clone mouse immunoglobulin G2A(κ); Pharmingen, San Diego, Calif.) in a final volume of 300 ml of PBS with 1% fetal bovine serum for 1 h at 4°C, washed, and fixed with 1% paraformaldehyde. Flow cytometric analysis was performed on a Coulter Elite fluorescence-activated cell sorter apparatus, using a 488-nm argon laser for excitation; 5,000 cells were counted for each sample. Relative CXCR4 expression was determined by calculating the increase in the mean fluorescence intensity relative to that of the control unstained cells for each cell type.
RESULTS
Heterologous expression of the FIV genome.
FIV 34TF10, a cell culture-adapted FIV clone with a broadly tropic envelope glycoprotein (39, 43), was modified for the present work. Although FIV 34TF10 productively infects CrFK cells, neither 34TF10 nor other domestic-cat strains or clones are grossly cytolytic in this cell line. Scattered, small, multinucleate giant cells can be detected, but extensive cell death and disruption of the CrFK monolayer do not occur and chronic infection is readily established (2–4, 39, 41).
To determine if substitution of an alternative promoter for the FIV LTR was feasible and would enable initiation of the productive phase of FIV replication in both human and feline cells, the 34TF10 molecular clone was modified as diagrammed in Fig. 1. The hCMVIEp was arranged to replace either the entire FIV LTR (in CF1) or only the 5′ U3 element by the precise fusion of the TATA box to the R repeat (plasmids CT5 and CT5Δpol). The fusion in CT5 aligns the FIV mRNA cap site downstream of the hCMVIEp TATA box such that the nucleotide spacing between the replaced FIV TATA box and the transcription start site is preserved.
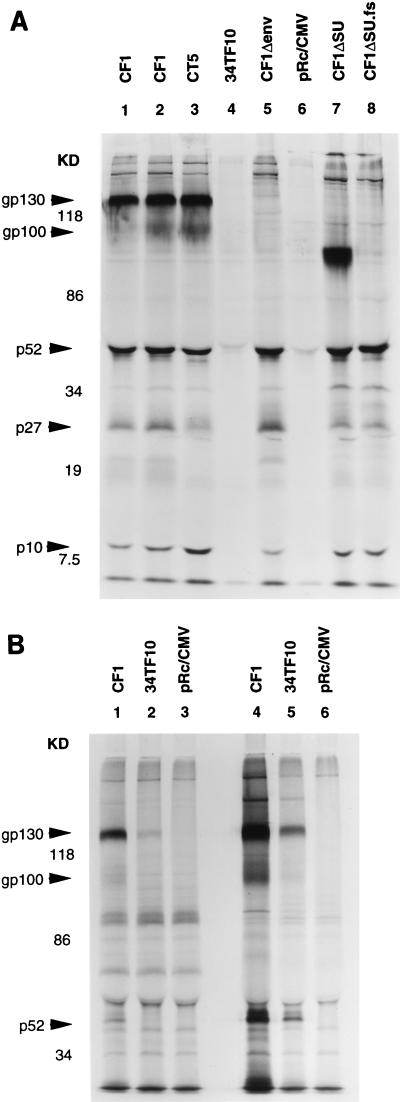
In both human and feline cells, transfection of CF1 or CT5 resulted in substantial FIV protein expression with a wild-type pattern as assessed by radioimmunoprecipitation (Fig. 2), high levels of pol-specific Mg2+-dependent RT activity (Fig. 3), and profuse syncytia. Expression by CF1 and CT5 exceeded LTR-directed expression by p34TF10 in cells of both feline and human origin. p34TF10 expression was undetectable in 293 cells even after prolonged film exposure (Fig. 2A). Expression of FIV proteins by p34TF10 in HeLa cells was detectable by radioimmunoprecipitation, but at levels lower than those of the hCMVIEp chimeras (Fig. 2B and 3).
FIG. 2.
Expression and processing of FIV proteins in transfected human and feline cells, assessed by radioimmunoprecipitation with FIV (Petaluma strain)-infected domestic-cat plasma. 293 cells (panel A, all 8 lanes), HeLa (panel B, lanes 1 to 3), and CrFK cells (panel B, lanes 4 to 6) were transfected with 5 μg of the indicated plasmids by calcium phosphate precipitation in 25-cm2 flasks. At 27 h (293 cells) or 44 h (HeLa and CrFK cells) after transfection, cells were radiolabeled and immunoprecipitated (see Materials and Methods). The HeLa cell lysate used for lane 1 in panel B was derived from approximately 15 to 25% of the amount of cells in the other lanes because of the loss of cells to extensive syncytial lysis. The data from one of two radioimmunoprecipitations performed are shown; each yielded the same results. Shown on the left are the positions of molecular mass markers (in kilodaltons).
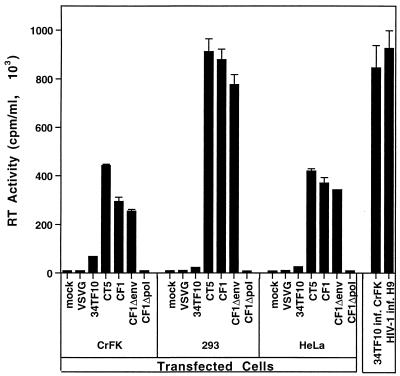
FIG. 3.
Supernatant Mg2+-dependent RT activity measured 36 h after transfection of the indicated plasmids in CrFK, HeLa, and 293 cells. Widespread syncytia were present in cells transfected with CT5, CF1, CF1Δpol, or pHCMV-G. The last two plasmids were included as controls for cell lysis to verify the viral specificity of the RT activity. Supernatants from chronically infected CrFK cells and H9 cells (infected 2 weeks earlier with HIV-1 at an MOI of 1) were also assayed for comparison (right side of figure). Each value is the mean of triplicate measurements ± the standard error of the mean.
