Abstract
Background
A recent study based on blood metabolomics analysis revealed inflammation‐associated mitochondrial dysfunction as a potential mechanism underlying acute‐on‐chronic liver failure (ACLF) in cirrhotic patients. Serine, glycine, and methionine serve to maintain a healthy immune system and adequately sustain mitochondrial functionality in hepatocytes for regulating redox homeostasis through the production of antioxidant glutathione (GSH). Based on this, we hypothesized that the circulatory levels of serine, glycine and methionine will be altered in ACLF patients due to acute worsening of hepatic function and may provide novel insights into the mitochondrial dysfunction as well.
Methods
The circulatory concentrations of serine, glycine, and methionine were estimated in the sera of 40 ACLF patients and 49 normal controls (NC) subject using 1D 1H‐CPMG NMR spectra recorded at 800 MHz NMR spectrometer. The resulting metabolite concentrations were compared using unpaired Student t‐test and p‐value < 0.05 was considered as the criterion of statistical significance. The diagnostic potential and statistical correlations were established using receiver‐operating‐characteristic (ROC) curve analysis and Pearson‐r method, respectively.
Results
Circulating levels of serine and glycine were significantly decreased in ACLF patients (Ser = 23.06 ± 1.67 µM and Gly = 83.11±7.52 µM) compared to NC subjects (Ser = 55.61 ± 2.28 µM and Gly = 156.9±7.16 µM) with p‐value < 0.0001, whereas those of methionine were significantly increased in ACLF (22.60 ± 2.49 µM) compared to NC subjects (=14.63 ± 0.85 µM) with p‐value < 0.0015. Further, the ROC analysis yielded satisfactory sensitivity and specificity for serine, glycine, and methionine‐to‐glycine ratio (MGR) with area under ROC (AUROC) curve values equal to: 0.95 [95%CI = 0.91‐0.99] for Ser; 0.87 [95%CI = 0.79‐0.95] for Gly; and 0.90 [95%CI = 0.83‐0.97] for MGR.
Conclusion
Compared to NC subjects, the sera of ACLF patients were characterized by hypermethioninemia and aberrantly decreased levels of serine and glycine suggesting mitochondrial dysfunction as the possible mechanism for disturbed redox homeostasis and therefore depressed immune system in ACLF.
Keywords: acute‐on‐chronic liver failure; circulatory profiling of serine, glycine and methionine; mitochondrial dysfunction
1. INTRODUCTION
Alcoholic hepatitis and chronic viral hepatitis are the most common underlying cause of liver cirrhosis 1 , 2 Acute‐on‐chronic liver failure (ACLF), which develops in patients with cirrhosis, is characterized by acute deterioration of liver function and intense systemic inflammation (owing to immune system dysfunction). 3 , 4 , 5 Due to various definitions of ACLF, the correct prevalence of ACLF cannot be assessed; but it is reported to vary between 5% to 30% among hospitalized cirrhotic patients, wherein 50% hospitalizations are due to infections. 6 , 7 Up to 40–50% of the cases of ACLF develop spontaneously, whereas in remaining patients, it develops after a precipitating event such as infection (sepsis), active alcoholism, and relapse of chronic viral hepatitis. 1 , 4 , 8 , 9 , 10 Despite the best possible treatments, ACLF has poor prognostic outcomes and is often associated with extra‐hepatic organ failure(s) and high short‐term mortality (estimated between 45% and 90%). 1 , 4 , 11 , 12 Therefore, the clear understanding of metabolic aberrations underlying the pathogenesis of ACLF and associated comorbidities can greatly decrease the mortality and morbidity of patients through predicting outcome and serving as surrogate endpoints for evaluating treatment response.
An excessive systemic inflammatory response seems to play a crucial role in the development of ACLF and recently, an extensive and rigorous blood metabolomics study revealed inflammation‐associated mitochondrial dysfunction as a potential mechanism underlying ACLF. 5 Recent studies have demonstrated the role of serine and glycine in maintaining the mitochondrial function and dynamics through regulating lipid metabolism. 13 , 14 Both serine and glycine are non‐essential amino acids and provide substrates for production of antioxidant glutathione (GSH) and supporting methionine cycle. Thus, both these directly affect cellular antioxidative capacity and help to reduce oxidative stress underlying liver diseases. 15 , 16 , 17 In alcohol‐induced fatty liver mouse model, serine intake has been reported to reduce the hepatic level of triglyceride and neutral lipid accumulation by enhancing homocysteine metabolism. 18 Further, glycine treatment improved mitochondrial membrane potential and restored liver mitochondrial ATP, 14 whereas serine deficiency is known to cause mitochondrial fragmentation. 13 As prolong ethanol consumption impairs several of the multiple steps in methionine metabolism which in turn lead to progressive liver injury. 19 Compelling evidence support that nutritional deficiency and glutathione (GSH) depletion in hepatocyte mitochondria represent the most important mechanisms underlying progressive liver damage in ALD patients. 20 However, the patients with alcoholic cirrhosis (or ALD) often have hypermethioninemia due to reduced activity of methionine adenosyltransferase (MAT). 20 The activity of MAT is crucial for the synthesis of S‐adenosylmethionine (SAM, Figure 1A) that serves as the main methylating agent and exerts many key functions in the liver. 21 As evident from Figure 1A, SAM, serine, and glycine are important precursors for the synthesis glutathione (GSH). Based on this, we hypothesized that the circulatory levels of serine and glycine in ACLF patients will be significantly decreased, whereas those of methionine will be increased and this metabolic derangement in ALD patient's may in part will attribute to hepatic mitochondrial dysfunction.
FIGURE 1.
