Abstract
Clinical high risk for psychosis (CHR) is a transdiagnostic risk state. However, it is unclear how risk states such as CHR fit within broad transdiagnostic models such as the Hierarchical Taxonomy of Psychopathology (HiTOP). In this study, a hierarchical dimensional symptom structure was defined by unfolding factor analysis of self-report data from 3,460 young adults (mage=20.3). A subsample (n=436) completed clinical interviews, 85 of whom met CHR criteria. Regression models examined relationships between symptom dimensions, CHR status, and clinician-rated symptoms. CHR status was best explained by a reality distortion dimension, with contributions from internalizing dimensions. Positive and negative attenuated psychotic symptoms were best explained by multiple psychotic and nonpsychotic symptom dimensions including reality distortion, distress, fear, detachment, and mania. Attenuated psychotic symptoms are a complex presenting problem warranting comprehensive assessment. HiTOP can provide both diagnostic precision and broad transdiagnostic coverage, making it a valuable resource for use with at-risk individuals.
Keywords: Psychosis, clinical high risk for psychosis (CHR), transdiagnostic, hierarchical taxonomy of psychopathology (HiTOP), clinical staging
Tens of millions of people worldwide—0.5 to 1% of the global population—live with schizophrenia, a serious mental illness characterized by positive symptoms (e.g., delusions, hallucinations, paranoia) and negative symptoms (e.g., loss of motivation, blunted emotional expression, social withdrawal) (American Psychiatric Association, 2013). Schizophrenia and related psychotic disorders tend to manifest in young adulthood and cause chronic and severe functional impairment: in fact, schizophrenia’s global disease burden is the third-largest of any mental illness in terms of years lived with disability (behind only major depressive disorder and anxiety disorders) (Vos et al., 2015). Due to their early incidence and chronic course, psychotic disorders are prime targets for early identification and intervention. Psychotic disorders are often preceded by a prodromal stage marked by subclinical or attenuated psychotic symptoms. Early identifications strategies largely focus on detecting these attenuated symptoms (e.g., perplexed mood, suspiciousness, transient or unformed perceptual abnormalities) in youth who do not currently have a diagnosable psychotic disorder (Fusar-Poli et al., 2013). Youth meet criteria for a clinical high risk for psychosis (CHR) syndrome if they exhibit attenuated psychotic symptoms; brief, intermittent frank psychotic symptoms; and/or genetic risk and functional decline (Fusar-Poli et al., 2016; Miller et al., 1999; Yung et al., 2005).1 A determination of CHR status is primarily based on attenuated positive symptoms of psychosis, although attenuated negative symptoms are also commonly observed in CHR (Strauss et al., 2020). Individuals meeting CHR criteria have greatly elevated risk for psychotic disorders, with roughly 25% developing a psychotic disorder within 2 years of identification (Fusar-Poli et al., 2013).
The CHR syndrome is a heterogenous and transdiagnostic group. This syndrome confers risk for various psychotic diagnoses (e.g., schizophrenia, delusional disorder, mood disorder with psychotic features), indicating that it reflects risk for a broad psychosis spectrum rather than a specific psychotic disorder. Moreover, most individuals meeting CHR criteria also meet criteria for at least one nonpsychotic psychiatric diagnosis—typically mood, anxiety, or substance use disorders (Addington et al., 2017; Albert et al., 2018; Fusar-Poli et al., 2014; Lim et al., 2015; Salokangas et al., 2012), although personality disorders are also common (Boldrini et al., 2019). Finally, individuals who meet CHR criteria but do not go on to develop psychotic disorders (“nonconverters”) experience high rates of nonpsychotic disorders causing cognitive and functional impairment (Addington et al., 2017). Psychosis risk—operationalized by the CHR syndrome—is evidently heterogenous and transdiagnostic. It is therefore important to have accurate and efficient models for understanding the co-occurrence of psychotic and nonpsychotic symptoms in this at-risk population.
There are two broad approaches to integrating disparate psychiatric symptoms. One approach is represented by the Diagnostic and Statistical Manual of Mental Disorders-5 and its predecessors, in which symptoms have been clustered into psychiatric disorders using a mixture of research, clinical observation, and expert consensus. Notably, DSM disorders have tended to evolve independently of one another, which has led to disorders co-occurring and sharing overlapping symptoms. Interestingly, within DSM disorders, it is also often the case the symptoms are heterogeneous and individuals with the same disorder have dissimilar clinical presentations (e.g., representing subgroups). This is certainly the case in schizophrenia, which has long been recognized as a heterogenous disorder with many possible presentations (Andreasen, 1993).
Increasingly, researchers have used more quantitatively-driven approaches, in which co-occurring symptoms are thought to reflect underlying psychopathology dimensions that cut across DSM disorders. Because transdiagnostic assessment typically measures psychiatric symptoms quantitatively, latent dimensions can be defined at various levels of analysis—from broad psychopathology spectra (e.g., psychosis) to specific symptom clusters (e.g., paranoia). Increasingly, momentum is building toward organizing these latent dimensional constructs in the Hierarchical Taxonomy of Psychopathology (HiTOP; Kotov et al., 2017, 2021). HiTOP proposes a nested hierarchy of transdiagnostic symptom factors, with comorbidity explained by variation in higher-order psychopathology dimensions (e.g., internalizing) which manifest across multiple lower-order dimensions (e.g., social anxiety and panic-like symptoms) (Kotov et al., 2021).
In CHR samples, comorbid symptoms have traditionally been modeled through DSM categorical diagnoses (e.g., Addington et al., 2017; Fusar-Poli et al., 2014). More recently, clinical staging studies have suggested that transdiagnostic factors play important roles in psychosis risk (Addington et al., 2019; McGorry et al., 2018). We recently conducted a small pilot study to investigate symptoms in a CHR sample through quantitative transdiagnostic factors (Cowan & Mittal, 2021). That study identified several transdiagnostic dimensions cutting across symptom categories and showed that depression and hypomania were relevant for multiple dimensions. However, that study was a case-control study of 71 CHR and 73 healthy comparison youth, which constrained analyses to a simple latent variable model (exploratory factor analysis of scale means). The CHR syndrome is rare in the general population, and CHR sample sizes tend to be relatively small as a result (Addington et al., 2008). However, psychosis occurs on a continuum with normative experience (DeRosse & Karlsgodt, 2015; Johns & van Os, 2001; Nelson et al., 2013). When this is the case, large population-based structural studies can address some limitations of traditional case-control studies (Kotov et al., 2022; Preacher et al., 2005). Specifically, if a CHR sample is drawn from a large, representative reference group of demographically matched youth, more complex hierarchical structures can be defined by item-level analyses in the reference group and then applied in the CHR sample.
As evidence accumulates for transdiagnostic models of psychosis risk, there are considerable advantages to analyzing CHR symptom data from a transdiagnostic and hierarchical perspective. First, a hierarchical transdiagnostic approach allows new evidence from hard-to-reach CHR samples to be integrated into the larger picture of psychiatric comorbidity. Second, this approach can determine which transdiagnostic dimensions, at which levels of analysis, are most important for understanding psychosis risk. A hierarchical, transdiagnostic model of CHR symptomatology can then guide research or clinical decision-making, for example in how broadly vs. specifically to assess various symptoms. Third, this approach can determine whether nonpsychotic symptoms directly contribute to psychosis risk. For instance, outcomes in the CHR syndrome (e.g., conversion to a psychotic disorder) might only relate to psychotic symptom dimensions (and not nonpsychotic symptom dimensions). This would imply that nonpsychotic symptoms—though important for individuals’ functioning and well being—are co-occurring problems unrelated to psychosis risk. By contrast, if outcomes relate to psychotic and nonpsychotic dimensions, this would suggest that nonpsychotic symptoms directly contribute to psychosis risk.
The current study examines the transdiagnostic phenotypic profile of CHR symptomatology in a hierarchical dimensional framework. A hierarchical dimensional structure was established based on self-report measures of psychotic and nonpsychotic symptoms in a large community youth sample (N = 3,460). Nonpsychotic symptoms included various categories that have been shown to be relevant for psychosis risk including internalizing (Fusar-Poli et al., 2014), mania (Correll et al., 2007), dissociation (Gibson et al., 2019; Williams et al., 2018), and substance use (Addington et al., 2017). Relationships were then examined between symptom dimensions and key psychosis risk variables (CHR status, attenuated positive symptoms, and attenuated negative symptoms) assessed in clinical interviews with a subset of participants (n = 436, 85 of whom met CHR criteria). These data-driven analyses addressed three research questions. First, what is the best level of specificity in the dimensional hierarchy to explain each psychosis risk variable? Second, at that level of specificity, which symptom dimensions relate to each psychosis risk variable? Third, do nonpsychotic symptom dimensions directly contribute to psychosis risk variables, indicated by incremental variance explained above and beyond the effect of psychotic symptom dimensions? We hypothesized that more fine-grained levels of the symptom hierarchy would explain more variance in interview-rated psychosis risk variables, and that both psychotic and nonpsychotic symptom dimensions would relate to attenuated psychotic symptoms.
Transparency and Openness
Preregistration
This study’s analyses were not preregistered and are clearly labelled as exploratory.
Data, Materials, Code, and Online Resources
Study data are uploaded periodically to a publicly accessible data repository, following the data sharing plan approved by all study sites’ institutional review boards, to which all participants consented. Data can be accessed at https://nda.nih.gov/edit_collection.html?id=2783. Study materials are commonly used and easily accessed questionnaires and clinical interviews. Study analysis code is available online and can be accessed at https://github.com/hrcowan/HiTOP-MAP.
Reporting
We report how we determined our sample size, all data exclusions, all manipulations, and all measures in the study.
Ethical Approval
The study protocol was approved by institutional review boards at all study sites. Participants gave written informed consent including consent to the data sharing plan included in the protocol. The study was carried out in accordance with the World Medical Association Declaration of Helsinki, as amended in 2013. The study protocol was made publicly available prior to data collection (Ellman et al., 2020).
Method
Participants
The reference sample comprised 3,460 community youth who took part in the Multi-Site Assessment of Psychosis-Risk (MAP) Study (for full study details, see Ellman et al., 2020). Participants were recruited from several large metropolitan areas in the eastern and midwestern United States. Eligibility requirements were age 16 – 30, English proficiency to complete online questionnaires, and normal or corrected-normal vision. Participants were not excluded on any clinical or demographic characteristics. All participants completed an online questionnaire battery including demographic variables, and measures of psychotic-like experiences, depression, anxiety, mania, dissociation, and substance use. A subsample of 439 individuals drawn from the reference sample attended an in-person clinical interview (see Figure 1). Three of these participants were excluded (see below), leaving an effective interview sample size of 436. Demographic characteristics of the reference sample and interview subsample are shown in Table 1. Sample size was determined by estimating population prevalence of CHR based on pilot data and prior population estimates (van Os et al., 2009) to obtain sufficient participants meeting CHR criteria for all planned analyses (for full details, see Ellman et al., 2020).
