Abstract
Heart failure (HF) is a sophisticated syndrome with structural or functional impairment of ventricular filling or ejection of blood, either causing symptoms and signs or being asymptomatic. HF is a major global health issue affecting about 64.3 million people worldwide. The gut microbiota refers to the complex ecosystem of microorganisms, mainly bacteria, in the gut. Studies have revealed that the gut microbiota is associated with many diseases ranging from neurodegenerative diseases to inflammatory bowel disease and cardiovascular diseases. The gut hypothesis of HF suggests that low cardiac output and systemic circulation congestion would cause insufficient intestinal perfusion, leading to ischemia and intestinal barrier dysfunction. The resulting bacterial translocation would contribute to inflammation. Recent studies have refined the hypothesis that changes of metabolites in the gut microbiota have a close relationship with HF. Thus, the gut microbiota has emerged as a potential therapeutic target for HF due to both its critical role in regulating host physiology and metabolism and its pivotal role in the development of HF. This review article aims to provide an overview of the current understanding of the gut microbiota's involvement in HF, including the introduction of the gut hypothesis of HF, its association with HF progression, the potential mechanisms involved mediated by the gut microbiota metabolites, and the impact of various interventions on the gut microbiota, including dietary interventions, probiotic therapy, fecal microbiota transplantation, antibiotics, and so on. While the gut hypothesis of HF is refined with up‐to‐date knowledge and the gut microbiota presents a promising target for HF therapy, further research is still needed to further understand the underlying mechanisms between gut microbiota and HF, the efficacy of these interventions, and contribute to the health of HF patients.
Keywords: gut microbiota, heart failure, short‐chain fatty acids, trimethylamine N‐oxide
Heart failure is associated with the gut microbiota, while the gut hypothesis of heart failure is less discussed in a thorough manner. We reviewed how changes in metabolites of the gut microbiota contribute to heart failure and possible underlying mechanisms. We also reviewed potential interventions for heart failure targeting the gut microbiota, including dietary interventions, probiotic therapy, fecal microbiota transplantation, antibiotics, and other approaches.
Highlights
Heart failure is associated with the gut microbiota, while the gut hypothesis of heart failure is less discussed in a thorough manner.
We reviewed how changes in metabolites of the gut microbiota contribute to heart failure and possible underlying mechanisms.
We also reviewed potential interventions for heart failure targeting the gut microbiota, including dietary interventions, probiotic therapy, fecal microbiota transplantation, antibiotics, and other approaches.
INTRODUCTION
Research on the gut microbiota has been flourishing in recent years, with numerous studies reporting its relationship with diseases such as type 2 diabetes, obesity, fatty liver disease, gastrointestinal diseases, and certain types of cancer [1]. Worldwide, there are approximately 64.3 million heart failure (HF) patients, with HF patients accounting for 1%–2% of adults in developed countries [2, 3]. The definition of HF was reached in 1983, it is suggested that “Heart failure is the state of any heart disease in which, despite adequate ventricular filling, the heart's output is decreased or in which the heart is unable to pump blood at a rate adequate for satisfying the requirements of the tissues with function parameters remaining within normal limits” [4]. The 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) guideline indicates that “HF is a complex clinical syndrome with symptoms and signs that result from any structural or functional impairment of ventricular filling or ejection of blood,” and asymptomatic stages with either cardiomyopathies or structural heart disease are considered at‐risk for HF or pre‐HF [5]. HF is characterized by circulatory congestion, which can lead to intestinal swelling and damage to the intestinal barrier. This can exacerbate inflammation through bacterial translocation, highlighting the potential role of the gut microbiota in HF [6].
Prevention of HF is important, especially for patients at at‐risk for HF or pre‐HF. Primary preventions cover controlling blood pressure, usage of sodium‐glucose cotransporter 2 inhibitors (SGLT2i) for patients with type 2 diabetes, and a healthy lifestyle with no cigarettes [5]. Treatments for HF mainly include pharmacological treatment, device and interventional therapies, mechanical circulatory support (MCS), and heart transplantation [5]. Commonly used drugs contain renin–angiotensin system inhibitors such as angiotensin‐converting enzyme inhibitors (ACEi) or angiotensin (II) receptor blockers (ARB) or angiotensin receptor‐neprilysin inhibitors (ARNi), beta‐blockers, mineralocorticoid receptor antagonists, SGLT2i, hydralazine, isosorbide dinitrate, and other drugs [5]. Device and interventional therapies mainly refer to implantable cardioverter defibrillator and cardiac resynchronization therapy to prevent sudden cardiac death. The most widely used MCS is the left ventricular assist device, which is regarded as both a bridge to transplantation and as destination therapy [7]. End‐stage HF patients satisfying certain criteria may undergo heart transplantation [8].
According to an estimation, the microbes in bodies could collectively consist of as many as 100 trillion cells, which is 10‐fold the number of human cells. It is also pointed out that microbes could encode 100‐fold more unique genes than the human genome [9]. It is believed that the gut holds the majority of microbes [10]. By examining fecal samples of 124 European individuals, it is found that each habored at least 160 bacterial species [11]. Besides HF, the gut microbiota is closely related to many diseases, including autism spectrum disorder [12], neurodegenerative diseases such as Alzheimer's disease [13] and Parkinson's disease [14], inflammatory bowel disease (IBD) [15], cardiovascular diseases (CVDs) such as atherosclerosis [16] and ischemic heart disease [17], and so on. As for HF, the gut microbiota acts like an endocrine organ, as several metabolites generated by its metabolism are involved in the disease status of HF [6].
The whole story originated from the gut hypothesis. By searching the Pubmed with keywords “(gut hypothesis) AND (heart failure),” the first article discussing the gut hypothesis is in 1999 by Niebauer et al. [18]. Actually, the concept of the gut hypothesis in heart failure could be traced back to no later than 1997, implying the role of chronic heart failure (CHF) in leading to increased bowel permeability and consequently bacterial translocation and release of endotoxin [19]. Overall, the inflammatory response would be triggered in HF patients [20]. After that, more researchers focused on the relationship between the gut microbiota and HF. To explore the complex interactions between the gut microbiota, a series of studies have been performed in discovering changes in the gut microbiota in HF patients [21], researching the effects of metabolites by the gut microbiota in contributing to HF [22], and possible interventions concerning the gut microbiota or the metabolites in alleviating HF [23]. Metabolites, mainly trimethylamine N‐oxide (TMAO) and short‐chain fatty acids (SCFAs), play an important role in the interaction with HF. Certain daily diet intakes will be transformed into trimethylamine (TMA) by the gut microbiota and eventually converted into TMAO in the liver. SCFAs are generated from dietary fibers and contribute to providing energy to the failing heart [24]. Other substances including N,N,Ntrimethyl‐5‐aminovaleric acid (TMAVA) is also produced during gut metabolism from trimethyllysine and contribute to HF.
In this review, we aim to briefly introduce “the gut hypothesis of heart failure,” the role of gut microbiota metabolites in HF, and related interventions.
GUT HYPOTHESIS OF HEART FAILURE AND IMPAIRMENT OF INTESTINAL BARRIER
The “gut hypothesis of HF” has gained popularity in recent years, with increasing amounts of studies supporting its validity. This hypothesis proposes that low cardiac output and circulation congestion in the system lead to reduced intestinal perfusion, resulting in ischemia and damage to the intestinal barrier. The intestinal barrier is mainly formed by the intestinal epithelium mechanically linked with each adjacent cell with selective permeability to enable the absorption of nutrients, electrolytes, and water, and disable the invasion of toxins, antigens, and enteric flora [25]. Ischemia of the intestines would generate a series of pathological changes such as transmural necrosis and reversible mucosal injury, and the severeness depends on the severity and duration of ischemia, whether it is occlusive or non‐occlusive, where and to what content is occluded and how long it takes to start pathological examination after ischemia [26]. In severe cases, digestive enzymes may enter the intestinal wall during the ischemic period as a result of intestinal barrier dysfunction [27].
Back in 1997, Anker et al. [19] hypothesized that mesenteric venous congestion in CHF leads to an increase in bowel permeability, thus contributing to bacterial translocation and release of endotoxin deteriorating inflammation. In 1999, Anker's team went further, Niebauer et al. [18] proved the hypothesis that there would be an increase in bacterial translocation and endotoxemia caused by altered gut permeability in CHF patients with edema. A damaged barrier can increase permeability, allowing bacterial translocation and endotoxins to enter the bloodstream, contributing to inflammatory responses in HF patients (Figure 1) [18, 20, 28]. This relationship has been further explored and refined to be more concrete. Besides bacterial translocation and endotoxemia, metabolites of the gut microbiota also have an impact on HF. The concept of “refining the gut hypothesis” is first used in a research article indicating the prognostic value of elevated levels of TMAO in HF and suggesting a potential link between the gut microbiota pathway and poor prognosis in HF patients [29]. Besides TMAO, SCFAs, and other metabolites such as TMAVA and phenylacetylgutamine (PAGln) also play an important role in the interaction of the gut microbiota and HF [30, 31, 32]. Up to now, a more specific correlation has been confirmed and the hypothesis has been refined: Congestion in HF would cause increased bowel permeability, followed by bacterial translocation and inflammation, and alterations in the gut microbiota can exacerbate HF through metabolites, mainly TMAO, SFCAs, and resulting in a vicious cycle.
Figure 1.
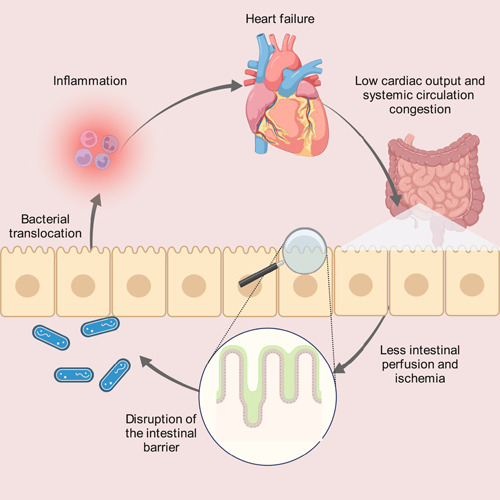
The gut hypothesis of heart failure. The gut hypothesis of HF proposes that low cardiac output and systemic circulation congestion lead to reduced intestinal perfusion, resulting in ischemia and consequently intestinal barrier disruption. A damaged barrier with increased permeability allows bacterial translocation and endotoxins to release into the bloodstream, contributing to inflammation and worsening HF.
