Abstract
Purpose of review
Predictive monitoring is an exciting new field involving analysis of physiologic data to detect abnormal patterns associated with critical illness. The first example of predictive monitoring being taken from inception (proof of concept) to reality (demonstration of improved outcomes) is the use of heart rate characteristics (HRC) monitoring to detect sepsis in infants in the neonatal ICU. The commercially available ‘HeRO’ monitor analyzes electrocardiogram data from existing bedside monitors for decreased HR variability and transient decelerations associated with sepsis, and converts these changes into a score (the HRC index or HeRO score). This score is the fold increase in probability that a patient will have a clinical deterioration from sepsis within 24 h. This review focuses on HRC monitoring and discusses future directions in predictive monitoring of ICU patients.
Recent findings
In a randomized trial of 3003 very low birthweight infants, display of the HeRO score reduced mortality more than 20%. Ongoing research aims to combine respiratory and HR analysis to optimize care of ICU patients.
Summary
Predictive monitoring has recently been shown to save lives. Harnessing and analyzing the vast amounts of physiologic data constantly displayed in ICU patients will lead to improved algorithms for early detection, prognosis, and therapy of critical illnesses.
Keywords: heart rate variability, ICU, neonate, predictive monitoring, prematurity, sepsis
INTRODUCTION
The toll of sepsis among preterm infants is high, with a mortality that approaches 20%. Furthermore, there is a high risk for permanent neurologic impairment in survivors. Early goal-directed therapy has been shown to improve survival and other outcomes in adults presenting with signs and symptoms of sepsis [1], and it stands to reason that the same would apply to neonates. An even greater improvement in outcomes could no doubt be achieved if serious infections could be detected before obvious clinical signs of illness are present. Predictive monitoring, or continuous analysis of vital signs to detect changes that occur early in the course of a systemic inflammatory response, can achieve this goal, as demonstrated in the case presentations that follow.
NEONATAL SEPSIS: A TALE OF TWO BABIES
2008: a 26-week preterm infant, now 3 weeks old, is stable on nasal continuous positive airway pressure (CPAP) and advancing feeds. He has increased apnea but looks well and receives a bolus dose of caffeine. The following day he has worsening apnea requiring intubation and is started on antibiotics after undergoing asepsis workup. Complete blood count reveals neutropenia and analysis of cerebrospinal fluid reveals pleocytosis. He is treated with granulocyte-colony stimulating factor and intravenous immunoglobulin (IVIG). He develops hypotension unresponsive to fluids and dopamine, but his blood pressure improves after administration of hydrocortisone. The blood culture is positive for Klebsiella pneumoniae. He survives but has a prolonged neonatal ICU (NICU) stay and exhibits developmental delay at 2 years of age.
2012: a26-weekpreterm infant, now3weeksold and stable on nasal CPAP and advancing feeds, has a large and abrupt increase in his heart rate characteristics (HRC) index. Other than a slight increase in apnea he is acting well. Because of the elevated HRC index, a complete blood count with differential is obtained that reveals an increased number of band forms, prompting a sepsis workup and antibiotic therapy. The blood and urine cultures are positive for E. coli. He remains hemodynamically stable and recovers uneventfully.
COMPLEXITY AND VARIABILITY ANALYSIS: IMPLICATIONS FOR ICU PREDICTIVE MONITORING FOR CATASTROPHIC ILLNESS
In healthy individuals, complex physiologic adaptations and interactions occur constantly, resulting in variability of heart rate, breathing, blood pressure, and temperature [2–3]. Decomplexification (loss of variability or of interactions between these physiologic processes) signals pathology, and represents an opportunity for early detection of potentially catastrophic events through predictive monitoring (Fig. 1) [4–6]. Vital signs are continuously monitored in all patients in ICUs, but absolute values or even trends in the numbers over time have limited diagnostic utility. New research shows that continuous automated detection of abnormal variability of vital signs, through mathematical algorithms incorporating measures such as entropy, can alert clinicians to impending clinical deterioration and allow earlier intervention [7■]. This review will highlight research into use of continuous physiologic monitoring to identify ICU patients in the early stages of life-threatening illnesses, with a particular focus on cardiorespiratory predictive monitoring for sepsis in preterm infants.
FIGURE 1.