Figure 1.
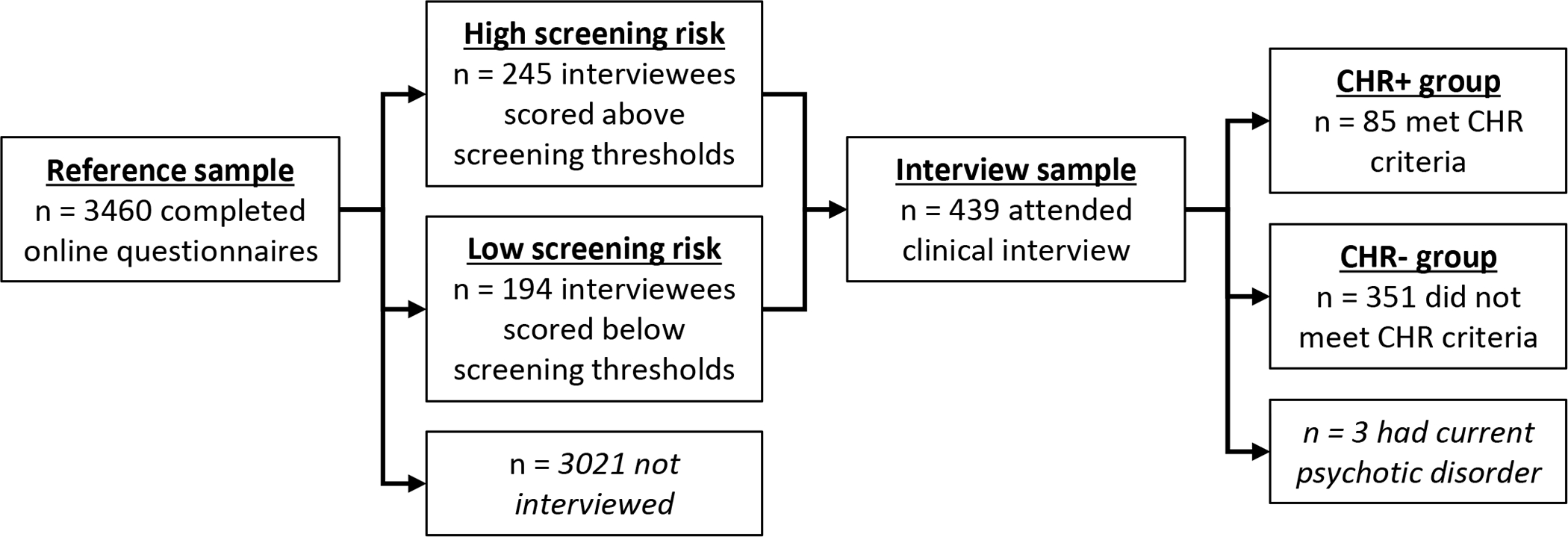
Study flowchart. See Table 1 for participant characteristics in the reference sample and interview sample.
Table 1.
Participant Characteristics
| Reference sample | Interview subsample (CHR+) | Interview subsample (CHR−) | CHR+ vs. CHR− comparison | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| N or mean | % or SD | N or mean | % or SD | N or mean | % or SD | χ2 or t | p | |
|
| ||||||||
| N | 3,460 | 85 | 351 | |||||
| Age | 20.29 | 2.26 | 20.28 | 2.02 | 20.12 | 1.84 | ||
| Gender (female) | 2358 | 68.9% | 61 | 71.8% | 273 | 77.8% | 1.00 | .317 |
| Family income | ||||||||
| Under $35,000 | 563 | 18.8% | 19 | 26.4% | 70 | 19.9% | ||
| $35,000 – $99,999 | 1384 | 46.3% | 32 | 44.4% | 152 | 43.3% | ||
| $100,000 and over | 1043 | 34.9% | 21 | 29.2% | 91 | 25.9% | 0.62 | .734 |
| Race | ||||||||
| White | 1960 | 56.6% | 52 | 61.2% | 194 | 55.3% | ||
| Asian | 962 | 27.8% | 21 | 24.7% | 106 | 30.2% | ||
| Black | 556 | 16.1% | 15 | 17.6% | 60 | 17.1% | ||
| Native/Pacific islander | 68 | 1.9% | 3 | 3.5% | 4 | 1.1% | 3.44 | .329 |
| Ethnicity (Hispanic) | 350 | 10.1% | 6 | 7.1% | 38 | 10.8% | 0.69 | .404 |
| Substance use | ||||||||
| Alcohol | ||||||||
| Never | 972 | 29.4% | 27 | 32.5% | 101 | 28.8% | ||
| Less than 1×/week | 1555 | 47.1% | 37 | 44.6% | 167 | 47.6% | ||
| 1×/week or more | 774 | 23.5% | 18 | 21.7% | 73 | 20.8% | 0.45 | .798 |
| Cannabis | ||||||||
| Never | 2290 | 68.1% | 48 | 57.1% | 227 | 64.7% | ||
| Less than 1×/week | 768 | 22.8% | 19 | 22.6% | 99 | 28.2% | ||
| 1×/week or more | 305 | 9.1% | 17 | 20.2% | 30 | 8.5% | 10.0 | .007 |
| Other | ||||||||
| Never | 2783 | 82.5% | 53 | 63.1% | 284 | 80.9% | ||
| Less than 1×/week | 473 | 14.0% | 24 | 28.6% | 48 | 13.7% | ||
| 1×/week or more | 115 | 3.4% | 7 | 8.3% | 16 | 4.6% | 13.6 | .001 |
Procedures
As shown in Figure 1, participants in the reference sample completed online questionnaires assessing multiple forms of psychotic and nonpsychotic psychopathology. An interview subsample was then drawn from the reference sample. The sampling strategy was designed to produce a fully dimensional sample enriched for psychosis risk. All individuals who scored above established screening thresholds on two measures of psychotic-like experiences were invited to participate in interviews (high screening risk, n = 245). Thresholds were Prodromal Questionnaire ≥ 8 items rated as “distressing” (Gibson et al., 2014; Loewy et al., 2005, 2007) or PRIME screen ≥ 2 items rated as “somewhat” or “definitely” agree (Kline et al., 2012). See “Measures” below for details of these questionnaires. To obtain a fully dimensional interview subsample, randomly selected individuals scoring below screening thresholds were also invited to participate in interviews (low screening risk, n = 194). Clinical interviews assessed CHR status and positive and negative attenuated psychosis symptoms (see Measures below). Individuals who met criteria for a current psychotic disorder at the time of the interview (n = 3) were excluded from analysis of interview data, leaving an effective interview sample size of 436 participants.
Measures
All participants completed questionnaires including measures of psychotic-like experiences, depression, anxiety, mania, dissociation, and substance use. Psychotic-like experiences were assessed by the Prodromal Questionnaire (PQ; Loewy et al., 2005), the PRIME screen (Kline et al., 2012), and the PROD screen (Heinimaa et al., 2003), three commonly used measures of psychosis risk. Depression was assessed by a shortened 14-item version of the Center for Epidemiological Studies-Depression Scale (CES-D; Kohout et al., 1993), a measure of depression severity in the past week. Anxiety was assessed by a 7-item version of the State-Trait Anxiety Inventory—Trait Form that removes items overlapping with depression (STAI; Bieling et al., 1998); and the Social Phobia Scale (SPS; Mattick & Clarke, 1998), a 20-item measure of social anxiety. Mania was assessed by the General Behavior Inventory—Patient Version (GBI; Youngstrom et al., 2008), a 10-item measure of hypomanic or manic elevations in mood or behavior over the past year. Dissociation was assessed by an abbreviated 14-item version of the Dissociative Experiences Scale (DES; Bernstein & Putnam, 1986), with some items removed to decrease participant burden (primarily items related to rare dissociative identity disorder symptoms). Substance use was assessed by the Drug Use Frequency scale (DUF; O’Farrell et al., 2003), a measure of substance use frequency in the past three months.
Participants in the interview subsample completed two structured clinical interviews following the questionnaire administration. CHR status and positive symptom severity were assessed by the Structured Interview for Psychosis-Risk Syndromes (SIPS; Miller et al., 1999), a clinician-rated interview which measures the severity of positive, negative, disorganized, and general symptoms and classifies participants who currently meet CHR syndrome criteria. CHR criteria are based on the presence of attenuated positive symptoms; brief intermittent psychotic symptoms; and/or genetic risk and recent functional decline.
Negative symptom severity was assessed by the Prodromal Inventory of Negative Symptoms (PINS; Pelletier-Baldelli et al., 2017; Strauss et al., 2020). The PINS is a clinician-rated interview designed to assess negative symptoms in the CHR syndrome, including anhedonia, avolition, and asociality across social, role, and recreation domains, as well as blunted affect and alogia.
Data Analysis
A series of exploratory factor analyses (EFA) were performed on the items in the nine self-report scales (see “Measures” above) using an unfolding or “bass-ackwards” method (Goldberg, 2006). We computed a series of EFAs with 1, 2, …, k factors, where k was the largest number of factors for which: (1) parallel analysis showed eigenvalues larger than those generated from random data; and (2) all factors were identified with at least two items (i.e., at least two items loaded ≥ .30 on the factor). EFAs used minimum residual factoring and varimax rotation, producing orthogonal factors in each factor model. Orthogonal rotation is typical in unfolding analyses (e.g., Kim & Eaton, 2015) because it produces interpretable cross-level correlations between latent variables (Goldberg, 2006). The number of participants (N = 3,460) gave an adequate 17.0:1 subject:item ratio. These factor models represent increasingly complex data-driven dimensional transdiagnostic models which should theoretically approximate higher to lower levels of the HiTOP transdiagnostic hierarchy as the number of factors increases (superspectra → spectra → subfactors) (Kotov et al., 2021). Regression-based factor scores were saved for all factors in all factor models. Pearson correlations between factor scores were examined to approximate relationships between factors at adjacent levels of the factor hierarchy. Missing data were infrequent (missing data < 6% for all items). Missing data were handled by pairwise deletion (factor analysis) and median imputation (regression-based factor scores). Simulations have shown that various methods of handling missing data perform similarly when sample size is large (n >1000) and when missing data rates are low (~5%) (Enders & Bandalos, 2001; Nassiri et al., 2018), as was the case in the current study.
