Abstract
Salmonella Typhimurium creates an intracellular niche for its replication by utilizing a large cohort of effectors, including several that function to interfere with host ubiquitin signaling. Although the mechanism of action of many such effectors has been elucidated, how the interplay between the host ubiquitin network and bacterial virulence factors dictates the outcome of infection largely remains undefined. In this study, we found that the SPI‐2 effector SseK3 inhibits SNARE pairing to promote the formation of a Salmonella‐induced filament by Arg‐GlcNAcylation of SNARE proteins, including SNAP25, VAMP8, and Syntaxin. Further study reveals that host cells counteract the activity of SseK3 by inducing the expression of the E3 ubiquitin ligase TRIM32, which catalyzes K48‐linked ubiquitination on SseK3 and targets its membrane‐associated portion for degradation. Hence, TRIM32 antagonizes SNAP25 Arg‐GlcNAcylation induced by SseK3 to restrict Salmonella‐induced filament biogenesis and Salmonella replication. Our study reveals a mechanism by which host cells inhibit bacterial replication by eliminating specific virulence factors.
Keywords: E3 ligase, Salmonella, SIF biogenesis, T3SS SPI‐2 effector
Impact statement
Bacterial pathogens residing in mammals often utilize type III secretion systems (T3SS) to deliver various effectors and establish a secure environment for replication. However, our understanding of how host proteins counteract these effectors is limited. In this study, we explored the role of a T3SS effector from Salmonella Typhimurium in shaping its survival within host cells. Our findings indicate that a host ubiquitin E3 ligase TRIM32 acts as a countermeasure, degrading the virulence effector and competing against the bacteria. These insights deepen our knowledge of the complex interplay between host cells and bacteria in determining the success of bacterial replication.
INTRODUCTION
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a facultative intracellular pathogen capable of infecting multiple hosts to cause salmonellosis 1 . S. Typhimurium encodes two type III secretion systems (T3SSs), SPI‐1 and SPI‐2, that inject a large cohort of effector proteins into host cells 2 . Whereas the function of SPI‐1 primarily is to promote invasion of nonphagocytic intestinal epithelial cells to establish a nascent phagosome, effectors translocated by SPI‐2 function to remodel the phagosome into a niche permissive for bacteria replication called the Salmonella‐containing vacuole (SCV) 3 . A number of SPI‐2 effectors are dedicated to subverting the endolysosomal system, including the formation of a complex and highly dynamic structure termed Salmonella‐induced filaments (SIFs) 4 , 5 . Although many pathogens have the ability to create bacteria‐containing vacuoles to support their intracellular replication, the formation of the SIF network is unique to S. Typhimurium 6 . The ability of this pathogen to form SIF strongly correlates with its ability to cause diseases in animal infection models 7 . It has been hypothesized that SIF structures facilitate the bacterium to gain access to nutrients and/or to evade host immunity 8 , 9 . Thus, understanding the mechanisms of SIF biogenesis and maintenance will not only lead to a better appreciation of the S. Typhimurium pathogenesis and host cell biology but also provide clues for its disruption as novel interference strategies against salmonellosis.
Ubiquitin (Ub) signaling plays an essential role in immunity by regulating various cellular processes, particularly protein turnover and vesicle trafficking 10 . Consistent with the existence of multiple mechanisms designed to sense and eliminate invading S. Typhimurium, the bacterium has evolved several virulence factors to counteract such surveillance by directly interfering with Ub signaling. At least four Salmonella effectors have been shown to function as E3 Ub ligases to modulate host immunity 11 . Among them, SopA is a HECT‐like E3 ligase that modifies and targets TRIM56 and TRIM65, two host E3 ligases for degradation 12 , 13 , 14 . The two IpaH‐type E3 enzymes SspH1 and SspH2 target protein kinase 1 and NO1, respectively 15 , 16 , 17 . SlrP promotes host cell death by targeting thioredoxin and the Hsp40/DnaJ chaperone family associated with the endoplasmic reticulum (ER) 18 , 19 . In addition, the two deubiquitinases, AvrA and SseL, have been found to regulate host immunity, particularly the nuclear factor‐κB pathway 20 , 21 , 22 . The fate of S. Typhimurium that escape the phagosome to reach the cytosol differs greatly from those residing in the membrane‐bound vacuoles; these bacteria are first ubiquitinated by the E3 ligase RNF213, which strikingly and directly recognizes and modifies the lipid A moiety of bacterial lipopolysaccharide (LPS) 23 . Several other E3 ligases coordinate to build the Ub coat on the bacterial surface to initiate xenophagy 11 , a process that was recently found to be inhibited by the effector SopF that ADP‐ribosylates the ATP6V0C subunit of the v‐ATPase complex 24 .
Another cohort of S. Typhimurium effectors contributes to the development of SCV by modulating vesicle trafficking 25 . Although the cellular function of these effectors has been extensively studied 26 , how the host cell counteracts their activity largely remains elusive. In the present study, we identified the host E3 ligase TRIM32 as a regulator for the activity of the SPI‐2 effector SseK3. We found that SseK3 catalyzes Arg‐GlcNAcylation on SNAP25 and promotes SIF biogenesis mediated by the SseK3–SNARE axis and that infection by S. Typhimurium induces the expression of TRIM32, which antagonizes the activity of SseK3 by ubiquitination‐mediated degradation.
RESULTS
SseK3 promotes SIF formation during S. Typhimurium infection
Effectors translocated by the SPI‐2 are required for the formation of SIFs 5 . Among these, the three SseK proteins, SseK1, SseK2, and SseK3, are arginine GlcNAc transferases that are important for S. Typhimurium virulence 27 . To test whether any of these effectors are involved in SIF biogenesis or maintenance, we examined the phenotypes by infecting a cell line derived from HeLa that expresses GFP‐VAMP8 with several relevant S. Typhimurium strains, including the wild‐type, ΔsseK1/2/3, and ΔssaV, which is defective in the SPI‐2. Samples were assessed for the integrity of the SCV membrane and SIF formation by confocal microscopy. At 2 h postinvasion, the frequency of VAMP8‐coated SCVs for strain ΔsseK1/2/3 was similar to that of the wild‐type and the ΔssaV mutant, with rates at 81 ± 2%, 83 ± 6%, and 83.1 ± 4%, respectively (Figure S1). Thus, SseKs do not contribute to the formation of nascent SCVs, which is consistent with the observation that Arg‐GlcNAcylation induced by members of the SseK family does not occur until after 6 h postinfection 27 . At 10 h postinvasion, we observed a significant decrease in the frequency of SIFs in cells infected with the ΔsseK1/2/3 strain when compared to those infected with wild‐type S. Typhimurium. Furthermore, expression of SseK3, but not SseK1, SseK2, or its enzymatically inactive mutant SseK3D226A/D228A, restored the development of SIF to wild‐type levels (Figures 1A,B and S2). These results indicate that SseK3 plays a role in promoting SIF formation during S. Typhimurium infection.
Figure 1.
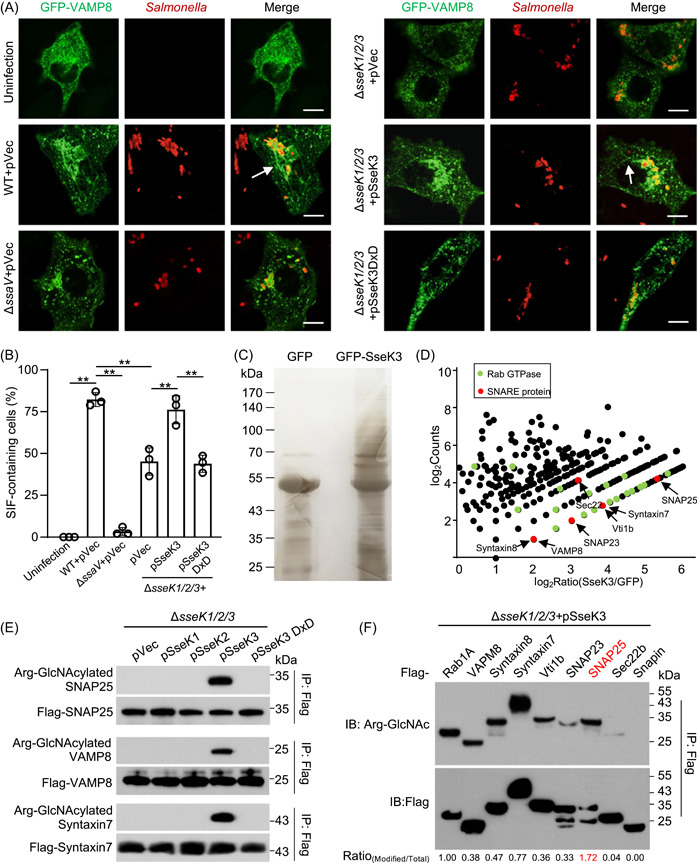
SseK3 promotes Salmonella‐induced filament (SIF) formation by modifying SNARE proteins during Salmonella Typhimurium infection. (A and B) SseK3 promotes SIF formation during S. Typhimurium infection. HeLa cells stably expressing EGFP‐VAMP8 were infected with the indicated S. Typhimurium strains for 10 h and analyzed for SIFs. Arrow indicates the SIF structure. pVec indicates the pET28a empty vector. The pSseK3 DxD indicates the pET28a plasmid expressing glycosyltransferase motif mutant of SseK3. The ΔssaV (an SPI‐2‐deficient mutant) strain was used as a control in this model. (A) Representative images showing the distribution of VAMP8 (green) and S. Typhimurium (red). Scale bar, 10 μm. (B) The rates of VAMP8‐positive tubules for each sample are indicated. At least 50 cells were counted for samples from experiments conducted in triplicate. **p < 0.01. (C) Detection of enriched Arg‐GlcNAcylated proteins by silver staining. Lysates of 293T cells transfected to express GFP or GFP‐SseK3 were subjected to immunoprecipitation (IP) with Arg‐GlcNAc‐specific antibodies, and precipitates separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) were detected by silver staining. (D) Scatter plots of protein ratios as a function of their relative abundance. Proteins immunoprecipitated with an anti‐Arg‐GlcNAc antibody were subjected to liquid chromatography with tandem mass spectrometry (LC‐MS/MS) analysis. The ratio was calculated as spectral counts in SseK3‐transfected samples divided by those in GFP‐transfected samples. Large ratios indicate preferential detection and modification in 293T cells transfected to express SseK3. Red dots correspond to SNARE proteins, and green dots correspond to Rab GTPase proteins. (E) SseK3 but not SseK1 or SseK2 modifies SNARE proteins during S. Typhimurium infection. The 293T cells expressing the indicated Flag‐tagged SNARE proteins were infected with relevant S. Typhimurium strains. Lysates immunoprecipitated with the Flag‐specific antibody were detected by immunoblotting with the indicated antibodies. Similar results were obtained from at least three independent experiments. (F) SNARE proteins are modified by SseK3 during S. Typhimurium infection. The 293T cells expressing the indicated Flag‐tagged SNARE‐associated proteins were infected with S. Typhimurium strain ΔsseK1/2/3(pSseK3). The level of Arg‐GlcNAcylation was obtained by measuring the band intensity of Arg‐GlcNAcylated proteins to total protein using ImageJ software.
