Abstract
Mycophilic or fungicolous fungi can be found wherever fungi exist since they are able to colonize other fungi, which occupy a diverse range of habitats. Some fungicolous species cause important diseases on Basidiomycetes, and thus, they are the main reason for the destruction of mushroom cultivations. Nonetheless, despite their ecological significance, their genomic data remain limited. Cladobotryum mycophilum is one of the most aggressive species of the genus, destroying the economically important Agaricus bisporus cultivations. The 40.7 Mb whole genome of the Greek isolate ATHUM6906 is assembled in 16 fragments, including the mitochondrial genome and 2 small circular mitochondrial plasmids, in this study. This genome includes a comprehensive set of 12,282 protein coding, 56 rRNA, and 273 tRNA genes. Transposable elements, CAZymes, and pathogenicity related genes were also examined. The genome of C. mycophilum contained a diverse arsenal of genes involved in secondary metabolism, forming 106 biosynthetic gene clusters, which renders this genome as one of the most BGC abundant among fungicolous species. Comparative analyses were performed for genomes of species of the family Hypocreaceae. Some BGCs identified in C. mycophilum genome exhibited similarities to clusters found in the family Hypocreaceae, suggesting vertical heritage. In contrast, certain BGCs showed a scattered distribution among Hypocreaceae species or were solely found in Cladobotryum genomes. This work provides evidence of extensive BGC losses, horizontal gene transfer events, and formation of novel BGCs during evolution, potentially driven by neutral or even positive selection pressures. These events may increase Cladobotryum fitness under various environmental conditions and potentially during host–fungus interaction.
Keywords: Cladobotryum mycophilum, genome, fungicolous fungi, biosynthetic gene cluster (BGC), secondary metabolism, horizontal gene transfer (HGT)
Introduction
Hypocrealean fungi present a plethora of biological interactions in the environment, exhibiting various symbiotic modes of life, positively or negatively affecting the host (Redman et al. 2001). Some of them, especially species belonging to the family Hypocreaceae, established a fungicolous (also known as mycophilic) mode of life, due to their stable association with other fungi (Gams et al. 2004). Their hosts are principally Basidiomycetes; however, in certain cases, it has been found to be ascomycetes (Gams et al. 2004). Some Hypocrealean species are the most important mycopathogens of cultivated mushrooms causing pathological conditions, i.e. cobweb (Cladobotryum spp.), dry bubble (Lecanicillium fungicola), green mold (Trichoderma aggressivum), and wet bubble disease (Mycogone spp.; Gea et al. 2021). The rate of the reported infections is increased in a wide range of edible mushrooms, i.e. Agaricus bisporus, Pleurotus ostreatus, Pleurotus eryngii, Ganoderma sichuanensis, Flammulina velutipes, and Hypsizygus marmoreus (Grogan and Gaze 2000; Back et al. 2012; Zuo et al. 2016; Gea et al. 2019).
Cladobotryum species are highly adaptable with a broad host range, affecting the sporophores of cultivated and wild Basidiomycetes, but also those of ascomycetes (Adie et al. 2006; Carrasco et al. 2017; Lakkireddy et al. 2020; Milic et al. 2022). Cladobotryum mycophilum, Cladobotryum dendroides, Cladobotryum protrusum, and Cladobotryum varium are some of the most common pathogenic agents in mushroom farms. Cladobotryum mycophilum is a highly important member, reported to cause cobweb disease primarily on Agaricales including the cultivated strains of white button (A. bisporus) and king oyster (P. eryngii) mushrooms (Gea et al. 2011, 2012; Kim et al. 2012; Muhammad et al. 2019; Milic et al. 2022). The sexual state of certain Cladobotryum species is associated with the genus Hypomyces, while for the rest, their anamorphs (conidial or asexual states) are not correlated with any teleomorphs (Rehner and Samuels 1995). Studies of C. mycophilum, the anamorph of Hypomyces odoratus, are focused on its diversity, distribution, and phylogenetic positioning within the genus, using gene regions of the internal transcribed spacer (ITS), the RNA polymerase II subunit (RPB1 and RPB2), and the translation elongation factor 1-alpha (TEF1) for barcoding, in addition to secondary metabolite profiling (Põldmaa 2011; Milic et al. 2022).
The genomic analyses of Cladobotryum species and in extent the genomes' contribution to the biotechnological importance of this genus are currently in need, since finding new environmentally friendly solutions for preventing mushroom cultivation damage is fundamental. Additionally, the potential of C. mycophilum as a biological control agent against fungal phytopathogens has been examined, and the initial outcomes appeared promising (Santos et al. 2019). All these factors necessitate the genetic elucidation of fungal–fungal interactions. To date, there is limited knowledge regarding the genetic mechanisms underlying Cladobotryum's colonization in respective hosts, which is crucial for enhancing cultivation efficiency through environmentally friendly methodologies.
Filamentous fungi produce a wide range of secondary metabolic compounds, which play a crucial role in fungal development and shape their interactions of fungi with other organisms (Keller 2019; Zhang et al. 2020). In some cases, the production of these metabolites is associated with host colonization and their mode of life (Lysøe et al. 2011; Pedrini 2018; Cheng et al. 2020; Henríquez-Urrutia et al. 2022). The genus Cladobotryum is notable for its abundant and diverse secondary metabolism, including the production of red pigments (Põldmaa 2011), cladobotric acids G–I (Dao et al. 2022), cyclopeptides (Zhou et al. 2021), parnafungins and their open-ring forms (Bills et al. 2009), flavipucine and brunnescin (Wagner et al. 1995), and various other secondary metabolites (Milic et al. 2022). In contrast to primary metabolite synthesis, genes responsible for the production of secondary metabolites are often organized in contiguous biosynthetic gene clusters (BGCs) (Bradshaw et al. 2013; Keller 2019). BGC genes encode synthases, tailor enzymes, transcription factors, proteins with toxic properties, and other regulatory functions. BGCs are often coregulated, controlling the production of the secondary metabolites, and as a result, they play an important role in species ecological functions (Keller 2019; Gotting et al. 2022).
The elucidation of fungicolous interactions through the identification of secondary metabolic pathways and other pathogenicity-related genes can be achieved by comparative genomics. Until now, the genomes of C. dendroides and C. protrusum along with the causal agent of wet bubble disease in A. bisporus, i.e. Mycogone perniciosa, are available, revealing a diverse array of secondary metabolic BGCs (Li et al. 2019; Sossah et al. 2019; Xu et al. 2020). Identification of BGCs implicated to the secretion of secondary metabolic compounds, pathogen–host interaction (PHI) genes, transposable elements (TEs), carbohydrate-active enzymes (CAZymes), secretory proteins, membrane transport proteins, and cytochromes P450 will provide the necessary information in order to understand the mycophilic mode of life of C. mycophilum. Comparative genomics are essential to reveal the molecular mechanisms and processes involved in the fungicolous mode of life in Hypocreales, illuminating its evolutionary ancestry.
The present work entails the de novo sequencing of the whole genome of the Greek isolate C. mycophilum ATHUM6906, utilizing Nanopore technology, for the purposes of (1) presenting a high-quality reference genome for C. mycophilum ATHUM6906, (2) identifying all genes and genetic elements potentially related to mycophilic behavior, (3) exploring its secondary metabolic range and plasticity, and (4) conducting a comparative secondary metabolism analysis in genomic and in extend evolutionary level, when the existing genomes of species within the Hypocreaceae family are under scrutiny. Overall, the current effort establishes the groundwork for a deeper understanding of the fungicolous adaptation of C. mycophilum and in extent of genus Cladobotryum, with the future goal to employ this knowledge for biocontrol of mushroom's cobweb disease.
Materials and methods
Fungal material and culture conditions
Cladobotryum mycophilum ATHUM6906 was collected from Hypholoma sp., a species belonging to the order Agaricales, in Castanea sp. forest, Mt. Pilio, Magnisia, Greece, 2009 (Milic et al. 2022). The fungus was deposited at the Mycetotheca ATHUM in the Dried Specimen Collection and Culture Collection of Fungi of the National and Kapodistrian University of Athens (NKUA, Athens, Greece). The isolate was cultured in Petri dishes (diameter: 90 mm) on potato dextrose broth with 1% yeast extract, incubated in a shaker (200 rpm) at 25°C in a natural day/night photoperiod. Preliminary in-house experiments showed that it is capable of infecting cultivations of the edible mushroom A. bisporus.
DNA extraction and sequencing
Mycelium was collected by vacuum filtration, and the total DNA isolation was performed using 100 mg of fungal material, using the HigherPurity Plant DNA Purification Kit (Canvax Reagents S.L., Valladolid, Spain) according to manufacturer's instructions. The extracted DNA was checked for quality and quantity using a NanoDrop (Thermo Fisher Scientific, Waltham, MA) and the Qubit broad range DNA assay kit (Thermo Fisher Scientific Waltham, MA), respectively.
One microgram of the total genomic DNA was used for Nanopore library preparation using the 1D Ligation Sequencing Kit (SQK-LSK110, Oxford Nanopore Technologies, Oxford, UK). Sequencing was performed using the R9.4.1 flow cell on a MinION device (Oxford Nanopore Technologies, Oxford, UK). Base calling was performed offline with ONT's Guppy software pipeline version 3.4.5, enabling the --pt_scaling flag and setting the --trim_strategy flag to DNA.
Long read filtering, correction, and assembly
Adapter trimming of the raw sequences was performed by Porechop version 0.2.4 (www.github.com/rrwick/Porechop), setting the --adapter_threshold to 96 and enabling the --no_split flag. Setting the genome size to 40 Mb, the trimmed reads were further trimmed and corrected using Canu version 2.2 (Koren et al. 2017), enabling the -trim and -correct flags, respectively. Genome assembly was created using the Flye version 2.9.1 (Kolmogorov et al. 2019) using the --nano-corr flag, setting the genome size to 40 Mb and enabling the --trestle flag. Bandage v.0.9.0 (Wick et al. 2015) was used to visualize assembly graphs and search for telomere sequences by using the built-in blast function to search the telomere sequence (TTAGGGT)n5–15. In order to evaluate the completeness of the final genome assembly, Benchmarking Universal Single-Copy Orthologs (BUSCO) analyses were performed with BUSCO version 5.4.7, using hypocreales_odb10 lineage gene set (Manni et al. 2021).
Gene prediction and functional annotation
Assembly annotations were performed using GenSAS v.6.0 (Humann et al. 2019), unless otherwise stated. Interspersed repeats, low complexity DNA sequences, and TEs were detected and masked by RepeatModeler v.2.0.1 and RepeatMasker v.4.1.1 (http://www.repeatmasker.org/) setting the DNA source to fungi. RNAmmer version 1.2 (Lagesen et al. 2007) and tRNAscan-SE version 2.0.7 (Lowe and Chan 2016) were used to detect the ribosomal RNA and tRNA genes, respectively. In order to primarily identify genomic regions with putative protein genes, transcript alignments were performed with BLAST nucleotide (blastn) tool version 2.12.0 using transcript database NCBI refseq fungi (Camacho et al. 2009) and BLAT tool version v2.5 using Transcripts FASTA file: NCBI refseq fungi (Kent 2002) and protein alignments were performed using DIAMOND proteins version 2.0.11 against Protein Data Set: NCBI refseq fungi (Protein; Buchfink et al. 2021). De novo gene prediction was performed using the following tools with default parameters: (1) AUGUSTUS tool version S3.4.0 with the reference gene data set of Fusarium graminearum (Stanke and Morgenstern 2005), (2) GeneMarkES version 4.48 (Ter-Hovhannisyan et al. 2008), and (3) GlimmerM tool version 2.5.1 selecting Aspergillus reference organism (Delcher et al. 2007). The tool EvidenceModeler was used to create a consensus gene set using the output files of the previously distributed tools (Haas et al. 2008). The ab initio official gene set (OGS) was evaluated with BUSCO analysis. Functional analysis of the OGS was performed using (1) BLAST protein vs protein (blastp) against Protein Data Set: NCBI refseq fungi (Protein; Camacho et al. 2009), (2) DIAMOND Functional version 2.0.11 against Protein Data Set: NCBI refseq fungi (Protein; Buchfink et al. 2021), and (3) InterProScan version 5.53-87.0 (Jones et al. 2014). The presence and location of signal peptide cleavage sites in amino acid sequence were identified using SignalP version 5.0b setting the -org flag to eukaryote (Petersen et al. 2011). The annotation of the OGS was performed using local BLASTp (e-value 1 × 10−50) against nonredundant (NR) protein sequence (Sayers et al. 2022), Swiss-Prot (Bateman et al. 2017), KEGG (Kanehisa et al. 2016), Gene Ontology (GO; Harris et al. 2004), clusters of orthologous groups for eukaryotic complete genomes (COG; Harris et al. 2004), PHI (Urban et al. 2022), CAZymes (Drula et al. 2022), MEROPS (Rawlings et al. 2014), PredGPI prediction server (Pierleoni et al. 2008), and Transporter Classification (TCdb; Saier et al. 2021) databases. TMHMM v2.0 was used to identify transmembrane proteins based on a hidden Markov model for transmembrane helices (Krogh et al. 2001).
Mitochondrial DNA and mating-type idiomorph characterization
The mitochondrial contig was annotated as follows: the protein coding, ribosomal (rRNA), and tRNA genes were identified using BLASTx, BLASTn (Johnson et al. 2008), and tRNAscan-SE version 2.0.7 (Lowe and Chan 2016), respectively. The genetic code employed was “The Mold, Protozoan, and Coelenterate Mitochondrial Code and the Mycoplasma/Spiroplasma Code” (NCBI transl_table=4). The mitochondrial genome and plasmid were visualized using OrganellarGenomeDRAW (OGDRAW) version 1.3.1 (Greiner et al. 2019). The mating-type genes for C. mycophilum were determined by tBLASTx against the respective ones of the related species C. dendroides and C. protrusum (Sossah et al. 2019; Xu et al. 2020), and this locus was visualized by Lasergene's MegAlign v.11 program (Burland 2000).
Comparative genomics
To study the evolution and genetic diversity of C. mycophilum's genome, OrthoVenn3 tool (Sun et al. 2023) was used to identify and annotate orthologous clusters and infer phylogenetic relationships among selected species representing Hypocreaceae family, i.e. Trichoderma harzianum, Trichoderma virens, C. dendroides, C. protrusum, Escovopsis sp., and Hypomyces perniciosus, with the entomopathogenic species Beauveria bassiana used as outgroup (NCBI assembly accessions: GCF_003025095.1; GCF_000170995.1; GCA_011799845.1; GCA_004303015.1; GCA_003055185.1; GCA_008477525.1; and GCA_000280675.1, respectively). The OrthoFinder algorithm (Emms and Kelly 2019) was selected enabling annotation, protein similarity analysis, and cluster relationship network, using default parameters. Maximum likelihood phylogenetic analysis of the selected species was also conducted in OrthoVenn3 interference using the program FastTree2 and the evolution model JTT + CAT (Price et al. 2010), and the reliability of each node was determined by the Shimodaira-Hasegawa (SH) test. CAFE5 Software was used to calculate the contraction and expansion in gene family size (Mendes et al. 2021). The divergence times were set 133 million years ago (MYA) for B. bassiana and T. harzianum and 76 MYA among Escovopsis sp. and T. harzianum, as found in TimeTree 5 web portal (Kumar et al. 2022). Synteny analyses among ATHUM6906 and the genomes of C. dendroides and C. protrusum were performed comparing using i-ADHoRe3 (Proost et al. 2012), with default parameters, and the visualization was performed locally using Circos version 0.69-9 (Krzywinski et al. 2009).