To examine the placement of clinical variables in the dimensional symptom hierarchy, we calculated regression models with factor scores entered as IVs and clinical variables (CHR status, positive symptoms, or negative symptoms) entered as DVs. Clinical variables were entered as means of symptom items. Continuous variables (positive and negative symptoms) were analyzed through linear regression. The categorical variable of CHR status was analyzed through logistic regression. For each clinical variable, a separate regression model was calculated for each level of the factor hierarchy (3 clinical variables x 10 factor levels = 30 total regression models). To determine which factor level provided the best fit for each clinical variable, overall fit statistics were compared for regression models at various factor levels (adjusted R2 for linear regression models and McFadden R2 for logistic regression models), and improvements in model fit from one level to the next were examined through likelihood ratio tests. Best fit was defined as the most complex factor level which achieved a statistically significant improvement in model fit. We examined standardized regression coefficients in the best-fitting regression model to determine which symptom dimensions had independent associations with the clinical variable of interest. Finally, we also calculated linear regression models for CHR status (dummy coded as 0 = absent and 1 = present) to facilitate direct comparisons of adjusted R2 and coefficients between CHR status and positive/negative symptoms (these appear in Supplemental Material, Figure S1).
Whereas an empirical approach to deriving symptom scores (e.g., exploratory factor analysis of symptom items) may be more precise, in research and clinical practice the SIPS and PINS are commonly analyzed and interpreted as means or total scores. To be consistent with prior research and clinical practice in this area, the present study analyzed scale means rather than latent variables for interview-rated symptoms.
Results
Preliminary Analyses
Demographic variables for the reference sample and the interview subsample are shown in Table 1. Chi-squared tests and t-tests found no significant differences in demographic variables between participants who met CHR criteria at the diagnostic interview (n = 85) vs. those who did not (n = 351). As shown in Table 1, participants meeting CHR criteria were more likely to use cannabis and other non-alcohol substances (e.g., cocaine, hallucinogens).
Descriptive statistics for full-scale symptom scores are shown in Supplemental Material Table S1. All symptoms were elevated in participants meeting CHR criteria (all p < .019). Following conventional effect size magnitudes (small: Cohen’s d <.50; medium: d = .50 - .80, large: d >.80), effect sizes ranged from small (depression, drug use frequency, negative symptoms) to medium (state-trait anxiety, social anxiety, dissociation, mania, PROD screen) to large (PQ, PRIME screen, SIPS positive symptoms). Descriptive statistics by screening risk (high vs. low) and CHR status are shown in Supplemental Material, Table S2. High screening risk CHR- participants scored higher than low screening risk CHR- participants on all symptom measures. By contrast, CHR+ participants scored higher than high screening risk CHR- participants only on the PQ, PRIME, and SIPS positive, but not on any other symptom measures.
Bivariate correlations between symptoms are shown in Supplemental Material Table S3. All correlations were significant at p < .05 after FDR-correction except for several correlations between drug use frequency and other scales (PRIME screen, DES, and SIPS attenuated positive symptoms). The mean correlation between study variables was r = .360, suggesting a meaningful amount of shared variance.
Hierarchical Symptom Dimensions
Figure 2 shows the hierarchical factor structure of the items in the self-report scales, with lines indicating Pearson correlations across factor levels. For models with 1 through 10 factors, parallel analysis indicated eigenvalues greater than chance and all factors had at least two items loading ≥ .30. New factors introduced in the 11- and 12-factor solutions did not have two items loading ≥ .30; therefore, the hierarchical factor analysis was conducted for 1 through 10 factors. See Table 2 for the highest-loading items on each factor in the 10-factor solution; Supplemental Material, Table S4.1 through S4.10 for the full item names of the highest-loading items at all levels of the factor hierarchy; and Supplemental Material, Table S5 for the item loadings of all items in the 10-factor solution.
Figure 2.
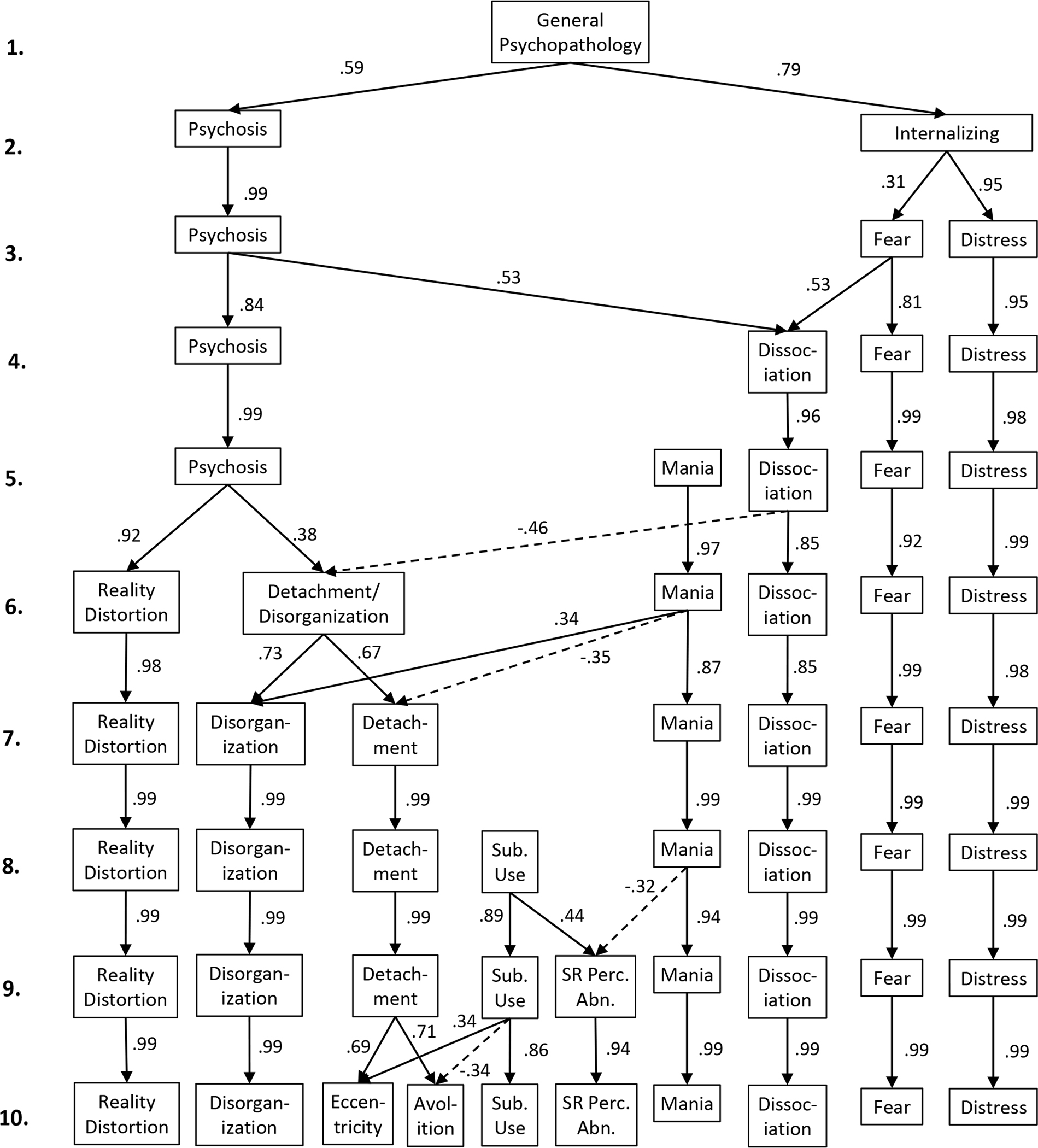
Hierarchy of self-reported symptom dimensions in the reference sample of 3460 community young adults (mage = 20.3). Factor analysis was performed for each number of factors (1 through 10) using Varimax rotation. Arrows indicate cross-level correlations (Pearson correlations between estimated factor scores). Correlations r ≤ .30 are omitted for clarity. Sub. Use. = Substance use; SR Perc. Abn. = Substance-related perceptual abnormalities.
Table 2.
Highest-Loading Items on Each Factor in the 10-Factor Solution: Abridged Item Names and Factor Loadings
| Factor | Item | Loading | Factor | Item | Loading |
|---|---|---|---|---|---|
|
| |||||
| Substance use | DUF 15: Frequency of using cocaine. | .49 | Perceptual abnormalities | PQ20: Things appear visually different. | .34 |
| DUF 20: Frequency of using PCP. | .49 | PQ50: Suddenly distracted by distant sounds. | .34 | ||
| DUF 19: Frequency of using hallucinogenics. | .47 | PQ18: Heard unusual sounds. | .32 | ||
| DUF 16: Frequency of using tranquilizers/downers. | .44 | PQ34: Felt unusually sensitive to noise. | .32 | ||
| Reality distortion | PRIME 1: Odd or unusual things going on. | .60 | Disorganization | PROD 5: Difficulty thinking clearly, interfering thoughts. | .51 |
| PRIME 8: Special natural or supernatural gifts. | .59 | PQ 10: Difficulty concentrating, listening or reading. | .50 | ||
| PRIME 9: Mind is “playing tricks” on me. | .59 | PROD 3: Bodily restlessness. | .46 | ||
| PRIME 6: I/others read minds. | .58 | PQ 1: Distracted by noises. | .44 | ||
| Eccentricity | PQ 31: Others thought I was strange. | .56 | Avolition | PQ43: Avoided social activities. | .48 |
| PQ 45: I am an odd, unusual person. | .48 | PQ21: Kept in the background on social occasions. | .47 | ||
| PQ 40: Unusual mannerisms and habits. | .47 | PQ78: Little interest getting to know other people. | .47 | ||
| PQ 90: People have found it hard to understand me. | .41 | PQ33: Nothing to say or very little to say. | .44 | ||
| Dissociation | DES 5: Not recognizing self in mirror. | .74 | Mania | GBI 4: Extreme happiness and intense energy. | .68 |
| DES 4: Standing next to and watching myself. | .74 | GBI 9: Irritable or angry. | .63 | ||
| DES 3: Don’t remember putting on clothes. | .74 | GBI 8: Periods depressed and periods manic. | .61 | ||
| DES 7: Body does not belong to me. | .72 | GBI 6: Difficulty getting to sleep. | .60 | ||
| Fear | SPS 20: Awkward and tense if people are watching. | .73 | Distress | CESD 6: I felt depressed. | .79 |
| SPS 16: Tense if people look at me in elevator. | .71 | PQ28: I felt unhappy or depressed. | .78 | ||
| SPS 13: Tense in a crowded cafeteria. | .71 | CESD 3: I could not shake off the blues. | .74 | ||
| SPS 15: Worried I might attract attention. | .69 | CESD 13: I felt sad. | .72 | ||
At level 1, a single general psychopathology dimension serves as a baseline for comparison. At level 2, psychosis and internalizing dimensions split apart. From levels 3 through 5, the structure of nonpsychotic symptoms differentiates into separate dimensions for dissociation, mania, fear, and distress, while psychosis remains a single dimension. Notably, at level 4, the new dissociation factor contains variance from both psychosis and fearful internalizing. From levels 6 through 10, psychosis splits into progressively finer-grained dimensions, with substance use appearing at level 8 and a substance-related perceptual abnormalities factor appearing at level 9.
