Abstract
Sanguinoderma infundibulare is a newly discovered species of Ganodermataceae known to have high medicinal and ecological values. In this study, the whole-genome sequencing and comparative genomic analyses were conducted to further understand Ganodermataceae's genomic structural and functional characteristics. Using the Illumina NovaSeq and PacBio Sequel platforms, 88 scaffolds were assembled to obtain a 48.99-Mb high-quality genome of S. infundibulare. A total of 14,146 protein-coding genes were annotated in the whole genome, with 98.6% of complete benchmarking universal single-copy orthologs (BUSCO) scores. Comparative genomic analyses were conducted among S. infundibulare, Sanguinoderma rugosum, Ganoderma lucidum, and Ganoderma sinense to determine their intergeneric differences. The 4 species were found to share 4,011 orthogroups, and 24 specific gene families were detected in the genus Sanguinoderma. The gene families associated with carbohydrate esterase in S. infundibulare were significantly abundant, which was reported to be involved in hemicellulose degradation. One specific gene family in Sanguinoderma was annotated with siroheme synthase, which may be related to the typical characteristics of fresh pore surface changing to blood red when bruised. This study enriched the available genome data for the genus Sanguinoderma, elucidated the differences between Ganoderma and Sanguinoderma, and provided insights into the characteristics of the genome structure and function of S. infundibulare.
Keywords: Sanguinoderma, Ganodermataceae, medicinal fungi, comparative genomic analyses, next-generation sequencing
Introduction
Ganodermataceae is an important family of macrofungi with high economic and ecological values. Recently, its species diversity, taxonomic system, and molecular phylogeny have been extensively studied (Sun et al. 2020; Sun, Xing, et al. 2022). The genus Sanguinoderma Y.F. Sun, D.H. Costa, & B.K. Cui was established in 2020 belonging to Ganodermataceae, and its species are characterized by the color of their fresh pore surface changing to blood red when bruised (Sun et al. 2020). Currently, 18 species have been included in the genus Sanguinoderma worldwide (Sun, Lebreton, et al. 2022). Among them, Sanguinoderma infundibulare B.K. Cui & Y.F. Sun is distinctive due to its annual basidiomata with thin and funnel-shaped pileus (Sun et al. 2020).
Sanguinoderma species have been found to contain a large number of secondary metabolites, such as polysaccharides, sterols and unsaturated fatty acids, that are extremely effective against tumor progression (Shiu et al. 2022), cardiovascular disease (Li et al. 2022), inflammation (Mai et al. 2022), antiproliferative activities (Chen, Zhang, et al. 2021), and many other disorders (Zheng et al. 2022). In addition, Ganodermataceae species can also secrete enzymes to degrade lignin and cellulose, playing important roles in wood decomposition and bleaching, and can be used for environmental protection and economic applications (Zhou et al. 2013). The laccase enzyme in Ganoderma lucidum Pat. was found to have excellent effects on the degradation of chlorine strife (Deng et al. 2022) and polycyclic aromatic hydrocarbons (Agrawal et al. 2018). However, there are no relevant studies on the degradation ability of Sanguinoderma species.
Whole-genome sequencing has been completed for an increasing number of Ganodermataceae species, which contributes to a deeper understanding of their biological and genetic characteristics from the genomic perspective. In Ganodermataceae, G. lucidum was the first species to be subjected to whole-genome sequencing on Roche 454 and Illumina platforms. The study specifically investigated the synergistic role of cytochrome P450s (CYPs), transporters, and regulatory proteins in secondary metabolism (Chen et al. 2012). Owing to the extensive genomic studies conducted on G. lucidum, this species has become a model organism for the investigation of secondary metabolic pathways and their regulation in medicinal fungi. The whole-genome sequencing, DNA methylation patterns, and small RNA transcriptome analyses of Ganoderma sinense J.D. Zhao, L.W. Hsu, & X.Q. Zhang were conducted to investigate mainly secondary metabolism and genome defense processes (Zhu et al. 2015). Ganoderma leucocontextum T.H. Li, W.Q. Deng, Sheng H. Wu, Dong M. Wang, & H.P. Hu, Ganoderma australe Pat., Ganoderma boninense Pat., and Ganoderma lingzhi Sheng H. Wu, Y. Cao, & Y.C. Dai were also sequenced to further explore the secondary metabolic synthesis pathways and its related genes in the Ganodermataceae (Utomo et al. 2018; Agudelo-Valencia et al. 2020; Liu et al. 2021; Sun, Fang, et al. 2022). However, most genomic studies focused on Ganoderma species, and currently only Sanguinoderma rugosum Y.F. Sun, D.H. Costa, & B.K. Cui has been sequenced and annotated (Lin et al. 2021). The sequencing results suggested that S. rugosum has a large number of gene clusters related to carbohydrate-active enzymes and biosynthetic secondary metabolites, laying a foundation for the further understanding of the genomic differences between the genera Sanguinoderma and Ganoderma.
In this study, next-generation sequencing and third-generation sequencing were combined to obtain high-quality whole-genome data for S. infundibulare. The protein-coding genes of S. infundibulare were annotated based on several functional databases. Comparative genomic analyses were conducted among S. infundibulare, S. rugosum, G. lucidum, and G. sinense. This study enriched the available genome data for Sanguinoderma species, and it also contributes to the analyses and utilization of secondary metabolites produced by Ganodermataceae species.
Materials and methods
Strains and DNA extraction
Fruiting bodies of S. infundibulare Cui 17238 were collected from Guangdong Province, southern China. The dikaryotic strain was isolated directly from the fruiting bodies, and it was deposited at the Institute of Microbiology, Beijing Forestry University. It is available upon request. The strain used for whole-genome sequencing was identified following Sun, Xing, et al. (2022). The S. infundibulare strain Cui 17238 was inoculated in liquid medium (malt extract medium, 2% malt powder, 1% glucose, and 0.3% KH2PO4), and cultured in a shake flask at 26°C and 150 rpm/min for 7 days. Then, the mycelia were collected by filtration under aseptic conditions and snap frozen in liquid nitrogen. Genomic DNA extraction using the modified cetyltrimethylammonium bromide extraction method and RNA was extracted by Magnetic Tissue Total RNA Kit (Tiangen, Beijing, China).
Genome sequencing and assembly
The standard Illumina TruSeq Nano DNA LT library preparation procedure (Illumina TruSeq DNA Sample Preparation Guide) and the TruSeqTM DNA Sample Prep Kit (Illumina, San Diego, USA) were used to construct the genomic upload library for next-generation sequencing, while the standard PacBio Template Prep Kit 1.0 library preparation procedure (20-kb Template Preparation Using BluePippin Size Selection) and the SMRTbellTM Template Prep Kit 1.0 (Pacific Biosciences, California, USA) were used to construct the genomic upload library for third-generation sequencing.