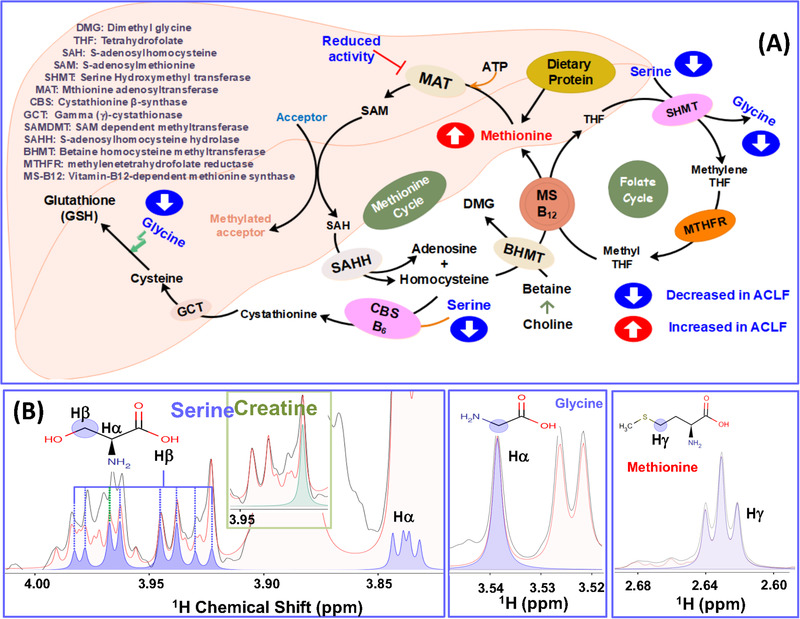
(A) Schematic showing reactions of serine, glycine and methionine metabolism in the liver and as depicted the amino acids serine and glycine are biosynthetically linked through serinehydroxymethyltransferase (SHMT). (B) Selected regions from the 1D 1H CPMG NMR spectra of serum samples displaying the serine (in blue), glycine (in middle box, blue) and methionine (in right box, blue) peaks selected for concentration profiling in NMR suite of CHENOMX software.
In this regard, we employed targeted NMR‐based metabolomics approach and estimated the circulatory concentrations of serine, glycine, and methionine in the serum samples of ACLF patients and normal control (NC) subjects following the procedure as described previously. 22 The resulted circulatory metabolic concentrations and metabolic ratios, that is, the serine to glycine ratio (SGR) and methionine to glycine ratio (MGR) were then compared between the ACLF and NC groups. We further sought to find their association in the pathogenesis of ACLF through evaluating their correlation with clinically used scores for hepatic impairment and assessment of disease severity.
2. MATERIALS AND METHODS
2.1. Recruitment of subjects
The serum samples were collected as per the study protocol approved by the institutional research and ethical committee, SGPGIMS, Lucknow, UP | India (IEC Code: 2017‐186‐DM‐99(B); File No.: PGI/BE/804/2017; Dated: October 30, 2017). An informed written consent was obtained from the guardians/kin of the patients after informing them the purpose of study and subjects were recruited following the norms of World Medical Association (WMA) declaration of Helsinki. Serum samples were obtained from ALD patients (N = 40, 100% male with ascites) within 1‐3 days after the patient is admitted in critical/intensive care unit (ICU) for the management of ACLF (with Grade ≥II based on CLIF‐SOFA score). 23 The clinical and demographic details were collected in a custom‐designed questionnaire. Only patients with alcoholic acute hepatitis and alcoholic cirrhosis were considered and ACLF was diagnosed as per APASL 24 and ACLF grades as per CLIF – SOFA criteria. 25 Exclusion criteria were age > 65 year, severe cardiopulmonary disease, chronic kidney disease (CKD) on dialysis, evidence of infection with the human immunodeficiency (HIV) virus, hepatitis B or C viruses, hepatic malignancy (or hepatocarcinoma), and a past history of acute decompensation during the previous 6 months. For comparative analysis, the serum samples from 49 age‐matched normal control (NC) male subjects were collected after taking an informed consent. In each case, it was confirmed that the NC subjects are normotensive with no cardiovascular abnormalities and satisfying the above exclusion criteria. The serum was extracted as per the established protocol. 26 Briefly, the blood collected in a plain vacutainer tube is allowed to clot at room temperature for 30‐40 minutes. The liquid fraction (designated serum) was separated from the clot performing centrifugation at 1200 × g for 10 min in a refrigerated centrifuge. The resulting supernatant after the processing is immediately transferred into a sterile 1.5 ml microcentrifuge tube (MCT) and stored at −80°C until the NMR experiments were performed.
2.2. NMR measurements and concentration profiling
Before starting NMR data collection, the stored serum samples were thawed and centrifuged at 10 000 rpm for 5 min to remove precipitates. In each case, 0.3 ml of serum sample was used to prepare the final NMR sample following the procedure as described previously. 27 , 28 The resulted samples (with pH value ranging from 7.0 to 7.4) were used to record transverse relaxation‐edited 1D 1H CPMG (Carr–Purcell–Meiboom–Gill) NMR spectra at high‐field 800 MHz NMR spectrometer as per the acquisition parameters described previously. 28 The recorded CPMG NMR spectra were manually corrected for phase and baseline distortions using PROCESSOR module of commercial software CHENOMX NMR Suite (v8.4, Chenomx Inc., Edmonton, Canada). Following the procedure described previously, 22 all spectra were calibrated with respect to formate δ8.43 (used here as an internal reference) and the concentration of formate was set to 10 micromolar (i.e., nearly close to the detection limit of 800 MHz NMR as well explained in our previous studies). 22 , 29 To be mentioned here is that the circulatory concentration of formate may vary from 10‐100 uM depending upon the health or diseased condition of the human subject 30 , 31 ; and so the varying concentration of circulatory proteins in the serum. The phase and baseline corrected spectra were imported into PROFILER‐Module of CHENOMX for concentration profiling of circulatory metabolites (serine, glycine and methionine). Before starting the concentration profiling, the metabolite specific assignments of peaks in the 1D 1H CPMG NMR spectrum were rigorously confirmed making composite use of two‐dimensional NMR experiments i.e. 1H‐13C heteronuclear single quantum coherence (HSQC) spectra, 1H‐1H total correlation spectroscopy (TOCSY) and J‐resolved spectroscopy (JRES) details are provided in the Supporting Information (Annexure‐I and segments of Figure S1).
