Abstract
Chinese herbal medicines (CHM) have been used to cure diseases for thousands of years. However, the bioactive ingredients of CHM are complex, and some CHM natural products cannot be directly absorbed by humans and animals. Moreover, the contents of most bioactive ingredients in CHM are low, and some natural products are toxic to humans and animals. Fermentation of CHM could enhance CHM bioactivities and decrease the potential toxicities. The compositions and functions of the microorganisms play essential roles in CHM fermentation, which can affect the fermentation metabolites and pharmaceutical activities of the final fermentation products. During CHM fermentation, probiotics not only increase the contents of bioactive natural products, but also are beneficial for the host gut microbiota and immune system. This review summarizes the advantages of fermentation of CHM using probiotics, fermentation techniques, probiotic strains, and future development for CHM fermentation. Cutting‐edge microbiome and synthetic biology tools would harness microbial cell factories to produce large amounts of bioactive natural products derived from CHM with low‐cost, which would help speed up modern CHM biomanufacturing.
Keywords: Chinese herbal medicine, probiotics, fermentation, microbiome, synthetic biology
This review summarizes the advantages of probiotic fermentation of Chinese herbal medicine (CHM), fermentation techniques, probiotic strains, and future development of CHM fermentation. Besides, synthetic biology would harness microbial cell factories to de novo produce large amounts of CHM bioactive natural products with low‐cost, and it might be an opportunity to bring CHM into modern biomanufacturing.
Highlights
Fermentation of Chinese herbal medicines (CHM) using probiotics can generate easily absorbed bioactive substances and reduce toxicities.
Probiotic fermentation techniques for CHM are described and discussed.
Microbiome, synthetic biology, and other cutting‐edge biotechnologies improve probiotic fermentation of CHM.
INTRODUCTION
Traditional Chinese medicine is one of the oldest healing systems, including herbal medicines, acupuncture, moxibustion, massage, food therapy, and a few other therapeutic strategies [1]. Chinese herbal medicines (CHM) refer to natural medicines and their processed products, and are mainly composed with plant medicines (including root, stem, leaf, and fruit) and mineral medicines [2]. Most CHM are derived from medicinal plants, and they have been used to treat human diseases in China and other Asian countries for thousands of years [3]. CHM contain hundreds of different components with diverse physiochemical properties based on metabonomic analysis [4, 5]. The artemisinin, one bioactive compound extracted from Artemisia annua, has been used for the treatment of malaria and other diseases [6, 7]. In the 3 years from 2018 to 2020, artemisinin‐based combination therapies had been used to treat more than 454 million malaria cases. Besides, some classical Chinese medicinal prescriptions based on CHM have been applied for the treatment of anxiety, insomnia, cognitive impairment, and other diverse difficult diseases [8].
The contents of some bioactive ingredients in CHM are lower than 1% [9, 10, 11, 12], and some CHM components are toxic to humans and animals [13, 14]. Microbial fermentation is one of the traditional CHM processing techniques, which reacts under proper temperature, humidity, and moisture conditions [15, 16]. CHM fermentation could increase pharmaceutical efficacy, reduce toxicity, produce new chemical components, and protect wild herb resources [15, 17]. The records of fermented CHM and its products were available in “Qi Min Yao Shu,” “Shen Nong Ben Cao Jing,” “Ben Cao Gang Mu,” and “Pharmacopoeia of the People's Republic of China,” including Pinelliae Rhizoma Qu, Shen Qu, Jian Shen Qu, Cai Yun Qu, Chen Xiang Qu, Semen Sojae Praeparatum, Bai Yao Jian, and Pien Tze Huang [15]. Moreover, some fermented CHM have been applied in animal feeding, and they are demonstrated to be beneficial for animal health. For example, Massa Medicata Fermentata (Shenqu or Liushenqu) improves intestinal homeostasis during piglets weaning [18], and probiotics‐fermented herbal blend can improve the growth performance of Salmonella pullorum‐infected chicks [19].
Normally, chemical compositions and contents of CHM were changed after microbial fermentation. Some effective ingredients of CHM can only be transformed and absorbed by the gut microbiota [17]. As the gut microbiota composition of hosts and their drug absorption capacity are personalized [20], in vitro fermentation could standardize CHM products and enhance the clinical efficacy of CHM [21, 22, 23]. Actually, a few fermented CHM have better pharmacological activity than the nonfermented CHM [15, 16]. Probiotics are live microorganisms that have demonstrated beneficial effects on human health [24]. Both probiotics and some CHM are beneficial for human gut microbes [25], intestinal epithelial barrier [26], and immune system [27], thus, fermentation of some CHM with probiotics is of great interest.
Some clinical trials on the use of probiotics‐fermented CHM showed promising clinical effects. Fermented milk containing Lactobacillus paracasei and the CHM Glycyrrhiza glabra is beneficial for patients infected with Helicobacter pylori; the treatment group significantly improved gastrointestinal symptoms and quality of life, and no serious adverse events were observed [28]. An open‐label, randomized, single‐dose, two‐period, and crossover study of the main ginsenoside metabolites, compound K, was conducted in 12 Japanese healthy subjects, showing that the absorption of compound K increased significantly after the intake of fermented ginseng compared with nonfermented ginseng [29]. Additionally, ginseng fermented by L. paracasei A221 improved the first‐night effect in humans [30]. Fermented red ginseng lowered postprandial glucose levels in subjects with impaired fasting glucose or type 2 diabetes [31], and improved nasal congestion symptoms and quality of life in patients with perennial allergic rhinitis [32].
Though CHM fermentation has been applied to herbal drug preparation, the underlying biotransformation mechanisms of most CHM fermentation are unclear. Therefore, global and systematic analyses of CHM fermentation are necessary. In this review, we focus on the summary and discussion of current probiotic fermentation of CHM, including the potential mechanisms of CHM fermentation, the CHM fermentation advantages, the probiotics used for CHM fermentation, and modern microbial fermentation technologies. Moreover, future microbiome strategies for CHM fermentation using probiotics and the application of synthetic biology in the production of CHM bioactive ingredients are discussed.
MECHANISMS OF CHM FERMENTATION BY PROBIOTICS
Compared with traditional CHM processing methods, fermentation of CHM with probiotics can improve CHM bioactivity under mild processing conditions [33]. First, some CHM natural products are difficult to absorb and utilize in vivo. In the meanwhile, several hydrolases produced by probiotics during CHM fermentation can destroy plant cell walls and promote the release of bioactive ingredients in CHM [34]. Streptococcus lactis could efficiently degrade the cellulose, and the fermentation of Astragalus with S. lactis increased the contents of crude polysaccharides, total flavonoids, and total saponins in Astragalus roots, stems, and leaves [35]. Secondly, most herbal medicines are orally administered to humans, and CHM components can be transformed by gut microbiota before absorption [20, 36]. The enzymes secreted by gut probiotics can hydrolyze and remove glycosyl groups from CHM natural products, which increases their lipophilicity and improves the absorption rate in the gastrointestinal tract. After oral ingestion of liquorice, glycyrrhizin is converted to glycyrrhizic acid, and subsequently converted to glycyrrhetinic acid by gut microbiota [37]. In addition, probiotic fermentation can reduce or degrade the toxicity of some CHM [38].
Some effective natural products in CHM can be acted as prebiotics, which promote the proliferation of beneficial microorganisms in hosts [39]. The intake of yam significantly changed mice' gut microbiota, and the numbers of Bifidobacterium and Lactobacillus increased in mice [40]. Astragalus, Angelica, cowherb seed, Codonopsis, Licorice, and ligustici wallichii could individually stimulate the proliferation of probiotics, such as Bacillus subtilis, Lactobacillus acidophilus and yeasts, in a dose‐related manner [41]. Both red ginseng and Semen Coicis promoted the growth of Bifidobacterium and Lactobacillus in vitro, and improved the gut microbiota and relieved the symptoms of ulcerative colitis in vivo (Figure 1) [42]. Flos lonicerae has a significant regulatory effect on gut dysbiosis of mice, which could promote the recovery of gut microbiota dysbiosis [43]. Thus, the synergistic effect of CHM fermented with probiotics might enhance the effectiveness of CHM.
Figure 1.
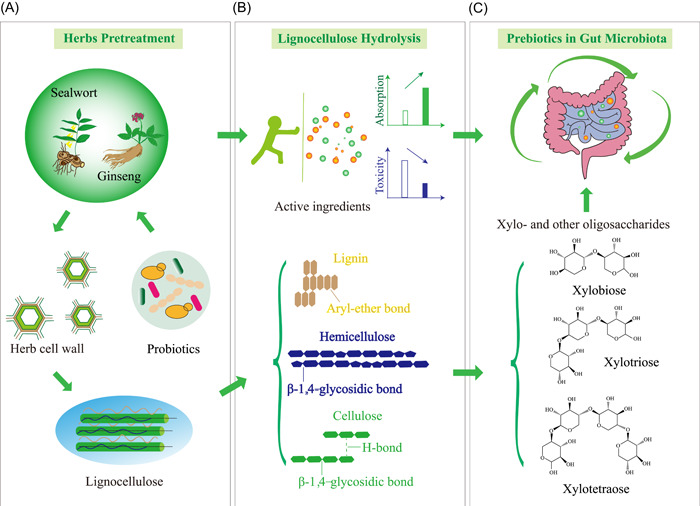
Lignocellulases and their functions in sealwort, ginseng, and other Chinese herbal medicine (CHM) fermentation. (A) The lignocellulose might prevent the release of bioactive ingredients of CHM, and lignocellulases derived from probiotics or other microbes can be used to degrade herb lignocellulose. (B) Lignocellulose hydrolysis releases bioactive ingredients in herbs, and leads to the generation of oligosaccharides prebiotics. (C) Bioactive ingredients and oligosaccharides are beneficial for the gut microbiota of humans and animals.
ADVANTAGES OF PROBIOTIC FERMENTATION OF CHM
Promoting the release of effective ingredients and improving the pharmacological activities of CHM
The effective ingredients of CHM are mostly distributed in the cytoplasm of root, stem, and leaf cells of plant biomass. The plant cell wall structure is tight, and is mainly composed of cellulose, hemicellulose, and lignin, which hinders the release of bioactive natural products and results in low absorption and utilization of CHM bioactive natural products [44]. Probiotics can produce a variety of hydrolytic enzymes, especially lignocellulases, to degrade plant cell wall and promote the release of bioactive natural products in CHM [45, 46] (Figure 1A,B). These released bioactive natural products include flavonoids, glycosides, anthraquinones, terpenoids, alkaloids, and organic acids (Table 1). Moreover, the lignocellulases can help generate oligosaccharide prebiotics for the gut microbiota of humans and animals (Figure 1C). Therefore, probiotic fermentation can improve the pharmacological activity of CHM [47].
Table 1.