CHANGES IN GUT MICROBIOTA COMPOSITION
The human gut is colonized by a sophisticated ecosystem of microorganisms, including bacteria, viruses, and fungi, collectively known as the gut microbiota [33]. In this context, we will discuss the changes that occur in the gut microbiota of patients with HF (Table 1). Multiple studies have revealed that the gut microbiota composition is different between HF patients and healthy controls (Table 2) [28, 34, 35, 36, 37, 38, 39, 40]. For instance, certain bacteria, such as Bacteroides/Prevotellain, Eubacterium rectale, and Fusobacterium prausnitzii, are found to be more frequent in HF patients, while others, such as Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae, are decreased [28, 36]. These changes can also impact systemic conditions, including persistent T‐cell activation and increased susceptibility to Clostridium difficile infection [40, 41]. Furthermore, the diversity of the gut microbiota is reduced in HF patients.
Table 1.
Changes of gut microbiota in heart failure patients.
| Increase | Decrease | |
|---|---|---|
| Phylum | — | Firmicutes |
| Family | Enterococcaceae | Lachnospiraceae, Rumminococcaceae |
| Genus | Bacteroides/Prevotellain, Campylobacter, Shigella, Salmonella, Prevotella, Hungatella, Succinclasticum Enterococcus, Synergistete, Lactobacillus | Blautia, Collinsella, uncl. Erysipelotrichaceae, uncl. Ruminococcaceae, Faecalibacterium, Ruminococcaceae UCG‐004, Ruminococcaceae UCG‐002, Lachnospiraceae FCS020 group, Butyricicoccus, Sutterella, Lachnospira, Ruminiclostridium |
| Species | Eubacterium rectale a, Fusobacterium prausnitzii, Yersinia enterocolitic | Eubacterium rectale a, Dorealongicatena |
| Fungi | Candida, Candida species | — |
Abbreviation: uncl., unclassified.
Eubacterium rectale is found to increase in HF patients by Sandek et al., while it is also reported to decrease by Kamo et al.
Table 2.
Summary of studies on changes in the gut microbiota in heart failure.
| Source | Time | Sample sizea | Microbiota | Results | Other |
|---|---|---|---|---|---|
| Sandek et al. [28] | 2007 | 22 CHF and 22 controls | Bacteroides/Prevotellain, Eubacterium rectale, and Fusobacterium prausnitzii | Increase | Bacteria were adherent to the mucosa more often |
| Sandek et al. [34] | 2014 | 21 CHF and 17 control | Both anaerobic and aerobic bacteria | Similar | Bacteria were restricted to the juxtamucosal zone more often |
| Pasini et al. [35] | 2016 | 60 CHF (NYHA I–II 30, III–IV 30) and 20 controls | Pathogenic bacteria and Candida such as Campylobacter, Salmonella, Shigella, Yersinia enterocolitica, and Candida species | Increase | Abundancy was different between two NYHA groups |
| Luedde et al. [36] | 2017 | 20 HFrEF and 20 controls | Blautia, Collinsella, uncl. Erysipelotrichaceae and uncl. Ruminococcaceae. | Decrease | Diversity decreased |
| Kamo et al. [37] | 2017 | 12 HF and 12 controls (age‐matched) | Eubacterium rectale and Dorea longicatena | Decrease | Older HF patients have less Bacteroidetes and more Proteobacteria |
| Kummen et al. [38] | 2018 | 84 stable HFrEF (40 discovery, and 44 validation (NYHA II–IV) and 266 controls | Genus Prevotella, Hungatella and Succinclasticum | Increase | Bacterial richness decreases in HF patients after adjustment |
| Lachnospiraceae family,b Rumminococcaceae Faecalibacterium and Bifidobactericeae Bifidobacterium | Decrease | ||||
| Sun et al. [39] | 2021 | 29 Severe CHF (NYHA III–IV) and 30 controls | Enterococcus and Enterococcaceae | Increase | Lower bacterial richness in chronic HF patients. Remarkable decrease in bacteria generating SCFAs. Increased production of lactic acid. |
| Phylum Firmicutes, genera Ruminococcaceae UCG‐002, Ruminococcaceae UCG‐004, Lachnospiraceae FCS020 group | Decrease | ||||
| Huang et al. [40] | 2021 | 30 HFpEF and 30 controls | Phylum Synergistetes, genus Enterococcus and Lactobacillus | Increase | Increase of microbiota linked with inflammation and decrease of microbiota linked with anti‐inflammatory effects |
| Genus Butyricicoccus, Sutterella, Lachnospira, and Ruminiclostridium | Decrease |
Abbreviations: CHF, chronic heart failure; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
Only includes samples used to identify microbiota changes.
Includes Anaerostipes, Blautia, Coprococcus (3), Fusicatenibacter, Lachnospiraceae FCS020, NCS2004, ND3007, and Pseudobutyrivibrio.
Studies have found that certain bacteria, such as Bacteroides/Prevotellain, Eubacterium rectale, and Fusobacterium prausnitzii, are more frequently found in CHF patients than in controls [28]. Additionally, these bacteria were found to adhere more often to the intestinal mucosa [28]. However, another study by Sandek et al. [34] suggested that more bacteria are restricted to the juxtamucosal zone, after examining both anaerobic and aerobic bacteria in stool. Pasini et al. [35] reported an increase in Campylobacter, Candida, Salmonella, Shigella, Yersinia enterocolitica, and Candida species in the entire CHF group. Specifically, considering colony‐forming units/mL (×105) of stool, Candida (37.2 ± 4.4 vs. 2.9 ± 1.1), Campylobacter (164.0 ± 6.1 vs. 8.3 ± 1.3), Shigella (70.4 ± 17.2 vs. 7.9 ± 1.7), and Salmonella (37.6 ± 13.1 vs. 20.2 ± 4.9) were found to be higher in New York Heart Association (NYHA) III to IV than in NYHA I to II CHF patients, while Yersinia enterocolitica (24.8 ± 7.5 vs. 23.1 ± 5.9) was similar between the two groups [35]. Elevation of the genera Enterococcus and Enterococcaceae is also observed in HF patients, leading to an increased lactic acid level [39].
In addition to the increased bacteria in HF patients, certain bacteria are decreased. Studies have shown that the abundance of Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae families is lower in HF patients than in healthy controls when evaluating individual core measurable microbiota (CMM). Blautia, Collinsella, unclassified Erysipelotrichaceae, and unclassified Ruminococcaceae also were found less in HF patients [36]. Furthermore, gut microbiota diversity is found to be significantly lower in HF patients [36]. Another study suggested that HF patients have lower levels of Eubacterium rectale and Dorea longicatena than healthy controls [37]. The trend of Eubacterium rectale in HF seems contradictory, with both an increase and decrease in the gut microbiota being reported. The inconsistency may be ascribed to the varieties of the underlying causes for HF. In patients with coronary heart disease, the relative abundance of Eubacterium rectale was reported to be remarkably higher compared with the healthy controls [42]. Thus, maybe the abundance of Eubacterium rectale is also influenced by the underlying disease that causes HF. Further research is needed for clarifying this contradictory finding. The abundance of Bacteroidetes is higher, while Proteobacteria is lower in young compared with older HF patients [37]. The richness of gut microbiota also decreases in HF patients, and they have lower levels of Lachnospiraceae family, Ruminococcaceae Faecalibacterium, and Bifidobactericeae Bifidobacterium [38]. Additionally, phylum Firmicutes and bacteria that generate SCFAs are also decreased in the HF group [39].
A direction toward inflammation occurs in changes in gut microbiota, with promoting bacteria flourishing and anti‐inflammatory bacteria diminishing, as confirmed in another study [40]. Moreover, the gut microbiota is more prone to be affected in HF patients, with hospitalized HF patients being more frequently affected by Clostridium difficile infection, which is linked with in‐hospital mortality [41]. Not only do changes in gut microbiota affect its composition, but they also impact systemic conditions. Persistent T‐cell activation is shown to be connected with gut microbiota changes in HF patients, which could result in activation of the immune system [40].
Overall, these findings suggest that gut microbiota plays a significant role in the pathophysiology of HF and has the potential of being a therapeutic target.
CHANGES IN METABOLITES CONTRIBUTING TO HEART FAILURE
It has been discovered that when food is broken down in the gut, it produces trimethylamine N‐oxide (TMAO), SCFAs, and endotoxins under the co‐metabolism between microbiota and host [16]. In individuals with HF, there is an increase in the production of TMAO and endotoxins, which contribute to myocardial fibrosis and hypertrophy. The intestinal barrier is also compromised, leading to inflammation that worsens HF [43]. Furthermore, there is a decrease in the abundance of SCFAs‐producing bacteria, which results in lower levels of SCFAs in HF patients. Other metabolites like TMAVA and PAGln are also involved in HF (Figure 2).
Figure 2.
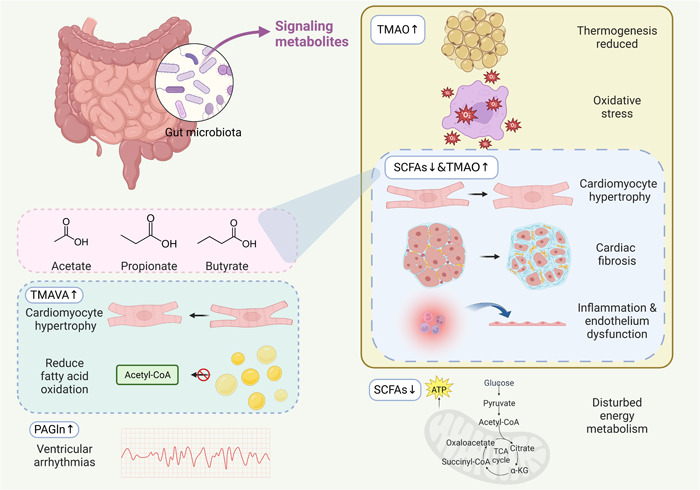
Metabolite changes contribute to HF. TMAO contributes to HF by promoting cardiac fibrosis and hypertrophy, boosting inflammation and endothelial dysfunction, inducing oxidation, and disturbing thermogenesis. A decrease in SCFA levels has been linked to HF, as SCFAs play an important role in preventing cardiac hypertrophy and fibrosis, reducing inflammation, and satisfying energy metabolism. Therefore, maintaining sufficient levels of SCFAs in the body may be beneficial in the prevention and treatment of HF. TMAVA promotes cardiac hypertrophy and reduces fatty acid oxidation. PAGln increases the susceptibility of ventricular arrhythmias in HF. HF, heart failure; PAGln, phenylacetylgutamine; SCFAs, short‐chain fatty acids; TMAO, trimethylamine N‐oxide; TMAVA, N,N,N‐trimethyl‐5‐aminovaleric acid.
