Abstract
Objectives:
Brachial plexus injuries (BPI), although rare, often results in significant morbidity. Stem cell was thought to be one of BPI treatment modalities because of their nerve-forming regeneration potential. Although there is a possibility for the use of mesenchymal stem cells as one of BPI treatment, it is still limited on animal studies. Therefore, this systematic review aimed to analyze the role of mesenchymal stem cells in nerve regeneration in animal models of brachial plexus injury.
Method:
This study is a systematic review with PROSPERO registration number CRD4202128321. Literature searching was conducted using keywords experimental, animal, brachial plexus injury, mesenchymal stem cell implantation, clinical outcomes, electrophysiological outcomes, and histologic outcomes. Searches were performed in the PubMed, Scopus, and ScienceDirect databases. The risk of bias was assessed using SYRCLE's risk of bias tool for animal studies. The data obtained were described and in-depth analysis was performed.
Result:
Four studies were included in this study involving 183 animals from different species those are rats and rabbits. There was an increase in muscle weight and shortened initial onset time of muscle contraction in the group treated with stem cells. Electrophysiological results showed that mesenchymal stem cells exhibited higher (Compound muscle action potential) CMAP amplitude and shorter CMAP latency than control but not better than autograft. Histological outcomes showed an increase in axon density, axon number, and the formation of connections between nerve cells and target muscles.
Conclusion:
Mesenchymal stem cell implantation to animals with brachial plexus injury showed its ability to regenerate nerve cells as evidenced by clinical, electrophysiological, and histopathological results. However, this systematic study involved experimental animals from various species so that the results cannot be uniformed, and conclusion should be drawn cautiously.
Key Words: Animal study, Brachial plexus injury, Mesenchymal stem cell, Nerve, Regeneration
Introduction
Brachial plexus injury (BPI) is a rare peripheral nerve injury which can cause serious and significant morbidity in patients.1 Based on epidemiological data BPI occurs in 1.2% of multiple trauma case. The number is nine times less than cervical trauma and sixty times less than head injury. However, BPI can result in disorder in motoric, sensorics, pain and functional disability impacts on patient’s quality of life.2-4
In general, BPI without spontaneous improvement was treated surgically.5 Surgery is aimed at restoring the function of the patient’s arm and hands and reducing pain as much as possible.6-8 However, there is no therapeutic modality that can guarantee complete regeneration and muscle reinnervation.9-13
The use of mesenchymal stem cells as a one of modalities of regenerative medicine is increasingly being used and gives satisfactory results.14-17 Researches on the regeneration of organs with mesenchymal stem cells, such as brain, eye, kidney, bone, cartilage, heart, intervertebral disc and also nerve have yielded good results.18-22 Some authors have revealed that mesenchymal stem cells have the effect of promoting neural tissue repair activity at the microcellular level.23,24 Animal study conducted by Bingham et al., showed that use of mesenchymal stem cells in peripheral nerve injury enhances nerve regeneration, treats muscle contractures, and promote improvement.25 Hogendoorn et al., suggested that local injection of autologous mesenchymal stem cells from bone marrow in partially denervated muscles can increase muscle reinnervation and regeneration.26 Rapid nerve regeneration is expected to shorten the time of nerve denervation so that is useful in maintaining neuromuscular junction.10,27,28
Several animal studies have yielded positive results regarding the use of mesenchymal stem cells in animal models of brachial plexus injury. Yang et al. suggested that the use of adipose stem cells combined with allograft improves nerve healing in brachial plexus injury model, and increase the number of motor end plates in target muscles.29,30 Guo et al., in their study stated that the use of mesenchymal stem cells derived from bone marrow can repair brachial plexus neuron and restore their physiological function. However, these studies limited to case report, preclinical and in vivo study.31
Based on the description above, research on brachial plexus injury cases associated with the use of mesenchymal stem cells as regenerative medicine has not been widely carried out. Mesenchymal stem cells therapy is an emerging and promising modality, therefore systematic review of existing preclinical studies is needed to determine the effect, safety and in the end guide the futures studies in human. The main purpose of this review was to systematically analyze the role of mesenchymal stem cells in nerve regeneration in animal models of brachial plexus injury. It was hypothesized that the use of mesenchymal stem cells would enhance regeneration of nerve cells as evidenced by clinical, electrophysiological, and histopathological results.
Methods
This study conducted in accordance to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) methods32
Eligibility criteria
The eligibility criteria for this review consisted of full text article, experimental study in animal (in vivo study), brachial plexus injury model, interventions of any application of mesenchymal stem cells, any functional or clinical, electrophysiological and histological outcomes measured, and not limited in any language. Duplicates, review studies, in vitro studies, different stem cells derivate, and irrelevant articles were excluded.
Literature search and study selection
The Search was comprehensively performed accordance to the Preferred Reporting Items for Systematic Review and
Meta-Analysis (PRISMA). The search was conducted in Three online database such as PubMed, Scopus, and Science Direct Journals and Books firstly in November 2021. We have also performed re-search for updating our results every 6 months to make our research reliable. The term Brachial plexus injur* OR brachial plexus neuropath* OR brachial plexus disorder* AND Stem cell* OR Mesenchymal cell* OR umbilical cord stem cell* OR adipose tissue stem cell* AND muscle weight ratio OR muscle to fat ratio OR behavioral analy* OR behavioural analy* OR compound muscle action potential OR CMAP OR electrophysiol* OR axon densit* OR motor end plate OR histopathol* were used as the search keywords. For more detail, literature search keywords based on PICO methods described in [Table 1].
