Abstract
The influence of diabetes on susceptibility to influenza virus infection was examined in a mouse model in which RIP-Kb transgenic mice and their nontransgenic littermates were used as the diabetic and nondiabetic hosts, respectively. Influenza virus A/Phil/82 (H3N2) grew to significantly higher titers in the lungs of diabetic than nondiabetic mice. The extent of viral replication in the lungs was proportional to blood glucose levels in the mice at the time of infection, and the enhanced susceptibility of diabetic mice was reversed with insulin. Growth of A/HKx31 (H3N2) virus was also enhanced in diabetic mice, whereas the highly virulent strain A/PR/8/34 (H1N1) showed no difference in virus yields in diabetic and nondiabetic mice, even with low inocula. A/Phil/82 and A/HKx31 are sensitive to neutralization in vitro by the pulmonary collectin surfactant protein D (SP-D), whereas A/PR/8/34 is essentially resistant. Glucose is a ligand for SP-D, and neutralization of A/Phil/82 virus by SP-D was abolished in the presence of glucose at levels commonly found in diabetic mice. These findings suggest that in mice, and perhaps in humans, diabetes predisposes to influenza virus infection through compromise of collectin-mediated host defense of the lung by glucose.
Influenza virus infections are associated with higher morbidity and mortality in diabetic patients than in nondiabetic patients (4, 6, 15). Known risks for diabetic patients with influenza include loss of metabolic control, development of ketoacidosis, and an increased susceptibility to secondary bacterial pneumonia due to diabetes-related immune defects or physiological abnormalities affecting lung function (6, 15). Whether diabetes increases susceptibility to influenza virus infection per se is not clear. In the present study we examined this question using the RIP-Kb transgenic mouse as the diabetic model.
The collectins are a family of soluble collagenous lectins believed to function in host defense against a variety of microorganisms (8, 12, 13). Our recent studies with mice have indicated an important role for collectins, in particular lung surfactant protein D (SP-D), in innate defense against influenza virus (20). SP-D binds through its lectin domain to oligosaccharide on influenza virus glycoproteins and neutralizes virus infectivity in vitro, more heavily glycosylated strains of virus being the most sensitive. Among human type A influenza viruses of the H3N2 subtype, which differ in their levels of glycosylation, we observed a marked inverse correlation between sensitivity to SP-D in vitro and the ability of a virus to replicate in the mouse lung. Furthermore, growth of the more highly glycosylated strains in the lung was enhanced if saccharide inhibitors of SP-D (yeast mannan or α-methyl mannoside) were included in the virus inoculum. Glucose is one of the preferred ligands for SP-D (19) and hence a potential inhibitor of SP-D function in diabetes. The objective of this study, therefore, was to determine whether diabetic mice are more susceptible to influenza virus infection and, if so, whether this could be attributed to the effect of glucose on lectin-mediated host defenses.
RIP-Kb transgenic mice were derived from the 50-1 line on a C57BL/6bm1 background (1, 2). These mice overexpress the major histocompatibility complex class I heavy chain H-2Kb in islet β cells under the influence of the rat insulin promoter and become diabetic at 3 to 4 weeks of age due to impaired insulin secretion without immune involvement. The diabetes is relatively mild, and mice can survive for up to 6 months without insulin injections. Nontransgenic, nondiabetic littermates were used as controls and were matched for age and sex with the diabetic mice in each experiment.
The influenza viruses used in this study were A/Phil/82 (H3N2), A/HKx31 (H3N2), and A/PR/8/34 (H1N1) (Mt. Sinai strain). A/Phil/82 and A/HKx31 are high-yielding reassortants of A/Philippines/2/82 (H3N2) and A/Aichi/2/68 (H3N2), respectively, with A/PR/8/34 and bear the surface glycoproteins of the H3N2 parent viruses (9, 14). The three viruses differ in the degrees of glycosylation of their hemagglutinin molecules, in their sensitivities to collectins, and in their abilities to replicate in the respiratory tract of normal mice (20). The viruses were grown in eggs by standard procedures (3).