TMAO
The gut microbiota is involved in the conversion of phosphatidylcholine/choline, l‐carnitine, and betaine from the daily diet into TMA. This TMA is then released into the body circulation, in which it is metabolized into TMAO through oxidization by hepatic flavin monooxygenase (FMO) family members, with FMO3 as the rate‐limiting enzyme [44]. In the metabolism, most TMAO is eliminated by the kidney [45].
Through metabolomics studies, it was reported in 2011 that TMAO, produced by gut microbiota, has the ability to predict CVD risk, showing an elevated risk of cardiovascular events in patients with cardiac diseases taking elective coronary angiography [46]. Since then, further research has been conducted, and TMAO has been found to be associated with multiple CVDs, including HF [43], myocardial infarction [47], hypertension [48], and diabetes [49]. Higher levels of TMAO are confirmed to be associated with a higher long‐term mortality risk [29]. Nine strains have been identified with the capability of generating TMAO in vitro, including two phyla, Proteobacteria and Firmicutes, and six genera: Providencia rettgeri, Anaerococcus hydrogenalis, Clostridium hathewayi, Clostridium asparagiforme, Clostridium sporogenes, Edwardsiella tarda, Escherichia fergusonii, and Proteus penneri [50].
As shown in Table 3, TMAO can contribute to HF development at molecular and organ/tissue levels through various and complex interactions, ultimately leading to cardiac fibrosis and hypertrophy, promoting inflammation and endothelial dysfunction, affecting oxidation, and even disrupting thermogenesis.
Table 3.
Summary of studies about mechanisms of TMAO contributing to HF by time.
| Source | Year | Species | Level | Pathway | Effect |
|---|---|---|---|---|---|
| Organ et al. [51] | 2016 | C57BL6/J mice | Organ/system | — | Leads to pulmonary edema, enlargement of heart, increased BNP, decreased left ventricular ejection fraction and myocardial fibrosis |
| Seldin et al. [52] | 2016 | Human endothelial cells, LDLR (−/−) mice | Molecule and gene | NF‐κB pathway | Elevated inflammatory gene expression in mice, promotes recruitment of activated leukocytes to endothelial cells |
| Sun et al. [53] | 2016 | Human umbilical vein endothelial cells | Molecule | — | Induces inflammation and endothelial dysfunction through ROS‐TXNIP‐NLRP3 inflammasome activation |
| Chen et al. [54] | 2017 | Human umbilical vein endothelial cells, aortas from ApoE−/− mice | Molecule | SIRT3–SOD2–mitochondrial ROS signaling pathway (inhibition) | Boosts vascular inflammation through NLRP3 inflammasome activation |
| Makrecka‐Kuka et al. [55] | 2017 | ICR mice | Organ/system | — | Impairs β‐oxidation in cardiac mitochondria, promotes cardiac energy metabolism disturbances, and decreases pyruvate metabolism by impairing substrate flux |
| Li et al. [56] | 2019 | Sprague‐Dawley rats | Molecule | Smad3 pathway | Promotes myocardial hypertrophy and fibrosis |
| Brunt et al. [57] | 2020 | Human and mice | Organ/system | — | Promotes age‐related vascular oxidative stress and endothelial dysfunction |
| Yoshida et al. [58] | 2022 | Mice | Molecule | — | Induces decrease of phosphocreatine and ATP levels in heart tissue by suppressing mitochondrial complex IV activity |
Abbreviations: ATP, adenosine triphosphate; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
TMAO can promote cardiac fibrosis and hypertrophy, leading to myocardial damage. Studies have shown that a TMAO‐rich diet can cause pulmonary edema, enlarged heart, lowered left ventricular ejection fraction, and myocardial fibrosis in mice [51]. Moreover, TMAO treatment has been reported to induce cardiac hypertrophy in cardiomyocytes in vitro and promote cardiac hypertrophy and fibrosis in Sprague‐Dawley rats. The level of atrial natriuretic peptide (ANP) and beta‐myosin heavy chain (β‐MHC) also increased, while the size of cardiomyocytes decreased after blocking the Smad3 pathway using a pharmacological inhibitor SIS3 [56].
TMAO exposure triggers an inflammatory response and endothelial dysfunction. TMAO is closely related to inflammatory status and increased inflammatory gene expression has been observed in mice. TMAO has been reported to increase inflammation in peritoneal dialysis patients [59]. To be more specific, the production of P‐selectin induced by tumor necrosis factor‐alpha (TNF‐α) was found to increase in mesothelial cells by TMAO and TMAO promoted TNF‐α induced by high glucose and expression of CCL2 in endothelial cells [59]. In addition, activated leukocytes are recruited to endothelial cells through the NF‐κB pathway in mice [52]. TMAO has also been found to induce both endothelial dysfunction and inflammation by activating the ROS–TXNIP–NLRP3 inflammasome [53]. Furthermore, TMAO activates the NLRP3 inflammasome by inhibition of the SIRT3–SOD2–mitochondrial ROS signaling pathway [54].
Furthermore, TMAO alters the oxidation process, leading to disturbances in energy metabolism. In both mice and healthy individuals, TMAO accelerates age‐related vascular oxidative stress and endothelial dysfunction. This is evidenced by the associations between TMAO and higher nitrotyrosine abundance in endothelial cells after biopsy, as well as oxidative stress‐related dysfunction of endothelium [57]. Additionally, TMAO could impair β‐oxidation in cardiac mitochondria, promote cardiac energy metabolism disturbances, and decrease pyruvate metabolism by impairing substrate flux, according to another study [55].
Interestingly, TMAO can also affect thermogenesis, which in turn may promote HF. Brown adipose tissue (BAT) is known for its thermogenic properties, but it also performs other functions. Metabolomic analysis has shown that elevated plasma TMAO levels are related to reduced BAT thermogenesis [58]. Experiments on mice have also revealed that TMAO can decrease phosphocreatine and adenosine triphosphate (ATP) levels in heart tissue by suppressing activity of mitochondrial complex IV [58]. Moreover, patients with dilated cardiomyopathy have been found to have elevated TMAO levels and low body temperature, which is associated with poor prognosis of HF. This suggests that TMAO may cause dysfunction of BAT, thus promoting HF [58].
SCFA
SCFAs are saturated aliphatic organic acids composed of 1 to 6 carbon atoms, with acetate (C2), propionate (C3), and butyrate (C4) being the most abundant (≥95%) [60, 61]. SCFAs are mainly produced from dietary fiber by the gut microbiota. Unmetabolized SCFAs would enter portal circulation, with a small number of SCFAs reaching the systemic circulation (Figure 3). In failing hearts, energy starvation occurs due to impaired oxidation of long‐chain fatty acids caused by reduced activity of carnitine palmitoyltransferase 1 (CPT1) on the outer mitochondrial membrane. However, studies have reported that SCFAs can bypass CPT1 and provide energy to the failing heart [24]. SCFAs are transported into colonocytes by monocarboxylate transporters (MCT), with MCT1 being the most widely distributed subtype, in addition to passive distribution [30].
Figure 3.
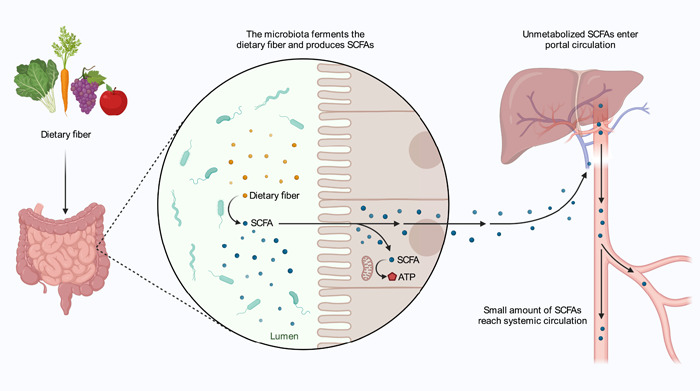
Metabolism of short‐chain fatty acids (SCFAs). SCFAs are mainly produced from dietary fiber by the gut microbiota and used as an energy source by the mitochondria. Unmetabolized SCFAs enter portal circulation, and a small amount of SCFAs reach systemic circulation.
Studies have shown a decrease in SCFA‐producing bacteria in hypertensive HF rats, which is also observed in CHF patients [39, 62]. Subsequent research into SCFA subtypes has revealed lower levels of plasma propionate, butyrate, and isovalerate in HF patients, while no difference is observed in acetate and valerate levels [63]. Interestingly, in patients with congestive HF, higher levels of SCFAs, especially propionate and butyrate, are associated with better cardiac function [30].
SCFAs play a crucial role in the regulation of proliferation, differentiation, and functions of intestinal epithelial cells (IECs) [64]. As the primary energy source of IECs, butyrate consumes up to 3/4 of oxygen for human colonocytes and the converted into ketone bodies [64]. Besides providing energy for healthy cells, butyrate inhibits the expansion of cancerous cells, which is known as the Warburg effect or butyrate paradox [65]. The junctional integrity of IECs could also be promoted by butyrate [66]. Additionally, SFCA can prevent pathophysiological changes in the heart, including cardiac fibrosis, inflammation, and energy metabolism disturbances.
In both mouse models of hypertensive cardiac damage and atherosclerosis, propionate has been found to mitigate cardiac fibrosis, hypertrophy, and vascular dysfunction. Additionally, SCFAs have been linked with an immune‐regulatory role, as propionate has been shown to attenuate systemic inflammation with fewer effector memory T cells and T helper 17 cells in the spleen, and less severe local infiltration of cardiac immune cells [67]. In fiber‐depleted mice, SCFAs have been found to have protective effects on cardiac hypertrophy and fibrosis, which are mediated by SCFAs receptors G‐protein‐coupled receptors (GPCR) 43/GPCR109A, and regulated by the level of L‐3,4‐dihydroxyphenylalanine and DNA‐methylation modulated regulatory T cells [68].