Table 1.
Literature search keywords
| Participants | Brachial plexus injur* OR brachial plexus neuropath* OR brachial plexus disorder* |
| Interventions | Stem cell* OR Mesenchymal cell* OR umbilical cord stem cell* OR adipose tissue stem cell* |
| Comparison | - |
| Outputs | muscle weight ratio OR muscle to fat ratio OR behavioral analy* OR behavioural analy* OR compound muscle action potential OR CMAP OR electrophysiol* OR axon densit* OR motor end plate OR histopathol* |
After excluding the duplicates, titles and abstract were reviewed by two authors, M.W.K and I.K.P for eligibility. Selected studies with full text availability were assessed to apply the inclusion criteria. Any discrepancy to agreement between authors was resolved by discussion and involving other authors, W.W and I.S.W.
Methodological quality assessment and risk of bias
Internal validity or risk of bias was performed using Systematic Review Center for Laboratory Animal Experimentation’s (SYRCLES’s) risk of bias tool consist of selection bias, performance bias, detection bias, attrition bias, reporting bias and other source of bias assessment. Two authors, M.W.K and I.K.P performed all the assessment independently and any discrepancy to agreement between authors was resolved by discussion and involving other authors, W.W and I.S.W.
Data extraction and synthesis
Data that has been collected was extracted using predetermined selection criteria. The following data were extracted: study design; type of animal for experimental study; sample size; duration of observation; type of mesenchymal stem cells (MSCs); procedure or intervention; study groups; comparison; functional or clinical outcomes; electrophysiological outcomes; histological outcomes; significant difference between outcomes in study groups; and other outcomes.
The data collection of experimental animal study was collected. In this review, meta-analysis could not be conducted due to the heterogenicity of the studies involved (i.e., subject animals, MSCs source, group and control groups, duration and outcomes).
Results
Study selection
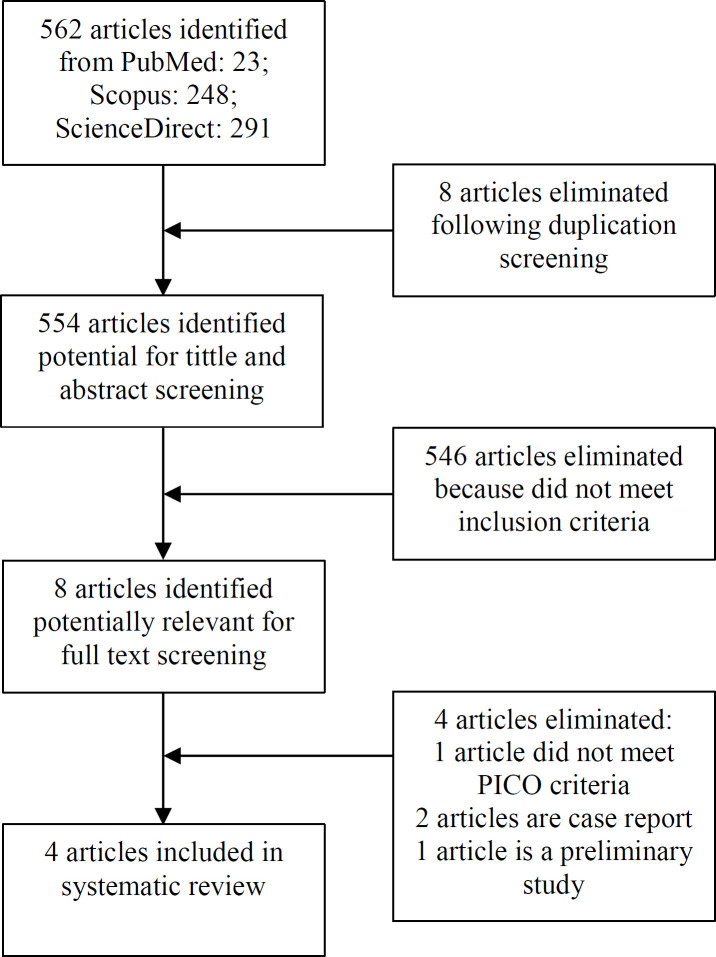
The study selection was summarized in PRISMA flow diagram as follows. A total of 562 studies were obtained from the three specified database. After performing abstracts and titles screening, eight articles were included for further evaluation. After full-text reading and assessment, four articles were included for this systematic review. The flow diagram described in [Figure 1].
Figure 1.
Flow Chart of Study Selection Process
Assessment of methodology quality
Study characteristics
All four works of literature involved used small animals. Three of them used mice and the other one used rabbits as their animal model consisted of 88 mice and 45 rabbits involved in this review. All samples were treated as a model of brachial plexus injury with different procedures in each study. Guo et al. performed an avulsion model of the brachial plexus by tearing the dorsal and ventral root of C5-T1. Huanxing et al. performed root avulsion of C5-C7 to detach the musculocutaneous nerve. Rodriguez et al. modeled the brachial plexus injury by excising three mm of the C5-C6
nerve segment. The brachial plexus injury model by Yang et al. was completed by performing moderate traction of the plexus away from the intervertebral foramen with micro-hemostat forceps.