2.3. Univariate statistical and correlation analysis
The circulatory levels of metabolites were compared between the groups using unpaired Student t‐test and the changes with p‐value ≤ 0.05 were considered as statistically significant. The boxplot representation was used to visualize the variation in the levels of significantly altered metabolites. The key metabolic changes were further evaluated for diagnostic potential using receiver operating characteristic (ROC) curve analysis (performed using GraphPad Prism v6.01 for Windows) and the area under the ROC (AUROC) curve value more than 0.85 were considered the criterion for diagnostic significance. Continuous variables were expressed as the mean ± SD and categorical variables as percentage. The correlation analysis and Bonferroni‐corrected P‐values (i.e., false discovery rate (FDR)) were estimated using Statistical analysis module of Metaboanalyst (an open access web‐based metabolomics data processing tool: https://www.metaboanalyst.ca/MetaboAnalyst/home.xhtml). 32
3. RESULTS
Metabolomics is an emerging application of NMR used for identification of metabolic disturbances in body fluids (such as blood plasma/serum, urine, etc.) in response to a disease or therapeutic intervention and the identified metabolic alterations are then used to improve clinical diagnostic and prognostic screening in human diseases. 33 , 34 The present study aims to compare the circulatory levels of serine, glycine and serine‐to‐glycine ratio (SGR) between ACLF patients and normal control (NC) subjects. The clinical and demographic characteristics of 89 subjects selected in this study are shown in Table 1. Of 89, 40 were ACLF patients (with mean age 41.7±7.54) and 49 are normal control (NC; with mean age 45±6.73 years). The main diagnoses for ACLF patients included in this study were sepsis (20%), hepatic encephalopathy, ascites (100%), and jaundice, whereas the main etiologic precipitant for ACLF was alcohol intake (within 4 weeks) with or without bacterial infection.
TABLE 1.
The biochemical, clinical and demographic characteristics of ALD‐ACLF patients and control cohorts recorded at inclusion
| Variables/Parameters | Case (n = 40) | Normal Control (n = 49) |
|---|---|---|
| Male gender | 100% | 100% |
| Alcoholic | 40 (100%) | 10 (20%) |
| Age (in years) | 41.7± 7.54 | 45 ± 6.73 |
| Ascites | 38 (95%) | – |
| Complete ascites mobilization | 32 (80%) | – |
| Precipitant | ||
| Active alcoholism | 32 (80%) | – |
| Sepsis (SBP) | 8 (20%) | |
| HE grading > 2 | 27 (67.5%) | – |
| ACLF Grade > 2 | 28 (70%) | – |
| Blood Urea (mg/dL) | 39.5 ± 19.06 | – |
| Hemoglobin g/dL | 9.19 ± 1.68 | – |
| Gastrointestinal bleeding (GIB) | 2 (5%) | |
| 28 days non‐survival | 10 | – |
| CRP/ESR | 3.0 ± 2.9/36.4 ± 25.5 | – |
| Total bilirubin (mg/dL) | 17.77 ± 11.83 | – |
| TLC (cells/mm3) | 11.25 ± 6.35 | – |
| Platelet count (G/L) | 87.69 ± 36.47 | – |
| Serum Sodium (Serum Potassium) | 133.62 ± 5.54 (4.01 ± 0.44) | – |
| Creatinine (mg/dL) (INR) | 1.53 ± 0.99 (2.79 ± 0.77) | – |
| Albumin (g/dL) | 2.6 ± 0.5 | – |
| SGOT (SGPT) U/L | 108.13 ± 66.927 (48.32 ± 26.31) | – |
| U Na (U K) | 34.66 ± 26.18 (48.2 ± 51.19) | – |
| MELD | 28.0 ± 7.0 | – |
| CTP | 12.2 ± 1.34 | – |
| Total OF | 1.76 ± 0.72 | – |
| CLIF SOFA | 10.35 ± 1.66 | – |
| CLIF C ACLF score | 52.4 ± 6.4 | – |
Note: Gender, age, the presence of ascites or hepatic encephalopathy, serum albumin, bilirubin, the International Normalized Ratio, serum glutamic‐oxaloacetic transaminase (SGOT) activity, Serum glutamic pyruvic transaminase (SGPT) activity, and serum urea and creatinine levels were recorded at inclusion. The abbreviations used in the table represent, respectively: ml: mililitre, mq/dL: milligram per decilitre, g/dl: gram per deciliter; U/L: Units per litre; G/L: Giga/Litre; TLC: total leucocyte count, SGOT: Serum Glutamic‐oxalacetic transaminase (also known as enzyme aspartate aminotransferase, AST), SGPT: Serum glutamic‐pyruvic transaminase (also known as alanine aminotransferase, ALT), ALP: Alkaline phosphate, INR: International Normalized Ratio, SBP: spontaneous bacterial peritonitis, CTP score: Child‐Turcotte‐Pugh score, MELD score: Model for End‐Stage Liver Disease score. 38 , 39 , 53 SOFA: Sequential Organ Failure Assessment, (CLIF) EASL‐Chronic Liver Failure.
Figure 1B shows the spectral regions of 1D 1H CPMG NMR spectrum recorded on normal control serum sample containing NMR signals of serine, glycine, and methionine identified and assigned by comparing the chemical shift and peak patterns with the 800 MHz database library of CHENOMX NMR suite. 35 The concentrations were explicitly measured with respect to formate as an internal reference so that to minimize the analytical variations and this is like we use creatinine in urine‐based metabolomics analysis. 36 Other specific advantages of using formate as an internal reference are: (a) formate does not interact with serum proteins, 36 (b) the singlet NMR signal of formate appears most downfield in the NMR spectrum of serum, does not overlap with NMR signals of other metabolites and (c) on top of this, the CHENOMX NMR suite provides the option to use formate as a calibration standard.