BGCs and secondary metabolism comparative analysis
Forty genomes from species belonging to Hypocreaceae family were selected for comparative analysis of secondary metabolism (Table 1). In all species, BGCs were identified using antiSMASH 7.0 (Blin et al. 2023) enabling the cluster border prediction based on transcription factor binding sites (CASSIS) selection. All genes that were identified as part of BGCs were analyzed in OrthoFinder v2.5.5 (Emms and Kelly 2019), in order to examine the shared and unique secondary metabolism–related protein-coding genes (PCGs) and BGCs in Hypocreaceae family. The rooted species tree was created setting the “-M msa” parameter, which enables MAFFT to create multiple sequence alignments and FastTree to construct a maximum likelihood secondary metabolism–based tree. The similarity network analysis and the exploration of BGC diversity within Hypocreaceae family were conducted using the BiG-SCAPE program (Navarro-Muñoz et al. 2020). BiG-SCAPE is a genome mining designed for the rapid and interactive examination of BGCs across multiple genomes, facilitates the development of similarity networks, and enables the classification of BGCs into families. To identify similarities with known metabolites, all reference BGCs from the MIBiG database were included in the analysis (Terlouw et al. 2023). Horizontal gene transfer (HGT) analysis and Alien Index (AI) scores of candidate HGTs.
Table 1.
Selected species and strains belonging to Hypocreaceae family, included in the secondary metabolism comparative analysis of this study.
| Number | Species | Strain | GenBank assembly accession |
|---|---|---|---|
| 1 | Cladobotryum dendroides | CCMJ2808 | GCA_011799845.1 |
| 2 | Cladobotryum mycophiluma | ATHUM6906 | JAVFKD000000000 |
| 3 | Cladobotryum protrusum | CCMJ2080 | GCA_004303015.1 |
| 4 | Escovopsis sp. | TC | GCA_003055185.1 |
| 5 | Escovopsis sp. | Ae724 | GCA_003055165.1 |
| 6 | Escovopsis weberi | — | GCA_001278495.1 |
| 7 | Hypocreaceae sp. | CTeuk-1143 | GCA_029290895.1 |
| 8 | Hypomyces perniciosus | HP10 | GCA_008477525.1 |
| 9 | Mycogone perniciosa | MgR1 | GCA_025331125.1 |
| 10 | Sphaerostilbella broomeana | TFC201724 | GCA_930272545.1 |
| 11 | Trichoderma afroharzianum | IIPRTh-33 | GCA_020736905.1 |
| 12 | Trichoderma arundinaceum | TP19.13 | GCA_025919625.1 |
| 13 | Trichoderma asperelloides | T203 | GCA_021066465.1 |
| 14 | Trichoderma asperellum | CBS 433.97 | GCA_003025105.1 |
| 15 | Trichoderma atrobrunneum | ITEM 908 | GCA_003439915.1 |
| 16 | Trichoderma atroviride | IMI 206040 | GCA_000171015.2 |
| 17 | Trichoderma breve | T069 | GCA_028502605.1 |
| 18 | Trichoderma brevicompactum | IBT 40841 | GCA_003012085.1 |
| 19 | Trichoderma brevicrassum | TC967 | GCA_017311225.1 |
| 20 | Trichoderma citrinoviride | TUCIM 6016 | GCA_003025115.1 |
| 21 | Trichoderma cornu-damae | KA19-0412C | GCA_020631695.1 |
| 22 | Trichoderma erinaceum | CRRI-T2N1 | GCA_013365115.1 |
| 23 | Trichoderma gamsii | T6085 | GCA_001481775.2 |
| 24 | Trichoderma gracile | HK011-1 | GCA_020002365.1 |
| 25 | Trichoderma hamatum | GD12 | GCA_000331835.2 |
| 26 | Trichoderma harzianum | CBS 226.95 | GCA_003025095.1 |
| 27 | Trichoderma koningii | JCM 1883 | GCA_001950475.1 |
| 28 | Trichoderma koningiopsis | RA3a | GCA_022985005.1 |
| 29 | Trichoderma lentiforme | CFAM-422 | GCA_011066345.1 |
| 30 | Trichoderma lixii | MUT 3171 | GCA_014468695.1 |
| 31 | Trichoderma longibrachiatum | FL-4 | GCA_026259275.1 |
| 32 | Trichoderma oligosporum | CGMCC 3.17527 | GCA_015266385.1 |
| 33 | Trichoderma parareesei | CBS 125925 | GCA_001050175.1 |
| 34 | Trichoderma pleuroti | TPhu1 | GCA_001721665.1 |
| 35 | Trichoderma pseudokoningii | — | GCA_943193705.1 |
| 36 | Trichoderma reesei | QM6a | GCA_000167675.1 |
| 37 | Trichoderma semiorbis | FJ059 | GCA_020045945.2 |
| 38 | Trichoderma simmonsii | GH-Sj1 | GCA_019565615.1 |
| 39 | Trichoderma virens | Gv29-8 | GCA_000170995.1 |
| 40 | Trichoderma viride | Tv-1511 | GCA_007896495.1 |
Their GenBank assembly accession numbers are also provided.
a Current work.
In order to determine if the core genes of the NI-siderophore, type 3 polyketide synthase (T3PKS), and non-alpha poly-amino acid (NAPAA) BGCs, which were found only in Cladobotryum genomes, are a result of HGT, the analyses described below were performed.
For each BGC core gene, a Blastp search was performed against the NR database, with default parameters. The first 250 hits with the highest score (e-value and identity) were included in the creation of an amino acid alignment. All alignments were acquired using the MAFFT online tool (Katoh et al. 2019) using default parameters. As a result, 3 matrices were created, henceforward called “NI-siderophore-matrix,” “T3PKS-matrix,” and “NAPAA-matrix” for NI-siderophore, T3PKS, and NAPAA core biosynthetic genes, respectively. For each matrix, amino acid model selection was performed in IQ-TREE (Kalyaanamoorthy et al. 2017). Maximum likelihood phylogenetic trees were constructed in IQ-TREE with the ultrafast bootstrap with the selected amino acid model (Nguyen et al. 2015).
In order to define the origin of the terpene synthase gene (cl_my.00g000130), a Blastp search was performed against the NR database. Primarily, the first aligned amino acid sequences with the highest score (e-value and identity) belonged to Basidiomycetes. In order to ensure that the results are true and independent, a second search excluding sequences derived from Basidiomycetes was performed. As a result, 250 sequences of Basidiomycetes and 250 sequences of other fungi were selected to produce the “terpene-matrix.” Additionally, Hypocreaceae terpene synthases, which were identified in the current work, were included. The “terpene-matrix” and phylogenetic tree was constructed as described previously.
To assess potential HGT events, the AI score was calculated for all candidate HGT genes included in the BGCs mentioned above. This quantitative method was employed to measure how well the C. mycophilum protein sequences align with those from non-Hypocrealean vs Hypocrealean. The AI score for each gene was computed by utilizing the e-value derived from the best sequence alignment of C. mycophilum proteins against all Hypocrealean and non-Hypocrealean sequences present in the NCBI NR database. The formula proposed by Matriano et al. (2021) was applied to calculate the AI score, allowing for a comprehensive evaluation of potential gene transfer events.
Results and discussion
Genome features and gene prediction
Genome sequencing of C. mycophilum ATHUM6906 produced a total of 1,581 Mb clean data which were further assembled into a 40.7 Mb draft genome with 37× mean coverage and 47.8 GC % (Table 2). This consisted of 16 contigs with N50 of approximately 5.07 Mb. The completeness of the genome was evaluated by mapping the BUSCOs against fungi_odb10 and hypocreales_odb10 data sets. This analysis indicated a high-quality genome, identifying 97.4 and 96.7% complete fungal and hypocrealean BUSCOs, respectively.
Table 2.
Whole genome sequencing statistics and genomic features of C. mycophilum ATHUM6906.
| ATHUM6906 genome statistics and features | |
|---|---|
| Total length (bp) | 40,679,719 |
| Number of fragments | 16 |
| N50 (bp) | 5,067,140 |
| Largest fragment (bp) | 7,644,443 |
| Mean genome coverage | 37 |
| Number of predicted tRNA genes | 273 |
| Number of predicted rRNA genes | 56 |
| Number of predicted PCGs | 12,282 |
| Transposable elements (%) | 3.72 |
| Mitochondrial genome size (bp) | 76,524 |
| Mitochondrial plasmid size (bp) | 11,721 |
In filamentous fungi, the telomeric repeats were identified at the end of the linear chromosomes (Cohn et al. 2005). Among the 13 linear contigs of this genome, 8 contigs contained telomere structures at least at the one end and 9 telomeric sequences were identified in total. Three out of 16 contigs had circular topology (contigs 24, 28, and 29). In detail, the telomeres consisted of the (TTAGGGT)n telomeric repeat followed by KolobokH DNA transposon sequences, which presented sizes of ca. 10,470 bp (Fig. 1a). KolobokH included 2 RecQ-like DNA helicase PCGs and an uncharacterized PCG, which is a similar structure with the one primarily found in a fungus of Glomeromycota, i.e. Rhizophagus irregularis (Kojima and Bao 2022). G-quadruplex (G4) analysis showed that the telomeric repeats of C. mycophilum could form G4 structures, which are implicated in telomere maintenance (Williamson et al. 1989; Zhao et al. 2010). Since 9 telomere structures were predicted, the genome of C. mycophilum consisted of at least 5 potential chromosomes. In C. dendroides genome, the 8 contigs are part of 6 potential chromosomes. Other Hypocrealean species, like Trichoderma spp. (3–11 chromosomes; Kubicek et al. 2019), Metarhizium spp. (7 chromosomes; Saud et al. 2021), Epichloë spp. (7 chromosomes; Treindl et al. 2021), and Fusarium spp. (4–15 chromosomes), possess from 3 to 15 haploid chromosomes.
Fig. 1.
a) Structure of telomeres in the genome of C. mycophilum ATHUM6906, including the (TTAGGGT)5–15 telomeric repeat and the KoloboxH DNA transposon. b) Comparison of predicted PCG annotation results among Swiss-Prot, PDB, CAZy, PHI, and COG databases. c) The complete mitochondrial genomes of ATHUM6906. The genes related to complex I, III, IV, and ATP synthase, tRNA genes and rRNA genes are indicated in the mitochodrial genome map. d) The mitochondrial plasmid where 3 copies of polB2 gene are located. e) Schematic representation of the structure of mating-type loci (MAT 1-1-1) in contig 1 of C. mycophilum ATHUM6906 genome. The arrows indicate the gene transcription orientation.
Two hundred and seventy-one tRNA genes were located in almost all contigs with the exception of the 5 smallest fragments. On the contrary, the 56 identified rRNA genes are located in scaffolds 12 and 17 (Table 2).
A total of 12,282 PCGs were predicted by the combination of homology- and de novo–based gene prediction methods (Fig. 1b; Supplementary Table 1). Among them, 11,830 (96.31%) genes were homologous in the NCBI NR database, followed by InterPro database (11,089 genes—90.27%), Swiss-Prot database (8,090 genes—65.87%), KEGG database (4,074 genes—33.2%), GO database (8,033 genes—65.40%), COG database (4,326 genes—35.22%), PHI database (3,956 genes—32.21%), and CAZyme database (420 genes—3.42%). In C. mycophilum genome, 1,111 secretory proteins were predicted. TMHMM v2.0 predicted at least 1 transmembrane helix in 2,339 PCGs. Interestingly, according to TCdb, 1,004 genes were aligned with genes encoding transporters (Supplementary Table 1).
TEs were found on 3.72% of the C. mycophilum genome. Class I TEs (retroelements) are more abundant covering 2.14%, and most of them are classified as non-LTR (LINEs) at 2.13%, while LTRs make up 0.01% of the total genome (Supplementary File 1). Class II TEs are less common (0.95%) followed by unclassified repeats (0.63%). Similar coverage in TEs can be found in other species of the genus, i.e. C. protrusum (2.59%) and C. dendroides (4.37%), while in smaller genomes, TEs tend to be more abundant, like the fungicolous species H. perniciosus (23.25%) or Escovopsis sp. (5.89%; Li et al. 2019; Sossah et al. 2019). Eighteen percent (456 out of 2,469) of the TEs of the C. mycophilum genome were located within coding regions. Seventy percent of those TEs are located within a single exon while the rest span over multiple exons and introns.
Circular molecules and identification of mating-type idiomorphs
The mitochondrial genome of C. mycophilum (contig 24; NCBI Acc. No: OP928225) was a circular molecule of 76,524 bp containing the 14 PCGs responsible for the oxidative phosphorylation and ATP production (cox1-3, nad1-6, nad4L, cob, atp6, and atp8-9), 2 rRNA genes (rns and rnl), and 26 tRNA genes (trns; Table 2 and Fig. 1c). More than 54.68% of the mtDNA corresponded to intronic regions. In total, 28 introns of group I were identified, in most cases hosting GIY-YIG and LAGLIDADG homing endonuclease genes. Only atp8, nad2, nad3, nad6, and rns genes did not possess introns. High intraspecies variation was identified among C. mycophilum ATHUM6906 (76,524 bp) and the respective genome published by Chen et al. (2020) (78,529 bp—GenBank accession no. NC_054243.1). Although gene order is identical for these mitochondrial genomes, the one of ATHUM6906 was approximately 2 kb smaller (Chen et al. 2020). Intron indels and transpositions along with variation in their intergenic regions were identified. In detail, mt genes of strain ATHUM6906 had gained (i.e. in nad5, cob, cox1, nad1, nad4, and rnl genes) or lost intronic region (i.e. in nad2, nad3, cox2, atp6, and cox3) genes. Another circular molecule of 11,721 bp (contig 28) was identified (Table 2 and Fig. 1d). Based on its gene content, contig 28 seems to be a mitochondrial plasmid, according to its similarity with mt plasmids found in Cryphonectria parasitica and Neurospora intermedia (Hausner 2012). It contained the mitochondrial DNA polymerase gene in 3 copies, with high sequence identity to the respective gene of C. parasitica's pCRY1 plasmid (Monteiro-Vitorello et al. 2000). This is a plasmid that reduces pathogenicity in C. parasitica, when present (Monteiro-Vitorello et al. 2000). Therefore, the C. mycophilum plasmid may play a similar role. However, more experiments for this plasmid have to be performed before concluding about its role. A third small circular molecule (contig 29) did not align with any known genes or genetic elements.