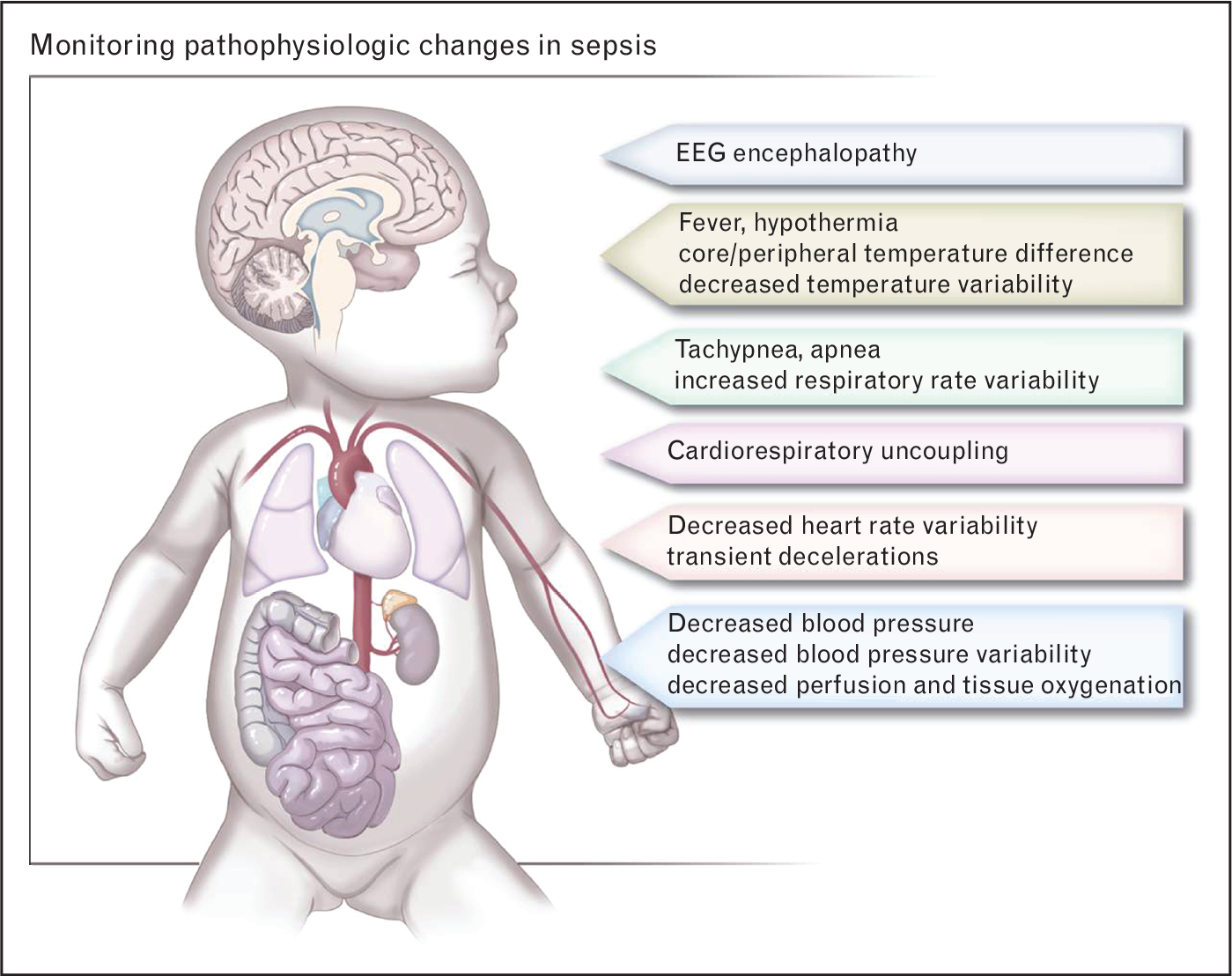
Pathophysiologic changes that can be monitored in sepsis. The systemic inflammatory response to severe infection leading to sepsis causes complex changes in multiple vital signs that are routinely monitored in ICU patients. Predictive monitoring (analysis of pathophysiologic changes using automated algorithms) can alert clinicians to the high risk for clinical deterioration, leading to earlier treatment and improved outcomes.
HEART RATE CHARACTERISTICS MONITORING FOR SEPSIS
The biggest advance in the relatively new field of predictive monitoring is early detection of sepsis in NICU patients through continuous HRC monitoring [8]. Heart rate is regulated by the autonomic nervous system, through neurotransmitter release at sinoatrial node pacemaker cells. Sympathetic activation increases the heart rate via norepinephrine binding to adrenergic receptors, whereas parasympathetic (vagal) activation slows the heart rate via acetylcholine binding to cholinergic receptors. In sepsis and other pathologic conditions such as fetal asphyxia, decreased heart rate variability occurs, with fewer normal small decelerations and accelerations. Superimposed on this overall decreased variability are transient decelerations of heart rate, larger than normal decelerations but usually not deep enough to trigger bradycardia alarms on bedside monitors. Decreased heart rate variability and decelerations are mediated in part by cytokines released during the systemic inflammatory response to infection [9–10]. Heart rate decelerations may be associated with apnea in some septic infants, but are also frequently noted during regular spontaneous breathing and in infants on mechanical ventilation. Evidence suggests that the decelerations are caused by pathogen-induced vagus nerve firing [11], likely as part of a protective cholinergic anti-inflammatory response [12].
