Abstract
Background:
Recent epidemiologic research shows many environmental chemicals exhibit endocrine disrupting effects on the female reproductive system. Few studies have examined exposure at reproductive organs. Our aim was to perform a preliminary untargeted metabolomic characterization of menstrual blood, a novel biofluid, to identify environmental toxins present in the endometrium and evaluate the suitability of this sample type for exposome research.
Methods:
Whole blood menstrual samples were collected from four women using a menstrual cup. Samples were analyzed for small molecules that include both environmental chemicals and endogenous metabolites using untargeted liquid chromatography with high-resolution mass spectrometry (LC-HRMS). Principal component analysis (PCA) and ANOVA was used to identify differences within and between individuals’ menstrual blood metabolomic profiles, and the influence of the sample processing method. To assess the presence of environmental exposures, LC-HRMS chemical profiles were matched to the ToxCast chemical database, which includes 4557 commonly used commercial chemicals. Select compounds were confirmed by comparison to reference standards.
Results:
PCA of metabolome profiles showed analysis of menstrual blood samples were highly reproducible, with high variability in detected metabolites between participants and low variability between analytical replicates of an individual’s sample. Endogenous metabolites detected in menstrual blood samples achieved good coverage of the human blood metabolome. We found 1748 annotations for environmental chemicals, including suspected reproductive toxicants such as phenols, parabens, phthalates, and organochlorines. Storage temperature for the first 24 h did not significantly influence global metabolomic profiles.
Conclusion:
Our results show chemical exposures linked to reproductive toxicity and endocrine disruption are present in menstrual blood, a sampling medium for the endometrium.
Keywords: Menstrual blood, Metabolomics, Menstruation, Biomarkers, Exposome
Graphical Abstract

1. Introduction
A wide variety of chemicals found in our environment have been linked to adverse reproductive and obstetrical outcomes including decreased fecundity, endometriosis, poor oocyte quality, poor fetal growth, pregnancy loss, and earlier delivery.(Segal and Giudice, 2019; Zlatnik, 2016) However, these studies have shown mixed results, and exposure burden in the reproductive organ is not considered.(Mínguez-Alarcón and Gaskins, 2017) To better understand the relationship between environment and reproductive health, there is a critical need to study exposure patterns at the reproductive organs, and see how these are related to early biological effects and reproductive outcomes. Recent studies have evaluated toxicant concentration in reproductive tissues and fluids including follicular fluid, amniotic fluid, and fetal cord blood; however, these studies are often limited to a small number of known chemicals and do not account for the complex mixtures of exposures that contributes to the human exposome. Furthermore, menstrual blood has not been extensively evaluated. (Iribarne-Durán et al., 2020) Menstrual blood assays offer several advantages for sampling toxicant exposure and metabolomic analysis; menstrual blood is easier to collect than peripheral serum, can be collected monthly allowing for longitudinal analysis, and provides a snapshot of exposure to exogenous chemicals and endogenous metabolite alterations at the endometrium, an important site for obstetrical and gynecological health.
Menstruation is triggered by a sharp decline in progesterone levels at the end of the secretory phase in a menstrual cycle that does not result in conception.(Evans and Salamonsen, 2012) The drop in progesterone results in upregulation of local inflammatory processes at the endometrium, inducing tissue break down and shedding of the functional layer of the endometrium. (Maybin and Critchley, 2015) Menstrual blood is composed of blood, vaginal fluid, and the endometrial cells in the uterine lining before menstruation. Because menstrual blood from early in a cycle may provide insight into the local endometrial environment immediately prior to menstruation, sampling of menstrual blood could provide a non-invasive method for repeated exposure and biological response measurement in longitudinal studies. The endometrial microenvironment is relevant for embryo implantation and development throughout pregnancy, as well as for various adverse health outcomes including endometrial hyperplasia, cancer, and fibroids. Recent advances in high throughput analytical strategies allow for efficient, low cost characterization of the human metabolome in blood, urine, biopsies, and other fluids.(Walker et al., 2019) The human metabolome includes all low-molecular weight endogenous metabolites, products from human-environment interaction, and any metabolites resulting from interactions and chemical reactions of these chemicals in the body, and offers a measure of the human exposome at a single point in time. This allows for evaluation of the exposome at the menstrual endometrium. Exposomic analysis of menstrual blood could offer important insight into understanding the role of environmental exposures in the uterine environment with broad applications including infertility and endometrial pathology.
To our knowledge, only one group has evaluated toxicants in menstrual blood, conducting targeted analysis of parabens and benzophenones in a cohort of Spanish women.(Jiménez-Díaz et al., 2016) Comparison with peripheral blood samples showed uncorrelated within-person toxicant levels, further highlighting the importance of local exposure measurement for reproductive toxicants.(Iribarne-Durán et al., 2020) Menstrual blood provides a novel, less invasive method for sampling exposures at the endometrium that could be utilized in longitudinal studies considering endogenous metabolite changes and disease progression over time. Our paper aims to show that whole menstrual blood samples collected from a pilot population of four women is suitable for untargeted metabolomic analysis, allowing for detection of exogenous chemicals, including environmental chemicals and drugs, and endogenous metabolites present at the endometrium, a biologically relevant tissue for reproductive health.
2. Methods
2.1. Recruitment
Four women were recruited through the ongoing IRB-approved Research Outsmarts Endometriosis (ROSE) study (IRB #13–376A) at the Feinstein Institutes. After consenting and completing questionnaires to collect demographic, lifestyle and health information, participants received a menstrual blood collection kit shipped by mail, along with instructions on how to collect their menstrual blood, as previously described (Warren et al., 2018; Nayyar et al., 2020). Women recruited for the study were of reproductive age, and had natural regular menstrual cycles (i.e., did not use oral or implantable contraceptives/hormones).
