Abstract
Immunity to adenoviruses is an important hurdle to be overcome for successful gene therapy. The presence of antibodies to the capsid proteins prevents efficacious adenovirus vector administration in vivo. We tested whether immunity to a particular serotype of adenovirus (Ad5) may be overcome with a vector that encodes the hexon sequences from a different adenovirus serotype (Ad12). We successfully constructed an adenovirus vector with a chimeric Ad5-Ad12 hexon which was not neutralized by plasma from C57BL/6 mice immunized with Ad5. The vector was also capable of transducing the livers of C57BL/6 mice previously immunized with Ad5.
Adenovirus vectors have great utility for the development of gene therapy protocols because they are capable of transducing a wide variety of cell types and mediating efficient gene transfer in vivo. However, one of the major obstacles to their use is the strong host humoral immune response to the capsid components, which has been shown to block vector efficacy following intratracheal or intravenous administration (13, 18, 23, 27). Antibodies against adenovirus vectors could be derived from two sources: a previous adenovirus-mediated common cold or, in the clinical setting, a previous treatment with an adenovirus vector. In animal models, the humoral response to the initial exposure to the vector has been shown to be sufficient to prevent readministration unless the animal is immunocompromised by pharmacological or immunological treatments (6, 19, 21, 22, 24–26) or made tolerant to viral capsid components (11, 12). This has cast serious doubt on the future use of adenoviruses as gene therapy vectors in patients with preexisting anti-vector antibodies or for any condition requiring multiple administrations.
One experimental approach to circumvent the problem of circulating antibodies against the vector capsid involves the development of multiple vectors, each derived from a different adenovirus serotype. The vectors would be used sequentially, each evading the antibodies generated by the previous types (12, 14). However, this strategy is limited by the need to verify efficacy and safety with multiple vectors. An alternative, more conservative strategy, described in the present study, involves generating vectors in which only the immunodominant capsid epitopes are altered.
Adenovirus capsids have three principal protein components: the hexon, the penton, and the fiber. The hexon contributes the majority of the structure, which is composed of 240 trimeric hexon capsomeres and 12 pentameric penton capsomeres. The trimeric fiber protein produces a knobbed rod-like structure with one copy embedded in each of the 12 penton capsomeres located at the vertices of the icosahedral capsid. At least 49 different serotypes of adenoviruses have been described, and these are classified into six different subgroups based on hemagglutination characteristics and DNA homology (10). Humoral immunity resulting from infection is restricted to a particular subgroup, and immunity to a particular serotype does not result in cross-immunity to an adenovirus serotype belonging to a different subgroup (9). Based on experiments using antibodies raised against purified hexon, penton, and fiber, it has been shown that the fiber and the hexon harbor type-specific determinants (15). Anti-fiber antibodies neutralize infectivity in vitro by a blocking mechanism (20) but have been shown to be inadequate in preventing transduction in an animal model (7). In contrast, anti-hexon antibodies neutralize infectivity by an efficient single-hit mechanism. One anti-hexon antibody molecule per virion is sufficient to effect loss of infectivity (20), possibly by preventing the conformational changes necessary for endosomal rupture.
Each hexon capsomere is a homotrimer of an approximately 900-amino-acid-long polypeptide. X-ray crystallography of the Ad2 hexon trimer (1, 16) has revealed a hexagonal “pedestal” base from which a “tower” region projects outward into the solvent. Three surface loops, L1, L2, and L4, from each monomer interdigitate to form the tower domain. A comparison of hexon sequences from several different serotypes indicates that the sequences encoding the pedestal are highly conserved whereas those encoding the outwardly disposed loops show considerable variability. The sequence encoding the L1 loop exhibits the greatest diversity (2).
