Abstract
The small GTPase RhoA and the downstream Rho kinase (ROCK) regulate several cell functions and pathological processes in the vascular system that contribute to the age‐dependent risk of cardiovascular disease, including endothelial dysfunction, excessive permeability, inflammation, impaired angiogenesis, abnormal vasoconstriction, decreased nitric oxide production and apoptosis. Frailty is a loss of physiological reserve and adaptive capacity with advanced age and is accompanied by a pro‐inflammatory and pro‐oxidative state that promotes vascular dysfunction and thrombosis. This review summarises the role of the RhoA/Rho kinase signalling pathway in endothelial dysfunction, the acquisition of the pro‐thrombotic state and vascular ageing. We also discuss the possible role of RhoA/Rho kinase signalling as a promising therapeutic target for the prevention and treatment of age‐related cardiovascular disease.
Keywords: ageing, cardiovascular disease, endothelial dysfunction, frailty, RhoA/rho kinase pathway, thrombosis
1. INTRODUCTION
The progressive ageing of the world population is resulting in a higher prevalence of age‐related disorders, including frailty and cardiovascular disease (CVD). 1 , 2 In recent decades, frailty has emerged as an underlying health condition that largely explains the most concerning health problems in older adults, which include hospitalisation, disability, falls and a high mortality risk. 3 Initially confined to the elderly, frailty now increasingly affects younger individuals, in whom it shows a strong association with multimorbidity and, as in the elderly population, is an important risk factor for harmful events. 4 These findings position frailty as an important focus of public health efforts.
Frailty syndrome describes a clinically recognisable complex state in older adults who exhibit increased vulnerability and dependency caused by gradual and progressive abnormal functioning of multiple organ systems. 5 In recent years, there has been a growing interest in the two‐way relationship between frailty syndrome and CVD. 6 , 7 The disability precipitated by frailty contributes to the appearance of CVD in the elderly; conversely, clinical and subclinical vascular disease are risk factors for frailty. 6 , 7 This interaction between vascular alterations and frailty appears to operate from early stages, with the risk of frailty showing an association with elevated levels of the endothelial dysfunction marker ADMA (asymmetric dimethylarginine) in individuals without atherosclerotic disease. 8 ADMA is a metabolic byproduct of the continuous metabolism of proteins in the organism and acts as a competitive inhibitor of the enzyme eNOS. Therefore, it is commonly used as endogenous marker of endothelial dysfunction. 9 This association suggest a relevant role of vascular system dysfunction as one of the main mechanisms leading to frailty. Progression from endothelial dysfunction to thrombus formation is dependent on platelet adhesion, since the alteration of endothelium‐platelet interaction is a well‐recognized contributor to prothrombotic states. 10 However, there has been little research into the relationship between endothelial dysfunction in frail elderly people and the early actions of platelets before thrombotic cardiovascular events such as myocardial infarction and stroke. Identification of the mechanisms underlying endothelial and/or platelet alterations that lead to cardiovascular events in frailty could contribute to measures to detect, treat and prevent CVD risk in frail elderly people.
Rho GTPases regulate multiple cellular processes, such as cytoskeletal reorganisation, cell migration, microtubule dynamics, signal transduction and gene expression, 11 and there is a large body of evidence that activation of the RhoA/Rho kinase pathway plays a major role in various forms of CVD and acts as a convergent node in the pathogenesis of endothelial dysfunction. 12 , 13 , 14 Endothelial injury in rats and RhoA/Rho kinase activation in stress‐treated EC cultures are associated with increased levels of biomarkers found in frail human adults, such as ADMA, endothelin 1 and 8‐isoprostane, suggesting a possible implication of the RhoA/Rho kinase pathway in the endothelial dysfunction occurring in frailty. 15 , 16 , 17 However, studies to date have not specifically addressed the direct contribution of RhoA/Rho kinase pathway activation to frailty syndrome or its role in the increased thrombosis risk in frail older adults.
This review summarises the role of RhoA/Rho kinase signalling in endothelial dysfunction, thrombosis and vascular aging and its possible role as a promising therapeutic target for the prevention and treatment of CVD in the elderly population.
2. POPULATION AGING
The world population is ageing rapidly due to increased life expectancy and decreased fertility rates. 18 , 19 World Health Organization figures show that the world population older than 60 years more than doubled from 382 million in 1980 to 962 million in 2017 (11% of the total world population). This figure is projected to double again by 2050 to reach nearly 2.1 billion, representing an estimated 22% of the total population. 1 , 20 This trend presents an immense challenge for health care systems because aging, particularly unhealthy aging, entails the loss of intrinsic biological capacity and an increased risk of developing chronic diseases (including dementia, diabetes, hypertension, obesity and kidney disease) and the combination of frailty and dependency characteristic of frailty syndrome 21 , 22 (Figure 1). This compendium of ageing‐related changes results in significant loss of life quality and social activity and a high risk of disability. 22 , 23 , 24 , 25
FIGURE 1.
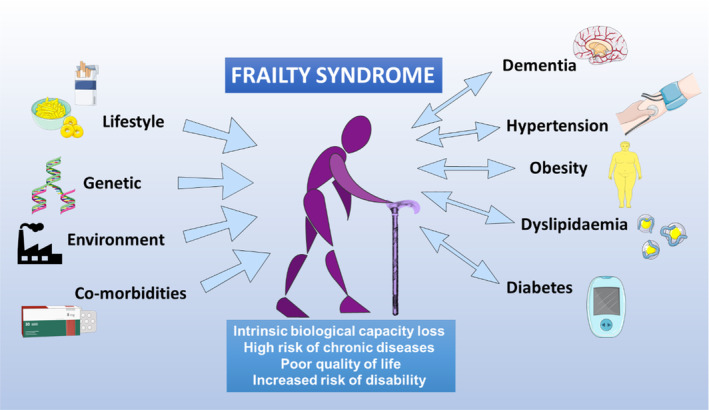
Frailty syndrome. Frailty is characterised by a significant decrease among elderly people in their biological capacity to confront the challenges of daily life. The multiple factors that can trigger the appearance of frailty include lifestyle, genetic background, environmental quality and the existence of co‐morbidities. Frailty status is an important risk factor for chronic diseases associated with old age, which themselves promote the development of frailty.