Contents of effective Chinese herbal medicine ingredients increased after microbial fermentation.
| Herbs/herb formula | Microorganism | Increased bioactive natural products | Pharmacological effects | References |
|---|---|---|---|---|
| Hwangryun‐haedok‐tang | Lactobacillus curvatus | Baicalin | Ovariectomy‐induced bone loss ↓ | [48] |
| Condonpsis lanceolata | Bifidobacterium longum, Lactobacillus acidophilus, Leuconostoc mesenteroides | Gallic acid and vanillic acid |
Neuroprotective effect ↑ Cognitive enhancing activity ↑ |
[49] |
| Artemisia princeps Pampanini | Lactobacillus plantarum | Catechol and seco‐tanapartholide C | Anti‐inflammatory activity ↑ | [50] |
| Panax notoginseng | Lactobacillus helveticus, Lactobacillus rhamnosus, L. acidophilus | Ginsenoside Rg3 and Rh1 | Anti‐hepatocarcinoma activity ↑ | [51] |
| Astragalus membranaceus | Enterococcus faecium, L. plantarum | Astragalus polysaccharide, total saponins, and flavonoids | Not determined | [52] |
| Polygonum cuspidatum | Aspergillus niger, yeast | Resveratrol | Not determined | [53] |
| Radix astragali | Aspergillus oryzae | 3,4‐Di(4′‐hydroxyphenyl) isobutyric acid | Antioxidant activity ↑ | [54] |
| Red ginseng (the steamed ginseng) | Phellinus linteus | Ginsenosides Rg3, Rg5, Rk1, compound K, Rh1, F2, and Rg2 | Skin permeability ↑ | [55] |
| Lactobacillus brevis | Ginsenosides Rg3, Rg5, Rk1, compound K, Rh1, F2, Rg2, and flavonoids |
Antiwrinkle efficacy ↑ Skin sensitization ↓ |
[56] | |
| L. plantarum | Ginsenoside Rd and total phenolic | Antioxidant activities ↑ | [57] | |
| Lactobacillus paracasei, B. longum | Ginsenosides Rg3, F2, Rh1, Rh2, and Rg2 | Ovalbumin‐induced inflammation ↓ | [58] | |
| Panax ginseng | Ganoderma lucidum mycelium | Polysaccharides | Immunological activity ↑ | [59] |
| Lactobacillus fermentum | Rare ginsenosides (Rg2, Rg3, Rh1, Rh2, F2, and Ro) |
Hyperlipidemia ↓ liver injury ↓ |
[60] | |
| Dendrobium officinale | Bacillus sp. DU‐106 | Polysaccharides with high proportion of mannose | Immunoregulatory activities ↑ | [61] |
After fermentation of CHM with the probiotics, such as Lactobacillus casei, Enterococcus faecalis, and Candida utilis, the contents of soluble total flavonoids, total alkaloids, crude polysaccharides, and total saponins in the fermented Chinese herbs of Semen vaccariae and Leonurus artemisia increased by 55.14%, 127.28%, 55.42%, and 49.21%, respectively, compared with the natural herbs [62]. After fermented by Lactobacillus pentosus, the contents of quercetin and kaempferol in the extracts of Lespedeza cuneata G. Don increased by 242.9% and 266.7%, respectively, which improved potential antioxidative and antiaging functions of the herb [63]. After fermentation with Bifidobactericum breve strain CCRC 14061, the contents of daidzein and genistein in Puerariae Radix increased 785% and 1010%, respectively, which can stimulate hyaluronic acid production in NHEK cells [64]. Fermenting Cordyceps militaris with Pediococcus pentosaceus (GRC‐ON89A) enhanced phagocytic activity of RAW 264.7 cells and primary cultured murine macrophages; the enhanced immune activity of C. militaris was attributed to the increased content of β‐glucan, cordycepin, and short‐chain fatty acids after fermentation [65]. The microbial fermentation, especially probiotic fermentation, can significantly increase the contents of bioactive natural products and improve the pharmacological effects of CHM (Table 1).
Reducing toxicities and side effects of CHM
Some CHM have certain toxicities to humans and animals, and direct oral intake of them would generate serious toxic effects [66]. Probiotics can degrade or modify the toxic components, thus, reduce the toxicities or side effects of CHM [67, 68]. Conjugated anthraquinones are the main components leading to severe diarrhea of rhubarb. Fermentation of rhubarb with Kluyveromyces marxianus KM12 could convert conjugated anthraquinone to free anthraquinone, and the side effects of severe diarrhea generated by rhubarb were alleviated [69]. Compared with the original crude Croton tiglium, fermentation of C. tiglium with Ganoderma lucidum and Beauveria bassiana could decrease acute oral toxicity by about four times, and have no inflammation effect and hemocytolysis [70]. Fermentation of Tripterygium wilfordii with G. lucidum reduced the hepatotoxicity of T. wilfordii, which was due to the decrease of wilforlide after fermentation [71].
Generating new bioactive substances and enhancing the bioavailability of CHM
Probiotic fermentation transforms CHM ingredients to new bioactive compounds, and this might bring new pharmacological characteristics to CHM (Figure 2). Ginsenosides are the main physiologically bioactive natural products of ginseng, and ginsenosides Rb1, Rb2, Rc, Re, and Rg1 constitute more than 80% of the total ginsenosides in Panax ginseng [72]. Some rare ginsenosides, such as F2 and Rd, are demonstrated to have high bioavailability and bioactivity. However, their contents in natural P. ginseng are extremely low, and some of them, such as compound K (CK), are not available until P. ginseng biomass is transformed in human body [72]. The probiotic, Bifidobacterium animalis subsp. lactis LT 19‐2, can effectively convert main ginsenosides Rb2 and Rb3 in red ginseng extracts to rare ginsenosides of Rd, Rh1, F2, and Rg3 (Figure 2C) [73]. Probiotic fermentation of red ginseng increased RAW 264.7 cells macrophage activity significantly and activated primary immune cells, including splenic cells and bone marrow‐derived macrophages, suggesting that fermentation with B. animalis subsp. lactis LT 19‐2 can improve the immunomodulatory function of red ginseng [73]. The probiotics‐fermented red ginseng significantly increased Th1 and Treg cell differentiation, which could activate macrophages in mice to alleviate cyclophosphamide‐induced immunosuppression and 2,4,6‐trinitrobenzenesulfonic acid‐induced colitis [74]. The probiotics‐fermented herbal blend can enhance the immune ability of chicks infected with S. pullorum [19]. In another study, fermentation of P. ginseng extracts with B. lactis and Lactobacillus rhamnosus HN001 transformed ginsenosides of Rb1, Rc, and Rb2 to Rd (Figure 2B,C) [75]. The probiotics fermentation or enzymatic catalysis can generate bioactive rare ginsenosides (Figure 2). Fermentation of Dioscorea opposita Thunb. with Saccharomyces boulardii generates a series of novel low‐molecular‐weight polysaccharides, and these polysaccharides are easy to digest and have improved antioxidant activity and radioprotection effects [76]. Probiotics enabled the production of novel bioactive substances during fermentation. Further insights into the functional mechanisms of probiotics‐fermented CHM would pave the way to rational design of proper fermentation strategies.
Figure 2.
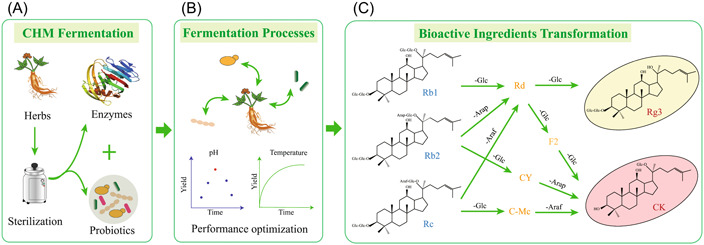
Biotransformation of ginsenosides to active rare ginsenosides using efficient enzymes or probiotics. (A) The herbs of Panax ginseng are sterilized for probiotic fermentation, and the enzymes and probiotics are the main driving forces for CHM fermentation. (B) Probiotic performance during ginseng fermentation can be optimized to improve bioactive ingredient yield. (C) Ginsenosides can be transformed to the bioactive rare ginsenosides during CHM fermentation. CHM, Chinese herbal medicines.
Reducing production costs and protecting environments
Probiotic CHM fermentation can increase the contents of effective ingredients and decrease the consumption of CHM. Many natural CHM resources, such as wild Panax and Glycyrrhiza resources, decreased in the past few years [60, 77]. Rare ginsenosides have been used to produce anticancer drugs, foods, and health care products [78], and probiotic fermentation could reduce the consumption of P. ginseng and the production costs of rare ginsenosides.
During CHM processing, large amounts of CHM residues were generated, direct abandonment or incineration of the residues would waste resources and generate environmental pollutions [79]. Huazhenghuisheng oral liquid (HOL), a clinical anti‐lung and liver cancer drug, is produced with 35 kinds of CHM. Fermentation of HOL residues with Aspergillus cristatus CB10002 could produce valuable compounds of anthraquinones [80]. The Lactobacillus plantarum HM218749 was used to ferment herb residues generated during the production of Jianweixiaoshi tablets, and the fermentation supernatant showed strong anti‐H. pylori activity in mice [81]. The fermented residues of one CHM formula composed of Pulsatilla, Rhizoma Coptidis, Cortex Phellodendri, Cortex Fraxini, Rhizoma Atractylodis, Rhizoma Artactylodis macrocephalae, and Granati Pericarpium, could improve the antioxidant capacity and immunity in weaned piglets, showing the fermented residues have potentials to be used as substitutes for antibiotics in piglets' feeding [82]. Probiotic fermentation can help reduce CHM consumption and provide a green recycling strategy of herb residues, which would save natural CHM sources, reduce production costs, and protect environments.
PROBIOTICS COMMONLY USED IN CHM FERMENTATION
A total of 35 species or subspecies microbes have been approved in China as edible probiotics [17], and some of them have been used to ferment CHM (Table 2). Lactobacillus is the most used probiotic genus in CHM fermentation. Lactobacillus has been used to ferment P. ginseng [21], Rhizoma A. macrocephalae [83], Anoectochilus formosanus Hayata [84], L. cuneata G. Don [63], Danshen [85], and some herb formulas, including Soshiho‐tang [86], Jaeumganghwa‐tang [87], and Hwangryun‐haedok‐tang [88]. Bifidobacterium species have been used to ferment Radix Puerariae [64] and A. formosanus Hayata [84]. Bacillus species have been used to ferment Danshen [85], ginseng seed [89], and Rhizoma A. macrocephalae (Table 2) [90]. Some fungi, especially medicinal fungi, have been applied in CHM or herb formulas fermentation. Saccharomyces have been used to ferment Glycyrrhiza uralensis Fisch [91] and Gegen Qinlian decoction [92]; and G. lucidum has been used to ferment Artemisia capillaris leaves [93] (Table 2). Currently, most CHM fermentation is still limited to a single‐strain fermentation of single Chinese herb, and few studies on fermentation of CHM with multiple probiotics or synthetic microbiota were reported [19, 94, 95]. As diverse probiotics or probiotic combinations are available in nature, screening novel probiotic strains or building synthetic probiotic microbiota might improve CHM fermentation [96, 97].
Table 2.
List of probiotics, medicinal fungi, and a few industrial fungi used for Chinese herbal medicine fermentation.
| Category | Genus | Species | Herbs/Herb formulas used for fermentation | References |
|---|---|---|---|---|
| Bacteria | Lactobacillus | L. plantarum | Red ginseng; Jianweixiaoshi tablets; Soshiho‐tang; Rhizoma Artactylodis macrocephalae | [57, 81, 83, 86] |
| L. acidophilus | Anoectochilus formosanus Hayata; Jaeumganghwa‐tang | [84, 87] | ||
| L. casei | A. formosanus Hayata; Hwangryun‐haedok‐tang | [84, 88] | ||
| L. paracasei | Red ginseng | [98] | ||
| L. pentosus | Lespedeza cuneata G. Don | [63] | ||
| L. rhamnosus | Panax ginseng; Salvia miltiorrhiza Bunge | [75, 85] | ||
| L. gasseri | Ginseng seed | [89] | ||
| L. fermentum | P. ginseng | [21] | ||
| Bifidobacterium | B. breve | Radix Puerariae | [64] | |
| B. longum | A. formosanus Hayata; Red ginseng | [84, 98] | ||
| B. lactis | P. ginseng | [75] | ||
| B. animalis subsp. lactis | Red ginseng | [73] | ||
| Bacillus | B. subtilis | S. miltiorrhiza Bunge; Ginseng seed; Deer antler; White ginseng roots | [85, 89, 99, 100] | |
| B. licheniformis | Rhizoma A. macrocephalae | [90] | ||
| Alcaligenes | A. spiechaudii | Rhodiola rosea; Lonicera japonica | [101] | |
| Lactococcus | L. lactis | P. ginseng | [98] | |
| Streptococcus | S. thermophiles | Cyclopia intermedia | [102] | |
| Leuconostoc | L. mesenteroides | R. coptidis | [103] | |
| Pediococcus | P. pentosaceus | Ginseng seed | [89] | |
| Fungi | Saccharomyces | S. cerevisiae | Glycyrrhiza uralensis Fisch; Gegen Qinlian decoction | [91, 92] |
| S. boulardii | Dioscorea opposita Thunb | [76] | ||
| Kluyveromyces | K. marxianus | Rhubarb | [69] | |
| Trichoderma | T. reesei | White ginseng roots | [100] | |
| Ganoderma | G. lucidum | Croton tiglium; Tripterygium wilfordii; Artemisia capillaris leaves | [70, 71, 93] | |
| Trametes | T. robiniophila Murr | Radix isatidis | [104] | |
| Grifola | G. frondosa | Rhizoma gastrodiae | [105] | |
| Coprinus | C. comatus | Sophora flavescens | [106] |
PROBIOTIC FERMENTATION TECHNIQUES FOR CHM
Traditional CHM fermentation technique is solid‐state fermentation, which uses wild‐type microorganisms in the environments to complete the fermentation process without accurate control of ambient temperature and humidity. The fermentation endpoint of solid‐state fermentation is often determined by individual experience. Therefore, the efficacy, safety, and stability of traditional fermented CHM are not stable, and this might due to insufficient strain purity, uncontrollable fermentation conditions, and lack of standardized fermentation process and appropriate monitoring indicators. Compared with traditional fermentation technique, modern fermentation technology integrates microbial ecology, fermentation engineering, and bioengineering, leading to new CHM fermentation techniques [15]. On the basis of fermentation forms, modern fermentation techniques can be divided into solid fermentation, liquid fermentation, and bidirectional fermentation with medicinal fungi (Figure 3).