Before sequencing, the libraries were quality checked on an Agilent Bioanalyzer using the Agilent High Sensitivity DNA Kit (Agilent, California, USA). After passing the quality check, the libraries were quantified using the Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher, Waltham, USA) on a Promega QuantiFluor fluorescence quantification system.
The whole-genome shotgun strategy was adopted separately using next-generation sequencing technology on an Illumina NovaSeq (Illumina, San Diego, USA) sequencing platform and using third-generation single-molecule sequencing technology on a PacBio Sequel (Pacific Biosciences, California, USA) sequencing platform.
Further filtering of downstream data was required to ensure the quality of subsequent data analyses. Joint contamination was removed from the 3′ end of raw data using AdapterRemoval v2 (Schubert et al. 2016), and all reads were quality corrected based on K-mer frequencies using SOAPec v2.0 (Luo et al. 2012). Sequencing data were assembled from scratch using Falcon (https://pb-falcon.readthedocs.io/en/latest/), Canu (Koren et al. 2017) was used to construct contigs and scaffolds, and the results were corrected in Pilon v1.18 (Walker et al. 2014). Finally, the benchmarking universal single-copy orthologs (BUSCO v3.0.2) tool with the Fungi odb10 data set was used to evaluate the integrity of the genome assembly (Simão et al. 2015).
Genomic structure analyses
Repeat sequences prediction
Repeat sequences were identified using the conserved model sequences of putative scattered repeats obtained in RepeatModler v1.0.4 (http://repeatmasker.org/RepeatModeler/). Then, these sequences were compared with the Swiss-Prot database (Boeckmann et al. 2003) for sequence alignment, and the similar protein-coding genes in this database were removed. The remaining sequences were considered as a library to search for new scattered repeat sequences using RepeatMasker v4.0.5 (http://www.repeatmasker.org/RepeatMasker/).
Noncoding RNA analyses
Noncoding RNA predictions were conducted using tRNAscan-SE v1.3.1 (http://lowelab.ucsc.edu/tRNAscan-SE/) to predict tRNAs and RNAmmer v1.2 (https://services.healthtech.dtu.dk/services/RNAmmer-1.2/) to predict rRNAs, and the rest of noncoding RNA predictions were predicted in Rfam database (https://rfam.org/).
Protein-coding gene prediction
To improve the accuracy of protein-coding gene predictions, Augustus v3.03 (Stanke and Morgenstern 2005), glimmerHMM v3.0.1 (Majoros et al. 2004), and GeneMark-ES v4.35 (Ter-Hovhannisyan et al. 2008) were used to predict the genomic model de novo, and Exonerate v2.2.0 (http://www.ebi.ac.uk/about/vertebrate-genomics/software/) was used to obtain the corresponding gene predictions based on the protein sequences in closely related species. At the same time, the program to assemble spliced alignments (Haas et al. 2003) was used to annotate gene structure and obtain the corresponding gene predictions based on transcriptome data. Finally, the prediction results derived from the above 3 methods were integrated using EVidenceModeler v2012.06.25 (Haas et al. 2008).
Collinearity and ortholog analyses
S. rugosum, G. lucidum, and G. sinense were selected for comparative genomic analyses with S. infundibulare. Genome data for the former 3 species were downloaded from the Joint Genomics Institute (https://genome.jgi.doe.gov/) and the National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/). The genomic data of the 4 species were first formatted and analyzed using MCscan v1.2.13 (Wang et al. 2012), and the results were subsequently plotted. Finally, the generated graphs were edited and visually improved in Adobe Illustrator (https://www.adobe.com/cn/products/illustrator.html).
The sequences of encoded proteins were formatted, and the orthogroups for the 4 genomes were constructed using OrthoFinder v2.5.4 (Emms and Kelly 2019).
Functional annotation of the genome
All predicted protein-coding genes of S. infundibulare were annotated by comparing them with various databases using Diamond v0.9.10.11 (Buchfink et al. 2021) and Blast+ v2.5.0 (https://blast.ncbi.nlm.nih.gov/blast/Blast.cgi). These databases were the evolutionary genealogy of genes: Non-supervised Orthologous Groups database (eggNOG, Huerta-Cepas et al. 2019), Gene Ontology (GO, Ashburner et al. 2000), the Kyoto Encyclopedia of Genes and Genomes (KEGG, Kanehisa et al. 2012), the Non-Redundant Protein Sequence Database (NR, https://ftp.ncbi.nlm.nih.gov/blast/db/FASTA/nr.gz), the transport classification database (TCDB, http://www.tcdb.org), and the Protein family (Pfam) databases (El-Gebali et al. 2019), Swiss-Prot (Boeckmann et al. 2003). In addition, genomic functional annotation was also performed on the Carbohydrate-Active enzymes (CAZymes) database (http://www.cazy.org/) and Antibiotics and Secondary Metabolites Analysis Shell (antiSMASH, Blin et al. 2019) between S. infundibulare and S. rugosum, G. lucidum, and G. sinense.
Results and discussion
Genome sequence and structure analyses
In the whole-genome sequencing of S. infundibulare, a total of 6,595,573,200- and 35,867,172,578-bp raw data were obtained using the Illumina NovaSeq and PacBio Sequel platforms. After quality control, the whole genome of S. infundibulare was 48,989,895 bp in length, in which the longest sequence was 4,906,148 bp and the shortest sequence was 11,236 bp (Table 1). The sequences were assembled into 88 scaffolds with a GC content of 55.84% and N50 of 3,461,589 bp. The quality of the S. infundibulare genome, which showed a good assembly integrity, was determined at 98.6% of complete BUSCO genes (95.4% complete and single-copy BUSCOs, 3.2% complete and duplicated BUSCOs, 1.3% missing BUSCOs, and 0.1% fragmented BUSCOs).
Table 1.
Structural characteristics of S. infundibulare genome.
| Size (bp) | 48,989,895 |
| GC content (%) | 55.84% |
| Scaffold number | 88 |
| Shortest scaffold (bp) | 11,236 |
| Longest scaffold (bp) | 4,906,148 |
| N50 (bp) | 3,461,589 |
| N90 (bp) | 684,864 |
| Complete BUSCOs (%) | 98.6% |
| Fragmented BUSCOs (%) | 0.1% |
| Missing BUSCOs (%) | 1.3% |
| Total interspersed repeats (bp) | 4,686,040 |
| Number of ncRNAs | 145 |
| Number of rRNAs | 130 |
| Number of tRNAs | 409 |
Additionally, a total of 4,685,915-bp repeat sequences were found in the S. infundibulare genome, accounting for 9.71%. Of these, long terminal repeats accounted for the highest percentage at 3.25%, while short interspersed nuclear elements, long interspersed nuclear elements, and DNA transposons accounted for 0.00%, 0.19%, and 1.23%, respectively. A total of 145 ncRNAs, 130 rRNAs, and 409 tRNAs were also predicted in the genome (Table 1).