3. FRAILTY SYNDROME IN OLDER ADULTS
Older adults are a highly heterogeneous group, with different genetic, biological and environmental backgrounds and life histories. Consequently, older adults of the same chronological age can have different biological ages. 22 , 26 Personal biological age can be estimated from a person's frailty index, which is a sensitive predictor of survival. 27 , 28 , 29 The frailty index is a continuous grading of age‐related deficit accumulation that provides a threshold above which the loss of physiological reserve and adaptation capacity manifests as functional deterioration. 30 , 31 Since several of the biological processes of ageing are modifiable, identifying and preventing frailty syndrome in older people is essential for extending healthy lifespan. 32 , 33 The term frailty thus describes a subset of older adults who appear weaker and more vulnerable than their age‐matched counterparts, despite having similar comorbidities and demographic characteristics. 34
The most widely used measure of frailty is that proposed by Linda Fried et al. 35 for a population older than 65 years and defined as the frailty phenotype. Older adults with frailty syndrome have a markedly elevated risk of falls, hospitalisation and death, with frailty being one of the best predictors of worsening mobility or difficulty in performing activities of daily living (ADL disabilities). 22 Recently, a frailty syndrome diagnosis and clinical follow‐up scale has been developed, called “frailty trait scale”. This scale is based on Fried's frailty phenotype but expands its range of evaluation to include all domains of the syndrome, providing a helpful tool for research and clinical practice. 36 , 37
In an observational study, frailty syndrome was present in 25%–50% of men and women 65 years and older with chronic diseases such as hypertension, obesity, dyslipidemia, dementia and diabetes. 38 Frailty syndrome has been proposed as a prognostic and risk stratification factor for coronary heart disease and slow gait speed (an increased time that a person takes to walk a specified distance on a surface over a short distance), showing a higher association than other parameters (OR: 3.8). 35 , 39 , 40 , 41 In a systematic review of 9 studies encompassing 54,250 patients older than 60 years with severe coronary artery disease or heart failure, the prevalence of frailty was 50%–54%, and this was associated with an OR of 1.6 to 4.0 for all‐cause mortality over a mean weighted follow‐up of 6.2 years. 42
A meta‐analysis of frailty syndrome among persons older than 60 years in Latin America and the Caribbean detected a mean prevalence of 19.6% (95%CI 15.4–24.3), with values ranging from 7.7% to 42.6%. 43 The highest prevalence in this range (42.6%) was detected in a cohort of 1301 older adults from Santiago, Chile, analysed in 2008. 44 However, other studies in Chilean adults older than 60 years in the Maule and Santiago Metropolitan regions reported prevalence values of 24.6% and 13.9%, respectively, 45 , 46 with the latter value similar to the Latin American average. Nevertheless, these values are much higher than those observed in European countries such as Spain (8.4%) and Germany (2.8%). 2 , 47 The latter could be related to the socioeconomic and quality of life differences between Latin America and Europe, since frailty is related with cognitive functioning, educational level and nutritional status in older adults.
4. FRAILTY SYNDROME AND VASCULAR INJURY
Ageing is associated with a series of structural and molecular changes in the vasculature, independently of other cardiovascular risk factors. 48 These changes involve a disruption of the balance between vasoconstrictor and vasodilator molecules, which leads to a decrease in nitric oxide (NO) availability and an increase in the production of reactive oxygen species (ROS) associated with mitochondrial dysfunction. 49 , 50 , 51 , 52 , 53 There is also strong experimental and clinical evidence that aging is accompanied by low‐grade inflammation, termed inflammaging. 54 Inflammaging has been detected in numerous mouse models and in older human adults and is characterized by increased circulating levels of pro‐inflammatory interleukins such as interleukin (IL)‐6 and IL‐1β. 55 , 56
Frailty syndrome has been widely characterised as a pro‐inflammatory and oxidative phenomenon that promotes vascular dysfunction, 8 , 51 , 57 , 58 , 59 , 60 , 61 , 62 triggering platelet activation and adhesion to activated endothelium through increased cytokine release and expression of adhesion molecules. 63 However, knowledge is limited about molecular and cellular mechanisms through which frail adults develop endothelial dysfunction and its potential role in platelet activation. Next, we present the main findings found in frail older people.
4.1. Platelet activation and thrombosis risk
Thrombosis risk increases significantly with age, and thrombosis is a common risk factor of morbidity and mortality in frail older people aged 65 years and older, 64 , 65 , 66 who have an elevated risk of thrombotic events (OR: 1.79, 95%CI 1.02–3.13). 66 In the initial stage of atherosclerosis, platelets adhere to the damaged endothelium and secrete molecules that amplify endothelial dysfunction, such as inflammatory mediators, chemokines, TNF superfamily factors and adhesion proteins. 67 , 68 In the final stage, after plaque rupture, platelets adhere to the damaged endothelium and aggregate to form a thrombus, blocking tissue irrigation and oxygenation. 69 The pathological mechanisms underlying the elevated thrombosis risk in frail older adults are not fully understood. 34 , 70
Evidence acquired in recent years links frailty syndrome to abnormalities in platelets that trigger their activation. 71 , 72 , 73 Compared with healthy young people and non‐frail older adults, frail patients have higher levels of platelet activation, demonstrated by increased P‐selectin expression 58 , 74 and a significantly higher increase in the binding of PAC‐1 (active glycoprotein [GP] IIb/IIIa) upon stimulation with 1 μM adenosine diphosphate (ADP). 72
The diagnosis of frailty is normally based on specific clinical criteria, and there is a clear need to identify and validate robust biomarkers for this condition. 34 Recent work by our group showed that frail adults older than 64 years have higher levels of platelet aggregation and activation (indexed by P‐selectin exposure and activated GPIIb/IIIa) than age‐matched non‐frail older adults, as well as higher plasma levels of thromboxane B2, 8‐isoprostane and growth differentiation factor (GDF)‐15 (a biomarker of mitochondrial dysfunction and cellular senescence). 75 We have also shown that frail older adults have higher concentrations of platelet‐derived microvesicles (P2RY12+/AV+). 76 This increased platelet activity in frail adults is associated with a decreased response to the antiplatelet drug acetylsalicylic acid. 77 , 78
Moreover, there is increasing evidence that platelets play a key role in the pathogenesis of vascular injury. Circulating activated platelets secrete a wide variety of molecules that favour the onset and progression of endothelial damage, such as cytokines, chemokines, TNF superfamily ligands, metalloproteinases, and other mediators. 10 , 79
4.2. Endothelial dysfunction
Endothelial dysfunction is an important contributor to atherosclerosis 80 and its thrombotic complications. 67 , 81 , 82 Endothelial cells (ECs) are a principal target through which ageing promotes vascular deterioration. 83 Frailty has been linked to endothelial dysfunction, 8 evidenced by increases in key markers: adhesion intercellular molecule 1 (ICAM‐1), 78 , 84 endothelin‐1, 85 Von Willebrand factor (VWF), 86 thrombomodulin, 87 ADMA, 8 IL‐6 88 and c‐reactive protein. 89 ICAM‐1 and VWF favour platelet adhesion, whereas endothelin‐1 induces platelet activation in patients with coronary heart disease or myocardial infarction. 90 As previously mentioned, ADMA is an endogenous inhibitor of NO synthase (NOS) and an independent cardiovascular risk factor, 91 , 92 and frailty has been associated with increasing levels of ADMA in subjects without atherosclerotic disease. 8 Likewise, IL‐6 and C‐reactive protein contribute to the prothrombotic state, 93 and IL‐6 has been positively associated with frailty in men. 94 Besides, C‐reactive protein increases with age, and increased plasma levels have been proposed as a biological component of frailty. 95
The term endothelial dysfunction encompasses several forms of abnormal endothelial activity, including impaired production of messenger molecules and increased expression of proinflammatory molecules. 96 A hallmark of endothelial dysfunction is decreased NO availability, due either to enhanced inactivation or to reduced synthesis. 97 One of the most important contributors to endothelial dysfunction is oxidative stress, which is characterised by an imbalance between the generation of endogenous ROS and antioxidant defence mechanisms. 98 In fact, frailty and pre‐frailty seem to be associated with higher oxidative stress, 59 , 99 thus supporting a possible mechanistic basis for associating frailty with endothelial dysfunction.