The circulatory concentrations of serine, glycine, and methionine were estimated first and then these were used to estimate the metabolic ratios: serine‐to‐glycine ratio (SGR) and methionine‐to‐glycine ratio (MGR). The circulatory levels of these metabolites and metabolic ratios were then compared between ACLF patients and NC subjects (see the respective box plots shown in Figure 2A–E). The circulating levels of serine, glycine, and SGR values were significantly decreased in ACLF patients (Ser = 23.06 ± 1.67 µM, Gly = 83.11 ± 7.52 µM, SGR = 0.31 ± 0.02) compared to NC subjects (Ser = 55.61 ± 2.28 µM, Gly = 156.9 ± 7.16 µM and SGR = 0.37 ± 0.02) with p‐value < 0.01. These findings were found well consistent with previous serum metabolic characteristics of liver cirrhotic patients with ascites 37 suggesting an augmented utilization of these metabolites rendering their depletion in the circulatory system. On the other hand, the circulating levels of methionine and MGR values were significantly increased in ACLF patients (Met = 22.60 ± 2.49 µM and MGR = 0.32 ± 0.04) compared to NC subjects (Met = 14.63 ± 0.85 µM and MGR = 0.10 ± 0.01) with p‐value < 0.01.
FIGURE 2.
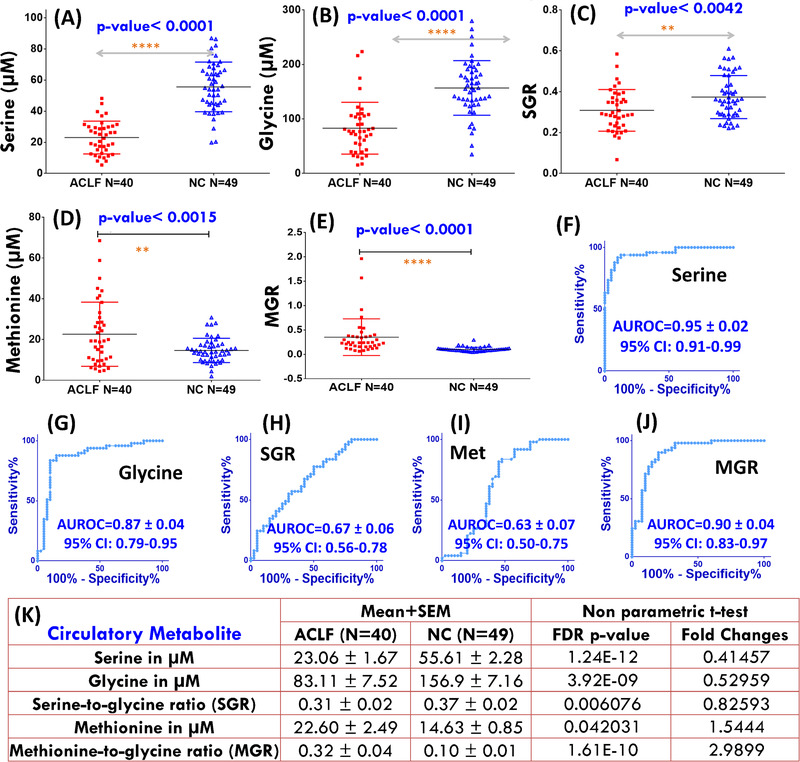
Box plots (A‐E) showing comparison of circulatory levels of serine (A), glycine (B), serine‐to‐glycine ratio (C) methionine (D) and methionine‐to‐glycine ratio (E) in ACLF patients compared to healthy normal control (NC) subjects. For each box plot, the boxes denote interquartile ranges, horizontal line inside the box denote the median, and bottom and top boundaries of boxes are 25th and 75th percentiles, respectively. Lower and upper whiskers are 5th and 95th percentiles, respectively. The p‐value summary derived from the unpaired statistical t‐test is highlighted in blue text for each metabolic comparison. The symbols ****, ***, **, and * represent the p‐values < 0.0001, < 0.001, < 0.01, and < 0.05, respectively. The plots in (F‐J) represent the corresponding receiver operating characteristic (ROC) curve analysis performed for evaluating their diagnostic potential in screening ACLF patients from NC subjects. The area under ROC curve (AUROC) values (with standard error and 95% confidence interval (CI) are shown in blue text for each ROC plot). The mean values of these circulatory metabolites (with their corresponding standard error in mean, SEM) and the explicit p‐values adjusted to control the false discovery rate below 0.05) are shown in tabulated form in (K)
Next, these circulatory levels were evaluated for their diagnostic potential and the results of ROC curve analysis are summarized in Figure 2F–J. Clearly evident that serine, glycine, and MGR exhibited the highest diagnostic potential in the discrimination of ACLF from NC with area under ROC (AUROC) curve values equal to: 0.95 [95% CI = 0.91‐0.99] for Ser; 0.87 [95% CI = 0.79‐0.95] for Gly; and 0.90 [95% CI = 0.83‐0.97] for MGR (Figure 2K).