Transfection of CT5 into human cells resulted in the production of infectious FIV. Transfection of either 34TF10 or CT5 into CrFK cells, or cell-free passage of virus from CT5-transfected human cells to CrFK cells, led to the establishment of a persistent infection with high levels of RT production (>5 × 105 cpm/ml) by days 7 to 14. However, consistent with previous studies (2–4, 39, 41), a minimal cytopathic effect was seen during either acute or chronic infection of CrFK cells (mean ± standard deviation, 6 to 12 ± 4 syncytia, with four to eight nuclei each, per 9.6-cm2 well of a six-well plate). Transfection of CT5 in human 293 cells yielded supernatant containing nearly 106 cpm of RT activity per ml (Fig. 3) and infectious FIV with titers of >105 focus forming units (FFU)/ml on CrFK cells, as determined by a previously described FIV-specific FIA (34).
CF1, which is designed to supply FIV proteins in trans, lacks the basis for reverse transcription and integration because of the deletion of both LTRs. CF1 produced high levels of RT activity (Fig. 3) but did not produce infectious virus. Passage of >107 cpm of RT activity from CF1-transfected 293T cells to 5 × 106 CrFK cells or to human cells (HeLa, 293, H9, Molt4, SupT1, or U937) resulted in a lack of syncytia and no RT production. The adherent cell lines were also examined and found to be negative by the immunoperoxidase FIA, which has a sensitivity of <5 infectious units per ml on CrFK cells (34).
Syncytium induction in human and feline cells.
Enabling efficient FIV expression in human cells revealed surprising cytopathicity of the FIV envelope glycoprotein. Transfection of CF1, CF1Δpol, or CT5, but not 34TF10, into human cells resulted in explosive syncytium formation within 12 to 18 h. HeLa and 293 cell monolayers were reproducibly 90 to 95% destroyed by syncytial lysis within 36 to 72 h after transfection of CF1 (Fig. 4A and B and data not shown). In CrFK cells, transfection of the four plasmids consistently produced >2 log units fewer and smaller (4 to 12 nuclei) syncytia without disruption of the monolayer.
FIG. 4.
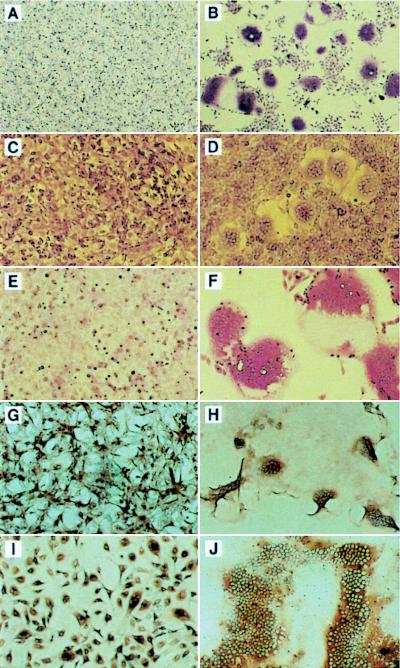
Fusogenic activity of FIV envelope in human, feline, and rodent cell lines and effect of stable human CXCR4 coexpression. Each left-right photo pair is presented for comparison. (A to F) Cells transfected with plasmids (indicated below) and stained with crystal violet 24 to 48 h later. (A) HeLa cells transfected with CF1Δenv; (B) HeLa cells transfected with CF1; (C to F) CF1-transfected 3T3 cells, 3T3.CXCR4 cells, HOS cells, and HOS.CXCR4 cells, respectively. (G to J) Cells infected at equal MOI with VSV-G-pseudotyped env expression vector CT5Δpol and stained by the FIV Env-specific immunoperoxidase assay 24 to 48 h later. (G) U87MG cells; (H) U87MG.CXCR4 cells; (I) CrFK cells; (J) CrFK.CXCR4 cells. Generation and characterization of CXCR4-expressing cell lines are described in the legend to Fig. 6. In addition to the syncytia seen, large areas of panels B, F, H, and J are denuded because of detachment of syncytia prior to or during staining. Transfections were controlled for efficiency as described in Materials and Methods.
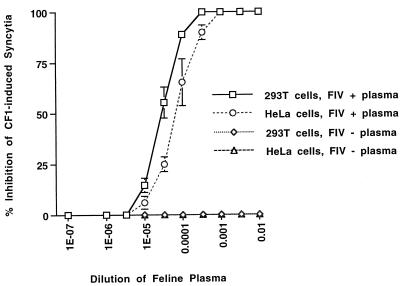
To determine if syncytium induction by CF1 and CT5 in human and feline cells was mediated specifically by the FIV envelope glycoprotein, env mutants with deletions either spanning both SU and TM domains (CF1Δenv) or only in the SU domain (CF1ΔSU and CF1ΔSU.fs) were constructed (Fig. 1). The two frameshifting env deletions (CF1Δenv and CF1ΔSU.fs) abrogated immunoprecipitable Env but not Gag-Pol production (Fig. 2A, lanes 5 and 8), while the frame-preserving SU deletion of CF1ΔSU resulted in expression of the predicted truncated envelope precursor (Fig. 2A, lane 7). All of the env mutants expressed high levels of RT and other immunoprecipitable FIV proteins (Fig. 2 and 3), but none produced any syncytia. In addition, as shown in Fig. 5, syncytium production in CF1-transfected human cells was potently blocked by FIV Petamula-infected domestic-cat plasma, with 50% inhibition in 293T and HeLa cells at 1:32,000-fold and 1:12,700-fold dilutions, respectively, while preimmune domestic-cat plasma had no effect on syncytium formation at any dilution, even 1:10. In contrast to transfection of CF1 or CT5, only rare syncytia containing four to six nuclei were detectable in HeLa cells 48 to 72 h after transfection of p34TF10. This result is consistent with the low level of LTR-directed expression in HeLa cells (Fig. 2B, lane 2).
FIG. 5.
Specific inhibition of CF1-induced syncytium production in 293T and HeLa cells by FIV-infected domestic-cat plasma. Dilutions of either FIV-positive (+) (squares and circles) or FIV-negative (−) (diamonds and triangles) plasma specimens were added to cells at the time of transfection in 12-well plates (1 μg of DNA/well) and again with a change of medium 14 h later. Syncytia were scored at 48 h (as foci per well containing at least eight nuclei) by crystal violet staining of methanol-fixed cells; values are means ± standard deviations of data from three experiments.