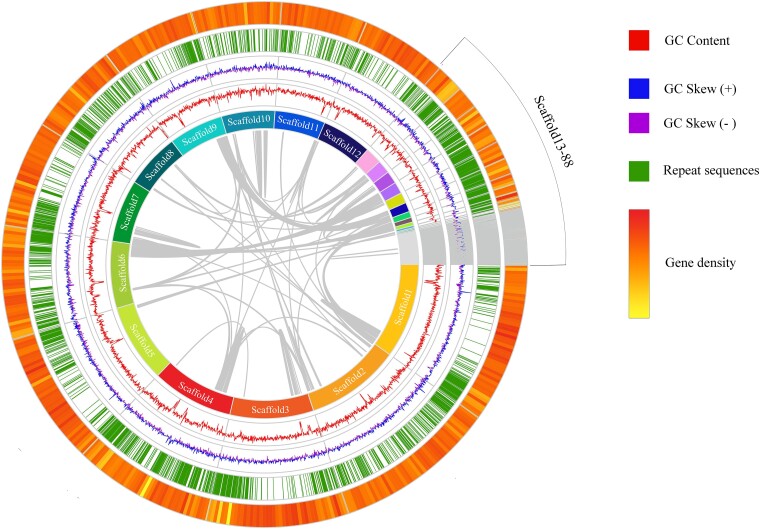
The collinearity analyses were performed on 88 scaffolds of S. infundibulare, and the basic genomic features and gene density were included in the circular genome map (Fig. 1). From the collinearity graph, it can be seen that there is no evidence for whole-genome or segmental duplications in S. infundibulare.
Fig. 1.
Circos of S. infundibulare. From inside to outside, the first circle is collinearity analyses, the second circle is a scaffold composition map, the third circle is a line chart of GC content, the fourth circle is a line chart of GC Skew, the fifth circle is a histogram of the repeat sequence, and the sixth circle is a heat map of gene density.
Collinearity and ortholog analyses
The comparative analyses in genome structure were conducted among S. infundibulare, S. rugosum, G. lucidum, and G. sinense (Table 2). The genome size of 4 species ranges from 40.66 to 48.99 Mb, with the genome of S. infundibulare being the largest (48.99 Mb), which also had the best integrity tested via BUSCO analyses (98.6%). The GC content in the S. infundibulare genome (55.84%) was more similar to that in the genomes of G. lucidum (55.90%) and G. sinense (55.59%) but higher than that in the S. rugosum genome (50.91%). The GC content in coding sequences (CDSs) has shown a positive correlation with the length of CDSs (Wang et al. 2019), and it may explain the longer genome length of S. infundibulare. Teng et al. (2023) suggested that GC content may be related to the different adaptation processes of ancestors to environmental changes, while the genome-wide GC content increases faster in species with higher recombination rates per time unit (Weber et al. 2014). The predicted tRNA sequences of S. infundibulare (409) were also considerably longer than those of the other fungi.
Table 2.
Genome comparison of S. infundibulare, S. rugosum, G. lucidum, and G. sinense
| Items | S. infundibulare | S. rugosum | G. lucidum | G. sinense |
|---|---|---|---|---|
| Genome size (Mb) | 48.99 | 40.66 | 43.30 | 48.96 |
| Scaffolds | 88 | 31 | 13a | 69 |
| GC content (%) | 55.84% | 50.91% | 55.90% | 55.59% |
| Complete BUSCOs (%) | 98.6% | 97.8% | 95.7% | 98.1% |
| Number of protein-coding sequences | 14,146 | 10,181 | 16,113 | 15,688 |
| Number of tRNAs | 409 | 67 | — | 202 |
| Source | Our study | Lin et al. (2021) | Chen et al. (2012) | Zhu et al. (2015) |
a Chromosome level.
The collinearity analyses between S. infundibulare and S. rugosum, G. lucidum, and G. sinense separately were conducted, and the high consistency was obtained for all three of them (Fig. 2), which can explained by the common ancestors of Ganodermataceae. This hypothesis would be in line with the inferences of previous studies (Sun et al. 2020). However, S. infundibulare showed a more concentrated collinearity with S. rugosum, and a more fragmented collinearity with the 2 Ganoderma species. This suggested that the 2 Sanguinoderma species may have diverged for a shorter period of time and have not yet developed more distinct characteristics.
Fig. 2.
Collinearity analyses between S. infundibulare and S. rugosum, G. lucidum, and G. sinense.
Ortholog analyses were carried out for S. infundibulare, S. rugosum, G. lucidum, and G. sinense using genome sequence data (Fig. 3). A total of 4,011 orthogroups, of which 3,712 were single-copy, were selected from the 4 species. S. infundibulare possessed 157 specific gene families. Concurrently, the genera Sanguinoderma and Ganoderma had 24 and 345 specific gene families, respectively.
Fig. 3.
Orthologous analyses between S. infundibulare, S. rugosum, G. lucidum, and G. sinense.
Functional annotation
A total of 14,146 protein-coding genes were predicted in S. infundibulare, with an average length of 2,050.7 bp and an average of 3 exons per gene, accounting for 59.22% of the whole genome. These protein-coding genes were highly annotated in tested databases: 92.45% of them were annotated in the NR database (Supplementary Table 1), 53.27% in the Swiss-Prot database (Supplementary Table 2), and 58.23% in the Pfam database (Supplementary Table 3). In total protein-coding genes, 13,788 of them were found to relate with the CYP superfamily, while only 2,659 genes were searched after controlling E-value less than 1e−5 (Supplementary Table 4), and 1,733 protein-coding genes were annotated as transporter proteins in the TCDB (Supplementary Table 5).
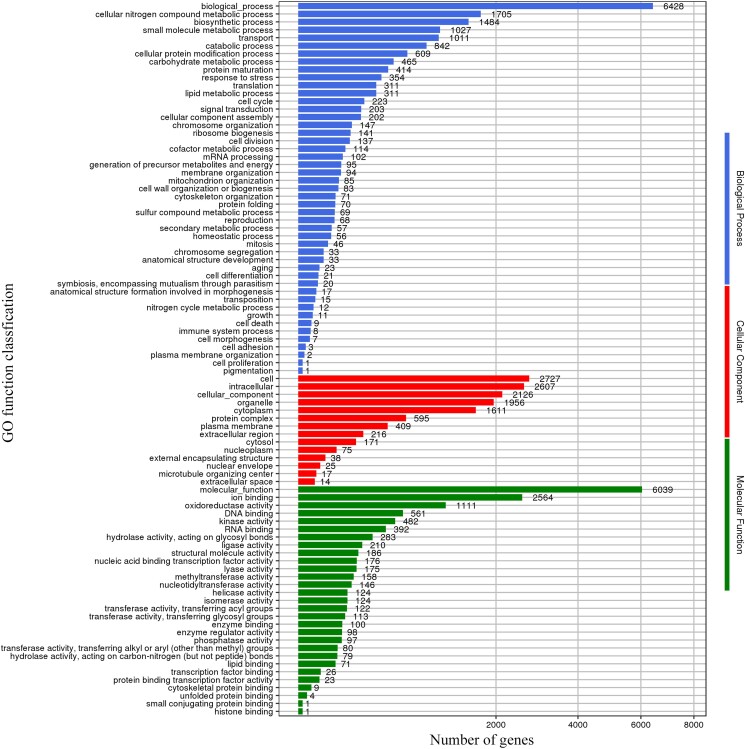
Genomic analyses of GO annotation
The GO database is a widely used bioinformatic resource database that provides a standardized language for describing the functions of genes. A total of 7,147 genes, accounting for 50.52% of all protein-coding genes, were annotated in the GO database (Fig. 4). Most genes were annotated in “biological process,” i.e. biological processes (6,428), cellular nitrogen compound metabolic process (1,705), and biosynthetic processes (1,484); “cellular component,” i.e. cell (2,727), intracellular (2,607), and cellular components (2,126); and “molecular function,” i.e. molecular functions (6,039), ion binding (2,564), and oxidoreductase activity (1,111). Especially, the abundant genes associated with oxidoreductase activity terms may reveal that S. infundibulare had strong cellulose and lignin degradation abilities (Tian et al. 2021).