MAT1-1 mating-type idiomorph was identified in the genome of C. mycophilum ATHUM6906 in 1 copy located on contig 1, in agreement with the fungicolous species C. dendroides and in contrast to C. protrusum and Hypomyces perniciosa, which possess the MAT1-2 idiomorph (Fig. 1e). The existence of MAT1-1 mating type in strain ATHUM6906 does not exclude the possibility that both mating types may be present at C. mycophilum population level. Complex I intermediate–associated protein 30 kDa (CIA30), anaphase-promoting complex subunit 5 (APC5), cytochrome C oxidase subunit 13 (COX13), AP DNA endonuclease 2 (APC5), DNA-binding mating-type M-specific polypeptide Mc (MATMC), endocytosis protein end4 (SLA2), and S ribosomal protein L21-A (RL21A) genes were located upstream and downstream of MAT1-1-2 idiomorph, almost the same gene content compared to C. dendroides, C. protrusum, and H. perniciosa (Li et al. 2019; Sossah et al. 2019; Xu et al. 2020).
Comparative genomics
Comparative whole genome analysis among ATHUM6906 and other representatives of Hypocreaceae family, whose genomes are available and fully characterized, was performed to study the evolutionary changes among organisms and to identify the conserved and unique genes of the species examined (as described in M&M). Clustering all predicted PCGs among the representatives of Hypocreaceae family, 12,033 orthogroups were formed (Supplementary File 2). Interestingly, even though species are closely related, only half of the predicted orthogroups (i.e. 5,824) were shared in all examined species. This is due to Escovospis sp. Ae724, whose genome size is significantly smaller than the rest Hypocreaceae (approximately 30 Mb with only 7,231 PCGs). When genomic sequences of Ae724 were excluded, 999 more orthologous clusters were identified in the rest species examined, resulting in a total core set of 6,823 protein clusters (Fig. 2a). The PCGs of C. mycophilum were grouped in 9,883 clusters and 646 singletons. It shared more orthogroups with C. protrusum (728 clusters) than C. dendoides (463 clusters; Fig. 2b). Nevertheless, based on the current whole genome phylogenetic analysis, C. mycophilum was closer to C. dendroides, as they diverged 9.9 MYA, while C. protrusum was more phylogenetically distant, as it branched off 19.13 MYA (Fig. 2c). This positioning is also supported by other single locus-based phylogenetic analyses, in which the ITS was mainly used (Põldmaa 2011; Milic et al. 2022). One hundred and fourteen gene family expansions and 79 gene family contractions were found in C. mycophilum, related to genes with transferase activity, oxidoreductase activity, hydrolase activity, and other enzymatic or binding molecular functions (Fig. 2c; Supplementary Fig. 1). Mycogone perniciosa and Cladobotryum spp. (and their Hypomyces teleomorphs), both mushroom-associated fungicolous species sharing a variety of hosts, were diverged 67 MYA (C. mycophilum—H. perniciosus), forming a clade that also includes the fungicolous genus Escovopsis (Fig. 2c). According to the current comparative study, massive gene family contraction had happened during the formation of genus Escovopsis (i.e. 572 families), and only 7 gene families were expanded (Fig. 2a and c). Overall, the Hypocreaceae family, which includes mycophilic species, appears to diverge from the rest of Hypocreales approximately 130 MYA, which is in accordance with a respective study focused on Trichoderma spp. that estimated that this event had happened among 100–140 MYA (Kubicek et al. 2019).
Fig. 2.
Genome comparison of C. mycophilum ATHUM6906. a) The chart presenting the number of orthologous clusters, which are shared among species. The numbers of orthogroups, which are shared within a group of species, as shown by their connected dots, are represented by the respective bar. b) The number of shared orthogroups among Cladobotryum species. c) The phylogenetic timetree of Hypocreaceae representatives based on their whole genome data. The tree was produced by the maximum likelihood method using the JTT + CAT evolutionary model. The numbers of contracted and expanded gene families are indicated in each node. d) Synteny comparison of C. mycophilum genome with C. dendroides (top) and C. protrusum (bottom). The Circo plots represent syntenic blocks. The outer numbers indicate chromosome numbers.
Cladobotryum spp. appeared to share a core set of genes, if compared to other fungicolous species. The formation of Cladobotryum clade happened approximately 19.2 MYA, and it was accompanied by the expansion of 61 gene families. These unique families and protein effectors may be related to their adaptation in Agaricomycetes (Fig. 2c). Gene order was conserved among C. mycophilum and C. dendroides genomes, and only small syntenic blocks were transposed (Fig. 2d). According to chromosome 7 of C. dendroides, contigs 3 and 10, which both have telomeric sequences only in one end, may form a single chromosome. On the contrary, more transposition events were identified, when comparing C. mycophilum and C. protrusum gene order. For example, contig 7 of C. mycophilum consists of partial synteny blocks of contigs 2 and 3 of C. protrusum. Similar events were observed in contigs 10, 12, and 24, with contig 10 to include gene blocks from 4 contigs of C. protrusum.
CAZymes and pathogenicity-related genes
In the first stages of infection, the pathogenic fungi use proteins of the CAZyme families to decompose the complex polysaccharides in the host cell wall (Bashyal et al. 2017). In C. mycophilum, 420 CAZymes were identified (Supplementary Table 1), with 214 genes encoding glycoside hydrolases (GHs), 97 genes encoding glycosyl transferases (GTs), 29 genes encoding carbohydrate-binding modules (CBMs), and 54 genes encoding auxiliary activities (AAs). From the 6 categories of CAZymes, the carbohydrate esterases (CEs; 12 genes) and polysaccharide lyases (PLs; 4 genes) were the most underrepresented. Cladobotryum mycophilum showed a significant abundance of CAZymes when compared to other species, which colonize basidiomycetous hosts like A. bisporus, i.e. 412 in C. protrusum (Sossah et al. 2019), 327 in C. dendroides (Xu et al. 2020), and 338 in H. perniciosus (Li et al. 2019). In the 4 fungicolous species mentioned above, GHs and GTs were the most commonly found enzymes, probably due to their role in the host cell wall degradation during the stages of infection.
The plethora of genes encoding cell wall–degrading enzymes is common in other fungicolous genera like Trichoderma in which genes encoding CAZymes were acquired through massive lateral transfer of genes from plant-associated fungi, a phenomenon which was not observed to the respective genes of Escovopsis sp. (Druzhinina et al. 2018; Kubicek et al. 2019). Druzhinina et al. (2018) did not include other fungicolous genera like Cladobotryum or Mycogone, due to the lack of genomic data at the time. According to Kubicek et al. in 2011, fungicolous behavior was an ancestral mode of life in genus Trichoderma, an ability lost in some species due to gene losses (Kubicek et al. 2011; Chaverri and Samuels 2013). All the above along with the plethora of CAZymes observed in C. mycophilum, C. protrusum, C. dendroides, and H. perniciosus suggest that the expansion in cell wall–degrading families may be related to the evolution of the fungicolous mode of life in Hypocreaceae.
Since chitin and β-(1,3) glucan are the core polysaccharides comprising fungal cell walls (Gow et al. 2017), chitinases and glucanases could be considered as core enzymes in host colonization process (Qin et al. 2020; Mart Nez-Cruz et al. 2021). In the genome of C. mycophilum, 26 chitinases were identified, all belonging to GH18 CAZyme category (Supplementary Table 2). An evolutionary study of chitinases showed that they are categorized in 3 groups, i.e. A, B, and C, with group B to be involved in fungicolous mode of life and entomopathogenicity of Sordariomycetes (Wang et al. 2021). Early-stage experiments in fungicolous fungi, like T. harzianum, have shown that some chitinases are upregulated during their interaction with a phytopathogenic fungus in vitro (Carsolio et al. 1994). This indicates the importance of GH18 chitinases in mycophilic behavior. Similarly, 37 PCGs were related to glucan degradation. These PCGs were found to be more variable since they belong to 13 different GH subfamilies and 1 AA family (Supplementary Table 3).
In fungi, secreted enzymes and other auxiliary proteins are highly important, especially in species that colonize the host surface (Bradley et al. 2022; Nazar Pour et al. 2022). In C. mycophilum genome, 1,111 secreted proteins were predicted (Supplementary Table 4), more than the respective ones in C. protrusum and C. dendroides genomes. Out of them, 339 PCGs had no match in any expertly curated database. From the rest of secretory proteins, 344 (31%) were predicted to encode pathogenicity–host-related genes (PHI), 181 (16.3%) CAZymes, 121 (10.9%) proteases (MEROPS database), and 31 GPI-anchored proteins (PredGPI prediction server), and only 15 genes were predicted to encode transporters (TCdb; Fig. 3a). In 15 chitinases and 18 glucan degradation enzymes, a Sec/SPI signal for secretory translocation was found, making them important candidates implicated in fungicolous mode of life.
Fig. 3.
a) PCGs with secretory signal for secretory translocation found in each database compared to the total number of PCGs with SP signal for C. mycophilum ATHUM6906. b) Pathogenicity-related genes of C. mycophilum ATHUM6906, as found by PHI, and classified according to their molecular function (top) and their biological process (bottom) in GO database. PCGs with more than 1 biological domain received a GO number for each identified domain. c) Number of matched genes per cellular function of the primary and secondary metabolism among Cladobotryum species, as predicted in KEGG database, i.e. Cladobotryum dendroides (top bar), C. protrusum (middle bar), and C. mycophilum (bottom bar).
Interestingly, according to PHI database, 32.21% PCGs of C. mycophilum ATHUM6906 could be related with pathogenicity (Supplementary Table 5). According to GO characterization, the identified biological domains of these putative pathogenicity-related genes were included in 3 functional categories (Fig. 3b). PCGs with binding and catalytic functions were the most abundant, as expected (Cairns et al. 2016). Furthermore, in C. mycophilum, more than 15% of pathogenicity-related genes encoded transporters highlighting their importance. Classified in respect to their biological process, most biological domains of pathogenicity-related PCGs were associated with metabolic processes of both primary and secondary metabolism (Fig. 3b). Preliminary analysis against KEGG database showed that more than 4,000 PCGs were associated with C. mycophilum primary and secondary metabolism, and 79 complete and 125 incomplete metabolic pathways were found. Some of them were implicated in biosynthesis of isoprenoids, the metabolism of cofactors and vitamins, in terpenoid, polyketides, and other secondary metabolite biosynthesis, and in xenobiotic biodegradation and metabolism (Fig. 3c). This indicated the necessity to further examine and characterize all genes related to the production of secondary metabolites.
BGCs in C. mycophilum
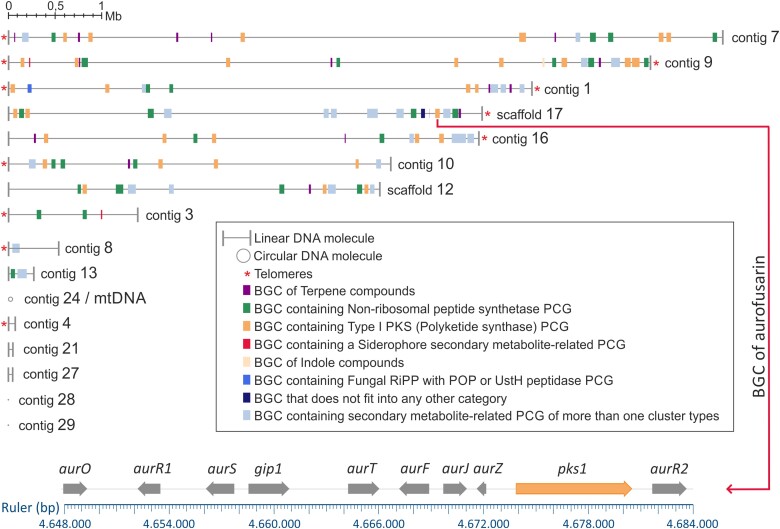
In fungi, multiple genes contributing to a secondary metabolic pathway are often clustered together in coregulated BGCs (Keller 2019). In C. mycophilum genome, 106 BGCs were identified, confirming its abundant secondary metabolism (Fig. 4). In total, 54 PCGs encoding type 1 polyketide synthases (T1PKSs), 48 nonribosomal polypeptide synthetases (NRPSs), 23 PCGs encoding terpene synthases, 3 fungal RiPP PCGs, 2 siderophore PCGs, 1 NAPAA like e-polylysine PCG, 1 indole PCG, and 1 T3PKS were found (Supplementary Table 6). In some cases, more than 1 synthase/synthetase genes from different categories were included in a single BGC (e.g. the NRPS-T1PKS BGC that has 72% similarity to BGC of chaetoglobosins from Chaetomium globosum CBS 148.51). In that case, clusters were overlapping each other, and in the common region, some regulatory or accessory genes exist and may play a role in both subclusters that form these BGCs. The shared auxiliary genes among these BGCs might possess a conserved nature, potentially serving as regulatory elements that have evolved from ancient fungal ancestors. This hypothesis could be an extension of the findings of a recent publication, which indicated that fungal genomes exhibit a delayed loss of ancestral gene families while rapidly duplicating certain gene groups, such as extracellular proteins and transcription factors (Merényi et al. 2023). Moreover, some BGCs had more than 70% similarity with well-known fungal biosynthetic clusters (i.e. chaetoglobosins, cichorine, wortmanamides A and B, clavaric acid, chrysogine, alternariol, dimethylcoprogen, pyranonigrin E, verticillin, naphthopyrone, aurofusarin, and depudecin).
Fig. 4.
Schematic representation of secondary metabolite BGC location in the 16 predicted fragments of C. mycophilum genome. The size of BGCs of terpene, NRPS, T1PKS, siderophore, indole, fungal RiPP, other that does not fit into any other category, and clusters containing more than 1 type of synthase is proportional to the genome size. Asterisk (*) in the end of the DNA fragments indicates the presence of telomeric sequences. Genetic locus of aurofusarin T1PKS BGC in scaffold 17 is also presented in detail (bottom).
Notably, the BGC of aurofusarin, the most well-known metabolite characterizing the red-colored Cladobotryum species (Milic et al. 2022), was located in scaffold 17 (Fig. 4). Aurofusarin is a dimeric polyketide belonging to the aromatic ones, also named naphthoquinones (Shibata et al. 1966). It was firstly found as a pigment in Fusarium culmorum (Ashley et al. 1937). The presence of aurofusarin's BGC was expected in C. mycophilum strain ATHUM6906, since a previous work in genus Cladobotryum showed that C. mycophilum is basal to the monophyletic clade formed by species producing this metabolite (Milic et al. 2022). The BGC of aurofusarin (Fig. 5) contained the polyketide synthase 1 gene (pks1) along with other genes encoding modification enzymes (aurO, gip1, aurF, aurJ, and aurZ), aurofusarin biosynthesis cluster protein S (aurS), regulatory proteins (aurR1, aurR2), and a rubrofusarin-specific efflux pump (aurT; Hoffmeister and Keller 2007). According to relevant studies in the phytopathogenic fungus F. graminearum, aurofusarin is able to increase its competitive saprophytic ability because of its antibiotic properties, but it is not implicated in host colonization (Malz et al. 2005; Cambaza 2018).