Figure 3.
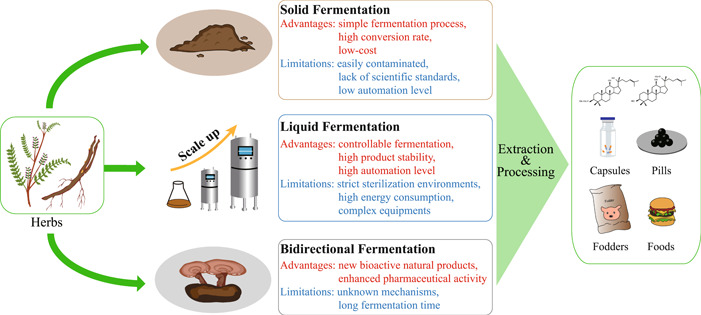
Different probiotic CHM fermentation strategies and their characterization. The liquid, solid, and bidirectional fermentation were used for CHM fermentation. After extraction and purification, the final products of CHM fermentation could be used as drugs, fodders, and foods. CHM, Chinese herbal medicines.
Solid fermentation
Solid fermentation uses one or several probiotic strains to ferment CHM biomass under low‐moisture or almost no free‐water conditions [17]. The solid fermentation system is naturally open, and sterilization of the substrates is not necessary (Figure 3). Moreover, solid fermentation generates small amounts of wastewater [107]. The cost of solid fermentation is low, and the procedure is relatively simple [108]. Solid fermentation has the advantages of high conversion rate and high yield. The solid fermentation converts 20%–30% CHM substrates to novel products, while the transformation efficiency of liquid fermentation is only about 5% [86]. However, solid fermentation has some limitations, including frequent contamination by miscellaneous bacteria due to the open fermentation system, slow fermentation rate, lack of scientific standards for fermentation endpoint and quality control, and low automation level [109].
In addition to the traditional starter‐making technology, a variety of novel solid probiotic fermentation systems for CHM have been developed [110]. After solid fermentation of ginseng seeds with Bacillus, Lactobacillus, and Pediococcus strains, respectively, the contents of total sugars, acidic polysaccharides, and phenolic compounds were higher than that of the nonfermented control [89]. Moreover, the antioxidant activity of ginseng seeds improved after probiotic solid fermentation [89]. Solid fermentation of Astragalus membranaceus with L. plantarum and Enterococcus faecium greatly improves the production of health‐promoting biological compounds, including polysaccharides, total saponins, and flavonoids [52].
Liquid fermentation
Liquid fermentation, also known as liquid‐submerged fermentation, is derived from the antibiotics production process [111]. Liquid fermentation technique inoculates the activated microorganisms into the medium composed with CHM extracts and proper microbial nutrients (Figure 3). The fermentation process was implemented under suitable temperature and pH value. Compared with solid fermentation, liquid fermentation has the advantages of high product stability, quantified production conditions, and high automation level. Moreover, liquid fermentation can be efficiently applied in large‐scale CHM fermentation [15]. Liquid fermentation requires strict sterilization environments, and the fermentation process is high energy consumption; moreover, the equipment is complex [15]. Thus, it is necessary to optimize the liquid fermentation process, especially fermentation devices and conditions, to improve active ingredient conversion rate and reduce pollution.
Some probiotic liquid fermentation strategies for CHM were applied. For example, liquid fermentation of hydroponic ginseng with Lactococcus lactis KC24 increased the antioxidant activity of ginseng [98]. Red ginseng fermented with L. paracasei and Bifidobacterium longum could efficiently alleviate ovalbumin‐induced inflammation in mice [58]. The hematopoietic activity of deer antler increased after the liquid fermentation with B. subtilis [99].
Bidirectional fermentation with medicinal fungi
Bidirectional fermentation with medicinal fungi includes liquid fermentation and solid fermentation; the former is the combination of basic culture medium, CHM extracts, and fungi in closed environment, while the latter is the combination of CHM and fungi in open environments. Bidirectional solid fermentation was established in the 1980s [112]. It is a new Chinese herbal fermentation technique that CHM substrates are fermented by medicinal fungi (Figure 3). During bidirectional fermentation, CHM substrates provide the nutrients for medicinal fungi growth, and the fungal fermentation increases the bioactive natural product composition of the CHM substrates [17]. Bidirectional fermentation could produce a large number of new bioactive fermentation metabolites. Insight into the fermentation process and fungal enzymatic systems would give clues to the bidirectional fermentation mechanisms [113].
Fresh ginseng fermented with G. lucidum mycelium in solid‐state culture could enhance its immunomodulatory activity [59]. The solid‐state bidirectional fermentation of A. capillaris leaves with G. lucidum enhanced the anti‐inflammatory effects in a mice model with atopic dermatitis [93]. Products of Trametes robiniophila Murr fermented with Radix isatidis strongly inhibited the cell proliferation of breast cancer cells [104]. Compared with the control, when Ginkgo biloba leaves were fermented with G. lucidum by bidirectional liquid fermentation, the yield of polysaccharides, triterpenes, and total flavonoids increased by 2.38, 1.96, and 2.10 times, respectively, which leads to higher antioxidation activity of the fermentation products [114]. However, the bidirectional fermentation rate is slow, and the application of the cutting‐edge genetic engineering tools is limited [115].
PROBIOTIC FERMENTATION MODES FOR CHM FERMENTATION
Single probiotic strain fermentation
Single probiotic strain fermentation is the most commonly used fermentation mode, and the probiotic fermentation modifies the structure of specific substrates by enzymatic catalysis [116, 117]. The strains of Lactobacillus, Bifidobacterium, Bacillus, and some medicinal fungi are often used for single‐strain CHM fermentation. The metabolites of P. ginseng fermented with Lactobacillus fermentum can treat antibiotic‐associated diarrhea symptoms and colon inflammation [21]. Moreover, the fermentation metabolites could transfer the gut microbiota disturbances to healthy state in rat [21]. Fermentation of Artemisia princeps Pampanini with L. plantarum SN13T increased the amounts of bioactive compounds of catechol and seco‐tanapartholide C [50]. The fermentation of red ginseng with B. animalis subsp. lactis LT 19‐2 isolated from the feces of infants could enhance immunomodulatory function of red ginseng [73]. Fermentation of Cynanchi atrati Radix with Lactobacillus increased the anti‐melanin activity [118]. As single probiotic strain fermentation can increase the performance of CHM, more probiotics might be applied in future CHM fermentation.
Multispecies fermentation
Compared with single‐strain fermentation, multispecies fermentation can provide diverse and redundant enzymatic systems. Multispecies fermentation has potential to improve the utilization rate and increase biotransformation efficiency of CHM [17]. Synthetic microbiota with bacteria, fungi, or bacteria‐fungi has been used for CHM fermentation. Salvia miltiorrhiza Bunge (Danshen) fermented by L. rhamnosus (F‐B4‐1) and B. subtilis Natto (F‐A7‐1) relieved dextran sulfate sodium‐induced ulcerative colitis in mice more effectively than raw Danshen [85]. Fermented white ginseng roots with B. subtilis and Trichoderma reesei enhanced the biotransformation yield from ginsenosides to rare ginsenosides, for these two species have nonsynchronous cell growth and different metabolic pathways [100]. The coimmobilized edible Aspergillus niger and yeast produced 11‐fold resveratrol from polydatin in Polygonum cuspidatum roots than that of the untreated sample [53]. At present, few multispecies fermentations of CHM are available, which might due to the complex control and modulation of multispecies fermentation. In the future, optimization of multispecies fermentation process and recovery of the underlying mechanisms are of great value to CHM fermentation.
APPLICATION OF MICROBIOME AND SYNTHETIC BIOLOGY STRATEGIES FOR EFFICIENT PROBIOTIC FERMENTATION OF CHM
CHM fermentation has the advantages of increasing pharmacological activity, reducing toxicity, and producing new bioactive ingredients. Understanding the underlying mechanisms of CHM fermentation lays the foundation for the optimization of probiotic fermentation [119]. High‐quality and safe fermentation strains are the basis and keystone for CHM fermentation [120]. Currently, most probiotic strains used for CHM fermentation derived from fermented dairy products and the animal fecal microbiota [121, 122]. The probiotic types are very limited, which are mainly assigned to Lactobacillus, Bifidobacterium, Bacillus, and yeasts [123]. With the development of synthetic microbiology technologies, efficient and affordable high‐throughput sequencing technologies help recover probiotics in diverse environments (Figure 4A,B) [124, 125, 126]. The microbiome strategies have been applied to reveal the microbial variations during the manufacturing process of Fu brick tea, the spontaneous fermentation periods of light‐flavor Baijiu, and the fermentation of Huafeng Dan Yaomu [127, 128, 129]. Though most microorganisms are uncultured, the new developed culturomics provide tools to isolate and screen proper probiotics for CHM fermentation (Figure 4C) [130, 131, 132].
Figure 4.
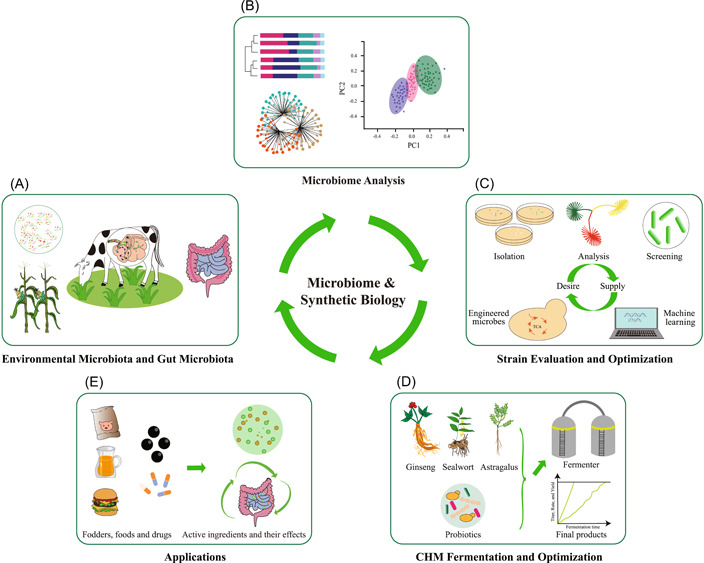
Application of microbiome and synthetic biology strategies for efficient probiotic CHM fermentation. (A) Environmental microbiota, and human and animal gut microbiota are potential microbial sources for CHM fermentation. (B) Environmental microbiota and gut microbiota can be analyzed by microbiome strategies, and screened for probiotics. (C) Efficient probiotics can be isolated, analyzed, and screened for CHM fermentation. Moreover, the machine learning and metabolic engineering technologies can provide further efficient enzymes or microbes for CHM fermentation. (D) Fermentation process can be optimized, which can lead to the production of bioactive ingredients with high yield. (E) The obtained CHM fermentation products can be applied in foods, animal feeds, drugs, or other industries. The active ingredients would produce beneficial effects for humans and animals. CHM, Chinese herbal medicines.
