Abstract
Once thought to be sterile, the human lung is now well recognized to harbor a consortium of microorganisms collectively known as the lung microbiome. The lung microbiome is altered in an array of lung diseases, including chronic lung diseases such as chronic obstructive pulmonary disease, asthma, and bronchiectasis, acute lung diseases caused by pneumonia, sepsis, and COVID‐19, and other lung complications such as those related to lung transplantation, lung cancer, and human immunodeficiency virus. The effects of lung microbiome in modulating host immunity and inflammation in the lung and distal organs are being elucidated. However, the precise mechanism by which members of microbiota produce structural ligands that interact with host genes and pathways remains largely uncharacterized. Multiple unique challenges, both technically and biologically, exist in the field of lung microbiome, necessitating the development of tailored experimental and analytical approaches to overcome the bottlenecks. In this review, we first provide an overview of the principles and methodologies in studying the lung microbiome. We next review current knowledge of the roles of lung microbiome in human diseases, highlighting mechanistic insights. We finally discuss critical challenges in the field and share our thoughts on broad topics for future investigation.
Keywords: gut–lung axis, lung microbiome, microbiome–host interaction, respiratory diseases
Highlights
Sputum, bronchoalveolar lavage, bronchial brushing, and lung tissue are the routine sample types for the lung microbiome. Multiomics have been increasingly applied for characterizing the lung microbiome.
Lung microbiome is broadly implicated in chronic and acute lung diseases, lung cancer, and other lung diseases, with potential mechanistic implications.
Current challenges of the lung microbiome studies include low microbial‐to‐host ratio, high disease heterogeneity, and the difficulty in precisely manipulating and culturing the lung microbiome.
Future potential topics for lung microbiome studies include understanding the diagnostic potential of the lung microbiome, its spatial dynamics, its mechanistic interaction with the host, and the crosstalk between the lung and distal organs.
Broad topics of the human lung microbiome are reviewed, including its principles and methodologies, applications to human diseases, mechanistic insights, current challenges, and future potential research avenues.

INTRODUCTION
As the “second genome” of the human body, the human microbiome plays a crucial role in human health and diseases, and has received extensive attention over the past decades [1]. Compared to the topic of gut microbiota, which has dominated the human microbiome studies, much less attention has been paid to the microbiome of the human respiratory tract, partly due to historical consideration of the healthy lung as a sterile organ over a century. The dogma of lung sterility has been overturned with the advent of culture‐independent sequencing techniques that led to the first discovery of a microbial community in the airway by Hilty et al. [2]. The field of lung microbiome has since witnessed exponential growth. Compelling evidence from human studies has demonstrated that the lung microbiome is altered in a broad range of lung diseases, such as chronic lung diseases (i.e., asthma, chronic obstructive pulmonary disease [COPD], bronchiectasis, and idiopathic pulmonary fibrosis [IPF]), acute lung diseases (i.e., pneumonia, sepsis, acute respiratory distress syndrome [ARDS], and COVID‐19), and complications postlung transplantation, human immunodeficiency virus (HIV), tuberculosis, and lung cancer. Emerging animal studies have further revealed a mechanistic implication of the lung microbiome in regulating host pathophysiological processes both locally and distally, together uncovering a hidden link between the lung microbiome and human diseases. Nevertheless, compared to the rapid advancement of gut microbiome studies, the field of lung microbiome is still in its infancy and facing a series of critical challenges stemming from the unique anatomy of the lung and the microbial biomass in the lung that is orders of magnitude lower than that in the gut, necessitating the development of novel approaches tailored for the lung microbiome. Here, we review the broad topic of the human lung or lower respiratory tract microbiome, including its principles and methodologies, applications to human diseases, current challenges, and future potential research avenues, in the hope that this review will serve as a catalyst to stimulate greater interest in the burgeoning field of human lung microbiome.