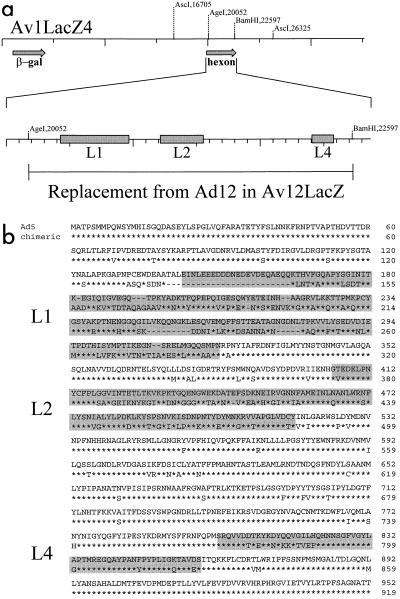
Adenovirus vectors used for gene transfer protocols are frequently prepared in a viral backbone derived from serotype 5 (Ad5). Because serotype-specific sequences are located on the variable regions of the loops (4), and because hexon neutralization epitopes are not shared between serotypes belonging to different subgroups, we hypothesized that removing the serotype-specific epitopes from an Ad5-based vector and replacing them with the analogous epitopes from adenovirus serotype 12 (Ad12) would yield a novel vector capable of transducing tissues in vivo in the presence of neutralizing antibodies to Ad5. To test the hypothesis, an Ad5-based vector, Av1LacZ4, which encodes β-galactosidase (18), was modified so that the region spanning loops L1 through L4 was replaced with the corresponding segment from Ad12 (Fig. 1) to yield the vector Av12LacZ, harboring a chimeric hexon. PCR primers with the sequences GCG ACC GGT CGC AGC GTC TGA CGC TGC GT and GTG AAT GCG TAC CAC GTC GAA were used to amplify a 2,507-bp fragment corresponding to this region from the wild-type Ad12 (obtained from the American Type Culture Collection) genome. The PCR product was used to replace the native Ad5 sequence between the AgeI and BamHI restriction enzyme sites in a plasmid harboring the Ad5 region between the two AscI restriction sites. In order to incorporate the modified hexon sequence into the vector Av12LacZ, a novel strategy was utilized in which the viral E2a gene was used as a selectable marker. The AscI restriction fragment, containing the chimeric hexon as well as the E2a gene, was incorporated into a virus genome by ligating it to an Av3nBg genome (identical to Av1LacZ4 except for an additional deletion in the E2a region [8]) digested with AscI. The ligation mixture was used to transfect 293 cells, which complement adenovirus E1 functions but not those of E2a. Recombinant virus containing the chimeric hexon and the E2a gene would be expected to grow in 293 cells, whereas the parental virus lacking the E2a gene would not. Plaques were picked, propagated, and analyzed by Southern hybridization for the desired virus—Av12LacZ, harboring a chimeric hexon gene. Due to the high degree of sequence identity between Ad5 and Ad12 in the region of the hexon flanking the substitution, the resulting chimeric hexon was 99.2% identical to the hexon of Ad12. The vector could be plaque purified, amplified, and isolated by isopycnic banding in a cesium chloride gradient. When evaluated by electron microscopy, Av12LacZ exhibited a normal adenovirus morphology (data not shown). However, virus yield as determined by plaque titers was approximately 100-fold lower than those of similar preparations of Av1LacZ4. The particle-to-PFU ratio in preparations of Av12LacZ obtained from 293 ranged between 40 and 100, values which are similar to those obtained for Av1LacZ.
FIG. 1.
(a) Hexon replacement strategy. A map of the genome of Av1LacZ4 is shown. The locations of the relevant restriction enzyme sites used for the manipulation of the genome are indicated. Arrows indicate the positions of the regions coding for the hexon and that for the β-galactosidase reporter gene. The locations of the L1, L2, and L4 loops within the hexon are shown. (b) Alignment of the complete Ad5 hexon amino acid sequence with that of the chimeric hexon of Av12LacZ (done with DNASTAR software). The replacement of the Ad5 sequence shown in panel a results in replacement of the Ad5 native sequence between amino acids 59 and 907. Identical amino acids are indicated by asterisks. Gaps in the sequence alignment are indicated by dashes. L1, L2, and L4 regions are shaded.