Associations with Psychosis Risk Variables
Figure 3a shows model fit for models with clinical variables (CHR status, positive and negative attenuated psychosis symptoms) entered as dependent variables and symptom dimensions for progressively more complex levels of the factor hierarchy (factor levels 1 through 10) entered as independent variables.2 For all three clinical variables, model fit improved at more complex levels of the factor hierarchy. Overall model fit was best for positive symptoms (best-fitting model Adj. R2 = .405), followed by CHR status (best-fitting model McFadden R2 = .248), then negative symptoms (best-fitting model Adj. R2 = .173). Overall F-tests were significant in all regression models (p < .001).
Figure 3.
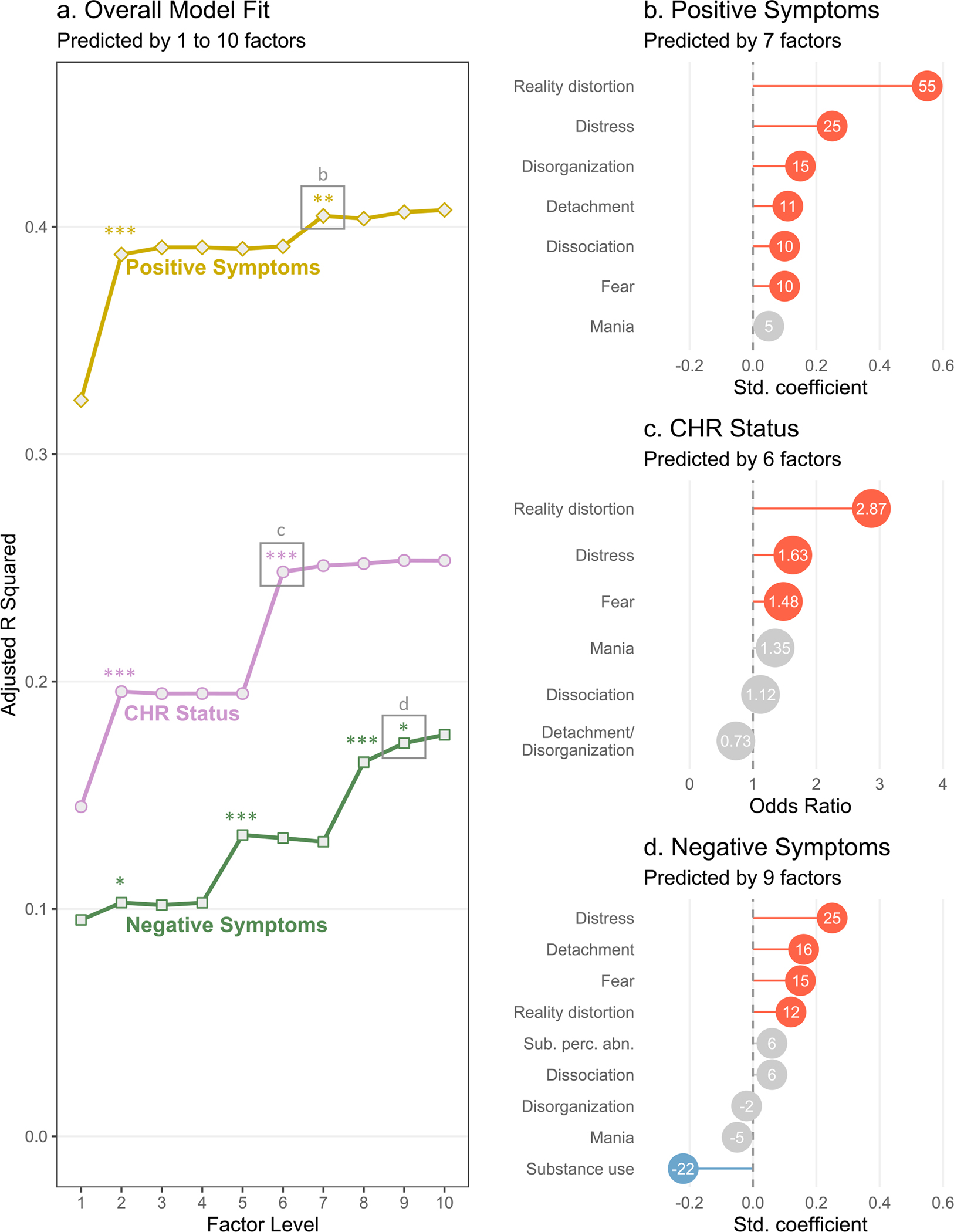
Associations between hierarchical transdiagnostic factors (see Figure 2) and interview-rated psychosis risk variables (n = 436). a. Overall fit of regression models with each psychosis risk variable entered as the dependent variable (CHR status, positive symptoms, and negative symptoms) at each level of the factor hierarchy. Note that model fit is expressed as adjusted R2 for linear regression models (positive and negative symptoms) and as McFadden R2 for logistic regression models (CHR status). All models were significant with p < .001 in all overall F tests. Significant improvements in model fit (F-tests of residual sums of squares) are shown as *p < .05, **p < .01, ***p < .001. b-d. Standardized regression coefficients or odds ratios from the most complex regression models that achieved a significant increase in model fit. Red indicates a positive coefficient (p < .05), blue indicates a negative coefficient (p < .05), and grey indicates a nonsignificant coefficient (p ≥ .05). Decimal points are omitted from linear regression coefficients for clarity. Sub. perc. abn. = substance-related perceptual abnormalities.
CHR Status
As shown in Figure 3a, model fit for CHR status significantly improved when internalizing split from psychosis (moving from level 1 to level 2 in the factor hierarchy), then improved again when psychosis split into separate dimensions for reality distortion and detachment/disorganization (level 6). There were no further improvements in model fit at more complex levels of the hierarchy.
Odds ratios from the best-fitting model for CHR status (level 6) are shown in Figure 3c. CHR status was primarily associated with reality distortion, OR = 2.87, p < .001, and somewhat associated with distress, OR = 1.63, p = .006, and fear, OR = 1.48, p = .030. CHR status was not associated with mania, dissociation, or detachment/disorganization.
Attenuated Positive Symptoms
As shown in Figure 3a, model fit for attenuated positive symptoms significantly improved when internalizing split from psychosis at factor level 2, and when disorganization split from detachment at level 7.
Standardized regression coefficients from the best-fitting model for positive symptoms (level 7) are shown in Figure 3b. Positive symptoms were most associated with reality distortion, β = .554, p < .001, followed by distress, β = .251, p < .001, disorganization, β = .149, p < .001, detachment, β = .108, p = .005, dissociation, β = .105, p = .006, and fear, β = .104, p = .007. The only factor unrelated to positive symptoms was mania.
Of note, model fit predicting positive symptoms improved marginally from level 8 to level 9 when a substance-related perceptual abnormalities factor emerged, Δ sum of squares = .039, F(1, 414) = 3.02, p = .083. Given this marginal improvement and the theoretical relevance of perceptual abnormalities in characterizing attenuated positive symptoms, we also examined the 9-factor model for positive symptoms. As shown in Supplemental Material, Figure S2, results largely resembled the 7-factor model with the addition of a significant coefficient for substance-related perceptual abnormalities, β = .151, p < .001, and a nonsignificant coefficient for substance use, β = .032, p = .406.
Attenuated Negative Symptoms
As shown in Figure 3d, model fit for attenuated negative symptoms significantly improved when internalizing split from psychosis at factor level 2, when mania appeared at level 5, when substance use appeared at level 8, and when substance-related perceptual abnormalities split from substance use at level 9. Of note, some improvements in model fit were driven by negative coefficients from mania and substance use. The improvement in model fit at level 5 coincided with a negative coefficient for mania, β = −.128, p = .006, while the improvement in model fit at level 8 coincided with a negative coefficient for substance use, β = −.186, p < .001. Note that the coefficient for mania is no longer significant in the 9-factor model shown in Figure 3d. This coefficient decreased in magnitude and became nonsignificant at lower levels of the factor hierarchy as psychotic symptom clusters absorbed some of the variance in the mania dimension (as shown in Figure 2).
Standardized regression coefficients from the best-fitting model for negative symptoms (level 9) are shown in Figure 3d. Negative symptoms were most associated with distress, β = .249, p < .001, followed by detachment, β = .163, p < .001, fear, β = .145, p = .001, and reality distortion, β = .115, p = .011. Negative symptoms were inversely associated with substance use, β = −.224, p < .001, and were not associated with substance-related perceptual abnormalities, dissociation, disorganization, or mania.
Discussion
Consistent with previous findings (Cowan & Mittal, 2021), this new sample of CHR youth reported broad psychopathology across every measured symptom category. A hierarchical unfolding factor analysis modeled this complex comorbidity picture by defining data-driven symptom dimensions at multiple levels of analysis. Ten progressively finer-grained factors unfolded in the data, from general psychopathological distress to specific symptom clusters for various kinds of psychotic and nonpsychotic psychopathology. This analysis revealed complex relationships between different kinds of symptoms in a large community youth sample—showing, for example, that dissociation, mania, and substance use each related to different aspects of psychosis. Moreover, the symptom dimensions provided the framework for a transdiagnostic phenotypic profile of CHR symptomatology which supplied important insights into characterizing attenuated psychotic symptoms, differentiating attenuated psychotic symptoms from mood symptoms, integrating psychosis risk symptom data into current theoretical models of psychopathology, improving the efficiency of psychosis risk assessment, and enhancing step-based care for individuals meeting CHR criteria.