Mesenchymal stem cells that were used in those studies also varied. Two studies used bone marrow-derived MSCs, other two studies used umbilical cord and adipose MSCs respectively. As for the route of MSCs administration, one study performed intraperitoneal injection (compared with the normal saline and sham group), one study performed injection into the musculocutaneous nerve (compared with vehicle injection), and two other studies performed extraplexal nerve transfer and applied mesenchymal stem cells to the nerve junction (compared with autograft and acellular nerve autograft (ANA)). A summary of study characteristics involved in this review is described in [Table 2].
Table 2.
Study Characteristics
| Author, year | Des ign | Sample size | Subject Criteria | Follow-Up | Procedure | Group | Outcomes |
|---|---|---|---|---|---|---|---|
| Guo et al. 2020 | E | 45 | Big-ear rabbit from Laboratory Animal Research Center of the Hospital | 3 weeks | Intraparitoneal injection |
|
|
| Huanxing et al. 2013 | E | 24 | Adult female Sprague-Dawley rat weight 220 – 250 grams | 16 weeks | Musculocutaneus nerve injection |
|
|
| Rodriguez et al. 2021 | E | 42 | Adult male Wistar Lewis rat 335.13 ± 35.71 grams and 80 ± 10 days old | 12 weeks | Phrenicus transfer with Musculocutaneous graft |
|
|
| Yang et al. 2019 | E | 18 | Adult male Sprague-Dawley rat weight 200-300 grams six weeks old | 16 weeks | Contralateral transfer of C7 to C5-C6 |
|
E: experimental
BMSCs: bone marrow mesenchymal stem cells
CMAP: compound muscle action potential
hi-UMC: human induced umbilical cord mesenchymal cells
EMG: electromyography
NMJ: neuromuscular junction
ANA: acellular nerve allograft
dASCs: differentiated adipose stem cells
IHC: immunohistochemistry
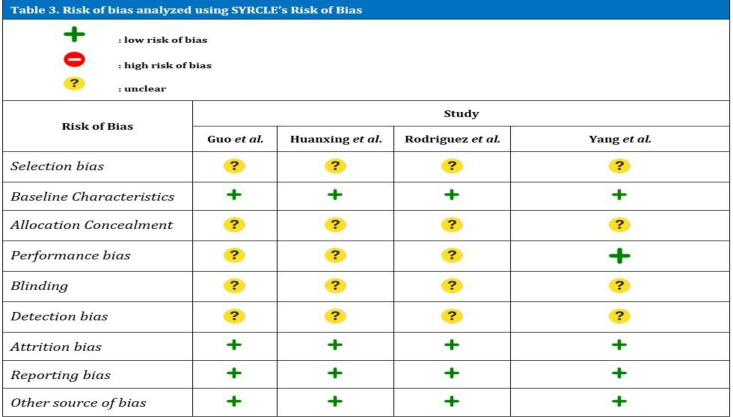
Risk of bias
These various studies were then analyzed for internal validity using the SYRCLE's Risk of Bias Tool described in [Table 3]. In general, all four involved studies have limitations in terms of validity. All of these studies had unexplained subject selection problems with details. Existing studies stated that research subjects are divided into predetermined groups, but it was not explained whether random allocation was carried out. One study explained that randomization was carried out in the process of allocating research subjects.34 The basic characteristics of the research subjects were well explained. The whole study describes the types of research animals used.35
Table 3.
Risk of bias analyzed using SYRCLE’s Risk of Bias
The process of allocation and concealment of allocations were not described in all studies. None of the studies above describe the process of masking. In the aspect of uniformity of room for research subjects, only one study describes the process. One study mentioned conditions of temperature, humidity, light and dark cycles, and access to water and food.34
Assessment of detection bias is done by selecting animals at random to measure the outcome. In all these studies, the selection of animals for outward assessment was not explained. However, all studies wrote down the final results of the study in full and there were no hidden or unreported data.
Study outcomes
Outcome measurement in this review is based on functional or clinical, electrophysiological, and histological evaluation in each study. There are no studies that have the same outcome measures as other studies. Therefore, we tried to simplify and group the comparable outcome measures of each of the involved studies. Functional or clinical improvement was evaluated by the onset of involuntary movement of the affected extremity, and two studies describe comparable results. Based on those studies one study showed faster onset for MSCs combined with the ANA group compared to ANA only. However, there was no significant difference between the groups. Another study showed a slower onset of the MSCs group compared to others with no significant difference [Table 4].
Table 4.
Clinical Outcomes
| Author, year | Group | Clinical output | P Values |
|---|---|---|---|
| Rodriguez et al. 2021 | Autograft (control) ANA ANA + BMSCs |
Onset of involuntary elbow flexion 20.69 ± 2.14 days 21.08 ± 2.61 days 21.28 ± 2.49 days |
- >005 >005 |
| Yang et al. 2019 | Autograft (control) ANA ANA + dASCs |
Onset of arm lifting function 45.40 ± 7.30 days 61.40 ± 6.80 days 53.20 ± 3.35 days |
>005 |
BMSCs: bone marrow mesenchymal stem cells
ANA: acellular nerve allograft
dASCs: differentiated adipose stem cells
Electrophysiological outcomes were evaluated and stratified according to amplitude of CMAP and latency of CMAP. Three studies describe electrophysiological outcomes. Guo et al. showed a significant difference in the MSCs group compared to the control with a higher amplitude of CMAP and faster latency of CMAP. Yang et al. only examined the amplitude of CMAP and found that there was a significant difference in the MSCs + ANA group compared to the ANA- only group. Rodriguez et al. failed to demonstrate supporting results from the MSCs group compared to other groups [Table 5].
Table 5.