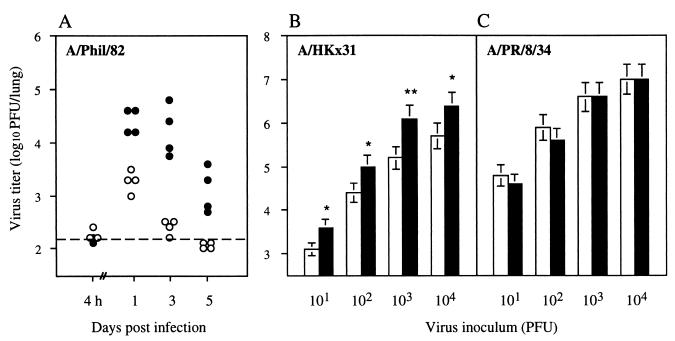
A/Phil/82 (H3N2) bears a highly glycosylated hemagglutinin, is very sensitive to murine collectins, and replicates poorly in mouse lungs (20). To compare the susceptibilities of diabetic and nondiabetic mice to infection with A/Phil/82, mice were inoculated intranasally (i.n.) with 105 PFU of virus in 50 μl of phosphate-buffered saline, and viral titers in the lungs were determined at different times postinfection. Preparation of lung homogenates and titration of virus by plaquing were carried out as described previously (20). Significantly higher titers of virus were recovered from diabetic than from nondiabetic mice (Fig. 1A). A 10-fold difference in virus yield was evident as early as 24 h postinfection, indicating a deficiency in some aspect of innate immunity in the diabetic mice. Subsequent viral clearance, which is known to be T cell mediated (7), appeared to be normal.
FIG. 1.
Replication of influenza A viruses in the lungs of diabetic and nondiabetic mice. (A) Virus titers in the lungs of individual diabetic mice (•) and nondiabetic littermates (○) at various times after i.n. inoculation with 105 PFU of A/Phil/82 virus. The dashed line represents the lower limit of detection of virus in lung homogenates. At all time points except 4 h postinfection, virus titers for diabetic mice were significantly higher than those for nondiabetic mice (P < 0.01, Student’s t test). (B and C) Virus titers in the lungs of diabetic (▪) and nondiabetic (□) mice 3 days after i.n. inoculation with different doses of A/HKx31 (B) or A/PR/8/34 (C) influenza virus. Data are the mean lung virus titers (± 1 standard error) for groups of 3 mice. Asterisks indicate lung virus titers of diabetic mice that were significantly different from those of the corresponding nondiabetic controls. ∗, P < 0.02; ∗∗, P < 0.01, Student’s t test.
To determine whether these findings extended to other strains of influenza virus, the replication of A/HKx31 and A/PR/8/34 viruses in diabetic and nondiabetic mice was examined. A/HKx31 is sensitive to collectins, but less so than A/Phil/82 (20), and replicates to moderately high titers in the lungs of normal mice. At all doses tested, the titers of A/HKx31 virus recovered from the lungs 3 days postinfection were significantly higher (three- to eightfold) for diabetic than for nondiabetic mice (Fig. 1B). In contrast, yields of A/PR/8/34 virus, which is poorly glycosylated, resistant to collectins (20), and highly virulent for mice, showed no difference in diabetic and nondiabetic mice, even at an inoculum dose as low as 10 PFU per mouse (Fig. 1C). The increased susceptibility of diabetic mice to A/Phil/82 and A/HKx31 viruses is thus due to impairment of an early defense mechanism which, in normal mice, is ineffective against the virulent A/PR/8/34 virus. These observations are consistent with the impairment affecting a collectin-mediated mechanism.
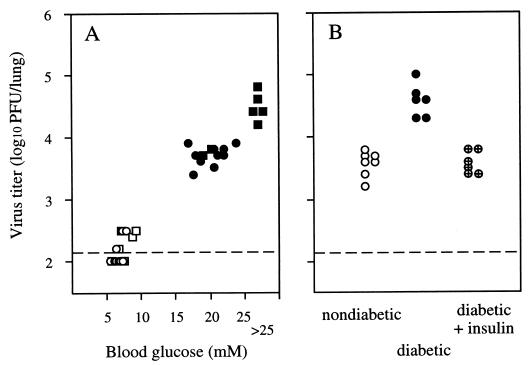
To assess the relationship between blood glucose levels and the ability of influenza virus to replicate in the lung, mice were bled for glucose determination 4 h before i.n. infection with A/Phil/82 virus, and virus titers in the lungs were determined after 3 days. Blood glucose levels were determined by using BM-test Glycemie test strips (Boehringer Mannheim, Indianapolis, Ind.) and a Reflolux 11 reflectometer. Diabetic mice had blood glucose levels of >17 mM, whereas those for nondiabetic controls were in the range from 5 to 10 mM. Male RIP-Kb mice tend to develop a more severe diabetes than do females, and the highest titers of virus were recovered from the lungs of five males in which the blood glucose levels exceeded 25 mM (Fig. 2A). Within the diabetic group overall, there was a significant correlation between blood glucose levels and titers of virus recovered from the lung 3 days postinfection (Spearman rank correlation coefficient, rs = 0.73; P < 0.01).