Fig. 4.
Annotation of S. infundibulare based on GO database.
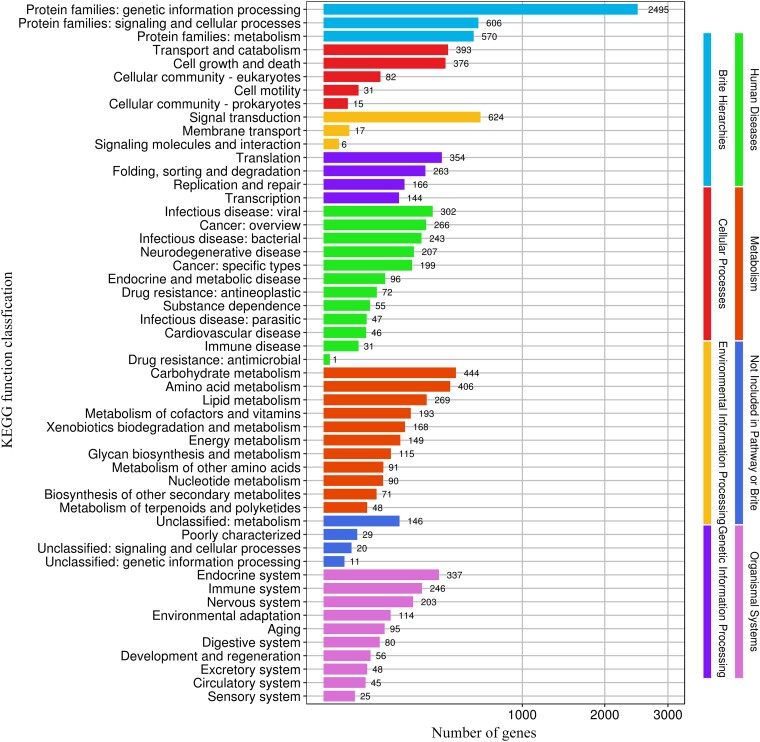
Genomic analyses of KEGG annotation
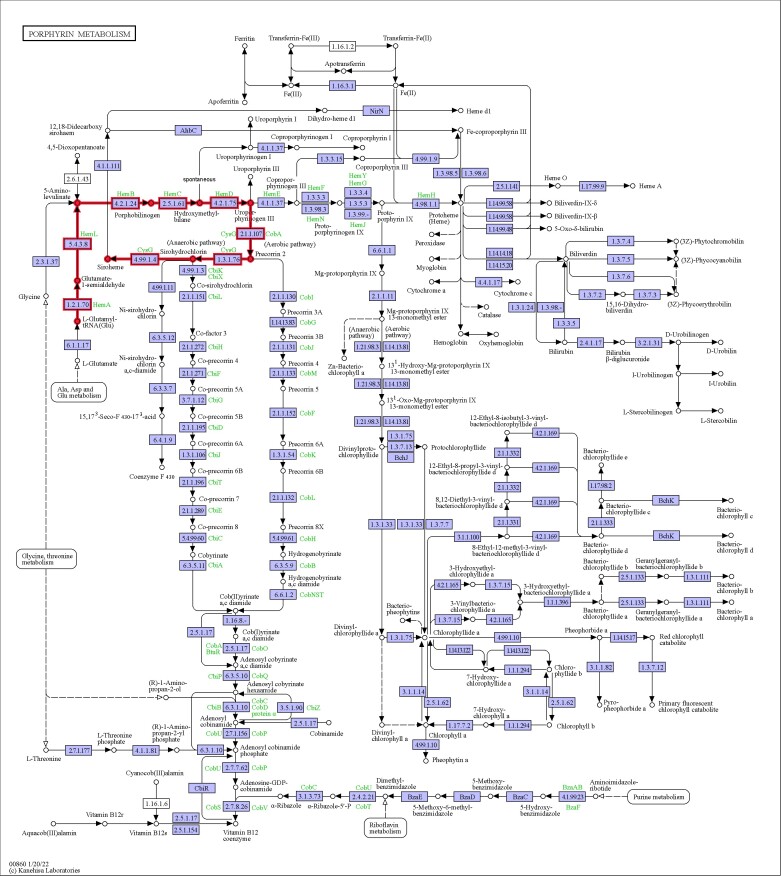
KEGG database annotations play an important role in understanding the detailed function of genes involved in biological systems, especially the category corresponding to metabolic pathways. In the KEGG database, 3,856 genes of S. infundibulare accounted for 27.26% of the whole genome, which were annotated in 8 physiological processes. In the second layer of KEGG pathway terms, the main protein-coding genes were annotated in genetic information processing (2,495), signal transduction (624), signaling and cellular processes (606), and metabolism pathways (570, Fig. 5). Besides, there is a specific gene family of Sanguinoderma that is included in the siroheme biosynthesis pathway. It is associated with the synthesis of compounds that can produce a blood red color (Spencer et al. 1993). Based on previous research, siroheme has been found in Saccharomyces cerevisiae and is related to the nicotinamide adenine dinucleotide-binding process (Schubert et al. 2002), which is consistent with our annotation in the Pfam database. In KEGG pathways, siroheme biosynthesis of S. infundibulare was a part of the porphyrin metabolism pathway (Fig. 6). Porphyrins belong to a kind of macrocyclic compound that was applied in cancer diagnosis and treatment (Chen, Zhu, et al. 2021). The porphyrin metabolism pathway can generate heme and siroheme. Dailey et al. (2017) found that this pathway is the most ancient, and it can convert siroheme to protoheme in Archaea without oxygen. Siroheme is a type of heme cofactor that has been shown to be involved in the formation of ammonia and sulfides in microorganisms and plants (Raux et al. 2003). It is a synthetic product of uroporphyrinogen III, generated after 2 steps of methylation, 1 step of dehydrogenation, and 1 step of chelation reaction (Zhu et al. 2020).