5. THE RhoA/RHO KINASE PATHWAY
The RhoA is one of the best‐known members of a large family of small GTPases that includes Rho, Rac and Cdc42 members. RhoA and its downstream targets and effector proteins, the Rho kinases, play important roles in many cellular functions, particularly cellular cytoskeletal reorganization 100 (Figure 2). The two known Rho kinase isoforms are ROCK1 (ROCK β) and ROCK 2 (ROCK α), which share 65% sequence homology. Both isoforms are expressed in ECs, with ROCK1 localized in plasma membrane and ROCK2 in the cytoplasm. 101 In ECs, the RhoA/Rho kinase pathway inhibits NO production, 102 and excessive pathway activity induces oxidative stress and promotes CVD development. 102 ROCK1 and ROCK 2 are both upregulated by angiotensin II and interleukin 1β, but whereas ROCK1 is cleaved by caspase 3 (an important step in erythroblast development), ROCK2 is cleaved by granzyme B released by cytotoxic lymphocytes, basophils, mast cells and vascular smooth muscle cells (VSMCs), implicating thus the inflammatory action of granzyme B in the activation of ROCK2. Also, ROCK2 is the main Rho kinase isoform in cells of the cardiovascular system. 102 Previous work from our group has shown that abnormal RhoA/Rho kinase pathway activation contributes to multiple pathological processes associated with thrombotic complications, such as metabolic syndrome, 13 inflammatory bowel disease 103 and cocaine‐related cardiovascular pathology. 104
FIGURE 2.
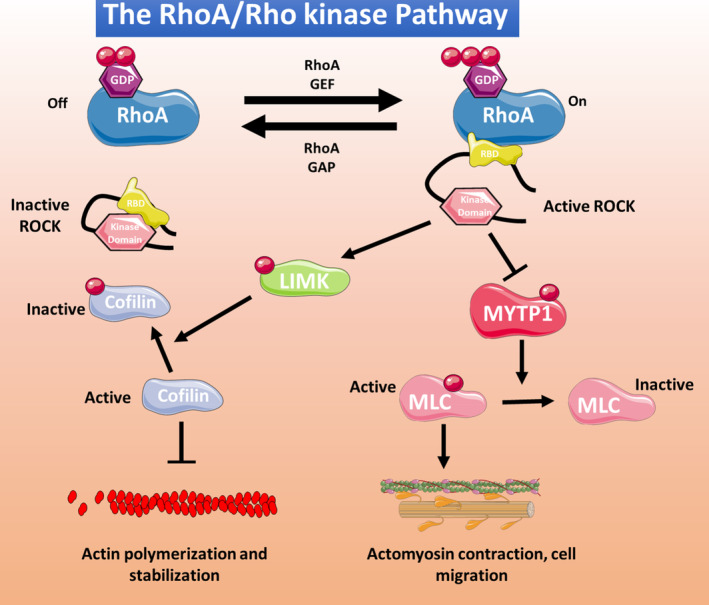
Classical RhoA/Rho kinase pathway. Different stimuli can induce RhoA activation, through guanosine exchange factors (GEF) or inhibition through GTPase‐activating protein (GAP). Activated RhoA interacts with the Rho‐binding domain (RBD) domain of Rho kinase, releasing and activating the kinase domain. Activated Rho kinase phosphorylates multiple cell targets, including LIM kinase (LMK) and myosin phosphatase target subunit 1 (MYTP1). Phosphorylated LIMK phosphorylates, and thus inactivates, cofilin, inducing actin polymerization and stabilization. Phosphorylated MYTP1 is inactivated, resulting in the stabilization of the phosphorylated and active form of myosin light chain (MLC), thus promoting actomyosin contraction and cell migration.
6. THE RhoA/RHO KINASE PATHWAY IN AGE‐RELATED FEATURES OF VASCULAR DYSFUNCTION
6.1. RhoA/Rho kinase in endothelial dysfunction and prothrombotic conditions
The RhoA/Rho kinase pathway is important for normal endothelial homeostasis, although studies with endothelial‐specific RhoA knockouts mice demonstrate that its lack of function can be compensated during the embryonic development. 11 Nevertheless, abnormal activity can lead to EC dysfunction. 105 RhoA/Rho kinase signalling plays a pivotal mechanosensory role in actin dynamics in ECs, promoting cell contraction and endothelial sensitivity. 106 Moreover, it has been proposed that the basal activity of RhoA/Rho kinase signalling mediates normal intrinsic barrier‐protective activity at EC margins, but abnormal activation by vasoactive agents (as thrombin) can disrupt this barrier, facilitating the breakdown of intercellular junctions and increasing endothelial permeability. 107
The RhoA/Rho kinase pathway is an important suppressor of endothelial NO synthase (eNOS) and increases oxidative stress. 108 , 109 RhoA/Rho kinase activation also upregulates the proapoptotic protein Bax through the tumour suppressor protein p53 to induce a mitochondrial death pathway. 110 There is evidence that RhoA/Rho kinase pathway activation by PKC underlies the increase in arginase I expression and activity induced by oxidative stress in bovine aortic ECs, related to a decrease in NO production due to the competition with eNOS for the substrate arginine. 111
The RhoA/Rho kinase pathway is involved in endothelial microvasculature damage caused by lipopolysaccharide (LPS). The anti‐inflammatory effect of catalpol, the major active compound in Rehmannia glutinosa, protects against LPS‐induced blood–brain barrier disruption by decreasing RhoA and ROCK2 mRNA and protein expression, reversing LPS‐induced cytoskeletal actin disaggregation in brain microvascular mouse ECs. 112 In rat pulmonary microvascular ECs, the pro‐apoptotic effect of LPS appears to be mediated by Rho/Rho kinase with JNK and p38 MAPKs as downstream effectors, since the ROCK inhibitor fasudil blocked JNK and p38 activation and the appearance of apoptosis markers. 113
The RhoA/Rho kinase pathway is also involved in impaired angiogenesis and focal adhesion dysregulation. 105 , 114 Angiogenesis is essential for physiological vascular function and recovery from ischemic conditions. In fact, there is a relationship between endothelial dysfunction and impaired NO production with angiogenic impairment, which contributes to age‐related decline in microvascular density, decreased myocardial blood supply, impaired capacity to adapt at hypoxia, and exacerbated ischemic tissue injury. 83 Thus, impaired angiogenesis is closely related to endothelial dysfunction and CVD. Endothelial homeostasis is crucially regulated by the Gα‐coupled heptahelical thromboxane A2 receptor, and the thromboxane A2 receptor/Gα13/RhoA/C/Rho kinase/LIMK2 pathway inhibits VEGF‐mediated human umbilical vein EC sprouting and promotes EC tension and focal adhesion dysregulation. 114