Electrophysiological Outcomes
| Author, year | Group | Amplitudo of CMAP | P Values | Latency of CMAP | P Values |
|---|---|---|---|---|---|
| Guo et al. 2020 | Normal saline BMSCs Sham |
15,50 2,1 28 1,25 32 0,54 |
0,046 | 3,54 0,31 2,07 0,24 1,32 0,56 |
0,004 |
| Rodriguez et al. 2021 | Autograft (control) ANA ANA + BMSCs |
0,04388 0,02 0,02525 0,01 0,02275 0,02 |
0,093* 0,026* |
2,48 0,47 4,38 0,78 4,08 0,85 |
0,001* 0,002* |
| Yang et al. 2019 | Autograft (control) ANA ANA + dASCs |
51,5 35,5 43,5 |
< 0,05** | Not evaluated |
* compared with control
**compared with ANA group
BMSCs: bone marrow mesenchymal stem cells
ANA: acellular nerve allograft
dASCs: differentiated adipose stem cells
Based on the histological outcome, all involved studies evaluated histologically either nerve or muscle targets or both. The study by Guo et al. and Huanxing et al. showed a significant difference between the MSCs groups compared to other groups and the study of Yang et al. who investigated biomarkers of the survival of implanted mesenchymal stem cells. On the other hand, the study of Rodriguez et al. did not show a significant difference among groups. The results of these studies can be seen in [Table 6] below. Generally, the results supported preferable outcomes related to MSC use.
Table 6.
Histopathological Outcomes
| Author, year | Group | Outcome | P Values |
|---|---|---|---|
| Guo et al. 2020 | Model Treat Sham |
Axons and myelinated nerves decrease, nerve density decreases and there is axonal atrophy and axonal degeneration The number of myelinated axons and nerves increases, the density of nerve fibers increases Large nerve fibers are myelinated and have densely packed endoneurium |
- |
| Hunxiang et al. 2013 | Cell transplantation group Vehicle injection group |
Many nerve grafts survive and form functional connections with target muscles. More neuromuscular junction (NMJ) was obtained, ie 8.1 ± 2.4 per mm2 There is no functional relationship with the target muscle which shows no EMG response. NMJ density 1.3 ± 1.2 per mm2 |
<0.05 |
| Rodriguez et al. 2021 | Autograft (control) ANA ANA+BMSCs |
There was no significant difference in histology between the control group, ANA, and ANA+BMSCs | - |
| Yang et al. 2019 | Autograft (control) ANA ANA + dASCs |
The percentage of NF-200 and S100 positive on autograft and ANA + dASCs was significantly higher than in the ANA group but there was no significant difference between the ANA group with autograft | < 0,05* >0.05** |
* comparison between the autograft group and ANA + dASCs groups compared to the ANA group
** comparison between the autograft group with ANA group
NMJ: neuromuscular junction
BMSCs: bone marrow mesenchymal stem cells
ANA: acellular nerve allograft
dASCs: differentiated adipose stem cells
NF: neurofilament
Discussion
Based on all four studies involved in this review, we found the results were varied. There was no similarity in the species
included, intervention performed, grouping, observation time, and outcomes. We have tried to evaluate the outcome measurement based on clinical, electrophysiological, and histological and we found there are no studies that have similar outcomes. In general, the studies included the uses of mesenchymal stem cells in increasing the regeneration of brachial plexus injury gave good results.
Brachial plexus injury is an intractable peripheral nerve injury that can cause permanent disability in the patient.36,37 Not only in humans but brachial plexus injury in experimental animals also shows poor functional outcomes in various nerve regeneration processes.38,39 It is very difficult to restore normal nerve function because of nerve necrosis and changes in the microenvironment to become less permissive to nerve regeneration.40,41 In addition, mature nerves cannot regenerate and multiply so they cannot be replaced by new nerve cells.42,43 This is why the brachial plexus injury recovery process takes a long time.44,45 Mesenchymal stem cells can proliferate and repair themselves so that they have the potential to regenerate damaged nerves.46-48 Under certain conditions, these mesenchymal stem cells can differentiate into neurons, astrocytes, and oligodendrocytes which are the main types of nerves.42,49-51
The functional outcomes obtained in this study varied. In a study conducted by Yang and Rodriguez, it was seen that mesenchymal stem cell implantation combined with acellular grafts was able to induce involuntary flexion and elevation of the upper extremities. However, the results shown are not as good as those produced by autografts. This is because acellular grafts do not have a microenvironment that supports axon regeneration so they do not provide maximum recovery potential.52,53 Zheng et al. demonstrated the importance of this microenvironment by examining protein-rich plasma (platelet-rich plasma) which stimulates stem cells to proliferate and migrate, thereby increasing the expression of nerve growth factor and glial-derived neurotrophic factor.54-56
In addition to affecting the occurrence of involuntary muscle contractions and elevation of the upper extremities, mesenchymal stem cell implantation can increase muscle weight.57,58 This is following a study by Guo et al. who found that in the group given mesenchymal stem cells, overall biceps muscle weight showed improvement compared to the group without cells.59 This can be explained, one of which is the effect of mesenchymal stem cells being able to restore functional relationships between nerves with the target muscle thereby preventing muscle atrophy.60,61 The clinical improvement was also supported by the research of Jun et al. The study showed that rats with mesenchymal stem cell implantation, either one week or two months after brachial plexus injury, showed better upper extremity function outcomes compared to rats without cells. This can happen because the implanted cells can survive and form a functional relationship with the target muscle.40,62