CXCR4 dependence of syncytium induction.
To investigate the molecular mechanism of fusion mediated by the FIV envelope, human cell lines which differ in their expression of the principal chemokine receptors involved in HIV or SIV infection (5, 15) were studied. Among all human cell lines tested, only U87MG and SK-N-MC cells failed to produce syncytia after transfection of CF1 (n = 9). Transfection of rodent cells (NIH 3T3, CHO, and rat 208F) also failed to produce syncytia (n = 8; efficiencies, ≥10%). However, CF1-transfected rat 208F cells readily formed syncytia when mixed with a variety of human cell lines (HeLa, HeLaT4, 293, 293T, Molt4, SupT1, H9, and Jurkat) and with CrFK cells but did not form syncytia with U87MG or SK-N-MC cells (data not shown). Because CXCR4, the coreceptor for T-cell-line-adapted strains of HIV (16), is expressed in all of the tested human cell lines except U87MG and SK-N-MC, these results suggested involvement of CXCR4 in FIV envelope glycoprotein-mediated fusion.
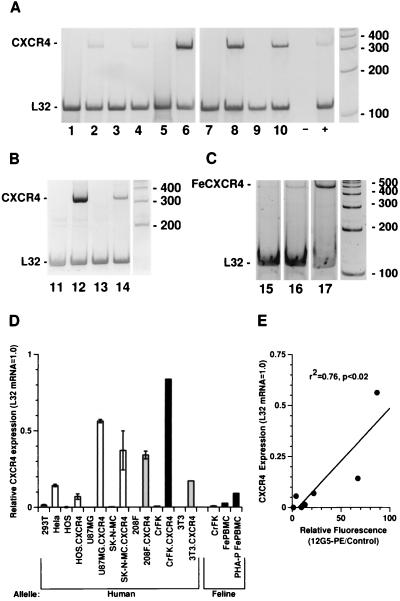
To confirm that CXCR4 was specifically responsible for fusion, a CXCR4-expressing Moloney murine leukemia virus-based retroviral vector (pZ.CXCR4) was constructed and used to generate a panel of G418-selected cell lines of human, rodent, and feline origin that stably express human CXCR4 and neomycin phosphotransferase from a bicistronic mRNA. This vector resulted in consistent, sustained expression of CXCR4 in all G418-selected cell lines, which are differentiated from control lines by the suffix CXCR4. Figure 6 shows the results of an analysis of human CXCR4 expression in these lines and control cell lines, as well as the relative levels of feline CXCR4 mRNA expression determined for feline peripheral blood mononuclear cells (PBMC) and CrFK cells. Cell surface expression of CXCR4, as measured by fluorescence-activated cell sorter analysis, correlated closely with mRNA expression (Fig. 6E).
FIG. 6.
Reverse transcription-PCR analysis of human and feline CXCR4 expression. Cell lines stably expressing human CXCR4 are designated by the suffix CXCR4 and were generated by G418 selection after transduction with Moloney murine leukemia virus-based retroviral vector pZ.CXCR4. RNA was isolated, treated with DNase, and analyzed by semiquantitative reverse transcription-PCR for human or feline CXCR4 with internal coamplification of a conserved cellular message (ribosomal protein L32 mRNA). Means of three or more independent measurements are plotted in panel D, while representative gels are shown in panels A to C. (A and B) Reverse transcription-PCR of human CXCR4 (332-bp band) and L32 (120-bp band) for human (open bars in panel D), rodent (shaded bars in panel D), and feline (closed bars in panel D) cells. Lane 1, 293T cells; lane 2, HeLa cells; lane 3, HOS cells (human osteosarcoma); lane 4, HOS.CXCR4 cells; lane 5, U87MG cells; lane 6, U87MG.CXCR4 cells; lane 7, SK-N-MC cells; lane 8, SK-N-MC.CXCR4 cells; lane 9, 208F rat fibroblasts; lane 10, 208F.CXCR4 cells; lane 11, CrFK cells; lane 12, CrFK.CXCR4 cells; lane 13, 3T3 cells; lane 14, 3T3.CXCR4 cells. −, RNA extraction blank; +, repeat HeLa cell positive control. (C) Feline CXCR4 (497-bp band) and L32 (120-bp band) expression. Lane 15, CrFK cells; lane 16, fresh Ficoll-purified F. catus PBMC (FePBMC); lane 17, F. catus PBMC stimulated for 48 h with phytohemagglutinin P in RPMI with 15% bovine serum (PHA-P FePBMC). (D) Expression of CXCR4 RNA plotted relative to L32 mRNA expression for each sample, with subtraction of local background from each window and correction for relative band product sizes. Error bars represent standard deviations of three or more independent measurements. For each sample, PCR was also performed without reverse transcription to exclude any contribution of DNA to products, and no bands were detected (data not shown). (E) CXCR4 mRNA expression, determined above, as described, was plotted against cell surface expression of human CXCR4, expressed as the ratio of mean fluorescence intensity of 12G5-PE stained and unstained control cells. RNA expression was found to be linearly related to CXCR4 expression on the cell surface (r2 = 0.76, P < 0.02).
Transfection of CF1 or CT5 into stable U87MG.CXCR4, SK-N-MC.CXCR4, rat 208F.CXCR4, and murine 3T3.CXCR4 cell lines derived with pZ.CXCR4 led to the production of extensive syncytia, while simultaneous transfection into the respective parental lines or into control lines generated with parental vector JZ308 yielded no syncytia (Fig. 4C and D). Moreover, while only rare, four- to eight-nucleus syncytia were seen after transfection of CF1 into HOS cells, parallel transfection of CF1 into HOS.CXCR4 cells resulted in extensive lysis, with multinucleated giant cells containing more than 100 nuclei (Fig. 4E and F).