The RhoA activity exerts an inhibitory effect on the angiogenic capacity. The expression of constitutively active RhoA (G14V/Q63L) in HUVEC inhibits endothelial proliferation, migration, tube formation and in vitro angiogenic sprouting, which I abrogated with a non‐active dominant‐negative version of RhoA (T19N). However, this induction of endothelial dysfunction and antiangiogenic effects by active RhoA seems to be independent of its downstream effectors, ROCK and LIMK. 105 On the other hand, RhoA/ROCK pathway may be involved in pathological angiogenesis. Xueke et al. 115 showed that the compound erianin inhibits pathological angiogenesis in vitro and neovascularization in vivo in a hypoxia‐induced retinopathy in adults and embryonic zebrafish, by inhibiting collagen binding to α2 and β1 integrins and suppressing the intracellular RhoA/ROCK1 signalling pathway. In the same line, Yoshifumi et al. 116 reported that ripasudil (ROCK inhibitor) prevented retinal edema, reduced the size of the nonperfusion area and improved retinal blood flow in a murine model of retinal vein occlusion by suppressing retinal phosphorylation of MYPT‐1 and inhibited disorganisation of tight junctions 1 in human retinal microvascular endothelial cells. The increase in vascular resistance and rigidity is associated with vascular stiffness, and when it is deregulated in vascular smooth muscle cells, it is a major cause of cardiovascular disorders. 117
This range of actions establishes RhoA/Rho kinase pathway activation and dysregulation as a major cause of ageing‐associated vascular dysfunction and suggests that it may be an attractive therapeutic target. 12 Studies with specific RhoA/Rho kinase inhibitors have shown multiple benefits, for example in the control of blood pressure, decreased cardiac damage in ischemia/reperfusion models, an enhanced vascular antioxidant response and normalization of vascular parameters and NO production in a mouse model of age‐induced endothelial dysfunction. 118 , 119 , 120 , 121 , 122 , 123 In an ex vivo study, Pereira et al. demonstrated an increased circulating endothelial cells and an increased activity of RhoA kinase activity by MYPT1‐P/T phosphorylated in circulating leukocytes from cocaine‐dependent individuals and in aortic cells from cocaine‐treated rats. Atorvastatin and the Rho kinase inhibitor Y‐27632 protect endothelial function in vitro by inhibiting pro‐adhesive and prothrombotic changes induced by cocaine or plasma from chronic cocaine consumers. 104 , 124 These data suggest that activation of RhoA/Rho kinase pathway plays a key role in endothelial dysfunction induced by injuries like cocaine consumption and that inhibition of this pathway may provide therapeutic benefits.
6.2. RhoA/Rho kinase in increased vasoconstriction and hypertension
The RhoA/Rho kinase activation has been observed in patients with heart failure, a population with high prevalence of frailty (~79%), and the Rho kinase inhibitor fasudil reduces vascular resistance and improves vasodilation. 125 , 126 RhoA/Rho kinase activation in arteries has also been shown in mouse models of aging 127 and has been suggested to contribute to age‐related blood pressure elevation, possibly via greater peripheral vasoconstrictor tone in older adults. 128 There are also several studies linking RhoA/Rho kinase to both natural and induced senescence in various cell types, including annulus fibrosus cells, 129 kidney cells, 130 internal anal sphincter smooth muscle cells, 131 corpus callosum cells 132 and mesenteric arterial smooth muscle. 133
There is also abundant evidence implicating the RhoA/Rho kinase pathway in VSMC hypercontraction, VSMC proliferation and migration in the media, inflammatory cell accumulation in the adventitia, inhibition of NO production and increased oxidative stress. 134 , 135 , 136 , 137 In another study, the vascular proteome of wild‐type male C57BL/6 mice was analysed by hierarchical clustering to detect proteins showing significant age‐related changes. In this analysis, several proteins associated with the RhoA/Rho kinase pathway showed changes consistent with hypertension and cerebral perfusion dysregulation, suggesting that the RhoA/Rho kinase pathway is an important target for age‐dependent hypertension. 138
The RhoA/Rho kinase pathway has been linked to age‐dependent VSMC dysfunction, including the age‐associated decrease in contractile function. In soleus muscle feed arteries from aged (24‐month‐old) rats, elevated levels of pROCK1 and pROCK2 were associated with depressed contractile ability, α‐actin stress fibres, recruitment of proteins to cell‐matrix adhesions and an increase in integrin adhesion to the matrix related with increased cell stiffness. 139 In mouse VSMCs, a non‐canonical Wnt5a/RhoA activation pathway shows a putative association with age‐related salt‐sensitive hypertension by increasing calcium sensitivity triggered by the decline in the protein Klotho with age. 140 The RhoA/Rho kinase pathway has also been linked to vascular reactivity and dysregulation associated with phenylephrine‐induced vasoconstriction. In aging spontaneously hypertensive rats, ROCK‐2 expression and activity are excessively increased, accompanied by decreased myosin light chain phosphatase (MLCP) activity and an increase in phosphorylated MLC, leading in turn to increased α1‐adrenergic‐induced vasoconstriction. 133
7. CONCLUSIONS
The RhoA/Rho kinase pathway is a crucial signalling component in the vascular system and particularly in ECs and has been extensively studied in diverse pathophysiological settings, including endothelial dysfunction, impaired angiogenesis, inflammation and apoptosis. In recent years, attention has focused on the emerging association between RhoA/Rho kinase signalling components and cell senescence and ageing in diverse cell types. However, further research is needed to define the role of the RhoA/Rho kinase pathway in vascular aging. The findings reviewed here suggest that RhoA/Rho kinase pathway activity in endothelial dysfunction is highly relevant to frailty syndrome and could provide a promising route to the development of therapeutic interventions to prevent vascular aging (Figure 3).
FIGURE 3.
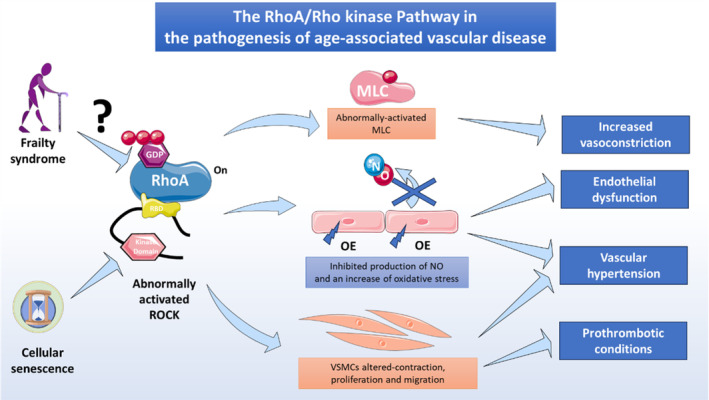
RhoA/Rho kinase pathway activation in age‐related vascular disease. Abnormal activation of the RhoA/Rho kinase pathway has been linked to aging and cellular senescence. Frailty syndrome, directly related with unhealthy ageing, may increase the risk of abnormal RhoA/Rho kinase activation, in turn triggering pathological cell mechanisms that lead to age‐related vascular disease and promote a prothrombotic status in elderly people. MLC, myosin light chain; OE, oxidative stress; VSMCs, vascular smooth muscle cells.