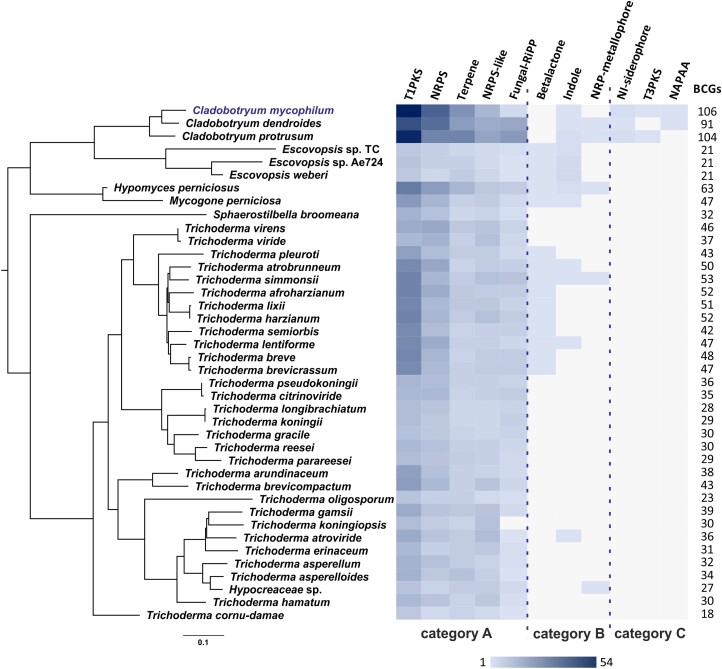
Fig. 5.
Heat map of BGC presence in 40 Hypocreaceae genomes in relation to their secondary metabolism phylogenetic tree. BGCs are grouped into 3 categories according to their distribution in the Hypocreaceae family. In detail, category A includes BGCs with wide distribution, category B BGCs with scattered distribution, and category C BGCs found only in Cladobotryum spp. The phylogenetic tree was produced by ML method in OrthoFinder v2.5.5 setting the “-M msa” parameter, which enables MAFFT to create multiple sequence alignments and FastTree to construct a maximum likelihood tree. All nodes are 100% supported. The number of the total BGCs of each species is indicated on the right.
Interestingly enough, the BGC cluster of a plant pathogenic metabolite, i.e. depudecin, was also retrieved in this mycophilic genome. Depudecin is an 11-carbon linear polyketide acting as an inhibitor of histone deacetylase (HDAC), which has been related with the virulence of the plant pathogenic fungus Alternaria brassicicola (Wight et al. 2009). The presence of this BGC prompts the question of whether it stands as an evolutionary vestige or if it plays a role in the mode of life of C. mycophilum.
Comparative analysis of Hypocreaceae secondary metabolism
Comparative genomics were used to investigate the diversity and distribution of genes and BGCs responsible for the synthesis of secondary metabolites among 40 genomes of Hypocreaceae family (Table 1). High variation in gene cluster presence and absence was found among the genomes (Fig. 5). Among the 40 available genomes of species belonging to Hypocreaceae family, C. mycophilum exhibited the highest abundance of “core” secondary metabolic–related PCGs and in extent BGCs, followed by the rest of Cladobotryum species. Interestingly, in genomes of genus Escovopsis, which is phylogenetically positioned as sister clade to Cladobotryum spp., an extended contraction of BGCs was found, which is probably directly related with their small genome size (approximately 27 Mb), which is in accordance with the study of Scott et al. (2023), which showed that BGC abundance is correlated with genome size in Trichoderma.
Cluster network analysis (Big-SCAPE program) linked the BGCs of C. mycophilum with those of the other species under investigation, organizing these BGCs into families. Out of the 106 BGCs in C. mycophilum, 58 were found to be singletons. Overall, the BGCs found in the examined genomes were clustered in 3 categories according to their distribution, i.e. the common BGCs in all examined species, hereafter called category A, the “mixed” distribution (random existence) of BGCs, named here as category B, and finally, the BGCs that existed only in genus Cladobotryum, category C (Fig. 5).
T1PKS, NRPS and NRPS-like, terpene, and fungal RiPP BGCs were identified in all examined species, comprising category A (Fig. 5). In detail, T1PKS type included 537 BGCs (Supplementary Fig. 2). BGC network analysis organized them in 251 families including 172 singletons distributed in all examined species. The most abundant family was FAM_00378, which includes 16 BGCs of genus Trichoderma. Notably, some clusters exhibited a scattered distribution across various genera within Hypocreaceae, suggesting potential losses in several species. For instance, family FAM_01528 (Table 3), which comprises BGCs found in 2 Trichoderma species, 3 Cladobotryum species, and H. perniciosus, was similar to the linear tetracyclic aromatic polyketide naphthacenedione “TAN-1612” cluster, originally identified in Aspergillus niger (Li et al. 2011). Conversely, certain T1PKS families were exclusively identified within Cladobotryum species, like FAM_01527 and FAM_01550, which are implicated in the production of depudecin and cichorine, respectively (Wight et al. 2009; Ahuja et al. 2012; Sanchez et al. 2012). A total of 723 NRPS BGCs were identified in the studied species (Supplementary Fig. 2). The largest family, FAM_00369, encompassed 16 BGCs found in Sphaerostilbella broomeana and 15 Trichoderma species. NRPS FAM_01191 (Table 3) included Cladobotryum species and H. perniciosus, sharing a 50% identity with the chrysogine BGC in F. graminearum (Wollenberg et al. 2017). Families FAM_01459, FAM_01615, FAM_0124, and FAM_01271 (Table 3) exclusively comprised BGCs from Cladobotryum species and exhibited similarities to BGCs responsible for producing the cyclic tetrapeptide apicidin, pyranonigrin E, swainsonine, and ascochlorin, respectively (Jin et al. 2010; Andersen et al. 2011; Cook et al. 2017; Araki et al. 2019). A total of 299 BGCs were associated with the biosynthesis of terpenes, and they were classified into 132 families, with 93 of them being singletons (Supplementary Fig. 2). While FAM_00237 (Table 3) represented the largest terpene family, comprising 23 BGCs from all Hypocreaceae genera, the specific terpene metabolite production associated with it could not be identified. Finally, a total of 181 BGCs were identified as fungal RiPPs, categorized into 122 families, with 92 of them existing as singletons (Supplementary Fig. 2). FAM_01311, a fusion of a fungal RiPP and NRPS, emerged as the most populous family, comprising 10 members exclusive to the genus Trichoderma, all sharing a 100% identity with the choline BGC from Aspergillus nidulans (Hai et al. 2019). Cladobotryum mycophilum possessed 3 RiPP BGCs, with 1 belonging to FAM_01522 (Table 3), aligning with the respective BGC of C. dendroides, and 2 as standalone singletons. Nevertheless, it was not possible to determine the potential products generated by C. mycophilum RiPPs due to lack of reference data.
Table 3.
Overview of BGCs in C. mycophilum ATHUM6906, detailing their type and genomic location.
| Number | BGC | Type | Location [from–to] | Family | Most similar known cluster (MIBiG accession) |
|---|---|---|---|---|---|
| 1 | contig1.region001 | T1PKS | Contig 1 [20,937–68,853] | FAM_01521 | |
| 2 | contig1.region002 | Fungal RiPP | Contig 1 [204,455–246,483] | FAM_01522 | |
| 3 | contig1.region003 | T1PKS | Contig 1 [1,036,058–1,079,067] | FAM_01523a | |
| 4 | contig1.region004 | NRPS-like, terpene | Contig 1 [1,427,122–1,468,197] | FAM_01524a | EQ-4 (BGC0001668) |
| 5 | contig1.region005 | NRPS | Contig 1 [1,470,220–1,514,188] | FAM_01525 | |
| 6 | contig1.region006 | NRPS-like | Contig 1 [1,721,962–1,761,887] | FAM_01526a | |
| 7 | contig1.region007 | T1PKS | Contig 1 [4,897,237–4,943,226] | FAM_01527 | Depudecin (BGC0000046) |
| 8 | contig1.region008 | T1PKS | Contig 1 [4,994,689–5,030,604] | FAM_01528 | |
| 9 | contig1.region009 | Terpene | Contig 1 [5,138,026–5,155,781] | FAM_01529 | |
| 10 | contig1.region010 | T1PKS, terpene | Contig 1 [5,158,976–5,238,693] | FAM_01530a | |
| 11 | contig1.region011 | Terpene, NRPS | Contig 1 [5,266,986–5,324,387] | FAM_01531a | |
| 12 | contig1.region012 | Terpene | Contig 1 [5,362,297–5,384,604] | FAM_01532a | |
| 13 | contig1.region013 | NRPS, T1PKS | Contig 1 [5,470,781–5,522,004] | FAM_01533a | |
| 14 | contig10.region001 | NRPS, T1PKS | Contig 10 [219,692–291,584] | FAM_01534a | TAN-1612 (BGC0000156) |
| 15 | contig10.region002 | T1PKS | Contig 10 [368,207–413,059] | FAM_01535a | Neurosporin A (BGC0001668) |
| 16 | contig10.region003 | NRPS | Contig 10 [460,675–503,357] | FAM_01536a | |
| 17 | contig10.region004 | NRPS | Contig 10 [558,013–605,851] | FAM_01537a | Ustiloxin B (BGC0000627) |
| 18 | contig10.region005 | Terpene | Contig 10 [1,278,927–1,300,991] | FAM_01514 | |
| 19 | contig10.region006 | NRPS | Contig 10 [1,334,843–1,379,470] | FAM_01539a | |
| 20 | contig10.region007 | T1PKS | Contig 10 [1,606,196–1,651,183] | FAM_01540a | |
| 21 | contig10.region008 | T1PKS | Contig 10 [2,199,826–2,243,385] | FAM_01541a | |
| 22 | contig10.region009 | T1PKS | Contig 10 [3,716,849–3,748,753] | FAM_01542a | |
| 23 | contig10.region010 | T1PKS, NRPS | Contig 10 [3,936,152–3,988,125] | FAM_01543a | |
| 24 | contig13.region001 | NRPS-like | Contig 13 [24,41–69,081] | FAM_01544 | |
| 25 | contig13.region002 | NRPS, T1PKS | Contig 13 [95,903–196,559] | FAM_01545a | Chaetoglobosins (BGC0001182) |
| 26 | contig16.region001 | Terpene | Contig 16 [272,867–293,287] | FAM_01546 | |
| 27 | contig16.region002 | T1PKS | Contig 16 [380,593–425,348] | FAM_01547a | |
| 28 | contig16.region003 | T3PKS | Contig 16 [1,649,997–1,690,854] | FAM_01548a | |
| 29 | contig16.region004 | NRPS | Contig 16 [1,980,369–2,022,000] | FAM_01191 | |
| 30 | contig16.region005 | T1PKS | Contig 16 [2,179,010–2,222,991] | FAM_01550 | Cichorine (BGC0000037) |
| 31 | contig16.region006 | Terpene | Contig 16 [3,599,879–3,610,426] | FAM_01205 | |
| 32 | contig16.region007 | NRPS | Contig 16 [3,972,935–4,023,015] | FAM_01206 | |
| 33 | contig16.region008 | T1PKS, NRPS | Contig 16 [4,292,252–4,339,457] | FAM_01553 | Cytochalasin E/K (BGC0000983) |
| 34 | contig16.region009 | T1PKS | Contig 16 [4,350,647–4,396,720] | FAM_01554a | |
| 35 | contig16.region010 | T1PKS | Contig 16 [4,610,190–4,659,198] | FAM_01491 | |
| 36 | contig16.region011 | T1PKS, NRPS-like, NRPS, fungal RiPP | Contig 16 [4,744,292–4,896,083] | FAM_01556a | Imizoquin-A/-B/-C/-D|TMC-2A/-2B (BGC0001621) |
| 37 | contig16.region012 | T1PKS, terpene | Contig 16 [4,913,043–4,982,503] | FAM_01493 | |
| 38 | contig3.region001 | NRPS | Contig 3 [302,615–351,334] | FAM_01558a | |
| 39 | contig3.region002 | NRPS-like | Contig 3 [795,142–838,465] | FAM_01559a | |
| 40 | contig3.region003 | Siderophore | Contig 3 [990,497–1,004,040] | FAM_01560a | |
| 41 | contig7.region001 | Terpene | Contig 7 [59,882–70,098] | FAM_01561a | |
| 42 | contig7.region002 | Fungal RiPP, terpene, T1PKS | Contig 7 [144,815–214,625] | FAM_01562a | Ustiloxin B (BGC0000627) |
| 43 | contig7.region003 | NRPS | Contig 7 [461,278–502,739] | FAM_01563a | |
| 44 | contig7.region004 | T1PKS | Contig 7 [586,782–624,931] | FAM_01564 | Fumagillin|β-trans-bergamotene|fumagillol (BGC0001067) |
| 45 | contig7.region005 | Terpene | Contig 7 [743,071–762,455] | FAM_01565a | |
| 46 | contig7.region006 | T1PKS | Contig 7 [854,062–899,633] | FAM_01566a | |
| 47 | contig7.region007 | Terpene | Contig 7 [1,795,286–1,816,474] | FAM_00237 | |
| 48 | contig7.region008 | Terpene | Contig 7 [2,166,178–2,178,846] | FAM_01137 | Squalestatin S1 (BGC0001839) |
| 49 | contig7.region009 | T1PKS | Contig 7 [2,484,124–2,529,280] | FAM_01448 | Wortmanamide -A/-B (BGC0001954) |
| 50 | contig7.region010 | T1PKS | Contig 7 [5,466,710–5,537,203] | FAM_01570 | |
| 51 | contig7.region011 | Terpene | Contig 7 [5,843,774–5,859,003] | FAM_01571a | |
| 52 | contig7.region012 | Terpene, T1PKS | Contig 7 [6,069,426–6,119,692] | FAM_01572 | Clavaric acid (BGC0001248) |
| 53 | contig7.region013 | NRPS | Contig 7 [6,222,719–6,285,023] | FAM_01573 | |
| 54 | contig7.region014 | NRPS | Contig 7 [6,418,277–6,472,527] | FAM_01199 | |
| 55 | contig7.region015 | T1PKS | Contig 7 [6,960,878–7,008,551] | FAM_01575a | |
| 56 | contig7.region016 | T1PKS | Contig 7 [7,041,422–7,089,522] | FAM_01576 | Solanapyrone D (BGC0000146) |
| 57 | contig7.region017 | NRPS | Contig 7 [7,536,613–7,583,924] | FAM_01577a | Chrysogine (BGC0001545) |
| 58 | contig8.region001 | T1PKS, NRPS-like | Congit 8 [38,977–117,909] | FAM_01578a | |
| 59 | contig9.region001 | T1PKS | Contig 9 [130,274–168,905] | FAM_01579a | |
| 60 | contig9.region002 | Siderophore | Contig 9 [220,046–231,546] | FAM_01580a | |
| 61 | contig9.region003 | T1PKS | Contig 9 [709,705–748,491] | FAM_01581 | Alternariol (BGC0000013) |
| 62 | contig9.region004 | Terpene | Contig 9 [753,035–766,04] | FAM_01260 | |
| 63 | contig9.region005 | NRPS, NRPS-like | Contig 9 [781,396–847,887] | FAM_01583 | |
| 64 | contig9.region006 | T1PKS | Contig 9 [2,328,638–2,372,546] | FAM_01584 | |
| 65 | contig9.region007 | Terpene | Contig 9 [3,448,248–3,465,323] | FAM_01585a | |
| 66 | contig9.region008 | NRPS-like | Contig 9 [3,510,825–3,548,097] | FAM_01586a | |
| 67 | contig9.region009 | T1PKS | Contig 9 [4,771,912–4,812,897] | FAM_01262 | |
| 68 | contig9.region010 | T1PKS | Contig 9 [5,251,051–5,297,281] | FAM_01588a | |
| 69 | contig9.region011 | Indole | Contig 9 [5,715,029–5,736,413] | FAM_01589a | |
| 70 | contig9.region012 | NRPS-like | Contig 9 [5,819,784–5,862,074] | FAM_01502 | |
| 71 | contig9.region013 | T1PKS | Contig 9 [5,919,206–5,978,029] | FAM_01591 | |
| 72 | contig9.region014 | NRPS, T1PKS | Contig 9 [6,129,121–6,199,192] | FAM_01592a | Leporin B (BGC0001445) |
| 73 | contig9.region015 | NRPS | Contig 9 [6,205,218–6,267,181] | FAM_01593a | Destruxin A (BGC0000337) |
| 74 | contig9.region016 | Terpene | Contig 9 [6,317,976–6,338,626] | FAM_01594a | |
| 75 | contig9.region017 | NRPS-like, T1PKS, NRPS | Contig 9 [6,449,891–6,543,804] | FAM_01271 | Ascochlorin (BGC0001923) |
| 76 | contig9.region018 | T1PKS | Contig 9 [6,595,828–6,658,103] | FAM_01596a | |
| 77 | contig9.region019 | T1PKS | Contig 9 [6,672,756–6,752,769] | FAM_01597a | |
| 78 | contig9.region020 | NRPS | Contig 9 [6,801,947–6,848,793] | FAM_01598a | |
| 79 | scaffold12.region001 | NRPS | Scaffold 12 [739,653–774,62] | FAM_01016 | Dimethylcoprogen (BGC0001249) |
| 80 | scaffold12.region002 | T1PKS | Scaffold 12 [792,59–838,101] | FAM_01600a | |
| 81 | scaffold12.region003 | NRPS | Scaffold 12 [1,147,823–1,226,560] | FAM_01601a | |
| 82 | scaffold12.region004 | NRPS, T1PKS | Scaffold 12 [1,278,228–1,361,597] | FAM_01231 | |
| 83 | scaffold12.region005 | NRPS, T1PKS | Scaffold 12 [1,716,476–1,767,455] | FAM_01230 | |