These results were specific for CXCR4. In contrast to the observed strict concordance of cell fusion with CXCR4 expression, cell lines stably expressing human CCR5 from a similarly constructed retroviral vector were not susceptible to FIV 34TF10 envelope glycoprotein-induced fusion (data not shown).
Single-round infection of human cells by an env-expressing, pol-deleted FIV provirus.
To verify the CXCR4 dependence of IV envelope glycoprotein-induced cell fusion in a single-round viral infection, 34TF10 env was expressed in HeLa, U87MG, and U87MG.CXCR4 cells by using a replication-defective FIV vector. CT5Δpol and CF1Δenv were cotransfected into 293T cells with the VSV-G expression plasmid pHCMV-G, generating vector CT5Δpol(VSV-G). Particles generated in this manner contain both FIV envelope and VSV-G glycoproteins on their surfaces but encode only the FIV envelope glycoprotein. As shown in infectivity experiments (see below), the much greater infectivity of VSV-G allows single-round infection of CXCR4-negative and CXCR4-positive cell lines with equal efficiency. Each cell line was infected (multiplicity of infection [MOI], 0.2) with filtered CT5Δpol(VSV-G) and examined by FIV-specific immunoperoxidase staining 34 h later. The FIV LTR was transcriptionally active in U87MG cells, since CT5Δpol(VSV-G)-infected U87MG cells expressed FIV proteins heavily. However, these cells formed no syncytia (Fig. 4G). In contrast, simultaneously infected U87MG.CXCR4 cells displayed equivalent immunostaining but exhibited marked syncytium formation (Fig. 4H). HeLa cells also exuberantly formed FIV immunoperoxidase FIA-positive syncytia after CT5Δpol(VSV-G) infection (data not shown).
Infected-cell mixing studies.
Feline cat lymphoma cells chronically infected with FIV (3201-FIV cells) were mixed with U87MG, SK-N-MC, HOS, and 3T3 cells, their respective pZ.CXCR4 vector-selected counterparts, and HeLa cells. Between 12 and 24 h, postinfection, large ballooning syncytia involving 40 to 80% of the monolayer were observed in the HeLa cells and in each CXCR4-expressing line, while no syncytia were seen at any time point in control U87MG, SK-N-MC, HOS, or 3T3 cells. The data from these cell mixing experiments corroborate the results of the intracellular expression experiments and indicate that CXCR4-specific fusogenesis is not limited to the FIV 34TF10 clone.
CXCR4-dependent syncytium formation in feline cells.
To examine whether feline cells were also dependent on CXCR4 for FIV envelope-mediated syncytium formation, transient transfection, virus infection, and infection with replication-defective CT5Δpol(VSV-G) were studied. CrFK cells infected with replication-defective CT5Δpol(VSV-G) at a high MOI (2.0) were strongly positive by immunoperoxidase staining for FIV Env at 36 h. However, as shown in Fig. 4I, these cells developed syncytia containing only two to eight nuclei, confirming the limited fusogenic capacity (2–4, 39, 41) of this cell line. In marked contrast, CrFK.CXCR4 cells infected with CT5Δpol(VSV-G) at the same MOI showed equal antigen-specific staining but developed dramatically more syncytia, containing hundreds of nuclei, resulting in coalescent fusion of the entire monolayer by 36 h (Fig. 4J). Transient transfection of CF1 into CrFK and CrFK.CXCR4 cells (n = 6) produced equally discrepant results (data not shown). Consistent with these results, Fig. 6C shows that CrFK cells express detectable but low levels of feline CXCR4 mRNA. Note that CrFK.CXCR4 cells express over 100-fold more human than feline CXCR4 message, relative to L32 RNA (83.6% versus 0.8%), and the expression of feline CXCR4 is over 10-fold greater in phytohemagglutinin P-stimulated feline PBMC (9.0%) than in CrFK cells. The abilities of the cell lines used in this study to support FIV envelope glycoprotein-induced syncytium production are summarized in Table 1.
TABLE 1.
Summary of syncytium induction by FIV envelope glycoprotein in human, feline, and rodent cell linesa
| Cell line | Syncytium induction resulting fromb:
|
||
|---|---|---|---|
| Transfection of CF1 or CT5 | Infection with CT5Δpol(VSV-G) | Coculture with 3201-FIV cells | |
| U87MG | None | None | None |
| SK-N-MC | None | None | None |
| 3T3 | None | None | None |
| 208F | None | None | None |
| CrFK | + | + | + |
| HOS | + | ND | None |
| CHO | None | ND | None |
| U87MG.CXCR4 | ++++ | ++++ | ++++ |
| SK-N-MC.CXCR4 | ++++ | ++++ | ++++ |
| 3T3.CXCR4 | ++++ | ND | ++++ |
| 208F.CXCR4 | ++ | ND | ++ |
| CrFK.CXCR4 | ++++ | ++++ | ++++ |
| HOS.CXCR4 | ++++ | ND | ++++ |
| HeLa | ++++ | ++++ | ++++ |
| 293 | ++++ | ND | ++++ |
| 293T | ++++ | ND | ++++ |
| Molt4 clone 8 | ND | ND | ++++ |
| SupT1 | ND | ND | ++++ |
| H9 | ND | ND | ++++ |
See Fig. 4 for representative photographs and text for quantitative scoring of syncytia, details of transfection, infection with replication-defective CT5Δpol(VSV-G), and number of experiments performed. Transfection efficiency controls are described in Materials and Methods.
++++, syncytia too numerous to count, or lytic destruction of monolayer; ++, >5 syncytia containing more than 8 nuclei each per cm2; +, <5 such syncytia per cm2; None, no synctia seen; ND, not done.
Effects of CXCR4 expression on FIV replication kinetics.