A Transdiagnostic Perspective on Attenuated Positive Symptoms and CHR Status
Clinician-rated attenuated positive symptoms were related to all psychotic and internalizing dimensions in the 7-factor model. Notably, distressed internalizing accounted for the second-most variance after reality distortion, implicating distress as a factor directly impacting attenuated positive psychotic symptoms. Transdiagnostic dimensions also provided a new perspective on attenuated negative symptoms, which are consistently shown to predict transition to psychosis in CHR samples (Alderman et al., 2015; Cowan & Mittal, 2021; Metzler et al., 2016; Oliver et al., 2020; Piskulic et al., 2012). Negative symptoms were most related to internalizing dimensions (distress and fear), psychotic dimensions (detachment and reality distortion), and the absence of substance use. Substance use may have been less likely to co-occur with negative symptoms due to the social context of substance use. Youth substance use primarily occurs in groups of socially motivated peers (Dumas et al., 2020; Kuntsche et al., 2005). Youth experiencing significant negative symptoms would be less likely to participate in these social group contexts, possibly resulting in less access and/or less social motivation to use substances.
Internalizing and negative symptoms are not easily distinguishable in the early clinical stages of risk for serious mental illness (Addington et al., 2019; Azar et al., 2018; Cowan et al., 2019; Cowan & Mittal, 2021). This study identified distinct self-reported symptom factors for distressed internalizing and detachment, providing an inroad into differentiating these symptoms in CHR samples (although see “Measurement Approach” below for a discussion of potential limits on self-report of negative symptoms). Notably, these findings suggest that differential diagnosis of internalizing vs. negative symptoms can be improved using the explanatory power of symptoms that are unlikely to co-occur with attenuated negative symptoms—namely, mania and substance use. If individuals experiencing mania or substance use are less likely to experience negative symptoms, then it is more likely that any affective or volitional disturbances in these individuals would be better explained by internalizing rather than negative symptoms. Speculatively, this argument suggests that it may be possible to identify transdiagnostic risk profiles within the CHR syndrome. Individuals presenting with positive symptoms, hypomania, and/or substance use may be at risk for affective or stress-reactive psychosis (Myin-Germeys & van Os, 2007), whereas individuals presenting with positive and negative symptoms may be at risk for anhedonic or deficit-syndrome psychosis (Kirkpatrick & Buchanan, 1990).
With respect to CHR status, comorbidity patterns were not obvious when symptoms were modeled as questionnaire mean scores (see Supplemental Material Table S1 and S2). CHR+ participants scored higher than CHR- participants on all symptom measures. When screening risk was taken into account, CHR+ participants scored higher than high screening risk CHR- participants only on a few measures tapping positive symptoms (PQ, PRIME, and SIPS positive). However, when symptoms were modeled through latent transdiagnostic dimensions, clear and specific associations emerged between CHR status and internalizing symptoms.
In the prevailing structured clinical interviews for detecting psychosis risk syndromes (Miller et al., 1999; Yung et al., 2005), CHR status is largely determined by the presence of positive symptoms. Genetic, neuropsychological, social, and environmental evidence support a fully dimensional model of psychosis in which a single psychosis continuum spans the entire general population, with severity of psychotic-like experiences differentiating normative experience from attenuated psychotic symptoms and clinical psychotic symptoms (Cowan & Mittal, 2020; DeRosse & Karlsgodt, 2015; Johns & van Os, 2001; Nelson et al., 2013). A determination of CHR status is in a sense a decision about where a given individual falls in the population distribution of positive psychotic-like experiences. In other words, CHR status can be seen as a dichotomization of a continuous variable. Dichotomous variables tend to be less precise and less statistically powerful than continuous variables, which may explain the weaker observed effects for CHR status in the present study.
Based on current diagnostic criteria for CHR status, which do not include attenuated negative symptoms, attenuated positive symptom severity is probably the best dimensional indicator of CHR symptomatology. The current study showed that attenuated positive symptom severity was a transdiagnostic factor linked to various psychotic and nonpsychotic self-reported symptom dimensions. If future diagnostic criteria for CHR status were to include negative symptoms, then an appropriately weighted composite of standardized positive and negative symptom severity would be a more appropriate choice for a dimensional indicator of CHR symptomatology.
Insights for Transdiagnostic Models
The symptom dimensions identified in this study provide further information on the placement of dissociation, mania, substance use, perceptual abnormalities, and disorganization in HiTOP.
Dissociation
Although transdiagnostic evidence on dissociative symptoms is limited (Kotov et al., 2021), these symptoms have been linked to internalizing, externalizing, and psychosis (Ellickson-Larew et al., 2020). In HiTOP, dissociation has been provisionally grouped with the thought disorder subfactor of psychosis pending further structural evidence (Kotov et al., 2020). In the present study, dissociation bridged fearful internalizing and psychosis factors, providing further evidence for dissociation’s relevance across HiTOP superspectra. Furthermore, dissociation related to positive but not negative symptoms, indicated by two pieces of evidence. First, dissociation positively correlated with the higher-order psychosis factor and negatively correlated with the lower-order detachment/disorganization factor. Second, dissociation had a small independent relationship with interview-rated positive symptoms (Fig 2b). These findings are consistent with prior empirical work (Longden et al., 2020) and reinforce dissociation’s links to the HiTOP thought disorder subfactor (Kotov et al., 2020). In sum, the current findings support dissociation’s placement as a transdiagnostic factor associated with multiple psychopathology spectra while also reinforcing dissociation’s links to positive psychotic symptoms within the psychosis spectrum.
Mania
Mania is another dimension whose placement in transdiagnostic models is not yet settled. Mania has been linked to both the internalizing and psychosis spectra, and several placements have been proposed relative to these two spectra. Mania may fit within internalizing or psychosis, blend elements of both, or form its own independent dimension (Kotov et al., 2020, 2021). Building on known relationships between mania and psychosis, our previous study in a smaller CHR sample linked hypomania to more positive symptoms but less negative symptoms (Cowan & Mittal, 2021). Because the hierarchical model in the current study was defined based on a community sample, the mania dimension likely captures hypomania more than true manic symptoms. This mania dimension appeared as an independent factor in the unfolding factor analysis, not splitting off from psychosis or internalizing. It also had a positive cross-level correlation with disorganization and negative cross-level correlations with detachment and substance-related perceptual abnormalities. Mania’s negative association with detachment is noteworthy because mania was also associated with lower levels of attenuated negative symptoms in the clinical interview (see Results: Attenuated Negative Symptoms above). In sum, in the current study, hypomania appeared to be an independent dimension that co-occurred with higher levels of disorganization and lower levels of detachment.
Substance Use and Perceptual Abnormalities
A substance-related perceptual abnormalities factor appeared with a cross-level correlation with substance use and items cross-loading from reality distortion. The substances with highest loadings on the substance use factor included PCP and hallucinogens (see Table 2), whose active effects include perceptual abnormalities. The substance-related perceptual abnormalities factor had highest loading items from the Prodromal Questionnaire, which asks participants not to include experiences while using alcohol or substances (Loewy et al., 2005). However, many participants may not read and follow survey instructions (Oppenheimer et al., 2009; Ramsey et al., 2016), and some participants in the current study likely included perceptual abnormalities experienced while intoxicated. Nevertheless, substance-related perceptual abnormalities also had an independent positive relationship with interview-rated attenuated positive symptoms, β = .151, p < .001, in the 9-factor positive symptoms model (see Supplemental Material, Figure S2). SIPS interviewers are trained not to rate perceptual abnormalities experienced while intoxicated, so there may in fact be an empirical association between substance-related perceptual abnormalities and overall attenuated positive symptoms. Future studies that can fully disambiguate perceptual abnormalities experienced while using vs. not using substances would be valuable to explore this relationship in more detail.
Additionally, there were other potential links between substance use and CHR symptomatology. Individuals meeting CHR criteria were more likely to use non-alcohol substances (although note that the majority—57% for cannabis and 63% for other substances—reported never using these substances). This is in line with research showing high rates of substance use in CHR samples and suggesting substance use as a risk factor for conversion to a psychotic disorder (Addington et al., 2014). Externalizing psychopathology more broadly—which includes disordered substance use (Kotov et al., 2021)—also appears to be a risk factor for future psychotic disorders (Gin et al., 2021). Interestingly, despite the elevated levels of substance use observed in the current study’s CHR sample, substance use was associated with lower levels of interview-rated negative symptoms and also differentiated lower-order detachment symptoms (eccentricity vs. avolition). Speculatively, this pattern of evidence suggests that substance use may differentiate two subgroups within the CHR syndrome: one subgroup with higher levels of substance use, more perceptual abnormalities, more eccentricity, and a less severe negative symptom profile; and another subgroup with lower levels of substance use, fewer perceptual abnormalities, and a more severe negative symptom profile marked by asociality and avolition. In future studies, subgroup analyses within CHR samples could explore this possibility.
Disorganization
Interestingly, the factor we labelled “disorganization” included items tapping into distractibility and inattention. We chose to label this factor “disorganization” rather than “inattention” to recognize its placement in relation to the broad psychosis factor and the detachment factor. However, this factor was only a minor contributor to clinician-rated psychosis risk variables, relating to attenuated positive symptoms, β = .149, p < .001, but not CHR status or attenuated negative symptoms. Further research is warranted on the potential for self-report scales to detect subclinical disorganization symptoms.
Practical and Theoretical Implications
Practically, a transdiagnostic model of psychosis risk can potentially save time and effort by making assessment more efficient in two ways. First, rather than independently assessing for each categorical diagnosis, a researcher or clinician can assess a single dimension that captures the overlapping features of these diagnoses. For instance, the current study showed that the distress dimension related to both positive and negative symptoms. Various DSM-5 diagnoses are consistent with a high loading on distress, including Major Depressive Disorder, Persistent Depressive Disorder, Generalized Anxiety Disorder, Posttraumatic Stress Disorder, Insomnia Disorder, Borderline Personality Disorder, and probably Bipolar Disorder (Kotov et al., 2021). Rather than assessing all DSM-5 categorical diagnoses, researchers may be able to focus on a handful of dimensions. Second, clinicians and researchers can take advantage of the hierarchical nature of HiTOP to gain further efficiency. In some contexts, a fine-grained assessment of externalizing subdimensions may be important, but in other cases simply assessing general externalizing symptoms might be sufficient.