A monitor was developed that analyzes electrocardiogram data from existing NICU bedside monitors and detects abnormal HRC of decreased variability and decelerations (HeRO monitor, Medical Predictive Science Corporation, Charlottesville, VA, USA) (Fig. 2). Using a mathematical algorithm, the monitor continuously reports the HRC index or HeRO score, which is the fold-increase in probability that the patient will experience a clinical deterioration consistent with sepsis in the ensuing 24-h period. The HRC index underwent the following rigorous process of development and testing:
FIGURE 2.
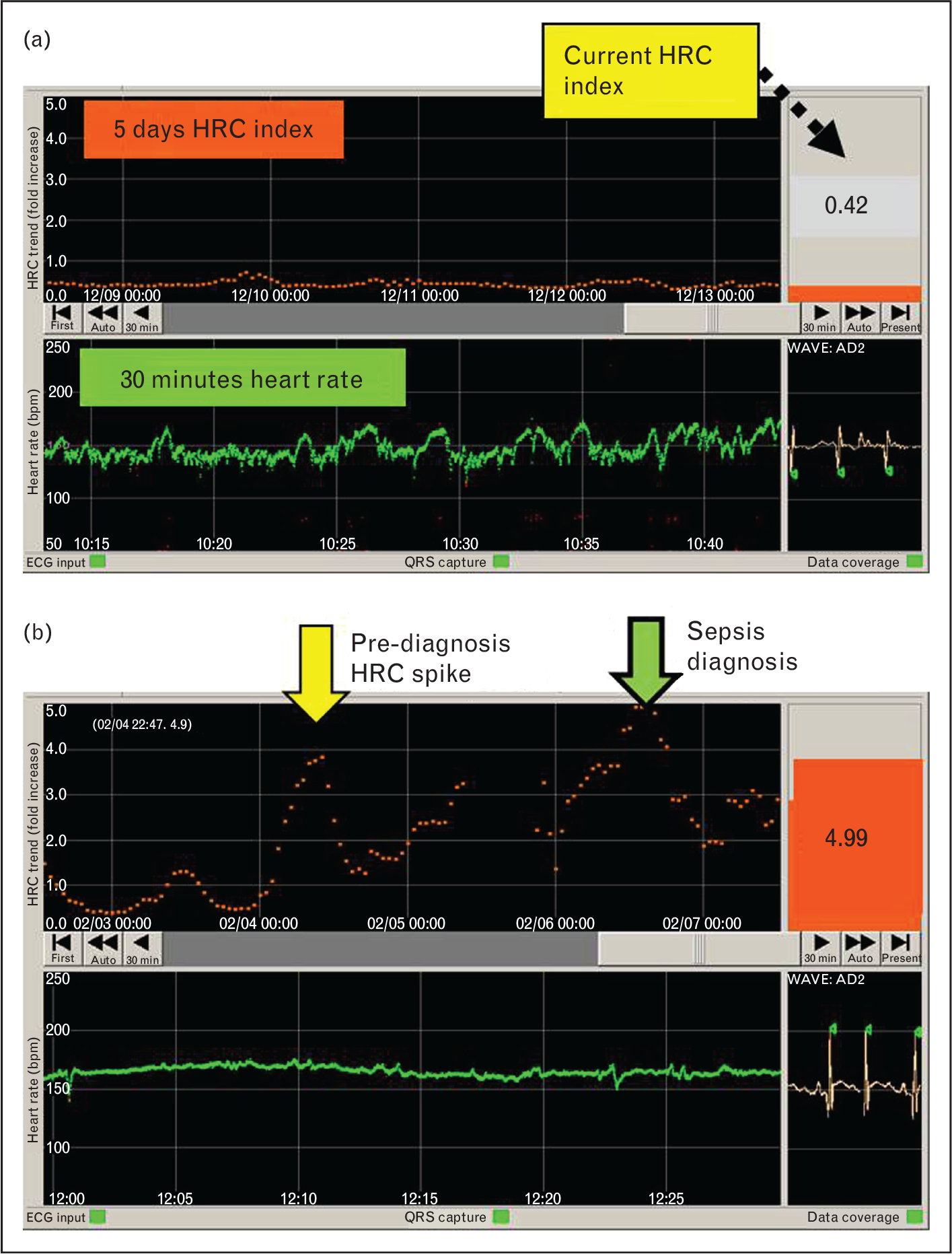
Heart rate characteristics monitor showing normal and abnormal heart rate characteristics. a). A screen shot of the HeRO monitor displaying the heart rate characteristics (HRC) of a neonatal ICU (NICU) patient. The bottom panel shows the last 30 min of heart rate (green, beats per minute) and the top panel shows the HRC index over the last 5 days (orange). The current HRC index (0.42), which reflects HR variability and decelerations and is updated hourly, is shown in the right panel. In this case, the infant’s heart rate has normal small accelerations and decelerations, and the HRC index has been low for the last 5 days, indicating a low chance of imminent clinical deterioration due to sepsis. (b) HRC monitor screen showing abnormal HRC (decreased HR variability) reflected in the high HRC index (4.99). Of note, the patient had clinical signs of sepsis at the time indicated by the green arrow, but had worsening HRC 2 days prior, at the time indicated by the yellow arrow. This prediagnosis HRC spike may reflect a subclinical sepsis prodrome and an opportunity for early intervention to avoid worsening illness.