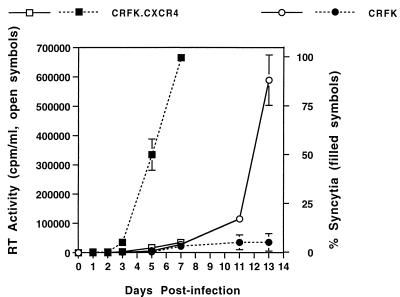
The effect of CXCR4 on the replication kinetics of replication-competent FIV was investigated by infecting CrFK and CrFK.CXCR4 cells with 34TF10 virus (produced in CrFK cells) and simultaneously monitoring RT production and syncytium induction. As shown in Fig. 7, the induction of syncytia by 34TF10 was dramatically increased in CrFK.CXCR4 cells, resulting in termination on day 7 because of >99% cell death. Although early-time-point RT values are compressed in the lower part of the arithmetic ordinate, RT production on days 3 and 5 was also 2.5- and 2.7-fold greater, respectively, in CrFK.CXCR4 cells than in CrFK cells despite the rapid loss of CrFK.CXCR4 cells. However, the peak level of RT production was much lower in CrFK.CXCR4 cells because of this rapid cytolysis (Fig. 7).
FIG. 7.
Effect of CXCR4 expression on FIV 34TF10 replication. RT activity and syncytium formation were simultaneously measured in cultures of CrFK cells (circles) and CrFK.CXCR4 cells (squares). Open symbols, RT values; closed symbols, percent syncytium formation. Results are means ± standard errors of the means of data from four separate experiments, with RT activity measured in duplicate for each sample. A total of 2 × 106 cells of each line were simultaneously plated (day −1) and infected (day 0) with virus produced in CrFK cells (MOI, 0.01). The difference in cytopathicity was dramatic, with CrFK.CXCR4 cultures terminated on day 7 because of >99% cell death.
Effects of CXCR4 on FIV infectivity.
To determine whether CXCR4 expression also correlated with an increase in viral infectivity, the infectivities of wild-type virus and a replication-defective FIV-enveloped vector on CrFK and CrFK.CXCR4 cells were measured (Table 2). The infectivities of replication-competent FIV 34TF10 for CrFK cells was compared with its infectivity for CrFK.CXCR4 cells by two methods: FIA (34) and, separately, endpoint dilution. By FIA, 34TF10 was 8.2-fold more infectious on CrFK.CXCR4 cells than on CrFK cells (mean ± standard deviation, 9.75 × 104 ± 0.83 × 104 versus 1.19 × 104 ± 0.89 × 104 FFU/ml; P = 0.002, signed Wilcoxon rank sum test). This method might have resulted in the underestimation of the infectivity ratio, since rounded, floating cells that stained by the immunoperoxidase method were detected in the infected CrFK.CXCR4 wells as early as 42 h. Such cells were not seen in infected or uninfected CrFK cells, suggesting that they represented rapidly killed, infected cells which were not counted in the FIA. A comparative endpoint dilution titration of 34TF10 virus on the two cell lines was therefore performed, and it showed that 34TF10 was 22.6-fold more infective on CrFK.CXCR4 cells than on CrFK cells (1.15 × 106 versus 5.12 × 104 FFU/ml; P = 0.0001, signed Wilcoxon rank sum test).
TABLE 2.
Effect of CXCR4 on infectivity of the FIV envelope glycoproteina
| Virus | Titration method | Infectivity (FFU/ml) on:
|
CrFK/CrFK.CXCR4 infectivity ratio | |
|---|---|---|---|---|
| CrFK | CrFK.CXCR4 | |||
| FIV 34TF10 | FIAb | 1.19 × 104 ± 0.89 × 104 | 9.75 × 104 ± 0.83 × 104 | 8.2 |
| Endpoint dilutionb | 5.12 × 104 | 1.15 × 106 | 22.6 | |
| CT5Δpol(FIV) | FIA | 5.3 × 104 ± 0.4 × 103 | 6.5 × 104 ± 0.9 × 104 | 12.5 |
| CT5Δpol(VSV-G) | FIA | 1.95 × 104 ± 0.1 × 105 | 1.87 × 104 ± 0.1 × 105 | 0.95 |
See Materials and Methods and Results for experimental details. CrFK versus CrFK.CXCR4 comparisons were performed simultaneously with the same viral preparation.
Different FIV preparations were used for the FIA and endpoint dilution experiments with infectious FIV.
The infectivity on CrFK cells of a replication-defective FIV-enveloped provirus that mimics a single round of viral infection was also compared with its infectivity for CrFK.CXCR4 cells by FIA. Vector, CT5Δpol generated by cotransfection with CF1Δenv in 293T cells, yielded a 12.5-fold-higher titer on CrFK.CXCR4 than on CrFK cells (6.5 × 104 ± 0.9 × 104 versus 5.3 × 103 ± 0.4 × 103 FFU/ml; P < 0.01, signed Wilcoxon rank sum test). The mean infectivity ratio (CrFK.CXCR4/CrFK) for the three sets of experiments was 14.4. In contrast, no infectivity difference was seen with VSV-G-pseudotyped CT5Δpol (Fig. 4I and J and Table 2).
FIV infection of human cells.
In agreement with previous studies, sustained FIV replication was not observed in human cells. Infection of HeLa or U87MG.CXCR4 cells (but not U87MG cells) with >105 U of RT from CT5-transfected 293T cells produced only transient syncytium and RT production. Similarly, transfection of CT5 or p34TF10 into human and rodent cell lines failed to result in a sustained, productive FIV infection of cells that did or did not express CXCR4. Virus produced by CT5 transfection of human cells could only generate sustained replication by passage to CrFK or CrFK.CXCR4 cells. Human cell cultures were RT negative by the second split after transfection, and they were immunoperoxidase FIA negative (except for rare positive cells) by the fourth split. In contrast, all of these measures produced sustained productive infection of CrFK cells and CrFK.CXCR4 cells (0.5 to 5% of CrFK.CXCR4 cells survived viral infection and became chronic producers of greater than 105 U of RT/ml).