All of the included studies evaluated the improvement in function using not only clinical and histopathological parameters but also electrophysiological parameters. This is because often the restoration of function occurs sub-clinically.63,64 Barman et al. suggested that one of the objective examinations to assess the earliest signs of improvement in the motor unit is by electrodiagnostic examination, one of which is electromyography (EMG).15,65,66
Electrophysiological examination serves to see the amplitude and latency of the muscle. The study of Guo et al. presented the Treat group had a significantly increased CMAP amplitude and shortened latency. This can be explained because MSC implantation can form a functional relationship with the target muscle and prevent muscle atrophy. Because of these two things, the CMAP amplitude in the MSCs implantation group was better than in the other groups.40
Research with different control groups was conducted by Yang et al. and Rodriguez et al. The two research groups compared mesenchymal stem cell administration with autograft and ANA. The result revealed that the CMAP amplitude was greater and lower latency in the autograft group than in the MSCs group. This is supported by the histological appearance data by Yang et al. which showed that there was a significant difference in the number of surviving axons and nerves.34, 67
The whole-cell patch clamp evaluation by Huanxing et al. showed that these differentiated nerve cells had mature motoneuron characteristics. In addition, the study demonstrated that successful prolongation of muscle atrophy events in denervated muscles can maintain endogenous motoneuron terminals and allow the establishment of functional connections later in life between regenerating axons and denervated muscles.62, 68
Several studies using other mesenchymal stem cells in the peripheral nervous system have shown supporting results. According to Yarar et al. who studied the administering mesenchymal stem cells at the end-to-end nerve junction, the results showed that there was a significant improvement in the evaluation of nerve conduction velocity and an effect on improving function.69 A meta-analysis conducted by Hundepool et al. also showed that the use of mesenchymal stem cells in experimental animals has a positive effect on the evaluation of electrophysiological parameters.70, 71
In this systematic study, there were two main results of tissue histology carried out by MSCs implantation or other similar procedures, namely the histological analysis of nerve tissue and target muscle tissue. The studies of Guo et a, Huanxing, et al., and Yang et al. showed that there were differences between the groups that were treated with mesenchymal stem cells compared to the control group of each study.32,34,62
Several other studies have shown positive results of reinnervation in the administration of mesenchymal stem cell implantation but in the sciatic nerve. In a study conducted by Kurwale et al., research subjects in the form of rats showed significant improvement in nerve injury seen from the parameters of axon diameter, nerve thickness, and thickness of myelin sheath diameter within 60 days after mesenchymal stem cell implantation.72, 73
However, different results were obtained in the study of Rodriguez et al. who showed no significant histologic differences between the autograft, ANA, and ANA + stem cell groups. This can happen because the comparison in this study is an autograft, different from other studies. Nevertheless, Rodriguez's research shows that acellular neural tissue plays an important role as a support for the growth of nerve stem cells. Other studies such as Goel et al. and Kurwale et al. showed that there were significant differences between the stem cell and no stem cell groups.72, 74, 75
Of the four studies involved, in general, three studies support the use of mesenchymal stem cells in increasing the regeneration of brachial plexus nerves in experimental animals. The studies of Guo et al., Huanxing et al., and Rodriguez et al. all showed significant improvements in clinical, electrophysiological, and histological outcomes. On the other hand, there is one study that does not support the use of mesenchymal stem cells in increasing the regeneration of brachial plexus nerves in experimental animals. Rodriguez et al. stated that mesenchymal stem cells failed to show significant improvement in the assessed neural regeneration parameters. The existence of this discrepancy may be due to different research methods ranging from research subjects, length of observation, procedures, allocation of research groups, outcome evaluation methods, and types of stem cells used.32,67,62
Study limitations
This systematic review has been structured in such a way that it can cover important studies in the field of brachial plexus and mesenchymal stem cells. However, there are some weaknesses in this research. This study still includes studies that have the potential for bias in the study because there are few references regarding the administration of mesenchymal stem cells to experimental animals in cases of brachial plexus injury. There are no specific inclusion criteria for certain types of animals because of the limitations of the research that was sought and found. This means there are two types of experimental animals in this systematic study, namely rabbits and mice. These species differences are of course a special concern when concluding this systematic study.
Conclusion
Mesenchymal stem cell implantation in animals with brachial plexus injury showed its ability to regenerate nerve cells as evidenced by clinical, electrophysiological, and histopathological results. However, this systematic study involved experimental animals from various species so the results cannot be uniform, and conclusions should be drawn cautiously.
Acknowledgment
No acknowledgment in this systematic review.