2.2. Collection of menstrual blood
Menstrual blood samples were collected using the DivaCup menstrual cup (International Organization for Standards 13,845 certified). Menstrual blood was collected on the day of peak flow (cycle day 1, corresponding to first day of full flow, or cycle day 2) from 4 women during the month of August. All women completed a brief questionnaire at the time of collection indicating whether they experienced pain in the collection cycle, the levels of pain (none, mild, moderate, or severe) and their use of pain relievers (see Table 1). Participants were instructed to collect menstrual blood for 4–10 h. Participants transferred their menstrual blood collection into 50 ml, bisphenol A (BPA)-free sterile containers containing antimycotics and antibiotics with screw-top caps and shipped on wet ice to the Boas Center Biorepository at the Feinstein Institutes, where it was accessioned and immediately processed. To test differences by storage technique, one portion of whole menstrual blood was immediately frozen in a cryovial at −20 °C for 24 h and the one portion was kept at 4 °C overnight in a cryovial for 24 h; both samples were then stored at −80 °C until analysis. Menstrual blood samples were collected by participants at home and stored in vials at the designated temperature until shipment to the laboratory for processing. Samples were mixed by gentle vortexing before aliquoting.
Table 1.
Pilot participant characteristics.
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | |
|---|---|---|---|---|
| Age, yrs | 31 | 33 | 37 | 35 |
| BMI, kg/m2 | 26.6 | 24.9 | 22.6 | 24.3 |
| Race | Caucasian | Caucasian, | Puerto Rican | Caucasian |
| Ethnicity | Non-Hispanic | Non-Hispanic | Hispanic | Non-Hispanic |
| Month collected | August | August | August | August |
| Age at menarche, yrs | 12 | 12 | 14 | 13 |
| Typical Cycle Length (time from D1 to D1), days - self report | 26–31 | 26–31 | [Getting confirmation] | [Getting confirmation] |
| Typical bleed length, days | 7–9 | 3–5 | 6 | 4 |
| Typical menstrual flowa | Heavy | Moderate | Heavy | Heavy |
| Pain at time of collection (Y/N) | N | Y | Y | Y |
| Pain severity at time of collection | N/A | Severe | Mild | Moderate |
| Pain medication used at collection (Y/A) | N | Y | Y | Y |
| Medications/Drugs at time of collection | N/A | Ibuprofen | Naproxen | Ibuprofen |
| Gynecological conditions (Yes/No) | ||||
| Endometriosis | N | N | Y | Y |
| Adenomysosis | N | N | N | N |
| Pelvic inflammatory disease | N | N | N | N |
| PCOS | N | N | N | N |
Light (using 10 or less pads/tampons per period) Moderate (11–20 pads/tampons per period) Moderately heavy (21–30 pads/tampons per period) Heavy (>30 pads/tampons per period).
2.3. Metabolomics analysis
The menstrual blood metabolome was characterized using untargeted liquid chromatography with high-resolution mass spectrometry to provide detection of a wide range of environmental chemicals and endogenous metabolites(Soltow et al., 2013; Liu et al., 2020). Six replicates for each menstrual blood sample were prepared by treating 50 ul of whole menstrual blood with 100 ul of acetonitrile containing a series of internal standards that include multiple 13C-labelled environmental and endogenous metabolites. Because samples were collected and stored without separating red blood cells from plasma, the metabolomic profiles represents a combined measure of circulating metabolites and metabolites from red blood cells. (Chaleckis et al., 2016) Treated samples were vortex mixed, allowed to equilibrate for 30 min on ice, and centrifuged at 18.2 xg. The supernatant was transferred to an autosampler containing a low volume insert and maintained at 4 °C until analysis.
Sample extracts were analyzed using a dual column chromatography system (Thermo Scientific Vanquish Duo) interfaced to a Thermo Scientific Q Exactive HF-X Orbitrap high-resolution mass spectrometer. Each extract was analyzed twice using C18 chromatography (Higgins Analytical TARGA C18 5 μm 50 × 2.1 mm) with mobile phases optimized for positive or negative electrospray ionization. The mobile phase for positive analysis included water containing 0.1 % formic acid (B) and acetonitrile containing 0.1 % formic acid (A); and 10 mM ammonium acetate in water (B) and 100 % acetonitrile (A) for negative mode. The flow rate was 0.40 ml/min and the total run time was 7.5 min. The HRMS was operated in full-scan MS at resolution of 120,000 full width at half maximum (FWHM) and scan range 85 to 1275 m/z. The spray voltage was set at 3.5 kV. The capillary temperature and aux gas heater temperature were set at 300 and 250 °C, respectively. Sheath gas and aux gas flow rate were set at 45 and 25 (in arbitrary units), respectively. The funnel RF level was set to 35. Mass spectral features were extracted and aligned separately for each mode using apLCMS with modifications by xMSanalyzer; the resulting feature tables were then filtered to remove all peaks not detected in at least 25 % of study samples (i.e., consistently detected in analytical replicates of at least one participant’s sample). Prior to statistical analysis, peak intensities were log2 transformed.