To examine FIV envelope glycoprotein-mediated infection of human cells quantitatively, while excluding a confounding role for endogenous feline retrovirus pseudotypes, filtered supernatant from 293T cells cotransfected with CT5Δpol and CF1Δenv was used to infect human cells that support FIV LTR-directed transcription. Simultaneous infection of U87MG, U87MG.CXCR4, and HeLa cells with this FIV-enveloped, replication-defective vector yielded titers of <1, 199 ± 16, and 1,096 ± 110 FFU/ml, respectively (n = 3). This supernatant yielded 330- and 60-fold-higher titers on CRFK.CXCR4 cells than on U87MG.CXCR4 and HeLa cells, respectively. In control experiments, no foci were seen in any of these lines exposed to supernatant from 293T producer cells transfected with CT5Δpol alone or when a 1:250 dilution of FIV serum was added during infection.
DISCUSSION
Differences in the tropism, epidemiology, and pathogenetic features of the primate and nonprimate lentiviruses have made the utility of nonprimate lentivirus infections as models for HIV pathogenesis uncertain. The present systematic study of the molecular prerequisites for FIV expression and infection in both host and nonhost cells indicates that the life cycles of the primate lentiviruses and FIV share notable core molecular features.
We conclude that poor promoter activity of the FIV U3 element is the only block to the productive phase of viral replication in human cells. Previous reports suggested that more-complex blocks to the productive phase existed, including poor Rev activity (40). In the present study, replacement of the FIV U3 LTR element with a heterologous promoter was found to be sufficient for efficient production of infectious virus, demonstrating that the Rev-Rev response element (RRE) regulatory axis and other productive-phase mechanisms occur efficiently when the obstacle to efficient transcription is overcome.
The effects of CXCR4 on infection, fusion, and replication of FIV were separately examined in the present work. The results, which were obtained through direct expression of FIV in human and rodent cells for the first time, corroborate and extend a previous, methodologically different study that examined mixing CXCR4-expressing human cell lines with FIV-infected CrFK cells (45). The data indicate that human CXCR4, but not human CCR5, is sufficient to specifically mediate infection as well as cell fusion by the FIV 34TF10 SU protein, demonstrating that a common cell surface receptor is involved in these processes in two distantly related groups of lentiviruses. Enabling efficient production of infectious FIV in nonfeline cells also permitted a conclusive study of the molecular prerequisites for cell fusion and infection free of the possibility of pseudotyping by xenotropic retroviruses.
We found a comparatively low level of feline CXCR4 expression in CrFK cells. This finding contrasts with the results of Willett et al. (45) but is consistent with our finding that FIV Env has a limited fusogenic capacity in these cells and with the observation by us and others (2–4, 39, 41) that chronic FIV infection of CrFK cells is readily established with minimal cell death. Whether this low-level feline CXCR4 expression and limited cytopathicity result from heterogeneous CXCR4 expression within the monolayer or from uniformly low-level expression is uncertain. Also in contrast to the results obtained by Willett et al. with a different FIV strain (45), FIV 34TF10 envelope-mediated fusion of most CXCR4-expressing human cell lines was not inhibited by a monoclonal antibody (MAb) directed against CXCR4 (MAb 12G5, kindly provided by J. Hoxie through the NIH AIDS Research Reagent Program). MAb 12G5 did inhibit the fusogenic activity of CF1 and CT5 in transfected 293 cells, but only at a high concentration (100 μg/ml for 50% inhibition). This difference in results may reflect the fact that the activity of 12G5 for inhibition of HIV-1-induced cell fusion is both viral strain and cell type dependent (21).
The implications of these data for the reconstruction of lentivirus evolution are striking: the use of CXCR4, but not of CD4, has either been conserved since divergence of FIV and primate lentiviruses from a common ancestor or has evolved independently (convergently). By either scenario, this common usage is indicative of a fundamental role for this chemokine receptor in the replication and cytopathicity of AIDS-causing lentiviruses. Shared utilization of CXCR4 is particularly intriguing in view of the broad tropism of FIV for B cells, CD8+ T cells, and CD4+ T cells and its lack of use of CD4 as a receptor. The uncoupling of selective CD4 depletion from use of the CD4 molecule for entry is an important distinction between FIV and HIV infection and suggests that there exist basic lentivirus pathogenetic mechanisms that are CD4 molecule independent yet affect this T-cell subset preferentially. Therefore, conservation of CXCR4 utilization in the two naturally occurring lentivirus infections that cause immunodeficiency characterized by progressive CD4 depletion suggests that CXCR4 may be central to this and other aspects of disease causation by both animal and human lentiviruses. In this regard, emergence of CXCR4-utilizing virus HIV strains in vivo has been correlated with more-rapid disease progression (19).
The cross-species function of human CXCR4 for infection and syncytium formation by FIV is also remarkable. This observation is consistent with recently acquired evidence that C-X-C chemokine receptors can be functionally substituted not only between species but also between mammalian families. For example, coexpression of murine (6, 37) or rat (7, 32) CXCR4 (but not CCR5) together with human CD4 has been shown to permit fusion and entry mediated by some HIV-1, but not HIV-2 (33), envelope glycoproteins. Moreover, F. catus CXCR4 (GenBank accession no. U92795) is more homologous to human CXCR4 (95% homology at the amino acid level) than to either rodent molecule (murine, 89%; rat, 91%).
These data demonstrate that few restrictions to the life cycle of this anciently divergent (11) lentivirus exist in cultured human cells. Clarification of the precise mechanisms restricting sustained replication will require a more-detailed study of chimeric viruses and establishment of whether a primary receptor for FIV exists. Entry may be mediated by CXCR4 alone, as has been described for HIV-2 (15). Alternatively, a primary receptor molecule could be ubiquitously present on human cells, although the human homolog may not be used as efficiently for viral entry as the feline homolog. The effects of repairing ORF2 (43) should also be examined. The minimal transcriptional activity of the FIV LTR in human cells in the present study is consistent with previous reports (17). Restrictions to transcription in human cells may reside in several motifs, including upstream CTF/NF-1 (−200) and GATA-1 (−160) sites, an AP-1 site (−116) reported to be responsible for basal activity in feline cells, and a more proximal ATF/CRE element (−51) (17, 18, 23–25).