| 84 | scaffold12.region006 | NAPAA, NRPS | Scaffold 12 [2,898,411–2,951,408] | FAM_01604a | |
| 85 | scaffold12.region007 | Terpene | Scaffold 12 [3,214,332–3,235,701] | FAM_01605a | |
| 86 | scaffold12.region008 | T1PKS | Scaffold 12 [3,363,240–3,402,845] | FAM_01606a | |
| 87 | scaffold12.region009 | T1PKS, NRPS | Scaffold 12 [3,418,472–3,502,365] | FAM_01607 | |
| 88 | scaffold12.region010 | NRPS | Scaffold 12 [3,729,321–3,784,772] | FAM_01459 | Apicidin (BGC0000304) |
| 89 | scaffold12.region011 | T1PKS | Scaffold 12 [3,809,533–3,850,712] | FAM_01609 | |
| 90 | scaffold12.region012 | NRPS-like, T1PKS | Scaffold 12 [3,869,049–3,916,556] | FAM_01247 | Swainsonine (BGC0001793) |
| 91 | scaffold17.region001 | T1PKS | Scaffold 17 [50,606–93,739] | FAM_01611a | |
| 92 | scaffold17.region002 | NRPS | Scaffold 17 [113,167–165,135] | FAM_01481 | |
| 93 | scaffold17.region003 | T1PKS | Scaffold 17 [181,389–227,613] | FAM_01613a | |
| 94 | scaffold17.region004 | NRPS | Scaffold 17 [1,490,267–1,553,136] | FAM_01480 | |
| 95 | scaffold17.region005 | T1PKS, NRPS | Scaffold 17 [1,664,879–1,744,092] | FAM_01615 | Pyranonigrin E (BGC0001124) |
| 96 | scaffold17.region006 | NRPS, T1PKS | Scaffold 17 [3,373,385–3,426,940] | FAM_01616a | |
| 97 | scaffold17.region007 | NRPS, terpene | Scaffold 17 [3,451,272–3,512,235] | FAM_01617 | |
| 98 | scaffold17.region008 | T1PKS, NRPS | Scaffold 17 [3,837,995–3,950,986] | FAM_01618a | Verticillin (BGC0001820) |
| 99 | scaffold17.region009 | T1PKS, NRPS | Scaffold 17 [4,151,159–4,228,823] | FAM_01475 | |
| 100 | scaffold17.region010 | NRPS-like | Scaffold 17 [4,307,093–4,364,974] | FAM_01620a | |
| 101 | scaffold17.region011 | Other | Scaffold 17 [4,416,112–4,457,005] | FAM_01621a | |
| 102 | scaffold17.region012 | Terpene | Scaffold 17 [4,503,384–4,504,782] | FAM_01622a | |
| 103 | scaffold17.region013 | T1PKS | Scaffold 17 [4,566,858–4,615,436] | FAM_01623 | Aurofusarin (BGC0002709) |
| 104 | scaffold17.region014 | T1PKS, NRPS-like | Scaffold 17 [4,653,857–4,729,974] | FAM_01474 | Naphthopyrone (BGC0000107) |
| 105 | scaffold17.region015 | NRPS | Scaffold 17 [4,750,437–4,815,420] | FAM_01474 | |
| 106 | scaffold17.region016 | Terpene | Scaffold 17 [4,820,426–4,842,031] | FAM_01626a |
Their family classification is provided. Additionally, the most closely related known cluster and its corresponding MIBiG accession are shown.
a Singletons.
Category B included the beta-lactone, indole, and NRP metallophore BGCs since all of them presented a scattered distribution among Hypocreaceae (Fig. 5). However, based on the distribution of these BGCs in the genomes of the species analyzed in this work, it was found that some of these BGCs could not be related to the mycophilic mode of life, while the rest could potentially be involved. In specific, beta-lactone BGC was absent in C. mycophium but found in Escovopsis, Mycogone spp., and T. harzianum clades that include fungicolous species, comprising 3 different families, respectively (Fig. 5). Similar BGCs have been identified in other fungi, like Stereum spp., Boreostereum vibrans, Cephalosporium sp., Fusarium sp., Scopulariopsis sp., A. niger, and Penicillum polonicum (Robinson et al. 2019; Wen et al. 2020; Wang et al. 2022), while it was firstly isolated from the bacterium Stenotrophomonas maltophilia (Mercuri et al. 2002). The diversity of beta-lactone BGCs, along with their presence in nonmycophilic species, suggests that they may not be directly linked to the fungicolous mode of life. On the contrary, BGCs of indole compound production exhibit discontinuous distribution and may be related to the fungicolous mode of life since Escovopsis weberi can produce various compounds, including shearinine terpene-indole alkaloids, which have an impact on ant behavior (Batey et al. 2020). Additionally, it synthesizes diketopiperazines to counteract defensive bacteria and produces other small molecules that inhibit the fungal cultivar. Indolic-derived compounds have also been associated with plant growth promotion by Trichoderma spp. (Contreras-Cornejo et al. 2016). According to BGC network analysis, Cladobotryum indole BGCs were singletons, without similarity to any known BGC (Table 3). Finally, NRP metallophore BGC was spread among Hypocreaceae, and although it was found in Cladobotryum clade, this BGC was lost in C. mycophilum.
Interestingly, BGCs of NI-siderophore (i.e. NRPS-independent, IucA/IucC-like siderophore), T3PKS, and NAPAA were found only in Cladobotryum genomes (category C), with T3PKS BGC to be lost in C. dendroides (Fig. 5). Although a NI-siderophore BGC was identified, the related secondary metabolite is yet unknown. NAPAA BGC found in Cladobotryum spp. was characterized, and it was found that it was responsible for the production of the NAPAA, ε-poly-l-lysine, with 100% identity with the respective BGC of the endophytic fungus Epichloë festucae (Clavisipitaceae; Purev et al. 2020). Purev et al. (2020) identified this BGC and isolated the metabolite, proving the inhibitory activity of ε-poly-l-lysine against spore germination of the fungal and oomycete plant pathogens Drechslera erythrospila, Botrytis cinerea, and Phytophthora infestans (Purev et al. 2020). However, whether a similar mechanism was expected from Cladobotryum genomes against their fungal hosts remains a subject of future investigation, and thus, the present study could only hypothesize about this potential interaction.
Results of this comparative analysis became highly interesting when all PCGs comprising the examined BGCs of Hypocreaceae were analyzed, including genes encoding the core synthases, tailor enzymes, and BGC-specific transcription factors along with protective and hypothetical genes, and they were included in the orthologous comparative analysis (Supplementary Table 7). A total of 2,875 orthogroups were identified, but only 3 of them included PCGs from all the examined species. In detail, the orthogroup OG0000000 includes an LCL-type condensation (C) domain of NRPS, OG0000001 family 58-like fungal cytochrome P450 genes (CYP58, also known as Tri4 and trichodiene oxygenase), and OG0000002 genes encoding ABC-type multidrug transport system, with ATPase and permease components. Two hundred and seven orthogroups were found only in Cladobotryum species. Out of them, 93 were characterized and mapped against the GO database, and according to their molecular function, they could be clustered into 2 main groups (Supplementary Table 8). The first category included PCGs with catalytic activity (GO:0003824) and, in detail, isomerase (i.e. aspartate racemase activity), l-pipecolate oxidase, dioxygenase, acid–amino acid ligase, hydrolase acting on glycosyl bonds, and 5′ methylthioadenosine deaminase activities. The second category included PCGs, the binding properties (GO:0005488) on Flavin Adenine dinucleotide (FAD), metal ion, 4 iron and 4 sulfur clusters, and phosphorpantetheine.
Interestingly, OG0001232 orthogroup was annotated as necrosis-inducing secreted protein similar to the respective one, i.e. NIS1, found in phytopathogen Colletotrichum orbiculare (Yoshino et al. 2012), and according to GO mapping, it was targeted to host cell cytoplasm (C:GO:0005576; C:GO:0030430). In C. mycophilum, the respective PCG was included in the NRPS/T1PKS hybrid BGC identified in contig 10 (location: 3,987,099–3,987,744; total: 468 bp excluding introns). The produced protein is expected to interact with the lost receptor-like kinases (RLKs and RLCKs) inhibiting their activity, finally inducing host cell death (Irieda et al. 2019; Yoshino et al. 2012). But the question if the existence of this PCG is related with host pathogenicity and effectiveness of Cladobotryum's fungicolous mode of life is still to be addressed in the future.
Diversity and evolution of BGCs in Hypocreaceae
The distribution patterns of BGCs in Hypocreaceae genomes, as illustrated in Fig. 5, and the cluster network analysis suggested the possibility of new cluster formation, cluster loss, and HGT events. These findings also revealed interesting insights into the evolutionary history of secondary metabolite production in these fungi.
The presence of a significant number of singletons suggested that these singletons might confer unique or specialized metabolic pathways on C. mycophilum, setting it apart from the other examined species. Additionally, this phenomenon was not restricted only in C. mycophilum’s BGCs but also expanded in the other examined Hypocreaceae species, and it signified an ongoing rapid evolution of these BGCs, leading to the formation of entirely novel and distinct clusters. The diverse arrays of biosynthetic genes and their genetic variability could contribute to fungicolous ecological interactions and adaptations.
BGCs of category B, i.e. associated with beta-lactone, indole, and NRP metallophore compounds, exhibited a scattered distribution in the phylogenetic tree, indicating potential multiply independent gene loss or acquisition events over time. Furthermore, beta-lactone compounds have been identified not only in Hypocreaceae but also in various other fungal genera, such as Fusarium, Cephalosporium, Aspergillus, Scopulariopsis, and Penicillium (Liu et al. 2018; Robinson et al. 2019; Wen et al. 2020; Tang et al. 2022; Wang et al. 2022). Similarly, indole-based alkaloids have been reported not only in Hypocreaceae but also in other Hypocreales species such as Ophiocordyceps xuefengensis and Clonostachys rosea (Jiang et al. 2021; Qin et al. 2021). This wide distribution underscored the ecological importance of these compounds and their potential roles in shaping the interactions of fungi with their environments and other organisms. Their presence in other fungi, along with their scattered distribution within the Hypocreaceae family, strongly suggested that these clusters might have been lost multiple times during the evolutionary history of Hypocreales. This scenario of multiple cluster losses in different lineages highlighted the dynamic nature of fungal secondary metabolism and the plasticity of these genetic pathways over evolutionary time. It also indicated that the production of certain secondary metabolites might not provide a selective advantage under specific conditions, leading to the loss of the corresponding BGCs in certain fungal lineages.
Initially, there was limited genomic evidence of HGT in fungi BGCs (Rosewich and Kistler 2000). However, the noncontinuous distribution of BGCs among fungi raised doubts about their strict vertical inheritance. It has been suggested that in BGCs, the genes grouped together with linked functions may increase fungal fitness, because of the added advantage they provide in adapting to novel hosts after HGT (Slot 2017). The acquisition of BGC category C, i.e. the NI-siderophore, T3PKS, and NAPAA, only in Cladobotryum spp. raised the question if these clusters resulted from HGT. To investigate the origin of category C BGCs, phylogenetic tree construction for the core biosynthetic genes and AI analysis including all PCGs belonging to BGCs of category C were conducted (Supplementary Table 9).
In detail, an in-depth analysis revealed the identification of 2 NI-siderophore BGCs in C. mycophilum and C. protrusum, while C. dendroides harbored only 1, resulting in the characterization of 5 NI-siderophore BGCs in total. The phylogenetic tree constructed from this “NI-siderophore-matrix” showed their division into 2 distinct subgroups (Supplementary File 3). NI-siderophore BGCs belonging to subgroup A were identified in C. mycophilum and C. protrusum, but not in C. dendroides, while the NI-siderophore BGC found in genus Cladobotryum is designated as subgroup B. Although subgroup A was similar concerning its content as BGC between these 2 species, in C. protrusum, it was found to be partially overlapped by T1PKS and fungal RiPP–like clusters, while in C. mycophilum, it was located in between 2 T1PKS BGCs (Supplementary File 3). A Blastp search of both subgroups showed identity only with PCGs from non-Hypocreaceae species, which are not close relatives or phylogenetically linked with the members of this genus. An AI analysis was conducted to investigate the origin of all PCGs belonging to the 2 NI-siderophore BGCs. In subgroup A of NI-siderophore BGCs, 3 out of 5 PCGs were found to have foreign or indeterminate origins, including the PCG encoding the siderophore synthase. Notably, species within the genus Aspergillus appeared to be potential donors of these genes. The subgroup B of Cladobotryum NI-siderophore BGC included some genes, which have also been found in Trichoderma species. However, it is worth mentioning that the siderophore synthase and hydroxylase/desaturase genes lacked homologs within other species of the Hypocreaceae family. Instead, they exhibited the highest similarity to genes from the genus Hirsutella, which belongs to the Hypocreales order but in a different family (Ophiocordycipitaceae). This observation leads to intriguing questions about the origin of these genes (Supplementary Table 9). Specifically, it raises the possibility of HGT events, wherein these genes may have been acquired from potential donor species like Hirsutella. Alternatively, it prompts consideration of whether the presence of these genes exclusively in the Cladobotryum genus might be a result of extensive gene losses within the Hypocreaceae family (Supplementary File 3).