Developed in more than 300 infants in the University of Virginia Neonatal ICU. A rise in the HRC index was shown to be associated with late-onset neonatal sepsis [13] and mortality [14], and was shown to be superior to and additive with clinical [15] and laboratory [16] findings for sepsis diagnosis.
Externally validated for sepsis detection in more than 300 infants in the Wake Forest University NICU [13].
Tested for its impact on outcomes in the largest randomized clinical trial conducted to date in very low birth weight infants[17■■]. In the HeRO trial, 3003 very low birth weight infants in nine NICUs in the United States underwent continuous HRC monitoring and were randomized to having their HRC index displayed to clinicians or not. There was a statistically significant and clinically important 22% relative reduction in mortality in infants whose HRC index was displayed (8.1 versus 10.2%; P=0.04). The tradeoff for lower mortality was 10% more blood cultures obtained, and 5% more days on antibiotics in the group with HRC monitor display.
In the HeRO trial, there was no difference in the incidence of late-onset septicemia (LOS) between the two study groups (about one out of four infants in the HRC display and nondisplay groups had at least one episode of LOS). The HRC index rose significantly around the time of diagnosis of septicemia with Gram-positive and Gram-negative bacteria and Candida. Mortality within 30 days of LOS with each organism group was reduced in HRC display versus nondisplay infants.
The reason for sepsis mortality reduction with HRC monitoring is likely due to earlier detection and treatment of sepsis. This cannot be proven with certainty, as the exact time that an infant develops a bloodstream infection leading to sepsis cannot be known. Of note, however, the average HRC index was lower and there were fewer abrupt large increases (spikes) in the HRC index in the week prior to diagnosis of LOS in patients in the HRC display group. These prediagnosis spikes have previously been described [13,18] and an example is shown in Fig. 2b. Waxing and waning of abnormal HRC prior to clinical deterioration may represent host defenses temporarily containing an infection, alterations in autonomic systems regulating heart rate, or other physiologic adaptations related to host–pathogen interactions. Whatever the mechanism, these abnormal HRC spikes may indicate a subclinical sepsis prodrome and displaying them to clinicians may be an opportunity for earlier testing and treatment, perhaps averting progression to severe illness.
Systematic evaluation of HRC monitoring as a novel risk marker for neonatal sepsis showed that it passed all phases of risk marker development [19], including proof of concept [20–22], development and validation in independent populations [13], documentation of incremental information when added to standard risk markers [16,23], and assessment of effects on patient management and outcomes [17■■]. Figure 3 shows that, in infants enrolled in the randomized trial, the HRC index rose significantly prior to diagnosis of sepsis. On the contrary, an analysis of the same patients based simply on standard risk factors (birthweight, gestational and postmenstrual age, and presence of mechanical ventilation) does not aid in determining whether, within the next 24h, an infant will experience a clinical deterioration from sepsis.
FIGURE 3.
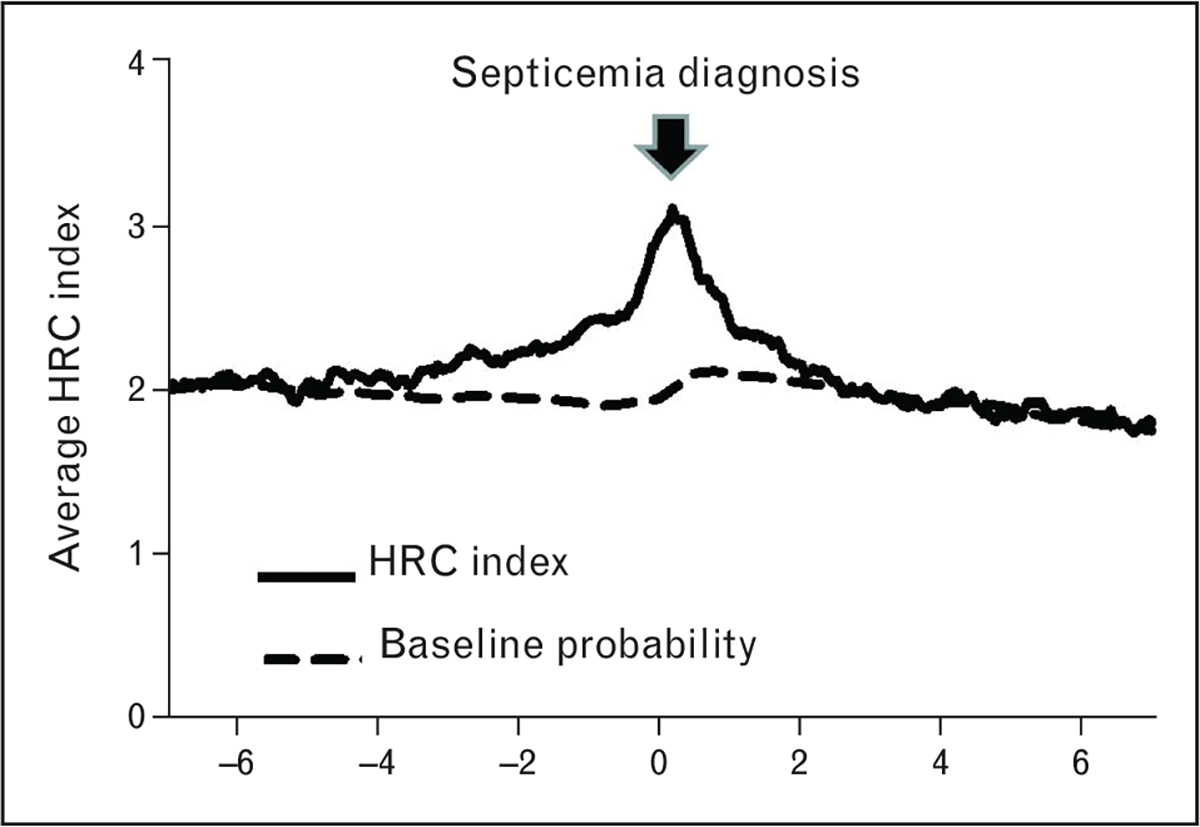
Heart rate characteristics index in cases of septicemia compared with risk factor-based probability of sepsis. In evaluating a new risk marker, it is important to compare its diagnostic utility with standard risk indicators. In this graph, average heart rate characteristics (HRC) index is shown for all cases of late-onset septicemia (LOS, >3 days of age) in infants in the randomized trial of HRC monitoring. The HRC index (solid line) rose prior to the clinical diagnosis of LOS (day 0, arrow). For comparison, probability of sepsis based on known risk factors of gestational and postmenstrual age, birth weight, and mechanical ventilation is shown for infants in the trial undergoing conventional monitoring (dashed line).