SseK3 attacks SNARE proteins by Arg‐GlcNAcylation
We next investigated the mechanism underlying SseK3‐induced SIF development. Our previous studies have shown that SseK3 catalyzes Arg‐GlcNAcylation on death domain‐containing receptor proteins and members of the Rab small GTPases 27 , 28 . Yet, despite extensive efforts, we did not detect SseK3‐induced modification of most of the Rabs involved in SIF formation, including Rab11, Rab9, and Rab7, in cells infected with S. Typhimurium 27 . We thus attempted to identify SseK3 targets involved in SIF biogenesis by Arg‐GlcNAc 29 , followed by mass spectrometry (MS) analysis. After immunoaffinity enrichment with the Arg‐GlcNAc antibody, a range of substrates was detected by silver staining (Figure 1C). KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis of the putative SseK3‐modified proteins revealed five significantly enriched pathways. Among them, 19 proteins are involved in SNARE interactions in the vesicular transport pathway, including SNAP23, SNAP25, VAMP8, Vti1b, Syntaxin7, Syntaxin8, and Sec22b (Figures 1D and S3, red dots). Further comparative analyses between SseK3 samples and controls led to the identification of several GTPase proteins that had been previously identified (green dots, e.g., Rab1 and Rab8), demonstrating the effectiveness of this strategy (Figures 1D and S3, green dots). Complementation experiments with SseK1, SseK2, or SseK3 showed that SseK3 was the sole enzyme responsible for modifying SNARE proteins during infection (Figure 1E). Further experiments established that several SNARE proteins, including VAMP8, Syntaxin7, Syntaxin8, Vti1b, Sec22b, SNAP23, and SNAP25, are modified by SseK3 in cells infected with S. Typhimurium (Figure 1F). Among these, SNAP25 was most robustly modified by SseK3, suggesting that this protein is its preferred substrate (Figures 1F and S4). Taken together, these results indicate that SNARE proteins are new cellular targets of SseK3.
SseK3 induces GlcNAcylation of SNAP25 on Arg30 and Arg31, two residues important for its interaction with VAMPs
SNARE proteins share a common coiled‐coil motif of 60–70 residues essential for membrane fusion (Figure 2A) 31 . By deletion mutagenesis, we found that the N‐terminal domain (1–90 aa, NTD) containing the coiled‐coil motif of SNAP25 can be modified by SseK3 in cells infected with S. Typhimurium (Figure 2B). To precisely map the modification site(s), we affinity‐purified Flag‐SNAP25 from 293T cells co‐transfected with either wild‐type SseK3 or empty vector. By tandem MS (MS/MS) analysis, we detected two GluC‐LysC endoproteinases produced peptides with a mass shift of 203 Da and one peptide 28STRRMLQLVEE38 with a mass shift of 406 Da only in samples from cells that co‐expressed SseK3. The 203 Da increase in mass corresponds to the attachment of one GlcNAc moiety. Quantitative analysis revealed that over 70% of the peptides 28STRRMLQLVEE38 were modified. In contrast, the percentage of modification for other modified peptides was below 10% (Figures 2C and S5). MS/MS analyses assigned the major modification sites to Arg30 and Arg31 (Figure 2D), which were further verified by mutagenesis analysis. Modification signals were significantly attenuated in samples expressing the SNAP25 R30K/R31K mutant (R2K) (Figure 2E). Importantly, both Arg30 and Arg31 are located within the coiled‐coil domain of the t‐SNARE, which is involved in SNAP25 self‐association and in interactions with its binding proteins (Figure 2F,G). To determine the impact of SseK3 on the binding of SNAP25 to its interacting partners, we used MS to analyze proteins pulled down by SNAP25 under conditions with and without SseK3, which revealed that VAMP8 was less abundant in the pull‐down products from samples that expressed SseK3 (Figure 2H). This phenomenon can be recapitulated by experiments in which such binding was detected by immunoblotting of samples that expressed enzymatically inactive SseK3 (Figure 2I).
Figure 2.
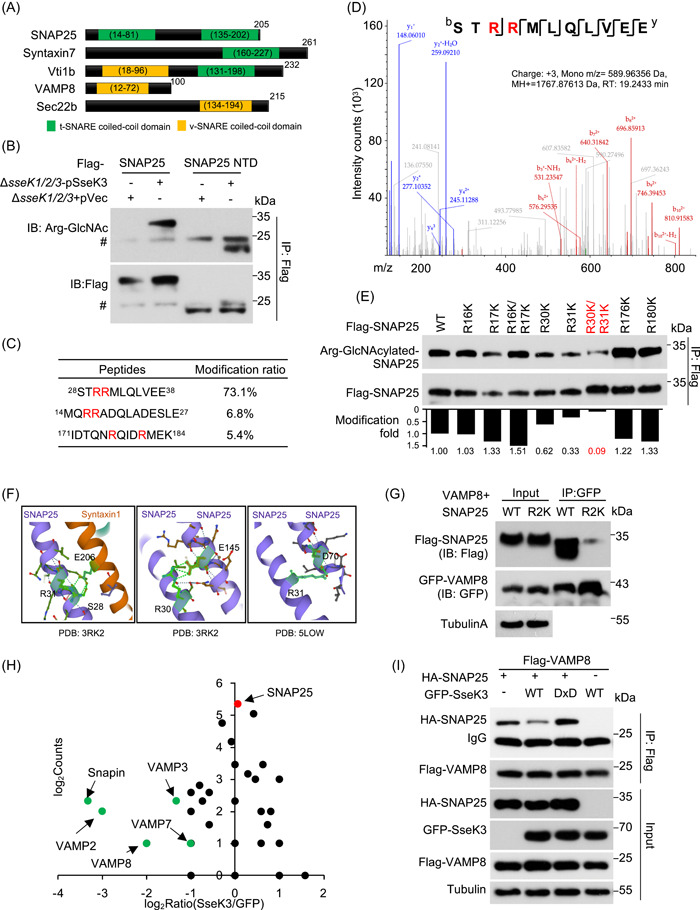
SseK3 interferes with the interactions between SNAP25 and VAMPs. (A) Schematic representation of the domain structure of known SNARE proteins. Each protein was represented by a rectangular bar and the localization of the t‐SNARE coiled‐coil domains and the v‐SNARE coiled‐coil domains are shown. (B) SseK3 modifies SNAP25 within the N‐terminal domain during Salmonella Typhimurium infection. Cells expressing the indicated domains of SNAP25 were infected with the indicated S. Typhimurium strains and the modification was detected by immunoblotting (IB). #IgG in the IP blot. (C) Mass spectrometric (MS) detection of modified peptides of SNAP25. The modification sites are indicated in red. Extracted ion chromatograms are shown with peak intensities indicating the relative amounts of either the modified or unmodified peptides in Figure S5. (D) Determination of modification sites by MS analysis. The tandem MS (MS/MS) spectrum of modified peptide 28STRRMLQLVEE30 is shown. The fragment ions b6 to b10 have a mass increase of 406 Da corresponding to the addition of two GlcNAc, while y1 to y6 fragments lack such a mass shift. SseK3 catalyzes GlyNAcylation on arginines and the 203‐Da increase corresponds to the attachment of one GlcNAc molecule, so the modification sites were mapped to Arg30 and Arg31 by MS/MS analyses. (E) Validation of Arg30 and Arg31 as the main modification sites of SNAP25 by SseK3. The 293T cells expressing the indicated Flag‐tagged SNAP25 mutants were infected with S. Typhimurium strain ΔsseK1/2/3(pSseK3); samples lysed were immunoprecipitated (IP) with anti‐Flag beads and detected with antibodies specific for the Arg‐GlcNAcylation. The levels of modification were quantified by measuring the ratio of band intensity for modified and total proteins with ImageJ. (F) Three‐dimensional (3D) structure visualization of Arg30 and Arg31 in SNAP25 (PBD number: 5LOW) and in the SNAP25–Syntaxin1 complex (PBD number: 3RK2). Note the role of the two residues in interactions between these two proteins. (G) The R30/R31 SNAP25 mutation inhibited the binding of SNAP25 with VAMP8. The 293T cells were transfected to express GFP‐VAMP8 and Flag‐SNAP25 or its R30/R31K mutant. At 18 h after transfection, co‐immunoprecipitation was performed with anti‐GFP antibodies, followed by standard immunoblotting analysis with the indicated antibodies. (H) Quantification of SNAP25‐binding proteins in cells expressing SseK3. Proteins that potentially bind SNAP25 were obtained by IP from cells transfected to express SseK3 and were analyzed by MS; cells transfected with the vector were used as controls. Scatter plots of protein ratios as a function of their relative abundance (denoted by MS/MS spectral counts). The ratio is calculated as spectral counts in SseK3‐transfected samples divided by those in controls. Lower ratios indicate decreased binding efficiency with SNAP25. Green dots correspond to Snapin and VAMPs, and the red dot corresponds to immunoprecipitated SNAP25. Results shown are representative of three independent experiments with similar results. (I) SseK3 interferes with the interactions between SNAP25 with VAMP8. Lysates of cells transfected to express the indicated protein combinations were subjected to IP with a Flag‐specific antibody. The products were detected for the presence of the binding partners by immunoblotting. Similar results were obtained in three independent experiments. WT, wild type.