The use of CXCR4 without the constraint of CD4 as a coreceptor or in combination with a more widely expressed primary receptor may contribute to the broader lymphocyte tropism of FIV in vivo. Additional chemokine receptor molecules may be utilized by FIV. The disparity between the susceptibility of F. catus and other felids to FIV disease could reflect differences in chemokine receptor interactions. Investigation of ungulate lentiviruses will clarify whether CXCR4 use is a universal lentivirus property.
ACKNOWLEDGMENTS
We thank M. C. Barr for feline plasma, J. Elder and R. Talbott for p34TF10, J. Zhang and H. Temin for pJZ308, N. Landau and R. Doms for information about CXCR4 expression in human cell lines, T. North for advice about FIV infectivity assays, W. Witke for excellent technical assistance, F. Mannino for cat blood, and F. Wong-Staal and the UCSD Center for AIDS Research for shared reagents and equipment.
This work was supported in part by NIH grants 1R01CA6739403, 1U19 AI3661203 (SPIRAT), 3K12DK01408-10S1, and 2P30AI3621404 (CFAR).
REFERENCES
- 1.Bachmann M H, Mathiason-Dubard C, Learn G H, Rodrigo A G, Sodora D L, Mazzetti P, Hoover E A, Mullins J I. Genetic diversity of feline immunodeficiency virus: dual infection, recombination, and distinct evolutionary rates among envelope sequence clades. J Virol. 1997;71:4241–4253. doi: 10.1128/jvi.71.6.4241-4253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandecchi P, Pistello M, Matteucci D, Lombardi S, Bendinelli M, Tozzini F. Examination of variables affecting syncytium formation by, and serum neutralization of, feline immunodeficiency virus on CrFK cells. New Microbiol. 1995;18:241–252. [PubMed] [Google Scholar]
- 3.Barr M C, Zou L, Holzschu D L, Phillips L, Scott F W, Casey J W, Avery R J. Isolation of a highly cytopathic lentivirus from a nondomestic cat. J Virol. 1995;69:7371–7374. doi: 10.1128/jvi.69.11.7371-7374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr M C, Zou L, Long F, Hoose W A, Avery R J. Proviral organization and sequence analysis of feline immunodeficiency virus isolated from a Pallas’ cat. Virology. 1997;228:84–91. doi: 10.1006/viro.1996.8358. [DOI] [PubMed] [Google Scholar]
- 5.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieniasz P D, Fridell R A, Anthony K, Cullen B R. Murine CXCR-4 is a functional coreceptor for T-cell-tropic and dual-tropic strains of human immunodeficiency virus type 1. J Virol. 1997;71:7097–7100. doi: 10.1128/jvi.71.9.7097-7100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brelot A, Heveker N, Pleskoff O, Sol N, Alizon M. Role of the first and third extracellular domains of CXCR-4 in human immunodeficiency virus coreceptor activity. J Virol. 1997;71:4744–4751. doi: 10.1128/jvi.71.6.4744-4751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown E W, Yuhki N, Packer C, O’Brien S J. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J Virol. 1994;68:5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown W C, Bissey L, Logan K S, Pedersen N C, Elder J H, Collisson E W. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991;65:3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpenter M A, O’Brien S J. Coadaptation and immunodeficiency virus: lessons from the Felidae. Curr Opin Genet Dev. 1995;5:739–745. doi: 10.1016/0959-437x(95)80006-q. [DOI] [PubMed] [Google Scholar]
- 12.Clements J E, Zink M C. Molecular biology and pathogenesis of animal lentivirus infections. Clin Microbiol Rev. 1996;9:100–117. doi: 10.1128/cmr.9.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Parseval A, Lerner D L, Borrow P, Willett B J, Elder J H. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J Virol. 1997;71:5742–5749. doi: 10.1128/jvi.71.8.5742-5749.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elder J H, Phillips T R. Molecular properties of feline immunodeficiency virus (FIV) Infect Agents Dis. 1993;2:361–374. [PubMed] [Google Scholar]
- 15.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Tomonaga K, Kawaguchi Y, Kohmoto M, Inoshimu Y, Tohya Y, Miyazawa T, Kai C, Mikami T. Feline immunodeficiency virus can infect a human cell line (MOLT-4) but establishes a state of latency in the cells. J Gen Virol. 1996;77:1623–1630. doi: 10.1099/0022-1317-77-8-1623. [DOI] [PubMed] [Google Scholar]
- 18.Inoshima Y, Kohmoto M, Ikeda Y, Yamada H, Kawaguchi Y, Tomonaga K, Miyazawa T, Kai C, Umemura T, Mikami T. Roles of the auxiliary genes and AP-1 binding site in the long terminal repeat of feline immunodeficiency virus in the early stage of infection in cats. J Virol. 1996;70:8518–8526. doi: 10.1128/jvi.70.12.8518-8526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z, Berson J F, Chen Y, Turner J D, Zhang T, Sharron M, Jenks M H, Wang Z, Kim J, Rucker J, Hoxie J A, Peiper S C, Doms R W. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc Natl Acad Sci USA. 1997;94:6426–6431. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAllister R M, Nicolson M, Gardner M B, Rongey R W, Rasheed S, Sarma P S, Huebner R J, Hatanaka M, Oroszlan S, Gilden R V, Kabigting A, Vernon L. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972;235:3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- 21.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazawa T, Kawaguchi Y, Kohmoto M, Sakuragi J, Adachi A, Fukasawa M, Mikami T. Production of feline immunodeficiency virus in feline and non-feline non-lymphoid cell lines by transfection of an infectious molecular clone. J Gen Virol. 1992;73:1543–1546. doi: 10.1099/0022-1317-73-6-1543. [DOI] [PubMed] [Google Scholar]