FIG. 2.
Relationship between blood glucose levels and the ability of influenza virus to replicate in the lungs of mice. (A) Blood glucose levels of male (▪ and □) and female (• and ○) mice were determined 4 h before i.n. infection with 105 PFU of A/Phil/82, and virus titers in the lungs were determined at 3 days postinfection. Diabetic animals are indicated by closed symbols, and nondiabetic animals are indicated by open symbols. (B) Effect of insulin treatment on replication of influenza virus in the lungs of diabetic mice. Data are the lung virus titers for individual diabetic mice (•), diabetic mice given subcutaneous injections of insulin every 6 h (⊕), and nondiabetic controls (○) 24 h after i.n. infection with 105 PFU of A/Phil/82 virus. The dashed line represents the lower limit of detection of virus in lung homogenates.
In another experiment, glucose levels in diabetic mice were reduced by insulin injection and the effect on virus replication in the lungs over the first 24 h was examined. The mice were injected with 1 U of Ultratard human monocomponent insulin (Novo Nordisk Pharmaceuticals Pty. Ltd., North Rocks, New South Wales, Australia) every 6 h subcutaneously in the flank. In preliminary experiments, this regime was shown to maintain blood glucose levels below 10 mM over a 24-h period without visible adverse effects on the health of the mice. The first insulin injection was given 30 min prior to i.n. inoculation with virus. As shown in Fig. 2B, in the insulin-treated diabetic mice the growth of A/Phil/82 virus in the lungs was restricted to the same extent as in the nondiabetic mice. To rule out the possibility that insulin itself had a direct antiviral effect, the replication of A/PR/8/34 virus in the lung was also examined and shown to be unaffected by insulin treatment of the mice (data not shown). These results further indicate a close association between elevated glucose levels and failure to control growth of influenza virus A/Phil/82 in diabetic animals.
To determine whether these findings might be attributed to inhibition of the pulmonary collectin SP-D by glucose, we examined the levels of SP-D in diabetic and nondiabetic mice and the effects of different concentrations of glucose on efficiency of neutralization of influenza virus by SP-D in vitro. Diabetic (n = 5) and nondiabetic (n = 6) mice showed no significant difference in the levels of SP-D in bronchoalveolar lavage fluids assayed by enzyme-linked immunosorbent assay as described in reference 20 (808 ± 198 and 686 ± 192 ng/ml, respectively; P > 0.3, Student’s t test). The actual concentration of SP-D in lung surfactant will be severalfold higher than this due to the dilution involved in the lavage process. The effect of glucose on neutralization by SP-D at concentrations of 2 and 10 μg/ml was therefore determined.
Neutralization of influenza virus by recombinant rat SP-D (5) was measured by fluorescent focus assay on Madin-Darby canine kidney (MDCK) cell monolayers in 96-well plates. A/Phil/82 virus in allantoic fluid was diluted 1/20 and incubated for 60 min at 37°C in complement fixation test (CFT) buffer (barbitone-buffered saline [pH 7.2]–0.25 mM CaCl2–1.8 mM MgCl2; Oxoid, London, United Kingdom) or in CFT buffer containing SP-D in the absence or presence of increasing concentrations of d-glucose. Residual infectivity of the samples was determined by titration on MDCK cell monolayers and detection of infected cells as fluorescent foci at 9 h postinfection with the anti-influenza virus nucleoprotein monoclonal antibody A-3 and fluorescein-conjugated sheep anti-mouse immunoglobulin, as described previously (20). Plates were viewed under ×128 magnification, and the dilution of each sample giving 50 to 100 fluorescent foci per field was determined. The number of fluorescent foci in four fields was counted and used to calculate the infectivity titer of each sample, expressed as focus-forming units per milliliter ± 1 standard error.