Like any clinical test or predictive monitoring tool, the HRC index must be interpreted in the individual clinical context. In the HRC randomized trial, there was no mandated intervention for a rising HRC index; clinicians were simply instructed to evaluate the patient and use the HRC index together with other clinical signs, physical exam findings, and available laboratory tests to make decisions about further testing and therapy. Clearly, infants with significant clinical signs of sepsis should receive antibiotics irrespective of the HRC index, at least until sepsis can be ruled out with negative cultures and another explanation found for the clinical signs. On the contrary, in the case of an infant with subtle, nonspecific signs, a low HRC index could contribute to a decision to wait and watch, or to send blood tests but not initiate antibiotics, or to limit duration of antibiotic therapy.
OTHER CAUSES OF ABNORMAL HEART RATE CHARACTERISTICS
The most common cause of a large, abrupt increase in the HRC index in preterm infants in the NICU is sepsis. Other serious infectious or inflammatory conditions such as urinary tract infection and necrotizing enterocolitis also cause abnormal HRC. Acute respiratory deterioration, with or without signs of pneumonia, may also lead to an abrupt increase in the HRC index. In this case, hypoxia, hypercapnia, or lung inflammation may contribute to decreased heart rate variability or transient decelerations. Some infants with acute brain injury such as severe intraventricular hemorrhage have intermittent elevation in their HRC index without other apparent cause, possibly reflecting a prolonged systemic or focal inflammatory process or autonomic nervous system dysfunction. Preliminary studies suggest that a high cumulative HRC index during the NICU stay is associated with adverse neurodevelopmental outcomes, independent of gestational age [24], suggesting that the HRC index may be a useful brain injury biomarker.
Most medications do not significantly impact the HRC index, with the exception of paralytics, anesthetics, and anticholinergics. Thus, patients undergoing surgery or evaluation or treatment for retinopathy of prematurity often display an acute increase in their HRC index. It is noteworthy that dexamethasone dramatically improves heart rate variability and lowers the HRC index, likely in part through anti-inflammatory effects.
BEYOND HEART RATE CHARACTERISTICS: CARDIORESPIRATORY PREDICTIVE MONITORING
The HRC monitor is the first Food and Drug Administration 510(k)-cleared commercially available tool for predictive monitoring in the NICU, but this is not the end of the story. It is possible that even greater diagnostic accuracy can be achieved by combining respiratory analysis with heart rate analysis. Focal or systemic infections are well known to impact respiratory rate and respiratory patterns. In adults, tachypnea is a common sign of sepsis, and reduced respiratory rate variability has also been described [2,25]. In preterm infants, in contrast, the most common presenting sign of late-onset sepsis is apnea [26]. Mechanistic studies in neonatal rats showed that administration of endotoxin from Gram-negative bacteria induces expression of interleukin-1 β within the respiratory control centers of the brainstem and leads to decreased hypoxic ventilatory responses and apnea [27]. Even a relatively low-virulence pathogen such as coagulase-negative staphylococcus can cause a dramatic increase in central apnea in preterm infants, sometimes to the point of requiring mechanical ventilation. Detecting an increase in apnea over a patient’s baseline could, at least theoretically, lead to earlier suspicion of sepsis, testing, and treatment.