METHODOLOGIES ON THE LUNG MICROBIOME
Sampling the lung microbiome
Despite sharing most principles established for the gut microbiome in terms of sequencing and data analyses, the lung microbiome has its unique aspects in methodologies particularly with respect to sampling (Figure 1). In essence, it is impractical to directly obtain the human lung tissue unless surgically justified (i.e., lung transplantation, tumor resection). As such, several noninvasive and invasive procedures have been implemented as a surrogate or proxy to sampling the lung environment. Of them, sputum has been one of the most commonly used specimens for studying the airway microbiota, due to its noninvasive nature, which facilitates sample collection particularly for patients with chronic lung diseases who are often able to produce sputum spontaneously. For patients or healthy individuals who are unable to do so, sputum induction using nebulized saline is a routine procedure that is clinically safe and effective [3]. Therefore, sputum remains the most viable option to study the airway microbiome for healthy individuals. However, the extent of sputum samples in representing the lower airways is the subject of debate, given its inherent admixture of materials from upper, lower airways and the oral cavity [4]. As such, a process for separating sputum plugs (the mucous part of a sputum) from saliva, followed by a quality assessment (i.e., via microscopy inspection of the leukocyte/squamous epithelial cell ratio), should be conducted for sputum samples to minimize oral contamination [5]. In addition, the concurrent oral rinse sample from the same individual can be used as a control to assess oral contamination [6].
Figure 1.
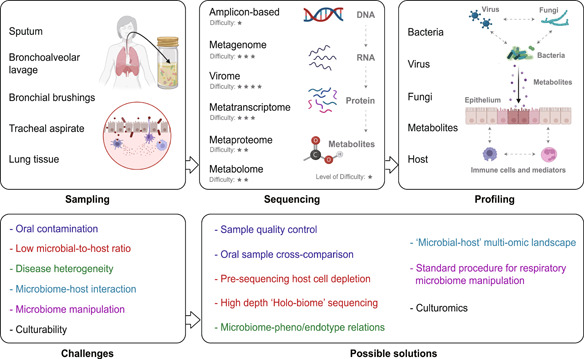
The principles and methodologies of studying the human lung microbiome, including sampling approaches, sequencing strategies, and the microbiome and host profiles that can be obtained. For each type of sequencing, the level of difficulty is scored based on the empirical assessment of technical challenges. The challenges in the field of the lung microbiome as well as possible solutions to address each of them are also shown.
Bronchoalveolar lavage (BAL) is another frequently used approach to sample the lung microbiome. Operated via bronchoscopy, BAL is invasive and more costly and time‐consuming than sputum sampling, posing a challenge for longitudinal sample collection. However, the clear advantage of BAL over sputum lies in its better resemblance to lower airways, with limited upper airway or oral contamination. Other approaches in sampling the lung microbiome include bronchial brushing and tracheal aspirate, which has been applied on a limited basis [7, 8, 9, 10]. Theoretically, lung tissue is the most ideal specimen to study the lung microbiome and has the unique merit in capturing the topographical distribution of microbial communities [11]. However, the inability to obtain lung tissue in most clinical conditions has limited its application only to patients receiving lung resection, cancer‐related surgery, or biopsy.
Sequencing the lung microbiome
The substantially low microbial biomass in the respiratory tract compared to that in gut and oral cavity calls for special attention to sampling, processing, and analysis of the lung microbiome (Figure 1). For sample processing, bacterial genomic DNA is present in reagents used for DNA extraction and polymerase chain reaction (PCR) [12]. This impact of reagents will further magnify when the concentration of the source DNA is low. Therefore, while negative reagent controls have often been neglected for gut microbiome studies, it is a standard practice for all lung microbiome studies to include negative reagent controls, in which nuclease‐free water is used in place of the real samples throughout DNA extraction, PCR, and sequencing [13]. The identified bacterial taxa in the reagent controls are often removed from the real samples in downstream analyses. Alternatively, they can be explicitly flagged and reported as contaminants and be retained in the real data, as simply excluding them could also remove potentially “true” signals and bias the overall observation, given the compositional nature of microbiome data [14].