The current study builds upon these general advantages of HiTOP through identifying dimensions specifically relevant to psychosis risk. In particular, the HiTOP subfactors of reality distortion, detachment, distress, fear, and mania captured the most relevant symptom variance in the present study. These symptom dimensions come from both internalizing and psychosis super spectra, suggesting a broad assessment of psychopathology dimensions can inform case conceptualization. For example, the same positive symptom scores on the SIPS may be interpreted differently if they are accompanied by associated features of mania and substance use in one case vs. distress and detachment in another. In practice, CHR individuals are typically identified when attenuated positive symptoms become concerning to the individual or their family members. Based on the current study, attenuated positive symptoms can be conceptualized as transdiagnostic expressions of interactions between multiple latent psychopathology dimensions. Practically, attenuated positive symptoms are therefore a complex presenting problem warranting comprehensive transdiagnostic assessment (for instance, using the HiTOP-DAT digital tool; Jonas et al., 2021). Based on comprehensive assessment, an individualized phenotypic symptom profile can guide case conceptualization and treatment. Future research defining subtypes of CHR symptom presentation based on co-occurring psychopathology dimensions would be highly useful in this respect.
Theoretically, a hierarchical transdiagnostic model of psychosis risk also allows results from hard-to-reach CHR samples to be integrated into the HiTOP framework, leveraging the growing body of knowledge on transdiagnostic psychopathology (e.g., Kotov et al., 2020, 2021) to deepen our understanding of psychosis risk states. The current study was cross-sectional and does not provide any evidence on longitudinal changes in symptoms. Hierarchical approaches, however, may allow for more precise modeling of symptom expression in difference clinical stages. Early stages of incipient psychopathology are characterized by relatively nonspecific indicators of psychological distress, which can include psychotic-like experiences as well as other symptoms (Addington et al., 2019; McGorry et al., 2018). In later clinical stages, symptoms crystalize into primary disorders. This hypothesis is difficult to test in the DSM framework, as all diagnoses are defined at the same level of specificity. By contrast, from a hierarchical transdiagnostic perspective, an individual’s symptom profile might initially show disturbances in broad dimensions in earlier stages which crystalize into more specific symptom clusters in later stages. One study has found that dimensional measures of psychopathology are already elevated at age seven in the children of adults with schizophrenia or bipolar disorder (Ellersgaard et al., 2018), highlighting the potential of dimensional approaches to inform clinical staging models. The HiTOP model therefore may facilitate more rigorous tests of clinical staging models of the development of mental illness, through longitudinal studies or cross-sectional studies of individuals in different clinical stages.
The current results also support step-based treatment suggested by clinical staging models (Hartmann et al., 2021; McGorry et al., 2018; Scott et al., 2013). Transdiagnostic treatments such as the Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (Barlow et al., 2010) likewise may be most effective for broad symptom dimensions while specialized treatments such as Cognitive Behavioral Therapy (CBT) for psychosis risk (Gaag et al., 2013) may be most effective for specific symptom dimensions.
Measurement Approach
In the current study, symptoms were measured on a variety of time scales ranging from past week to past year. The time scale instructions matched those commonly used for each measure, to keep findings as consistent as possible with how these measures are used in research and clinical practice. Notably, keeping the original time scales of the three psychosis screeners preserved the validity of their established screening cutoffs. However, differing time scales could introduce an additional source of variance. It is currently unknown on what time scales state symptom measures begin to approximate trait measures (DeYoung et al., 2022). While state and trait measures often show differing results in schizophrenia (e.g., in state vs. trait anhedonia, Cowan et al., 2020; Dickinson et al., 2018; Strauss & Gold, 2012), past-week measures already capture considerably more trait-like variance than true state measures (DeYoung et al., 2022). For instance, past-week and entire-lifetime measures of depression in schizophrenia correlate with r > .60 (Chiappelli et al., 2014). While there would still have been some value in harmonizing the time frames of assessment to improve the current study’s measurement precision (e.g., changing all instructions to “past month” to match the clinician-rated variables), this approach would have come with the downsides of making results less compatible with prior research using the same measures.
Symptoms were also assessed by different modalities in the reference sample (self-report) and the interview subsample (clinician-rated interviews). By examining outcomes assessed in a different modality, the current study minimizes common method variance which could inflate estimated relationships between symptom dimensions and clinician-rated outcomes (Podsakoff et al., 2003). Clinician-rated outcomes were also rated on an intermediate time scale (past month), which would be expected to relate similarly to shorter (e.g., past week) and longer (e.g., past year) self-report timescales. At the same time, there may be important nuances to the use of self-report and clinician-rated assessments in the current study. Notably, self-report items tapping anhedonic or blunted affect aspects of negative symptoms (e.g., PQ 89: “I have felt uninterested in the things I used to enjoy”; PQ 82: “I have left like…I was no longer able to feel sadness, or…I could not feel happy”) loaded onto the distress factor rather than the detachment factor or its subfactors (see Supplemental Material, Table S5). These items correspond to those forming a “dissociative/depressive” factor in a recent exploratory factor analysis of PQ negative symptom items (Pierce et al., 2021). In that study, the dissociative/depressive factor correlated strongly (r ≥ .62) with depression and anxiety.
These converging findings could reflect issues with measuring negative symptoms—that is, that available self-report measures identify some negative symptom factors (e.g., asociality) more cleanly than others (e.g., anhedonia, blunted affect). Alternatively, these findings could indicate that distressed internalizing and anhedonia/blunted affect cannot be distinguished in community youth samples, perhaps due to phenotypic overlap between distress and negative symptoms in the early clinical stages of developing psychopathology. Future research including more targeted measures of negative symptoms (e.g., Self-Evaluation of Negative Symptoms scale) could shed light on these issues. Similarly, insight or symptom severity may also affect young people’s ability to accurately report on their own symptoms. Future research could explore this possibility by including measures of cognitive or clinical insight.
Strengths and Limitations
This study’s strengths included its large sample size in the reference group, which allowed for a complex data-driven hierarchy of symptom dimensions without sacrificing statistical power; its high-resolution assessment of psychotic-like experiences; and its validation of self-reported symptom dimensions by clinical interviews. The study also had some limitations. Participants were recruited from large metropolitan areas in the central and eastern United States, leaving the possibility that results would not generalize to youth participants recruited in different contexts. Mitigating this concern, SIPS positive symptom scores for CHR participants in this study (mean total score = 9.68, SD = 3.30) were typical of SIPS scores for CHR samples recruited across many contexts in 38 studies worldwide (median total score = 9.9, interquartile range = 9.0 – 11.6) (Woods et al., 2019). Another potential limitation concerns sample size in the CHR+ subgroup. While the overall study leveraged structural findings from a large reference group, future studies with larger CHR sample sizes would be required to examine hierarchical symptom dimensions within a pure CHR sample. Externalizing symptoms were also underrepresented in the symptom questionnaires, with coverage limited to substance use. It is unclear, for example, whether the link between substance use and lower negative symptoms reflects a unique effect for substance use, or a shared effect for externalizing symptoms more broadly. Similarly, there were potential confounds with perceptual abnormalities experienced while intoxicated (see “Substance use and perceptual abnormalities” above). This study also did not assess cognitive deficits, which have been shown to relate to detachment and disorganization symptom dimensions in psychotic disorders (Kotov et al., 2020). Further research linking cognitive deficits to hierarchical symptom dimensions in CHR would be valuable. Finally, this study was cross-sectional rather than longitudinal, which did not allow analyses of participants in distinct clinical stages or participants who went on to develop psychotic disorders.
Conclusion
Psychosis risk symptoms were revealed to be transdiagnostic constructs when mapped onto hierarchical symptom dimensions. These data allow new evidence from CHR samples to be integrated into the larger picture of psychiatric comorbidity. Continued research can build on these results to explore the relative contribution of various symptom dimensions to psychosis risk; identity subgroups in psychosis risk samples with different phenotypic symptom profiles; and implement transdiagnostic assessment and treatment strategies for individuals at risk for psychosis.
Supplementary Material
Funding
This work was supported in part by the Canadian Institutes of Health Research (DFS-152268 to HRC) and by the National Institutes of Health (R01 MH118545, R01 MH120091, R01 MH112613 to LME; R01 MH112612 to JS; and R01 MH112545, R01 MH116039 and R01 MH120088 to VAM).
Footnotes
These individuals are also commonly described as having an at-risk mental state (ARMS), when they are diagnosed using a different clinical interview (the CAARMS) with very similar diagnostic criteria to those used in the current study (Fusar-Poli et al., 2016). For simplicity, this study uses CHR terminology throughout.
Variance explained is shown as adjusted R2, which is adjusted downward according to the degrees of freedom in the model. Because of this adjustment, adjusted R2 can decrease when new variables are added to a regression model if the adjustment for lost degrees of freedom outweighs a marginal improvement in R2.
Conflicts of Interest
The authors declare that there were no conflicts of interest with respect to the authorship or the publication of this article.
Supplemental Material
A supplemental material file has been provided to be posted alongside this manuscript.