The efficient hydrolase and other CHM biomass hydrolysis enzymes, especially lignocellulases, transform CHM substrates to bioactive natural products and generate/produce prebiotics from lignocellulose (Figures 1C and 4D,E). Thus, recovery and characterization of efficient enzymes for CHM fermentation are essential. For example, ginsenosides are believed to be the primary beneficial components of ginseng, but its oral bioavailability is low. Ginsenoside transformed by human gut microbiota could increase biological activity and bioavailability in vivo [133]. The biotransformation mechanism of human gut microbiota is hydrolysis of sugar moieties of ginsenosides by β‐glucosidase derived from gut microbiota to produce rare ginsenosides (Figure 2C) [133]. An A. niger XD101 strain could transform Rb1 to easily absorbed ginsenoside CK by its extracellular β‐glucosidase [134]. In addition, a variety of probiotics with high β‐glucosidase activity have been screened for P. ginseng fermentation, including B. lactis Bi‐07 [75], L. rhamnosus HN001 [75], and Lentilactobacillus buchneri URN103L [134]. Baicalin (baicalein 7‐O‐β‐d‐glucuronide) is one of the major flavonoids in Scutellaria baicalensis. Baicalein, the aglycone of baicalin, is easier to be absorbed and more effective than baicalin, but the content of baicalein in S. baicalensis is relatively low. Lactobacillus brevis subsp. coagulans can convert baicalin to baicalein using its β‐glucuronidase [135]. More than 90,000 genes/gene fragments encoding for carbohydrate‐active enzymes were recovered from diverse cellulolytic microorganisms [136]. Further enzymatic characterization identified some xylanase and pectinolytic enzymes [137, 138], suggesting that efficient hydrolase for CHM fermentation could be recovered from natural environments using microbiome strategies.
Synthetic biology provides valuable tools for the optimization of enzymes and strains with efficient CHM fermentation ability. The protein engineering and metabolic engineering based on machine learning can improve hydrolase activities and other performances, which would provide efficient engineered enzymes/microbes for CHM fermentation (Figure 4C) [139]. The CHM bioactive natural product yield can be improved by optimization of synthetic fermentation microbiota and fermentation parameters (Figure 4D). Fermentation with B. subtilis and T. reesei promoted biotransformation efficiency of ginsenosides in white ginseng roots, and the inoculation proportion of B. subtilis and T. reesei at 1:4 resulted in the highest rare ginsenoside yield [100]. Pretreatment of P. cuspidatum root with immobilized β‐glucosidases could improve the conversion of polydatin to resveratrol at proper fermentation environment [140]. Designing and building synthetic microbiota with wild‐type probiotics, and optimizing fermentation parameters, including pH value, temperature, and incubation time, could improve the yield of bioactive natural products generated by CHM fermentation [141] (Figure 4D). Production of terpenoids, lipids and other plant natural products by engineered yeasts has been achieved [142, 143, 144, 145], and synthetic biology could design and reprogram of microorganisms to de novo produce various bioactive natural products [139, 146, 147, 148, 149, 150]. In the future, production of CHM bioactive natural products by synthetic biology technology might be an alternative strategy for probiotic CHM fermentation, and the products can be further applied in foods, animal feed, and other industries [151].
FUTURE PERSPECTIVES
The contents of many bioactive ingredients in CHM are low, and some CHM components are toxic. Probiotic fermentation of CHM can generate easily absorbed bioactive compounds and reduce toxicities. Therefore, discovering efficient and safe probiotic strains and developing novel fermentation strategies for CHM fermentation are of great interest. Insights into the generation pathway of active ingredients could accelerate to screen efficient enzymes and probiotics for CHM fermentation. Optimization of fermentation equipments and parameters are necessary to obtain high titer, rate, and yield of CHM bioactive products. Although probiotics are safe for the human body, the products of probiotic fermentation should accept a comprehensive and scientific safety evaluation. Thus, CHM fermentation standards should be drafted and optimized before application. Probiotic fermentation of CHM would not only offer opportunities to recover underlying mechanisms for bioactive natural product generation, but also provide healthy products for humans and animals. In the future, the development of synthetic biology would lead to the production of CHM bioactive natural products with efficient microbial cell factories.
AUTHOR CONTRIBUTIONS
Yongjun Wei, Lingbo Qu, and Yulong Yin conceived the study. Xiaoling Zhang, Qin Miao, Yongjun Wei, and Chenxue Pan drafted and revised the manuscript. Xiaoling Zhang, Qin Miao, and Yongjun Wei prepared the figures and tables. Jia Yin, Leli Wang, Lingbo Qu, and Yulong Yin revised the manuscript. Yongjun Wei and Xiaoling Zhang designed the study. All the authors read and approved the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (No. 2019YFB1503904), and the National Natural Science Foundation of China (Nos. 32101003 and 32111530179).
Zhang, Xiaoling , Miao Qin, Pan Chengxue, Yin Jia, Wang Leli, Qu Lingbo, Yin Yulong, and Wei Yongjun. 2023. “Research advances in probiotic fermentation of Chinese herbal medicines.” iMeta 2, e93. 10.1002/imt2.93
Contributor Information
Yulong Yin, Email: yinyulong@isa.ac.cn.
Yongjun Wei, Email: yongjunwei@zzu.edu.cn.
DATA AVAILABILITY STATEMENT
Supplementary materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.
REFERENCES
- 1. Tang, Jin‐Ling , Liu Bao‐Yan, and Ma Kan‐Wen. 2008. “Traditional Chinese Medicine.” The Lancet 372: 1938–40. 10.1016/S0140-6736(08)61354-9 [DOI] [PubMed] [Google Scholar]
- 2. Hao, Da‐cheng , and Xiao Pei‐gen. 2020. “Pharmaceutical Resource Discovery from Traditional Medicinal Plants: Pharmacophylogeny and Pharmacophylogenomics.” Chinese Herbal Medicines 12: 104–17. 10.1016/j.chmed.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niazian, Mohsen . 2019. “Application of Genetics and Biotechnology for Improving Medicinal Plants.” Planta 249: 953–73. 10.1007/s00425-019-03099-1 [DOI] [PubMed] [Google Scholar]
- 4. Zhou, Ping . 2010. “Traditional Chinese Medicine.” Combinatorial Chemistry & High Throughput Screening 13: 836. 10.2174/138620710793360329 [DOI] [PubMed] [Google Scholar]
- 5. Wu, Wei , Jiao Chuanxi, Li Hui, Ma Yue, Jiao Lili, and Liu Shuying. 2018. “LC‐MS Based Metabolic and Metabonomic Studies of Panax ginseng .” Phytochemical Analysis 29: 331–40. 10.1002/pca.2752 [DOI] [PubMed] [Google Scholar]
- 6. Tu, Youyou . 2016. “Artemisinin—A Gift from Traditional Chinese Medicine to the World (Nobel Lecture).” Angewandte Chemie International Edition 55: 10210–26. 10.1002/anie.201601967 [DOI] [PubMed] [Google Scholar]
- 7. Efferth, Thomas , and Oesch Franz. 2021. “The Immunosuppressive Activity of Artemisinin‐type Drugs Towards Inflammatory and Autoimmune Diseases.” Medicinal Research Reviews 41: 3023–61. 10.1002/med.21842 [DOI] [PubMed] [Google Scholar]
- 8. Zhang, Wenting , Yan Yonghuang, Wu Yujie, Yang Han, Zhu Peixuan, Yan Fang, Zhao Ruixue, et al. 2022. “Medicinal Herbs for the Treatment of Anxiety: A Systematic Review and Network Meta‐analysis.” Pharmacological Research 179: 106204. 10.1016/j.phrs.2022.106204 [DOI] [PubMed] [Google Scholar]
- 9. Yan, Xing , Fan Yun, Wei Wei, Wang Pingping, Liu Qunfang, Wei Yongjun, Zhang Lei, et al. 2014. “Production of Bioactive Ginsenoside Compound K in Metabolically Engineered Yeast.” Cell Research 24: 770–3. 10.1038/cr.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao, Linggai , Wu Hao, Zhang He, Zhao Quan, Yin Xue, Zheng Dongran, Li Chuanwang, et al. 2020. “Highly Efficient Production of Diverse Rare Ginsenosides Using Combinatorial Biotechnology.” Biotechnology and Bioengineering 117: 1615–27. 10.1002/bit.27325 [DOI] [PubMed] [Google Scholar]
- 11. Zhao, Le , Zhu Yunhao, Jia Haoyu, Han Yongguang, Zheng Xiaoke, Wang Min, and Feng Weisheng. 2022. “From Plant to Yeast‐advances in Biosynthesis of Artemisinin.” Molecules 27: 6888. 10.3390/molecules27206888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shao, Fenjuan . 2021. “The Research Progress of Taxol in Taxus .” Current Pharmaceutical Biotechnology 22: 360–6. 10.2174/18734316MTA3oNTEc1 [DOI] [PubMed] [Google Scholar]
- 13. Yang, Bo , Xie Yun, Guo Maojuan, Rosner Mitchell H., Yang Hongtao, and Ronco Claudio. 2018. “Nephrotoxicity and Chinese Herbal Medicine.” Clinical Journal of the American Society of Nephrology 13: 1605–11. 10.2215/cjn.11571017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charen, Elliot , and Harbord Nikolas. 2020. “Toxicity of Herbs, Vitamins, and Supplements.” Advances in Chronic Kidney Disease 27: 67–71. 10.1053/j.ackd.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 15. Li, Lin , Wang Li, Fan Wenxiang, Jiang Yun, Zhang Chao, Li Jianghua, Peng Wei, and Wu Chunjie. 2020. “The Application of Fermentation Technology in Traditional Chinese Medicine: A Review.” The American Journal of Chinese Medicine 48: 899–921. 10.1142/s0192415x20500433 [DOI] [PubMed] [Google Scholar]
- 16. Zhang, Lixia , Gao Wenyuan, and Wang Haiyang. 2012. “Review of Traditional Chinese Medicine Processed by Fermentation.” China Journal of Chinese Materia Medica 37: 3695–700. [PubMed] [Google Scholar]
- 17. Liu, Bo , Zhang Pengyi, Meng Xiangjing, Zhang Xiangkui, Li Min, Duan Chonggang, Zhang Lanying, Zhang Daizhou, and Ling Peixue. 2020. “Research Progress in Probiotics Fermentation Methods of Traditional Chinese Medicine and Its Application.” Modern Chinese Medicine 22: 1741–50. [Google Scholar]
- 18. Wang, Yanbo , Xie Qiuhong, Sun Sheng, Huang Baojia, Zhang Ying, Xu Yun, Zhang Shumin, and Xiang Hongyu. 2018. “Probiotics‐fermented Massa Medicata Fermentata Ameliorates Weaning Stress in Piglets Related to Improving Intestinal Homeostasis.” Applied Microbiology and Biotechnology 102: 10713–27. 10.1007/s00253-018-9438-y [DOI] [PubMed] [Google Scholar]