AUTHOR CONTRIBUTIONS
Iván Palomo: Conceptualization (equal); investigation (equal); writing – original draft (equal). Sergio Wehinger: Investigation (equal); writing – original draft (equal). Vicente Andrés: Conceptualization (equal); writing – review and editing (equal). Francisco J. García‐García: Investigation (equal); writing – review and editing (equal). Eduardo Fuentes: Conceptualization (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
ACKNOWLEDGEMENTS
We thank Simon Bartlett for English editing. This research was funded by ANID/FONDECYT grant no. 1220339, ANID/FONDECYT grant no. 1211136 and ANID‐FONDEQUIP No. EQM200049 (Flow Cytometry). We would also like to thank ANID‐Ring ACT210097 (MIBI: Interdisciplinary Group on Mitochondrial Targeting and Bioenergetics), ANID‐FOVI210024, ANID‐FOVI210022, ANID‐FOVI220021, the Spanish Ministerio de Ciencia, Innovación y Universidades and Agencia Estatal de Investigación (MICIN/AEI/10.13039/501100033, grant no. PID2022‐141211OB‐I00), with co‐funding from the European Social Fund (ESF) (The ESF‐Investing in your future), and Fundación Ramón Areces (grant no. CIVP21S13281). The CNIC is supported by the Ministerio de Ciencia e Innovación, the Instituto de Salud Carlos III, the Pro‐CNIC Foundation and is a Severo Ochoa Center of Excellence (grant number CEX2020‐001041‐S funded by MCIN/AEI/10.13039/501100011033).
Palomo I, Wehinger S, Andrés V, García‐García FJ, Fuentes E. RhoA/rho kinase pathway activation in age‐associated endothelial cell dysfunction and thrombosis. J Cell Mol Med. 2024;28:e18153. doi: 10.1111/jcmm.18153
DATA AVAILABILITY STATEMENT
Data derived from public domain resources.
REFERENCES
- 1. United Nations Department of Economic and Social Affairs Population Division . World Population Ageing 2017 – Highlights (ST/ESA/SER.A/397). 2017.
- 2. Garcia‐Garcia FJ, Gutierrez Avila G, Alfaro‐Acha A, et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo study for healthy aging. J Nutr Health Aging. 2011;15(10):852‐856. [DOI] [PubMed] [Google Scholar]
- 3. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365‐1375. [DOI] [PubMed] [Google Scholar]
- 4. Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre‐frailty in middle‐aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK biobank participants. Lancet Public Health. 2018;3(7):e323‐e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Uchmanowicz I, Mlynarska A, Lisiak M, et al. Heart failure and problems with frailty syndrome: why it is time to care about frailty syndrome in heart failure. Card Fail Rev. 2019;5(1):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alonso‐Bouzón C, Carcaillon L, García‐García FJ, Amor‐Andrés MS, El Assar M, Rodríguez‐Mañas L. Association between endothelial dysfunction and frailty: the Toledo study for healthy aging. Age. 2014;36(1):495‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stuhlinger MC, Oka RK, Graf EE, et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108(8):933‐938. [DOI] [PubMed] [Google Scholar]
- 10. Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: from pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. 2018;8(5):568‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zahra FT, Sajib MS, Ichiyama Y, et al. Endothelial RhoA GTPase is essential for in vitro endothelial functions but dispensable for physiological in vivo angiogenesis. Sci Rep. 2019;9(1):11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao L, Romero MJ, Toque HA, Yang G, Caldwell RB, Caldwell RW. The role of RhoA/rho kinase pathway in endothelial dysfunction. J Cardiovasc Dis Res. 2010;1(4):165‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leguina‐Ruzzi A, Pereira J, Pereira‐Flores K, et al. Increased RhoA/rho‐kinase activity and markers of endothelial dysfunction in young adult subjects with metabolic syndrome. Metab Syndr Relat Disord. 2015;13(9):373‐380. [DOI] [PubMed] [Google Scholar]
- 14. Satoh K, Fukumoto Y, Shimokawa H. Rho‐kinase: important new therapeutic target in cardiovascular diseases. Am J Physiol Heart and Circ Physiol. 2011;301(2):H287‐H296. [DOI] [PubMed] [Google Scholar]
- 15. Yi SL, Kantores C, Belcastro R, Cabacungan J, Tanswell AK, Jankov RP. 8‐Isoprostane‐induced endothelin‐1 production by infant rat pulmonary artery smooth muscle cells is mediated by rho‐kinase. Free Radic Biol Med. 2006;41(6):942‐949. [DOI] [PubMed] [Google Scholar]
- 16. Tsou PS, Amin MA, Campbell PL, et al. Activation of the thromboxane A2 receptor by 8‐Isoprostane inhibits the pro‐Angiogenic effect of vascular endothelial growth factor in scleroderma. J Invest Dermatol. 2015;135(12):3153‐3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao Y, Fang Y, Mu J, Liu X. High salt medium activates RhoA/ROCK and downregulates eNOS expression via the upregulation of ADMA. Mol Med Rep. 2016;14(1):606‐612. [DOI] [PubMed] [Google Scholar]
- 18. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. The Lancet. 2014;383(9913):245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716‐719. [DOI] [PubMed] [Google Scholar]
- 20. Bloom DE, Boersch‐Supan A, McGee P, Seike A. Population aging: facts, challenges, and responses. J Int Compens Benefits. 2011;41(1):22. [Google Scholar]
- 21. Liotta G, Gilardi F, Orlando S, et al. Cost of hospital care for the older adults according to their level of frailty. A cohort study in the Lazio region, Italy. PloS One. 2019;14(6):e0217829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kojima G, Liljas AEM, Iliffe S. Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen CY, Gan P, How CH. Approach to frailty in the elderly in primary care and the community. Singapore Med J. 2018;59(5):240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang F, Gu D, Mitnitski A. Frailty and life satisfaction in Shanghai older adults: the roles of age and social vulnerability. Arch Gerontol Geriatr. 2016;67:68‐73. [DOI] [PubMed] [Google Scholar]
- 25. Fairhall N, Sherrington C, Kurrle SE, et al. Economic evaluation of a multifactorial, interdisciplinary intervention versus usual care to reduce frailty in frail older people. J Am Med Dir Assoc. 2015;16(1):41‐48. [DOI] [PubMed] [Google Scholar]
- 26. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):919‐930. [DOI] [PubMed] [Google Scholar]
- 27. Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late‐life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bersani FS, Canevelli M, Cesari M, et al. Frailty index as a clinical measure of biological age in psychiatry. J Affect Disord. 2020;268:183‐187. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20‐years follow‐up. Elife. 2020;9:e51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell A, Converse PE, Rodgers WL. The Quality of American Life: Perceptions, Evaluations, and Satisfactions. Russell Sage Foundation; 1976. [Google Scholar]