Similarly, in the case of T3PKS, BGCs were exclusively identified in the genomes of C. mycophilum and C. protrusum, but not in C. dendroides (Supplementary File 4). This cluster exhibits a similar structure in both species, and interestingly, in the case of C. protrusum, the T3PKS cluster partially overlaps with a T1PKS cluster, while in the case C. mycophilum, it is located among T1PKS and NRPS BGCs (Supplementary File 4). Due to these structural differences and BGC fusion, cluster network analysis classified T3PKS BGCs as singletons. After performing a phylogenetic tree analysis based on the “T3PKS-matrix” (Supplementary File 4), it became evident that the identified T3PKS genes in Cladobotryum were not closely related to any PCGs within the Hypocreaceae family. Instead, they showed a closer phylogenetic relationship with T3PKS from other members of the Hypocreales order, particularly with the respective genes of genera Ophiocordyceps and Sarocladium. Another observation worth mentioning was the presence of homologs of many additional biosynthetic and regulatory genes associated with the T3PKS BGC in the genus Trichoderma (Supplementary Table 9). However, it is important to note that in Trichoderma, these genes were not clustered within a single BGC, and some of them were not part of any contiguous BGC due to their lack of contiguity with core biosynthetic genes. Based on these findings, we propose that the T3PKS BGC likely originated through a combination of native biosynthetic and regulatory genes from the Hypocreaceae family, along with the incorporation of the core T3PKS gene, possibly through HGT with a non-Hypocreaceae donor-like species belonging to Ophiocordyceps or Sarocladium. This result aligns with a previous study that showed a scattered distribution of T3PKS in fungi due to HGT events (Navarro-Muñoz and Collemare 2020).
NAPAA, as described earlier, is considered a subgroup of NRPS-like BGCs responsible for producing NAPAAs, such as ε-poly-l-lysine (Purev et al. 2020). The NAPAA BGC was identified in the genomes of C. dendroides and C. mycophilum, while they were absent in C. protrusum (Supplementary File 5). This observation suggests that this BGC might have been acquired later in the evolution of the Cladobotryum genus. In both species in which NAPAA BGC was present, it overlapped with an NRPS cluster. Notably, in C. dendroides, this region additionally contained a T1PKS cluster. Due to the structural distinctions arising from the fusion of the C. dendroides BGC with a T1PKS cluster, the NAPAA BGCs were categorized as singletons. Through sequence similarity and phylogenetic analysis (see M&M:“NAPAA-matrix”), it was observed that the NAPAA core gene was closely related to NRPS-like genes found in various Hypocrealean species (Supplementary File 5). However, no similarity was found with species belonging to the Hypocreaceae family, except for a PCG of S. broomeana, which was characterized as NRPS-like. Interestingly, the clade containing NAPAA genes from Cladobotryum and S. broomeana was not monophyletic. It also included NRPS-like PCGs from other fungal genera, such as Claviceps, Aspergillus, Metarhizium, Beauveria, Hirsutella, Ophiocordyceps, and, as expected, the ε-poly-l-lysine synthetase of E. festucae (Purev et al. 2020; NCBI Acc. No. BBU42014). These findings align with the AI analysis of NAPAA gene, which revealed a null AI value among both Hypocrealean and non-Hypocrealean species (Supplementary Table 9). The presence of NAPAA BGCs in some Cladobotryum species and their close relationships with genes from diverse fungal lineages raises possibilities regarding the evolution of NAPAA biosynthesis. This phenomenon suggests 2 plausible scenarios: the occurrence of potential HGT events involving the acquisition of NAPAA BGCs or an alternative scenario characterized by extensive gene losses within Hypocrealean genera that could result in the presence of NAPAA BGC only in some taxa. Further research into the functions and roles of NAPAA BGCs in Cladobotryum and related fungi can provide valuable insights into the ecological adaptations and metabolic diversity of these organisms.
Therefore, the presence of numerous singletons, both in C. mycophilum and other Hypocreaceae species, suggests that these unique gene clusters may encode specialized metabolic pathways, showcasing ongoing BGC evolution and the potential emergence of novel metabolites. Additionally, the scattered distribution of category B BGCs in the phylogenetic tree implies multiple independent gene loss or acquisition events over time, reflecting the dynamic nature of fungal secondary metabolism. Some Cladobotryum BGCs, mostly of category C, unveil potential HGT events that enriched the arsenal of the organism’s secondary metabolites. Recent research has enlightened the advantages of incorporating core PCGs of secondary metabolites through HGT events, as demonstrated in cases such as Thinopyrum elongatum with its Fhb7 gene (Wang et al. 2020) and Malassezia sympodialis with its flavohemoglobin-encoding genes (Ianiri et al. 2020). In the former case, HGT from the endophytic Epichloë granted the plant the ability to deepoxidize trichothecenes produced by phytopathogenic Fusarium species (Wang et al. 2020). In the latter example, genes were incorporated into the M. sympodialis genome from diverse donor bacteria that are part of the mammalian microbiome, through independent HGT events, and facilitated the role of M. sympodialis as a predominant inhabitant of animal skin (Ianiri et al. 2020). In Cladobotryum species explored in this study, similar HGT events, which appear to be of a stochastic phenomenon in nature, contributed to the evolution of the Cladobotryum genus and purifying selection led to the survival of the best fit species. Nevertheless, the BGCs of category C cannot be directly correlated to Cladobotryum's fungicolous mode of life without further experimental work. In other words, these HGT-acquired BGCs may directly or indirectly influence the fungicolous mode of life, yet their primary role appears to provide survival advantages to Cladobotryum species under other varying environmental conditions.
Interphylum HGT
In addition to the earlier mentioned acquisition of BGCs in Cladobotryum species, a particularly interesting HGT event shaped the presence of a core PCG encoding a terpene synthase with a cyclase metal-binding domain. This terpene BGC (region 1), found within contig 7 of the C. mycophilum genome (Fig. 6a), captivated our attention due to its donor, which was identified as Basidiomycetes. Additionally, it was classified as a singleton by cluster network analysis. In-depth examination revealed that the phylogenetic analysis of all terpene synthase genes positioned this specific PCG within a clade exclusively comprising terpene synthases of Basidiomycetous origin (i.e. Agaricomycetes; Fig. 6b). In contrast, the remaining 3 Cladobotryum terpene synthases were positioned among Ascomycetes (Fig. 6b). According to the AI (AI = 88), this terpene synthase PCG seemed to be foreign and had emerged for HGT with Agaricomycetes donor (Supplementary Table 9). Notably, there was not any homologous gene present in the rest examined Cladobotryum genomes, indicating that this potential transfer was a recent event. Upstream and downstream of this gene, there was an rnd-4_family-643 repeat belonging to LINE/I retroelements (Fig. 6a). This observation indicated that the acquisition of this terpene synthase was facilitated by an HGT event involving a retrotransposon vector, during the later stages of C. mycophilum's evolution. This event served as clear evidence of an ongoing and continuous interaction between mycophilic species and their hosts.
Fig. 6.
a) The BGC of the terpene BGC (region 1) located in contig 7. The BGC includes a synthase/cyclase with metal-binding domain core gene and upstream an EEP and RNaseH PCG. The locations of LINE/I retrotransposons (repeat rnd-4 family-643) within terpene BGC locus of contig 7 are also indicated with arrows. b) The terpene synthase phylogenetic tree produced by the ML method in IQ-TREE. Clades corresponding to Basidiomycetes, Sordariomycetes, Leotiomycetes, Eurotiomycetes, Lecanoromycetes, Dothideomycetes, Chytridiomycetes, and Glomeromycetes are also indicated. Clades indicated with arrows represent terpene synthases of Cladobotryum species. The core terpene synthase of region 1, contig 7 BGC (see detailed presentation in a), which is found among terpene synthases of Basidiomycetous origin, is indicated with an asterisk (*). The bootstrap values other than 100% are indicated in the nodes of the phylogenetic tree.
HGT is a mechanism believed to significantly shape genomes by rapidly introducing beneficial traits and expanding fungal metabolic capabilities, enhancing their adaptive strategies (Slot and Rokas 2010). Studies investigating the origin, evolution, and significance of gene clusters within fungal genomes, particularly focusing on the galactose utilization pathway, have demonstrated the independent emergence of these clusters in diverse lineages. These gene clusters play a pivotal role in fungal adaptation, enabling the dynamic acquisition and loss of metabolic capacities in response to changing environments (Slot and Rokas 2010). Furthermore, the acquisition of horizontally transferred genes in early-diverging fungal phyla, specifically those involved in nucleic acid synthesis and salvage, has been found to play a critical role in mediating interactions between the metabolic networks of hosts and pathogens (Alexander et al. 2016). Notably, other HGT events among Hypocrealean fungi and their hosts have been published. For instance, the evolutionary transition of Metarhizium robertsii from a plant endophyte ancestor to an insect pathogen has been linked to the acquisition of a sterol carrier gene through HGT from an insect (Zhao et al. 2014). Similarly, other examined HGT events, such as the transfer of high-affinity nitrate assimilation genes from Basidiomycetes to another Ascomycetous organism (Trichoderma reesei), underscore the vital role of HGT in facilitating the adaptation and success of Dikarya on land (Slot and Hibbett 2007). Collectively, these findings highlighted the significance of HGT in shaping fungal genomes, driving metabolic diversification, and contributing to their evolutionary fitness in changing ecological contexts. In the current case, the acquisition of a terpene synthase with Basidiomycetous origin did not seem to be related with any distinct advantage against C. mycophilum host, since the Basidiomycetous host carries a homologous PCG. Nonetheless, this event underscored the complex dynamics underlying the fungus–fungus interactions.
Conclusion
The comprehensive examination of C. mycophilum's whole genome, with an emphasis on the BGCs associated with secondary metabolism, has provided compelling insights into the distribution and potential loss of BGCs within fungal genomes of the Hypocreaceae family. Cluster network analysis indicated a dynamic evolution of BGCs in these fungi. The presence of a significant number of singletons in C. mycophilum’s BGCs suggested a level of genetic and metabolic diversity that might contribute to its ecological success and adaptability. Further, it was shown that C. mycophilum as well as the rest Cladobotryum species share unique BGCs, a phenomenon that can be elucidated by massive cluster losses or independent HGT events. In depth, the presence of the basidiomycetous origin terpene synthase underscored the complex dynamics of host–fungus interactions and the multifaceted role of HGT in shaping fungal genomes. These events appeared to deviate from a deterministic pattern governing the formation of BGCs that would unequivocally confer a survival advantage upon Cladobotryum. Instead, it is more likely that the assimilation of these acquired genes transpired in a stochastic manner, driven by neutral or positive selection pressures. However, further analyses including transcriptomics and metabolomics should be performed in the near future, to confirm if the horizontally acquired BGCs were related to C. mycophilum's fitness in various environments and in extent in the fungicolous mode of life.
Acknowledgments
The authors would like to thank Assistant Professor Dr. Zacharoula Gonou-Zagou, the curator of Mycetotheca ATHUM Culture Collection of Fungi of the National and Kapodistrian University of Athens (NKUA, Athens, Greece; http://mycetotheca.biol.uoa.gr/), for providing the Greek isolate C. mycophilum ATHUM6906 analyzed in the current work (Milic et al. 2022).
Contributor Information
Anastasia C Christinaki, Section of Genetics and Biotechnology, Department of Biology, National and Kapodistrian University of Athens, Athens 15771, Greece.
Antonis I Myridakis, Section of Genetics and Biotechnology, Department of Biology, National and Kapodistrian University of Athens, Athens 15771, Greece.
Vassili N Kouvelis, Section of Genetics and Biotechnology, Department of Biology, National and Kapodistrian University of Athens, Athens 15771, Greece.
Data availability
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAVFKD000000000. The provided BioProject and BioSample accessions for the genome of Cladobotryum mycophilum ATHUM6906 are PRJNA933814 and SAMN33249331, respectively. Supplemental material available in Figshare (DOI: https://doi.org/10.6084/m9.figshare.23936796.v2).
Funding
The research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 4th Call for HFRI PhD Fellowships (Fellowship Number: 19620).