- 19. Wang, Yiming , Li Jiayi, Xie Yuanhong, Zhang Hongxing, Jin Junhua, Xiong Lixia, and Liu Hui. 2021. “Effects of a Probiotic‐fermented Herbal Blend on the Growth Performance, Intestinal Flora and Immune Function of Chicks Infected with Salmonella pullorum .” Poultry Science 100: 101196. 10.1016/j.psj.2021.101196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie, Yuan , Hu Fangdi, Xiang Dawei, Lu Hui, Li Wenbin, Zhao Anpeng, Huang Longji, and Wang Rong. 2020. “The Metabolic Effect of Gut Microbiota on Drugs.” Drug Metabolism Reviews 52: 139–56. 10.1080/03602532.2020.1718691 [DOI] [PubMed] [Google Scholar]
- 21. Qu, Qingsong , Yang Fang, Zhao Chongyan, Liu Xing, Yang Pengshuo, Li Zhixun, Han Lu, and Shi Xinyuan. 2021. “Effects of Fermented Ginseng on the Gut Microbiota and Immunity of Rats with Antibiotic‐associated Diarrhea.” Journal of Ethnopharmacology 267: 113594. 10.1016/j.jep.2020.113594 [DOI] [PubMed] [Google Scholar]
- 22. Chen, Pin , Sun Jinwei, Liang Zhiqiang, Xu Hanxue, Du Peng, Li Aili, Meng Yueyue, et al. 2022. “The Bioavailability of Soy Isoflavones In Vitro and Their Effects on Gut Microbiota in the Simulator of the Human Intestinal Microbial Ecosystem.” Food Research International 152: 110868. 10.1016/j.foodres.2021.110868 [DOI] [PubMed] [Google Scholar]
- 23. Guo, Rui , Guo Shuchen, Gao Xiong, Wang Huaiyou, Hu Weihui, Duan Ran, Dong Tina T. X., and Tsim Karl W. K.. 2020. “Fermentation of Danggui Buxue Tang, an Ancient Chinese Herbal Mixture, Together with Lactobacillus plantarum Enhances the Anti‐diabetic Functions of Herbal Product.” Chinese Medicine 15: 98. 10.1186/s13020-020-00379-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suez, Jotham , Zmora Niv, Segal Eran, and Elinav Eran. 2019. “The Pros, Cons, and Many Unknowns of Probiotics.” Nature Medicine 25: 716–29. 10.1038/s41591-019-0439-x [DOI] [PubMed] [Google Scholar]
- 25. Wieërs, Grégoire , Belkhir Leila, Enaud Raphaël, Leclercq Sophie, Philippart de Foy Jean‐Michel, Dequenne Isabelle, de Timary Philippe, and Cani Patrice D.. 2019. “How Probiotics Affect the Microbiota.” Frontiers in Cellular and Infection Microbiology 9: 454. 10.3389/fcimb.2019.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu, Qing , Yu Zhiming, Tian Fengwei, Zhao Jianxin, Zhang Hao, Zhai Qixiao, and Chen Wei. 2020. “Surface Components and Metabolites of Probiotics for Regulation of Intestinal Epithelial Barrier.” Microbial Cell Factories 19: 23. 10.1186/s12934-020-1289-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang, Chen‐Xing , Wang Hui‐Yu, and Chen Tong‐Xin. 2019. “Interactions Between Intestinal Microflora/Probiotics and the Immune System.” BioMed Research International 2019: 6764919. 10.1155/2019/6764919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon, Jin Young , Cha Jae Myung, Hong Seong Soo, Kim Hyung Kyung, Kwak Min Seob, Jeon Jung Won, and Shin Hyun Phil. 2019. “Fermented Milk Containing Lactobacillus paracasei and Glycyrrhiza glabra Has a Beneficial Effect in Patients with Helicobacter pylori Infection: A Randomized, Double‐blind, Placebo‐controlled Study.” Medicine (Baltimore) 98: e16601. 10.1097/md.0000000000016601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fukami, Hiroyuki , Ueda Taro, and Matsuoka Nobuya. 2019. “Pharmacokinetic Study of Compound K in Japanese Subjects After Ingestion of Panax ginseng Fermented by Lactobacillus paracasei A221 Reveals Significant Increase of Absorption into Blood.” Journal of Medicinal Food 22: 257–63. 10.1089/jmf.2018.4271 [DOI] [PubMed] [Google Scholar]
- 30. Kitaoka, Kazuyoshi , Uchida Kaoru, Okamoto Naoko, Chikahisa Sachiko, Miyazaki Toshitsugu, Takeda Eiji, and Séi Hiroyoshi. 2009. “Fermented Ginseng Improves the First‐night Effect in Humans.” Sleep 32: 413–21. 10.1093/sleep/32.3.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oh, Mi‐Ra , Park Soo‐Hyun, Kim Sun‐Young, Back Hyang‐Im, Kim Min‐Gul, Jeon Ji‐Young, Ha Ki‐Chan, et al. 2014. “Postprandial Glucose‐lowering Effects of Fermented Red Ginseng in Subjects with Impaired Fasting Glucose or Type 2 Diabetes: A Randomized, Double‐blind, Placebo‐controlled Clinical Trial.” BMC Complementary and Alternative Medicine 14: 237. 10.1186/1472-6882-14-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jung, Jae‐Woo , Kang Hye‐Ryun, Ji Geun‐Eog, Park Myeong‐Soo, Song Woo‐Jung, Kim Min‐Hye, Kwon Jae‐Woo, et al. 2011. “Therapeutic Effects of Fermented Red Ginseng in Allergic Rhinitis: A Randomized, Double‐blind, Placebo‐controlled Study.” Allergy, Asthma & Immunology Research 3: 103–10. 10.4168/aair.2011.3.2.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussain, Ahtesham , Bose Shambhunath, Wang Jing‐Hua, Yadav Mukesh Kumar, Mahajan Girish B., and Kim Hojun. 2016. “Fermentation, a Feasible Strategy for Enhancing Bioactivity of Herbal Medicines.” Food Research International 81: 1–16. 10.1016/j.foodres.2015.12.026 [DOI] [Google Scholar]
- 34. Wang, Lu , Wei Wenhao, Tian Xiaofei, Shi Kan, and Wu Zhenqiang. 2016. “Improving Bioactivities of Polyphenol Extracts from Psidium guajava L. Leaves Through Co‐fermentation of Monascus anka GIM 3.592 and Saccharomyces cerevisiae GIM 2.139.” Industrial Crops & Products 94: 206–15. [Google Scholar]
- 35. Su, Guilong , Zhang Jingyan, Zhang Kai, Wang Lei, Zhang Kang, Wang Xuezhi, Yang Zhiqiang, and Li Jianxi. 2017. “Study on Improving Active Ingredients of Astragalus Root, Stem and Leaf by Probiotic Fermentation.” China Animal Husbandry & Veterinary Medicine 44: 1877–83. [Google Scholar]
- 36. Gong, Xue , Li Xue, Bo Agula, Shi Ru‐Yu, Li Qin‐Yu, Lei Lu‐Jing, Zhang Lei, and Li Min‐Hui. 2020. “The Interactions Between Gut Microbiota and Bioactive Ingredients of Traditional Chinese Medicines: A Review.” Pharmacological Research 157: 104824. 10.1016/j.phrs.2020.104824 [DOI] [PubMed] [Google Scholar]
- 37. Kwon, Yu‐Jin , Son Da‐Hye, Chung Tae‐Ha, and Lee Yong‐Jae. 2020. “A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings.” Journal of Medicinal Food 23: 12–20. 10.1089/jmf.2019.4459 [DOI] [PubMed] [Google Scholar]
- 38. Wang, Yuchen , Tao Yang, Zhang Xinyan, Shao Shengjie, Han Yongbin, Chu Dinh‐Toi, Xie Guangjie, and Ye Xiaosong. 2019. “Metabolic Profile of Ginkgo Kernel Juice Fermented with Lactic Aicd Bacteria: A Potential Way to Degrade Ginkgolic Acids and Enrich Terpene Lactones and Phenolics.” Process Biochemistry 76: 25–33. 10.1016/j.procbio.2018.11.006 [DOI] [Google Scholar]
- 39. Gao, Lu‐Lu , Ma Jia‐Min, Fan Yan‐Na, Zhang Yan‐Nan, Ge Rui, Tao Xiu‐Juan, Zhang Meng‐Wei, Gao Qing‐Han, and Yang Jian‐Jun. 2021. “ Lycium barbarum Polysaccharide Combined with Aerobic Exercise Ameliorated Nonalcoholic Fatty Liver Disease Through Restoring Gut Microbiota, Intestinal Barrier and Inhibiting Hepatic Inflammation.” International Journal of Biological Macromolecules 183: 1379–92. 10.1016/j.ijbiomac.2021.05.066 [DOI] [PubMed] [Google Scholar]
- 40. Hsu, Cheng‐Chin , Huang Yi‐Chia, Yin Mei‐Chin, and Lin Shyh‐Jye. 2006. “Effect of Yam (Dioscorea alata compared to Dioscorea japonica) on Gastrointestinal Function and Antioxidant Activity in Mice.” Journal of Food Science 71: S513–6. 10.1111/j.1750-3841.2006.00113.x [DOI] [Google Scholar]
- 41. Wang, Xiang , Xie Haijun, Liu Fu, and Wang Yuhong. 2017. “Production Performance, Immunity, and Heat Stress Resistance in Jersey Cattle Fed a Concentrate Fermented with Probiotics in the Presence of a Chinese Herbal Combination.” Animal Feed Science and Technology 228: 59–65. 10.1016/j.anifeedsci.2017.03.015 [DOI] [Google Scholar]
- 42. Guo, Mingzhang , Ding Shuo, Zhao Changhui, Gu Xinxi, He Xiaoyun, Huang Kunlun, Luo Yunbo, et al. 2015. “Red Ginseng and Semen Coicis Can Improve the Structure of Gut Microbiota and Relieve the Symptoms of Ulcerative Colitis.” Journal of Ethnopharmacology 162: 7–13. 10.1016/j.jep.2014.12.029 [DOI] [PubMed] [Google Scholar]
- 43. Yao, Xiao‐Hua , Tang Li, Gao Fei, Li Wei‐Hua, Zhang Guo‐Bin, Liu Yin‐Hui, Li Hua‐Jun, et al. 2014. “Effect of Flos lonicerae on the Intestinal Dysbiosis of Mice.” Chinese Journal of Microecology 26: 886–92. [Google Scholar]
- 44. Zhao, Shuna , Baik Oon‐Doo, Choi Young Jin, and Kim Sang‐Moo. 2014. “Pretreatments for the Efficient Extraction of Bioactive Compounds from Plant‐based Biomaterials.” Critical Reviews in Food Science and Nutrition 54: 1283–97. 10.1080/10408398.2011.632698 [DOI] [PubMed] [Google Scholar]
- 45. Berikashvili, Violet , Sokhadze Kakha, Kachlishvili Eva, Elisashvili Vladimir, and Chikindas Michael L.. 2018. “ Bacillus amyloliquefaciens Spore Production Under Solid‐state Fermentation of Lignocellulosic Residues.” Probiotics and Antimicrobial Proteins 10: 755–61. 10.1007/s12602-017-9371-x [DOI] [PubMed] [Google Scholar]
- 46. Woldemariam Yohannes, Kalekristos , Wan Zhen, Yu Qinglin, Li Hongyan, Wei Xuetuan, Liu Yingli, Wang Jing, and Sun Baoguo. 2020. “Prebiotic, Probiotic, Antimicrobial, and Functional Food Applications of Bacillus amyloliquefaciens .” Journal of Agricultural and Food Chemistry 68: 14709–27. 10.1021/acs.jafc.0c06396 [DOI] [PubMed] [Google Scholar]
- 47. Dai, Cheng‐En , Li Hai‐Long, He Xiao‐Ping, Zheng Fen‐Fen, Zhu Hua‐Liu, Liu Liang‐Feng, and Du Wei. 2018. “Research Advance in Metabolism of Effective Ingredients from Traditional Chinese Medicines by Probiotics.” China Journal of Chinese Materia Medica 43: 31–8. [DOI] [PubMed] [Google Scholar]
- 48. Shim, Ki‐Shuk , Kim Taesoo, Ha Hyunil, Lee Kwang Jin, Cho Chang‐Won, Kim Han Sung, Seo Dong‐Hyun, and Ma Jin Yeul. 2013. “ Lactobacillus Fermentation Enhances the Inhibitory Effect of Hwangryun‐haedok‐tang in an Ovariectomy‐induced Bone Loss.” BMC Complementary and Alternative Medicine 13: 106. 10.1186/1472-6882-13-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weon, Jin Bae , Yun Bo‐Ra, Lee Jiwoo, Eom Min Rye, Ko Hyun‐Jeong, Kim Ji Seon, Lee Hyeon Yong, et al. 2013. “Effect of Codonopsis lanceolata with Steamed and Fermented Process on Scopolamine‐induced Memory Impairment in Mice.” Biomolecules & Therapeutics 21: 405–10. 10.4062/biomolther.2013.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Okamoto, Tomoko , Sugimoto Sachiko, Noda Masafumi, Yokooji Tomoharu, Danshiitsoodol Narandalai, Higashikawa Fumiko, and Sugiyama Masanori. 2020. “Interleukin‐8 Release Inhibitors Generated by Fermentation of Artemisia princeps Pampanini Herb Extract with Lactobacillus plantarum SN13T.” Frontiers in Microbiology 11: 1159. 10.3389/fmicb.2020.01159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin, Yu‐Wei , Mou Yu‐Chen, Su Chen‐Chiang, and Chiang Been‐Huang. 2010. “Antihepatocarcinoma Activity of Lactic Acid Bacteria Fermented Panax Notoginseng .” Journal of Agricultural and Food Chemistry 58: 8528–34. 10.1021/jf101543k [DOI] [PubMed] [Google Scholar]