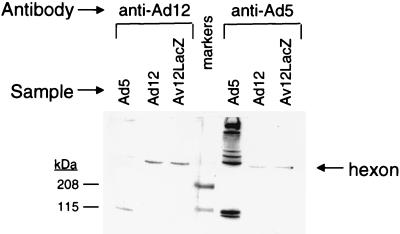
To assess antibody reactivity, preparations of Ad5, Ad12, and Av12LacZ were subjected to Western blot analyses. Purified Ad5, Ad12, and Av12LacZ (2 × 109 PFU of each) were electrophoresed (in duplicate) in standard Laemmli sample buffer without a reducing agent, and the samples were not heated prior to electrophoresis. Under these conditions the hexons migrate as trimers with molecular weights of ∼310,000 for Ad12 and Av12LacZ and 324,000 for Ad5. Following electrophoresis on a 4 to 15% polyacrylamide gradient gel, the separated proteins were electroblotted onto a polyvinylidene difluoride membrane. The blot was cut into two identical strips, each containing the three viruses. The strips were then subjected to immunodetection by standard protocols. The two strips were probed with serotype-specific rabbit polyclonal antibodies to Ad5 (ATCC VR-1082) and Ad12 (ATCC VR-1089), respectively, used at a 1:3,000 dilution. The blots were developed with secondary antibodies and reagents supplied in the Amersham ECL Western blotting kit by protocols recommended by the manufacturer. As shown in Fig. 2, a polyclonal antiserum raised against Ad5 reacted with greater avidity to the Ad5 hexon than to either the Ad12 hexon or the chimeric Av12LacZ hexon. Additionally, a polyclonal antiserum raised against Ad12 reacted more intensely to both the Ad12 and the Av12LacZ hexons than to the Ad5 hexon. These data suggest that incorporation of the chimeric hexon altered the vector’s antigenic footprint from that of Ad5 to one that more closely resembled Ad12.
FIG. 2.
Western blot showing reactivities of Ad5, Ad12, and the chimeric hexon to anti-Ad5 and anti-Ad12 antibodies. markers, molecular mass markers.
To determine whether Av12LacZ would successfully evade anti-Ad5 antibodies in a murine model, the vector was analyzed with a neutralization assay. Six C57BL/6 mice were immunized by intravenous administration via tail vein with 109 PFU of an Ad5-based vector, Av1ALAPH8 (3), and plasma was collected 14 days later. Anti-adenovirus titers against Av1LacZ4 and Av12LacZ in the plasma samples were determined as described previously (19). Briefly, the vectors were incubated with dilutions of heat-inactivated plasma and then used to transduce cells in culture. The following day, the cells were stained for β-galactosidase activity. The titer of neutralizing antibody was reported as the highest dilution with which less than 25% of the cells stained blue. As shown in Table 1, the plasma samples inactivated Av1LacZ4 at dilutions between 1:8 and 1:1,024. However, none of the plasma samples had any neutralizing activity against Av12LacZ at the highest concentration tested, a dilution of 1:2. This indicated that Av12LacZ could successfully infect cells in the presence of antibodies generated against an Ad5-based vector.
TABLE 1.
Neutralization titers of plasma from C57BL/6 mice immunized with Ad5
| Plasma sample no. | Neutralization titer for:
|
|
|---|---|---|
| Av1LacZ4 | Av12LacZ | |
| 1 | 256 | <2 |
| 2 | 8 | <2 |
| 3 | 64 | <2 |
| 4 | 256 | <2 |
| 5 | 256 | <2 |
| 6 | 1,024 | <2 |
We also used the in vitro neutralization assay described above to determine whether serum samples obtained from screened healthy human blood donors (kindly provided by Susan Leitman, National Institutes of Health, Rockville, Md.) harbor antibodies capable of reducing the infectivity of Av1LacZ4 and Av12LacZ. Of the 23 serum samples assayed, 9 had blocking or neutralizing antibodies against the Ad5-based Av1Lacz4 as well as wild-type Ad12 (as determined by a plaque assay). These samples also neutralized Av12LacZ. The other 14 samples did not neutralize Ad5, but 7 had detectable neutralizing activity against Av12LacZ (at a dilution of 1:8 or greater), as well as wild-type Ad12.