SseK3 limits the size of SNAP25‐decorated infection‐associated macropinosomes (IAMs) during the late stages of infection
SNAP25 is enriched on the fluid‐filled IAM 32 , and it plays a crucial role in homotypic fusion among IAMs and their heterotypic fusion with SCVs, an event that is required for the expansion of the bacterial phagosome shortly after phagocytosis 33 . To explore the possibility that SseK3‐induced GlcNAcylation may interfere with its ability to promote such fusion events and the expansion of the SCV, we measured the dimension of the SNAP25‐containing vacuoles (SNAP25‐CVs) that represent SCVs (with bacteria) and IAMs (without bacteria), respectively. Our results indicate that at 0.5 and 2 h infection time points, there was no significant difference between the diameter of SNAP25‐CVs found in cells infected with wild‐type bacteria and the ΔsseK1/2/3 mutant. Intriguingly, when the infection had proceeded for 6 h, the size of the SNAP25‐CVs was significantly smaller in cells infected with the wild‐type strain than that found in cells infected with the ∆sseK1/2/3 mutant (Figure 3A). This difference disappeared when SseK3 was expressed in strain ΔsseK1/2/3 from a plasmid (Figure 3B,C). In agreement with these results, in cells transfected to express SseK3 before infection, the size of vacuoles formed by strain ΔsseK1/2/3 decreased (Figure 3D,E). Furthermore, signals of protein Arg‐GlcNAcylation induced by SseK3 in infected cells showed clear co‐localization with SNAP25 on bacterial vacuoles (Figure 3F). The fluorescence intensity of Arg‐GlcNAcylation showed a similar pattern to that of SNAP25 (Figure 3G). Thus, SseK3 limits the size of the SNAP25‐containing IAM vacuole when the infection has proceeded for 6 h. Given the fact that SseK3 attenuates the interaction between SNAP25 and VAMPs (Figure 2H), such alternations in the size of the vacuoles may be caused by the inhibition of fusion among IAMs and between IAMs and SCVs. Because SNAREs are essential for SIF formation 34 , it is likely that SseK3 interferes with SNARE paring to impact SCV size and SIF formation at the late stage of infection (Figure 1A).
Figure 3.
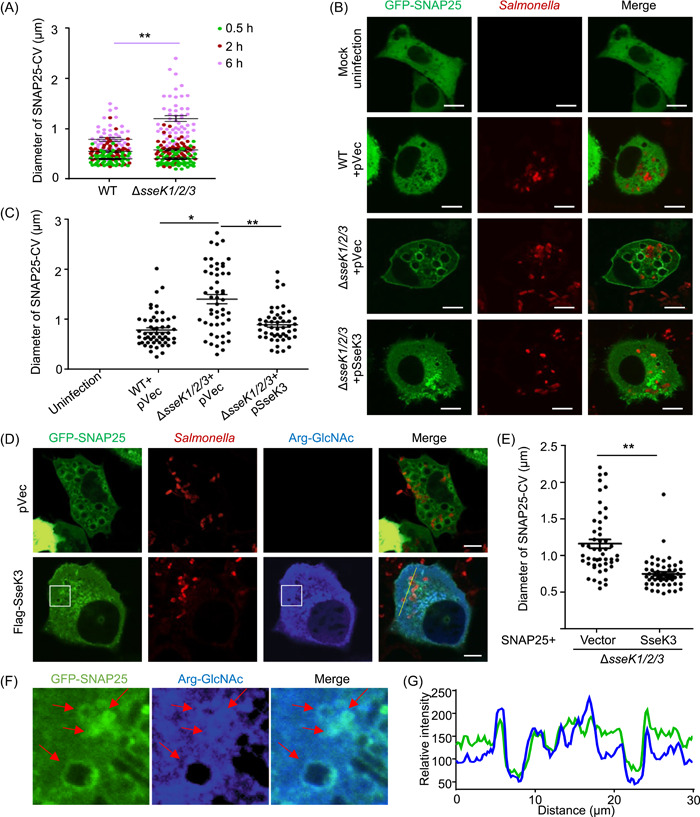
SseK3 limits the size of SNAP25‐labeled Salmonella‐containing vacuoles (SCVs). (A) Effects of the SseK family proteins on the size of the SNAP25‐containing vacuole (SNAP25‐CV) during S. Typhimurium infection. HeLa cells transfected to express GFP‐SNAP25 were infected with the indicated S. Typhimurium strains. The diameter of SNAP25‐CV was measured at the indicated time points. (B and C) Effects of SseK3 on the size of SNAP25‐CV. HeLa cells transfected to express GFP‐SNAP25 were infected with the indicated S. Typhimurium strains for 6 h. The distributions of SNAP25 (green) and S. Typhimurium (red) are shown in (B). Statistical data of the diameter of SCVs positive for GFP‐SNAP25 are shown in (C). (D and E) Ectopic expression of SseK3 limits the size of SNAP25‐CV. HeLa cells transfected to express GFP‐SNAP25 and Flag‐SseK3 were infected with the indicated S. Typhimurium strains for 6 h. The distribution of GFP‐SNAP25 (green), S. Typhimurium (red), and Arg‐GlcNAcylated proteins (blue) is shown (D). Statistical data of the diameter of the SNAP25‐positive SCVs are shown in (E). At least 30 cells in (A), (C), and (E) were analyzed for each experiment. Scale bar, 10 μm. *p < 0.05; **p < 0.01. (F) Enlarged co‐localization of SNAP25 with Arg‐GlcNAcylation is shown. Arrows indicate the co‐localization of SNAP25 and Arg‐GlcNAcylation on the vacuoles. (G) Line scan was obtained from images in (D). Data show the localization of Arg‐GlcNActlated proteins (blue) relative to SNAP25 (green). Line scan shows the fluorescence intensity along a portion of the yellow line overlaying the image in (D). WT, wild type.
Expression level of the host E3 Ub ligase TRIM32 is induced upon S. Typhimurium infection
To determine the mechanism that the host may use to counteract the activity of S. Typhimurium effectors, we reanalyzed the available transcriptomic data of host cells infected with strain SL1344 35 . These efforts led to the identification of TRIM32 that codes for an E3 Ub ligase as one of the significantly induced genes in response to S. Typhimurium challenge (Figure 4A). Further analyses by quantitative real‐time PCR (qRT‐PCR) and immunoblotting confirmed that both TRIM32 mRNA and TRIM32 protein levels were induced upon bacterial challenge (Figure 4B,C). Moreover, overexpression of TRIM32 led to a significant decrease in the frequency of the intracellular SIF structures found in cells infected with S. Typhimurium (Figure 4D,E).
Figure 4.
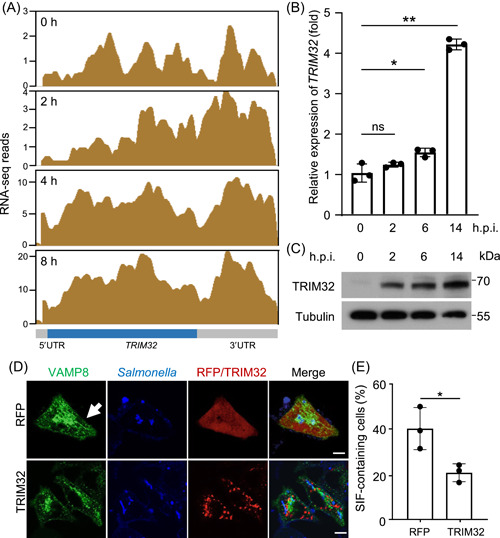
Expression of the host E3 ligase gene TRIM32 is induced in response to Salmonella Typhimurium infection. (A) Diagrams of the TRIM32 locus based on RNA‐sequencing (RNA‐seq) reads. Enriched RNA‐seq signals 35 visualized by Integrated Genome Browser are representative of three independent experiments. (B) Quantitative real‐time PCR (qRT‐PCR) detection of TRIM32 expression during S. Typhimurium infection. HeLa cells infected with S. Typhimurium strain SL1344 for the indicated time points were probed for the mRNA levels of TRIM32. The statistical data are expressed as means ± SD from three independent experiments. *p < 0.05; **p < 0.01. (C) Induction of TRIM32 in response to S. Typhimurium infection was measured by detecting proteins. HeLa cells infected with S. Typhimurium strain SL1344 for the indicated time points were probed with TRIM32‐specific antibodies. Tubulin was detected as a loading control. Data shown are one representative of three independent experiments with similar results. (D and E) Effects of TRIM32 on Salmonella‐induced filament (SIF) biogenesis. HeLa cells transfected to express GFP‐VAMP8 and the indicated proteins for 12 h were infected with the indicated S. Typhimurium for 10 h. (D) The distribution of GFP‐VAMP8 (green), S. Typhimurium (blue), and RFP or RFP‐TRIM32 is shown. (E) Quantification of cells showing VAMP8‐positive tubules is indicated. At least 50 cells were counted for each experiment and the statistical data shown are from three independent experiments. Arrows indicate the SIF structure. Scale bar, 10 μm. *p < 0.05. h.p.i., hours postinfection; ns, not significant; UTR, the untranslated region.
TRIM32 interacts with and ubiquitinates SseK3
An earlier study has demonstrated interactions between TRIM32 and SseK3 36 . Yet, SseK3 does not detectably modify TRIM32 by Arg‐GlcNAcylation, and the physiological significance of this interaction is unknown. TRIM32 consists of an N‐terminal RING domain, a type II B‐box domain, a coiled‐coil domain, and a carboxyl NHL domain (Figure 5A). To determine which of these regions is important for its interaction with SseK3, we generated a series of TRIM32 deletion mutants and examined their ability to bind the effector. Results from these experiments indicate that the NHL domain is essential for SseK3 binding (Figure 5B).