For sequencing strategies, 16S ribosomal RNA (rRNA) gene‐based amplicon sequencing is widely applied to lung microbiota studies due to its technical ease and robustness. The V4 hypervariable region of the 16S rRNA gene is the most frequent choice of sequencing, which is, however, incapable of providing in‐depth taxonomic resolution (i.e., mostly up to the genus level) [15]. Powered by third‐generation long‐read sequencing (i.e., PacBio), recent studies have characterized the species or even the strain level of the lung microbiome by sequencing the full‐length 16S rRNA gene, uncovering additional microbial diversity and heterogeneity [16, 17]. It is known that 16S rRNA gene sequencing has inherent biases, largely ascribed to the differential efficiency of PCR amplification of the 16S rRNA gene from individual bacterium [18]. The copy number variation of the 16S rRNA gene among bacterial species further leads to biased cell abundance estimation [19]. Metagenomic sequencing has demonstrated its strength in profiling the functional capacity of the microbiome, moving the scientific focus from “who is there” to “what can they do” [20]. The metagenomic approach is generally considered amplification‐free. However, whole‐genomic amplification may occasionally be applied to low‐biomass samples to increase the DNA quantity, which can introduce additional biases [21]. However, its application to the lung microbiome remains largely scarce, hindered by an intimidatingly high host‐to‐microbial DNA ratio in the lung compartments. As a result, the vast majority of metagenomic sequencing reads will come from the host. Certain methods and commercial kits have been developed to deplete host genomic DNA before sequencing [22, 23], which, however, have shown varied efficiency with a critical risk of concomitantly removing bacterial DNA. Sequencing the host‐microbial “holo‐biome” and filtering the host reads postsequencing represent a viable approach [24], and yet, the high sequencing depth required to achieve sufficient microbial coverage makes this approach cost‐inhibitive. The same limitation also applies to other amplification‐free sequencing approaches such as metatranscriptomics.
Compared to the bacterial microbiome, the fungal and viral components of the lung microbiome, despite their critical importance, remain largely unexplored until recently (Figure 1). The lung fungal microbiome (or mycobiome) can be characterized by sequencing the 18S rRNA gene or the internal transcribed spacer (ITS) region. Extraction of fungal DNA requires additional procedures (i.e., bead‐beating) to break the fungal cell wall [25]. The incomplete fungal taxonomic reference database is a technical bottleneck for airway mycobiome studies, resulting in suboptimal fungal taxonomic assignment [26]. The variable length of the ITS region across fungal species further complicates the procedure for sequencing reads processing and taxonomic identification [27]. Although respiratory pathogenic viruses are well characterized clinically (i.e., by multiplex quantitative PCR) [28], the overall viral community or virome in the lung remains poorly understood, largely due to the methodological challenges for virome sequencing. A purification and enrichment step is required to isolate the viral particles and eliminate nonviral components before viral DNA/RNA extraction. Implementation of this approach in airway samples is challenging, however, due to the unique features of sample types (i.e., high viscosity), and the low abundance and fragility of viral components. It is noteworthy that a recent study has shown the feasibility in characterizing the sputum virome, revealing a much stronger association between the virome and clinical parameters in asthma compared to bacteriome [29]. Finally, although largely understudied, archaea were also found to harbor the human lung, with members of Woesearchaeota (DPANN superphylum) identified as the dominating lung archaeal taxon [30].
The human microbiome studies have entered a multiomics era [31]. Integration of multiple omics along the microbiome–host axis, such as metagenomics, metatranscriptomics, metabolomics, and metaproteomics, allows researchers to gain a more comprehensive insight into the functions of microbiome and its interactions with host (Figure 1). While multiomics are increasingly being applied to gut microbiome studies [32, 33], its implementation in the lung microbiome remains sparse. Untargeted metabolomics characterization is routinely applied to airway samples (i.e., sputum, BAL) for exploratory and hypothesis‐generating purposes [34, 35]. The levels of key metabolites of interest are often validated using targeted metabolomics with a reference standard. Metaproteomics are a promising technique and increasingly being used to gain unique insights into microbiome–host interactions by characterizing functional proteins from specific microbial taxa and host [36]. However, due to its relatively high cost, the application of metaproteomics to respiratory studies remains scant [37, 38].
THE LUNG MICROBIOME IN HUMAN HEALTH AND DISEASES
The healthy lung microbiome
Due to the unique topographic structure of the lung, which is constantly exposed to the environment, the lung microbiome is in an ecologically dynamic state, inherently shaped by three factors: microbial immigration (i.e., via microaspiration from the upper respiratory tract), microbial emigration or clearance, and replication of the local microbes [39]. Firmicutes and Bacteroidetes are the predominant phyla in healthy lung microbiota, with Prevotella, Veillonella, and Streptococcus being the most common genera [11, 40]. In healthy individuals, the lung microbiome composition is determined by a balance between microbial immigration and emigration, with limited contribution from local microbial replication [41]. In the disease state, the alterations in the lung structure and the local microenvironment, including mucosa pH, oxygen gradient, nutrient availability, temperature, and inflammation, promote microbial growth, leading to an altered composition of lung microbiota (i.e., increased Proteobacteria).
Following the above‐mentioned principles and methodologies, numerous studies have characterized the lung microbiome in human health and diseases, in which a shift of the microbiome is found in association with diseases and key clinical parameters such as severity, exacerbation, phenotype, endotype, inflammation, and mortality. This section reviews current knowledge of the lung microbiome in human diseases, spanning across chronic, acute, and other types of lung diseases (Figure 2, Table 1).