Fig. 5.
Annotation of S. infundibulare based on KEGG database.
Fig. 6.
Porphyrin metabolism pathway of S. infundibulare (map00860) and the bold line part represent the siroheme biosynthesis pathway of S. infundibulare.
Genomic analyses of CAZymes annotation
Performing CAZymes database annotation in S. infundibulare helps identify and classify the protein-coding genes related to carbohydrate-active enzymes, which enables a better understanding of its functions and roles. A total of 629, 377, 513, and 489 protein-coding genes in S. infundibulare, S. rugosum, G. lucidum, and G. sinense, respectively, were annotated in the CAZymes database. The genomes of 4 species were dominated by the gene families of glycoside hydrolases (GH) and glycosyltransferases (GT). S. infundibulare and G. lucidum presented plentiful gene families of carbohydrate-binding module (CBM) than the other 2 species, and the former was mostly annotated with gene families of carbohydrate esterases (CEs, Fig. 7). The gene families of CE10 (62), CE16 (23), and CE1 (15) were detected in the genome of S. infundibulare. The gene families of CE10 were contained in aromatase, carboxylesterase, and acetylcholinesterase activities (de Vries and de Vries 2020), CE16 was contained in acetylesterase activity (Koutaniemi et al. 2013), and CE1 was contained in acetyl xylan esterase, cinnamic acid esterase, and ferulic acid esterase activities (Li et al. 2020). The past studies have shown that acetylesterase, acetyl xylan esterase, and cinnamic acid esterase contribute to the degradation of cellulose and hemicellulose (Tsujiyama and Ueno 2011; Yang et al. 2013; Mai-Gisondi et al. 2015). It suggested that S. infundibulare may be more active in the degradation of cellulose and hemicellulose. Moreover, the abundant gene families of CBM were detected in S. infundibulare (39) and G. lucidum (53), which can enhance the hydrolysis activity of cellulase (Byrt et al. 2012).
Fig. 7.
Annotation of S. infundibulare, S. rugosum, G. lucidum, and G. sinense based on CAZymes database.
The orthogroups found in S. infundibulare, S. rugosum, G. lucidum, and G. sinense were also annotated in the CAZymes database. Most orthogroups were annotated as GH and GT families, of which the most abundant were GH3, GH18, and GT2 (Supplementary Table 6). GH3 and GH18 are related to β-glucosidase, xylan 1,4-beta-xylosidase, and β-glucosylceramidase activities (Faure 2002), and the GT2 is related to cellulose synthase and chitin synthase activities (Breton et al. 2006), chitinase, lysosomal, and acetylamino glucosidase activities (Lienemann et al. 2009). Like most white-rot fungi, the CAZymes in S. infundibulare have outstanding lignocellulosic degradation ability, which may be due to the common saprophytic habit (Větrovský et al. 2013; Sista Kameshwar and Qin 2018). The specific gene families in Ganoderma were annotated with 5 GH18, while the specific gene families in Sanguinoderma were annotated with 2 auxiliary activities 9 and 2 CBM1, which were considered as typical cellulose decomposing enzyme families of white-rot fungi (Levasseur et al. 2014).
Genomic analyses of antiSMASH annotation
Conducting antiSMASH database annotation is essential for identifying and predicting biosynthetic gene clusters in fungal genomes, which in turn allows for the exploration and discovery of a wide range of biologically active compounds and potential drug molecules. In the antiSMASH database, 33, 36, 32, and 36 secondary metabolic gene clusters were annotated for S. infundibulare, S. rugosum, G. lucidum, and G. sinense, respectively (Supplementary Table 7). All 4 species have annotated a large number of terpene gene clusters and nonribosomal peptide synthetase (NRPS)-related gene clusters, among which 18 terpenes and 10 NRPS-related gene clusters (including 1 NRPS gene clusters and 9 NRPS-like gene clusters) have been annotated in S. infundibulare. Many studies have pointed out that fungi in Ganodermataceae contain a large amount of terpenoids, which were related to their medicinal value in anti-inflammatory and anticancer effects (Tang et al. 2010; Baby et al. 2015; Galappaththi et al. 2022), and some studies have pointed out that nonribosomal peptides catalyzed by NRPS also have anti-inflammatory and antitumor effects (Nikolouli and Mossialos 2012; Süssmuth and Mainz 2017; Iacovelli et al. 2021).
Genomic analyses of eggNOG annotation
The eggNOG database collects a large number of functional proteins from other organisms, and it can annotate the function of genes based on blasting similar sequences in the database. S. infundibulare presented 9,891 annotated genes in the eggNOG database (Supplementary Table 8), excluding the S (function unknown) functions, predominantly associated with O (posttranslational modification, protein turnover, and chaperones), G (carbohydrate transport and metabolism), and Q (secondary metabolites biosynthesis, transport, and catabolism) functions. The gene with O, G, and Q functions should be related to the secondary and primary metabolism of white-rot fungi on wooden substrates (Zhao et al. 2019). However, the annotation results of S. infundibulare were not completely consistent with previous study on G. lucidum (Yu et al. 2015). Both of these focus on the G function, but in other aspects, G. lucidum placed more emphasis on the E (amino acid transport and metabolism) and C (energy production and conversion) functions. The most annotated functions in the orthogroups of the 4 species, except for the S function, were the O, T (signal transduction mechanisms), and G functions. In addition, Q functions were the most annotated in the specific gene families of S. infundibulare (Fig. 8a). No significant differences were detected among the 4 Ganodermataceae species, except that S. infundibulare had more S functions (Fig. 8b). Compared with the genus Ganoderma, the specific gene families of Sanguinoderma had additional H (coenzyme transport and metabolism) functions associated with siroheme synthase, which corresponded to the annotation results of the KEGG database mentioned above.
Fig. 8.
a) Annotation of the genome and specific gene families of S. infundibulare and orthologroups of 4 species based on eggNOG database. b) Annotation of S. infundibulare, S. rugosum, G. lucidum, and G. sinense based on eggNOG database. Category functions: (A) RNA processing and modification; (B) chromatin structure and dynamics; (C) energy production and conversion; (D) cell cycle control, cell division, chromosome partitioning; (E) amino acid transport and metabolism; (F) nucleotide transport and metabolism; (G) carbohydrate transport and metabolism; (H) coenzyme transport and metabolism; (I) lipid transport and metabolism; (J) translation, ribosomal structure, and biogenesis; (K) transcription; (L) replication, recombination, and repair; (M) cell wall/membrane/envelope biogenesis; (N) cell motility; (O) posttranslational modification, protein turnover, and chaperones; (P) inorganic ion transport and metabolism; (Q) secondary metabolites biosynthesis, transport, and catabolism; (R) general function prediction only; (S) function unknown; (T) signal transduction mechanisms; (U) intracellular trafficking, secretion, and vesicular transport; (V) defense mechanisms; (W) extracellular structures; (Y) nuclear structure; and (Z) cytoskeleton.