- 52. Qiao, Hongxing , Zhang Xiaojing, Shi Hongtao, Song Yuzhen, Bian Chuanzhou, and Guo Aizhen. 2018. “Assessment of the Physicochemical Properties and Bacterial Composition of Lactobacillus plantarum and Enterococcus faecium‐fermented Astragalus membranaceus Using Single Molecule, Real‐time Sequencing Technology.” Scientific Reports 8: 11862. 10.1038/s41598-018-30288-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jin, Shuang , Luo Meng, Wang Wei, Zhao Chunjian, Gu Chengbo, Li Chunying, Zu Yuangang, Fu Yujie, and Guan Yue. 2013. “Biotransformation of Polydatin to Resveratrol in Polygonum cuspidatum Roots by Highly Immobilized Edible Aspergillus niger and Yeast.” Bioresource Technology 136: 766–70. 10.1016/j.biortech.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 54. Sheih, I‐Chuan , Fang Tony J., Wu Tung‐Kung, Chang Cheng‐Hsiang, and Chen Ru‐Yin. 2011. “Purification and Properties of a Novel Phenolic Antioxidant from Radix astragali Fermented by Aspergillus oryzae M29.” Journal of Agricultural and Food Chemistry 59: 6520–5. 10.1021/jf2011547 [DOI] [PubMed] [Google Scholar]
- 55. Ryu, Jae Sik , Lee Hyun Jung, Bae Song Hwan, Kim Sun Young, Park Yooheon, Suh Hyung Joo, and Jeong Yoon Hwa. 2013. “The Bioavailability of Red Ginseng Extract Fermented by Phellinus linteus .” Journal of Ginseng Research 37: 108–16. 10.5142/jgr.2013.37.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee, Hyun‐Sun , Kim Mi‐Ryung, Park Yooheon, Park Hyo Jung, Chang Un Jae, Kim Sun Young, and Suh Hyung Joo. 2012. “Fermenting Red Ginseng Enhances its Safety and Efficacy as a Novel Skin Care Anti‐aging Ingredient: In Vitro and Animal Study.” Journal of Medicinal Food 15: 1015–23. 10.1089/jmf.2012.2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jung, Jieun , Jang Hye Ji, Eom Su Jin, Choi Nam Soon, Lee Na‐Kyoung, and Paik Hyun‐Dong. 2019. “Fermentation of Red Ginseng Extract by the Probiotic Lactobacillus plantarum KCCM 11613P: Ginsenoside Conversion and Antioxidant Effects.” Journal of Ginseng Research 43: 20–6. 10.1016/j.jgr.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bae, Chu Hyun , Kim Jisoo, Nam Woo, Kim Hyeonji, Kim Jooyun, Nam Bora, Park Soodong, Lee Junglyoul, and Sim Jaehun. 2021. “Fermented Red Ginseng Alleviates Ovalbumin‐induced Inflammation in Mice by Suppressing Interleukin‐4 and Immunoglobulin E Expression.” Journal of Medicinal Food 24: 569–76. 10.1089/jmf.2020.4854 [DOI] [PubMed] [Google Scholar]
- 59. Kim, Hoon , Suh Hyung Joo, Kang Choong‐Min, Lee Kyung‐Haeng, Hwang Jong‐Hyun, and Yu Kwang‐Won. 2014. “Immunological Activity of Ginseng is Enhanced by Solid‐state Culture with Ganoderma lucidum Mycelium.” Journal of Medicinal Food 17: 150–60. 10.1089/jmf.2013.3063 [DOI] [PubMed] [Google Scholar]
- 60. Nan, Bo , Yanlong Liu, You Ying, Li Wancong, Fan Jingjing, Wang Yushan, Piao Chunhong, et al. 2018. “Protective Effects of Enhanced Minor Ginsenosides in Lactobacillus fermentum KP‐3‐fermented Ginseng in Mice Fed a High Fat Diet.” Food & Function 9: 6020–8. 10.1039/c8fo01056k [DOI] [PubMed] [Google Scholar]
- 61. Tian, Wenni , Dai Liwei, Lu Siming, Luo Zhifeng, Qiu Ziyou, Li Junjian, Li Pan, and Du Bing. 2019. “Effect of Bacillus sp. DU‐106 Fermentation on Dendrobium officinale Polysaccharide: Structure and Immunoregulatory Activities.” International Journal of Biological Macromolecules 135: 1034–42. 10.1016/j.ijbiomac.2019.05.203 [DOI] [PubMed] [Google Scholar]
- 62. Liu, Yang , Jin Shunyi, Chang Juan, Wang Ping, Liu Chaoqi, Yin Qingqiang, Gao Tianzeng, Zhu Qun, and Lu Fushan. 2017. “Changes of Active Ingredients Before and After Compound Probiotic Fermented Chinese Herbs.” Journal of Anhui Agricultural Sciences 45: 123–5. [Google Scholar]
- 63. Seong, Joon Seob , Xuan Song Hua, Park So Hyun, Lee Keon Soo, Park Young Min, and Park Soo Nam. 2017. “Antioxidative and Antiaging Activities and Component Analysis of Lespedeza cuneata G. Don Extracts Fermented with Lactobacillus pentosus .” Journal of Microbiology and Biotechnology 27: 1961–70. 10.4014/jmb.1706.06028 [DOI] [PubMed] [Google Scholar]
- 64. Wen, Kuo‐Ching , Lin Shiuan‐Pey, Yu Chung‐Ping, and Chiang Hsiu‐Mei. 2010. “Comparison of Puerariae Radix and Its Hydrolysate on Stimulation of Hyaluronic Acid Production in NHEK Cells.” The American Journal of Chinese Medicine 38: 143–55. 10.1142/s0192415x10007725 [DOI] [PubMed] [Google Scholar]
- 65. Kwon, Ha‐Kyoung , Jo Woo‐Ri, and Park Hye‐Jin. 2018. “Immune‐enhancing Activity of C. militaris Fermented with Pediococcus pentosaceus (GRC‐ON89A) in CY‐induced Immunosuppressed Model.” BMC Complementary and Alternative Medicine 18: 75. 10.1186/s12906-018-2133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ismail, Hassan Fahmi , Hashim Zanariah, Soon Wong Tet, Rahman Nur Syukriah Ab, Zainudin Ain Nabihah, and Majid Fadzilah Adibah Abdul. 2017. “Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo.” Journal of traditional and complementary medicine 7: 452–65. 10.1016/j.jtcme.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li, Kun , Xiaowen Li, Weimin He, Yuncai Xiao, Xiliang Wang, Dingren Bi, Peng Li, Jiaxiang Wang, and Zutao Zhou. 2017. “Application of Microecological Preparations of Chinese Medicine in Animal Production.” Animal Husbandry & Veterinary Medicine 49: 128–33. [Google Scholar]
- 68. Yang, Hye Jeong , Kwon Dae Young, Moon Na Rang, Jung Kim Min, Kang Hee Joo, Jung Do Yeon, and Park Sunmin. 2013. “Soybean Fermentation with Bacillus licheniformis Increases Insulin Sensitizing and Insulinotropic Activity.” Food & Function 4: 1675–84. 10.1039/c3fo60198f [DOI] [PubMed] [Google Scholar]
- 69. Ma, Chao , Hu Shan, Li Xueru, Zhang Bo, and Meng Tao. 2013. “Study on Conversion of Conjugated Anthraquinone in Radix et Rhizoma Rhei by Yeast Strain.” World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica 15: 1333–7. [Google Scholar]
- 70. Liu, Chunmei , Wu Xiaofeng, Pan Yang, Jiang Yaping, and Zhang Xian. 2011. “Comparison of Toxic Ingredient Contents of Fermented Croton tiglium, Crude C. Tiglium and Defatted C. Tiglium Seed Powder.” China Pharmacy 22: 4071–4. [Google Scholar]
- 71. He, Luanying , Lin Zichun, Lu Jiandong, Xiong Guoliang, and Wang Shihui. 2021. “Detoxification and Sustained Effects of Tripterygium wilfordii Based on Ganoderma lucidum Bi‐directional Solid Fermentation.” Journal of Beijing University of Chemical Technology (Natural Science) 48: 48–56. [Google Scholar]
- 72. Zhao, Jing , Duan Zhiguang, Ma Xiaoxuan, Liu Yannan, and Fan Daidi. 2021. “Recent Advances in Systemic and Local Delivery of Ginsenosides Using Nanoparticles and Nanofibers.” Chinese Journal of Chemical Engineering 30: 291–300. 10.1016/j.cjche.2020.11.012 [DOI] [Google Scholar]
- 73. Kim, Jae Hwan , Doo Eun‐Hee, Jeong Minju, Kim Seungil, Lee Yun‐Yeol, Yang Jaesik, Lee Ji Su, et al. 2019. “Enhancing Immunomodulatory Function of Red Ginseng Through Fermentation Using Bifidobacterium animalis subsp. lactis LT 19‐2.” Nutrients 11: 1481. 10.3390/nu11071481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim, Jeon‐Kyung , Kim Jae‐Young, Jang Se‐Eun, Choi Min‐Sun, Jang Hyo‐Min, Yoo Hae‐Hyun, and Kim Dong‐Hyun. 2018. “Fermented Red Ginseng Alleviates Cyclophosphamide‐induced Immunosuppression and 2,4,6‐Trinitrobenzenesulfonic Acid‐induced Colitis in Mice by Regulating Macrophage Activation and T Cell Differentiation.” The American Journal of Chinese Medicine 46: 1879–97. 10.1142/s0192415x18500945 [DOI] [PubMed] [Google Scholar]
- 75. Tan, Joanne Sh , Yeo Chia‐Rou, and Popovich David G. 2017. “Fermentation of Protopanaxadiol Type Ginsenosides (PD) with Probiotic Bifidobacterium lactis and Lactobacillus rhamnosus .” Applied Microbiology and Biotechnology 101: 5427–37. 10.1007/s00253-017-8295-4 [DOI] [PubMed] [Google Scholar]
- 76. Shao, Yiwen , Kang Qiaozhen, Zhu Jiaqing, Zhao Changcheng, Hao Limin, Huang Jinyong, Lu Jike, Jia Shiru, and Yi Juanjuan. 2022. “Antioxidant Properties and Digestion Behaviors of Polysaccharides from Chinese Yam Fermented by Saccharomyces boulardii .” LWT 154: 112752. 10.1016/j.lwt.2021.112752 [DOI] [Google Scholar]
- 77. Kim, Chul‐Joong , Ryu Hyeon‐Yeol, Lee Somin, Lee Han‐Joo, Chun Yoon‐Soek, Kim Jong‐Kyu, Yu Chang‐Yeon, Ghimire Bimal Kumar, and Lee Jae‐Geun. 2021. “Neuroprotective Effect and Antioxidant Potency of Fermented Cultured Wild Ginseng Root Extracts of Panax ginseng C.A. Meyer in Mice.” Molecules 26: 3001. 10.3390/molecules26103001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li, Weina , and Fan Daidi. 2020. “Biocatalytic Strategies for the Production of Ginsenosides Using Glycosidase: Current State and Perspectives.” Applied Microbiology and Biotechnology 104: 3807–23. 10.1007/s00253-020-10455-9 [DOI] [PubMed] [Google Scholar]
- 79. Wang, Xiaoguo . 2019. “Resource Utilization of Herb Medicine Residues.” China Resources Comprehensive Utilization 37: 81–4. [Google Scholar]
- 80. Kong, Wenping , Huang Chengshuang, Shi Jie, Li Yu, Jiang Xinxin, Duan Quwen, Huang Yong, Duan Yanwen, and Zhu Xiangcheng. 2019. “Recycling of Chinese Herb Residues by Endophytic and Probiotic Fungus Aspergillus cristatus CB10002 for the Production of Medicinal Valuable Anthraquinones.” Microbial Cell Factories 18: 102. 10.1186/s12934-019-1150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Meng, Fanjing , Yang Shaoguo, Wang Xin, Chen Tingtao, Wang Xiaolei, Tang Xianyao, Zhang Rongji, and Shen Liang. 2017. “Reclamation of Chinese Herb Residues Using Probiotics and Evaluation of Their Beneficial Effect on Pathogen Infection.” Journal of Infection and Public Health 10: 749–54. 10.1016/j.jiph.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 82. Hou, Haifeng , and Li Qian. 2018. “Effects of Fermented Chinese Herb Residues on Growth Performance, Serum Biochemical Parameters, Antioxidant Indexes and Immune Function of Weaned Piglets.” China Animal Husbandry & Veterinary Medicine 45: 947–52. [Google Scholar]