2.4. Annotation
The goal of annotation in this initial study was to 1) broadly explore the presence of endogenous metabolites that can be used to characterize biological response to exposure in the endometrium as found in menstrual blood and 2) screen for the presence of endocrine disrupting chemicals. Endogenous metabolites were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database and HUMANBLOOD chemical list on the EPA CompTox Chemistry Dashboard. Exogenous compounds were then annotated using the TOXCAST_V3 databases from the EPA CompTox Chemical Dashboard, a library of approximately 4400 commonly used commercial chemicals. Prior to annotation, each database was merged based upon chemical formula, and the structures corresponding to these formulas were sorted in descending order based upon the number of PubMed reference counts. These annotations were completed separately for each database and ionization mode using xMSannotator with common adducts for positive or negative mode and 5 ppm mass accuracy. We used these separate databases to characterize variability in chemical profiles in menstrual blood corresponding to endogenous metabolites and exogenous chemicals, which included environmental and commercial chemicals, although there is some overlap in each database. Annotated environmental chemicals were cross-referenced to lists of chemicals monitored by NHANES(United States Centers for Disease Control and Prevention, 2019) and personal care product (PCP) libraries(Chow and Mahalingaiah, 2016) to identify chemicals of health concern potentially present in menstrual blood samples. Methods for compiling personal care product libraries using ingredient lists from best-selling lip, face, and eye make-up products from top-rated beauty brands have been described in detail previously. (Chow and Mahalingaiah, 2016) When possible, detected environmental chemicals were identified by comparison of accurate mass and retention time to a database of authentic standards previously analyzed on the same platform.
2.5. Statistical analysis
Due to the small sample size of this study, we performed an exploratory analysis to evaluate the main sources of variability in the menstrual blood metabolomic profiles. Principal component analysis (PCA) was completed with centered and scaled data using the prcomp function in R. The resulting scores from PC1 and PC2 were evaluated using analysis of variance (ANOVA) were used to examine the effects of sample processing method and the differences within analytical replicates and between individuals’ menstrual blood metabolomic profiles to assess potential sources of variation Results showed minimal differences in menstrual samples frozen immediately or stored at 4 °C after collection; therefore, all further analysis was restricted to menstrual blood samples frozen immediately after collection. Differences in variability for endogenous metabolites, drugs, and environmental chemicals for the four participant samples were assessed using PCA for all annotations from the HUMANBLOOD and TOXCAST_V3 databases.
To evaluate intra- and inter-variation among all m/z features detected in menstrual blood samples, we performed a one-way ANOVA and pathway enrichment analysis to see how endogenous metabolites from metabolic pathways varied among participants. We determined both Bonferroni-adjusted p-values and F-values. Due to the small number of participants and repeat sampling, adjusted p-values are estimates and were only used as guidance to identify which features showed the most variability across the samples. To characterize biological response pathways present in the menstrual blood samples, we performed pathway enrichment for all metabolite features corresponding to the 95th percentile F-scores (F > 22.17). Pathways were identified using Mummichog. All statistical analyses were conducted using R 4.0.5.
3. Results
Research participants were between the ages of 31 and 37 years (average 34 years). Two women reported surgically diagnosed endometriosis (in the absence of pelvic inflammatory disease, adenomyosis, or PCOS) and two women reported no endometriosis, pelvic inflammatory disease, adenomyosis, or PCOS). The participants’ demographic, lifestyle and health characteristics are shown in Table 1.
Untargeted metabolomic profiling of menstrual blood samples detected 21,512 m/z features. Due to the potential for multiple adducts and isotopes arising from a single compound, this number of features does not define the number of chemicals detected and multiple annotation strategies were used to identify endogenous and exogenous metabolites. Accurate mass m/z were first annotated using the KEGG database, which provided 1701 Level 4 annotations (assignment of an unequivocal molecular formula) (Schymanski et al., 2014), from 126 pathways that included 5 or more detected endogenous metabolites. Ibuprofen was detected in all four participants and higher in participants who reported taking ibuprofen at the time of sample collection (Participants 2 and 4). Naproxen was not detected in any of the samples.
To evaluate the influence of sample storage after collection, we analyzed menstrual blood samples frozen immediately after collection and a second aliquot stored at 4 °C for 24 h prior to freezing. Comparison of global metabolomic profiles for samples stored at 4 °C in a refrigerator and at −20 °C prior to storage at −80 °C using PCA suggest processing prior to storage had minimal effects on overall sample quality (Fig. 1A) and suggest inter-individual differences were much greater than any variation due to initial sample storage methods, with respect to global profiles. PC1 scores showed a nominal association with storage methods (p = 0.06), while PC2 scores did not differ based upon initial sample processing temperature (Fig. 1B), however, statistical tests may not be a valid method for comparing differences in this small of a sample size. For simplicity, further analyses were restricted to the samples initially stored at −20 °C.
Fig. 1.

Principal component analysis (PCA) to evaluate effect of storage temperature of menstrual blood samples for 24 h prior to freezing at −80 °C.
To evaluate global metabolomic alterations due to inter-individual differences in menstrual blood metabolomic profiles, we evaluated PC1 and PC2 for all detected m/z features for each participant. PC1 and PC2 suggest individual menstrual blood metabolomic profiles differed among individuals, which is evident from the clear clustering among participants in Fig. 2A. Analysis of PC1 and PC2 using ANOVA to evaluate within and between group variability were highly different (p < 0.0001) (Fig. 2B). While none of the participant variables could explain the differences in PC1, comparison of scores for PC2 suggested a strong association with age (Fig. 2C). Due to the small number of samples in this study, these results are only suggestive and will have to be confirmed in a larger study showing a greater age range.
Fig. 2.