Incubation of A/Phil/82 virus with recombinant rat SP-D at concentrations of 2 and 10 μg/ml reduced the infectivity of the virus by factors of 3 and 4.8 log10 units, respectively. Glucose inhibited virus neutralization in a dose-dependent manner (Fig. 3); at glucose concentrations of 20 and 25 mM, levels commonly found in the blood of diabetic mice, neutralization by 2 μg of SP-D/ml was essentially abolished, whereas at concentrations characteristic of normal mice (5 to 10 mM), substantial neutralizing activity was retained. Neutralization by SP-D at 10 μg/ml was likewise strongly inhibited by glucose, the residual virus infectivity being 2,000-fold higher in the presence of 20 mM glucose than in its absence (data not shown). Assuming that the concentration of glucose present in lung surfactant approximates that in blood, these findings suggest that the activity of SP-D against influenza virus in vivo is compromised in diabetic mice.
FIG. 3.
Effect of glucose on neutralization of influenza virus by SP-D. A/Phil/82 virus was incubated with (•) or without (○) recombinant rat SP-D (final concentration, 2 μg/ml) in the absence or presence of increasing concentrations of glucose (5 to 25 mM) for 60 min at 37°C, and the residual infectivity of the samples was determined by fluorescent focus assay on MDCK cell monolayers.
The results of this study extend those of our previous study which indicated an important role for collectins in innate immunity to influenza virus infection (20). We have shown here (i) that diabetic mice are more susceptible to influenza virus infection than nondiabetic mice, (ii) that this difference in susceptibility applies to strains of virus that are sensitive to collectins but not to a virulent strain that is collectin resistant, (iii) that the susceptibility of the mice correlates directly with blood glucose levels, and (iv) that neutralization of influenza virus in vitro by the pulmonary collectin SP-D is inhibited by levels of glucose commonly found in the blood of diabetic mice. Together, these findings suggest a direct link between the increased susceptibility of diabetic mice to influenza virus infection and interference with collectin-mediated innate immune mechanisms by glucose.
The increased risk of influenza-associated morbidity and mortality in diabetic patients is well documented and is associated with loss of metabolic control, development of ketoacidosis, and a higher incidence of secondary bacterial pneumonia than in nondiabetics with influenza (4, 6, 15). The results of the present study suggest that susceptibility to influenza virus infection itself may also be higher for diabetic than for nondiabetic individuals, due to compromise of their collectin-mediated host defenses. Furthermore, in addition to mediating direct neutralization of influenza virus infectivity, the interaction of SP-D with influenza virus has been shown by Hartshorn et al. to block the depression of neutrophil function that is normally caused by this virus (10, 11). Inhibition of this activity of SP-D by glucose would enhance susceptibility to secondary bacterial pneumonia through influenza virus-induced impairment of neutrophil function.
Of additional interest is the fact that a number of respiratory pathogens to which diabetic patients show particular susceptibility (15) are known to bind and be agglutinated by SP-D. These include the yeast Cryptococcus neoformans (21) and gram-negative bacilli such as Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa (16, 17). The predisposition of diabetic patients to development of pneumonia caused by gram-negative bacilli has been attributed to an increased rate of upper airway colonization with these microorganisms compared to that in nondiabetic individuals (18). This pattern of colonization by gram-negative organisms may itself reflect the inhibitory effects of glucose on lectin-mediated host defense of the respiratory tract in diabetes.