Conclusion
This study provided the whole-genome sequencing data and basic genomic features of S. infundibulare, compared it with the genomes of S. rugosum, G. lucidum, and G. sinense for comparative genomic analysis, and revealed the potential functions of 4 species. S. infundibulare has a relatively large genome with high quality in Ganodermataceae, and it has more concentrated collinearity with S. rugosum. Based on the annotation of 14,146 protein-coding genes of S. infundibulare, it was found that S. infundibulare has high annotation rates in tested databases. In the genus Sanguinoderma, there is a specific gene family related to siroheme biosynthesis, and it may be associated with the color of injured pore surface that can change to blood red in this genus. In conclusion, this study contributes to enriching the genetic database for the family Ganodermataceae, further understanding its genomic structure and function and also providing a solid foundation for the further development and utilization of the genus Sanguinoderma in the future.
Supplementary Material
Acknowledgments
We are thankful to Shanghai Personal Biotechnology Co., Ltd. for the help in obtaining the sequencing data.
Contributor Information
Yuxuan Fang, State Key Laboratory of Efficient Production of Forest Resources, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing 100083, China.
Dongmei Wu, Xinjiang Production and Construction Group Key Laboratory of Crop Germplasm Enhancement and Gene Resources Utilization, Biotechnology Research Institute, Xinjiang Academy of Agricultural and Reclamation Sciences, Shihezi 832061, China.
Neng Gao, Xinjiang Production and Construction Group Key Laboratory of Crop Germplasm Enhancement and Gene Resources Utilization, Biotechnology Research Institute, Xinjiang Academy of Agricultural and Reclamation Sciences, Shihezi 832061, China.
Mengxue Lv, State Key Laboratory of Efficient Production of Forest Resources, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing 100083, China.
Miao Zhou, State Key Laboratory of Efficient Production of Forest Resources, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing 100083, China.
Chuangui Ma, Beijing Jingcheng Biotechnology Co., Ltd, Beijing 100083, China.
Yifei Sun, State Key Laboratory of Efficient Production of Forest Resources, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing 100083, China.
Baokai Cui, State Key Laboratory of Efficient Production of Forest Resources, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing 100083, China.
Data availability
The raw data of S. infundibulare have been deposited in NCBI under the BioProject number PRJNA994897, and the accession number was JAVLRN000000000. The annotations of genome are available at Figshare (http:/doi.org/10.6084/m9.figshare.24886239).
Supplemental material available at G3 online.
Funding
The research is supported by the Fundamental Research Funds for the Central Universities (Nos. BLX202251 and QNTD202307), the National Natural Science Foundation of China (Nos. 32325001, 32270010, and U2003211), and the Scientific and Technological Tackling Plan for the Key Fields of Xinjiang Production and Construction Corps (No. 2021AB004).
Literature cited
- Agrawal N, Verma P, Shahi SK. 2018. Degradation of polycyclic aromatic hydrocarbons (phenanthrene and pyrene) by the ligninolytic fungi Ganoderma lucidum isolated from the hardwood stump. Bioresour Bioprocess. 5(1):1–9. doi: 10.1186/s40643-018-0197-5. [DOI] [Google Scholar]
- Agudelo-Valencia D, Uribe-Echeverry PT, Betancur-Pérez JF. 2020. De novo assembly and annotation of the Ganoderma australe genome. Genomics. 112(1):930–933. doi: 10.1016/j.ygeno.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. The Gene Ontology Consortium 2000. Gene ontology: tool for the unification of biology. Nat Genet. 25(1):25–29. doi 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby S, Johnson AJ, Govindan B. 2015. Secondary metabolites from Ganoderma. Phytochemistry. 114:66–101. doi 10.1016/j.phytochem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T. 2019. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47(W1):W81–W87. doi: 10.1093/nar/gkz310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann B, Bairoch A, Apweiler R, Blatter MC, Estreicher A, Gasteiger E, Martin MJ, Michoud K, O'Donovan C, Phan I, et al. 2003. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 31(1):365–370. doi: 10.1093/nar/gkg095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton C, Snajdrová L, Jeanneau C, Koca J, Imberty A. 2006. Structures and mechanisms of glycosyltransferases. Glycobiol. 16(2):29R–37R. doi: 10.1093/glycob/cwj016. [DOI] [PubMed] [Google Scholar]
- Buchfink B, Reuter K, Drost H-G. 2021. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods. 18(4):366–368. doi: 10.1038/s41592-021-01101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrt C, Cahyanegara R, Grof C. 2012. Plant carbohydrate binding module enhances activity of hybrid microbial cellulase enzyme. Front Plant Sci. 19(3):254. doi: 10.3389/fpls.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Zhu YF, Kaskel S. 2021. Porphyrin-based metal-organic frameworks for biomedical applications. Angew Chem Int Ed Engl. 60(10):5010–5035. doi: 10.1002/anie.201909880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SD, Zhang YF, Yong TQ, Guan XY, Yang XB, Xie YZ. 2021. A mycochemical investigation of the black Lingzhi mushroom, Amauroderma rugosum (Agaricomycetes), reveals several lipidic compounds with anti-inflammatory and antiproliferative activities. Int J Med Mushrooms. 23(4):71–80. doi: 10.1615/IntJMedMushrooms.2021037977. [DOI] [PubMed] [Google Scholar]
- Chen SL, Xu J, Liu C, Zhu YJ, Nelson DR, Zhou SG, Li CF, Wang LZ, Guo X, Sun YZ, et al. 2012. Genome sequence of the model medicinal mushroom Ganoderm lucidum. Nat Commun. 3(1):913. doi: 10.1038/ncomms1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey HA, Dailey TA, Gerdes S, Jahn D, Jahn M, O'Brian MR, Warren MJ. 2017. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Microbiol Mol Biol R. 81(1):e00048-16. doi 10.1128/MMBR.00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries S, de Vries J. 2020. A global survey of carbohydrate esterase families 1 and 10 in oomycetes. Front Genet. 11:756. doi: 10.3389/fgene.2020.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Zhao W, Yang Y. 2022. Degradation and detoxification of chlorophenols with different structure by LAC-4 laccase purified from white-rot fungus Ganoderma lucidum. Int J Environ Res Public Health. 