- 31. Hazzard WR, Blass JP, Halter JB, Ouslander JG. Principles of Geriatric Medicine and Gerontology. McGraw‐Hill; 1990. [Google Scholar]
- 32. Bland JS. Age as a modifiable risk factor for chronic disease. Integr Med (Encinitas). 2018;17(4):16‐19. [PMC free article] [PubMed] [Google Scholar]
- 33. Voskamp MJH, Vermeer M, Molijn GJ, Cornel EB. The usefulness of the modified frailty index for muscle‐invasive bladder cancer patients treated with radical cystectomy. Curr Urol. 2020;14(1):32‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saedi AA, Feehan J, Phu S, Duque G. Current and emerging biomarkers of frailty in the elderly. Clin Interv Aging. 2019;14:389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146‐M157. [DOI] [PubMed] [Google Scholar]
- 36. Garcia‐Garcia FJ, Carcaillon L, Fernandez‐Tresguerres J, et al. A new operational definition of frailty: the frailty trait scale. J Am Med Dir Assoc. 2014;15(5):371 e7‐371 e13. [DOI] [PubMed] [Google Scholar]
- 37. García‐García FJ, Carnicero JA, Losa‐Reyna J, et al. Frailty trait scale‐short form: a frailty instrument for clinical practice. J Am Med Dir Assoc. 2020;21(9):1260‐1266.e2. [DOI] [PubMed] [Google Scholar]
- 38. Ierodiakonou D, Kampouraki M, Poulonirakis I, et al. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm Med. 2019;19(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sanchis J, Bonanad C, Ruiz V, et al. Frailty and other geriatric conditions for risk stratification of older patients with acute coronary syndrome. Am Heart J. 2014;168(5):784‐791. [DOI] [PubMed] [Google Scholar]
- 40. Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, Alexander KP. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54(11):1674‐1681. [DOI] [PubMed] [Google Scholar]
- 41. Stewart R. Cardiovascular disease and frailty: what are the mechanistic links? Clin Chem. 2019;65(1):80‐86. [DOI] [PubMed] [Google Scholar]
- 42. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103(11):1616‐1621. [DOI] [PubMed] [Google Scholar]
- 43. Da Mata FAF, da Silva Pereira PP, de Andrade KRC, Figueiredo ACMG, Silva MT, Pereira MG. Prevalence of frailty in Latin America and the Caribbean: a systematic review and meta‐analysis. PloS One. 2016;11(8):e0160019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alvarado BE, Zunzunegui M‐V, Béland F, Bamvita J‐M. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63(12):1399‐1406. [DOI] [PubMed] [Google Scholar]
- 45. Palomo I, Giacaman RA, Leon S, et al. Analysis of the characteristics and components for the frailty syndrome in older adults from central Chile. The PIEI‐ES Study. ereArch Gerontol Geriatr. 2019;80:70‐75. [DOI] [PubMed] [Google Scholar]
- 46. Albala C, Lera L, Sanchez H, et al. Frequency of frailty and its association with cognitive status and survival in older Chileans. Clin Interv Aging. 2017;12:995‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buttery AK, Busch MA, Gaertner B, Scheidt‐Nave C, Fuchs J. Prevalence and correlates of frailty among older adults: findings from the German health interview and examination survey. BMC Geriatr. 2015;15(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barodka VM, Joshi BL, Berkowitz DE, Hogue CW Jr, Nyhan D. Review article: implications of vascular aging. Anesth Analg. 2011;112(5):1048‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrari AU, Radaelli A, Centola M. Invited review: aging and the cardiovascular system. J Appl Physiol (1985). 2003;95(6):2591‐2597. [DOI] [PubMed] [Google Scholar]
- 50. Matz RL, Schott C, Stoclet JC, Andriantsitohaina R. Age‐related endothelial dysfunction with respect to nitric oxide, endothelium‐derived hyperpolarizing factor and cyclooxygenase products. Physiol Res. 2000;49(1):11‐18. [PubMed] [Google Scholar]
- 51. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti‐tumor necrosis factor‐alpha treatment in aging. Am J Pathol. 2007;170(1):388‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tang X, Luo Y‐X, Chen H‐Z, Liu D‐P. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 2014;5:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. El Assar M, Angulo J, Rodríguez‐Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013;65:380‐401. [DOI] [PubMed] [Google Scholar]
- 54. Franceschi C, Bonafè M, Valensin S, et al. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244‐254. [DOI] [PubMed] [Google Scholar]
- 55. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: a systematic review and meta‐analysis. Ageing Res Rev. 2016;31:1‐8. [DOI] [PubMed] [Google Scholar]
- 57. Vatic M, von Haehling S, Ebner N. Inflammatory biomarkers of frailty. Exp Gerontol. 2020;133:110858. [DOI] [PubMed] [Google Scholar]
- 58. Marzetti E, Picca A, Marini F, et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty “cytokinome” at its core. Exp Gerontol. 2019;122:129‐138. [DOI] [PubMed] [Google Scholar]
- 59. Alvarez‐Satta M, Berna‐Erro A, Carrasco‐Garcia E, et al. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging. 2020;12(10):9982‐9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. El Assar M, Angulo J, Rodríguez‐Mañas L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic Biol Med. 2019;149:72‐77. [DOI] [PubMed] [Google Scholar]
- 61. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17(1):21‐30. [DOI] [PubMed] [Google Scholar]
- 62. Arenas IA, Xu Y, Davidge ST. Age‐associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF‐alpha antagonism. Am J Physiol Heart Circ Physiol. 2006;290(3):H1259‐H1263. [DOI] [PubMed] [Google Scholar]
- 63. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363‐2371. [DOI] [PubMed] [Google Scholar]
- 65. Engbers MJ, van Hylckama VA, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost. 2010;8(10):2105‐2112. [DOI] [PubMed] [Google Scholar]
- 66. Folsom AR, Boland LL, Cushman M, Heckbert SR, Rosamond WD, Walston JD. Frailty and risk of venous thromboembolism in older adults. J Gerontol A Biol Sci Med Sci. 2007;62(1):79‐82. [DOI] [PubMed] [Google Scholar]
- 67. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. 2014;276(6):618‐632. [DOI] [PubMed] [Google Scholar]
- 68. Otterdal K, Smith C, Oie E, et al. Platelet‐derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood. 2006;108(3):928‐935. [DOI] [PubMed] [Google Scholar]
- 69. Fuentes QE, Fuentes QF, Andres V, Pello OM, Font de Mora J, Palomo GI. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets. 2012;24(4):255‐262. [DOI] [PubMed] [Google Scholar]
- 70. Yayan J, Bals R. Relative risk of deep vein thrombosis in very elderly patients compared with elderly patients. Clin Appl Thromb Hemost. 2016;22(1):77‐84. [DOI] [PubMed] [Google Scholar]