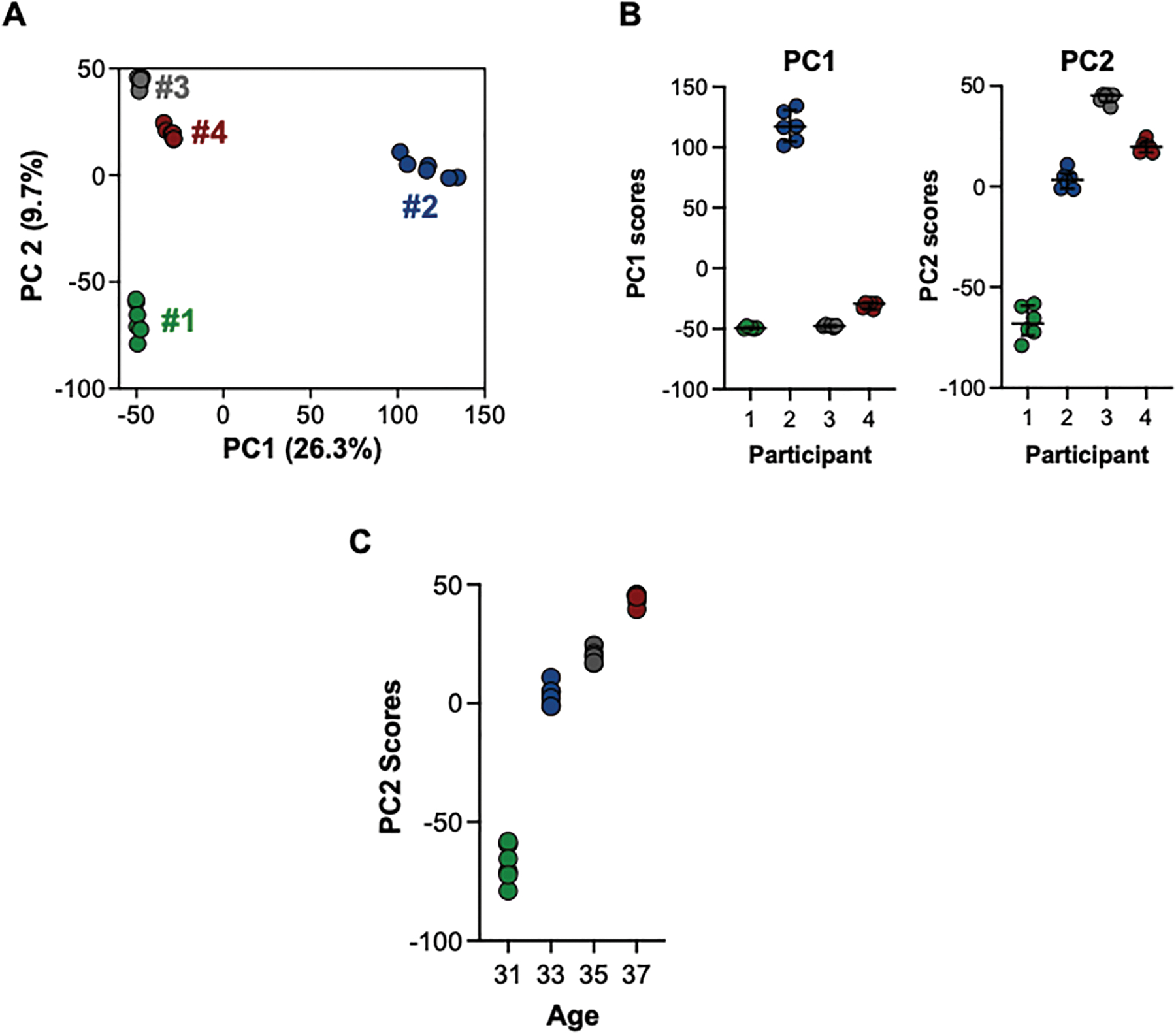
Principal component analysis (PCA) to evaluate alterations due to inter-individual differences in menstrual blood. Principal component 1 (PC1) and principal component 2 (PC2) were evaluated for association with global metabolomic profiles for each participant. A) PC1 and PC2 suggest individual menstrual blood metabolome profiles differed significantly among individuals, with minimum variation observed among replicate analysis of samples and large variation present among the samples from different participants. B) Analysis of PC1 and PC2 using ANOVA to evaluate within and between group variability were highly different (p < 0.0001). C) PC2 scores suggest a trend by age.
To evaluate differences in endogenous pathways among the samples, we performed ANOVA and pathway enrichment analysis to identify which metabolites showed the greatest variation among participants (and minimal analytical variation). Initial ANOVA results showed a large number m/z features that differed among the participants, (Fig. 3A) further supporting the ability to characterize distinct phenotypes within the metabolic profile. Because this study was designed as an exploratory analysis and only included four samples, the m/z features were sorted based upon the magnitude of the F-value, and the m/z features corresponding to the 95th percentile of F-scores were tested for the presence of enriched pathways using Mummichog. Results from this analysis show which metabolic pathways have the greatest potential for variation within the menstrual blood metabolome of our pilot population. A wide range of endogenous metabolic pathways differed among participants (Fig. 3B), including amino acid metabolism, antioxidants, fatty acids and lipids, co-factors and xenobiotic metabolism.
Fig. 3.

Intra-individual variations in all features and endogenous metabolites. A) One-way ANOVA identified a very large number of metabolites that were found to vary among participants. We determined both Bonferroni-adjusted p-values and F-value. Due to the very small number of participants and repeat sampling, adjusted p-values are estimates and are included for visualization purposes only. B) To determine which endogenous metabolites showed the greatest variation among participants, we took m/z corresponding to the 95th percentile F-scores (F > 22.17) and performed pathway enrichment using Mummichog.
To see if the menstrual blood metabolome was influenced by metabolite source, we performed additional analysis of metabolites annotated as endogenous or exogenous compounds using the HUMANBLOOD and ToxCast databases (Fig. S1). Comparison of PC1 scores for endogenous metabolites, and environmental chemicals among the participants suggest overall differences for these profiles are similar among the participants for each class of compounds, and whether the chemical is endogenous or environmental in origin does not influence the overall distribution.
Annotation of m/z features using both positive and negative mode data provided 1748 Level 4 annotations(Schymanski et al., 2014) to chemicals listed in the ToxCast database (Table S1). Of these, 61 annotated chemicals are monitored as part of NHANES or are known ingredients in PCPs (Table 2). These compounds included suspected endocrine disrupting compounds such as phthalates, phenols, parabens, organochlorines, and per- and polyfluoroalkyl substances (PFAS). While many of these are annotations are estimates based upon accurate mass matching and will need to be confirmed using authentic reference standards, this initial characterization suggests the presence of a wide range of environmental chemicals in menstrual blood.