Inflammation is closely related to endothelial cells, and these cells cooperate with immune cells in regulating inflammation, which could be activated by lipopolysaccharide (LPS) and TNF‐α [69]. Once activated, endothelial cells can strengthen the inflammatory response [70]. LPS is a major unit of the Gram‐negative bacteria's cell wall and could induce inflammation through several signaling pathways, which can be inhibited by SCFAs via GPCRs and histone deacetylases (HDACs) [71]. In general, SCFAs can downregulate pro‐inflammatory cytokines and up‐regulate anti‐inflammatory cytokines. For instance, by activating free fatty acid receptors, acetate can reduce the secretion of TNF‐α from mononuclear cells [72]. Butyrate and propionate can downregulate TNF expression and nitric oxide synthase (NOS) in neutrophils induced by LPS [73]. SCFAs also inhibit the generation of other pro‐inflammatory factors, such as IL‐6, IL‐8, and MCP‐1 [74, 75], and induce the release of IL‐10, which functions as an anti‐inflammatory factor, in monocytes [76]. It is suggested that SCFAs perform their anti‐inflammatory effects on LPS‐ or TNF‐α‐stimulated endothelial cells by activating GPCRs 41/43 and inhibiting HDACs [77]. Apart from SCFAs’ effect in modulating inflammation, SCFAs can interact with endothelial cells directly, and their production could improve vascular endothelial function [78].
SCFAs play a significant role in energy metabolism, accounting for about 10% of daily energy demand [79]. For colonic epithelium, SCFAs are the primary energy resource, contributing to approximately 75% of energy metabolism [80]. Acetate serves as a precursor for hepatic and adipocyte lipogenesis, while butyrate is associated with cell growth, differentiation, and mitochondrial activity, improves insulin sensitivity, prevents obesity induced by diet without causing hypophagia, and enhances intestinal barrier function [81, 82]. Propionate, on the other hand, is a necessary substrate for gluconeogenesis and has been shown to reduce food intake and cholesterol synthesis [81, 83]. SCFAs have been reported to support the failing heart since they can bypass CPT1 and be used as an energy source [24]. In summary, SCFAs’ metabolic rate is essential to energy balance, and they play critical roles in various metabolic processes.
Other agents
Change in gut microbiota is a complex process that interacts with HF in various ways. Besides TMAO and SCFAs, the gut microbiota also impacts HF through other agents, including TMAVA, PAGln, and other molecular actors. TMAVA is produced by the gut microbiota from trimethyllysine and has been found to be elevated and associated with an increased risk of cardiac mortality and transplantation [31]. Studies have shown that TMAVA can reduce fatty acid oxidation and promote cardiac hypertrophy in mouse models [31]. PAGln is generated by the gut microbiota and the human liver and has been identified as a risk factor and prognostic indicator of HF [84, 85]. It is related to the presence and severity of HF both clinically and mechanistically [32]. In an HF mouse model, PAGln increased the chance of ventricular arrhythmias by TLR4/AKT/mTOR signaling pathway activation [86].
GUT MICROBIOTA AND HEART FAILURE INTERVENTIONS/TREATMENTS
Recent research has highlighted the importance of gut microbiota in the development and progress of HF. This has led to the exploration of several dietary interventions, including the Dietary Approaches to Stop Hypertension (DASH) diet and Mediterranean diet, probiotic therapy, fecal microbiota transplantation, and antibiotics, as potential treatments for HF (Figure 4). In addition, other interventions, such as vitamin D, B vitamins, berberine, and 3,3‐dimethyl‐1‐butanol (DMB), have been investigated for their potential to reduce HF risk by targeting the gut microbiota. In this section, we will discuss findings regarding these interventions and their effects on the gut microbiota in the context of HF.
Figure 4.
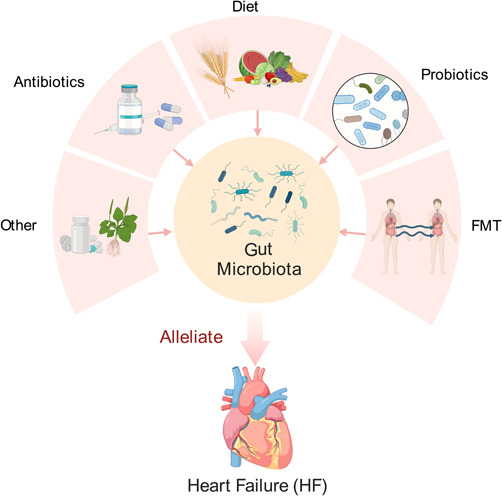
Intervention on the gut microbiota in HF. Interventions targeting the gut microbiota in HF include dietary interventions, such as the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diets, probiotic therapy, fecal microbiota transplantation, antibiotics, and other potential approaches.
Dietary interventions
Gut microbiota is closely related to daily diets, and even a short‐term adjustment of the diet, for only 5 days, is sufficient to alter the composition of gut microbiota and induce corresponding changes for adaptation [87]. As the name suggests, the DASH diet was designed for stopping hypertension through dietary approaches, and it has been regarded as an effective dietary intervention in lowering blood pressure, especially with reduced dietary sodium [88]. Compared with the daily diet, the DASH diet is richer in vegetables, fruits, and low‐fat dairy products [89]. In a cohort study involving 35,004 participants with a median follow‐up period of 22 years, it was suggested that the DASH diet could lower the risk of HF [90]. In HF patients, the DASH diet can also improve 6‐min walking test performance, compliance of artery, exercise capacity, and quality of life scores evaluated after an intervention for 3 months [91]. The Mediterranean diet describes the shared diet pattern among at least 16 countries bordering the Mediterranean Sea [92]. Besides being rich in fruits and vegetables, which is similar to that of the DASH diet, the Mediterranean diet is also characterized by bread, cereals of other forms, potatoes, beans, nuts, seeds, olive oil, little red meat, and low to moderate amounts of dairy products, fish, poultry and wine [92]. Although the Mediterranean diet was linked with lower all‐cause mortality in CVD patients, a pre‐specified secondary analysis from the PREvención con DIeta MEDiterránea (PREDIMED) trial did not find a significant decrease in HF incidence [93, 94]. However, further trials are needed to explore the effects of the Mediterranean diet on HF patients, as this analysis may not be powerful enough to provide solid conclusions [94]. In general, the DASH diet and the Mediterranean diet may assist in the prevention of HF, but high‐quality evidence is needed to establish their efficacy [95].
Probiotic therapy
The definition of a probiotic by the Food and Agriculture Organization of the United Nations and the World Health Organization (FAO/WHO) has been widely adopted, which describes a probiotic as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [96]. Pieces of literature have revealed that Lactobacillus rhamnosus GR‐1 can significantly attenuate hypertrophy and improve not only the systolic but also the diastolic function of the left ventricle, preserve LVEF and fractional shortening after a 6‐week follow‐up, indicating that probiotic therapy has the potential to attenuate HF [97]. In CHF patients, a multistrain probiotic has been reported to reduce sarcopenia and improve functional capacity by regulating the Wnt signaling pathway [98]. A randomized, double‐blind, placebo‐controlled pilot trial targeting HF patients using Saccharomyces boulardii for 3 months showed that it could improve LVEF, shorten left atrial diameter, and lower total cholesterol and uric acid levels [99]. Another randomized, triple‐blind, controlled trial suggested that probiotic yogurt might be helpful in relieving the inflammatory status in CHF patients by elevating sTWEAK levels [100]. However, the randomized Targeting Gut Microbiota to Treat Heart Failure (GutHeart) trial found that treatment with Saccharomyces boulardii or rifaximin for 3 months, on top of standard of care, had no significant effect on LVEF, diversity of microbiota, or the measured biomarkers in HFrEF patients [101]. Further research and studies are needed to figure out the potential effects and underlying mechanisms of probiotic therapy in HF.
Fecal microbiota transplantation
The procedure of transplanting stools from a healthy donor into another patient's intestine is known as fecal microbiota transplantation (FMT) or stool transplantation [102]. FMT has primarily been used to treat recurrent Clostridium difficile infection [102, 103]. However, studies have also focused on the potential of FMT in treating chronic diseases, and the super‐donor phenomenon has been observed, indicating that FMT may be more successful when using feces from specific donors [104]. Nonetheless, FMT can also carry risks, such as importing viral communities together with the necessary microbiota [105]. While the potential effects of FMT on HF are not well studied, it is important to consider both the benefits and risks associated with the procedure, and FMT may hold promise as a supplementary treatment for HF.
Antibiotics
The misuse of antibiotics can disrupt an individual's microbiota and cause harmful effects. However, in some cases, antibiotics may be helpful since microbial translocation can cause harmful events. For example, after an ST‐elevation myocardial infarction, microbial translocation can induce inflammation and cardiovascular events, which can be alleviated by antibiotics [106]. Rifaximin is also widely used to treat microbiota toxicity and translocation by performing anti‐inflammatory effects and promoting the growth of bifidobacteria and lactobacillus [107, 108]. Unfortunately, the effects of antibiotics on gut microbiota in HF have not been extensively studied. It is important to remember that antibiotics are a double‐edged sword, with potential benefits and risks that need to be carefully weighed.
Other interventions
Evidence implies that high TMAO levels are linked with a deficiency in vitamin D, indicating that vitamin D may help reduce TMAO levels in patients [109]. Moreover, a study has proposed that B vitamins + vitamin D can cause changes in choline metabolism, resulting in further lowering of TMAO levels when compared to vitamin D alone [110]. In addition, oral intake of berberine for 4 months has been shown to decrease TMAO production in animal intestines and lower TMA and TMAO levels in both the feces and plasma of patients through vitamin‐like effects [44]. Furthermore, DMB has been reported to raise cardiac function and alleviate cardiac remodeling in HF mice induced by pressure overload by downregulating plasma TMAO levels, which inhibits the TGF‐β1/Smad3 and p65 NF‐κB signaling pathway and attenuates cardiac hypertrophy, fibrosis, and inflammation [111]. Additionally, traditional Chinese medicine (TCM) has been shown to interact with gut microbiota, as TCM regulates metabolism and is metabolized by gut microbiota [112].