- 83. Wang, Jing‐Hua , Bose Shambhunath, Kim Hyung‐Gu, Han Kyung‐Sun, and Kim Hojun. 2015. “Fermented Rhizoma Atractylodis macrocephalae Alleviates High Fat Diet‐induced Obesity in Association with Regulation of Intestinal Permeability and Microbiota in Rats.” Scientific Reports 5: 8391. 10.1038/srep08391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ng, Chang‐Chai , Wang Chung‐Yi, Wang Ya‐Ping, Tzeng Wen‐Sheng, and Shyu Yuan‐Tay. 2011. “Lactic Acid Bacterial Fermentation on the Production of Functional Antioxidant Herbal Anoectochilus formosanus Hayata.” Journal of Bioscience and Bioengineering 111: 289–93. 10.1016/j.jbiosc.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 85. Su, Le , Su Yue, An Zaiyong, Zhang Ping, Yue Qiulin, Zhao Chen, and Sun Xin, et al. 2021. “Fermentation Products of Danshen Relieved Dextran Sulfate Sodium‐induced Experimental Ulcerative Colitis in Mice.” Scientific Reports 11: 16210. 10.1038/s41598-021-94594-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee, Jung‐Jin , Kwon Hyeeun, Lee Ji‐Hye, Kim Dong‐Gun, Jung Sang‐Hyuk, and Ma Jin Yeul. 2014. “Fermented Soshiho‐tang with Lactobacillus plantarum Enhances the Antiproliferative Activity in Vascular Smooth Muscle Cell.” BMC Complementary and Alternative Medicine 14: 78. 10.1186/1472-6882-14-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim, Aeyung , Im Minju, Hwang Youn‐Hwan, Yang Hye Jin, and Ma Jin Yeul. 2015. “Jaeumganghwa‐tang Induces Apoptosis Via the Mitochondrial Pathway and Lactobacillus Fermentation Enhances Its Anti‐cancer Activity in HT1080 Human Fibrosarcoma Cells.” PLoS ONE 10: e0127898. 10.1371/journal.pone.0127898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shim, Ki‐Shuk , Kim Taesoo, Ha Hyunil, Cho Chang‐Won, Kim Han Sung, Seo Dong‐Hyun, and Ma Jin Yeul. 2012. “Hwangryun‐haedok‐tang Fermented with Lactobacillus casei Suppresses Ovariectomy‐induced Bone Loss.” Evidence‐Based Complementary and Alternative Medicine 2012: 325791. 10.1155/2012/325791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee, Myung‐Hee , Lee Young‐Chul, Kim Sung‐Soo, Hong Hee‐Do, and Kim Kyung‐Tack. 2015. “Quality and Antioxidant Activity of Ginseng Seed Processed by Fermentation Strains.” Journal of Ginseng Research 39: 178–82. 10.1016/j.jgr.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bose, Shambhunath , and Kim Hojun. 2013. “Evaluation of In Vitro Anti‐inflammatory Activities and Protective Effect of Fermented Preparations of Rhizoma Atractylodis macrocephalae on Intestinal Barrier Function Against Lipopolysaccharide Insult.” Evidence‐Based Complementary and Alternative Medicine 2013: 363076. 10.1155/2013/363076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li, Jing , Wang Juan, Li Jinxin, Liu Dahui, Li Hongfa, Gao Wenyuan, Li Jianli, and Liu Shujie. 2016. “ Aspergillus niger Enhance Bioactive Compounds Biosynthesis as well as Expression of Functional Genes in Adventitious Roots of Glycyrrhiza uralensis Fisch.” Applied Biochemistry and Biotechnology 178: 576–93. 10.1007/s12010-015-1895-5 [DOI] [PubMed] [Google Scholar]
- 92. Du, Chenhui , Yan Yan, Feng Qianjin, and Song Qiang. 2016. “Research on Levels of Total Flavonoids and Total Alkaloids in Gegen Qinlian Decoction Before and After Fermentation.” China Journal of Traditional Chinese Medicine Pharmacy 31: 4850–3. [Google Scholar]
- 93. Son, Hyeong‐U , Lee Seul, Heo Jin‐Chul, and Lee Sang‐Han. 2017. “The Solid‐state Fermentation of Artemisia capillaris Leaves with Ganoderma lucidum Enhances the Anti‐inflammatory Effects in a Model of Atopic Dermatitis.” International Journal of Molecular Medicine 39: 1233–41. 10.3892/ijmm.2017.2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang, Guey‐Horng , Chen Chih‐Yu, Tsai Teh‐Hua, Chen Ching‐Kuo, Cheng Chiu‐Yu, Huang Yi‐Hsin, Hsieh Min‐Chi, and Chung Ying‐Chien. 2017. “Evaluation of Tyrosinase Inhibitory and Antioxidant Activities of Angelica dahurica Root Extracts for Four Different Probiotic Bacteria Fermentations.” Journal of Bioscience and Bioengineering 123: 679–84. 10.1016/j.jbiosc.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 95. Goluch, Zuzanna Sabina , Rybarczyk Artur, Drozd Arleta, and Drozd Radosław. 2022. “Fatty Acid Profile and Lipid Indices of the Porker Meat Supplemented with Pro‐health Herbal Probiotics, Ascorbic Acid and Allicin.” British Food Journal 124: 3841–54. 10.1108/BFJ-09-2021-0972 [DOI] [Google Scholar]
- 96. Liu, Meiling , Zhang Xiuxia, Hao Yunpeng, Ding Jinhua, Shen Jing, Xue Ziyu, Qi Wei, et al. 2019. “Protective Effects of a Novel Probiotic Strain, Lactococcus lactis ML2018, in Colitis: In Vivo and In Vitro Evidence.” Food & Function 10: 1132–45. [DOI] [PubMed] [Google Scholar]
- 97. Mabwi, Humphrey A. , Kim Eunjung, Song Dae‐Geun, Yoon Hyo Shin, Pan Cheol‐Ho, Komba Erick V. G., Ko GwangPyo, and Cha Kwang Hyun. 2021. “Synthetic Gut Microbiome: Advances and Challenges.” Computational and Structural Biotechnology Journal 19: 363–71. 10.1016/j.csbj.2020.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chung, Yerim , Park Ji‐Young, Lee Ji‐Eun, Kim Kee‐Tae, and Paik Hyun‐Dong. 2021. “Antioxidant Activity and Inhibitory Effect on Nitric Oxide Production of Hydroponic Ginseng Fermented with Lactococcus lactis KC24.” Antioxidants 10: 1614. 10.3390/antiox10101614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Park, Yooheon , Choi Hyeon‐Son, Lee Hyun‐Sun, and Suh Hyung Joo. 2015. “Hematopoietic Effect of Deer Antler Extract Fermented by Bacillus subtilis on Murine Marrow Cells.” Nutrition Research and Practice 9: 451–8. 10.4162/nrp.2015.9.5.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Xie, Guo , Guo Bian‐Qin, Li Xiao‐Min, Liu Shuai, Liu Hong‐Xia, and Wang Yong‐Zhong. 2021. “Enhancement of Biotransformation of Ginsenosides in White Ginseng Roots by Aerobic Co‐cultivation of Bacillus subtilis and Trichoderma reesei .” Applied Microbiology and Biotechnology 105: 8265–76. 10.1007/s00253-021-11631-1 [DOI] [PubMed] [Google Scholar]
- 101. Chen, Yuh Shuen , Liou Hua‐Chian, and Chan Chin Feng. 2013. “Tyrosinase Inhibitory Effect and Antioxidative Activities of Fermented and Ethanol Extracts of Rhodiola rosea and Lonicera japonica .” The Scientific World Journal 2013: 1–5. 10.1155/2013/612739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Im, A‐Rang , Song Jae Hyoung, Lee Mi Young, Yeon Sung Hum, Um Key An, and Chae Sungwook. 2014. “Anti‐wrinkle Effects of Fermented and Non‐fermented Cyclopia Intermedia in Hairless Mice.” BMC Complementary and Alternative Medicine 14: 424. 10.1186/1472-6882-14-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bose, Shambhunath , Jeon Songhee, Eom Taewoong, Song Mi‐young, and Kim Hojun. 2012. “Evaluation of the In Vitro and In Vivo Protective Effects of Unfermented and Fermented Rhizoma coptidis Formulations Against Lipopolysaccharide Insult.” Food Chemistry 135: 452–9. 10.1016/j.foodchem.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 104. Liu, Ziyao , Tang Yun, Zhou Rongrong, Shi Xiaosa, Zhang Hongmei, Liu Tengfei, Lian Zenglin, and Shi Xinyuan. 2018. “Bi‐directional Solid Fermentation Products of Trametes robiniophila Murr with Radix isatidis Inhibit Proliferation and Metastasis of Breast Cancer Cells.” Journal of the Chinese Medical Association 81: 520–30. 10.1016/j.jcma.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 105. Wang, Na , Wu Tianxiang, Zhang Yong, Xu Xiaobao, Tan Sha, and Fu Hongwei. 2013. “Experimental Analysis on the Main Contents of Rhizoma gastrodiae Extract and Inter‐transformation Throughout the Fermentation Process of Grifola frondosa .” Archives of Pharmacal Research 36: 314–21. 10.1007/s12272-013-0029-2 [DOI] [PubMed] [Google Scholar]
- 106. Han, Chunchao , Wei Hong, and Guo Jianyou. 2011. “Anti‐inflammatory Effects of Fermented and Non‐fermented Sophora flavescens: A Comparative Study.” BMC Complementary and Alternative Medicine 11: 100. 10.1186/1472-6882-11-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Garrido‐Galand, Sara , Asensio‐Grau Andrea, Calvo‐Lerma Joaquim, Heredia Ana, and Andrés Ana. 2021. “The Potential of Fermentation on Nutritional and Technological Improvement of Cereal and Legume Flours: A Review.” Food Research International 145: 110398. 10.1016/j.foodres.2021.110398 [DOI] [PubMed] [Google Scholar]
- 108. Cano y Postigo, Luis O. , Jacobo‐Velázquez Daniel A., Guajardo‐Flores Daniel, Garcia Amezquita Luis Eduardo, and García‐Cayuela Tomás. 2021. “Solid‐state Fermentation for Enhancing the Nutraceutical Content of Agrifood By‐products: Recent Advances and Its Industrial Feasibility.” Food Bioscience 41: 100926. 10.1016/j.fbio.2021.100926 [DOI] [Google Scholar]
- 109. Arora, Sidharth , Rani Richa, and Ghosh Sanjoy. 2018. “Bioreactors in Solid State Fermentation Technology: Design, Applications and Engineering Aspects.” Journal of Biotechnology 269: 16–34. 10.1016/j.jbiotec.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 110. Yang, Lijie , Zeng Xiangfang, and Qiao Shiyan. 2021. “Advances in Research on Solid‐state Fermented Feed and Its Utilization: The Pioneer of Private Customization for Intestinal Microorganisms.” Animal Nutrition 7: 905–16. 10.1016/j.aninu.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Atanasov, Atanas G. , Zotchev Sergey B., Dirsch Verena M., The International Natural Product Sciences Taskforce , Supuran Claudiu T.. 2021. “Natural Products in Drug Discovery: Advances and Opportunities.” Nature Reviews Drug Discovery 20: 200–16. 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhuang, Yi , Hong Jing, and Xu Youling. 2009. “Fungal Medicine in the Traditional Chinese Medicine.” World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica 11: 777–82. [Google Scholar]
- 113. Zou, Gen , Li Bo, Wang Ying, Yin Xin, Gong Ming, Shang Junjun, Wei Yongjun, Li Xiaoling, and Bao Dapeng. 2021. “Efficient Conversion of Spent Mushroom Substrate into a High Value‐added Anticancer Drug Pentostatin with Engineered Cordyceps militaris .” Green Chemistry 23: 10030–8. 10.1039/D1GC03594K [DOI] [Google Scholar]
- 114. Xin, Yanhua , Liang Bin, Wang Yingxia, Liu Yang, and Bai Xiaojing. 2017. “Optimization of the Ganoderma lucidum–Ginkgo biloba Bi‐directional Liquid Fermentation Condition and Antioxidation Properties of Its Products.” Mycosystema 36: 1427–35. 10.13346/j.mycosystema.170020 [DOI] [Google Scholar]