Quantitation of apnea in NICU patients is confounded by the relatively primitive reliance on busy NICU nurses to document events in the medical record. Major advances in computer storage and processing have made it possible for the research group at the University of Virginia to develop an automated system for quantifying central apnea of prematurity using chest impedance waveforms from standard NICU bedside monitors [28–30]. After extensive clinical validation, this system has been used to show that nursing records underreport and misreport apnea, that anemia is associated with apnea [30], and that apnea burden together with other cardiorespiratory indices can be used to predict acute respiratory failure as much as a day before a patient requires urgent intubation [31]. This new monitoring system will make it possible for clinicians to be alerted to an increase in number or severity of apneic events over a patient’s baseline, potentially leading to earlier interventions to avert further clinical deterioration, including requirement for mechanical ventilation. An example of HRC and apnea monitoring in a preterm NICU patient is shown in Fig. 4. Future work will determine whether combined cardiorespiratory analysis improves on the diagnostic utility of HRC analysis for detection of sepsis.
FIGURE 4.
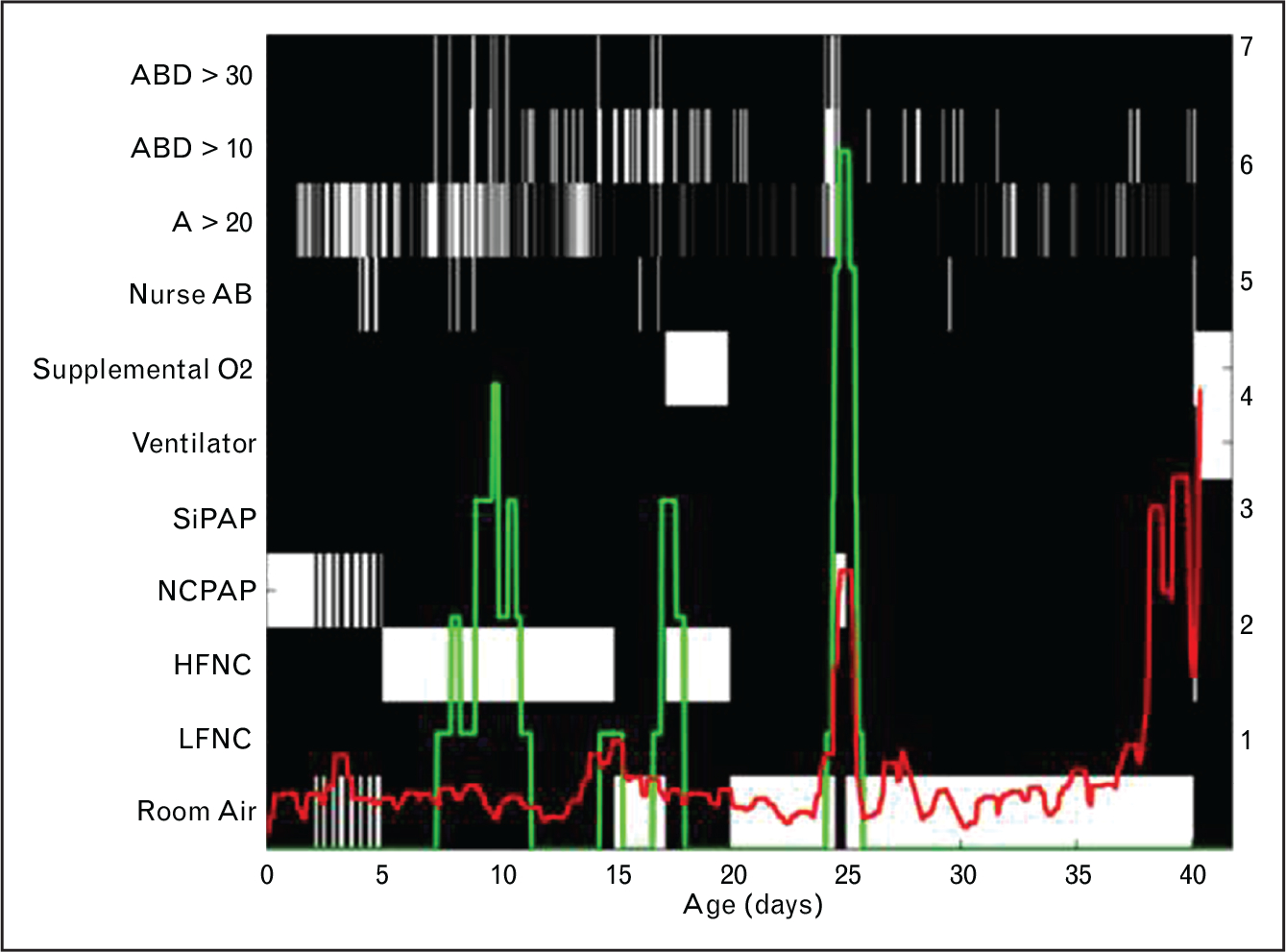
Apnea count and heart rate characteristics index. This map shows the heart rate characteristics (HRC) index (HeRO score, red) and count of 30 s apnea events accompanied by bradycardia and desaturation (ABD30, green) in a preterm infant throughout the NICU stay. The x axis shows days since birth, the left Y axis indicates clinical events (depicted on the map as white or gray boxes), and the right y axis indicates the HRC index or ABD30 count. Note that there are times when a high apnea count and high HRC index coincide, and other times when one occurs without the other. Algorithms incorporating heart rate, respiratory rate, and cardiorespiratory coupling analyses are under development for better predictive monitoring in the NICU.