As serine and glycine are biosynthetically linked, their circulatory levels are expected to correlate if the metabolic pathway is functional. The statistical correlation plots generated for serine, glycine, and SGR levels in ACLF patients using Pearson r method are shown in Supporting Information (Figure S2). Clearly evident that the circulatory levels of serine and glycine metabolites are positively and significantly correlated both in ACLF patients [r = 0.66 (95% CI: 0.43‐0.80); p‐value < 0.0001] and NC subjects [r = 0.59 (95% CI: 0.37‐0.75); p‐value < 0.0001] suggesting that serine and glycine are biosynthetically linked and the corresponding metabolic pathway is operative in both ACLF and NC subjects. To be mentioned here is that the correlation of circulatory serine with SGR was found to be very poor in ACLF patients [r = 0.10 (95% CI: ‐0.22 to 0.40); p‐value < 0.53; Figure S2C) compared to that in NC subjects [r = 0.28 (95% CI: ‐0.04 to 0.50); p‐value < 0.086; Figure S2F) suggesting altered utilization of these metabolites in ACLF. As, the metabolic alterations contributing to the development and progression of a disease are often correlated, we further sought to identify the correlations between these circulatory metabolic profiles and clinical scores used for evaluating disease severity and predicting clinical outcomes and survival such as The Model for End‐Stage Liver Disease (MELD), 38 , 39 Child‐Turcotte‐Pugh (CTP), and CLIF‐SOFA (i.e., chronic liver failure sequential organ failure assessment). 25 For this, we employed both the Pearson r and Spearman rank methods, and the resulted statistical correlation heat maps generated for various circulatory and clinical parameters estimated for ACLF patients are shown in Figures 3A and 3B. Among various circulatory metabolites, the MGR levels were found to be significantly associated with MELD (the commonly used severity score for liver diseases), as inferred from the Pearson r value of 0.31 (95% CI = 0.00 to 0.57) with p‐value equal to 0.0493 (though FDR adjusted p‐value was found to be 0.13, See Figure 3A,C). Similarly, the Spearman rank correlation between MGR and MELD was found to be adequate as evident from statistical correlation value equal to 0.3 with p‐value equal to 0.06 and FDR adjusted p‐value equal to 0.17. As a reference for future studies, the values of Pearson r coefficient obtained for correlation of SGR and MGR with other metabolic profiles and clinical scores are shown in Table 2A and 2B, respectively. Whereas, the explicit statistical correlation plots for ACLF patients generated using Spearson r method between concentration profiles and disease severity scores are shown in ESM, Figure S3. As evident, no significant correlation was found between selected circulatory metabolites and clinical scores. However, the circulatory levels of methionine and MGR were found to be correlated with MELD score with p‐value nearly close to the statistically significance (See Figure 3B and explicit details in Figure S3).
FIGURE 3.
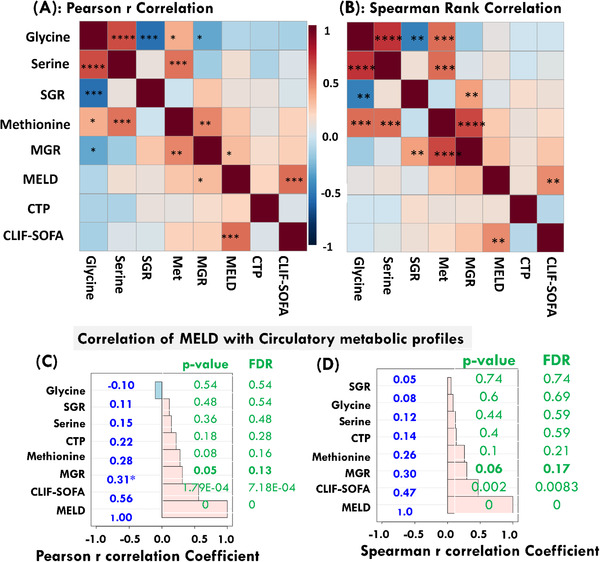
The metabolic profiles and clinical parameters estimated for ACLF patients subjected to correlation analysis using online software Metaboanalyst. The statistical correlation heat maps obtained based on Pearson r and Spearman rank correlation methods are shown in (A) and (B), respectively. A correlation with p‐value < 0.05 was considered to be statistically significant. The symbols ****, ***, **, and * represent the p‐values < 0.00001, < 0.0001, < 0.005, and < 0.05, respectively. (C and D) The correlation plots showing, respectively, the Pearson r and Spearman rank correlation of clinical MELD parameter with other clinical and circulatory levels of serine, glycine, SGR, methionine, and MGR. For each correlation bar, the p‐value and FDR adjusted p‐values are shown in green text
TABLE 2.
The Pearson r correlation estimated for circulatory levels of SGR (A) and MGR (B) with other circulatory parameters and clinical scores of severity estimated for ALD‐ACLF patients
| A. Correlation of SGR estimated for ACLF patients | Methionine | Glycine | Serine | MELD | CTP | CLIF‐SOFA |
|---|---|---|---|---|---|---|
| Pearson r | −0.01 | −0.55 | 0.10 | 0.12 | 0.11 | 0.04 |
| 95% confidence interval | −0.33 to 0.30 | −0.74 to −0.29 | −0.22 to 0.40 | −0.20 to 0.41 | −0.21 to 0.40 | −0.28 to 0.34 |
| R square | 0.00 | 0.30 | 0.01 | 0.13 | 0.01 | 0.00 |
| p (two‐tailed) summary (alpha = 0.05) | No | Yes*** | No | No | No | No |
| p‐Value | 0.9273 | 0.0002 | 0.5302 | 0.4799 | 0.5159 | 0.8214 |
| B. Correlation of MGR estimated for ACLF patients | Methionine | Glycine | Serine | MELD | CTP | CLIF‐SOFA |
|---|---|---|---|---|---|---|
| Pearson r | 0.49 | −0.31 | −0.15 | 0.31 | 0.17 | 0.24 |
| 95% confidence interval | 0.21 to 0.70 | −0.57 to −0.00 | −0.44 to 0.17 | 0.00 to 0.57 | −0.15 to 0.46 | −0.076 to 0.51 |
| R 2 | 0.24 | 0.10 | 0.02 | 0.10 | 0.03 | 0.06 |
| p (two‐tailed) summary (alpha = 0.05) | ** | * | ns | * | ns | ns |
| p‐Value | 0.0014 | 0.0478 | 0.3496 | 0.0493 | 0.2996 | 0.1334 |