Literature cited
- Adie B, Grogan H, Archer S, Mills PR. 2006. Temporal and spatial dispersal of Cladobotryum conidia in the controlled environment of a mushroom growing room. Appl Environ Microbiol. 72(11):7212–7217. doi: 10.1128/aem.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja M, Chiang Y-M, Chang S-L, Praseuth MB, Entwistle R, Sanchez JF, Lo H-C, Yeh H-H, Oakley BR, Wang CCC. 2012. Illuminating the diversity of aromatic polyketide synthases in Aspergillus nidulans. J Am Chem Soc. 134(19):8212–8221. doi: 10.1021/ja3016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WG, Wisecaver JH, Rokas A, Hittinger CT. 2016. Horizontally acquired genes in early-diverging pathogenic fungi enable the use of host nucleosides and nucleotides. Proc Natl Acad Sci U S A. 113(15):4116–4121. doi: 10.1073/pnas.1517242113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MR, Salazar MP, Schaap PJ, van de Vondervoort PJI, Culley D, Thykaer J, Frisvad JC, Nielsen KF, Albang R, Albermann K, et al. 2011. Comparative genomics of citric-acid-producing Aspergillus niger ATCC 1015 versus enzyme-producing CBS 513.88. Genome Res. 21(6):885–897. doi: 10.1101/gr.112169.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Awakawa T, Matsuzaki M, Cho R, Matsuda Y, Hoshino S, Shinohara Y, Yamamoto M, Kido Y, Inaoka DK, et al. 2019. Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum. Proc Natl Acad Sci U S A. 116(17):8269–8274. doi: 10.1073/pnas.1819254116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley JN, Hobbs BC, Raistrick H. 1937. Studies in the biochemistry of micro-organisms: the crystalline colouring matters of Fusarium culmorum (W. G. Smith) Sacc. and related forms. Biochem J. 31(3):385–397. doi: 10.1042/bj0310385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back C-G, Lee C-Y, Seo G-S, Jung H-Y. 2012. Characterization of species of Cladobotryum which cause cobweb disease in edible mushrooms grown in Korea. Mycobiology 40(3):189–194. doi: 10.5941/myco.2012.40.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashyal BM, Rawat K, Sharma S, Kulshreshtha D, Gopala Krishnan S, Singh AK, Dubey H, Solanke AU, Sharma T, Aggarwal R. 2017. Whole genome sequencing of Fusarium fujikuroi provides insight into the role of secretory proteins and cell wall degrading enzymes in causing bakanae disease of rice. Front Plant Sci. 8:2013. doi: 10.3389/fpls.2017.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Martin MJ, O’Donovan C, Magrane M, Alpi E, Antunes R, et al. 2017. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45(D1):D158–D169. doi: 10.1093/nar/gkw1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batey SFD, Greco C, Hutchings MI, Wilkinson B. 2020. Chemical warfare between fungus-growing ants and their pathogens. Curr Opin Chem Biol. 59:172–181. doi: 10.1016/j.cbpa.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills GF, Platas G, Overy DP, Collado J, Fillola A, Jiménez MR, Martín J, del Val AG, Vicente F, Tormo JR, et al. 2009. Discovery of the parnafungins, antifungal metabolites that inhibit mRNA polyadenylation, from the Fusarium larvarum complex and other Hypocrealean fungi. Mycologia 101(4):449–472. doi: 10.3852/08-163. [DOI] [PubMed] [Google Scholar]
- Blin K, Shaw S, Augustijn H, Reitz Z, Biermann F, Alanjary M, Fetter A, Terlouw B, Metcalf W, Helfrich E, et al. 2023. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 51(W1):W46–W50. doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EL, Ökmen B, Doehlemann G, Henrissat B, Bradshaw RE, Mesarich CH. 2022. Secreted glycoside hydrolase proteins as effectors and invasion patterns of plant-associated fungi and oomycetes. Front Plant Sci. 13:853106. doi: 10.3389/fpls.2022.853106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw RE, Slot JC, Moore GG, Chettri P, Wit PJGM, Ehrlich KC, Ganley ARD, Olson MA, Rokas A, Carbone I, et al. 2013. Fragmentation of an aflatoxin-like gene cluster in a forest pathogen. New Phytol. 198(2):525–535. doi: 10.1111/nph.12161. [DOI] [PubMed] [Google Scholar]
- Buchfink B, Reuter K, Drost H-G. 2021. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods. 18(4):366–368. doi: 10.1038/s41592-021-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland TG. 2000. DNASTAR's lasergene sequence analysis software. Methods Mol Biol. 132:71–91. doi: 10.1385/1-59259-192-2:71. [DOI] [PubMed] [Google Scholar]
- Cairns TC, Studholme DJ, Talbot NJ, Haynes K. 2016. New and improved techniques for the study of pathogenic fungi. Trends Microbiol. 24(1):35–50. doi: 10.1016/j.tim.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambaza E. 2018. Comprehensive description of Fusarium graminearum pigments and related compounds. Foods 7(10):165. doi: 10.3390/foods7100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco J, Navarro M-J, Gea FJ. 2017. Cobweb, a serious pathology in mushroom crops: a review. Span J Agric Res. 15(2):e10R01–e10R01. doi: 10.5424/sjar/2017152-10143. [DOI] [Google Scholar]
- Carsolio C, Gutiérrez A, Jiménez B, Van Montagu M, Herrera-Estrella A. 1994. Characterization of ech-42, a Trichoderma harzianum endochitinase gene expressed during mycoparasitism. Proc Natl Acad Sci U S A. 91(23):10903–10907. doi: 10.1073/pnas.91.23.10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaverri P, Samuels GJ. 2013. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 67(10):2823–2837. doi: 10.1111/evo.12169. [DOI] [PubMed] [Google Scholar]
- Chen C, Wang J, Fu R, Chen X, Chen X, Lu D. 2020. The complete mitochondrial genome of Cladobotryum mycophilum (Hypocreales: Sordariomycetes). Mitochondrial DNA B Resour. 5(3):2595–2596. doi: 10.1080/23802359.2020.1742600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Cao F, Chen X-A, Li Y, Mao X-M. 2020. Genomic and transcriptomic survey of an endophytic fungus Calcarisporium arbuscula NRRL 3705 and potential overview of its secondary metabolites. BMC Genomics 21(1):424. doi: 10.1186/s12864-020-06813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M, Liti G, Barton DB. 2005. Telomeres in fungi, editors. Comparative Genomics. Topics in Current Genetics. Berlin: Springer, vol. 15. p. 101–130. [Google Scholar]
- Contreras-Cornejo HÁ, Macías-Rodríguez L, del-Val E, Larsen J. 2016. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants. FEMS Microbiol Ecol. 92(4):fiw036. doi: 10.1093/femsec/fiw036. [DOI] [PubMed] [Google Scholar]
- Cook D, Donzelli BGG, Creamer R, Baucom DL, Gardner DR, Pan J, Moore N, Krasnoff SB, Jaromczyk JW, Schardl CL. 2017. Swainsonine biosynthesis genes in diverse symbiotic and pathogenic fungi. G3 (Bethesda). 7(6):1791–1797. doi: 10.1534/g3.117.041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao TT, Williams K, de Mattos-Shipley K, Song Z, Takebayashi Y, Simpson TJ, Spencer J, Bailey AM, Willis CL. 2022. Cladobotric acids: metabolites from cultures of Cladobotryum sp., semisynthetic analogues and antibacterial activity. J Nat Prod. 85(3):572–580. doi: 10.1021/acs.jnatprod.1c01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcher AL, Bratke KA, Powers EC, Salzberg SL. 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23(6):673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drula E, Garron M-L, Dogan S, Lombard V, Henrissat B, Terrapon N. 2022. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50(D1):D571–D577. doi: 10.1093/nar/gkab1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzhinina IS, Chenthamara K, Zhang J, Atanasova L, Yang D, Miao Y, Rahimi MT, Grujic M, Cai F, Pourmehdi S, et al. 2018. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 14(4):e1007322–e1007322. doi: 10.1371/journal.pgen.1007322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20(1):238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gams W, Diederich P, Poldmaa K. 2004. Fungicolous fungi. In: Mueller GM, Foster MS, Bills GF, editors. Biodiversity of Fungi. Burlington (VT): Elsevier. p. 343–392. [Google Scholar]
- Gea FJ, Navarro MJ, Carrasco J, González AJ, Suz LM. 2012. First report of cobweb on white button mushroom (Agaricus bisporus) in Spain caused by Cladobotryum mycophilum. Plant Disease 96(7):1067–1067. doi: 10.1094/pdis-02-12-0120-pdn. [DOI] [PubMed] [Google Scholar]
- Gea FJ, Navarro MJ, Santos M, Diánez F, Carrasco J. 2021. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: a review. Microorganisms 9(3):585. doi: 10.3390/microorganisms9030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gea FJ, Navarro MJ, Suz LM. 2011. First report of Cladobotryum mycophilum causing cobweb on cultivated king oyster mushroom in Spain. Plant Disease 95(8):1030–1030. doi: 10.1094/pdis-03-11-0255. [DOI] [PubMed] [Google Scholar]
- Gea FJ, Navarro MJ, Suz LM. 2019. Cobweb disease on oyster culinary-medicinal mushroom (Pleurotus ostreatus) caused by the mycoparasite Cladobotryum mycophilum. J Plant Pathol. 101(2):349–354. doi: 10.1007/s42161-018-0174-z. [DOI] [Google Scholar]
- Gotting K, May DS, Sosa-Calvo J, Khadempour L, Francoeur CB, Berasategui A, Thairu MW, Sandstrom S, Carlson CM, Chevrette MG, et al. 2022. Genomic diversification of the specialized parasite of the fungus-growing ant symbiosis. Proc Natl Acad Sci U S A. 119(51):e2213096119. doi: 10.1073/pnas.2213096119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NAR, Latge J-P, Munro CA. 2017. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 5(3). doi: 10.1128/microbiolspec.FUNK-0035-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner S, Lehwark P, Bock R. 2019. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 47(W1):W59–W64. doi: 10.1093/nar/gkz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan HM, Gaze RH. 2000. Fungicide resistance among Cladobotryum spp.—causal agents of cobweb disease of the edible mushroom Agaricus bisporus. Mycol Res. 104(3):357–364. doi: 10.1017/s0953756299001197. [DOI] [Google Scholar]
- Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, White O, Buell CR, Wortman JR. 2008. Automated eukaryotic gene structure annotation using EvidenceModeler and the program to assemble spliced alignments. Genome Biol. 9(1):R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai Y, Huang AM, Tang Y. 2019. Structure-guided function discovery of an NRPS-like glycine betaine reductase for choline biosynthesis in fungi. Proc Natl Acad Sci U S A. 116(21):10348–10353. doi: 10.1073/pnas.1903282116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Clark J, Ireland A, et al. 2004. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 32(90001):D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausner G. 2012. Introns, mobile elements, and plasmids. In: Bullerwell CE, editor. Organelle Genetics. Heidelberg: Springer. p. 329–357. [Google Scholar]
- Henríquez-Urrutia M, Spanner R, Olivares-Yánez C, Seguel-Avello A, Pérez-Lara R, Guillén-Alonso H, Winkler R, Herrera-Estrella A, Canessa P, Larrondo LF. 2022. Circadian oscillations in Trichoderma atroviride and the role of core clock components in secondary metabolism, development, and mycoparasitism against the phytopathogen Botrytis cinerea. eLife 11:e71358. doi: 10.7554/elife.71358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister D, Keller NP. 2007. Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep. 24(2):393–416. doi: 10.1039/b603084j. [DOI] [PubMed] [Google Scholar]
- Humann JL, Lee T, Ficklin SP, Main D. 2019. Structural and functional annotation of eukaryotic genomes with GenSAS. Methods Mol Biol. 1962:29–51. doi: 10.1007/978-1-4939-9173-0_3. [DOI] [PubMed] [Google Scholar]
- Ianiri G, Coelho MA, Ruchti F, Sparber F, McMahon TJ, Fu C, Bolejack M, Donovan O, Smutney H, Myler PJ, et al. 2020. HGT in the human and skin commensal Malassezia: a bacterially derived flavohemoglobin is required for NO resistance and host interaction. Proc Natl Acad Sci U S A. 117(27):15884–15894. doi: 10.1073/pnas.2003473117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irieda H, Inoue Y, Mori M, Yamada K, Oshikawa Y, Saitoh H, Uemura A, Terauchi R, Kitakura S, Kosaka A, et al. 2019. Conserved fungal effector suppresses PAMP-triggered immunity by targeting plant immune kinases. Proc Natl Acad Sci U S A. 116(2):496–505. doi: 10.1073/pnas.1807297116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Yu B, Miao Y-M, Ren H, Xu Q, Zhao CY, Tian L, Yu Z-Q, Zhou P, Wang X, et al. 2021. Indole alkaloids from a soil-derived Clonostachys rosea. J Nat Prod. 84(9):2468–2474. doi: 10.1021/acs.jnatprod.1c00457. [DOI] [PubMed] [Google Scholar]
- Jin J-M, Lee S, Lee J, Baek S-R, Kim J-C, Yun S-H, Park S-Y, Kang S, Lee Y-W. 2010. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. Mol Microbiol. 76(2):456–466. doi: 10.1111/j.1365-2958.2010.07109.x. [DOI] [PubMed] [Google Scholar]
- Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res. 36(Web Server):W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang H-Y, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, et al. 2014. InterProScan 5: genome-scale protein function classification. Bioinformatics 30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. 2016. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinformatics. 20(4):1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP. 2019. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 17(3):167–180. doi: 10.1038/s41579-018-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. 2002. BLAT—the BLAST-like alignment tool. Genome Res. 12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Lee YH, Cho KM, Lee JY. 2012. First report of cobweb disease caused by Cladobotryum mycophilum on the edible mushroom Pleurotus eryngii in Korea. Plant Dis. 96(9):1374–1374. doi: 10.1094/pdis-01-12-0015-pdn. [DOI] [PubMed] [Google Scholar]
- Kojima K, Bao W. 2022. A unique eukaryotic lineage of composite-like DNA transposons encoding a DDD/E transposase and a His-Me finger homing endonuclease. Mob DNA. 13(1):24. doi: 10.1186/s13100-022-00281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmogorov M, Yuan J, Lin Y, Pevzner PA. 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol. 37(5):540–546. doi: 10.1038/s41587-019-0072-8. [DOI] [PubMed] [Google Scholar]
- Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptivek-mer weighting and repeat separation. Genome Res. 27(5):722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. 2001. Predicting transmembrane protein topology with a hidden arkov model: application to complete genomes11Edited by F. Cohen. J Mol Biol. 305(3):567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. 2009. Circos: an information aesthetic for comparative genomics. Genome Res. 19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, Martinez DA, Druzhinina IS, Thon M, Zeilinger S, Casas-Flores S, Horwitz BA, Mukherjee PK, et al. 2011. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 12(4):R40. doi: 10.1186/gb-2011-12-4-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicek CP, Steindorff AS, Chenthamara K, Manganiello G, Henrissat B, Zhang J, Cai F, Kopchinskiy AG, Kubicek EM, Kuo A, et al. 2019. Evolution and comparative genomics of the most common Trichoderma species. BMC Genomics 20(1):485. doi: 10.1186/s12864-019-5680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suleski M, Craig JM, Kasprowicz AE, Sanderford M, Li M, Stecher G, Hedges SB. 2022. TimeTree 5: an expanded resource for species divergence times. Mol Biol Evol. 39(8):msac174. doi: 10.1093/molbev/msac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagesen K, Hallin P, Rødland EA, Stærfeldt H-H, Rognes T, Ussery DW. 2007. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35(9):3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkireddy K, Khonsuntia W, Kües U. 2020. Mycoparasite Hypomyces odoratus infests Agaricus xanthodermus fruiting bodies in nature. AMB Express 10(1):141. doi: 10.1186/s13568-020-01085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Sossah FL, Sun L, Fu Y, Yu L. 2019. Genome analysis of Hypomyces perniciosus, the causal agent of wet bubble disease of button mushroom (Agaricus bisporus). Genes (Basel). 10(6):417–417. doi: 10.3390/genes10060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chooi Y-H, Sheng Y, Valentine JS, Tang Y. 2011. Comparative characterization of fungal anthracenone and naphthacenedione biosynthetic pathways reveals an α-hydroxylation-dependent claisen-like cyclization catalyzed by a dimanganese thioesterase. J Am Chem Soc. 133(39):15773–15785. doi: 10.1021/ja206906d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S-Z, Yan X, Tang X-X, Lin J-G, Qiu Y-K. 2018. New bis-alkenoic acid derivatives from a marine-derived fungus Fusarium solani H915. Mar Drugs. 16(12):483. doi: 10.3390/md16120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe TM, Chan PP. 2016. tRNAscan-SE on-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 44(W1):W54–W57. doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysøe E, Seong K-Y, Kistler HC. 2011. The transcriptome of Fusarium graminearum during the infection of wheat. Mol Plant Microbe Interact. 24(9):995–1000. doi: 10.1094/mpmi-02-11-0038. [DOI] [PubMed] [Google Scholar]