Figure 5.
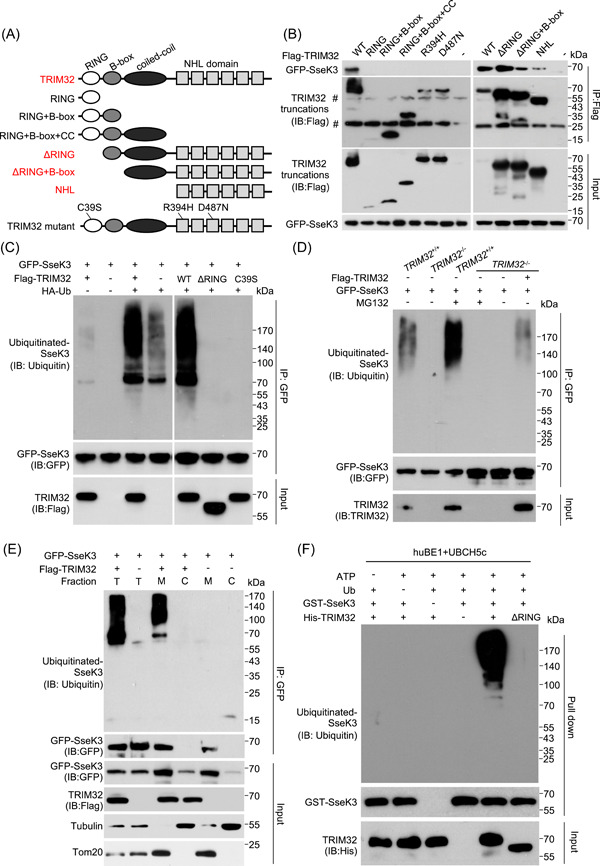
TRIM32 interacts with and ubiquitinates SseK3. (A) Schematic representation of TRIM32 domain structure and the several TRIM32 mutants used in this study. TRIM32 truncation mutants that retain the ability to interact with SseK3 are shown in red. (B) Mapping the domain important for TRIM32 to interact with SseK3. Flag‐tagged full‐length or several deletion mutants of TRIM32 co‐expressed with GFP‐SseK3 in 293T cells and the interactions were determined by immunoprecipitation (IP) with beads coated with the Flag‐specific antibody. Binding was detected by immunoblotting (IB) with GFP‐specific antibodies. #IgG in the IP blot. (C) Overexpression of wild‐type (WT) but not the mutant TRIM32 promotes ubiquitination of SseK3. The 293T cells were transfected to express GFP‐SseK3 and TRIM32 or its mutants in the presence or absence of HA‐ubiquitin (Ub). At 18 h after transfection, coimmunoprecipitation was performed with anti‐GFP antibodies, followed by standard immunoblotting analysis with the indicated antibodies. (D) TRIM32 is required for the ubiquitination of SseK3. The indicated cell lines, TRIM32 +/+, TRIM32 −/−, and TRIM32 −/− complemented with TRIM32 were transfected to express GFP‐SseK3 for 16 h. Cells were treated with or without 25 μM MG132 for 12 h before probing for the ubiquitination levels of GFP‐SseK3 by immunoblotting. (E) TRIM32‐mediated ubiquitination of SseK3 occurs at the membrane components of SseK3. The 293T cells were transfected with the indicated plasmids. Total membrane and cytosol proteins were isolated and immunoblotted with the corresponding antibodies. (F) TRIM32 catalyzes SseK3 ubiquitination in vitro. Recombinant His‐TRIM32, GST‐SseK3, ubiquitin (Ub), E1 (huBE1), and E2 (UBCH5c) were added as indicated for ubiquitination assays. After the GST pull‐down, ubiquitin‐conjugated proteins were detected by immunoblot with a ubiquitin‐specific antibody. The input levels of TRIM32 proteins were detected by immunoblots. Data shown are representative of three independent experiments with similar results. C, cytoplasmic fraction; M, membrane fraction; T, total protein.
TRIM32 is an E3 Ub ligase that contains a RING finger domain. Therefore, we examined whether TRIM32 can catalyze ubiquitination on SseK3. Co‐expression of wild‐type TRIM32 with SseK3 and HA‐Ub led to strong ubiquitination of the effector. In contrast, a weak ubiquitination signal was detected in samples from cells transfected to express emptor vector, RING‐deficient mutants (ΔRING and C39S, Figure 5C), or NHL‐deficient mutants (RING and D487N, Figure S6). This ubiquitination signal may be caused by endogenous TRIM32. Transfection of RING‐deficient mutants inhibited the ubiquitination of SseK3 caused by endogenous TRIM32. It is speculated that both of these mutants contain NHL domains and can competitively bind SseK3 with endogenous TRIM32 (Figures 5C and S6). In agreement with these results, knocking out TRIM32 diminished SseK3 ubiquitination in a way that can be complemented with the wild‐type TRIM32. Furthermore, treatment of the cells with the proteasome inhibitor MG132 considerably enhanced SseK3 ubiquitination (Figure 5D). SseK3 is a Golgi‐located protein 27 , and we found that TRIM32‐mediated ubiquitination occurred at the membrane components of SseK3 (Figure 5E). Finally, in biochemical assays, the inclusion of TRIM32 in reactions containing huBE1 (E1), UBCH5c (E2), Ub, and ATP led to robust SseK3 ubiquitination. Such modifications did not occur in reactions receiving TRIM32ΔRING (Figure 5F). The above data indicate that TRIM32 ubiquitinates SseK3 by its E3 ligase activity of RING domain and its NHL domain recognition.
TRIM32 catalyzes K48‐linked ubiquitination on SseK3 and targets its membrane‐associated portion for degradation
There are seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) in the Ub molecule, and each dictates the formation of a specific type of polyubiquitin chain that determines the biological consequence of the modification 37 . To further understand the implications of TRIM32‐induced ubiquitination on the activity of SseK3, we evaluated the chain type of the Ub polymers on SseK3 using a series of lysine mutants of Ub. Robust ubiquitination of SseK3 was observed in reactions receiving wild‐type or K48‐only Ub (a mutant in which all Lys residues except for Lys48 have been replaced with Arg). Conversely, in reactions containing the K48R ubiquitin mutant, no ubiquitination was detected (Figure 6A). In addition, the linkage has been confirmed by in vitro experiments with only one type of Ub mutant. When the recombinant K48R Ub mutant was added, the Ub chain on SseK3 was much weaker (Figure S7), suggesting that TRIM32 catalyzes ubiquitination on SseK3 mainly by K48 linkage. As polyubiquitination by K48 chain type typically results in protein degradation by the Ub‐proteasome system (UPS) 38 , we then investigated the stability of SseK3 in cellulo. Chase experiments with cycloheximide (CHX) showed that the levels of SseK3 associated with the membrane (M‐SseK3) were markedly reduced in a manner that can be blocked by MG132 (Figure 6B). These results strongly suggest that SseK3 was degraded via the UPS after being ubiquitinated by TRIM32.
Figure 6.
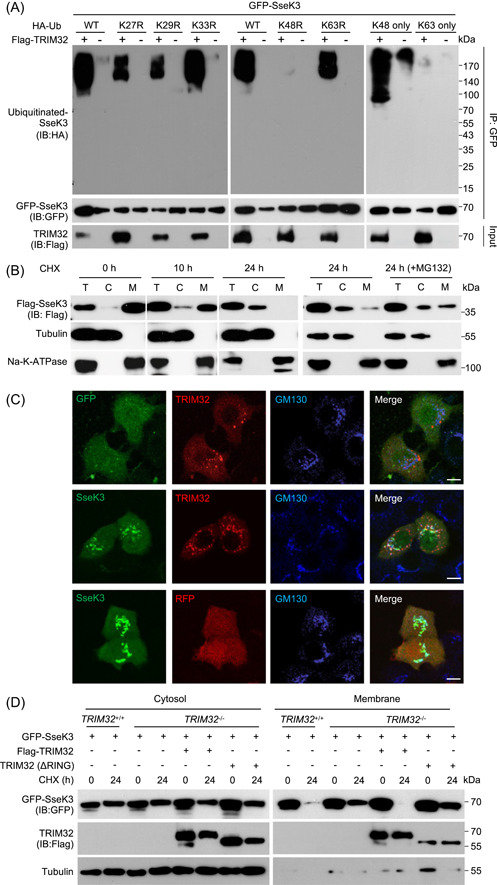
TRIM32 catalyzes K48‐linked ubiquitination to degrade membrane‐associated SseK3. (A) TRIM32 catalyzes K48‐linked polyubiquitination of SseK3. The 293T cells were transfected to express GFP‐SseK3 and the indicated proteins. Lysates of the samples were subjected to immunoprecipitation (IP) and immunoblot (IB) analysis with the indicated antibodies. (B) The stability of SseK3 was probed using the cycloheximide (CHX) pulse‐chase assay. The 293T cells transfected to express Flag‐SseK3 were treated with CHX for the indicated time points. Cells were treated with 25 μM MG132 for 12 h before cell lysis (the right panel). Total membrane and cytosol proteins were isolated and immunoblotted with the corresponding antibodies. (C) Co‐localization of TRIM32 and SseK3 on the Golgi apparatus in HeLa cells. HeLa cells transfected to express the indicated proteins were fixed with 4% paraformaldehyde and analyzed by confocal microscopy. The distribution of SseK3 or GFP (green), RFP‐TRIM32 or RFP (red), and GM130 (blue) is shown. Scale bar, 20 µm. (D) Effects of TRIM32 on the stability of SseK3. TRIM32 +/+, TRIM32 −/−, and TRIM32 −/− cells complemented with TRIM32 were transfected to express GFP‐SseK3. Samples were treated with 100 μg ml−1 CHX for 24 h and were separated into membrane and cytosolic fractions. The presence of the relevant proteins in these fractions was probed by immunoblotting with the appropriate antibodies. Data shown are representative of three independent experiments with similar results. C, cytoplasmic fraction; M, membrane fraction; T, total lysates; Ub, ubiquitin.