Cardiorespiratory predictive monitoring encompasses not only analysis of heart rate and respiratory rate patterns but also examining cardiorespiratory interactions. Cardiorespiratory coupling (CRC)refers to regular timing of heart beats in relation to the phase of breathing (degree of lung inflation) and is a sign of physiologic stability, reflecting sympathovagal balance [32]. Our group showed that CRC in preterm infants increases with maturation, independent of gestational age at birth, occurring 10% of the time in infants at 30 weeks postmenstrual age (PMA) and 25% of the time at 40 weeks PMA, similar to the fraction of time in CRC reported in healthy adults [29]. We also showed that cardiorespiratory uncoupling occurs in infants with evolving respiratory deterioration leading to nonelective intubation [31]. Future work will determine whether cardiorespiratory uncoupling occurs in infants in the early stages of sepsis, and adds to existing predictive monitoring algorithms.
OTHER PREDICTIVE MONITORING STRATEGIES
Although heart rate analysis has undergone the most research and development for predictive monitoring, other physiologic parameters measured in ICU patients may provide valuable clinical information (Fig. 1). Blood pressure, systemic perfusion, and tissue oxygenation decline in sepsis, and algorithms incorporating these parameters may identify patients with higher risk for morbidity and mortality [33] or assist in gaging need for or response to various therapies. Likewise, electroencephalographic monitoring can provide information about sepsis-associated encephalopathy [34] that has prognostic and perhaps therapeutic implications. Temperature instability (fever and hypothermia) is well described in sepsis, and continuous monitoring of core temperature, temperature variability and core–peripheral temperature differential have been proposed for detection of sepsis. In adult ICU patients, loss of the normal small fluctuations in body temperature is associated with increased morbidity and mortality [25], and limited data in infants suggests that monitoring the difference between central and peripheral skin temperature is a simple way to detect sepsis [35].
CONCLUSION
Predictive monitoring has enormous potential for improving outcomes of ICU patients through earlier detection and treatment of acute, potentially catastrophic illnesses such as sepsis. The first concrete example of the benefit of predictive monitoring is the reduction in sepsis-associated mortality in preterm infants through continuous monitoring and display of abnormal HRC. The accelerating pace of research in this field, in neonatal, pediatric, and adult ICUs, will lead to more advanced predictive algorithms with a wide range of diagnostic, prognostic, and therapeutic applications.
KEY POINTS.
Predictive monitoring involves analyzing vital signs and other physiologic data to predict impending clinical deterioration in ICU patients.
Monitoring for abnormal heart rate characteristics (HRC, decreased variability and transient decelerations) predicts sepsis in NICU patients, often before any clinical signs of illness are recognized.
In a randomized clinical trial of continuous HRC monitoring of 3003 very low birthweight infants, displaying patients’ HRC to clinicians reduced mortality more than 20%.
Ongoing research combining HRC and other physiologic data such as respiratory rate and oxygen saturation will lead to more advanced predictive algorithms for early detection of impending illness in ICU patients.
Acknowledgements
Grant NICHD 051609.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 276–277).
- 1.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 2.Dick TE, Molkov YI, Nieman G, et al. Linking inflammation, cardiorespiratory variability, and neural control in acute inflammation via computational modeling. Front Physiol 2012; 3:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seely AJ, Green GC, Bravi A. Continuous multiorgan variability monitoring in critically ill patients: complexity science at the bedside. Conf Proc IEEE Eng Med Biol Soc 2011; 2011:5503–5506. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad S, Tejuja A, Newman KD, et al. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care 2009; 13:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad S, Ramsay T, Huebsch L, et al. Continuous multiparameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One 2009; 4:e6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravi A, Longtin A, Seely AJ. Review and classification of variability analysis techniques with clinical applications. Biomed Eng Online 2011; 10:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.■. Moorman JR, Rusin CE, Lee H, et al. Predictive monitoring for early detection of subacute potentially catastrophic illnesses in critical care. Conf Proc IEEE Eng Med Biol Soc 2011; 2011:5515–5518. This comprehensive and colorful review highlights important developments and future directions in predictive monitoring of ICU patients. Key mathematical concepts for algorithm development are described. The importance of close collaborations between engineers, computer, math, and statistics experts and hard-working clinicians to advance research in predictive monitoring is stressed.