- Malz S, Grell MN, Thrane C, Maier FJ, Rosager P, Felk A, Albertsen KS, Salomon S, Bohn L, Schäfer W, et al. 2005. Identification of a gene cluster responsible for the biosynthesis of aurofusarin in the Fusarium graminearum species complex. Fungal Genet Biol. 42(5):420–433. doi: 10.1016/j.fgb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, Simão FA, Zdobnov EM. 2021. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 38(10):4647–4654. doi: 10.1093/molbev/msab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mart Nez-Cruz JS, Romero D, Hierrezuelo JS, Thon M, de Vicente A, Rez-Garc AP. 2021. Effectors with chitinase activity (EWCAs), a family of conserved, secreted fungal chitinases that suppress chitin-triggered immunity. Plant Cell. 33(4):1319–1340. doi: 10.1093/plcell/koab011. [DOI] [PubMed] [Google Scholar]
- Matriano DM, Alegado RA, Conaco C. 2021. Detection of horizontal gene transfer in the genome of the choanoflagellate Salpingoeca rosetta. Sci Rep. 11(1):5993. doi: 10.1038/s41598-021-85259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes FK, Vanderpool D, Fulton B, Hahn MW. 2021. CAFE 5 models variation in evolutionary rates among gene families. Bioinformatics 36(22-23):5516–5518. doi: 10.1093/bioinformatics/btaa1022. [DOI] [PubMed] [Google Scholar]
- Mercuri PS, Ishii Y, Ma L, Rossolini GM, Luzzaro F, Amicosante G, Franceschini N, Frere J-M, Galleni M. 2002. Clonal diversity and metallo-β-lactamase production in clinical isolates of Stenotrophomonas maltophilia. Microb Drug Resist. 8(3):193–200. doi: 10.1089/107662902760326904. [DOI] [PubMed] [Google Scholar]
- Merényi Z, Krizsán K, Sahu NP, Liu X, Bálint B, Stajich JE, Spatafora JW, Nagy LG. 2023. Genomes of fungi and relatives reveal delayed loss of ancestral gene families and evolution of key fungal traits. Nat Ecol Evol. 7(8):1221–1231. doi: 10.1038/s41559-023-02095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic N, Christinaki AC, Benaki D, Stavrou AA, Tsafantakis N, Fokialakis N, Kouvelis VN, Gonou-Zagou Z. 2022. Polyphasic systematics of the fungicolous genus Cladobotryum based on morphological, molecular and metabolomics data. J Fungi. 8(8):877. doi: 10.3390/jof8080877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Vitorello CB, Baidyaroy D, Bell JA, Hausner G, Fulbright DW, Bertrand H. 2000. A circular mitochondrial plasmid incites hypovirulence in some strains of Cryphonectria parasitica. Curr Genet. 37(4):242–256. doi: 10.1007/s002940050526. [DOI] [PubMed] [Google Scholar]
- Muhammad I, Sossah FL, Yang Y, Li D, Li S, Fu Y, Yu L. 2019. Identification of resistance to cobweb disease caused by Cladobotryum mycophilum in wild and cultivated strains of Agaricus bisporus and screening for bioactive botanicals. RSC Adv. 9(26):14758–14765. doi: 10.1039/c9ra00632j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Muñoz JC, Collemare J. 2020. Evolutionary histories of type III polyketide synthases in fungi. Front Microbiol. 10:3018. doi: 10.3389/fmicb.2019.03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Muñoz JC, Selem-Mojica N, Mullowney MW, Kautsar SA, Tryon JH, Parkinson EI, De Los Santos ELC, Yeong M, Cruz-Morales P, Abubucker S, et al. 2020. A computational framework to explore large-scale biosynthetic diversity. Nat Chem Biol. 16(1):60–68. doi: 10.1038/s41589-019-0400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar Pour F, Pedrosa B, Oliveira M, Fidalgo C, Devreese B, Driessche GV, Félix C, Rosa N, Alves A, Duarte AS, et al. 2022. Unveiling the secretome of the fungal plant pathogen Neofusicoccum parvum induced by in vitro host mimicry. J Fungi. 8(9):971. doi: 10.3390/jof8090971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini N. 2018. Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies. Fungal Biol. 122(6):538–545. doi: 10.1016/j.funbio.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. Signalp 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Pierleoni A, Martelli P, Casadio R. 2008. PredGPI: a GPI-anchor predictor. BMC Bioinformatics 9(1):392. doi: 10.1186/1471-2105-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Põldmaa K. 2011. Tropical species of Cladobotryum and Hypomyces producing red pigments. Stud Mycol. 68:1–34. doi: 10.3114/sim.2011.68.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MN, Dehal PS, Arkin AP. 2010. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One 5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proost S, Fostier J, De Witte D, Dhoedt B, Demeester P, Van de Peer Y, Vandepoele K. 2012. I-ADHoRe 3.0—fast and sensitive detection of genomic homology in extremely large data sets. Nucleic Acids Res. 40(2):e11–e11. doi: 10.1093/nar/gkr955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purev E, Kondo T, Takemoto D, Niones JT, Ojika M. 2020. Identification of ε-poly-L-lysine as an antimicrobial product from an Epichloë endophyte and isolation of fungal ε-PL synthetase gene. Molecules 25(5):1032. doi: 10.3390/molecules25051032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li B, Zhou S. 2020. A novel glycoside hydrolase 74 xyloglucanase CvGH74A is a virulence factor in Coniella vitis. J Integr Agric. 19(11):2725–2735. doi: 10.1016/s2095-3119(20)63254-3. [DOI] [Google Scholar]
- Qin Y, Zhou R, Jin J, Cheng F, Shen B, Zeng H, Wan D, Zhong C, Xie J, Shen J, et al. 2021. Indole-based alkaloids from Ophiocordyceps xuefengensis. Phytochemistry 181:112536. doi: 10.1016/j.phytochem.2020.112536. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Waller M, Barrett AJ, Bateman A. 2014. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 42(D1):D503–D509. doi: 10.1093/nar/gkt953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Dunigan DD, Rodriguez RJ. 2001. Fungal symbiosis from mutualism to parasitism: who controls the outcome, host or invader? New Phytol. 151(3):705–716. doi: 10.1046/j.0028-646x.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. 1995. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canad J Bot. 73(S1):816–823. doi: 10.1139/b95-327. [DOI] [Google Scholar]
- Robinson SL, Christenson JK, Wackett LP. 2019. Biosynthesis and chemical diversity of β-lactone natural products. Nat Prod Rep. 36(3):458–475. doi: 10.1039/c8np00052b. [DOI] [PubMed] [Google Scholar]
- Rosewich UL, Kistler HC. 2000. Role of horizontal gene transfer in the evolution of fungi. Annu Rev Phytopathol. 38(1):325–363. doi: 10.1146/annurev.phyto.38.1.325. [DOI] [PubMed] [Google Scholar]
- Saier MH, Reddy VS, Moreno-Hagelsieb G, Hendargo KJ, Zhang Y, Iddamsetty V, Lam K, Tian N, Russum S, Wang J, et al. 2021. The Transporter Classification Database (TCDB): 2021 update. Nucleic Acids Res. 49(D1):D461–D467. doi: 10.1093/nar/gkaa1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, Entwistle R, Corcoran D, Oakley BR, Wang CCC. 2012. Identification and molecular genetic analysis of the cichorine gene cluster in Aspergillus nidulans. MedChemComm. 3(8):997–997. doi: 10.1039/c2md20055d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M, Diánez F, Moreno-Gavíra A, Sánchez-Montesinos B, Gea FJ. 2019. Cladobotryum mycophilum as potential biocontrol agent. Agronomy 9(12):891. doi: 10.3390/agronomy9120891. [DOI] [Google Scholar]
- Saud Z, Kortsinoglou AM, Kouvelis VN, Butt TM. 2021. Telomere length de novo assembly of all 7 chromosomes and mitogenome sequencing of the model entomopathogenic fungus, Metarhizium brunneum, by means of a novel assembly pipeline. BMC Genomics 22(1):87. doi: 10.1186/s12864-021-07390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau D, Connor R, Funk K, Kelly C, Kim S, et al. 2022. Database resources of the national center for biotechnology information. Nucleic Acids Res. 50(D1):D20–D26. doi: 10.1093/nar/gkab1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Konkel Z, Gluck-Thaler E, David GEV, Simmt CF, Grootmyers D, Chaverri P, Slot JC. 2023. Endophyte genomes support greater metabolic gene cluster diversity compared with non-endophytes in Trichoderma. PloS one. 18(12): e0289280. doi: 10.1371/journal.pone.02892802023. [DOI] [PMC free article] [PubMed]
- Shibata S, Morishita E, Takeda T, Sakata K. 1966. The structure of aurofusarin. Tetrahedron Lett. 7(40):4855–4860. doi: 10.1016/s0040-4039(00)70103-1. [DOI] [Google Scholar]
- Slot JC. 2017. Fungal gene cluster diversity and evolution. Adv Genet. 100:141–178. doi: 10.1016/bs.adgen.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Slot JC, Hibbett DS. 2007. Horizontal transfer of a nitrate assimilation gene cluster and ecological transitions in fungi: a phylogenetic study. PLoS One 2(10):e1097. doi: 10.1371/journal.pone.0001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot JC, Rokas A. 2010. Multiple GAL pathway gene clusters evolved independently and by different mechanisms in fungi. Proc Natl Acad Sci U S A. 107(22):10136–10141. doi: 10.1073/pnas.0914418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossah FL, Liu Z, Yang C, Okorley BA, Sun L, Fu Y, Li Y. 2019. Genome sequencing of Cladobotryum protrusum provides insights into the evolution and pathogenic mechanisms of the cobweb disease pathogen on cultivated mushroom. Genes (Basel). 10(2):124–124. doi: 10.3390/genes10020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Morgenstern B. 2005. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 33(Web Server):W465–W467. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Lu F, Luo Y, Bie L, Xu L, Wang Y. 2023. OrthoVenn3: an integrated platform for exploring and visualizing orthologous data across genomes. Nucleic Acids Res. 51(W1):W397–W403. doi: 10.1093/nar/gkad313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Huang X, Cao M-H, Wang Z, Yu Z, Yan Y, Huang J-P, Wang L, Huang S-X. 2022. Mono-/bis-alkenoic acid derivatives from an endophytic fungus Scopulariopsis candelabrum and their antifungal activity. Front Chem. 9:812564. doi: 10.3389/fchem.2021.812564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Hovhannisyan V, Lomsadze A, Chernoff YO, Borodovsky M. 2008. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 18(12):1979–1990. doi: 10.1101/gr.081612.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlouw BR, Blin K, Navarro-Muñoz JC, Avalon NE, Chevrette MG, Egbert S, Lee S, Meijer D, Recchia MJJ, Reitz ZL, et al. 2023. MIBig 3.0: a community-driven effort to annotate experimentally validated biosynthetic gene clusters. Nucleic Acids Res. 51(D1):D603–D610. doi: 10.1093/nar/gkac1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treindl AD, Stapley J, Winter DJ, Cox MP, Leuchtmann A. 2021. Chromosome-level genomes provide insights into genome evolution, organization and size in Epichloe fungi. Genomics 113(6):4267–4275. doi: 10.1016/j.ygeno.2021.11.009. [DOI] [PubMed] [Google Scholar]
- Urban M, Cuzick A, Seager J, Wood V, Rutherford K, Venkatesh S, Sahu J, Iyer SV, Khamari L, De Silva N, et al. 2022. PHI-base in 2022: a multi-species phenotype database for pathogen–host interactions. Nucleic Acids Res. 50(D1):D837–D847. doi: 10.1093/nar/gkab1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Anke H, Besl H, Sterner O. 1995. Flavipucine and brunnescin, two antibiotics from cultures of the mycophilic fungus Cladobotryum rubrobrunnescens. Z Naturforsch C J Biosci. 50(5-6):358–364. doi: 10.1515/znc-1995-5-605. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun S, Ge W, Zhao L, Hou B, Wang K, Lyu Z, Chen L, Xu S, Guo J, et al. 2020. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 368(6493):eaba5435. doi: 10.1126/science.aba5435. [DOI] [PubMed] [Google Scholar]
- Wang P, Xu S, Tang Y, Wang H, Bai X, Zhang H. 2022. Genomic and antismash analyses of marine-sponge-derived strain Aspergillus niger L14 unveiling its vast potential of secondary metabolites biosynthesis. J Fungi. 8(6):591–591. doi: 10.3390/jof8060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zeng Z-Q, Zhuang W-Y. 2021. Comparative molecular evolution of chitinases in ascomycota with emphasis on mycoparasitism lifestyle. Microb Genom. 7(9):000646. doi: 10.1099/mgen.0.000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Lv Y, Hao J, Chen H, Huang Y, Liu C, Huang H, Ma Y, Yang X. 2020. Two new compounds of Penicillium polonicum, an endophytic fungus from Camptotheca acuminata Decne. Nat Prod Res. 34(13):1879–1883. doi: 10.1080/14786419.2019.1569003. [DOI] [PubMed] [Google Scholar]
- Wick RR, Schultz MB, Zobel J, Holt KE. 2015. Bandage: interactive visualization of de novo genome assemblies. Bioinformatics 31(20):3350–3352. doi: 10.1093/bioinformatics/btv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight WD, Kim K-H, Lawrence CB, Walton JD. 2009. Biosynthesis and role in virulence of the histone deacetylase inhibitor depudecin from Alternaria brassicicola. Mol Plant Microbe Interact. 22(10):1258–1267. doi: 10.1094/mpmi-22-10-1258. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Raghuraman MK, Cech TR. 1989. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell 59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Wollenberg RD, Saei W, Westphal KR, Klitgaard CS, Nielsen KL, Lysøe E, Gardiner DM, Wimmer R, Søndergaard TE, Sørensen JL. 2017. Chrysogine biosynthesis is mediated by a two-module nonribosomal peptide synthetase. J Nat Prod. 80(7):2131–2135. doi: 10.1021/acs.jnatprod.6b00822. [DOI] [PubMed] [Google Scholar]
- Xu R, Liu X, Peng B, Liu P, Li Z, Dai Y, Xiao S. 2020. Genomic features of Cladobotryum dendroides, which causes cobweb disease in edible mushrooms, and identification of genes related to pathogenicity and mycoparasitism. Pathogens 9(3):232–232. doi: 10.3390/pathogens9030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino K, Irieda H, Sugimoto F, Yoshioka H, Okuno T, Takano Y. 2012. Cell death of Nicotiana benthamiana is induced by secreted protein NIS1 of Colletotrichum orbiculare and is suppressed by a homologue of CgDN3. Mol Plant Microbe Interact. 25(5):625–636. doi: 10.1094/mpmi-12-11-0316. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fasoyin OE, Molnár I, Xu Y. 2020. Secondary metabolites from hypocrealean entomopathogenic fungi: novel bioactive compounds. Nat Prod Rep. 37(9):1181–1206. doi: 10.1039/c9np00065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Xu C, Lu H-L, Chen X, St. Leger RJ, Fang W. 2014. Host-to-pathogen gene transfer facilitated infection of insects by a pathogenic fungus. PLoS Pathog. 10(4):e1004009. doi: 10.1371/journal.ppat.1004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bacolla A, Wang G, Vasquez KM. 2010. Non-B DNA structure-induced genetic instability and evolution. Cell Mol Life Sci. 67(1):43–62. doi: 10.1007/s00018-009-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Katsuragawa M, Xing T, Fukaya K, Okuda T, Tokiwa T, Tashiro E, Imoto M, Oku N, Urabe D, et al. 2021. Cyclopeptides from the mushroom pathogen fungus Cladobotryum varium. J Nat Prod. 84(2):327–338. doi: 10.1021/acs.jnatprod.0c00980. [DOI] [PubMed] [Google Scholar]
- Zuo B, Lu BH, Liu XL, Wang Y, Ma GL, Gao J. 2016. First report of Cladobotryum mycophilum causing cobweb on Ganoderma lucidum cultivated in Jilin Province, China. Plant Disease 100(6):1239–1239. doi: 10.1094/pdis-12-15-1431-pdn. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JAVFKD000000000. The provided BioProject and BioSample accessions for the genome of Cladobotryum mycophilum ATHUM6906 are PRJNA933814 and SAMN33249331, respectively. Supplemental material available in Figshare (DOI: https://doi.org/10.6084/m9.figshare.23936796.v2).