Table 2.
List of annotated chemicals matched to NHANES surveyed chemicals, reproductive toxicants, and personal care product libraries.
| Chemical Class | Chemical Analyte | Confidence Levela |
|---|---|---|
| PFAS | Perfluoroheptanoic acid (PFHpA) | Level 1 |
| Perfluorohexane sulfonic acid (PFHxS) | Level 1 | |
| Perfluorooctanoic acid (PFOA) | Level 1 | |
| Perfluorononanoic acid (PFNA) | Level 1 | |
| Perfluorooctane sulfonic acid (PFOS) | Level 1 | |
| Perfluorodecanoic acid (PFDA) | Level 1 | |
| Pesticides, herbicides, fungicides | Ethylene thiourea | Level 4 |
| Nitrobenzene | Level 4 | |
| para-Nitrophenol | Level 4 | |
| 1-Hydroxynaphthalene (1-Naphthol) | Level 4 | |
| Diethylphosphate (DEP) | Level 4 | |
| 2,4-Dichlorophenol | Level 4 | |
| Desisopropyl atrazine | Level 4 | |
| Desethyl atrazine | Level 4 | |
| N,N-Diethyl-meta-toluamide (DEET) | Level 1 | |
| 3,5,6-Trichloro-2-pyridinol | Level 4 | |
| 3-Phenoxybenzoic acid | Level 4 | |
| 2,4-Dichlorophenoxyacetic acid | Level 4 | |
| Pentachlorophenol | Level 4 | |
| Thiacloprid | Level 1 | |
| Plasticizers | Fenthion | Level 4 |
| 1,2-Dichloroethane (Ethylene dichloride) | Level 4 | |
| Benzonitrile | Level 4 | |
| m-Xylene | Level 4 | |
| Dimethyl phthalate (DMP) | Level 4 | |
| Mono-butyl phthalate | Level 1 | |
| Bisphenol A | Level 1 | |
| Bisphenol S | Level 1 | |
| Diphenyl phosphate (DPhP) | Level 4 | |
| Mono-benzyl phthalate (MBzP) | Level 4 | |
| Di-n-butyl phthalate (DBP) | Level 4 | |
| Personal care product-related chemicals | Di-n-octyl phthalate (DOP) | Level 4 |
| Diethanolamine (DEA) | Level 4 | |
| 4-Nitro-12-phenylenediamine (Coal tar dyes) | Level 4 | |
| Hexamethyldisiloxane | Level 4 | |
| Ethyl paraben | Level 1 | |
| ortho-Phenylphenol | Level 4 | |
| Propyl paraben | Level 1 | |
| Benzophenone | Level 4 | |
| Butyl paraben | Level 4 | |
| Octylphenol | Level 1 | |
| Dibutyl phosphate (DBuP) | Level 4 | |
| Hexamethylcyclotrisiloxane | Level 4 | |
| HHCB | Level 4 | |
| 11,335,577-Octamethyltetrasiloxane | Level 4 | |
| Retinol | Level 4 | |
| Enterolactone | Level 4 | |
| Flame retardants | Retinoic acid | Level 4 |
| Dimethylphosphate (DMP) | Level 4 | |
| Tris(2-chloropropyl) phosphate | Level 1 | |
| Tricresyl phosphate | Level 1 | |
| Tobacco or nicotine-related chemicals | Glycidamide | Level 4 |
| 2,5-Dimethylfuran | Level 4 | |
| Cotinine | Level 4 | |
| NNAL | Level 4 | |
| Synthetic hormones | Diethylstilbesterol (DES) | Level 4 |
| Ethinyl estradiol | Level 4 | |
| Food preservatives, additives, or supplements | Limonene | Level 4 |
| Butylated hydroxytoluene (BHT) | Level 4 | |
| Equol | Level 4 | |
| Retinyl acetate | Level 4 |
Confidence levels assigned according to Schymanski et al., 2014 (Segal and Giudice, 2019). Level 1 identifications were confirmed by comparing accurate mass m/z and retention time to authentic standards run using identical analytical parameters. Level 4 annotations were assigned by accurate mass matching; the resulting structures corresponding to a given formula were then ranked by number of PubMed references, and the top compound is given.
A subset of environmental chemicals annotated using the ToxCast database and monitored by NHANES (n = 18) were confirmed as Level 1 annotations (confirmed structure) (Schymanski et al., 2014) by comparing accurate mass m/z and retention time to authentic standards run on the same LC-HRMS platform. Peak intensities for PFAS compounds (Fig. 4), pesticides (Fig. 5A), flame retardants (Fig. 5B), plasticizers (Fig. 5C), and PCP-related chemicals (Fig. 5D) varied across participants. Of the confirmed chemicals, coefficients of variation between samples were largest for ethyl paraben, monobutyl phthalate, bisphenol A, bisphenol S, N, N-diethyl-meta-toluamide (DEET), and thiacloprid (Table 3), suggesting these chemicals show the greatest variability in exposure patterns among the four participants.
Fig. 4.

Intensities for identified PFAS compounds in menstrual blood samples.
Fig. 5.

Intensities for identified A) pesticides, B) flame retardants, C) plasticizers, and D) personal care product related chemicals in menstrual blood samples.
Table 3.