- 71. Nguyen TN, Pepperell D, Morel‐Kopp M‐C, Cumming RG, Ward C, Hilmer SN. Effect of frailty and age on platelet aggregation and response to aspirin in older patients with atrial fibrillation: a pilot study. Cardiol Ther. 2016;5(1):51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shih L, Sanders N, Rondina S, et al. Activated platelet integrin alpha IIb Beta3 is increased in older adults with frailty but not in healthy aging. J Am Geriatr Soc. 2015;63:S110. [Google Scholar]
- 73. Hernández B, Fuentes E, Palomo I, Alarcón M. Increased platelet function during frailty. Exp Hematol. 2019;77:12‐25.e2. [DOI] [PubMed] [Google Scholar]
- 74. Layne K, Passacquale G, Ferro A. Chapter 4 – The role of platelets in the pathophysiology of atherosclerosis and its complications. In: Topaz O, ed. Cardiovascular Thrombus. Academic Press; 2018:51‐65. [Google Scholar]
- 75. Arauna D, Garcia F, Rodriguez‐Manas L, et al. Older adults with frailty syndrome present an altered platelet function and an increased level of circulating oxidative stress and mitochondrial dysfunction biomarker GDF‐15. Free Radic Biol Med. 2020;149:64‐71. [DOI] [PubMed] [Google Scholar]
- 76. Arauna D, Chiva‐Blanch G, Padró T, Fuentes E, Palomo I, Badimon L. Frail older adults show a distinct plasma microvesicle profile suggesting a prothrombotic and proinflammatory phenotype. J Cell Physiol. 2021;236(3):2099‐2108. [DOI] [PubMed] [Google Scholar]
- 77. Williams FM, Wynne H, Woodhouse KW, Rawlins MD. Plasma aspirin esterase: the influence of old age and frailty. Age Ageing. 1989;18(1):39‐42. [DOI] [PubMed] [Google Scholar]
- 78. Hubbard RE, O'Mahony MS, Calver BL, Woodhouse KW. Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008;64(9):895‐900. [DOI] [PubMed] [Google Scholar]
- 79. Becker RC, Sexton T, Smyth SS. Translational implications of platelets as vascular first responders. Circ Res. 2018;122(3):506‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gimbrone MA Jr, Garcia‐Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620‐636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kyrle PA, Eichinger S. Is Virchow's triad complete? Blood. 2009;114(6):1138‐1139. [DOI] [PubMed] [Google Scholar]
- 82. Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol. 2002;7(1):40‐53. [PMC free article] [PubMed] [Google Scholar]
- 83. Ungvari Z, Tarantini S, Kiss T, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15(9):555‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moore‐Carrasco R, Donoso W, Sutin F, et al. Expresión de ICAM‐1 en el endotelio de arterias humanas mediante inmunohistoquímica. Int J Morphol. 2011;29(4):1351‐1356. [Google Scholar]
- 85. Sáez CG, Olivares P, Pallavicini J, et al. Increased number of circulating endothelial cells and plasma markers of endothelial damage in chronic cocaine users. Thromb Res. 2011;128(4):e18‐e23. [DOI] [PubMed] [Google Scholar]
- 86. Mezzano D, Tagle R, Pais E, et al. Endothelial cell markers in chronic uremia: relationship with hemostatic defects and severity of renal failure. Thromb Res. 1997;88(6):465‐472. [DOI] [PubMed] [Google Scholar]
- 87. Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep. 2007;59(6):715‐720. [PubMed] [Google Scholar]
- 88. Namioka N, Hanyu H, Hirose D, Hatanaka H, Sato T, Shimizu S. Oxidative stress and inflammation are associated with physical frailty in patients with Alzheimer's disease. Geriatr Gerontol Int. 2016;17(6):913‐918. [DOI] [PubMed] [Google Scholar]
- 89. Zhu Y, Liu Z, Wang Y, et al. C‐reactive protein, frailty and overnight hospital admission in elderly individuals: a population‐based study. Arch Gerontol Geriatr. 2016;64:1‐5. [DOI] [PubMed] [Google Scholar]
- 90. Jagroop IA, Daskalopoulou SS, Mikhailidis DP. Endothelin‐1 and human platelets. Curr Vasc Pharmacol. 2005;3(4):393‐399. [DOI] [PubMed] [Google Scholar]
- 91. Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the "L‐arginine paradox" and acts as a novel cardiovascular risk factor. J Nutr. 2004;134(10 Suppl):2842S‐2847S. discussion 53S. [DOI] [PubMed] [Google Scholar]
- 92. Lin KY, Ito A, Asagami T, et al. Impaired nitric oxide synthase pathway in diabetes mellitus. Circulation. 2002;106(8):987‐992. [DOI] [PubMed] [Google Scholar]
- 93. Kanda T, Takahashi T. Interleukin‐6 and cardiovascular diseases. Jpn Heart J. 2004;45(2):183‐193. [DOI] [PubMed] [Google Scholar]
- 94. Tembo MC, Holloway‐Kew KL, Bortolasci CC, et al. Association between serum interleukin‐6 and frailty in older men: cross‐sectional data. Eur Geriatr Med. 2021;12(4):887‐892. [DOI] [PubMed] [Google Scholar]
- 95. Velissaris D, Pantzaris N, Koniari I, et al. C‐reactive protein and frailty in the elderly: a literature review. J Clin Med Res. 2017;9(6):461‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Le Brocq M, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10(9):1631‐1674. [DOI] [PubMed] [Google Scholar]
- 97. Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Archiv. 2010;459(6):923‐939. [DOI] [PubMed] [Google Scholar]
- 98. Higashi Y, Maruhashi T, Noma K, Kihara Y. Oxidative stress and endothelial dysfunction: clinical evidence and therapeutic implications. Trends Cardiovasc Med. 2014;24(4):165‐169. [DOI] [PubMed] [Google Scholar]
- 99. Soysal P, Isik AT, Carvalho AF, et al. Oxidative stress and frailty: a systematic review and synthesis of the best evidence. Maturitas. 2017;99:66‐72. [DOI] [PubMed] [Google Scholar]
- 100. Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446‐456. [DOI] [PubMed] [Google Scholar]
- 101. Liu J, Wada Y, Katsura M, et al. Rho‐associated coiled‐coil kinase (ROCK) in molecular regulation of angiogenesis. Theranostics. 2018;8(21):6053‐6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shimokawa H, Sunamura S, Satoh K. RhoA/rho‐kinase in the cardiovascular system. Circ Res. 2016;118(2):352‐366. [DOI] [PubMed] [Google Scholar]
- 103. Pereira J, Saez CG, Alvarez M, et al. Evidence of endothelial dysfunction and activation of RhoA/rho kinase pathway in inflammatory bowel disease. Blood. 2019;134(Supplement_1):3641. [Google Scholar]
- 104. Pereira J, Saez CG, Pallavicini J, et al. Cocaine‐induced endothelial dysfunction: role of RhoA/rho kinase pathway activation. Blood. 2012;120(21):2177. [Google Scholar]
- 105. Hauke M, Eckenstaler R, Ripperger A, Ender A, Braun H, Benndorf RA. Active RhoA exerts an inhibitory effect on the homeostasis and angiogenic capacity of human endothelial cells. J Am Heart Assoc. 2022;11(12):e025119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chapados R, Abe K, Ihida‐Stansbury K, et al. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ Res. 2006;99(8):837‐844. [DOI] [PubMed] [Google Scholar]
- 107. van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007;27(11):2332‐2339. [DOI] [PubMed] [Google Scholar]