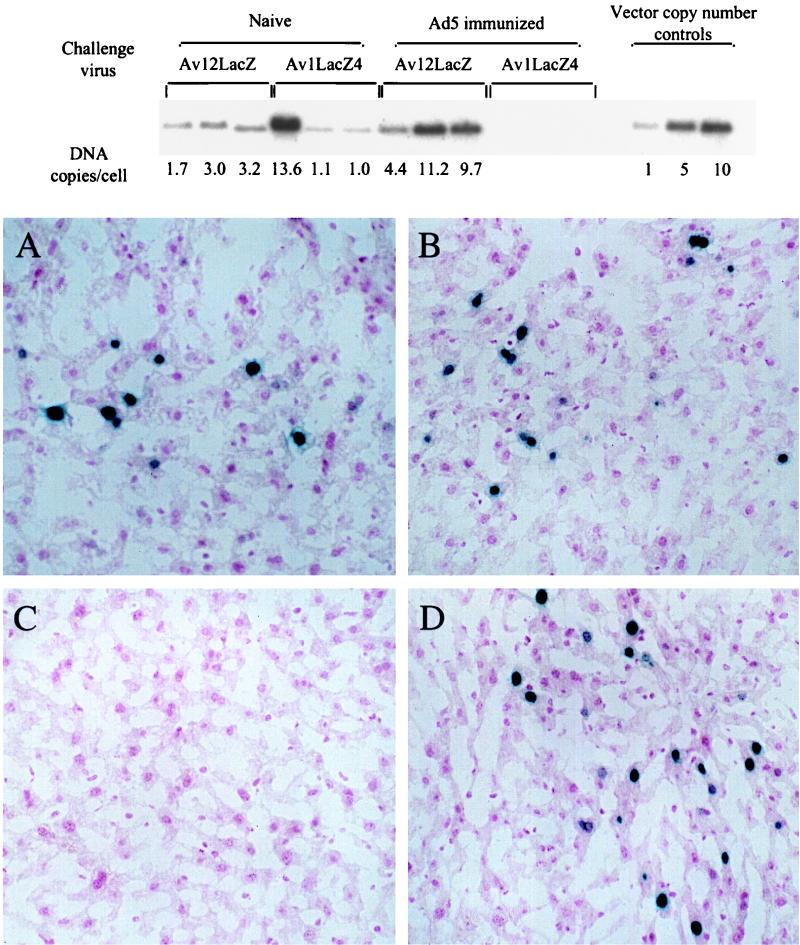
To confirm that mice immunized with Ad5 could be transduced with the vector Av12LacZ, cohorts of three C57BL/6 mice were immunized with a tail vein injection of 108 PFU of an Ad5-based vector, Av1ALAPH8 (3), a dose which had previously been determined to prevent readministration (19). After 1 month, the mice were challenged with 3 × 108 PFU each of either Av12LacZ or Av1LacZ4. Two days later the mice were sacrificed, and their livers were analyzed for vector transduction by histochemical staining for β-galactosidase activity (Fig. 3). The transduction efficiency in the liver of each of the experimental mice was quantitated by Southern hybridization of liver DNA with a β-galactosidase gene cDNA probe (Fig. 3). The Southern blot showed that both Av1LacZ4 and Av12LacZ efficiently transduced the livers of naive mice. However, only Av12LacZ could transduce the livers of the mice which had been immunized by a previous administration of an Ad5-based vector. The results of the histochemical analysis for β-galactosidase activity confirmed the Southern data. Blue-staining hepatocytes were seen with both vectors in naive mice but only with Av12LacZ in mice previously immunized with the Ad5 vector. Thus, the adenovirus vector harboring the chimeric hexon was efficacious in vivo in animals with circulating antibodies to Ad5.