Coefficient of variation for select environmental chemicals confirmed using tandem mass spectrometry with comparison to reference standards.
| Chemical Class | Chemical Analyte | Coefficient of Variationa (SD/mean) |
|---|---|---|
| PFAS | Perfluoroheptanoic acid (PFHpA) | 0.23 |
| Perfluorohexane sulfonic acid (PFHxS) | 0.73 | |
| Perfluorooctanoic acid (PFOA) | 0.59 | |
| Perfluorononanoic acid (PFNA) | 0.29 | |
| Perfluorooctane sulfonic acid (PFOS) | 0.42 | |
| Perfluorodecanoic acid (PFDA) | 0.73 | |
| Plasticizers | Bisphenol S | 1.34 |
| Bisphenol A | 1.33 | |
| Monobutyl phthalate | 1.39 | |
| Personal care product-related | Ethyl paraben | 1.67 |
| Propyl praben | 0.10 | |
| Octylphenol | 0.08 | |
| Pesticides | N,N-Diethyl-meta-toluamide (DEET) | 1.24 |
| Thiacloprid | 1.25 | |
| Flame retardants | Tris(2-chloropropyl) phosphate | 0.07 |
| Tricresyl phosphate | 0.85 |
Coefficient of variation was calculated from averaged levels of the chemical analyte for each participant (across analytical replicates).
4. Discussion
Our preliminary results demonstrate that fresh menstrual blood can feasibly be collected and utilized for untargeted analysis of small molecule profiles. Comparison of variation in analytical methods to inter-individual differences suggests this is due to differences in overall endogenous and exogenous metabolite profiles among the participants and is not from variation in the analytical method, demonstrating that our analytical method is robust enough to observe differences in metabolite profiles across participants. Analyses of samples were highly reproducible, with high variability between participants and minimum variability between analytical replicates of samples from the same participants. Taken together, these preliminary results highlight the value and quality of menstrual blood for metabolomic and exposome profiling and provide a non-surgically obtained sample for measuring key biochemical changes to the endometrial lining. While limited by the small sample size, our results support the usability of menstrual blood for untargeted metabolomics analysis and highlight its potential for use in larger population studies. The combination of menstrual blood and exposome profiling methods provide a key strategy for understanding environmental effects on the uterus and how external exposures may influence women’s reproductive health (Walker et al., 2016a).
No studies have previously determined the effects of menstrual blood storage at 4 °C or −20 °C to enable at-home collection from study participants, with storage at −80 °C the most commonly recommended storage temperature for biological samples used in metabolomic analysis (Walker et al., 2016b; Patel et al., 2015). We found that samples stored at either temperature for 24 h prior to freezing at −80 °C showed sufficient quality for analysis and that storage temperature had minimal effects on global metabolite profiles. This result has implications for future studies using menstrual blood samples and suggests at-home collection by participants is feasible without sacrificing sample quality. However, this comparison should be repeated focusing on groups of endogenous and exogenous chemicals of interest to ensure storage is not affecting a smaller subset of metabolites.
In this study, we present the first results from untargeted, high-resolution mass spectrometry analysis of freshly collected menstrual blood, a novel biofluid for studying environmental and biological effects in the endometrium. We observed some variation in overall endogenous metabolome profiles across the small sample size of 4 individuals and identified a large number of metabolites, corresponding to a number of endogenous pathways, that were found to vary among participants. Confirmed environmental chemicals included known endocrine disruptors, such as various PFAS, pesticides, flame retardants, phenols, phthalates, and parabens. Variation in levels of these chemicals between participants was higher for short half-life chemicals such as phenols and phthalates and lower for long-half-life PFAS. Our paper supports the concept that the body is interconnected and that key reproductive toxicants previously sampled in serum and urine bioassays are present in the endometrium, a biologically relevant tissue for reproductive health.
Only one prior group has evaluated environmental chemicals in menstrual blood (Iribarne-Durán et al., 2020; Jiménez-Díaz et al., 2016). We identified (Level 1 identification) two of the environmental chemicals previously measured by this group in menstrual blood: ethyl- and propyl-paraben. Jiménez-Díaz et al. additionally quantified methyl- and butyl-paraben as well as 6 benzophenones in menstrual blood collected using menstrual cups (Jiménez-Díaz et al., 2016). Our untargeted analysis annotated benzophenone and butyl- paraben as probable matches (Level 4 annotations), and we additionally identified several other endocrine-disrupting chemicals (EDCs) of interest. In their second paper, Iribarne-Dúran et al. showed that toxicant concentrations for these chemicals were related to age, use of PCPs, and diet (Iribarne-Durán et al., 2020). Although we did observe a suggestion of an age-related effect on global metabolome profiles, due to our small sample size, we were not able to assess associations between demographic variables and individual chemicals. In a subset of 12 women who volunteered to provide both menstrual blood and peripheral blood samples, Iribarne-Dúran et al. also found toxicant levels in menstrual blood samples had low correlation with levels in serum from peripheral blood drawn at the same time as menstrual blood collection (ρ = 0.20–0.51) (p-value >0.09) (Iribarne-Durán et al., 2020). These studies show that menstrual blood can be used for targeted analysis to obtain specific concentrations and show the importance of menstrual blood for measuring exposures at the endometrium and uterus.
Our ability to achieve good coverage of the human endogenous metabolome shows menstrual blood samples could be used to study endogenous metabolites and biological pathways related to reproductive health. Because menstruation occurs at the end of a reproductive cycle where the body is preparing for pregnancy, menstrual blood as a biofluid could provide insight into the metabolomic microenvironment within the uterus immediately prior to implantation. This method for endometrial sampling could be useful in the study of uterine factor infertility and endometrial disease progression. In addition, because menstrual blood can be collected using non-invasive methods repeatedly each month, samples could be utilized in longitudinal studies examining biological perturbations associated with fertility treatments or time-varying exposures. Epidemiologic studies that combine menstrual blood sampling with exposomic measurement methods have the potential to contribute significantly to women’s reproductive health by adding new insight into exposure induced disease pathogenesis at the endometrium.