4. DISCUSSION
Several liver diseases often result from perturbations in the metabolism of hepatocytes and gaining a precise understanding of such metabolic pathway perturbations is of utmost clinical interest for developing new diagnostic tools and nutritional strategies that will reduce the morbidity and mortality. Genome‐scale metabolic modeling of hepatocytes has revealed serine deficiency in patients with non‐alcoholic steatohepatitis (NASH). 40 Following this, the analysis of transcriptomic data of NASH patients in clinical trials confirmed the changes in several gene expressions by serine administration. 40 Another potential metabolic hallmark of cirrhotic liver diseases is the elevated plasma levels of methionine, the condition known as hypermethioninemia. 20 Methionine is largely catabolized in the liver through the formation of S‐adenosylmethionine (SAM, the principal biologic methyl donor). 41 The elevated levels of methionine in ACLF patients, therefore, might be indicative of impaired methionine catabolism in liver resulting into the decreased production of SAM. As depicted in Figure 1A, serine, glycine, and SAM serve as important precursors for cellular glutathione (GSH, the major physiologic defense mechanism against oxidative stress). 19 , 20 Therefore it is legitimate to state that depletion of SAM, serine, and glycine would cause reduced synthesis of GSH. 42 This in turn may cause selective decrease of mitochondrial pool of GSH (↓mGSH) and so causing mitochondrial dysfunction. 20 , 42 , 43
In this targeted NMR‐based metabolomics study, we estimated the circulatory levels of serine, glycine, SGR, methionine, and MGR in ACLF patients and compared with those of NC subjects. The analysis revealed that the sera of ACLF patients are characterized by aberrantly decreased circulatory levels of serine, glycine, and SGR (Figure 2A‐C). As both serine and glycine levels were significantly depleted in ACLF, the decreased SGR levels were further indicative of excessive serine depletion. These metabolic alterations might be related to excessive utilization of serine and glycine in ACLF due to systemic inflammatory response, 44 which is causally linked to profound immunosuppression or immunodysfunction (i.e., an imbalance between the systemic inflammatory response and the compensatory anti‐inflammatory response). 45 , 46 Compared to NC subjects, the circulatory methionine and MGR levels were found to be significantly elevated in ACLF patients suggesting progressive loss of liver functions and so impaired catabolism of methionine.
These AAs directly affect cellular antioxidative capacity through providing substrates for production of antioxidant glutathione (GSH) and S‐adenosylmethionine (SAM, see Figure 1A); thus help to reduce oxidative stress underlying alcoholic liver diseases. 15 , 16 , 17 Overall, these have critical roles in ensuring a healthy immune system. 47 Recently, the role of serine and glycine has been demonstrated in maintaining the mitochondrial function and dynamics through regulating lipid metabolism. 13 , 14 In alcohol‐induced fatty liver mouse model, serine intake has been reported to reduce the hepatic level of triglyceride and neutral lipid accumulation by enhancing homocysteine metabolism. 18 Serine like glycine, is an important functional amino acids (AAs) and participates in the biosynthesis of purines, pyrimidines, and other precursors metabolites used in the biosynthesis of proteins (including antibodies), nucleic acids, and lipids. 17 , 48 , 49 Further, glycine treatment improved mitochondrial membrane potential and restored liver mitochondrial ATP, 14 whereas serine deficiency is known to cause mitochondrial fragmentation. 13 Recently, inflammation‐associated mitochondrial dysfunction has been reported as a potential mechanism underlying Acute‐on‐Chronic liver failure (ACLF) patients. 5 As mitochondrial functionality has critical role to maintain healthy immune system, 50 therefore, severe deficiency of serine and glycine in the sera of ACLF patients and simultaneously impaired catabolism of methionine might be related to mitochondrial dysfunction demonstrated recently as a potential mechanism underlying ACLF. 5
As circulatory levels of serine, glycine, and SGR showed insignificant correlation with clinical scores of hepatic impairment (Figure 3 and Table 2A) suggesting decreased serum levels of these amino acids are not related to disease severity and possible underlies the pathophysiology of the ACLF. However, the circulatory MGR levels in ACLF patients found to be correlated to MELD suggesting these circulatory levels might be related to the severity of the disease and so to progressive liver damage as inferred from its positive correlation with MELD (Pearson r = 0.31, p‐value ∼ 0.05). Therefore, further validation studies on larger prospective cohorts of ACLF patients in a longitudinal manner are crucial to confirm these results and their association with clinical outcomes and severity of hepatic and extra‐hepatic impairment.