- 108. Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/rho‐kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes‐associated erectile dysfunction. Proc Natl Acad Sci. 2004;101(24):9121‐9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ming X‐F, Viswambharan H, Barandier C, et al. Rho GTPase/rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22(24):8467‐8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Del Re DP, Miyamoto S, Brown JH. RhoA/rho kinase up‐regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282(11):8069‐8078. [DOI] [PubMed] [Google Scholar]
- 111. Chandra S, Romero MJ, Shatanawi A, Alkilany AM, Caldwell RB, Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/rho kinase pathway. Br J Pharmacol. 2012;165(2):506‐519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Feng S, Zou L, Wang H, He R, Liu K, Zhu H. RhoA/ROCK‐2 pathway inhibition and tight junction protein upregulation by Catalpol suppresses Lipopolysaccaride‐induced disruption of blood‐brain barrier permeability. Molecules. 2018;23(9):2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu H, Chen X, Han Y, et al. Rho kinase inhibition by fasudil suppresses lipopolysaccharide‐induced apoptosis of rat pulmonary microvascular endothelial cells via JNK and p38 MAPK pathway. Biomed Pharmacother. 2014;68(3):267‐275. [DOI] [PubMed] [Google Scholar]
- 114. Eckenstaler R, Ripperger A, Hauke M, et al. Thromboxane A2 receptor activation via Gα13‐RhoA/C‐ROCK‐LIMK2‐dependent signal transduction inhibits angiogenic sprouting of human endothelial cells. Biochem Pharmacol. 2022;201:115069. [DOI] [PubMed] [Google Scholar]
- 115. Edwards DF, Russell RG. Probable vitamin K–deficient bleeding in two cats with malabsorption syndrome secondary to lymphocytic‐plasmacytic enteritis. J Vet Intern Med. 1987;1(3):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hida Y, Nakamura S, Nishinaka A, Inoue Y, Shimazawa M, Hara H. Effects of ripasudil, a ROCK inhibitor, on retinal edema and nonperfusion area in a retinal vein occlusion murine model. J Pharmacol Sci. 2018;137(2):129‐136. [DOI] [PubMed] [Google Scholar]
- 117. Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin. 2010;31(10):1267‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shi J, Wei L. Rho kinases in cardiovascular physiology and pathophysiology: the effect of fasudil. J Cardiovasc Pharmacol. 2013;62(4):341‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Surma M, Wei L, Shi J. Rho kinase as a therapeutic target in cardiovascular disease. Future Cardiol. 2011;7(5):657‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nohria A, Grunert ME, Rikitake Y, et al. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99(12):1426‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. De Silva TM, Modrick ML, Dabertrand F, Faraci FM. Changes in cerebral arteries and parenchymal arterioles with aging: role of rho kinase 2 and impact of genetic background. Hypertension. 2018;71(5):921‐927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shimokawa H, Takeshita A. Rho‐kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25(9):1767‐1775. [DOI] [PubMed] [Google Scholar]
- 123. Dong M, Yan BP, Liao JK, Lam Y‐Y, Yip GWK, Yu C‐M. Rho‐kinase inhibition: a novel therapeutic target for the treatment of cardiovascular diseases. Drug Discov Today. 2010;15(15–16):622‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Saez CG, Pereira‐Flores K, Ebensperger R, et al. Atorvastatin reduces the proadhesive and prothrombotic endothelial cell phenotype induced by cocaine and plasma from cocaine consumers in vitro. Arterioscler Thromb Vasc Biol. 2014;34(11):2439‐2448. [DOI] [PubMed] [Google Scholar]
- 125. Kishi T, Hirooka Y, Masumoto A, et al. Rho‐kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005;111(21):2741‐2747. [DOI] [PubMed] [Google Scholar]
- 126. Vitale C, Spoletini I, Rosano GM. Frailty in heart failure: implications for management. Card Fail Rev. 2018;4(2):104‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shin SY, Padgham S, Trache A, Woodman C. Effect of aging on rho‐kinase activity and vascular smooth muscle contractility in skeletal muscle resistance arteries. FASEB J. 2018;32(1_supplement):705. [Google Scholar]
- 128. Bachman NP, Terwoord JD, Racine ML, Ketelhut NB, Richards JC, Dinenno FA. Acute systemic rho‐kinase inhibition reduces blood pressure and influences peripheral vascular tone in healthy older adults. FASEB J. 2020;34(S1):1. [Google Scholar]
- 129. Ning L, Gao L, Zhang F, Li X, Wang T. Mechanical stretch induces annulus fibrosus cell senescence through activation of the RhoA/ROCK pathway. Biomed Res Int. 2021;2021:5321121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Xiang C, Yan Y, Zhang D. Alleviation of the doxorubicin‐induced nephrotoxicity by fasudil in vivo and in vitro. J Pharmacol Sci. 2021;145(1):6‐15. [DOI] [PubMed] [Google Scholar]
- 131. Singh A, Rattan S. BDNF rescues aging‐associated internal anal sphincter dysfunction. Am J Physiol Gastrointest Liver Physiol. 2021;321(1):G87‐G97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bao C, He C, Shu B, et al. Aerobic exercise training decreases cognitive impairment caused by demyelination by regulating ROCK signaling pathway in aging mice. Brain Res Bull. 2021;168:52‐62. [DOI] [PubMed] [Google Scholar]
- 133. Wei X, Lan T, Zhou Y, et al. Mechanism of α1‐adrenergic receptor‐induced increased contraction of rat mesenteric artery in aging hypertension rats. Gerontology. 2021;67(3):323‐337. [DOI] [PubMed] [Google Scholar]
- 134. Kandabashi T, Shimokawa H, Miyata K, et al. Evidence for protein kinase C‐mediated activation of rho‐kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23(12):2209‐2214. [DOI] [PubMed] [Google Scholar]
- 135. Yamakawa T, Tanaka S, Numaguchi K, et al. Involvement of rho‐kinase in angiotensin II‐induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35(1 Pt 2):313‐318. [DOI] [PubMed] [Google Scholar]
- 136. Löhn M, Steioff K, Bleich M, Busch AE, Ivashchenko Y. Inhibition of rho‐kinase stimulates nitric oxide‐independent vasorelaxation. Eur J Pharmacol. 2005;507(1–3):179‐186. [DOI] [PubMed] [Google Scholar]
- 137. Noma K, Goto C, Nishioka K, et al. Roles of rho‐associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49(6):698‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Rabaglino MB, Wakabayashi M, Pearson JT, Jensen LJ. Effect of age on the vascular proteome in middle cerebral arteries and mesenteric resistance arteries in mice. Mech Ageing Dev. 2021;200:111594. [DOI] [PubMed] [Google Scholar]
- 139. Seawright JW, Sreenivasappa H, Gibbs HC, et al. Vascular smooth muscle contractile function declines with age in skeletal muscle feed arteries. Front Physiol. 2018;9:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kawarazaki W, Mizuno R, Nishimoto M, et al. Salt causes aging‐associated hypertension via vascular Wnt5a under klotho deficiency. J Clin Invest. 2020;130(8):4152‐4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data derived from public domain resources.