SseK3 has been shown to localize to the cis‐Golgi apparatus. 27 Importantly, RFP‐TRIM32 also extensively targets the Golgi apparatus when co‐expressed with GFP‐SseK3 in HeLa cells (Figure 6C). We, therefore, examined whether TRIM32 induces the degradation of SseK3. Our results indicate that TRIM32 indeed causes the reduction of SseK3 that is associated with the membrane (Figure 6D). Consistently, knockout of TRIM32 led to elevated SseK3 in the membrane fraction, which can be reversed by expressing TRIM32 but not the ΔRING mutant. In contrast, the protein level of the cytosol fraction of SseK3 (C‐SseK3) was largely unaffected by TRIM32 (Figure 6D). Thus, TRIM32 induces K48‐linked ubiquitination on SseK3, which results in its degradation, particularly for a protein that is associated with the membrane.
TRIM32 antagonizes SNAP25 Arg‐GlcNAcylation induced by SseK3 to restrict SIF biogenesis and Salmonella replication
The observation that TRIM32 ubiquitinates SseK3 and targets it for degradation suggests that this E3 Ub ligase regulates the function of the effector. Indeed, we found that SseK3 injected into host cells by S. Typhimurium interacted with TRIM32 (Figure 7A). As expected, the level of GlcNAcylated SNAP25 decreased in the membrane fraction of the samples expressing TRIM32 but not the C39S mutant(Figure 7B). In line with these observations, overexpression of TRIM32 decreased the ability of SseK3 to promote SIF formation (Figure 7C,D). Considering that both SseK3 protein and SIF structure are required for Salmonella intracellular survival within macrophages 27 , 30 , we next determined the effects of TRIM32 on bacterial replication in macrophage cells. We synthesized three pairs of siRNA and found that the second pair had the best downregulation effect (Figure 7E). As expected, TRIM32 knockdown efficiently facilitated bacterial replication of SseK3‐expressing Salmonella, but not S. Typhimurium lacking sseKs in RAW264.7 macrophage cells (Figure 7F). Together, these results indicate that TRIM32 functions restrict the activity of SseK3 by targeting it for proteasome degradation, thus lowering Arg‐GlcNAcylation on SNAP25, which ultimately causes a reduction in SIF formation and bacterial virulence (Figure 8).
Figure 7.
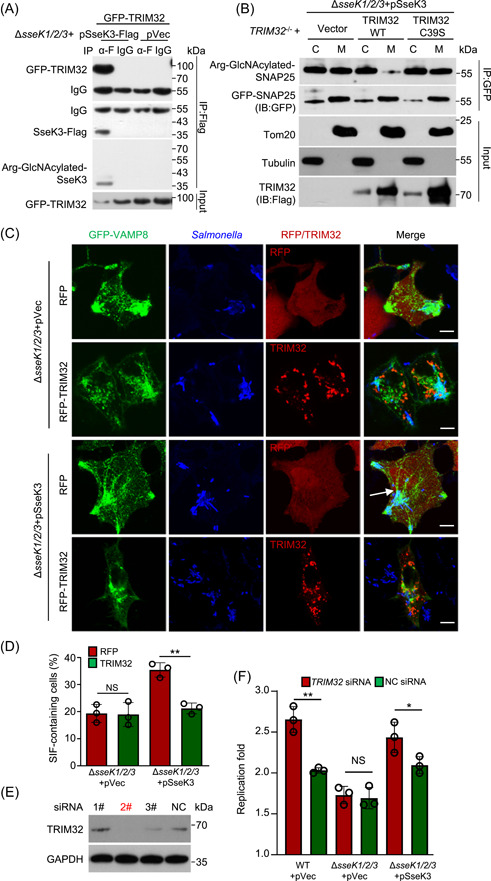
TRIM32 antagonizes SseK3‐catalyzed‐Arg‐GlcNAcylation on SNAP25 and restricts SseK3‐SNARE‐mediated Salmonella‐induced filament (SIF) biogenesis and Salmonella replication. (A) TRIM32 targets SseK3 during S. Typhimurium infection. The 293T cells transfected to express GFP‐TRIM32 were infected with the indicated bacterial strains for 16 h. The interactions between TRIM32 and SseK3 were detected by immunoprecipitation (IP). (B) Effects of TRIM32 on the SseK3‐catalyzed‐Arg‐GlcNAcylation on SNAP25 during S. Typhimurium infection. TRIM32 −/− cells transfected to express GFP‐SNAP25 and the indicated proteins were infected with strain ∆sseK1/2/3(pSseK3) for 10 h. Chloramphenicol was added to inhibit the bacteria protein synthesis for another 12 h. Lysed cells were separated into soluble and membrane fractions and the presence of SseK3 was probed by immunoblotting (IB). Data shown are representative of three independent experiments with similar results. (C and D) Effects of TRIM32 on SseK3‐SNARE‐mediated SIF biogenesis. HeLa cells transfected to express GFP‐VAMP8 and the indicated proteins for 12 h were infected with the indicated S. Typhimurium strains for 10 h. (C) The distribution of VAMP8 (green), S. Typhimurium (blue), and RFP or RFP‐TRIM32 was determined by confocal microscopic analysis. Arrows indicate the SIF structure. Scale bar, 10 μm. (D) Rates of cells showing VAMP8‐positive tubules are indicated. At least 50 cells were counted for each sample done in triplicate and the statistical data shown are from three independent experiments. **p < 0.01. (E and F) Effects of TRIM32 knockdown on Salmonella replication in macrophage cells. (E) Knockdown efficiency of TRIM32 siRNA was detected by immunoblotting. (F) RAW264.7 cells were transfected with 2# siRNA for TRIM32 for 48 h, and then subjected to infection with the indicated S. Typhimurium at a multiplicity of infection of 10. Replication fold was determined by comparing bacterial counts at 2 and 24 h postinfection. Results shown are mean values ± SD (error bar) from three independent experiments. *p < 0.05; **p < 0.01. α‐F, anti‐Flag antibody; NC, negative control; NS, not significant; WT, wild type.
Figure 8.
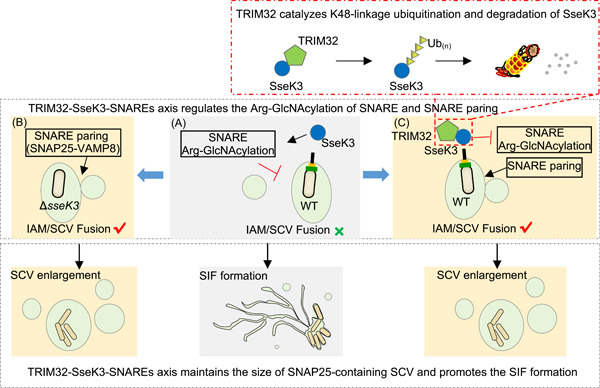
Schematic diagram of the regulation of Salmonella Typhimurium intracellular lifecycle by SseK3 and TRIM32. Soon after Salmonella entry, nascent Salmonella‐containing vacuole (SCV) fuses with surrounding infection‐associated macropinosomes (IAMs) to form a larger bacterial vacuole, which is mediated by SNAREs proteins (e.g., SNAP25–VAMP8 pairing). At the late stage of infection, SPI‐2 type III secretion system (T3SS) effector SseK3 Arg‐GlcNAcylates SNARE proteins, blocks SNARE pairing, and prevents further fusion events. The decrease in the SCV size may favor Salmonella‐induced filament (SIF) biogenesis and Salmonella replication (A). Lack of SseK3 leads to a significant reduction in this capacity (B). On the other hand, the expression of TRIM32 is induced during Salmonella infection. TRIM32 catalyzes K48‐linked ubiquitination on SseK3 and targets it for degradation, which counteracts SseK3's functions and restricts SIF biogenesis (C).
DISCUSSION
The outcome of bacterial infection is dictated by strong interactions between host factors and virulence factors 39 . Several members of the TRIM E3 Ub ligase family have been found to be involved in regulating pathogen infection, including some that confer resistance to the virus by directly targeting viral proteins 40 , 41 . TRIM56 and TRIM65 have been shown to be targeted by SopA, a HECT‐like E3 ligase 14 . Our discovery of TRIM32 as a host factor that functions to control S. Typhimurium virulence has expanded the role of these E3 Ub ligases in defense against bacterial infection.
Importantly, an earlier study has shown that Trim32 plays a role in defending against S. Typhimurium infection because Trim32 −/− mice are more sensitive to inflammatory death caused by this bacterium 42 . In this study, several lines of evidence show that TRIM32 is involved in the defense against S. Typhimurium by attacking the SPI‐2 effector SseK3. First, the expressions of TRIM32 and SseK3 are synchronously upregulated. SseK3 belongs to an SPI‐2 effector and begins to catalyze Arg‐GlcNAcylation after 6 h postinfection 27 . Both TRIM32 mRNA and TRIM32 protein levels were significantly induced at this time (Figure 4). Second, TRIM32 interacts with SseK3 expressed by transfected or injected into host cells by S. Typhimurium via its carboxyl NHL repeats. Third, TRIM32 catalyzes K48‐type polyubiquitin chains on SseK3 and targets its membrane‐associated SseK3 for degradation. Fourth, overexpression of this E3 ligase led to less SIF formation during S. Typhimurium infection, and TRIM32 knockdown facilitated Salmonella replication within macrophage cells.