5. CONCLUSION
The present targeted NMR‐based metabolomics study revealed aberrantly decreased serine and glycine levels in the sera of ACLF patients (Figure 2A–C), whereas the circulatory levels of methionine and MGR were significantly increased in ACLF (Figure 2D,E). The ROC analysis yielded satisfactory sensitivity and specificity for serine, glycine, and MGR suggesting their utility in clinical diagnosis and prognosis (Figure 2F,G,J). The Pearson r correlation analysis revealed that the decreased circulatory levels of serine, glycine and SGR in ACLF is possibly a pathophysiological manifestation as these were found not to be associated with any of the severity scores of the liver diseases (Figure 3A, Table 2A; Figure. S3). However, the positive correlation of circulatory MGR with MELD suggested its potential to serve as marker of progressive liver damage (Figure 3B, Table 2B) and possibility to improve MELD score for reliable prediction of mortality. 51 Practically, the use of MGR in clinical settings will require future studies on large patient cohorts in a longitudinal manner to validate these findings and their association with clinical outcomes and severity of hepatic impairment. The elevated serum levels of methionine in corroboration with aberrantly decreased serum‐levels of serine and glycine may cause reduced synthesis of GSH in liver cells (as evident from Figure 1A) which in turn may cause depressed functioning of mitochondria. Overall, the findings of this study are well in line with the recent blood metabolomics study demonstrating inflammation‐associated mitochondrial dysfunction as a potential mechanism underlying ACLF in cirrhotic patients. 5 The aberrantly decreased circulatory levels of serine, glycine, and SGR in ACLF patients might be caused by systemic inflammatory response which in part contribute to hepatic mitochondrial dysfunction and further explains the hepatoprotective effects of glycine reported previously in cholestatic animal models 14 and in the postoperative phase of liver transplantation. 52 Taken together, the hepatoprotective effects of serine, glycine, and SAM might be linked to their ability to preserve mitochondrial functionality, maintaining cellular redox balance, and preventing oxidative stress. The strong and statistically significant correlations established between circulatory serine and glycine levels in ACLF and NC subjects (see ESM, Fig. S2) also validated that the concentration profiling of serine and glycine performed here for the first time using 1H NMR based spectroscopy is adequately relevant for its future biomedical and clinical implications as well.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
GP and DK: Study design and study approval from the Institutional research committee and Institutional human ethics committee; SS, MD, and GP: Recruitment of patients, extraction of serum from blood obtained from each patient and proper storage of all the samples. Cataloguing and proper indexing of patient details; PA and UK: NMR data processing and metabolic profiling; RR: 2D NMR experiments for metabolite assignment including spiking experiments; DK and AG: Univariate statistical data analysis, preparing the manuscripts and all the Tables and Figures. Involved in the procurement of all the chemicals, glasswares and other labwares required for sample preparation
Supporting information
Supporting Information
ACKNOWLEDGMENTS
DK acknowledges the Department of Science and Technology for financial assistance under SERB EMR Scheme (Ref. No.: EMR/2016/001756). AG acknowledges the Department of Science and Technology (DST), Government of India for financial assistance under DST INSPIRE Faculty Award (Ref. No. DST/Inspire Faculty Award 2014/LSBM‐120) and SERB Women Excellence Award (Ref. No. SB/WEA‐08/2019). We would also like to acknowledge the Department of Medical Education, Govt. of Uttar Pradesh for supporting the High Field NMR Facility at Centre of Biomedical Research, Lucknow, India. UK acknowledges receipt of an SRF fellowship [ICMR sanction no.3/1/3/JRF‐2014/HRD‐100 (32508)] from The Indian Council of Medical Research (ICMR), New Delhi, India. RR acknowledges the receipt of an SRF fellowship from CSIR, INDIA.
Arya P, Kumar U, Sharma S, et al. Targeted NMR based serum metabolic profiling of serine, glycine and methionine in acute‐on‐chronic liver failure patients: possible insights into mitochondrial dysfunction. Anal Sci Adv. 2021;2:536–545. 10.1002/ansa.202000167
Contributor Information
Gaurav Pande, Email: drgauravpandey@yahoo.com.
Dinesh Kumar, Email: dineshcbmr@gmail.com, Email: dinesh@cbmr.res.in.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study has been uploaded on ZENODO (https://zenodo.org/record/4638623) and is available without undue reservation for further studies on request to the corresponding author.
REFERENCES
- 1. Hernaez R, Solá E, Moreau R, Ginès P. Acute‐on‐chronic liver failure: an update. Gut. 2017;66 (3):541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gustot T, Jalan R. Acute‐on‐chronic liver failure in patients with alcohol‐related liver disease. J Hepatol. 2019;70:319‐327. [DOI] [PubMed] [Google Scholar]
- 3. Alam A, Suen K, Ma D. Acute‐on‐chronic liver failure: recent update. J Biomed Res. 2016;31:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moreau R, Jalan R, Gines P, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426‐1437. [DOI] [PubMed] [Google Scholar]
- 5. Moreau R, Clària J, Aguilar F, et al. Blood metabolomics uncovers inflammation‐associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol. 2020;72:688‐701. [DOI] [PubMed] [Google Scholar]
- 6. Singal AK, Kamath PS. Acute on chronic liver failure in non‐alcoholic fatty liver and alcohol associated liver disease. Transl Gastroenterol Hepatol. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saxena N, Singhal A, Subramanian S, Yadav AK, Aathur A. Clinical profile of acute on chronic liver failure patients in a tertiary care centre. J Clin Diagn Res. 2020;14:12‐15. [Google Scholar]
- 8. Amathieu R, Triba MN, Nahon P, et al. Serum 1H‐NMR metabolomic fingerprints of acute‐on‐chronic liver failure in intensive care unit patients with alcoholic cirrhosis. PLoS One. 2014;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajaj JS, O'Leary JG, Reddy KR, et al. Survival in infection‐related acute‐on‐chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jampana SC, Khan R. Pathogenesis of alcoholic hepatitis: role of inflammatory signaling and oxidative stress. World J Hepatol. 2011;3:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen AM, Kim WR, Moriarty JP, Shah ND, Larson JJ, Kamath PS. Time trends in the health care burden and mortality of acute on chronic liver failure in the United States. Hepatology. 2016;64:2165‐2172. [DOI] [PubMed] [Google Scholar]