- 23.Miyazawa T, Kohmoto M, Kawaguchi Y, Tomonaga K, Toyosaki T, Ikuta K, Adachi A, Mikami T. The AP-1 binding site in the feline T lymphocytes. J Gen Virol. 1993;74:1573–1580. doi: 10.1099/0022-1317-74-8-1573. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa T, Tomonaga K, Kawaguchi Y, Kohmoto M, Inoshima Y, Maeda K, Mikami T, Maedadel K. Effects of insertion of multiple AP-1 binding sites into the U3 region of the long terminal repeat of feline immunodeficiency virus. Arch Virol. 1994;139:37–48. doi: 10.1007/BF01309453. . (Erratum, 140:621, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazawa T, Tomonaga K, Kawaguchi Y, Mikami T. The genome of feline immunodeficiency virus. Arch Virol. 1994;134:221–234. doi: 10.1007/BF01310563. [DOI] [PubMed] [Google Scholar]
- 26.Novotney C, English R V, Housman J, Davidson M G, Nasisse M P, Jeng C R, Davis W C, Tompkins M B. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS. 1990;4:1213–1218. doi: 10.1097/00002030-199012000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Olmsted R A, Barnes A K, Yamamoto J K, Hirsch V M, Purcell R H, Johnson P R. Molecular cloning of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:2448–2452. doi: 10.1073/pnas.86.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olmsted R A, Hirsch V M, Purcell R H, Johnson P R. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci USA. 1989;86:8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olmsted R A, Langley R, Roelke M E, Goeken R M, Adger-Johnson D, Goff J P, Albert J P, Packer C, Laurenson M K, Caro T M, Scheepers L, Wildt D E, Bush M, Martenson J S, O’Brien S J. Worldwide prevalence of lentivirus infection in wild feline species: epidemiologic and phylogenetic aspects. J Virol. 1992;66:6008–6018. doi: 10.1128/jvi.66.10.6008-6018.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen N C. The feline immunodeficiency virus. In: Levy J A, editor. The Retroviridae. New York, N.Y: Plenum Press; 1993. pp. 181–228. [Google Scholar]
- 31.Pedersen N C, Ho E W, Brown M L, Yamamoto J K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 32.Pleskoff O, Sol N, Labrosse B, Alizon M. Human immunodeficiency virus strains differ in their ability to infect CD4+ cells expressing the rat homolog of CXCR-4 (fusin) J Virol. 1997;71:3259–3262. doi: 10.1128/jvi.71.4.3259-3262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potempa S, Picard L, Reeves J D, Wilkinson D, Weiss R A, Talbot S J. CD4-independent infection by human immunodeficiency virus type 2 strain ROD/B: the role of the N-terminal domain of CXCR-4 in fusion and entry. J Virol. 1997;71:4419–4424. doi: 10.1128/jvi.71.6.4419-4424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remington K M, Chesebro B, Wehrly K, Pedersen N C, North T W. Mutants of feline immunodeficiency virus resistant to 3′-azido-3′-deoxythymidine. J Virol. 1991;65:308–312. doi: 10.1128/jvi.65.1.308-312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sparger E E, Shacklett B L, Renshaw-Gegg L, Barry P A, Pedersen N C, Elder J H, Luciw P A. Regulation of gene expression directed by the long terminal repeat of the feline immunodeficiency virus. Virology. 1992;187:165–177. doi: 10.1016/0042-6822(92)90305-9. [DOI] [PubMed] [Google Scholar]
- 36.Spearman C. The method of right and wrong cases (constant stimuli) without Gauss’s formulae. Br J Psychol. 1908;2:227–242. [Google Scholar]
- 37.Tachibana K, Nakajima T, Sato A, Igarashi K, Shida H, Iizasa H, Yoshida N, Yoshie O, Kishimoto T, Nagasawa T. CXCR4/fusin is not a species-specific barrier in murine cells for HIV-1 entry. J Exp Med. 1997;185:1865–1870. doi: 10.1084/jem.185.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi Y, Cosset F-L, Lachmann P J, Okada H, Weiss R A, Collins M K L. Type C retrovirus inactivation by human complement is determined by both the viral genome and the producer cell. J Virol. 1994;68:8001–8007. doi: 10.1128/jvi.68.12.8001-8007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talbott R L, Sparger E E, Lovelace K M, Fitch W M, Pedersen N C, Luciw P A, Elder J H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci USA. 1989;86:5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomonaga K, Miyazawa T, Kawaguchi Y, Kohmoto M, Inoshima Y, Mikami T. Comparison of the Rev transactivation of feline immunodeficiency virus in feline and non-feline cell lines. J Vet Med Sci. 1994;56:199–201. doi: 10.1292/jvms.56.199. [DOI] [PubMed] [Google Scholar]
- 41.Tozzini F, Matteucci D, Bandecchi P, Baldinotti F, Poli A, Pistello M, Siebelink K H, Ceccherini-Nelli L, Bendinelli M. Simple in vitro methods for titrating feline immunodeficiency virus (FIV) and FIV neutralizing antibodies. J Virol Methods. 1992;37:241–252. doi: 10.1016/0166-0934(92)90026-a. [DOI] [PubMed] [Google Scholar]
- 42.Wagaman P C, Hasselkus-Light C S, Henson M, Lerner D L, Phillips T R, Elder J H. Molecular cloning and characterization of deoxyuridine triphosphatase from feline immunodeficiency virus (FIV) Virology. 1993;196:451–457. doi: 10.1006/viro.1993.1501. [DOI] [PubMed] [Google Scholar]
- 43.Waters A K, De Parseval A P, Lerner D L, Neil J C, Thompson F J, Elder J H. Influence of ORF2 on host cell tropism of feline immunodeficiency virus. Virology. 1996;215:10–16. doi: 10.1006/viro.1996.0002. [DOI] [PubMed] [Google Scholar]
- 44.Willett B J, Hosie M J, Jarrett O, Neil J C. Identification of a putative cellular receptor for feline immunodeficiency virus as the feline homologue of CD9. Immunology. 1994;81:228–233. [PMC free article] [PubMed] [Google Scholar]
- 45.Willett B J, Picard L, Hosie M J, Turner J D, Adema K, Clapham P R. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J Virol. 1997;71:6407–6415. doi: 10.1128/jvi.71.9.6407-6415.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willey R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Temin H M. Rate and mechanism of nonhomologous recombination during a single cycle of retroviral replication. Science. 1993;259:234–238. doi: 10.1126/science.8421784. [DOI] [PubMed] [Google Scholar]