CONCLUSION
The gut hypothesis of HF highlights the potential of targeting gut microbiota for the interventions or treatments of HF. The composition and diversity of gut microbiota are altered in HF, and it produces more TMAO and fewer SCFAs compared to healthy individuals. TMAO promotes HF by promoting cardiac hypertrophy, fibrosis, inflammation, and endothelial dysfunction, while SCFAs have a protective role by preventing pathophysiological changes and satisfying energy metabolism. Other microbiota metabolites like TMAVA and PAGln may also play a role in HF. Dietary interventions and probiotic therapy have shown potential in attenuating HF and improving cardiac function. However, further research and studies are needed to determine the effectiveness of FMT and antibiotics in HF treatment. Overall, the gut microbiota represents a promising avenue for the development of novel HF treatments.
AUTHOR CONTRIBUTIONS
An‐Tian Chen wrote the manuscript. Jian Zhang and Yuhui Zhang supervised this project. All authors have read the final manuscript and approved it for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (grant number 2020‐I2M‐1‐002) and the High‐level Hospital Clinical Research Fund of Fuwai Hospital (grant number 2022‐GSP‐GG‐9). All figures are created using BioRender.com. Figure 3 is reprinted from “Mechanism of SCFAs,” by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates.
Chen, An‐Tian , Zhang Jian, and Zhang Yuhui. 2023. “Gut Microbiota in Heart Failure and Related Interventions.” iMeta 2, e125. 10.1002/imt2.125
Contributor Information
Jian Zhang, Email: fwzhangjian62@126.com.
Yuhui Zhang, Email: yuhuizhangjoy@163.com.
DATA AVAILABILITY STATEMENT
This manuscript does not generate any code or data. Supplementary materials (graphical abstract, slides, videos, Chinese translated version and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.
REFERENCES
- 1. de Vos, Willem M. , Tilg Herbert, Van Hul Matthias, and Cani Patrice D.. 2022. “Gut Microbiome and Health: Mechanistic Insights.” Gut 71: 1020–32. 10.1136/gutjnl-2021-326789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. James, Spencer L. , Abate Degu, Abate Kalkidan Hassen, Abay Solomon M., Abbafati Cristiana, Abbasi Nooshin, Abbastabar Hedayat, et al. 2018. “Global, Regional, and National Incidence, Prevalence, and Years Lived With Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017.” The Lancet 392: 1789–858. 10.1016/s0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groenewegen, Amy , Rutten Frans H., Mosterd Arend, and Hoes Arno W.. 2020. “Epidemiology of Heart Failure.” European Journal of Heart Failure 22: 1342–56. 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Denolin, H. , Kuhn H., Krayenbuehl H., Loogen F., and Reale A.. 1983. “The Defintion of Heart Failure.” European Heart Journal 4: 445–8. 10.1093/oxfordjournals.eurheartj.a061500 [DOI] [PubMed] [Google Scholar]
- 5. Heidenreich, Paul A. , Bozkurt Biykem, Aguilar David, Allen Larry A., Byun Joni J., Colvin Monica M., Deswal Anita, et al. 2022. “2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines.” Circulation 145: e895–1032. 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 6. Tang, W. H. Wilson , Li Daniel Y., and Hazen Stanley L.. 2019. “Dietary Metabolism, the Gut Microbiome, and Heart Failure.” Nature Reviews Cardiology 16: 137–54. 10.1038/s41569-018-0108-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gustafsson, Finn , and Rogers Joseph G.. 2017. “Left Ventricular Assist Device Therapy in Advanced Heart Failure: Patient Selection and Outcomes.” European Journal of Heart Failure 19: 595–602. 10.1002/ejhf.779 [DOI] [PubMed] [Google Scholar]
- 8. Chambers, Daniel C. , Perch Michael, Zuckermann Andreas, Cherikh Wida S., Harhay Michael O., Hayes Don, Hsich Eileen, et al. 2021. “The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐Eighth Adult Lung Transplantation Report—2021; Focus on Recipient Characteristics.” The Journal of Heart and Lung Transplantation 40: 1060–72. 10.1016/j.healun.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ley, Ruth E. , Peterson Daniel A., and Gordon Jeffrey I.. 2006. “Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine.” Cell 124: 837–48. 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 10. Dogra, Shaillay Kumar , Doré Joel, and Damak Sami. 2020. “Gut Microbiota Resilience: Definition, Link to Health and Strategies for Intervention.” Frontiers in Microbiology 11: 572921. 10.3389/fmicb.2020.572921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qin, Junjie , Li Ruiqiang, Raes Jeroen, Arumugam Manimozhiyan, Burgdorf Kristoffer Solvsten, Manichanh Chaysavanh, Nielsen Trine, et al. 2010. “A Human Gut Microbial Gene Catalogue Established ny Metagenomic Sequencing.” Nature 464: 59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharon, Gil , Cruz Nikki Jamie, Kang Dae‐Wook, Gandal Michael J., Wang Bo, Kim Young‐Mo, Zink Erika M., et al. 2019. “Human Gut Microbiota From Autism Spectrum Disorder Promote Behavioral Symptoms in Mice.” Cell 177: 1600–18.e17. 10.1016/j.cell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu, Xu , Wang Tao, and Jin Feng. 2016. “Alzheimer's Disease and Gut Microbiota.” Science China Life Sciences 59: 1006–23. 10.1007/s11427-016-5083-9 [DOI] [PubMed] [Google Scholar]
- 14. Quigley, Eamonn M. M . 2017. “Microbiota‐Brain‐Gut Axis and Neurodegenerative Diseases.” Current Neurology and Neuroscience Reports 17: 94. 10.1007/s11910-017-0802-6 [DOI] [PubMed] [Google Scholar]
- 15. Jiang, Shuaiming , Chen Denghui, Ma Chenchen, Liu Huanwei, Huang Shi, and Zhang Jiachao. 2022. “Establishing a Novel Inflammatory Bowel Disease Prediction Model Based on Gene Markers Identified From Single Nucleotide Variants of the Intestinal Microbiota.” iMeta 1: e40. 10.1002/imt2.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosa, Giulio La , and Biasucci Luigi Marzio. 2016. “The Gut Microbiota and Atherosclerosis: the State Of the Art and Novel Perspectives.” Cardiovascular Innovations and Applications 1: 433–42. 10.15212/CVIA.2016.0027 [DOI] [Google Scholar]
- 17. Fan, Yong , Ying Jiajun, Ma Hongchuang, and Cui Hanbin. 2023. “Microbiota‐Related Metabolites Fueling the Understanding of Ischemic Heart Disease.” iMeta 2: e94. 10.1002/imt2.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niebauer, Josef , Volk Hans‐Dieter, Kemp Michael, Dominguez Martin, Schumann Ralf R., Rauchhaus Mathias, Poole‐Wilson Philip A., Coats Andrew J. S., and Anker Stefan D.. 1999. “Endotoxin and Immune Activation in Chronic Heart Failure: a Prospective Cohort Study.” The Lancet 353: 1838–42. 10.1016/s0140-6736(98)09286-1 [DOI] [PubMed] [Google Scholar]
- 19. Anker, Stefan D. , Egerer Karl R., Volk Hans‐Dieter, Kox Wolfgang J., Poole‐Wilson Philip A., and Coats Andrew J. S.. 1997. “Elevated Soluble CD14 Receptors and Altered Cytokines in Chronic Heart Failure.” The American Journal of Cardiology 79: 1426–30. 10.1016/s0002-9149(97)00159-8 [DOI] [PubMed] [Google Scholar]
- 20. Nagatomo, Yuji , and Tang W. H. Wilson. 2015. “Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis.” Journal of Cardiac Failure 21: 973–80. 10.1016/j.cardfail.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues, Alexandre , Gonçalves Alexandre, Morais Juliana, Araujo Ricardo, and Falcão‐Pires Inês. 2023. “Diet‐Induced Microbiome's Impact on Heart Failure: A Double‐Edged Sword.” Nutrients 15: 1223. 10.3390/nu15051223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan, Xueqing , and Sun Zhijun. 2023. “The Role of Intestinal Flora and its Metabolites in Heart Failure.” Infection and Drug Resistance 16: 51–64. 10.2147/idr.S390582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mamic, Petra , Snyder Michael, and Tang W. H. Wilson. 2023. “Gut Microbiome‐Based Management of Patients with Heart Failure.” Journal of the American College of Cardiology 81: 1729–39. 10.1016/j.jacc.2023.02.045 [DOI] [PubMed] [Google Scholar]
- 24. Carley, Andrew N. , Maurya Santosh K., Fasano Matthew, Wang Yang, Selzman Craig H., Drakos Stavros G., and Lewandowski E. Douglas. 2021. “Short‐Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart.” Circulation 143: 1797–808. 10.1161/circulationaha.120.052671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groschwitz, Katherine R. , and Hogan Simon P.. 2009. “Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis.” Journal of Allergy and Clinical Immunology 124: 3–20. quiz 21‐22. 10.1016/j.jaci.2009.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitsudo, Sumi , and Brandt Lawrence J.. 1992. “Pathology of Intestinal Ischemia.” Surgical Clinics of North America 72: 43–63. 10.1016/s0039-6109(16)45627-6 [DOI] [PubMed] [Google Scholar]