Conflict of interest:
None
Funding:
None
Informed consent
None
Note:
This systematic review is already presented in 69th Continuing Orthopaedic Education (COE) of Indonesia Orthopaedic Association (IOA) in Makassar Indonesian May 28th 2022
References
- 1.Kaiser R, Waldauf P, Ullas G, Krajcová A. Epidemiology, etiology, and types of severe adult brachial plexus injuries requiring surgical repair: systematic review and meta-analysis. Neurosurg Rev. 2020;43(2):443–452. doi: 10.1007/s10143-018-1009-2. [DOI] [PubMed] [Google Scholar]
- 2.de Azevedo Filho FAS, Abdouni YA, Ogawa G, Couto de Sá CK, da Costa AC, de Moraes Barros Fucs PM. Functional outcome of oberlin procedure. Acta Ortop Bras. 2019;27(6):294–297. doi: 10.1590/1413-785220192706224552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thatte MR, Babhulkar S, Hiremath A. Brachial plexus injury in adults: Diagnosis and surgical treatment strategies. Ann Indian Acad Neurol. 2013;16(1):26–33. doi: 10.4103/0972-2327.107686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakellariou VI, Badilas NK, Mazis GA, et al. Brachial Plexus Injuries in Adults: Evaluation and Diagnostic Approach. ISRN Orthop. 2014;2014:1–9. doi: 10.1155/2014/726103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinner RJ, Shin AY, Elhassan BT, Bishop AT. Traumatic Brachial Plexus Injury. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, Cohen MS, editors. Green’s Operative Hand Surgery. 7th ed. Philadelphia: Elsevier Inc; 2017. pp. 1146–1203. [Google Scholar]
- 6.Iorio ML. The Role of Peripheral Nerve Surgery in Surgery. 2019:259–269. [Google Scholar]
- 7.Tjokorda M. Surgical Managementof Brachial Plexus Injury. 2018;11(December):2079–2084. [Google Scholar]
- 8.Forli A, Bouyer M, Aribert M, et al. Upper limb nerve transfers: A review. Hand Surg Rehabil. 2017;36(3):151–172. doi: 10.1016/j.hansur.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Moore AM, Novak CB. Advances in nerve transfer surgery. J Hand Ther. 2014;27(2):96–105. doi: 10.1016/j.jht.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Primaputra MRA, Widodo W. Clinical and Functional Outcome Analysis in Patients with Traumatic Brachial Plexus Injury after Nerve and Muscle. Procedure in Cipto Mangunkusumo Hospital 2010-2017. 2017 [Google Scholar]
- 11.Sakuma M, Gorski G, Sheu SH, et al. Lack of motor recovery after prolonged denervation of the neuromuscular junction is not due to regenerative failure. Eur J Neurosci. 2016;43(3):451–462. doi: 10.1111/ejn.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin E, Senders JT, DiRisio AC, Smith TR, Broekman MLD. Timing of surgery in traumatic brachial plexus injury: A systematic review. J Neurosurg. 2019;130(4):1333–1345. doi: 10.3171/2018.1.JNS172068. [DOI] [PubMed] [Google Scholar]
- 13.Moiyadi AV, Devi BI, Nair KPS. Brachial plexus injuries: Outcome following neurotization with intercostal nerve. J Neurosurg. 2007;107(2):308–313. doi: 10.3171/JNS-07/08/0308. [DOI] [PubMed] [Google Scholar]
- 14.Giuffre JL, Kakar S, Bishop AT, Spinner RJ, Shin AY. Current Concepts of the Treatment of Adult Brachial Plexus Injuries. J Hand Surg Am. 2010;35(4):678–688. doi: 10.1016/j.jhsa.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Barman A, Chatterjee A, Prakash H, Viswanathan A, Tharion G, Thomas R. Traumatic brachial plexus injury: Electrodiagnostic findings from 111 patients in a tertiary care hospital in India. Injury. 2012;43(11):1943–1948. doi: 10.1016/j.injury.2012.07.182. [DOI] [PubMed] [Google Scholar]
- 16.Rikhtegar R, Pezeshkian M, Dolati S, et al. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed Pharmacother. 2019;109( 2018):304–313. doi: 10.1016/j.biopha.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Takagi Y. History of neural stem cell research and its clinical application. Neurol Med Chir (Tokyo). 2016;56(3):110–124. doi: 10.2176/nmc.ra.2015-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berebichez-Fridman R, Gómez-García R, Granados-Montiel J, et al. The Holy Grail of Orthopedic Surgery: Mesenchymal Stem Cells - Their Current Uses and Potential Applications. Stem Cells Int. 2017:2017. doi: 10.1155/2017/2638305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dilogo IH, Phedy P, Kholinne E, et al. Autologous mesenchymal stem cell implantation, hydroxyapatite, bone morphogenetic protein-2, and internal fixation for treating critical-sized defects: a translational study. Int Orthop. 2019;43(6):1509–1519. doi: 10.1007/s00264-019-04307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dilogo IH, Kamal AF, Gunawan B, Rawung RV. Autologous mesenchymal stem cell (MSCs) transplantation for critical-sized bone defect following a wide excision of osteofibrous dysplasia. Int J Surg Case Rep. 2015;17:106–111. doi: 10.1016/j.ijscr.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahyussalim AJ, Saleh I, Kurniawati T, Lutfi APWY. Improvement of renal function after human umbilical cord mesenchymal stem cell treatment on chronic renal failure and thoracic spinal cord entrapment: A case report. J Med Case Rep. 2017;11(1):1–7. doi: 10.1186/s13256-017-1489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mead B, Berry M, Logan A, Scott RAH, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015;14(3):243–257. doi: 10.1016/j.scr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpentier A, Nimgaonkar I, Chu V, Xia Y, Hu Z, Liang TJ. Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Res. 2016;16(3):640–650. doi: 10.1016/j.scr.