Our findings have also provided novel insights into the role of effector‐induced Arg‐GlcNAcylation in S. Typhimurium infection. Additional proteins potentially modified by members of the SseK have been identified in cells ectopically expressing the effectors or in cells infected with S. Typhimurium strains expressing specific effectors 27 , 43 . These results are consistent with observations made in an earlier RNA interference screen, which revealed that the canonical mammalian late endo‐/lysosomal vesicle fusion machinery (SNARE and Rab GTPase) is involved in SIF biogenesis 34 . Our finding that multiple coiled‐coil containing‐domain proteins are Arg‐GlcNAcylated in infected cells suggests that SseKs may act in a coordinated manner to target endomembrane components to facilitate S. Typhimurium replication. In particular, SseK3 appears to directly participate in this process by catalyzing Arg‐GlcNAcylation on SNAP25, leading to inhibition of its pairing with VAMP8 and restriction of the expansion of SCVs. This activity of SseK3 also causes a reduction in the size of macropinosomes, but the physiological consequence of this reduction is not clear. A recent screen using proximity labeling (BioID) found that SPI‐2 effectors SseG, SopD2, PipB2, and SifA potentially interact with proteins in the SNARE complex 44 . Interestingly, these effectors have been suggested to be involved in SIF formation 5 . It is likely that multiple effectors, including SseK3, coordinate to regulate the dynamics of SNARE pairing to promote SIF biogenesis during S. Typhimurium infection. Future research aiming at dissecting the potential interplay among these effectors and how each is temporally and spatially regulated to ensure successful infection will yield more insights into their roles in the intracellular lifecycle of the pathogen.
A majority of proteins anchored in membrane‐containing organelles have been reported to be degraded via the cytosolic‐proteasome system 45 , 46 . The spatial separation between substrate selection and degradation requires either membrane‐anchored substrate extraction from the membrane or recruitment of 26S proteasomes to the membrane. A well‐studied example is the ER‐associated protein degradation pathway. Ubiquitinated proteins on ER are extracted from the membrane by the Cdc48p/p97 complex and transported to the proteasome 47 . Besides, FKBP38, residing in the ER and mitochondrial membranes, functions to anchor the 26S proteasome to the organellar membrane 48 . Recently, proteasomes have also been reported to be constitutively associated with the Golgi membranes by PSMD6 and mediate the degradation of the Golgi protein GM130 49 . SseK3 is a Golgi‐located protein, and here, we show that membrane counterparts of SseK3 are ubiquitinated and degraded in a proteasome‐dependent manner. The mechanism is likely similar to GM130, and further investigations are needed.
The NHL motif is a conserved protein domain that has been found at the C termini of four E3 ligase TRIMs, including TRIM2, TRIM3, TRIM32, and TRIM71. The NHL domain consists of five or six repeats of ~40 residues each and folds into a barrel‐like propeller structure, which has been generally regarded as structural units involved in RNA and protein binding 50 . Despite sharing a highly conserved three‐dimensional topology, different TRIM NHLs proteins show a low level of sequence similarity and have diverse interaction substrates. The crystal structure also revealed significant differences existing in the central NHL binding pockets, including considerable variations in shape, amino acid composition, and electrostatic potential, which distinguishes characteristics and potential recognition functions 51 . Although we did not determine whether other TRIM‐NHL proteins could bind and regulate SseK3, we speculate that TRIM32 is responsible for SseK3 ubiquitination among TRIM‐NHL proteins. First, on searching the mass spectral data, it is clear that TRIM32 is the only NHL‐containing protein that SseK3 can bind to 36 . Besides, the ubiquitination of SseK3 disappears by TRIM32 knockout (Figure 5D).
TRIM32 localizes to cytosolic perinuclear speckles as well as to the Golgi network 36 , 52 , 53 . However, the mechanism of its Golgi localization is still unclear. A recent report showed that TRIM32 was regulated by posttranslational acetylation modifications of three lysine residues (K50, K247, and K401) by MS. When lysine K247 was mutated to an acetylation‐mimic glutamine residue, TRIM32 has a strong tendency to aggregate the Golgi protein GM130, indicating that the Golgi localization of TRIM32 is associated with K247 acetylation 52 . Our previous study showed that the lysines on SseK3 could efficiently bind to phospholipid, which was crucial for the Golgi‐localization of SseK3 27 . However, whether K247 or other unrecognized amino acids within TRIM32 are also required for the interaction with phosphoinositides like SseK3 is to be elucidated. On the other hand, co‐transfection of SseK3 may increase the co‐localization of TRIM32 and Golgi (Figure 6C), indicating that TRIM32 protein may also partially be recruited to the Golgi network by binding to SseK3.
Based on our results, we propose a model for the regulatory role of the TRIM32–SseK3–SNARE–SIF axis in S. Typhimurium infection. A few minutes after bacterial entry, the SCV increases in size through fusions with the macropinosomes. The t‐SNARE protein SNAP25 localized on IAMs and the v‐SNARE protein VAMP8 are recruited to the SCV. The fusion between the SCV and IAMs allows the former to expand in size. As the infection proceeds to late stages (>6 h), SseK3 injected by the SPI‐2 attacks SNAP25 by Arg‐GlcNAcylation, leading to ablation of SNAP25–VAMP8 pairing and the SNARE fusion events, which may contribute to SIF formation. Meanwhile, infected cells sense the presence of S. Typhimurium and induce the expression of TRIM32 by an as yet unrecognized mechanism. Elevated TRIM32 captures membrane‐associated SseK3 for ubiquitination and subsequent proteasome degradation, which restricts SseK3‐SNARE‐mediated SIF biogenesis and inhibits the intracellular replication of Salmonella (Figure 8).
MATERIALS AND METHODS
Bacterial strains, cell culture, and infection
The S. Typhimurium strains (wild‐type SL1344 and its mutant derivatives) used in this study are listed in Table S1. pET28a vector‐based complementation plasmids were introduced into S. Typhimurium by electroporation (2.5 kV, 200 Ω, 25 μF, and 5 ms). Bacteria were cultured in LB broth at 37°C on a shaker (220 rpm min−1). When necessary, cultures were supplemented with antibiotics at the following final concentrations: streptomycin, 100 μg ml−1; ampicillin, 100 μg ml−1; and kanamycin, 50 μg ml−1.
The procedure for bacterial infection of mammalian cells was performed as previously described 27 . Briefly, wild‐type and mutant Salmonella were cultured overnight (approximately 16 h) at 37°C on a shaker (220 rpm min−1) and were then subcultured at a 1:33 dilution in LB without antibiotics for 3 h. Bacteria were diluted in serum‐free and antibiotic‐free Dulbecco's modified Eagle's medium (DMEM) and added to cells at a multiplicity of infection (MOI) of 100 for 30 min at 37°C. 24‐well plates were centrifuged at 700g for 5 min at room temperature to promote infection. Extracellular bacteria were removed by extensive washing with phosphate‐buffered saline (PBS), and the culture medium was replaced with a medium containing 100 μg ml−1 gentamicin. Cells were incubated at 37°C in a 5% CO2 incubator for a further 1.5 h, and the culture medium was replaced with a medium containing 20 μg ml−1 gentamicin. Infected cells were incubated to the indicated time at 37°C in a 5% CO2 incubator. Samples were processed further for immunoprecipitation or immunofluorescence.
To measure the S. Typhimurium replication fold, RAW264.7 cells were infected with indicated Salmonella strains at an MOI of 10. Infection was facilitated by centrifugation at 700g for 5 min at room temperature. After 30 min incubation at 37°C, cells were washed three times with PBS to remove extracellular bacteria and incubated with fresh DMEM containing 100 μg ml−1 gentamicin. At 2 h postinfection, the gentamicin concentration was reduced to 20 μg ml−1. At 2 and 24 h postinfection, cells were lysed in cold PBS containing 1% Triton X‐100, and colony‐forming units were determined by serial‐dilution plating on agar plates containing 100 μg ml−1 streptomycin and 50 μg ml−1 kanamycin. The replication fold was determined by dividing the number of intracellular bacteria at 24 h by the number at 2 h.
Plasmids, antibodies, and reagents
The plasmids used in this study are listed in Table S2. Genes coding for SseK1, SseK2, and SseK3 were amplified from genomic DNA of S. Typhimurium strain SL1344 and were inserted into pCS2‐EGFP, pCS2‐RFP, pCS2‐Flag, and pCS2‐HA, respectively, for transient expression in mammalian cells. For complementation in the S. Typhimurium ΔsseK1/2/3 strain, a DNA fragment containing genes encoding SseK1, SseK2, and SseK3, each together with their upstream promoter regions, was amplified from S. Typhimurium SL1344 genomic DNA and inserted into pET28a. cDNAs for SNAP23, SNAP25, VAMP8, Syntaxin7, Syntaxin8, Vti1b, Sec22b, Snapin, Rab1, and TRM32 were amplified from a cDNA library of HeLa cells. For mammalian expression, cDNAs were cloned into pCS2‐EGFP and pCS2‐Flag vectors. Truncation, deletion, and point‐mutation mutants were constructed using the standard PCR cloning strategy. All plasmids were verified by sequencing analysis. Antibodies and reagents are listed in Table S3.
Cell culture, transfection, and stable cell‐line construction
The 293T and HeLa cells were obtained from the American Type Culture Collection and were maintained in DMEM (HyClone) supplemented with 10% FBS (Gibco), 2 mM l‐glutamine, 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. Cells were cultivated in a humidified atmosphere of 5% CO2 at 37°C.
Transient transfection was performed using Vigofect (Vigorus) or Jetprime (Polyplus) reagents following the manufacturer's instructions. For siRNA knockdown, 200 pmol of siRNAs were transfected into 2 × 106 RAW264.7 cells. Sense sequences for the siRNAs used were as follows: TRIM32 1#, 5′‐CCATCTGCATGGAGTCCTTTT‐3′; TRIM32 2#, 5′‐CCAAGTGTTCAACCGCAAATT‐3′; TRIM32 3#, 5′‐GCTATCATCTGAGAAGATATT‐3′; and negative control (NC), 5′‐TTCTCCGAACGTGTCAC GT‐3′.