Acknowledgments
This work was supported by grant 970283 to E.M.A. and by a block grant to the Walter and Eliza Hall Institute, both from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Allison J, Campbell I L, Morahan T E, Mandel T E, Harrison L C, Miller J F A P. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic β cells. Nature. 1988;333:529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- 2.Allison J, Malcolm L, Culvenor J, Bartholomeusz R K, Holmberg K, Miller J F A P. Overexpression of β2-microglobulin in transgenic mouse islet β cells results in defective insulin secretion. Proc Natl Acad Sci USA. 1991;88:2070–2074. doi: 10.1073/pnas.88.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anders E M, Hartley C A, Jackson D C. Bovine and mouse serum β inhibitors of influenza A viruses are mannose-binding lectins. Proc Natl Acad Sci USA. 1990;87:4485–4489. doi: 10.1073/pnas.87.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouter K P, Diepersloot R J A, van Romunde L K J, Uitslager R, Masurel N, Hoekstra J B L, Erkelens D W. Effect of epidemic influenza on ketoacidosis, pneumonia and death in diabetes mellitus: a hospital register survey of 1976–1979 in The Netherlands. Diabetes Res Clin Pract. 1991;12:61–68. doi: 10.1016/0168-8227(91)90131-v. [DOI] [PubMed] [Google Scholar]
- 5.Crouch E C, Chang D, Rust K, Persson A, Heuser J. Recombinant pulmonary surfactant protein D. J Biol Chem. 1994;269:15808–15813. [PubMed] [Google Scholar]
- 6.Diepersloot R J, Bouter K P, Hoekstra J B. Influenza infection and diabetes mellitus. Case for annual vaccination. Diabetes Care. 1990;13:867–882. doi: 10.2337/diacare.13.8.876. [DOI] [PubMed] [Google Scholar]
- 7.Doherty P C, Allan W, Eichelberger M, Carding S R. Roles of αβ and γδ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 8.Ezekowitz R A B, Sastry K N, Reid K B M, editors. Collectins and innate immunity. R. G. Austin, Tex: Landes Company; 1996. [Google Scholar]
- 9.Hartley C A, Reading P C, Ward A C, Anders E M. Changes in the hemagglutinin molecule of influenza A (H3N2) virus associated with increased virulence for mice. Arch Virol. 1997;142:75–88. doi: 10.1007/s007050050060. [DOI] [PubMed] [Google Scholar]
- 10.Hartshorn K L, Crouch E C, White M R, Eggleton P, Tauber A I, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartshorn K L, Tauber A I. The influenza virus-infected phagocyte. A model of deactivation. Hematol Oncol Clin N Am. 1988;2:301–315. [PubMed] [Google Scholar]
- 12.Holmskov U, Malhotra R, Sim R B, Jensenius J. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe H-J, Reid K B M. Collectins—soluble proteins containing collagenous regions and lectin domains—and their role in innate immunity. Protein Sci. 1994;3:1143–1158. doi: 10.1002/pro.5560030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilbourne E D. Future influenza vaccines and the use of genetic recombinants. Bull W H O. 1969;41:643–645. [PMC free article] [PubMed] [Google Scholar]
- 15.Koziel H, Koziel M J. Pulmonary complications of diabetes mellitus: pneumonia. Infect Dis Clin N Am. 1995;9:65–95. [PubMed] [Google Scholar]
- 16.Kuan S-F, Rust K, Crouch E. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J Clin Invest. 1992;90:97–106. doi: 10.1172/JCI115861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim B-L, Wang J-Y, Holmskov U, Hoppe H-J, Reid K B M. Expression of the carbohydrate recognition domain of lung surfactant protein D and demonstration of its binding to lipopolysaccharides of gram-negative bacteria. Biochem Biophys Res Commun. 1994;202:1674–1680. doi: 10.1006/bbrc.1994.2127. [DOI] [PubMed] [Google Scholar]
- 18.Mackowiack P A, Martin R M, Jones S R, Smith J W. Pharyngeal colonization by Gram-negative bacilli in aspiration-prone persons. Arch Intern Med. 1978;138:1224–1227. [PubMed] [Google Scholar]
- 19.Persson A, Chang D, Crouch E. Surfactant protein D (SP-D) is a divalent cation-dependent carbohydrate-binding protein. J Biol Chem. 1990;265:5755–5760. [PubMed] [Google Scholar]
- 20.Reading P C, Morey L S, Crouch E C, Anders E M. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol. 1997;71:8204–8212. doi: 10.1128/jvi.71.11.8204-8212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schelenz S, Malhotra R, Sim R B, Holmskov U, Bancroft G J. Binding of host collectins to the pathogenic yeast Cryptococcus neoformans: human surfactant protein D acts as an agglutinin for acapsular yeast cells. Infect Immun. 1995;63:3360–3366. doi: 10.1128/iai.63.9.3360-3366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]