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma A, Sane H, Gokulchandran N, et al. Cellular Therapy for Chronic Traumatic Brachial Plexus Injury. Adv Biomed Res. 2018;7(1):51 . doi: 10.4103/2277-9175.228631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019;6(July) doi: 10.21037/sci.2019.06.04. doi:10.21037/sci.2019.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bingham JR, Kniery KR, Jorstad NL, Horkayne-Szakaly I, Hoffer ZS, Salgar SK. “Stem cell therapy to promote limb function recovery in peripheral nerve damage in a rat model” – Experimental research. Ann Med Surg. 2019;41(November 2018):20–28. doi: 10.1016/j.amsu.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogendoorn S, Duijnisveld BJ, Van Duinen SG, et al. Local injection of autologous bone marrow cells to regenerate muscle in patients with traumatic brachial plexus injury: A pilot study. Bone Jt Res. 2014;3(2):38–47. doi: 10.1302/2046-3758.32.2000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannerman P, James MA. Molecular mechanisms to improve nerve regeneration following damage to the immature peripheral nervous system. J Bone Jt Surg - Ser A. 2009;91(SUPPL. 4):87–89. doi: 10.2106/JBJS.I.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006;26(7-8):1235–1252. doi: 10.1007/s10571-006-9029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Q, Luo M, Li P, Jin H. Transplantation of human amniotic epithelial cells repairs brachial plexus injury : pathological and biomechanical analyses. 2014;9(24):2159–2163. doi: 10.4103/1673-5374.147947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayad S, Zaminy A. Stem cell therapy for nerve injury. World J Stem Cells. 2017;9(9):144–151. doi: 10.4252/wjsc.v9.i9.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo M, Li D, Wu L, Li M, Yang B. Bone marrow mesenchymal stem cells repair brachial plexus injury in rabbits through ERK pathway. y. :1515–1523. doi: 10.26355/eurrev_202002_20210. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009 [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Fang J, Li L, Chen G, Qin B, Gu L. Contralateral C7 transfer combined with acellular nerve allografts seeded with differentiated adipose stem cells for repairing upper brachial plexus injury in rats. 2016 doi: 10.4103/1673-5374.259626. doi:10.4103/1673-5374.259626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):1–9. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arzillo S, Gishen K, Askari M. Brachial plexus injury: Treatment options and outcomes. J Craniofac Surg. 2014;25(4):1200–1206. doi: 10.1097/SCS.0000000000000841. [DOI] [PubMed] [Google Scholar]
- 37.Sumarwoto T, Suroto H, Mahyudin F, et al. Brachial plexus injury: Recent diagnosis and management. Open Access Maced J Med Sci. 2021;9:13–24. [Google Scholar]
- 38.Xian H, Xie R, Luo C, Cong R. Comparison of Different in Vivo Animal Models of Brachial Plexus Avulsion and Its Application in Pain Study. Neural Plast. 2020;2020 :10. doi: 10.1155/2020/8875915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu C, Qian X, Jianxiong A, et al. A New Animal Model of Brachial Plexus Neuralgia Produced by Injection of Cobra Venom into the Lower Trunk in the Rat. Pain Med (United States). 2015;16(9):1680–1689. doi: 10.1111/pme.12722. [DOI] [PubMed] [Google Scholar]
- 40.Jun Z, Xinying Z, Yang C, Chunjiang F. Application of spinal cord progenitor cell transplantation in brachial plexus root avulsion injury in rats. Chinese J Rehabil Reconstr Surg. 2005;19:11. [Google Scholar]
- 41.Seddighi A, Nikouei A, Seddighi AS, Zali AR, Tabatabaei SM. Peripheral Nerve Injury: a Review Article. Int Clin Neurosci J. 2016;3(1):1–6. [Google Scholar]
- 42.Menorca RMG, Fussell TS, Elfar JC. Peripheral Nerve Trauma: Mechanisms of Injury and Recovery. Hand Clin. 2013;29(3):317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palispis WA, Gupta R. Surgical repair in humans after traumatic nerve injury provides limited functional neural regeneration in adults. Exp Neurol. 2017;290:106–114. doi: 10.1016/j.expneurol.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Lao J, Gao K, Gu Y, Zhao X. Functional outcome of nerve transfers for traumatic global brachial plexus avulsion. Injury. 2013;44(5):655–660. doi: 10.1016/j.injury.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Kachramanoglou C, Carlstedt T, Koltzenburg M, Choi D. Long-Term Outcome of Brachial Plexus Reimplantation After Complete Brachial Plexus Avulsion Injury. World Neurosurg. 2017;103:28–36. doi: 10.1016/j.wneu.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimoldi N, Colleoni F, Tiberio F, et al. Stem cell salvage of injured peripheral nerve. Cell Transplant. 2015;24(2):213–222. doi: 10.3727/096368913X675700. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W, Fang X, Zhang C, et al. Transplantation of embryonic spinal cord neurons to the injured distal nerve promotes axonal regeneration after delayed nerve repair. Eur J Neurosci. 2017;45(6):750–762. doi: 10.1111/ejn.13495. [DOI] [PubMed] [Google Scholar]
- 48.Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10(68):1–22. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang RC, Du WQ, Zhang JY, et al. Mesenchymal stem cell treatment for peripheral nerve injury: A narrative review. Neural Regen Res. 2021;16(11):2170–2176. doi: 10.4103/1673-5374.310941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooney DS, Wimmers EG, Ibrahim Z, et al. Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model. Sci Rep. 2016;6(July):1–12. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tatullo M, Gargiulo IC, Dipalma G, et al. Stem cells and regenerative medicine. Transl Syst Med Oral Dis. 2019:387–407. [Google Scholar]