To generate the cell line that stably expresses EGFP‐VAMP8, pcDNA4‐EGFP‐VAMP8 was transfected into 293T cells. Cells emitting green fluorescence obtained by fluorescence‐activated cell sorting were cultured in DMEM medium supplemented with 10% FBS, 1% v/v penicillin/streptomycin, and 50 μg ml−1 zeocin. Knockout cell lines were generated using the CRISPR‐Cas9 method as previously described 36 . Briefly, the pHKO plasmid containing the guide RNA targeting TRIM32 was co‐transfected with packaging plasmid psPAX2 and envelope plasmid pMD2.G into Cas9‐expressed 293T cells. The transfection cocktail was removed after 6 h and replaced by a fresh medium. After 72 h, the viral‐containing supernatant was collected and filtered with a 0.45‐μm membrane and stored at 4°C before transduction. The 293T cells were transduced with the lentiviral particles. Three days later, GFP‐positive cells were sorted into single clones in 96‐well plates by flow cytometry and knockout lines were identified by PCR and by Western blot with antibodies specific for TRIM32. The sequence for the guide RNA used for TRIM32 knockout is 5′‐CCAGTTTGTAGTAACCGATG‐3′.
Immunoprecipitation
For immunoprecipitation, 293T cells at a confluency of 60%–70% in six‐well plates were transfected with a total of 5 μg plasmids that code for the protein of interest. Twenty‐four hours after transfection, cells were washed once with PBS and lysed in buffer A containing 25 mM Tris‐HCl, pH 7.5, 150 mM NaCl, 10% glycerol, and 1% Triton X‐100, supplemented with a protease inhibitor mixture (Roche Molecular Biochemicals). Precleared lysates were subjected to anti‐Flag M2 or anti‐GFP immunoprecipitation following the manufacturer's instructions. The beads were washed four times with lysis buffer, and the immunoprecipitates were eluted with sodium dodecyl sulfate (SDS) sample buffer, followed by standard immunoblotting analysis. All the immunoprecipitation assays were performed more than three times, and representative results are shown. For enrichment of the arginine‐GlcNAcylated proteins from lysates of transfected cells, samples were washed three times in ice‐cold PBS and lysed in buffer A containing 25 mM Tris‐HCl, pH 7.5, 150 mM NaCl, 10% glycerol, and 1% Triton X‐100, supplemented with a protease inhibitor mixture (Roche Molecular Biochemicals). Precleared lysates were subjected to immunoprecipitation with the anti‐Arg‐GlcNAc antibodies 29 . The beads were washed four times with the lysis buffer, and the immunoprecipitates were dissolved by SDS sample buffer.
Immunofluorescence labeling and confocal microscopy
At the indicated time points post transfection or bacterial infection, cells were fixed for 10 min with 4% paraformaldehyde in PBS and permeabilized for 15 min with 0.2% Triton X‐100 in PBS. After the blockade of nonspecific binding by incubation of cells for 30 min with 2% bovine serum albumin in PBS, coverslips were incubated with the appropriate primary antibodies and subsequently with fluorescein‐labeled secondary antibodies (Thermo Fisher Scientific). Confocal fluorescence images were acquired under the confocal microscope (Spinning Disc; Leica). All image data shown are representative of at least three randomly selected fields.
Expression and purification of recombinant proteins
Protein expression was induced in Escherichia coli BL21(DE3) strain (Novagen) harboring the appropriate plasmid that expresses the protein of interest at 22°C for 15 h with 0.4 mM isopropyl‐β‐d‐thiogalactopyranoside after the cultures had reached an OD600 of 0.8–1.0. Affinity purification of GST‐SseK3 was performed using glutathione sepharose (GE Healthcare), and purification of 6×His‐SUMO‐hUBE1, 6×His‐TRIM32, 6×His‐TRIM32 (ΔRING), and 6×His‐Flag‐Ub was conducted using Ni‐NTA agarose (Qiagen) following the manufacturer's instructions. Proteins were concentrated in a buffer containing 20 mM HEPES, pH 7.5, 150 mM NaCl, and 5% glycerol. The protein concentration was determined using the Bradford method.
In vitro ubiquitination assays
To assay ubiquitination of SseK3 in vitro, Ub (5 μg), E1 (200 ng), UBCH5a (300 ng), His‐TRIM32 (0.8 μg), and GST‐SseK3 (2 μg) were incubated with 2 mM ATP at 37°C for 2 h in Ub assay buffer (20 mM Tris‐HCl, pH 7.5, 5 mM MgCl2, 2 mM dithiothreitol [DTT]). Reactions were stopped by adding 30 mM EDTA and 15 mM DTT. After GST pull‐down, the sample was washed with 1 M urea for 60 min to exclude potential binding of unanchored polyubiquitin, then the sample was placed in an SDS‐loading buffer, and finally boiled at 95°C for 5 min. Samples were subsequently analyzed by SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE), followed by Western blot analysis.
MS analyses
For identification of the GlcNAcylated arginine and arginine‐containing peptides, purified SNAP25 protein was subjected to digestion with GluC and LysC, and the resulting peptides were separated on an EASY‐nLC 1200 system (Thermo Fisher Scientific). The nano liquid chromatography gradient was as follows: 0%–8% B in 3 min, 8%–28% B in 42 min, 28%–38% B in 5 min, and 38%–100% B in 10 min (solvent A: 0.1% formic acid in water; solvent B: 80% CH3CN in 0.1% formic acid). Peptides eluted from the capillary column were applied directly onto a Q Exactive Plus mass spectrometer by electrospray (Thermo Fisher Scientific) for MS and MS/MS analyses. Mass spectrometry data were searched against the amino acid sequence of SNAP25 and were performed with cleavage specificity allowing four miscleavage events. Mass spectrometry data were searched with the variable modifications of oxidation of methionine, N‐acetyl‐hexosamine addition to arginine (Arg‐GlcNAc), and acetylation of protein N termini. For identification of the SNAP25‐binding protein, immunoprecipitates were separated using SDS‐PAGE, fixed, and visualized after silver staining as recommended by the manufacturer. An entire lane of bands was excised and subjected to in‐gel trypsin digestion and MS/MS detections as described above. Identification of proteins was carried out using the Proteome Discoverer 2.2 program. Mass spectrometry data were searched against the Human proteomes depending on the samples with carbamidomethylation of cysteine set as a fixed modification. The precursor mass tolerance was set to 10 ppm and the fragment mass tolerance was set to 0.02 Da. A maximum false discovery rate of 1.0% was set for protein and peptide identifications.
Quantification and statistical analysis
All results are presented as mean ± standard deviation containing a specified number of replicates. Data were analyzed using a Student's t‐test to compare two experimental groups. The comparison of multiple groups was conducted using one‐way analysis of variance. *p < 0.05 and **p < 0.01 were considered as statistically significant.
AUTHOR CONTRIBUTIONS
Kun Meng: Conceptualization (supporting); data curation (equal); formal analysis (lead); funding acquisition (supporting); investigation (lead); validation (lead); writing—original draft (equal); writing—review and editing (equal). Jin Yang: Data curation (equal); formal analysis (supporting); investigation (equal). Juan Xue: Formal analysis (supporting); investigation (supporting). Jun Lv: Investigation (supporting); resources (supporting). Ping Zhu: Formal analysis (supporting). Liuliu Shi: Formal analysis (supporting); writing—review and editing (supporting). Shan Li: Conceptualization (lead); funding acquisition (lead); project administration (lead); resources (lead); supervision (lead); writing—original draft (lead); writing—review and editing (lead).
ETHICS STATEMENT
This study did not involve human subjects and animals.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We thank the members of the Li laboratory and the central laboratory of Taihe Hospital for helpful discussions and technical assistance. We thank Prof. Hongbing Shu and Prof. Bo Zhong at Wuhan University (China) for providing the pRK‐HA‐Ub (K48 only) and pRK‐HA‐Ub (K63 only) plasmids. We also thank Prof. Gang Cao at the Huazhong Agricultural University (China) for providing the pHKO14‐Cas9‐Flag and pHKO‐GFP‐sgRNA plasmids. This work was supported by the National Key Research and Development Programs of China (2021YFD1800404 and 2018YFA0508000), the National Natural Science Foundation of China (32270197 and 32200156), the Natural Science Foundation of Hubei Provincial Department of Education (D20222104 and Q20212106), and the Hubei Provincial Natural Science Foundation (2021CFB472 and 2022CFB934).
Meng K, Yang J, Xue J, Lv J, Zhu P, Shi L, et al. A host E3 ubiquitin ligase regulates Salmonella virulence by targeting an SPI‐2 effector involved in SIF biogenesis. mLife. 2023;2:141–158. 10.1002/mlf2.12063
Edited by Zhao‐Qing Luo, Purdue University, USA
DATA AVAILABILITY
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the data set identifier PXD019062 and PXD039746. All other data supporting the findings of this study are available within the article and the Supporting Information: Data, or from the corresponding author upon reasonable request.