19(13):8150. doi: 10.3390/ijerph19138150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, et al. 2019. The Pfam protein families database in 2019. Nucleic Acids Res. 47(D1):D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20(1):238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure D. 2002. The family-3 glycoside hydrolases: from housekeeping functions to host-microbe interactions. Appl Environ Microbiol. 68(4):1485–1490. doi: 10.1128/AEM.68.4.1485-1490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galappaththi MCA, Patabendige NM, Premarathne BM, Hapuarachchi KK, Tibpromma S, Dai DQ, Suwannarach N, Rapior S, Karunarathna SC. 2022. A review of Ganoderma triterpenoids and their bioactivities. Biomolecules. 13(1):24. doi: 10.3390/biom13010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Delcher AL, Mount SM, Wortman JR, Smith RK, Hannick LI, Maiti R, Ronning CM, Rusch DB, Town CD, et al. 2003. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 31(19):5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Salzberg SL, Zhu W, Pertea M, Allen JE, Orvis J, White O, Buell CR, Wortman JR. 2008. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 9(1):R7. doi: 10.1186/gb-2008-9-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Cepas J, Szklarczyk D, Heller D, Hernández-Plaza A, Forslund SK, Cook H, Mende DR, Letunic I, Rattei T, Jensen LJ, et al. 2019. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47(D1):D309–D314. doi: 10.1093/nar/gky1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli R, Bovenberg RAL, Driessen AJM. 2021. Nonribosomal peptide synthetases and their biotechnological potential in Penicillium rubens. J Ind Microbiol Biotechnol. 48(7-8):kuab045. doi: 10.1093/jimb/kuab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. 2012. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40(D1):D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren S, Walenz BP, Berlin K, Miller JR, Bergman NH, Phillippy AM. 2017. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 27(5):722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutaniemi S, van Gool MP, Juvonen M, Jokela J, Hinz SW, Schols HA, Tenkanen M. 2013. Distinct roles of carbohydrate esterase family CE16 acetyl esterases and polymer-acting acetyl xylan esterases in xylan deacetylation. J Biotechnol. 168(4):684–692. doi: 10.1016/j.jbiotec.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Levasseur A, Lomascolo A, Chabrol O, Ruiz-Dueñas FJ, Boukhris-Uzan E, Piumi F, Kües U, Ram AF, Murat C, Haon M, et al. 2014. The genome of the white-rot fungus Pycnoporus cinnabarinus: a basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genomics. 15(1):486. doi: 10.1186/1471-2164-15-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Cheng YF, Li RK, Wu XP, Zheng CW, Shiu PH-T, Chan JC, Rangsinth P, Liu C, Leung SW, et al. 2022. Protective effects of Amauroderma rugosum on doxorubicin-induced cardiotoxicity through suppressing oxidative stress, mitochondrial dysfunction, apoptosis, and activating Akt/mTOR and Nrf2/HO-1 signaling pathways. Oxid Med Cell Longev. 2022:9266178. doi: 10.1155/2022/9266178. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li XX, Griffin K, Langeveld S, Frommhagen M, Underlin EN, Kabel MA, de Vries RP, Dilokpimol A. 2020. Functional validation of two fungal subfamilies in carbohydrate esterase family 1 by biochemical characterization of esterases from uncharacterized branches. Front Bioeng Biotechnol. 8:694. doi: 10.3389/fbioe.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienemann M, Boer H, Paananen A, Cottaz S, Koivula A. 2009. Toward understanding of carbohydrate binding and substrate specificity of a glycosyl hydrolase 18 family (GH-18) chitinase from Trichoderma harzianum. Glycobiology. 19(10):1116–1126. doi: 10.1093/glycob/cwp102. [DOI] [PubMed] [Google Scholar]
- Lin WP, Shi YH, Jia GT, Sun HY, Sun TY, Hou DH. 2021. Genome sequencing and annotation and phylogenomic analysis of the medicinal mushroom Amauroderma rugosum, a traditional medicinal species in the family Ganodermataceae. Mycologia. 113(2):268–277. doi: 10.1080/00275514.2020.1851135. [DOI] [PubMed] [Google Scholar]
- Liu YC, Huang LH, Hu HP, Cai MJ, Liang XW, Li XM, Zhang Z, Xie YZ, Xiao C, Chen SD, et al. 2021. Whole-genome assembly of Ganoderma leucocontextum (Ganodermataceae, Fungi) discovered from the Tibetan Plateau of China. G3 (Bethesda). 11(12):jkab337. doi: 10.1093/g3journal/jkab337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo RB, Liu BH, Xie YL, Li ZY, Huang WH, Yuan JY, He GZ, Chen YX, Pan Q, Liu YJ, et al. 2012. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 1(1):18. doi: 10.1186/2047-217X-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai YZ, Xu SY, Shen R, Feng BR, He H, Xu YF. 2022. Gastroprotective effects of water extract of domesticated Amauroderma rugosum against several gastric ulcer models in rats. Pharm Biol. 60(1):600–608. doi: 10.1080/13880209.2022.2047210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai-Gisondi G, Turunen O, Pastinen O, Pahimanolis N, Master ER. 2015. Enhancement of acetyl xylan esterase activity on cellulose acetate through fusion to a family 3 cellulose binding module. Enzyme Microb Tech. 79–80:27–33. doi: 10.1016/j.enzmictec.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Majoros WH, Pertea M, Salzberg SL. 2004. TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics. 20(16):2878–2879. doi: 10.1093/bioinformatics/bth315. [DOI] [PubMed] [Google Scholar]
- Nikolouli K, Mossialos D. 2012. Bioactive compounds synthesized by non-ribosomal peptide synthetases and type-I polyketide synthases discovered through genome-mining and metagenomics. Biotechnol Lett. 34(8):1393–1403. doi: 10.1007/s10529-012-0919-2. [DOI] [PubMed] [Google Scholar]
- Raux E, Leech HK, Beck R, Schubert HL, Santander PJ, Roessner CA, Scott AI, Martens JH, Jahn D, Thermes C, et al. 2003. Identification and functional analysis of enzymes required for precorrin-2 dehydrogenation and metal ion insertion in the biosynthesis of sirohaem and cobalamin in Bacillus megaterium. Biochem J. 370(2):505–516. doi: 10.1042/BJ20021443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert HL, Raux E, Brindley AA, Leech HK, Wilson KS, Hill CP, Warren MJ. 2002. The structure of Saccharomyces cerevisiae Met8p, a bifunctional dehydrogenase and ferrochelatase. EMBO J. 21(9):2068–2075. doi: 10.1093/emboj/21.9.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M, Lindgreen S, Orlando L. 2016. AdapterRemoval v2: rapid adapter trimming, identification, and read merging. BMC Res Notes. 9(1):88. doi: 10.1186/s13104-016-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu PH-T, Li JJ, Zheng CW, Rangsinth P, Li RK, Cheung QT-L, Lau AH-Y, Chan JC-K, Kwan YW, Cheung TM, et al. 2022. Amauroderma rugosum extract suppresses inflammatory responses in tumor necrosis factor alpha/interferon gamma-induced HaCat keratinocytes. Molecules. 27(19):6533. doi: 10.3390/molecules27196533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 31(19):3210–3212. doi: 10.1093/bioinformatics/btv351.32. [DOI] [PubMed] [Google Scholar]