- 52.Burnett MG, Zager EL, Urnett MARKGB, Ager ERICLZ. Pathophysiology of peripheral nerve injury : a brief review. Neurosurg Focus. 2004;16(5):1–7. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 53.Yi S, Zhang Y, Gu X, et al. Application of stem cells in peripheral nerve regeneration. Burn Trauma. 2020:8. doi: 10.1093/burnst/tkaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng C, Zhu Q, Liu X, et al. Improved peripheral nerve regeneration using acellular nerve allografts loaded with platelet-rich plasma. Tissue Eng - Part A. 2014;20(23-24):3228–3240. doi: 10.1089/ten.tea.2013.0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavorato A, Raimondo S, Boido M, et al. Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: Systematic review. Int J Mol Sci. 2021;22(2):1–22. doi: 10.3390/ijms22020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L, Lu LJ, Meng XT, Chen D, Zhang ZX, Yang F. Reimplantation combined with transplantation of transgenic neural stem cells for treatment of brachial plexus root avulsion. Chinese J Traumatol - English Ed. 2008;11(5):267–273. doi: 10.1016/s1008-1275(08)60054-1. [DOI] [PubMed] [Google Scholar]
- 57.Andrade BM, Baldanza MR, Ribeiro KC, et al. Bone marrow mesenchymal cells improve muscle function in a skeletal muscle re-injury model. PLoS One. 2015;10(6):1–13. doi: 10.1371/journal.pone.0127561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J. Mesenchymal Stem Cells Therapy for Muscular Atrophy and Sarcopenia. Epidemiol Int J. 2022;6:2. [Google Scholar]
- 59.Tos P, Crosio A, Pellegatta I, et al. Efficacy of anti-adhesion gel of carboxymethylcellulose with polyethylene oxide on peripheral nerve: Experimental results on a mouse model. Muscle and Nerve. . 2016;53(2):304–309. doi: 10.1002/mus.24739. [DOI] [PubMed] [Google Scholar]
- 60.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119(9):1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 61.Ayala-Cuellar AP, Kang JH, Jeung EB, Choi KC. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol Ther. 2019;27(1):25–33. doi: 10.4062/biomolther.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su H, Wang L, Cai J, et al. Transplanted motoneurons derived from human induced pluripotent stem cells form functional connections with target muscle. Stem Cell Res. 2013;11(1):529–539. doi: 10.1016/j.scr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Texakalidis P, Hardcastle N, Tora MS, Boulis NM. Functional restoration of elbow flexion in nonobstetric brachial plexus injuries: A meta-analysis of nerve transfers versus grafts. Microsurgery. 2020;40(2):261–267. doi: 10.1002/micr.30510. [DOI] [PubMed] [Google Scholar]
- 64.Moran SL, Steinmann SP, Shin AY. Adult brachial plexus injuries: Mechanism, patterns of injury, and physical diagnosis. Hand Clin. 2005;21(1):13–24. doi: 10.1016/j.hcl.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Mansukhani KA. Electrodiagnosis in traumatic brachial plexus injury. Ann Indian Acad Neurol. 2013;16(1):19–25. doi: 10.4103/0972-2327.107682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrante MA. Electrodiagnostic assessment of the brachial plexus. Neurol Clin. 2012;30(2):551–580. doi: 10.1016/j.ncl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez AG, Gonzales SA, Melero NC, Arufe MC. Acellular nerve graft enriched with mesenchymal stem cells in the transfer of the phrenic nerve to the musculocutaneous nerve in a C5-C6 brachial plexus avulsion in a rat model. Microsurgery. 2021;42:57–65. doi: 10.1002/micr.30829. [DOI] [PubMed] [Google Scholar]
- 68.Saeki S, Tokutake K, Takasu M, et al. Functional Reconstruction of Denervated Muscle by Xenotransplantation of Neural Cells from Porcine to Rat. Int J Mol Sci. 2022;23:15. doi: 10.3390/ijms23158773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yarar E, Kuruoglu E, Kocabıcak E, et al. Electrophysiological and histopathological effects of mesenchymal stem cells in treatment of experimental rat model of sciatic nerve injury. Int J Clin Exp Med. 2015;8(6):8776–8784. [PMC free article] [PubMed] [Google Scholar]
- 70.Hundepool CA, Nijhuis THJ, Mohseny B, Selles RW, Hovius SER. The effect of stem cells in bridging peripheral nerve defects: A meta-analysis - A review. J Neurosurg. 2014;121(1):195–209. doi: 10.3171/2014.4.JNS131260. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Li J, Zhang Y, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res Ther. 2014;16:2. doi: 10.1186/ar4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurwale NS, Suri V, Srivastava A, et al. Role of bone marrow derived pluripotent stem cells in peripheral nerve repair in adult rats: A morphometric evaluation. J Neurosci Rural Pract. 2015;6(2):152–159. doi: 10.4103/0976-3147.153218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dadon-Nachum M, Sadan O, Srugo I, Melamed E, Offen D. Differentiated Mesenchymal Stem Cells for Sciatic Nerve Injury. Stem Cell Rev Reports. 2011;7(3):664–671. doi: 10.1007/s12015-010-9227-1. [DOI] [PubMed] [Google Scholar]
- 74.Goel RK, Suri V, Suri A, et al. Effect of bone marrow-derived mononuclear cells on nerve regeneration in the transection model of the rat sciatic nerve. J Clin Neurosci. 2009;16(9):1211–1217. doi: 10.1016/j.jocn.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 75.Matsuse D, Kitada M, Kohama M, et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol. 2010;69(9):973–985. doi: 10.1097/NEN.0b013e3181eff6dc. [DOI] [PubMed] [Google Scholar]