FIG. 3.
(Top) Transduction of liver cells of C57BL/6 mice previously immunized with Ad5 by Av12LacZ. Genomic DNA prepared from the liver samples was digested with the restriction enzyme ClaI and subjected to Southern hybridization with a 2,256-bp ClaI DNA fragment from the β-galactosidase gene as a probe. Copy number controls represent the number of Av1LacZ4 copies per diploid genome spiked into normal mouse genomic DNA. The approximate numbers of copies of β-galactosidase gene DNA in the livers of the mice were determined by comparison with the copy number standards by a quantitative analysis of band intensities in the autoradiogram done with NIH-Image software. (Bottom) Liver samples were also stained for β-galactosidase activity with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as the substrate, as described previously (21). Representative microscopic sections show β-galactosidase activity in livers from naive mice injected with Av1LacZ4 (A) and Av12LacZ (B) or from Ad5-preimmunized mice injected with Av1LacZ4 (C) and Av12LacZ (D).
It is noteworthy that none of the plasma samples from the C57BL/6 mice had any neutralizing activity against the chimeric virus in vitro. Since Av1LacZ4 and Av12LacZ have identical capsid components apart from the hexon, antibodies against such components, principally against the fiber, may be expected to react with the chimeric virus. Although anti-fiber antibodies are not thought to possess true neutralizing activity (20), they do reduce titers measured in vitro by agglutination of virion particles. However, unless they are present at a very high titer, such antibodies may not be sufficient to prevent transduction following a systemic administration of a gene therapy vector. This has been observed to be the case in Sprague-Dawley rats in the presence of substantial blocking antibodies to the fiber (7), in a study where the authors concluded that under standard repeated administration conditions, the contribution to the prevention of transduction by anti-fiber antibodies was not significant. We have observed neutralizing activity against the chimeric virus Av12LacZ in vitro in plasma from mouse strains other than C57BL/6, and experiments are planned to test whether our observations for C57BL/6 mice hold true for other mouse strains.
Of the human adenoviruses, Ad12 is among the most phylogenetically distant from Ad5 (2). However, because of the high degree of sequence relatedness in the pedestal domains of the hexons of even serotypically distant adenoviruses, it seemed plausible that such hexons may be functionally interchangeable. Whereas cocultivation experiments with closely related adenoviruses have demonstrated intertypic recombinants, such recombinants between adenoviruses belonging to different subgroups have not been reported (5). However, we have demonstrated that replacement of the majority of the Ad5 hexon protein sequence with that of a phylogenetically distant adenovirus can result in a viable, albeit lower-titer, virus. It will be interesting to determine if the decrease in titer may be avoided by a more conservative approach of limiting the changes to the variable regions within the hexon loops.
The choice of Ad12 as the donor for the hexon switch was based not only on its phylogenetic distance from Ad5 but also on the reportedly low prevalence of Ad12 infections (17). However, a majority of the normal human sera we tested harbored antibodies capable of neutralizing Ad12. Thus, use of a hexon-switching strategy in a clinical setting will require screening of the target population to identify suitable hexon donor serotypes.
The finding that Av12LacZ could efficiently evade host immunity in mice underscores the importance of anti-hexon antibodies in effecting rapid adenovirus neutralization. Thus, vectors with modified hexon epitopes may be efficacious in patients previously exposed to Ad5, and sequential use of a battery of such vectors may enable repeated therapy.
Acknowledgments
Sera from screened healthy human voluntary blood donors were kindly provided by Susan Leitman, Chief of Blood Services, National Institutes of Health, Rockville, Md. We thank Christine Mech for preparing liver sections and for staining for β-galactosidase activity. We also thank Adam Shoemaker and Julie Andrews for help in conducting experiments.