- 12. Sarin SK, Kumar A, Almeida JA, et al. Acute‐on‐chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao X, Lee K, Reid MA, et al. Serine availability influences mitochondrial dynamics and function through lipid metabolism. Cell Rep. 2018;22:3507‐3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heidari R, Ghanbarinejad V, Mohammadi H, et al. Mitochondria protection as a mechanism underlying the hepatoprotective effects of glycine in cholestatic mice. Biomed Pharmacother. 2018;97:1086‐1095. [DOI] [PubMed] [Google Scholar]
- 15. Alves A, Bassot A, Bulteau AL, Pirola L, Morio B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients. 2019;11:1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Locasale JW. Serine, glycine and one‐carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee DY, Kim EH. Therapeutic effects of amino acids in liver diseases: current studies and future perspectives. J Canc Prev. 2019;24:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sim WC, Yin HQ, Choi HS, et al. L‐serine supplementation attenuates alcoholic fatty liver by enhancing homocysteine metabolism in mice and rats. J Nutr. 2015;145:260‐267. [DOI] [PubMed] [Google Scholar]
- 19. Kharbanda KK. Alcoholic liver disease and methionine metabolism. Semin Liver Dis. 2009;29:155‐165. [DOI] [PubMed] [Google Scholar]
- 20. Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335‐1349. [DOI] [PubMed] [Google Scholar]
- 21. Lieber CS. S‐adenosyl‐L‐methionine: its role in the treatment of liver disorders. Am J Clin Nutr. 2002;76:1183S‐1187S. [DOI] [PubMed] [Google Scholar]
- 22. Kumar U, Jain A, Guleria A, et al. Circulatory Glutamine/Glucose ratio for evaluating disease activity in Takayasu arteritis: a NMR based serum metabolomics study. J Pharm Biomed Anal. 2020;180:113080. [DOI] [PubMed] [Google Scholar]
- 23. Moreau R, Jalan R, Gines P, et al. Acute‐on‐chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426‐1437. [DOI] [PubMed] [Google Scholar]
- 24. Sarin SK, Choudhury A, Sharma MK, et al. Acute‐on‐chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jalan R, Saliba F, Pavesi M, et al. Development and validation of a prognostic score to predict mortality in patients with acute‐on‐chronic liver failure. J Hepatol. 2014;61:1038‐1047. [DOI] [PubMed] [Google Scholar]
- 26. Beckonert O, Keun HC, Ebbels TM, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692‐2703. [DOI] [PubMed] [Google Scholar]
- 27. Guleria A, Kumar A, Kumar U, Raj R, Kumar D. NMR based metabolomics: an exquisite and facile method for evaluating therapeutic efficacy and screening drug toxicity. Curr Top Med Chem. 2018;18:1827‐1849. [DOI] [PubMed] [Google Scholar]
- 28. Guleria A, Pratap A, Dubey D, et al, NMR based serum metabolomics reveals a distinctive signature in patients with Lupus Nephritis. Sci Rep. 2016. ; 6, 35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar U, Kumar A, Singh S, et al. An elaborative NMR based plasma metabolomics study revealed metabolic derangements in patients with mild cognitive impairment: a study on north Indian population. Metab Brain Dis. 2021;36:957‐968. [DOI] [PubMed] [Google Scholar]
- 30. Pietzke M, Meiser J, Vazquez A. Formate metabolism in health and disease. Mol Metab. 2020;33:23‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pietzke M, Arroyo SF, Sumpton D, et al. METTEN study group Stratification of cancer and diabetes based on circulating levels of formate and glucose. Cancer Metab. 2019;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia J, Wishart DS. Web‐based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc. 2011;6:743‐760. [DOI] [PubMed] [Google Scholar]
- 33. Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9:280‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guleria A, Kumar A, Kumar U, Raj R, Kumar D. NMR based metabolomics: an exquisite and facile method for evaluating therapeutic efficacy and screening drug toxicity. Curr Top Med Chem. 2018;18:1827‐1849. [DOI] [PubMed] [Google Scholar]
- 35. Wishart DS, Greiner R, Rosborough TA, et al. 2007. Automatic identification of compounds in a sample mixture by means of NMR spectroscopy. [Google Scholar]
- 36. Bouatra S, Aziat F, Mandal R, et al. The human urine metabolome. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang T, Zheng X, Xing F, Zhuo H, Liu C. Serum metabolomic characteristics of patients with liver cirrhotic ascites. Integrative Medicine International. 2014;1:136‐143. [Google Scholar]
- 38. Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end‐stage liver disease. Hepatology. 2001;33:464‐470. [DOI] [PubMed] [Google Scholar]
- 39. Kamath PS, Kim W. The model for end‐stage liver disease (MELD). Hepatology. 2007;45:797‐805. [DOI] [PubMed] [Google Scholar]
- 40. Mardinoglu A, Agren R, Kampf C, Asplund A, Uhlen M, Nielsen J. Genome‐scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non‐alcoholic fatty liver disease. Nat Commun. 2014;5:1‐11. [DOI] [PubMed] [Google Scholar]
- 41. Lu SC, Tsukamoto H, Mato JM. Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol. 2002;27:155‐162. [DOI] [PubMed] [Google Scholar]
- 42. Fernández‐Checa JC, Colell A, García‐Ruiz C. S‐Adenosyl‐L‐methionine and mitochondrial reduced glutathione depletion in alcoholic liver disease. Alcohol. 2002;27:179‐183. [DOI] [PubMed] [Google Scholar]
- 43. Marí M, Morales A, Colell A, García‐Ruiz C, Fernández‐Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid Redox Signaling. 2009;11:2685‐2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lange CM, Moreau R. Immunodysfunction in acute‐on‐chronic liver failure. Visc Med. 2018;34:276‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wasmuth HE, Kunz D, Yagmur E, et al. Patients with acute on chronic liver failure display sepsis‐like immune paralysis. J Hepatol. 2005;42:195‐201. [DOI] [PubMed] [Google Scholar]
- 46. Noor MT, Manoria P. Immune dysfunction in cirrhosis. J Clin Trans Hepatol. 2017;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma EH, Bantug G, Griss T, et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 2017;25:345‐357. [DOI] [PubMed] [Google Scholar]
- 48. Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1:31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39:191‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mehta MM, Weinberg SE, Chandel NS. Mitochondrial control of immunity: beyond ATP. Nat Rev Immunol. 2017;17:608. [DOI] [PubMed] [Google Scholar]
- 51. Hernaez R, Liu Y, Kramer JR, Rana A, El‐Serag HB, Kanwal F. Model for end‐stage liver disease‐sodium underestimates 90‐day mortality risk in patients with acute‐on‐chronic liver failure. J Hepatol. 2020;73:1425‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luntz SP, Unnebrink K, Seibert‐Grafe M, et al. HEGPOL: randomized, placebo controlled, multicenter, double‐blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312]. BMC Surg. 2005;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eriksson L, Johansson E, Kettaneh‐Wold N, Wold S. Umea: Sweden. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study has been uploaded on ZENODO (https://zenodo.org/record/4638623) and is available without undue reservation for further studies on request to the corresponding author.