- 8.Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin Perinatol 2010; 37:581–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairchild KD, Saucerman JJ, Raynor LL, et al. Endotoxin depresses heart rate variability in mice: cytokine and steroid effects. Am J Physiol Regul Integr Comp Physiol 2009; 297:R1019–R1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raynor LL, Saucerman JJ, Akinola MO, et al. Cytokine screening identifies NICU patients with Gram-negative bacteremia. Pediatr Res 2012; 71:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairchild KD, Srinivasan V, Moorman JR, et al. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol 2011; 300:R330–R339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med 2011; 269:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin MP, O’Shea TM, Bissonette EA, et al. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res 2003; 53:920–926. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MP, O’Shea TM, Bissonette EA, et al. Abnormal heart rate characteristics are associated with neonatal mortality. Pediatr Res 2004; 55:782–788. [DOI] [PubMed] [Google Scholar]
- 15.Griffin MP, Lake D, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res 2007; 61:222–227. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics 2005; 115:937–941. [DOI] [PubMed] [Google Scholar]
- 17.■■. Moorman JR, Carlo WA, Kattwinkel J, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr 2011; 159:900–906; e1. In this, the largest randomized clinical trial to date in very low birth weight infants (n= 3003), monitoring HRC and displaying the HRC index to clinicians led to a 22% relative reduction in mortality (P= 0.04). Sepsis-associated mortality was reduced by nearly 40%. This is the first clinical trial showing improved patient outcomes with predictive monitoring.
- 18.Moorman JR, Lake DE, Griffin MP. Heart rate characteristics monitoring for neonatal sepsis. IEEE Trans Biomed Eng 2006; 53:126–132. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009; 119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghili AA, Rizwan-uddin Griffin MP, Moorman JR. Scaling and ordering of neonatal heart rate variability. Phys Rev Lett 1995; 74:1254–1257. [DOI] [PubMed] [Google Scholar]
- 21.Cao H, Lake DE, Griffin MP, Moorman JR. Increased nonstationarity of neonatal heart rate before the clinical diagnosis of sepsis. Ann Biomed Eng 2004; 32:233–244. [DOI] [PubMed] [Google Scholar]
- 22.Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics 2001; 107:97–104. [DOI] [PubMed] [Google Scholar]
- 23.Griffin MP, Lake DE, O’Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res 2007; 61:222–227. [DOI] [PubMed] [Google Scholar]
- 24.Addison K, Griffin MP, Moorman JR, et al. Heart rate characteristics and neurodevelopmental outcome in very low birth weight infants. J Perinatol 2009; 29:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchan CA, Bravi A, Seely AJ. Variability analysis and the diagnosis, management, and treatment of sepsis. Curr Infect Dis Rep 2012; 14:512–521. [DOI] [PubMed] [Google Scholar]
- 26.Fanaroff AA, Korones SB, Wright LL, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 1998; 17:593–598. [DOI] [PubMed] [Google Scholar]
- 27.Balan KV, Kc P, Hoxha Z, et al. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal medulla oblongata. Respir Physiol Neurobiol 2011; 178:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Rusin CG, Lake DE, et al. A new algorithm for detecting central apnea in neonates. Physiol Meas 2011; 33:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark MT, Rusin CG, Hudson JL, et al. Breath-by-breath analysis of cardiorespiratory interaction for quantifying developmental maturity in premature infants. J Appl Physiol 2012; 112:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zagol K, Lake DE, Vergales B, et al. Anemia, apnea of prematurity, and blood transfusions. J Pediatr 2012; 161:417–421; e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark MT, Vergales BD, Paget-Brown AO, et al. Predictive monitoring for respiratory decompensation leading to urgent unplanned intubation in the neonatal intensive care unit. Pediatr Res 2013; 73:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartsch RP, Schumann AY, Kantelhardt JW, et al. Phase transitions in physiologic coupling. Proc Natl Acad Sci U S A 2012; 109:10181–10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro NI, Arnold R, Sherwin R, et al. The association of near-infrared spectroscopy-derived tissue oxygenation measurements with sepsis syndromes, organ dysfunction and mortality in emergency department patients with sepsis. Crit Care 2011; 15:R223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol 2012; 8:557–566. [DOI] [PubMed] [Google Scholar]
- 35.Messaritakis J, Anagnostakis D, Laskari H, Katerelos C. Rectal-skin temperature difference in septicaemic newborn infants. Arch Dis Child 1990; 65:380–382. [DOI] [PMC free article] [PubMed] [Google Scholar]