REFERENCES
- 1.Athappilly F K, Murali R, Rux J J, Cai Z, Burnett R M. The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 Å resolution. J Mol Biol. 1994;242:430–455. doi: 10.1006/jmbi.1994.1593. [DOI] [PubMed] [Google Scholar]
- 2.Bailey A, Mautner V. Phylogenetic relationships among adenovirus serotypes. Virology. 1994;205:438–452. doi: 10.1006/viro.1994.1664. [DOI] [PubMed] [Google Scholar]
- 3.Connelly S, Gardner J M, Lyons R M, McClelland A, Kaleko M. Sustained expression of therapeutic levels of human factor VIII in mice. Blood. 1996;87:4671–4677. [PubMed] [Google Scholar]
- 4.Crawford-Miksza L, Schnurr D P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70:1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford-Miksza L K, Schnurr D P. Adenovirus serotype evolution is driven by illegitimate recombination in the hypervariable regions of the hexon protein. Virology. 1996;224:357–367. doi: 10.1006/viro.1996.0543. [DOI] [PubMed] [Google Scholar]
- 6.Dai Y, Schwarz E M, Gu D, Zhang W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorziglia M I, Kadan M J, Yei S, Lim J, Lee G M, Luthra R, Trapnell B C. Elimination of both E1 and E2a from adenovirus vectors further improves prospects for in vivo human gene therapy. J Virol. 1996;70:4173–4178. doi: 10.1128/jvi.70.6.4173-4178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hierholzer J C, Stone Y O, Broderson J R. Antigenic relationships among the 47 human adenoviruses determined in reference horse antisera. Arch Virol. 1991;121:179–197. doi: 10.1007/BF01316753. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz M S. Adenoviridae and their replication. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1679–1721. [Google Scholar]
- 11.Kass-Eisler A, Falck-Pedersen E, Elfenbein D H, Alvira M, Buttrick P M, Leinwald L A. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 12.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pedersen E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 13.Kozarsky K F, McKinley D R, Austin L L, Raper S E, Stratford-Perricaudet L D, Wilson J M. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- 14.Mastrangeli A, Harvey B, Yao J, Wolff G, Kovesdi I, Crystal R G, Falck-Pedersen E. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7:79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 15.Norby E. The structural and functional diversity of adenovirus capsid components. J Gen Virol. 1969;5:221–236. doi: 10.1099/0022-1317-5-2-221. [DOI] [PubMed] [Google Scholar]
- 16.Roberts M M, White J L, Grütter M G, Burnett R M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986;232:1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz H, Wigand R, Heinrich W. Worldwide epidemiology of human adenovirus infections. Am J Epidemiol. 1983;117:455–466. doi: 10.1093/oxfordjournals.aje.a113563. [DOI] [PubMed] [Google Scholar]
- 18.Smith T A G, Mehaffey M G, Kayda D B, Saunders J M, Yei S, Trapnell B C, McClelland A, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX. Nat Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- 19.Smith T A G, White B D, Gardner J M, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 20.Wohlfart C. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J Virol. 1988;62:2321–2328. doi: 10.1128/jvi.62.7.2321-2328.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Greenough K, Wilson J M. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 1996;3:412–420. [PubMed] [Google Scholar]
- 22.Yang Y, Haecker S E, Su Q, Wilson J M. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum Mol Genet. 1996;5:1703–1712. doi: 10.1093/hmg/5.11.1703. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Su Q, Grewal I S, Schilz R, Flavell R A, Wilson J M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70:6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y, Wilson J M. CD40 ligand-dependent T cell activation: requirement of B7-CD28 signalling through CD40. Science. 1996;273:1862–1864. doi: 10.1126/science.273.5283.1862. [DOI] [PubMed] [Google Scholar]
- 27.Yei S, Mittereder N, Tang K, O’Sullivan C, Trapnell B C. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther. 1994;1:192–200. [PubMed] [Google Scholar]