References
- Addington J, Case N, Saleem MM, Auther AM, Cornblatt BA, & Cadenhead KS (2014). Substance use in clinical high risk for psychosis: A review of the literature. Early Intervention in Psychiatry, 8(2), 104–112. 10.1111/eip.12100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Epstein I, Reynolds A, Furimsky I, Rudy L, Mancini B, McMillan S, Kirsopp D, & Zipursky RB (2008). Early detection of psychosis: Finding those at clinical high risk. Early Intervention in Psychiatry, 2(3), 147–153. 10.1111/j.1751-7893.2008.00078.x [DOI] [PubMed] [Google Scholar]
- Addington J, Liu L, Goldstein BI, Wang J, Kennedy SH, Bray S, Lebel C, Stowkowy J, & MacQueen G (2019). Clinical staging for youth at-risk for serious mental illness. Early Intervention in Psychiatry, 13(6), 1416–1423. 10.1111/eip.12786 [DOI] [PubMed] [Google Scholar]
- Addington J, Piskulic D, Liu L, Lockwood J, Cadenhead KS, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Bearden CE, Mathalon DH, & Woods SW (2017). Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophrenia Research, 190, 90–95. 10.1016/j.schres.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert U, Tomassi S, Maina G, & Tosato S (2018). Prevalence of non-psychotic disorders in ultra-high risk individuals and transition to psychosis: A systematic review. Psychiatry Research, 270, 1–12. 10.1016/j.psychres.2018.09.028 [DOI] [PubMed] [Google Scholar]
- Alderman T, Addington J, Bearden C, Cannon TD, Cornblatt BA, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, & Cadenhead KS (2015). Negative symptoms and impaired social functioning predict later psychosis in Latino youth at clinical high risk in the North American prodromal longitudinal studies consortium. Early Intervention in Psychiatry, 9(6), 467–475. 10.1111/eip.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistican Manual of Mental Disorders (5th ed.). American Psychiatric Association Press. 10.1176/appi.books.9780890425596.UseofDSM5 [DOI] [Google Scholar]
- Andreasen NC (1993). Diagnosis and Classification of Schizophrenia. Schizophrenia Bulletin, 19(2), 199. 10.1093/schbul/19.2.199 [DOI] [PubMed] [Google Scholar]
- Azar M, Pruessner M, Baer LH, Iyer S, Malla AK, & Lepage M (2018). A study on negative and depressive symptom prevalence in individuals at ultra-high risk for psychosis. Early Intervention in Psychiatry, 12(5), 900–906. 10.1111/eip.12386 [DOI] [PubMed] [Google Scholar]
- Barlow DH, Ellard KK, Fairholme CP, Farchione TJ, Boisseau CL, Allen LB, & Ehrenreich-May JT (2010). The Unified Protocol for Transdiagnostic Treatment of Emotional Disorders. Oxford University Press. [Google Scholar]
- Bernstein EM, & Putnam FW (1986). Development, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease, 174(12), 727–735. 10.1097/00005053-198612000-00004 [DOI] [PubMed] [Google Scholar]
- Bieling PJ, Antony MM, & Swinson RP (1998). The State-Trait Anxiety Inventory, Trait version: Structure and content re-examined. Behaviour Research and Therapy, 36(7–8), 777–788. 10.1016/s0005-7967(98)00023-0 [DOI] [PubMed] [Google Scholar]
- Boldrini T, Tanzilli A, Pontillo M, Chirumbolo A, Vicari S, & Lingiardi V (2019). Comorbid Personality Disorders in Individuals With an At-Risk Mental State for Psychosis: A Meta-Analytic Review. Frontiers in Psychiatry, 10, 429. 10.3389/fpsyt.2019.00429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli J, Nugent KL, Thangavelu K, Searcy K, & Hong LE (2014). Assessment of Trait and State Aspects of Depression in Schizophrenia. Schizophrenia Bulletin, 40(1), 132–142. 10.1093/schbul/sbt069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Frederickson AM, Richter JJ, Auther AM, Smith CW, Kane JM, & Cornblatt BA (2007). Differentiation in the preonset phases of schizophrenia and mood disorders: Evidence in support of a bipolar mania prodrome. Schizophrenia Bulletin, 33(3), 703–714. 10.1093/schbul/sbm028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan HR, McAdams DP, & Mittal VA (2019). Core beliefs in healthy youth and youth at ultra high-risk for psychosis: Dimensionality and links to depression, anxiety, and attenuated psychotic symptoms. Development and Psychopathology, 31(1), 379–392. 10.1017/S0954579417001912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan HR, & Mittal VA (2020). Three types of psychotic-like experiences in youth at clinical high risk for psychosis. European Archives of Psychiatry and Clinical Neuroscience, 271, 733–744. 10.1007/s00406-020-01143-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan HR, & Mittal VA (2021). Transdiagnostic Dimensions of Psychiatric Comorbidity in Individuals at Clinical High Risk for Psychosis: A Preliminary Study Informed by HiTOP. Frontiers in Psychiatry, 11, 614710. 10.3389/fpsyt.2020.614710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan HR, Mittal VA, Allen DN, Gold JM, & Strauss GP (2020). Heterogeneity of emotional experience in schizophrenia: Trait affect profiles predict clinical presentation and functional outcome. Journal of Abnormal Psychology, 129(7), 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosse P, & Karlsgodt KH (2015). Examining the Psychosis Continuum. Current Behavioral Neuroscience Reports, 2(2), 80–89. 10.1007/s40473-015-0040-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Chmielewski M, Clark LA, Condon DM, Kotov R, Krueger RF, Lynam DR, Markon KE, Miller JD, Mullins-Sweatt SN, Samuel DB, Sellbom M, South SC, Thomas KM, Watson D, Watts AL, Widiger TA, Wright AGC, & Workgroup, the H. N. P. (2022). The distinction between symptoms and traits in the Hierarchical Taxonomy of Psychopathology (HiTOP). Journal of Personality, 90(1), 20–33. 10.1111/jopy.12593 [DOI] [PubMed] [Google Scholar]
- Dickinson D, Pratt DN, Giangrande EJ, Grunnagle M, Orel J, Weinberger DR, Callicott JH, & Berman KF (2018). Attacking Heterogeneity in Schizophrenia by Deriving Clinical Subgroups From Widely Available Symptom Data. Schizophrenia Bulletin, 44(1), 101–113. 10.1093/schbul/sbx039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TM, Ellis W, & Litt DM (2020). What Does Adolescent Substance Use Look Like During the COVID-19 Pandemic? Examining Changes in Frequency, Social Contexts, and Pandemic-Related Predictors. Journal of Adolescent Health, 67(3), 354–361. 10.1016/j.jadohealth.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellersgaard D, Jessica Plessen K, Richardt Jepsen J, Soeborg Spang K, Hemager N, Klee Burton B, Jerlang Christiani C, Gregersen M, Søndergaard A, Uddin MJ, Poulsen G, Greve A, Gantriis D, Mors O, Nordentoft M, & Elgaard Thorup AA (2018). Psychopathology in 7-year-old children with familial high risk of developing schizophrenia spectrum psychosis or bipolar disorder – The Danish High Risk and Resilience Study—VIA 7, a population-based cohort study. World Psychiatry, 17(2), 210–219. 10.1002/wps.20527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson-Larew S, Stasik-O’Brien SM, Stanton K, & Watson D (2020). Dissociation as a multidimensional transdiagnostic symptom. Psychology of Consciousness: Theory, Research, and Practice, 7(2), 126–150. 10.1037/cns0000218 [DOI] [Google Scholar]
- Ellman LM, Schiffman J, & Mittal VA (2020). Community Psychosis Risk Screening: An Instrument Development Investigation. Journal of Psychiatry and Brain Science, 5, e200019. 10.20900/jpbs.20200019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C, & Bandalos D (2001). The Relative Performance of Full Information Maximum Likelihood Estimation for Missing Data in Structural Equation Models. Structural Equation Modeling: A Multidisciplinary Journal, 8(3), 430–457. 10.1207/S15328007SEM0803_5 [DOI] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, Keshavan M, Wood S, Ruhrmann S, Seidman LJ, Valmaggia L, Cannon T, Velthorst E, Haan LD, Cornblatt B, Bonoldi I, Birchwood M, McGlashan T, Carpenter W, … Yung A (2013). The Psychosis High-Risk State: A Comprehensive State-of-the-Art Review. JAMA Psychiatry, 70(1), 107–120. 10.1001/jamapsychiatry.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Rutigliano G, Lee TY, Beverly Q, Bonoldi I, Lelli J, Kaar SJ, Gago E, Rocchetti M, Patel R, Bhavsar V, Tognin S, Badger S, Calem M, Lim K, Kwon JS, Perez J, & McGuire P (2016). Towards a Standard Psychometric Diagnostic Interview for Subjects at Ultra High Risk of Psychosis: CAARMS versus SIPS [Research Article]. Psychiatry Journal; Hindawi. 10.1155/2016/7146341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Nelson B, Valmaggia LR, Yung AR, & McGuire PK (2014). Comorbid Depressive and Anxiety Disorders in 509 Individuals With an At-Risk Mental State: Impact on Psychopathology and Transition to Psychosis. Schizophrenia Bulletin. 10.1093/schbul/sbs136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaag M. van der, Nieman D, & Berg D. van den. (2013). CBT for Those at Risk of a First Episode Psychosis: Evidence-based psychotherapy for people with an “At Risk Mental State.” Routledge. 10.4324/9780203503478 [DOI] [Google Scholar]
- Gibson LE, Anglin DM, Klugman JT, Reeves LE, Fineberg AM, Maxwell SD, Kerns CM, & Ellman LM (2014). Stress sensitivity mediates the relationship between traumatic life events and attenuated positive psychotic symptoms differentially by gender in a college population sample. Journal of Psychiatric Research, 53, 111–118. 10.1016/j.jpsychires.2014.02.020 [DOI] [PubMed] [Google Scholar]
- Gibson LE, Reeves LE, Cooper S, Olino TM, & Ellman LM (2019). Traumatic life event exposure and psychotic-like experiences: A multiple mediation model of cognitive-based mechanisms. Schizophrenia Research, 205, 15–22. 10.1016/j.schres.2018.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gin K, Stewart C, & Jolley S (2021). A systematic literature review of childhood externalizing psychopathology and later psychotic symptoms. Clinical Psychology & Psychotherapy, 28, 56–78. 10.1002/cpp.2493 [DOI] [PubMed] [Google Scholar]
- Goldberg LR (2006). Doing it all Bass-Ackwards: The development of hierarchical factor structures from the top down. Journal of Research in Personality, 40(4), 347–358. 10.1016/j.jrp.2006.01.001 [DOI] [Google Scholar]
- Hartmann JA, McGorry PD, Destree L, Amminger GP, Chanen AM, Davey CG, Ghieh R, Polari A, Ratheesh A, Yuen HP, & Nelson B (2021). Pluripotential Risk and Clinical Staging: Theoretical Considerations and Preliminary Data From a Transdiagnostic Risk Identification Approach. Frontiers in Psychiatry, 11. 10.3389/fpsyt.2020.553578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinimaa M, Salokangas RKR, Ristkari T, Plathin M, Huttunen J, Ilonen T, Suomela T, Korkeila J, & Mcglashan TH (2003). PROD-screen – a screen for prodromal symptoms of psychosis. International Journal of Methods in Psychiatric Research, 12(2), 92–104. 10.1002/mpr.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LC, & van Os J (2001). The continuity of psychotic experiences in the general population. Clinical Psychology Review, 21(8), 1125–1141. 10.1016/S0272-7358(01)00103-9 [DOI] [PubMed] [Google Scholar]