- 27. Chang, Marisol , Kistler Erik B., and Schmid‐Schönbein Geert W.. 2012. “Disruption of the Mucosal Barrier During Gut Ischemia Allows Entry of Digestive Enzymes into the Intestinal Wall.” Shock 37: 297–305. 10.1097/SHK.0b013e318240b59b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sandek, Anja , Bauditz Juergen, Swidsinski Alexander, Buhner Sabine, Weber‐Eibel Jutta, von Haehling Stephan, Schroedl Wieland, et al. 2007. “Altered Intestinal Function in Patients with Chronic Heart Failure.” Journal of the American College of Cardiology 50: 1561–9. 10.1016/j.jacc.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 29. Tang, W. H. Wilson , Wang Zeneng, Fan Yiying, Levison Bruce, Hazen Jennie E., Donahue Lillian M., Wu Yuping, and Hazen Stanley L.. 2014. “Prognostic Value of Elevated Levels of Intestinal Microbe‐Generated Metabolite Trimethylamine‐N‐Oxide in Patients with Heart Failure: Refining the Gut Hypothesis.” Journal of the American College of Cardiology 64: 1908–14. 10.1016/j.jacc.2014.02.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu, Tongtong , Wu Qingqing, Yao Qi, Jiang Kebing, Yu Jiabin, and Tang Qizhu. 2022. “Short‐Chain Fatty Acid Metabolism and Multiple Effects on Cardiovascular Diseases.” Ageing Research Reviews 81: 101706. 10.1016/j.arr.2022.101706 [DOI] [PubMed] [Google Scholar]
- 31. Zhao, Mingming , Wei Haoran, Li Chenze, Zhan Rui, Liu Changjie, Gao Jianing, Yi Yaodong, et al. 2022. “Gut Microbiota Production of trimethyl‐5‐aminovaleric Acid Reduces Fatty Acid Oxidation and Accelerates Cardiac Hypertrophy.” Nature Communications 13: 1757. 10.1038/s41467-022-29060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Romano, Kymberleigh A. , Nemet Ina, Prasad Saha Prasenjit, Haghikia Arash, Li Xinmin S., Mohan Maradumane L., Lovano Beth, et al. 2023. “Gut Microbiota‐Generated Phenylacetylglutamine and Heart Failure.” Circulation: Heart Failure 16: e009972. 10.1161/CIRCHEARTFAILURE.122.009972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sorboni, Shokufeh Ghasemian , Moghaddam Hanieh Shakeri, Jafarzadeh‐Esfehani Reza, and Soleimanpour Saman. 2022. “A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders.” Clinical Microbiology Reviews 35: e0033820. 10.1128/cmr.00338-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandek, Anja , Swidsinski Alexander, Schroedl Wieland, Watson Alastair, Valentova Miroslava, Herrmann Ralph, Scherbakov Nadja, et al. 2014. “Intestinal Blood Flow in Patients with Chronic Heart Failure.” Journal of the American College of Cardiology 64: 1092–102. 10.1016/j.jacc.2014.06.1179 [DOI] [PubMed] [Google Scholar]
- 35. Pasini, Evasio , Aquilani Roberto, Testa Cristian, Baiardi Paola, Angioletti Stefania, Boschi Federica, Verri Manuela, and Dioguardi Francesco. 2016. “Pathogenic Gut Flora in Patients with Chronic Heart Failure.” JACC: Heart Failure 4: 220–7. 10.1016/j.jchf.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 36. Luedde, Mark , Winkler Thorben, Heinsen Femke‐Anouska, Rühlemann Malte C., Spehlmann Martina E., Bajrovic Amer, Lieb Wolfgang, et al. 2017. “Heart Failure is Associated with Depletion of Core Intestinal Microbiota.” ESC Heart Failure 4: 282–90. 10.1002/ehf2.12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamo, Takehiro , Akazawa Hiroshi, Suda Wataru, Saga‐Kamo Akiko, Shimizu Yu, Yagi Hiroki, Liu Qing, et al. 2017. “Dysbiosis and Compositional Alterations with Aging in the Gut Microbiota of Patients with Heart Failure.” PLOS ONE 12: e0174099. 10.1371/journal.pone.0174099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kummen, Martin , Mayerhofer Cristiane C. K., Vestad Beate, Broch Kaspar, Awoyemi Ayodeji, Storm‐Larsen Christopher, Ueland Thor, et al. 2018. “Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts.” Journal of the American College of Cardiology 71: 1184–6. 10.1016/j.jacc.2017.12.057 [DOI] [PubMed] [Google Scholar]
- 39. Sun, Weiju , Du Debing, Fu Tongze, Han Ying, Li Peng, and Ju Hong. 2022. “Alterations of the Gut Microbiota in Patients With Severe Chronic Heart Failure.” Frontiers in Microbiology 12: 813289. 10.3389/fmicb.2021.813289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang, Ziyin , Mei Xiaofei, Jiang Yufeng, Chen Tan, and Zhou Yafeng. 2022. “Gut Microbiota in Heart Failure Patients With Preserved Ejection Fraction (GUMPTION Study).” Frontiers in Cardiovascular Medicine 8: 803744. 10.3389/fcvm.2021.803744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mamic, Petra , Heidenreich Paul A., Hedlin Haley, Tennakoon Lakshika, and Staudenmayer Kristan L.. 2016. “Hospitalized Patients with Heart Failure and Common Bacterial Infections: A Nationwide Analysis of Concomitant Clostridium Difficile Infection Rates and In‐Hospital Mortality.” Journal of Cardiac Failure 22: 891–900. 10.1016/j.cardfail.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 42. Li, Wenlong , Li Huijun, Wang Shaolan, Han Keyang, Liu Yuan, An Zhen, Wu Hui, et al. 2022. “Regional Pattern and Signatures of Gut Microbiota in Rural Residents with Coronary Heart Disease: A Metagenomic Analysis.” Frontiers in Cellular and Infection Microbiology 12: 1007161. 10.3389/fcimb.2022.1007161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jia, Qiujin , Wang Lirong, Zhang Xiaonan, Ding Yuejia, Li Hao, Yang Yingxi, Zhang Ao, et al. 2020. “Prevention and Treatment of Chronic Heart Failure Through Traditional Chinese Medicine: Role of the Gut Microbiota.” Pharmacological Research 151: 104552. 10.1016/j.phrs.2019.104552 [DOI] [PubMed] [Google Scholar]
- 44. Ma, Shu‐Rong , Tong Qian, Lin Yuan, Pan Li‐Bin, Fu Jie, Peng Ran, Zhang Xian‐Feng, et al. 2022. “Berberine Treats Atherosclerosis Via A Vitamine‐Like Effect Down‐Regulating Choline‐TMA‐TMAO Production Pathway in Gut Microbiota.” Signal Transduction and Targeted Therapy 7: 207. 10.1038/s41392-022-01027-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Canyelles, Marina , Borràs Carla, Rotllan Noemí, Tondo Mireia, Escolà‐Gil Joan Carles, and Blanco‐Vaca Francisco. 2023. “Gut Microbiota‐Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? International Journal of Molecular Sciences 24: 1940. 10.3390/ijms24031940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang, Zeneng , Klipfell Elizabeth, Bennett Brian J., Koeth Robert, Levison Bruce S., DuGar Brandon, Feldstein Ariel E., et al. 2011. “Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease.” Nature 472: 57–63. 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tan, Yu , Sheng Zhaoxue, Zhou Peng, Liu Chen, Zhao Hanjun, Song Li, Li Jiannan, et al. 2019. “Plasma Trimethylamine N‐Oxide as a Novel Biomarker for Plaque Rupture in Patients with ST‐Segment‐Elevation Myocardial Infarction.” Circulation: Cardiovascular Interventions 12: e007281. 10.1161/CIRCINTERVENTIONS.118.007281 [DOI] [PubMed] [Google Scholar]
- 48. Jiang, Shan , Shui Yongjie, Cui Yu, Tang Chun, Wang Xiaohua, Qiu Xingyu, Hu Weipeng, et al. 2021. “Gut Microbiota Dependent Trimethylamine N‐Oxide Aggravates Angiotensin II‐induced Hypertension.” Redox Biology 46: 102115. 10.1016/j.redox.2021.102115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li, Doudou , Lu Ying, Yuan Shuai, Cai Xiaxia, He Yuan, Chen Jie, Wu Qiong, et al. 2022. “Gut Microbiota‐Derived Metabolite Trimethylamine‐N‐Oxide and Multiple Health Outcomes: An Umbrella Review and Updated Meta‐Analysis.” The American Journal of Clinical Nutrition 116: 230–43. 10.1093/ajcn/nqac074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Romano Kymberleigh, A. , Eugenio I. Vivas, Amador‐Noguez Daniel, and Federico E. Rey. 2015. “Intestinal Microbiota Composition Modulates Choline Bioavailability From Diet and Accumulation of the Proatherogenic Metabolite Trimethylamine‐N‐Oxide.” MBio 6: e02481. 10.1128/mbio.02481-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Organ, Chelsea L. , Otsuka Hiroyuki, Bhushan Shashi, Wang Zeneng, Bradley Jessica, Trivedi Rishi, Polhemus David J., et al. 2016. “Choline Diet and its Gut Microbe‐Derived Metabolite, Trimethylamine N‐Oxide, Exacerbate Pressure Overload‐Induced Heart Failure.” Circulation: Heart Failure 9: e002314. 10.1161/circheartfailure.115.002314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seldin, Marcus M. , Meng Yonghong, Qi Hongxiu, Zhu WeiFei, Wang Zeneng, Hazen Stanley L., Lusis Aldons J., and Shih Diana M.. 2016. “Trimethylamine N‐Oxide Promotes Vascular Inflammation Through Signaling of Mitogen‐Activated Protein Kinase and Nuclear Factor‐κB.” Journal of the American Heart Association 5: e002767. 10.1161/jaha.115.002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sun, Xiaolei , Jiao Xuefei, Ma Yarong, Liu Yong, Zhang Lei, He Yanzheng, Chen Yunhui, and Chen Yunhui. 2016. “Trimethylamine N‐Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells Via Activating ROS‐TXNIP‐NLRP3 Inflammasome.” Biochemical and Biophysical Research Communications 481: 63–70. 10.1016/j.bbrc.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 54. Chen, Ming‐liang , Zhu Xiao‐hui, Ran Li, Lang He‐dong, Yi Long, and Mi Man‐tian. 2017. “Trimethylamine‐N‐Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3‐SOD2‐mtROS Signaling Pathway.” Journal of the American Heart Association 6: e006347. 10.1161/jaha.117.006347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Makrecka‐Kuka, Marina , Volska Kristine, Antone Unigunde, Vilskersts Reinis, Grinberga Solveiga, Bandere Dace, Liepinsh Edgars, and Dambrova Maija. 2017. “Trimethylamine N‐Oxide Impairs Pyruvate and Fatty Acid Oxidation In Cardiac Mitochondria.” Toxicology Letters 267: 32–8. 10.1016/j.toxlet.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 56. Li, Zehua , Wu Zhiye, Yan Jianyun, Liu Hailin, Liu Qicai, Deng Yi, Ou Caiwen, and Chen Minsheng. 2019. “Gut Microbe‐Derived Metabolite Trimethylamine N‐Oxide Induces Cardiac Hypertrophy and Fibrosis.” Laboratory Investigation 99: 346–57. 10.1038/s41374-018-0091-y [DOI] [PubMed] [Google Scholar]