REFERENCES
- 1. Acheson D, Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–9. [DOI] [PubMed] [Google Scholar]
- 2. Dos Santos AMP, Ferrari RG, Conte‐Junior CA. Type three secretion system in Salmonella Typhimurium: the key to infection. Genes Genom. 2020;42:495–506. [DOI] [PubMed] [Google Scholar]
- 3. Lou L, Zhang P, Piao R, Wang Y. Salmonella pathogenicity island 1 (SPI‐1) and its complex regulatory network. Front Cell Infect Microbiol. 2019;9:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jennings E, Thurston TLM, Holden DW. Salmonella SPI‐2 type III secretion system effectors: molecular mechanisms and physiological consequences. Cell Host Microbe. 2017;22:217–31. [DOI] [PubMed] [Google Scholar]
- 5. Knuff K, Finlay BB. What the SIF is happening—the role of intracellular Salmonella‐induced filaments. Front Cell Infect Microbiol. 2017;7:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santos JC, Enninga J. At the crossroads: communication of bacteria‐containing vacuoles with host organelles. Cell Microbiol. 2016;18:330–9. [DOI] [PubMed] [Google Scholar]
- 7. Stein MA, Leung KY, Zwick M, Portillo FG, Finlay BB. Identification of a Salmonella virulence gene required for formation of filamentous structures containing lysosomal membrane glycoproteins within epithelial cells. Mol Microbiol. 1996;20:151–64. [DOI] [PubMed] [Google Scholar]
- 8. Liss V, Swart AL, Kehl A, Hermanns N, Zhang Y, Chikkaballi D, et al. Salmonella enterica remodels the host cell endosomal system for efficient intravacuolar nutrition. Cell Host Microbe. 2017;21:390–402. [DOI] [PubMed] [Google Scholar]
- 9. Noster J, Chao T‐C, Sander N, Schulte M, Reuter T, Hansmeier N, et al. Proteomics of intracellular Salmonella enterica reveals roles of Salmonella pathogenicity island 2 in metabolism and antioxidant defense. PLoS Pathog. 2019;15:e1007741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dikic I. Proteasomal and autophagic degradation systems. Annu Rev Biochem. 2017;86:193–224. [DOI] [PubMed] [Google Scholar]
- 11. Herhaus L, Dikic I. Regulation of Salmonella–host cell interactions via the ubiquitin system. Indian J Med Microbiol. 2018;308:176–84. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Higashide WM, McCormick BA, Chen J, Zhou D. The inflammation‐associated Salmonella SopA is a HECT‐like E3 ubiquitin ligase. Mol Microbiol. 2006;62:786–93. [DOI] [PubMed] [Google Scholar]
- 13. Fiskin E, Bhogaraju S, Herhaus L, Kalayil S, Hahn M, Dikic I. Structural basis for the recognition and degradation of host TRIM proteins by Salmonella effector SopA. Nat Commun. 2017;8:14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamanova J, Sun H, Lara‐Tejero M, Galán JE. The Salmonella effector protein SopA modulates innate immune responses by targeting TRIM E3 ligase family members. PLoS Pathog. 2016;12:e1005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhavsar AP, Brown NF, Stoepel J, Wiermer M, Martin DDO, Hsu KJ, et al. The Salmonella type III effector SspH2 specifically exploits the NLR co‐chaperone activity of SGT1 to subvert immunity. PLoS Pathog. 2013;9:e1003518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haraga A, Miller SI. A Salmonella type III secretion effector interacts with the mammalian serine/threonine protein kinase PKN1. Cell Microbiol. 2006;8:837–46. [DOI] [PubMed] [Google Scholar]
- 17. Keszei AFA, Tang X, McCormick C, Zeqiraj E, Rohde JR, Tyers M, et al. Structure of an SspH1–PKN1 complex reveals the basis for host substrate recognition and mechanism of activation for a bacterial E3 ubiquitin ligase. Mol Cell Biol. 2014;34:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bernal‐Bayard J, Ramos‐Morales F. Salmonella type III secretion effector SlrP is an E3 ubiquitin ligase for mammalian thioredoxin. J Biol Chem. 2009;284:27587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernal‐Bayard J, Cardenal‐Muñoz E, Ramos‐Morales F. The Salmonella type III secretion effector, Salmonella leucine‐rich repeat protein (SlrP), targets the human chaperone ERdj3. J Biol Chem. 2010;285:16360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ye Z, Petrof EO, Boone D, Claud EC, Sun J. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol. 2007;171:882–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mesquita FS, Holden DW, Rolhion N. Lack of effect of the Salmonella deubiquitinase SseL on the NF‐κB pathway. PLoS One. 2013;8:e53064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hermanns T, Hofmann K. Bacterial DUBs: deubiquitination beyond the seven classes. Biochem Soc Trans. 2019;47:1857–66. [DOI] [PubMed] [Google Scholar]
- 23. Otten EG, Werner E, Crespillo‐Casado A, Boyle KB, Dharamdasani V, Pathe C, et al. Ubiquitylation of lipopolysaccharide by RNF213 during bacterial infection. Nature. 2021;594:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu Y, Zhou P, Cheng S, Lu Q, Nowak K, Hopp A‐K, et al. A bacterial effector reveals the V‐ATPase‐ATG16L1 axis that initiates xenophagy. Cell. 2019;178:552–66. [DOI] [PubMed] [Google Scholar]
- 25. Galán JE. Salmonella Typhimurium and inflammation: a pathogen‐centric affair. Nat Rev Microbiol. 2021;19:716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tuli A, Sharma M. How to do business with lysosomes: Salmonella leads the way. Curr Opin Microbiol. 2019;47:1–7. [DOI] [PubMed] [Google Scholar]
- 27. Meng K, Zhuang X, Peng T, Hu S, Yang J, Wang Z, et al. Arginine GlcNAcylation of Rab small GTPases by the pathogen Salmonella Typhimurium. Commun Biol. 2020;3:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan X, Luo J, Li S. Bacteria‐catalyzed arginine glycosylation in pathogens and host. Front Cell Infect Microbiol. 2020;10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pan M, Li S, Li X, Shao F, Liu L, Hu HG. Synthesis of and specific antibody generation for glycopeptides with arginine N‐GlcNAcylation. Angew Chem Int Ed. 2014;53:14517–21. [DOI] [PubMed] [Google Scholar]
- 30. Singh Y, Saxena A, Kumar R, Saxena MK. Virulence system of Salmonella with special reference to Salmonella enterica . In: Mascellino MT, editor. Salmonella: a reemerging pathogen. London: IntechOpen Limited; 2018. p. 41–53. [Google Scholar]
- 31. Harbury PA. Springs and zippers: coiled coils in SNARE‐mediated membrane fusion. Structure. 1998;6:1487–91. [DOI] [PubMed] [Google Scholar]
- 32. Stévenin V, Giai Gianetto Q, Duchateau M, Matondo M, Enninga J, Chang Y‐Y. Purification of infection‐associated macropinosomes by magnetic isolation for proteomic characterization. Nat Protoc. 2021;16:5220–49. [DOI] [PubMed] [Google Scholar]
- 33. Stévenin V, Chang Y‐Y, Le Toquin Y, Duchateau M, Gianetto QG, Luk CH, et al. Dynamic growth and shrinkage of the Salmonella‐containing vacuole determines the intracellular pathogen niche. Cell Rep. 2019;29:3958–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kehl A, Göser V, Reuter T, Liss V, Franke M, John C, et al. A trafficome‐wide RNAi screen reveals deployment of early and late secretory host proteins and the entire late endo‐/lysosomal vesicle fusion machinery by intracellular. PLoS Pathog. 2020;16:e1008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Avraham R, Haseley N, Brown D, Penaranda C, Jijon HB, Trombetta JJ, et al. Pathogen cell‐to‐cell variability drives heterogeneity in host immune responses. Cell. 2015;162:1309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang Z, Soderholm A, Lung TWF, Giogha C, Hill MM, Brown NF, et al. SseK3 is a Salmonella effector that binds TRIM32 and modulates the host's NF‐κB signalling activity. PLoS One. 2015;10:e0138529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC Biol. 2012;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clague MJ, Urbé S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–5. [DOI] [PubMed] [Google Scholar]
- 39. Tripathi‐Giesgen I, Behrends C, Alpi AF. The ubiquitin ligation machinery in the defense against bacterial pathogens. EMBO Rep. 2021;22:e52864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lazzari E, Meroni G. TRIM32 ubiquitin E3 ligase, one enzyme for several pathologies: from muscular dystrophy to tumours. Int J Biochem Cell Biol. 2016;79:469–77. [DOI] [PubMed] [Google Scholar]
- 41. Koepke L, Gack MU, Sparrer KM. The antiviral activities of TRIM proteins. Curr Opin Microbiol. 2021;59:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang Q, Liu T‐T, Lin H, Zhang M, Wei J, Luo W‐W, et al. TRIM32–TAX1BP1‐dependent selective autophagic degradation of TRIF negatively regulates TLR3/4‐mediated innate immune responses. PLoS Pathog. 2017;13:e1006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Newson JM, Scott N, Yeuk Wah Chung I, Wong Fok Lung T, Giogha C, Gan J, et al. Salmonella effectors SseK1 and SseK3 target death domain proteins in the TNF and TRAIL signaling pathways. Mol Cell Proteomics. 2019;18:1138–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. D'Costa VM, Coyaud E, Boddy KC, Laurent EMN, St‐Germain J, Li T, et al. BioID screen of Salmonella type 3 secreted effectors reveals host factors involved in vacuole positioning and stability during infection. Nat Microbiol. 2019;4:2511–22. [DOI] [PubMed] [Google Scholar]
- 45. Ramachandran KV, Fu JM, Schaffer TB, Na CH, Delannoy M, Margolis SS. Activity‐dependent degradation of the nascentome by the neuronal membrane proteasome. Mol Cell. 2018;71:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo X. Localized proteasomal degradation: from the nucleus to cell periphery. Biomolecules. 2022;12:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hwang J, Qi L. Quality control in the endoplasmic reticulum: crosstalk between ERAD and UPR pathways. Trends Biochem Sci. 2018;43:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakagawa T, Shirane M, Iemura S, Natsume T, Nakayama KI. Anchoring of the 26S proteasome to the organellar membrane by FKBP38. Genes Cells. 2007;12:709–19. [DOI] [PubMed] [Google Scholar]
- 49. Eisenberg‐Lerner A, Benyair R, Hizkiahou N, Nudel N, Maor R, Kramer MP, et al. Golgi organization is regulated by proteasomal degradation. Nat Commun. 2020;11:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tocchini C, Ciosk R. TRIM‐NHL proteins in development and disease. Semin Cell Dev Biol. 2015;47:52–9. [DOI] [PubMed] [Google Scholar]
- 51. Chaikuad A, Zhubi R, Tredup C, Knapp S. Comparative structural analyses of the NHL domains from the human E3 ligase TRIM–NHL family. IUCrJ. 2022;9:720–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garcia‐Garcia J, Overå KS, Khan W, Sjøttem E. Generation of the short TRIM32 isoform is regulated by Lys 247 acetylation and a PEST sequence. PLoS One. 2021;16:e0251279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Günster RA, Matthews SA, Holden DW, Thurston TLM. SseK1 and SseK3 type III secretion system effectors inhibit NF‐κB signaling and necroptotic cell death in Salmonella‐infected macrophages. Infect Immun. 2017;85:e00010‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the data set identifier PXD019062 and PXD039746. All other data supporting the findings of this study are available within the article and the Supporting Information: Data, or from the corresponding author upon reasonable request.