- Jonas K, Stanton K, Simms L, Mullins-Sweatt SN, Gillett D, Dainer E, Nelson B, Cohn JR, Guillot ST, & Kotov R (2021). HiTOP Digital Assessment and Tracker (HiTOP-DAT) Manual. OSF. 10.17605/OSF.IO/8HNGD [DOI] [Google Scholar]
- Kim H, & Eaton NR (2015). The hierarchical structure of common mental disorders: Connecting multiple levels of comorbidity, bifactor models, and predictive validity. Journal of Abnormal Psychology, 124(4), 1064–1078. 10.1037/abn0000113 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, & Buchanan RW (1990). Anhedonia and the deficit syndrome of schizophrenia. Psychiatry Research, 31(1), 25–30. 10.1016/0165-1781(90)90105-E [DOI] [PubMed] [Google Scholar]
- Kline E, Wilson C, Ereshefsky S, Denenny D, Thompson E, Pitts SC, Bussell K, Reeves G, & Schiffman J (2012). Psychosis risk screening in youth: A validation study of three self-report measures of attenuated psychosis symptoms. Schizophrenia Research, 141(1), 72–77. 10.1016/j.schres.2012.07.022 [DOI] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, & Cornoni-Huntley J (1993). Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. Journal of Aging and Health, 5(2), 179–193. 10.1177/089826439300500202 [DOI] [PubMed] [Google Scholar]
- Kotov R, Jonas KG, Carpenter WT, Dretsch MN, Eaton NR, Forbes MK, Forbush KT, Hobbs K, Reininghaus U, Slade T, South SC, Sunderland M, Waszczuk MA, Widiger TA, Wright AGC, Zald DH, Krueger RF, & Watson D (2020). Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry, 19(2), 151–172. 10.1002/wps.20730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Jonas KG, Lian W, Docherty AR, & Carpenter WT (2022). Reconceptualizing schizophrenia in the Hierarchical Taxonomy Of Psychopathology (HiTOP). Schizophrenia Research. 10.1016/j.schres.2022.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, Brown TA, Carpenter WT, Caspi A, Clark LA, Eaton NR, Forbes MK, Forbush KT, Goldberg D, Hasin D, Hyman SE, Ivanova MY, Lynam DR, Markon K, … Zimmerman M (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126(4), 454–477. 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Cicero DC, Conway CC, DeYoung CG, Eaton NR, Forbes MK, Hallquist MN, Latzman RD, Mullins-Sweatt SN, Ruggero CJ, Simms LJ, Waldman ID, Waszczuk MA, & Wright AGC (2021). The Hierarchical Taxonomy of Psychopathology (HiTOP): A Quantitative Nosology Based on Consensus of Evidence. Annual Review of Clinical Psychology, 17(1), null. 10.1146/annurev-clinpsy-081219-093304 [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, & Engels R (2005). Why do young people drink? A review of drinking motives. Clinical Psychology Review, 25(7), 841–861. 10.1016/j.cpr.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Lim J, Rekhi G, Rapisarda A, Lam M, Kraus M, Keefe RSE, & Lee J (2015). Impact of psychiatric comorbidity in individuals at Ultra High Risk of psychosis—Findings from the Longitudinal Youth at Risk Study (LYRIKS). Schizophrenia Research, 164(1), 8–14. 10.1016/j.schres.2015.03.007 [DOI] [PubMed] [Google Scholar]
- Loewy RL, Bearden CE, Johnson JK, Raine A, & Cannon TD (2005). The prodromal questionnaire (PQ): Preliminary validation of a self-report screening measure for prodromal and psychotic syndromes. Schizophrenia Research, 77(2–3), 141–149. 10.1016/j.schres.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Loewy RL, Johnson JK, & Cannon TD (2007). Self-report of attenuated psychotic experiences in a college population. Schizophrenia Research, 93(1–3), 144–151. 10.1016/j.schres.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden E, Branitsky A, Moskowitz A, Berry K, Bucci S, & Varese F (2020). The Relationship Between Dissociation and Symptoms of Psychosis: A Meta-analysis. Schizophrenia Bulletin, 46(5), 1104–1113. 10.1093/schbul/sbaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, & Clarke JC (1998). Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy, 36(4), 455–470. 10.1016/S0005-7967(97)10031-6 [DOI] [PubMed] [Google Scholar]
- McGorry PD, Hartmann JA, Spooner R, & Nelson B (2018). Beyond the “at risk mental state” concept: Transitioning to transdiagnostic psychiatry. World Psychiatry, 17(2), 133–142. 10.1002/wps.20514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler S, Dvorsky D, Wyss C, Nordt C, Walitza S, Heekeren K, Rössler W, & Theodoridou A (2016). Neurocognition in help-seeking individuals at risk for psychosis: Prediction of outcome after 24 months. Psychiatry Research, 246, 188–194. 10.1016/j.psychres.2016.08.065 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, Hoffman R, & Davidson L (1999). Symptom Assessment in Schizophrenic Prodromal States. Psychiatric Quarterly, 70(4), 273–287. 10.1023/A:1022034115078 [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, & van Os J (2007). Stress-reactivity in psychosis: Evidence for an affective pathway to psychosis. Clinical Psychology Review, 27(4), 409–424. 10.1016/j.cpr.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Nassiri V, Lovik A, Molenberghs G, & Verbeke G (2018). On using multiple imputation for exploratory factor analysis of incomplete data. Behavior Research Methods, 50(2), 501–517. 10.3758/s13428-017-1013-4 [DOI] [PubMed] [Google Scholar]
- Nelson MT, Seal ML, Pantelis C, & Phillips LJ (2013). Evidence of a dimensional relationship between schizotypy and schizophrenia: A systematic review. Neuroscience & Biobehavioral Reviews, 37(3), 317–327. 10.1016/j.neubiorev.2013.01.004 [DOI] [PubMed] [Google Scholar]
- O’Farrell TJ, Fals-Stewart W, & Murphy M (2003). Concurrent validity of a brief self-report Drug Use Frequency measure. Addictive Behaviors, 28(2), 327–337. 10.1016/s0306-4603(01)00226-x [DOI] [PubMed] [Google Scholar]
- Oliver D, Reilly TJ, Baccaredda Boy O, Petros N, Davies C, Borgwardt S, McGuire P, & Fusar-Poli P (2020). What Causes the Onset of Psychosis in Individuals at Clinical High Risk? A Meta-analysis of Risk and Protective Factors. Schizophrenia Bulletin, 46(1), 110–120. 10.1093/schbul/sbz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DM, Meyvis T, & Davidenko N (2009). Instructional manipulation checks: Detecting satisficing to increase statistical power. Journal of Experimental Social Psychology, 45(4), 867–872. 10.1016/j.jesp.2009.03.009 [DOI] [Google Scholar]
- Pelletier-Baldelli A, Strauss GP, Visser KH, & Mittal VA (2017). Initial development and preliminary psychometric properties of the Prodromal Inventory of Negative Symptoms (PINS). Schizophrenia Research, 189, 43–49. 10.1016/j.schres.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce KM, Maxwell SD, Olino TM, Cooper S, & Ellman LM (2021). Factor Structure, Convergent, and Divergent Validity of the Prodromal Questionnaire–Negative Symptom Subscale. Assessment, 28(1), 153–168. 10.1177/1073191119899981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskulic D, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, & McGlashan TH (2012). Negative symptoms in individuals at clinical high risk of psychosis. Psychiatry Research, 196(2), 220–224. 10.1016/j.psychres.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsakoff PM, MacKenzie SB, Lee J-Y, & Podsakoff NP (2003). Common method biases in behavioral research: A critical review of the literature and recommended remedies. Journal of Applied Psychology, 88(5), 879–903. 10.1037/0021-9010.88.5.879 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, MacCallum RC, & Nicewander WA (2005). Use of the Extreme Groups Approach: A Critical Reexamination and New Recommendations. Psychological Methods, 10(2), 178–192. 10.1037/1082-989X.10.2.178 [DOI] [PubMed] [Google Scholar]
- Ramsey SR, Thompson KL, McKenzie M, & Rosenbaum A (2016). Psychological research in the internet age: The quality of web-based data. Computers in Human Behavior, 58, 354–360. 10.1016/j.chb.2015.12.049 [DOI] [Google Scholar]
- Salokangas RKR, Ruhrmann S, von Reventlow HG, Heinimaa M, Svirskis T, From T, Luutonen S, Juckel G, Linszen D, Dingemans P, Birchwood M, Patterson P, Schultze-Lutter F, & Klosterkötter J (2012). Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: Prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophrenia Research, 138(2–3), 192–197. 10.1016/j.schres.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Scott J, Leboyer M, Hickie I, Berk M, Kapczinski F, Frank E, Kupfer D, & McGorry P (2013). Clinical staging in psychiatry: A cross-cutting model of diagnosis with heuristic and practical value. The British Journal of Psychiatry: The Journal of Mental Science, 202(4), 243–245. 10.1192/bjp.bp.112.110858 [DOI] [PubMed] [Google Scholar]
- Strauss GP, & Gold JM (2012). A New Perspective on Anhedonia in Schizophrenia. American Journal of Psychiatry, 169(4), 364–373. 10.1176/appi.ajp.2011.11030447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Pelletier-Baldelli A, Visser KF, Walker EF, & Mittal VA (2020). A review of negative symptom assessment strategies in youth at clinical high-risk for psychosis. Schizophrenia Research, 222, 104–112. 10.1016/j.schres.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, & Krabbendam L (2009). A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychological Medicine, 39(2), 179–195. 10.1017/S0033291708003814 [DOI] [PubMed] [Google Scholar]
- Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhardt L, Dicker D, Duan L, Erskine H, Feigin VL, Ferrari AJ, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, … Murray CJ (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 386(9995), 743–800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Bucci S, Berry K, & Varese F (2018). Psychological mediators of the association between childhood adversities and psychosis: A systematic review. Clinical Psychology Review, 65, 175–196. 10.1016/j.cpr.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Woods SW, Walsh BC, Powers AR, & McGlashan TH (2019). Reliability, Validity, Epidemiology, and Cultural Variation of the Structured Interview for Psychosis-Risk Syndromes (SIPS) and the Scale of Psychosis-Risk Symptoms (SOPS). In Li H, Shapiro DI, & Seidman LJ (Eds.), Handbook of Attenuated Psychosis Syndrome Across Cultures: International Perspectives on Early Identification and Intervention (pp. 85–113). Springer International Publishing. 10.1007/978-3-030-17336-4_5 [DOI] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, & Findling RL (2008). Developing a Ten Item Mania Scale from the Parent General Behavior Inventory for Children and Adolescents. The Journal of Clinical Psychiatry, 69(5), 831–839. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2777983/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, & Buckby J (2005). Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Australian and New Zealand Journal of Psychiatry, 39, 964–971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