- 57. Brunt, Vienna E. , Gioscia‐Ryan Rachel A., Casso Abigail G., VanDongen Nicholas S., Ziemba Brian P., Sapinsley Zachary J., Richey James J., et al. 2020. “Trimethylamine‐N‐Oxide Promotes Age‐Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans.” Hypertension 76: 101–12. 10.1161/hypertensionaha.120.14759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoshida, Yohko , Shimizu Ippei, Shimada Atsuhiro, Nakahara Keita, Yanagisawa Sachiko, Kubo Minoru, Fukuda Shinji, et al. 2022. “Brown Adipose Tissue Dysfunction Promotes Heart Failure Via a Trimethylamine N‐Oxide‐Dependent Mechanism.” Scientific Reports 12: 14883. 10.1038/s41598-022-19245-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang, L. E. I. , Xie Feifei, Tang Haie, Zhang Xinrong, Hu Jianxia, Zhong Xiaohong, Gong Nirong, et al. 2022. “Gut Microbial Metabolite TMAO Increases Peritoneal Inflammation and Peritonitis Risk in Peritoneal Dialysis Patients.” Translational Research 240: 50–63. 10.1016/j.trsl.2021.10.001 [DOI] [PubMed] [Google Scholar]
- 60. den Besten, Gijs , van Eunen Karen, Groen Albert K., Venema Koen, Reijngoud Dirk‐Jan, and Bakker Barbara M.. 2013. “The Role of Short‐Chain Fatty Acids in the Interplay Between Diet, Gut Microbiota, and Host Energy Metabolism.” Journal of Lipid Research 54: 2325–40. 10.1194/jlr.R036012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cook, Sellin . 1998. “Review Article: Short Chain Fatty Acids in Health and Disease.” Alimentary Pharmacology & Therapeutics 12: 499–507. 10.1046/j.1365-2036.1998.00337.x [DOI] [PubMed] [Google Scholar]
- 62. Li, Lin , Zhong Sen‐jie, Hu Si‐yuan, Cheng Bin, Qiu Hong, and Hu Zhi‐xi. 2021. “Changes of Gut Microbiome Composition and Metabolites Associated with Hypertensive Heart Failure Rats.” BMC Microbiology 21: 141. 10.1186/s12866-021-02202-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kirschner, Sarah K. , Deutz Nicolaas E. P., Rijnaarts Iris, Smit Tiffany J., Larsen Daniel J., and Engelen Mariëlle P. K. J.. 2022. “Impaired Intestinal Function is Associated With Lower Muscle and Cognitive Health and Well‐Being in Patients with Congestive Heart Failure.” Journal of Parenteral and Enteral Nutrition 46: 660–70. 10.1002/jpen.2193 [DOI] [PubMed] [Google Scholar]
- 64. Martin‐Gallausiaux, Camille , Marinelli Ludovica, Blottière Hervé M., Larraufie Pierre, and Lapaque Nicolas. 2021. “SCFA: Mechanisms and Functional Importance in the Gut.” Proceedings of the Nutrition Society 80: 37–49. 10.1017/S0029665120006916 [DOI] [PubMed] [Google Scholar]
- 65. Donohoe, Dallas R. , Collins Leonard B., Wali Aminah, Bigler Rebecca, Sun Wei, and Bultman Scott J.. 2012. “The Warburg Effect Dictates the Mechanism of Butyrate‐Mediated Histone Acetylation and Cell Proliferation.” Molecular Cell 48: 612–26. 10.1016/j.molcel.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mathewson, Nathan D. , Jenq Robert, Mathew Anna V., Koenigsknecht Mark, Hanash Alan, Toubai Tomomi, Oravecz‐Wilson Katherine, et al. 2016. “Gut Microbiome‐Derived Metabolites Modulate Intestinal Epithelial Cell Damage and Mitigate Graft‐Versus‐Host Disease.” Nature Immunology 17: 505–13. 10.1038/ni.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bartolomaeus, Hendrik , Balogh András, Yakoub Mina, Homann Susanne, Höges Lajos Sascha, Tsvetkov Dmitry, Krannich Alexander, et al. 2019. “Short‐Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage.” Circulation 139: 1407–21. 10.1161/circulationaha.118.036652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kaye, David M. , Shihata Waled A., Jama Hamdi A., Tsyganov Kirill, Ziemann Mark, Kiriazis Helen, Horlock Duncan, et al. 2020. “Deficiency of Prebiotic Fiber and Insufficient Signaling Through Gut Metabolite‐Sensing Receptors Leads to Cardiovascular Disease.” Circulation 141: 1393–403. 10.1161/circulationaha.119.043081 [DOI] [PubMed] [Google Scholar]
- 69. Li, Meng , van Esch Betty C. A. M., Wagenaar Gerry T. M., Garssen Johan, Folkerts Gert, and Henricks Paul A. J.. 2018. “Pro‐ and Anti‐Inflammatory Effects of Short Chain Fatty Acids On Immune and Endothelial Cells.” European Journal of Pharmacology 831: 52–9. 10.1016/j.ejphar.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 70. Chen, An‐tian , Wang Chen‐yu, Zhu Wen‐ling, and Chen Wei. 2022. “Coagulation Disorders and Thrombosis in COVID‐19 Patients and a Possible Mechanism Involving Endothelial Cells: A Review.” Aging and Disease 13: 144–56. 10.14336/ad.2021.0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. He, Jin , Zhang Peiwen, Shen Linyuan, Niu Lili, Tan Ya, Chen Lei, Zhao Ye, et al. 2020. “Short‐Chain Fatty Acids and their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism.” International Journal of Molecular Sciences 21: 6356. 10.3390/ijms21176356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Masui, Ryuta , Sasaki Makoto, Funaki Yasushi, Ogasawara Naotaka, Mizuno Mari, Iida Akihito, Izawa Shinya, et al. 2013. “G Protein‐Coupled Receptor 43 Moderates Gut Inflammation Through Cytokine Regulation From Mononuclear Cells.” Inflammatory Bowel Diseases 19: 2848–56. 10.1097/01.MIB.0000435444.14860.ea [DOI] [PubMed] [Google Scholar]
- 73. Vinolo, Marco A. R. , Rodrigues Hosana G., Hatanaka Elaine, Sato Fábio T., Sampaio Sandra C., and Curi Rui. 2011. “Suppressive Effect of Short‐Chain Fatty Acids on Production of Proinflammatory Mediators by Neutrophils.” The Journal of Nutritional Biochemistry 22: 849–55. 10.1016/j.jnutbio.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 74. Ohira, Hideo , Fujioka Yoshio, Katagiri Chikae, Mamoto Rie, Aoyama‐Ishikawa Michiko, Amako Katsumi, Izumi Yoshihiro, et al. 2013. “Butyrate Attenuates Inflammation and Lipolysis Generated by the Interaction of Adipocytes and Macrophages.” Journal of Atherosclerosis and Thrombosis 20: 425–42. 10.5551/jat.15065 [DOI] [PubMed] [Google Scholar]
- 75. Halnes, Isabel , Baines Katherine J., Berthon Bronwyn S., MacDonald‐Wicks Lesley K., Gibson Peter G., and Wood Lisa G.. 2017. “Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma.” Nutrients 9: 57. 10.3390/nu9010057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vinolo, Marco A. R. , Rodrigues Hosana G., Nachbar Renato T., and Curi Rui. 2011. “Regulation of Inflammation by Short Chain Fatty Acids.” Nutrients 3: 858–76. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li, Meng , van Esch Betty C. A. M., Henricks Paul A. J., Folkerts Gert, and Garssen Johan. 2018. “The Anti‐Inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide‐ or Tumor Necrosis Factor α‐Stimulated Endothelial Cells Via Activation of GPR41/43 and Inhibition of HDACs.” Frontiers in Pharmacology 9: 533. 10.3389/fphar.2018.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li, Bo , He Xinglishang, Jin Hai‐Ying, Wang Hui‐Ying, Zhou Fu‐Chen, Zhang Ning‐Yu, Jie Dong‐Ying, et al. 2021. “Beneficial Effects of Dendrobium Officinale on Metabolic Hypertensive Rats by Triggering the Enteric‐Origin SCFA‐GPCR43/41 Pathway.” Food & Function 12: 5524–38. 10.1039/d0fo02890h [DOI] [PubMed] [Google Scholar]
- 79. Amabebe, Emmanuel , Robert Faith O., Agbalalah Tarimoboere, and Orubu Ebiowei S. F.. 2020. “Microbial Dysbiosis‐Induced Obesity: Role of Gut Microbiota in Homoeostasis of Energy Metabolism.” British Journal of Nutrition 123: 1127–37. 10.1017/s0007114520000380 [DOI] [PubMed] [Google Scholar]
- 80. Rees, Douglas . 2017. “The Obesity Epidemic and Our Gut Microbiome–Could it All be Down to Our ‘Bugs’?” The Biochemist 39: 26–9. 10.1042/bio03902026 [DOI] [Google Scholar]
- 81. Chakraborti, Chandra Kanti . 2015. “New‐Found Link Between Microbiota and Obesity.” World Journal of Gastrointestinal Pathophysiology 6: 110–19. 10.4291/wjgp.v6.i4.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carvalho, Bruno Melo , and Abdalla Saad Mario Jose. 2013. “Influence of Gut Microbiota on Subclinical Inflammation and Insulin Resistance.” Mediators of Inflammation 2013: 986734. 10.1155/2013/986734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Davis, Cindy D. 2016. “The Gut Microbiome and its Role in Obesity.” Nutrition Today 51: 167–74. 10.1097/nt.0000000000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nemet, Ina , Saha Prasenjit Prasad, Gupta Nilaksh, Zhu Weifei, Romano Kymberleigh A., Skye Sarah M., Cajka Tomas, et al. 2020. “A Cardiovascular Disease‐Linked Gut Microbial Metabolite Acts Via Adrenergic Receptors.” Cell 180: 862–877.e822. 10.1016/j.cell.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zong, Xiao , Fan Qin, Yang Qian, Pan Roubai, Zhuang Lingfang, and Tao Rong. 2022. “Phenylacetylglutamine as a Risk Factor and Prognostic Indicator of Heart Failure.” ESC Heart Failure 9: 2645–53. 10.1002/ehf2.13989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fu, Hui , Kong Bin, Zhu Jun, Huang He, and Shuai Wei. 2023. “Phenylacetylglutamine Increases the Susceptibility of Ventricular Arrhythmias in Heart Failure Mice by Exacerbated Activation of the TLR4/AKT/mTOR Signaling Pathway.” International Immunopharmacology 116: 109795. 10.1016/j.intimp.2023.109795 [DOI] [PubMed] [Google Scholar]