- 115. Zou, Gen , Nielsen Jens B., and Wei Yongjun. 2022. “Harnessing Synthetic Biology for Mushroom Farming.” Trends in Biotechnology. 10.1016/j.tibtech.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 116. Ai, Su , Tang Wei, Guo Ruolin, Li Jiqian, Yang Wu, and He Zengguo. 2019. “Research Progress on Chinese Herbal Medicine Fermentation and Profile of Active Substances Derived.” China Journal of Chinese Materia Medica 44: 1110–8. [DOI] [PubMed] [Google Scholar]
- 117. Cao, Hui , Chen Xiaoqing, Jassbi Amir Reza, and Xiao Jianbo. 2015. “Microbial Biotransformation of Bioactive Flavonoids.” Biotechnology Advances 33: 214–23. 10.1016/j.biotechadv.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 118. Son, Chang‐Gue , Lee Sam‐Keun, Choi In‐Kyu, Jang Eun‐Su, and Bang Kee‐Jung. 2020. “Herbal Transformation by Fermentation.” Journal of Acupuncture and Meridian Studies 13: 167–8. 10.1016/j.jams.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 119. Palachum, Wilawan , Chisti Yusuf, and Choorit Wanna. 2018. “ In‐vitro Assessment of Probiotic Potential of Lactobacillus plantarum WU‐P19 Isolated from a Traditional Fermented Herb.” Annals of Microbiology 68: 79–91. 10.1007/s13213-017-1318-7 [DOI] [Google Scholar]
- 120. Mohammed, Sarhan , and Çon Ahmet Hilmi. 2021. “Isolation and Characterization of Potential Probiotic Lactic Acid Bacteria from Traditional Cheese.” LWT 152: 112319. 10.1016/j.lwt.2021.112319 [DOI] [Google Scholar]
- 121. Cunningham, Marla , Azcarate‐Peril M. Andrea, Barnard Alan, Benoit Valerie, Grimaldi Roberta, Guyonnet Denis, Holscher Hannah D., et al. 2021. “Shaping the Future of Probiotics and Prebiotics.” Trends in Microbiology 29: 667–85. 10.1016/j.tim.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 122. Albayrak, Çisem Bulut , and Duran Mustafa. 2021. “Isolation and Characterization of Aroma Producing Lactic Acid Bacteria from Artisanal White Cheese for Multifunctional Properties.” LWT 150: 112053. 10.1016/j.lwt.2021.112053 [DOI] [Google Scholar]
- 123. Ilango, Shankar , and Antony Usha. 2021. “Probiotic Microorganisms from Non‐dairy Traditional Fermented Foods.” Trends in Food Science & Technology 118: 617–38. [Google Scholar]
- 124. Liu, Hai , Jiang Shan, Ou Jintao, Tang Jinfeng, Lu Yang, and Wei Yongjun. 2022. “Investigation of Soil Microbiota Reveals Variable Dominant Species at Different Land Areas in China.” Biotechnology & Biotechnological Equipment 36: 245–55. 10.1080/13102818.2022.2071634 [DOI] [Google Scholar]
- 125. Liu, Sijia , Moon Christina D., Zheng Nan, Huws Sharon, Zhao Shengguo, and Wang Jiaqi. 2022. “Opportunities and Challenges of Using Metagenomic Data to Bring Uncultured Microbes into Cultivation.” Microbiome 10: 76. 10.1186/s40168-022-01272-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang, Yudie , Qu Lingbo, Mijakovic Ivan, and Wei Yongjun. 2022. “Advances in the Human Skin Microbiota and Its Roles in Cutaneous Diseases.” Microbial Cell Factories 21: 176. 10.1186/s12934-022-01901-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cao, Guoqiong , Ma Fengang, Xu Jian, and Zhang Yongping. 2020. “Microbial Community Succession and Toxic Alkaloids Change During Fermentation of Huafeng Dan Yaomu.” Letters in Applied Microbiology 70: 318–25. 10.1111/lam.13276 [DOI] [PubMed] [Google Scholar]
- 128. Li, Qin , Huang Jianan, Li Yongdi, Zhang Yiyang, Luo Yu, Chen Yuan, Lin Haiyan, Wang Kunbo, and Liu Zhonghua. 2017. “Fungal Community Succession and Major Components Change During Manufacturing Process of Fu Brick Tea.” Scientific Reports 7: 6947. 10.1038/s41598-017-07098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pang, Xiao‐Na , Han Bei‐Zhong, Huang Xiao‐Ning, Zhang Xin, Hou Lin‐Feng, Cao Ming, Gao Li‐Juan, Hu Guang‐Hui, and Chen Jing‐Yu. 2018. “Effect of the Environment Microbiota on the Flavour of Light‐flavour Baijiu During Spontaneous Fermentation.” Scientific Reports 8: 3396. 10.1038/s41598-018-21814-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lagier, Jean‐Christophe , Dubourg Grégory, Million Matthieu, Cadoret Frédéric, Bilen Melhem, Fenollar Florence, Levasseur Anthony, et al. 2018. “Culturing the Human Microbiota and Culturomics.” Nature Reviews Microbiology 16: 540–50. 10.1038/s41579-018-0041-0 [DOI] [PubMed] [Google Scholar]
- 131. O'Toole, Paul W. , Marchesi Julian R., and Hill Colin. 2017. “Next‐generation Probiotics: the Spectrum from Probiotics to Live Biotherapeutics.” Nature Microbiology 2: 17057. 10.1038/nmicrobiol.2017.57 [DOI] [PubMed] [Google Scholar]
- 132. Matar, Ghassan , and Bilen Melhem. 2022. “Culturomics, a Potential Approach Paving the Way Toward Bacteriotherapy.” Current Opinion in Microbiology 69: 102194. 10.1016/j.mib.2022.102194 [DOI] [PubMed] [Google Scholar]
- 133. Kim, Dong‐Hyun . 2018. “Gut Microbiota‐mediated Pharmacokinetics of Ginseng Saponins.” Journal of Ginseng Research 42: 255–63. 10.1016/j.jgr.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jiang, Yunyun , Li Weina, and Fan Daidi. 2021. “Biotransformation of Ginsenoside Rb1 to Ginsenoside CK by Strain XD101: A Safe Bioconversion Strategy.” Applied Biochemistry and Biotechnology 193: 2110–27. 10.1007/s12010-021-03485-0 [DOI] [PubMed] [Google Scholar]
- 135. Sakurama, Haruko , Kishino Shigenobu, Uchibori Yoshie, Yonejima Yasunori, Keiko Hisashi, Satomi Kita, Jun Takahashi, and Ashida Ogawa. 2014. “β‐Glucuronidase from Lactobacillus brevis Useful for Baicalin Hydrolysis Belongs to Glycoside Hydrolase Family 30.” Applied Microbiology and Biotechnology 98: 4021–32. 10.1007/s00253-013-5325-8 [DOI] [PubMed] [Google Scholar]
- 136. Liang, Jiawei , Mai Wenning, Wang Jia, Li Xiaoqi, Su Minhua, Du Jiaxu, Wu Yanwei, et al. 2021. “Performance and Microbial Communities of a Novel Integrated Industrial‐scale Pulp and Paper Wastewater Treatment Plant.” Journal of Cleaner Production 278: 123896. 10.1016/j.jclepro.2020.123896 [DOI] [Google Scholar]
- 137. Miao, Qin , Zhang Xiaoling, Wang Yitong, Li Xiaoqi, Wang Zheng, Tian Lingmin, Qu Lingbo, and Wei Yongjun. 2022. “Characterization of Novel Pectinolytic Enzymes Derived from the Efficient Lignocellulose Degradation Microbiota.” Biomolecules 12: 1388. 10.3390/biom12101388 [DOI] [PMC free article] [PubMed] [Google Scholar]; https://www.mdpi.com/2218-273X/12/10/1388
- 138. Wang, Jia , Liang Jiawei, Li Yonghong, Tian Lingmin, and Wei Yongjun. 2021. “Characterization of Efficient Xylanases from Industrial‐scale Pulp and Paper Wastewater Treatment Microbiota.” AMB Express 11: 19. 10.1186/s13568-020-01178-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gao, Qiyu , Wang Luan, Zhang Maosen, Wei Yongjun, and Lin Wei. 2020. “Recent Advances on Feasible Strategies for Monoterpenoid Production in Saccharomyces cerevisiae .” Frontiers in Bioengineering and Biotechnology 8: 609800. 10.3389/fbioe.2020.609800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wang, Chunqing , Liu Xiaolong, Zhang Mengle, Shao Haoyue, Zhang Manman, Wang Xiaomeng, Wang Qinghua, et al. 2019. “Efficient Enzyme‐assisted Extraction and Conversion of Polydatin to Resveratrol from Polygonum cuspidatum Using Thermostable Cellulase and immobilized β‐Glucosidase.” Frontiers in Microbiology 10: 445. 10.3389/fmicb.2019.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hu, Yongfei , Liu Dan, Jin Xiaolu, Feng Yuqing, and Guo Yuming. 2023. “Synthetic Microbiome for a Sustainable Poultry Industry.” The Innovation 4: 100357. 10.1016/j.xinn.2022.100357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wei, Yongjun , Bergenholm David, Gossing Michael, Siewers Verena, and Nielsen Jens. 2018. “Expression of Cocoa Genes in Saccharomyces cerevisiae Improves Cocoa Butter Production.” Microbial Cell Factories 17: 11. 10.1186/s12934-018-0866-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Wei, Yongjun , Ji Boyang, Siewers Verena, Xu Deyang, Halkier Barbara Ann, and Nielsen Jens. 2019. “Identification of Genes Involved in Shea Butter Biosynthesis from Vitellaria paradoxa Fruits Through Transcriptomics and Functional Heterologous Expression.” Applied Microbiology and Biotechnology 103: 3727–36. 10.1007/s00253-019-09720-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Jiang, Yuguo , Ma Jiangfan, Wei Yongjun, Liu Yining, Zhou Zhihua, Huang Yongping, Wang Pingping, and Yan Xing. 2022. “ De Novo Biosynthesis of Sex Pheromone Components of Helicoverpa armigera Through an Artificial Pathway in Yeast.” Green Chemistry 24: 767–78. 10.1039/D1GC02965G [DOI] [Google Scholar]
- 145. Zhang, Xiaoling , Miao Qin, Xu Xia, Ji Boyang, Qu Lingbo, and Wei Yongjun. 2021. “Developments in Fatty Acid‐derived Insect Pheromone Production Using Engineered Yeasts.” Frontiers in Microbiology 12: 759975. 10.3389/fmicb.2021.759975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Guan, Ruobing , Wang Mengge, Guan Zhonghua, Jin Chengyun, Lin Wei, Ji Xiaojun, and Wei Yongjun. 2020. “Metabolic Engineering for Glycyrrhetinic Acid Production in Saccharomyces cerevisiae .” Frontiers in Bioengineering and Biotechnology 8: 588255. 10.3389/fbioe.2020.588255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ma, Yirong , Li Wenjuan, Mai Jie, Wang Jinpeng, Wei Yongjun, Ledesma‐Amaro Rodrigo, and Ji Xiaojun. 2020. “Engineering Yarrowia lipolytica for Sustainable Production of the Chamomile Sesquiterpene (‐)‐α‐Bisabolol.” Green Chemistry 2: 23. 10.1039/D0GC03180A [DOI] [Google Scholar]
- 148. Peng, Jinjin , Wang Luan, Wang Mengge, Du Rui, Qin Shangshang, Jin Chengyun, and Wei Yongjun. 2021. “Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides.” Molecules 26: 1641. 10.3390/molecules26061641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang, Jinpeng , Ledesma‐Amaro Rodrigo, Wei Yongjun, Ji Boyang, and Ji Xiao‐Jun. 2020. “Metabolic Engineering for Increased Lipid Accumulation in Yarrowia lipolytica—A Review.” Bioresource Technology 313: 123707. 10.1016/j.biortech.2020.123707 [DOI] [PubMed] [Google Scholar]
- 150. Wang, Mengge , Wei Yongjun, Ji Boyang, and Nielsen Jens. 2020. “Advances in Metabolic Engineering of Saccharomyces cerevisiae for Cocoa Butter Equivalent Production.” Frontiers in Bioengineering and Biotechnology 8: 594081. 10.3389/fbioe.2020.594081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wei, Yongjun , Ji Boyang, Ledesma‐Amaro Rodrigo, Chen Tao, and Ji Xiao‐Jun. 2021. “Editorial: Engineering Yeast to Produce Plant Natural Products.” Frontiers in Bioengineering and Biotechnology 9: 798097. 10.3389/fbioe.2021.798097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supplementary materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.