This project was exploratory, and the goal was to identify the suitability of menstrual blood for untargeted analysis. Our pilot study included a small sample size and therefore is not adequately powered to characterize relationships between menstrual blood exposures and adverse health outcomes. We were able to demonstrate that menstrual blood can be successfully collected and provides a novel biofluid for untargeted small molecule analysis. Use of the menstrual cup as a collection tool is relatively new. However, the DivaCup is manufactured with medical-grade silicone, which is chemically and biologically inert and free of chemicals, plastics, latex, or dyes. Silicone materials have been shown to equilibrate to the concentrations of toxicants in the environment where they are placed and for this reason are often chosen as sampling mediums (Anderson et al., 2017). Participants were instructed to wash DivaCups with water or mild, unscented, oil-free soap. However, if participants washed the DivaCup with oil-containing soap, it may leach off the inner part of the cup during collection. Our collection method using the DivaCup and freezing and transportation methods allowed for successful metabolic phenotyping of menstrual blood. Another potential limitation is that untargeted analysis gives relative, rather than absolute, chemical analyte concentrations. Although this limits external comparability across studies, chemicals of particular concern can be further considered in future targeted analyses where absolute concentrations can be measured. While key environmental exposures were detected and confirmed in menstrual blood using authentic standards, most detected exposure biomarkers did not have available standards and were annotated using computational approaches that were based upon adduct and isotope matching, accurate mass, and reference counts. Annotation confidence can be further improved with MSMS; however, the intensity of environmental chemicals in blood samples are often too low to trigger collection of data dependent MSMS spectra, and the presence of high concentration endogenous metabolites cam result in inconclusive spectra when using data independent acquisition. The current study provides an initial characterization of the small molecule profiles of menstrual blood samples and the presence of suspected environmental chemicals in the endometrial lining. Future studies that combine ongoing efforts to establish exposome-scale libraries of environmental chemicals with untargeted analysis will be needed to further validate the identity of many of the exposures detected in menstrual blood (Liu et al., 2021). Most detected chemicals do not have authentic reference standards available to confirm identities, and collection of MSMS of low concentration exposure biomarkers is challenging.
Most existing epidemiological and toxicological studies on reproductive effects of environmental exposures do not measure exposures directly at the target organ. As has been noted in other studies using non-end organ biofluids such as serum or urine, understanding endometrial exposomics in relation to BPA, PFAS, phthalates, and flame retardants could be particularly helpful for studying uterine factor infertility or unexplained infertility with multiple implantations (Carignan et al., 2017; Mínguez-Alarcón et al., 2019; Wang et al., 2018; Fei et al., 2009; Vélez et al., 2015), as well as gynecological outcomes related to the endometrium such as endometriosis, endometrial cancer, and uterine fibroids (Sirohi et al., 2021; Dogan and Simsek, 2016; Mallozzi et al., 2017; Bariani et al., 2020; Lee et al., 2020). Our results support the need for toxicological examination of reproductive organs in animal studies and the need for more detailed and specific exposure assessment in epidemiological studies on women’s reproductive health. Future work should consider variability of exogenous and endogenous metabolites in menstrual blood across a woman’s menstrual cycles. Recruitment for this study is ongoing to expand this protocol to sample a larger cohort of women and will integrate data on metabolomics with data on menstrual cycle features and other covariates. This will allow more in-depth characterization of environmental exposures at the endometrium and their relationship with reproductive outcomes and progression of reproductive disorders, including endometriosis, a leading cause of infertility in reproductive-aged women. Our paper shows that environmental toxicants are present in the endometrium lining. Future longitudinal studies including bigger sample sizes are warranted to confirm that menstrual blood samples can be utilized for metabolomics studies on exposure to environmental chemicals and women’s health.
Supplementary Material
HIGHLIGHTS.
Menstrual blood successfully collected and used for exposome research.
Untargeted analysis reveals PFAS, phenols, phthalates, organochlorines in endometrium.
Menstrual blood identified as potential biofluid for endometrial exposure assessment.
Menstrual blood utilized for metabolomics research.
Acknowledgements
Thank you to Erika Chow for contributing directories of toxicants in cosmetics, Megan Fitzpatrick for her help cross-referencing annotations to chemical libraries, and Erika Rodriguez for supporting the initiation of the study.
Funding
Support for this project came from a pilot awarded to SM via NIEHS P30 ES023515 including support to CM, PG, and DIW. Support for DIW, ZCF and BPP was received from NIEHS, award numbers U2C ES030859 and P30 ES023515.
Footnotes
CRediT authorship contribution statement
Emily L. Silva: Writing - Original Draft, Formal analysis (compiling tables).
Douglas I. Walker: Conceptualization, Funding acquisition, Methodology, Formal analysis Writing - Review & Editing, Investigation (running samples), Visualization.
Zoe Coates Fuentes: Formal analysis, Investigation (running samples), Visualization.
Brismar Pinto Pacheco: Formal analysis, Investigation (running samples), Visualization.
Christine N. Metz: Conceptualization, Investigation (participant recruitment & sample collection), Resources, Writing - Review & Editing.
Peter K. Gregersen: Conceptualization, Investigation (participant recruitment & sample collection), Resources.
Shruthi Mahalingaiah: Conceptualization, Funding acquisition, Writing - Review & Editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.157005.