- 87. David, Lawrence A. , Maurice Corinne F., Carmody Rachel N., Gootenberg David B., Button Julie E., Wolfe Benjamin E., Ling Alisha V., et al. 2014. “Diet Rapidly and Reproducibly Alters the Human Gut Microbiome.” Nature 505: 559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Filippou, Christina D. , Tsioufis Costas P., Thomopoulos Costas G., Mihas Costas C., Dimitriadis Kyriakos S., Sotiropoulou Lida I., Chrysochoou Christina A., Nihoyannopoulos Petros I., and Tousoulis Dimitrios M.. 2020. “Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults With and Without Hypertension: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials.” Advances in Nutrition 11: 1150–60. 10.1093/advances/nmaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sacks, Frank M. , Svetkey Laura P., Vollmer William M., Appel Lawrence J., Bray George A., Harsha David, Obarzanek Eva, et al. 2001. “Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet.” New England Journal of Medicine 344: 3–10. 10.1056/nejm200101043440101 [DOI] [PubMed] [Google Scholar]
- 90. Ibsen, Daniel B. , Levitan Emily B., Åkesson Agneta, Gigante Bruna, and Wolk Alicja. 2022. “The DASH Diet is Associated with a Lower Risk of Heart Failure: A Cohort Study.” European Journal of Preventive Cardiology 29: 1114–23. 10.1093/eurjpc/zwac003 [DOI] [PubMed] [Google Scholar]
- 91. Rifai, Luay , Pisano Carol, Hayden Janel, Sulo Suela, and Silver Marc A.. 2015. “Impact of the DASH Diet on Endothelial Function, Exercise Capacity, and Quality of Life in Patients with Heart Failure.” Baylor University Medical Center Proceedings 28: 151–56. 10.1080/08998280.2015.11929216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kris‐Etherton, Penny , Eckel Robert H., Howard Barbara V., St. Jeor Sachiko, and Bazzarre Terry L.. 2001. “Lyon Diet Heart Study: Benefits of a Mediterranean‐Style, National Cholesterol Education Program/American Heart Association Step I Dietary Pattern on Cardiovascular Disease.” Circulation 103: 1823–25. 10.1161/01.cir.103.13.1823 [DOI] [PubMed] [Google Scholar]
- 93. Lopez‐Garcia, Esther , Rodriguez‐Artalejo Fernando, Li Tricia Y., Fung Teresa T., Li Shanshan, Willett Walter C., Rimm Eric B., and Hu Frank B.. 2014. “The Mediterranean‐Style Dietary Pattern and Mortality Among Men and Women with Cardiovascular Disease.” The American Journal of Clinical Nutrition 99: 172–80. 10.3945/ajcn.113.068106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Papadaki, Angeliki , Martínez‐González Miguel Ángel, Alonso‐Gómez Angel, Rekondo Javier, Salas‐Salvadó Jordi, Corella Dolores, Ros Emilio, et al. 2017. “Mediterranean Diet and Risk of Heart Failure: Results from the PREDIMED Randomized Controlled Trial.” European Journal of Heart Failure 19: 1179–85. 10.1002/ejhf.750 [DOI] [PubMed] [Google Scholar]
- 95. Sanches Machado d'Almeida, Karina , Ronchi Spillere Stefanny, Zuchinali Priccila, and Corrêa Souza Gabriela. 2018. “Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review.” Nutrients 10: 58. 10.3390/nu10010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hill, Colin , Guarner Francisco, Reid Gregor, Gibson Glenn R., Merenstein Daniel J., Pot Bruno, Morelli Lorenzo, et al. 2014. “Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic.” Nature Reviews Gastroenterology & Hepatology 11: 506–14. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 97. Gan, Xiaohong Tracey , Ettinger Grace, Huang Cathy X., Burton Jeremy P., Haist James V., Rajapurohitam Venkatesh, Sidaway James E., et al 2014. “Probiotic Administration Attenuates Myocardial Hypertrophy and Heart Failure After Myocardial Infarction in the Rat.” Circulation: Heart Failure 7: 491–9. 10.1161/circheartfailure.113.000978 [DOI] [PubMed] [Google Scholar]
- 98. Karim, Asima , Muhammad Tahir, Shah Islam, Khan Javaidullah, and Qaisar Rizwan. 2022. “A Multistrain Probiotic Reduces Sarcopenia By Modulating Wnt Signaling Biomarkers in Patients With Chronic Heart Failure.” Journal of Cardiology 80: 449–55. 10.1016/j.jjcc.2022.06.006 [DOI] [PubMed] [Google Scholar]
- 99. Costanza, Annelise C. , Moscavitch Samuel D., Faria Neto Hugo C. C., and Mesquita Evandro T.. 2015. “Probiotic Therapy With Saccharomyces Boulardii for Heart Failure Patients: A Randomized, Double‐Blind, Placebo‐Controlled Pilot Trial.” International Journal of Cardiology 179: 348–50. 10.1016/j.ijcard.2014.11.034 [DOI] [PubMed] [Google Scholar]
- 100. Pourrajab, Behnaz , Naderi Nasim, Janani Leila, Hajahmadi Marjan, Mofid Vahid, Dehnad Afsaneh, Sohouli Mohammad Hassan, Hosseini Sharieh, and Shidfar Farzad. 2022. “The Impact of Probiotic Yogurt Versus Ordinary Yogurt on Serum sTWEAK, sCD163, ADMA, LCAT and BUN in Patients with Chronic Heart Failure: A Randomized, Triple‐Blind, Controlled Trial.” Journal of the Science of Food and Agriculture 102: 6024–35. 10.1002/jsfa.11955 [DOI] [PubMed] [Google Scholar]
- 101. Awoyemi, Ayodeji , Mayerhofer Cristiane, Felix Alex S., Hov Johannes R., Moscavitch Samuel D., Lappegård Knut Tore, Hovland Anders, et al. 2021. “Rifaximin or Saccharomyces Boulardii in Heart Failure with Reduced Ejection Fraction: Results From the Randomized GutHeart Trial.” EBioMedicine 70: 103511. 10.1016/j.ebiom.2021.103511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gupta, Arjun , and Khanna Sahil. 2017. “Fecal Microbiota Transplantation.” JAMA 318: 102. 10.1001/jama.2017.6466 [DOI] [PubMed] [Google Scholar]
- 103. Jia, Qiujin , Li Hao, Zhou Huan, Zhang Xiaonan, Zhang Ao, Xie Yingyu, Li Yanyang, Lv Shichao, and Zhang Junping. 2019. “Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure.” Cardiovascular Therapeutics 2019: 1–10. 10.1155/2019/5164298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wilson, Brooke C. , Vatanen Tommi, Cutfield Wayne S., and O'Sullivan Justin M.. 2019. “The Super‐Donor Phenomenon in Fecal Microbiota Transplantation.” Frontiers in Cellular and Infection Microbiology 9: 2. 10.3389/fcimb.2019.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chehoud, Christel , Dryga Anatoly, Hwang Young, Nagy‐Szakal Dorottya, Hollister Emily B., Luna Ruth Ann, Versalovic James, Kellermayer Richard, and Bushman Frederic D.. 2016. “Transfer of Viral Communities Between Human Individuals During Fecal Microbiota Transplantation.” MBio 7: e00322. 10.1128/mBio.00322-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhou, Xin , Li Jing, Guo Junli, Geng Bin, Ji Wenjie, Zhao Qian, Li Jinlong, et al. 2018. “Gut‐Dependent Microbial Translocation Induces Inflammation and Cardiovascular Events After ST‐Elevation Myocardial Infarction.” Microbiome 6: 66. 10.1186/s40168-018-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ponziani, Francesca Romana , Zocco Maria Assunta, D'Aversa Francesca, Pompili Maurizio, and Gasbarrini Antonio. 2017. “Eubiotic Properties of Rifaximin: Disruption of the Traditional Concepts in Gut Microbiota Modulation.” World Journal of Gastroenterology 23: 4491–9. 10.3748/wjg.v23.i25.4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chen, Ming‐liang , Yi Long, Zhang Yong, Zhou Xi, Ran Li, Yang Jining, Zhu Jun‐dong, Zhang Qian‐yong, and Mi Man‐tian. 2016. “Resveratrol Attenuates Trimethylamine‐N‐Oxide (TMAO)‐Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism Via Remodeling of the Gut Microbiota.” MBio 7: e02210–5. 10.1128/mBio.02210-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Barrea, Luigi , Muscogiuri Giovanna, Annunziata Giuseppe, Laudisio Daniela, de Alteriis Giulia, Tenore Gian Carlo, Colao Annamaria, and Savastano Silvia. 2019. “A New Light on Vitamin D in Obesity: A Novel Association With Trimethylamine‐N‐Oxide (TMAO).” Nutrients 11: 1310. 10.3390/nu11061310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Obeid, Rima , Awwad Hussain M., Kirsch Susanne H., Waldura Christiane, Herrmann Wolfgang, Graeber Stefan, and Geisel Juergen. 2017. “Plasma Trimethylamine‐N‐Oxide Following Supplementation with Vitamin D or D Plus B Vitamins.” Molecular Nutrition & Food Research 61: 1600358. 10.1002/mnfr.201600358 [DOI] [PubMed] [Google Scholar]
- 111. Wang, Guangji , Kong Bin, Shuai Wei, Fu Hui, Jiang Xiaobo, and Huang He. 2020. “3,3‐Dimethyl‐1‐butanol Attenuates Cardiac Remodeling in Pressure‐Overload‐Induced Heart Failure Mice.” The Journal of Nutritional Biochemistry 78: 108341. 10.1016/j.jnutbio.2020.108341 [DOI] [PubMed] [Google Scholar]
- 112. Feng, Wuwen , Ao Hui, Peng Cheng, and Yan Dan. 2019. “Gut Microbiota, A New Frontier to Understand Traditional Chinese Medicines.” Pharmacological Research 142: 176–91. 10.1016/j.phrs.2019.02.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript does not generate any code or data. Supplementary materials (graphical abstract, slides, videos, Chinese translated version and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.