- Sista Kameshwar AK, Qin W. 2018. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology. 9(2):93–105. doi: 10.1080/21501203.2017.1419296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JB, Stolowich NJ, Roessner CA, Scott AI. 1993. The Escherichia coli cysG gene encodes the multifunctional protein, siroheme synthase. FEBS Lett. 335(1):57–60. doi: 10.1016/0014-5793(93)80438-z. [DOI] [PubMed] [Google Scholar]
- Stanke M, Morgenstern B. 2005. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 33(Web Server):W465–W467. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Costa-Rezende DH, Xing JH, Zhou JL, Zhang B, Gibertoni TB, Gates G, Glen M, Dai YC, Cui BK. 2020. Multi-gene phylogeny and taxonomy of Amauroderma s. lat. (Ganodermataceae). Persoonia. 44(1):206–239. doi: 10.3767/persoonia.2020.44.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Fang YX, Cui BK. 2022. Taxonomy and phylogeny of Sanguinoderma rugosum complex with descriptions of a new species and a new combination. Front Microbiol. 13:1087212. doi: 10.3389/fmicb.2022.1087212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Lebreton A, Xing JH, Fang YX, Si J, Morin E, Miyauchi S, Drula E, Ahrendt S, Cobaugh K, et al. 2022. Phylogenomics and comparative genomics highlight specific genetic features in Ganoderma species. J Fungi. 8(3):311. doi: 10.3390/jof8030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YF, Xing JH, He XL, Wu DM, Song CG, Liu S, Vlasák J, Gates G, Gibertoni TB, Cui BK. 2022. Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud Mycol. 101(1):287–415. doi: 10.3114/sim.2022.101.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Süssmuth RD, Mainz A. 2017. Nonribosomal peptide synthesis-principles and prospects. Angew Chem Int Ed Engl. 56(14):3770–3821. doi: 10.1002/anie.201609079. [DOI] [PubMed] [Google Scholar]
- Tang QJ, Ji Z, Hao RX, Liu YF, Yang Y, Zhang JS. 2010. Inhibition of tumor cell proliferation by a neutral triterpenoid fraction from Ganoderma lucidum. Acta Edulis Fungi. 17(01):60–64. doi: 10.16488/j.cnki.1005-9873.2010.01.017. [DOI] [Google Scholar]
- Teng WK, Liao B, Chen MY, Shu WS. 2023. Genomic legacies of ancient adaptation illuminate GC-content evolution in bacteria. Microbiol Spectr. 11(1):e0214522. doi: 10.1128/spectrum.02145-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Hovhannisyan V, Lomsadze A, Chernoff YO, Borodovsky M. 2008. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 18(12):1979–1990. doi: 10.1101/gr.081612.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YZ, Wang ZF, Liu YD, Zhang GZ, Li G. 2021. The whole-genome sequencing and analysis of a Ganoderma lucidum strain provide insights into the genetic basis of its high triterpene content. Genomics. 113(1):840–849. doi: 10.1016/j.ygeno.2020.10.015. [DOI] [PubMed] [Google Scholar]
- Tsujiyama S, Ueno H. 2011. Production of cellulolytic enzymes containing cinnamic acid esterase from Schizophyllum commune. J Gen Appl Microbiol. 57(6):309–317. doi: 10.2323/jgam.57.309. [DOI] [PubMed] [Google Scholar]
- Utomo C, Tanjung ZA, Aditama R, Buana RFN, Pratomo ADM, Tryono R, Liwang T. 2018. Draft genome sequence of the phytopathogenic fungus Ganoderma boninense, the causal agent of basal stem rot disease on oil palm. Genome Announc. 6(17):e00122-18. doi: 10.1128/genomeA.00122-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Větrovský T, Baldrian P, Gabriel J. 2013. Extracellular enzymes of the white-rot fungus fomes fomentarius and purification of 1,4-β-glucosidase. Appl Biochem Biotechnol. 169(1):100–109. doi 10.1007/s12010-012-9952-9. [DOI] [PubMed] [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng QD, Wortman J, Young SK, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 9(11):e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FP, Wang ZJ, Li YX. 2019. Comparison of GC contents in genomes of the three model microbes. Genom Appl Biol. 38:2215–2220. doi: 10.13417/j.gab.038.002215. [DOI] [Google Scholar]
- Wang YP, Tang HB, Debarry JD, Tan X, Li JP, Wang XY, Lee TH, Jin HZ, Marler B, Guo H, et al. 2012. MCScanx: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40(7):e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CC, Boussau B, Romiguier J, Jarvis ED, Ellegren H. 2014. Evidence for GC-biased gene conversion as a driver of between-lineage differences in avian base composition. Genome Biol. 15(12):549. doi: 10.1186/s13059-014-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SW, Zhao B, Chi X, Li JY, Chen YX. 2013. Enzymatic characteristics of anaerobic fungal series acetyl esterase. J Microbiol. 33(05):58–61. doi: 10.3969/j.issn.1005-7021.2013.05.012. [DOI] [Google Scholar]
- Yu GJ, Yin YL, Yu WH, Liu W, Jin YX, Shrestha A, Yang Q, Ye XD, Sun H. 2015. Proteome exploration to provide a resource for the investigation of Ganoderma lucidum. PLoS One. 10(3):e0119439. doi: 10.1371/journal.pone.0119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QQ, Chi Y, Zhang J, Feng LR. 2019. Transcriptome construction and related gene expression analysis of Lenzites gibbosa in woody environment. Scientia Silvae Sinicae. 55(8):11. doi:CNKI:SUN:LYKE.0.2019-08-011. [Google Scholar]
- Zheng CW, Cheung TMY, Leung GPH. 2022. A review of the phytochemical and pharmacological properties of Amauroderma rugosum. Kaohsiung J Med Sci. 38(6):509–516. doi: 10.1002/kjm2.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XW, Cong WR, Su K-Q, Zhang YM. 2013. Ligninolytic enzymes from Ganoderma spp: current status and potential applications. Crit Rev Microbiol. 39(4):416–426. doi: 10.3109/1040841X.2012.722606. [DOI] [PubMed] [Google Scholar]
- Zhu YJ, Xu J, Sun C, Zhou SG, Xu HB, Nelson DR, Qian J, Song JY, Luo HM, Xiang L, et al. 2015. Chromosome-level genome map provides insights into diverse defense mechanisms in the medicinal fungus Ganoderma sinense. Sci Rep. 5(1):11087. doi: 10.1038/srep11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZW, Zhang J, Wang Q, Cui ZY, Qi QS. 2020. Recent advances in the production of high-value products of porphyrins metabolism pathways and their microbial synthesis. Sci Sin Vitae. 50(12):1405–1417. doi: 10.1360/SSV-2002-0152. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data of S. infundibulare have been deposited in NCBI under the BioProject number PRJNA994897, and the accession number was JAVLRN000000000. The annotations of genome are available at Figshare (http:/doi.org/10.6084/m9.figshare.24886239).
Supplemental material available at G3 online.