References
- Anderson KA, Points GL, Donald CE, et al. , 2017. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Exposure Sci. Environ. Epidemiol 27 (6), 551–559. 10.1038/jes.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariani MV, Rangaswamy R, Siblini H, Yang Q, Al-Hendy A, Zota AR, 2020. The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Curr. Opin. Endocrinol., Diabetes Obes 27 (6), 380–387. 10.1097/MED.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carignan CC, Mínguez-Alarcón L, Butt CM, et al. , 2017. Urinary concentrations of organophosphate flame retardant metabolites and pregnancy outcomes among women undergoing in vitro fertilization. Environ. Health Perspect 125 (8), 087018. 10.1289/EHP1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M, 2016. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. U. S. A 113 (16), 4252–4259. 10.1073/pnas.1603023113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow ET, Mahalingaiah S, 2016. Cosmetics use and age at menopause: is there a connection? Fertil. Steril 106 (4), 978–990. 10.1016/j.fertnstert.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S, Simsek T, 2016. Possible relationship between endocrine disrupting chemicals and hormone dependent gynecologic cancers. Med. Hypotheses 92, 84–87. 10.1016/j.mehy.2016.04.041. [DOI] [PubMed] [Google Scholar]
- Evans J, Salamonsen LA, 2012. Inflammation, leukocytes and menstruation. Rev. Endocr. Metab. Disord 13 (4), 277–288. 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J, 2009. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod 24 (5), 1200–1205. 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]
- Iribarne-Durán LM, Domingo-Piñar S, Peinado F, et al. , 2020. Menstrual blood concentrations of parabens and benzophenones and related factors in a sample of Spanish women: an exploratory study. Environ. Res 183, 109228. 10.1016/j.envres.2020.109228. [DOI] [PubMed] [Google Scholar]
- Jiménez-Díaz I, Iribarne-Durán LM, Ocón O, et al. , 2016. Determination of personal care products –benzophenones and parabens– in human menstrual blood. J. Chromatogr. B 1035, 57–66. 10.1016/j.jchromb.2016.09.035. [DOI] [PubMed] [Google Scholar]
- Lee G, Kim S, Bastiaensen M, et al. , 2020. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ. Res 189, 109874. 10.1016/j.envres.2020.109874. [DOI] [PubMed] [Google Scholar]
- Liu KH, Nellis M, Uppal K, et al. , 2020. Reference standardization for quantification and harmonization of large-scale metabolomics. Anal. Chem 92 (13), 8836–8844. 10.1021/acs.analchem.0c00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Lee CM, Singer G, et al. , 2021. Large scale enzyme based xenobiotic identification for exposomics. Nat. Commun 12 (1), 5418. 10.1038/s41467-021-25698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallozzi M, Leone C, Manurita F, Bellati F, Caserta D, 2017. Endocrine disrupting chemicals and endometrial cancer: an overview of recent laboratory evidence and epidemiological studies. IJERPH 14 (3), 334. 10.3390/ijerph14030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maybin JA, Critchley HOD, 2015. Menstrual physiology: implications for endometrial pathology and beyond. Hum. Reprod. Update 21 (6), 748–761. 10.1093/humupd/dmv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Gaskins AJ, 2017. Female exposure to endocrine disrupting chemicals and fecundity: a review. Curr. Opin. Obstet. Gynecol 29 (4), 202–211. 10.1097/GCO.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Messerlian C, Bellavia A, et al. , 2019. Urinary concentrations of bisphenol a, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environ. Int 126, 355–362. 10.1016/j.envint.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayyar A, Saleem MI, Yilmaz M, et al. , 2020. Menstrual effluent provides a novel diagnostic window on the pathogenesis of endometriosis. Front. Reprod. Health 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RM, Roback JD, Uppal K, Yu T, Jones DP, Josephson CD, 2015. Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion 55 (3), 544–552. 10.1111/trf.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymanski EL, Jeon J, Gulde R, et al. , 2014. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ. Sci. Technol 48 (4), 2097–2098. 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- Segal TR, Giudice LC, 2019. Before the beginning: environmental exposures and reproductive and obstetrical outcomes. Fertil. Steril 112 (4), 613–621. 10.1016/j.fertnstert.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Sirohi D, Ramadhani RA, Knibbs LD, 2021. Environmental exposures to endocrine disrupting chemicals (EDCs) and their role in endometriosis: a systematic literature review. Rev. Environ. Health 36 (1), 101–115. 10.1515/reveh-2020-0046. [DOI] [PubMed] [Google Scholar]
- Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP, 2013. High-performance metabolic profiling with dual chromatography-fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics 9 (1 Suppl), S132–S143. 10.1007/s11306-011-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Centers for Disease Control and Prevention, 2019. Fourth National Report on human exposure to environmental chemicals and updated tables. Published February 5, 2020 Accessed November 23, 2020. https://www.cdc.gov/exposurereport/index.html.
- Vélez MP, Arbuckle TE, Fraser WD, 2015. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum. Reprod 30 (3), 701–709. 10.1093/humrep/deu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DI, Go YM, Liu K, Pennell KD, Jones DP, 2016. Chapter 7 - population screening for biological and environmental properties of the human metabolic phenotype: implications for personalized medicine. In: Holmes E, Nicholson JK, Darzi AW, Lindon JC (Eds.), Metabolic Phenotyping in Personalized and Public Healthcare. Academic Press, pp. 167–211. 10.1016/B978-0-12-800344-2.00007-0. [DOI] [Google Scholar]
- Walker DI, Pennell KD, Uppal K, et al. , 2016. Pilot metabolome-wide association study of Benzo(a)pyrene in serum from military personnel. J. Occup. Environ. Med 58 (8 Suppl 1), S44–S52. 10.1097/JOM.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DI, Valvi D, Rothman N, Lan Q, Miller GW, Jones DP, 2019. The metabolome: a key measure for exposome research in epidemiology. Curr. Epidemiol. Rep 6, 93–103. [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhou W, Zhu W, et al. , 2018. Associations of female exposure to bisphenol a with fecundability: evidence from a preconception cohort study. Environ. Int 117, 139–145. 10.1016/j.envint.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Warren LA, Shih A, Marquez Renteira S, et al. , 2018. Analysis of menstrual effluent: diagnostic potential for endometriosis. Mol. Med 24 (1). 10.1186/s10020-018-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatnik MG, 2016. Endocrine-disrupting chemicals and reproductive health. J. Midwifery Womens Health 61 (4), 442–455. 10.1111/jmwh.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