Figure 2.
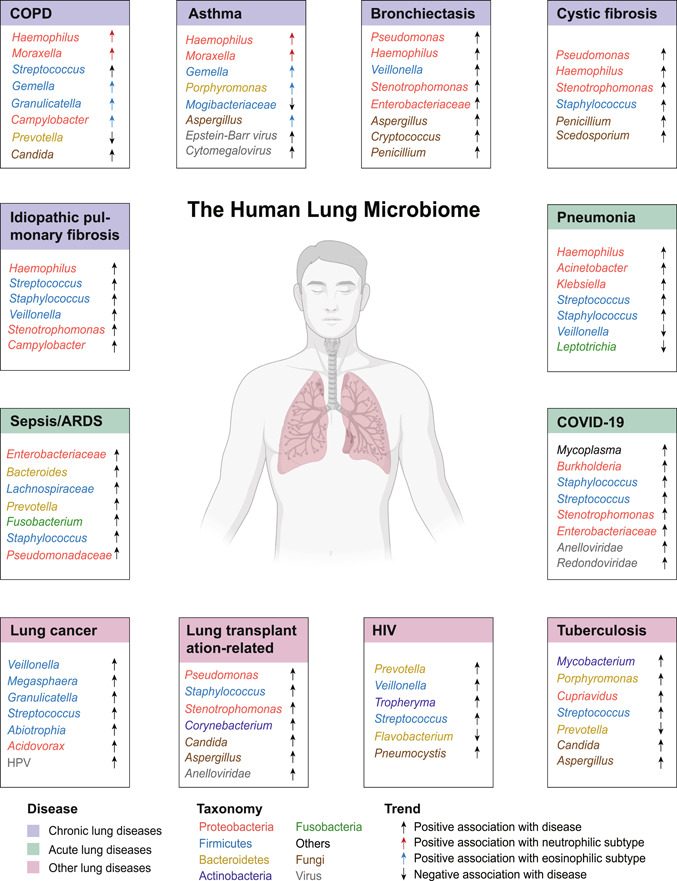
Applications of the human lung microbiome to disease areas, categorized by chronic lung diseases, acute lung diseases, and other lung diseases. For each disease, the bacteria, viral, or fungi taxa positively (either enriched in disease vs. controls or positively associated with key clinical features such as exacerbation, inflammation, or mortality) or negatively (either depleted in disease vs. controls or negatively associated with key clinical features) are indicated by arrows pointing upward or downward, respectively. For COPD and asthma, bacteria taxa associated with a specific inflammatory endotype, namely, neutrophilic or eosinophilic inflammation, are indicated by red or blue arrows, respectively. ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease.
Table 1.
Summary of key studies on the lung microbiota in chronic, acute, and other types of lung diseases
| Disease | Study | Specimen | Design and sample size | Methods | Key findings |
|---|---|---|---|---|---|
| COPD | Wang et al. (2016) | Sputum | Stable, exacerbations, posttherapy, recovery, 476 samples, 87 patients | 16S V3–V5 |
|
| COPD | Wang et al. (2018) | Sputum | Stable, exacerbations, 715 samples, 287 patients | 16S V4 |
|
| COPD | Dicker et al. (2020) | Sputum | Stable, exacerbations, 252 patients | 16S V4 |
|
| COPD | Wang et al. (2020) | Sputum | 1666 Microbiome samples, 1340 human transcriptome samples | 16S, metagenomics, host transcriptome |
|
| COPD | Wang et al. (2021) | Sputum | Stable, exacerbations, 1706 sputum samples, 510 patients | 16S V4, V3–V5 |
|
| Asthma | Huang et al. (2015) | Bronchial brushing | 40 Patients | 16S phylochip, host transcriptomics |
|
| Asthma | Taylor et al. (2018) | Sputum | 167 Patients | 16S V1–V3 |
|
| Asthma | Abdel‐Aziz et al. (2020) | Sputum | 100 Severe and 24 mild‐moderate asthma | 16S, metagenomics |
|
| Asthma | Sharma et al. (2019) | BAL, endobronchial brush | 39 Asthma and 19 controls | 16S V4, ITS |
|
| Bronchiectasis | Guan et al. (2018) | Sputum | 106 Patients and 17 controls | 16S V4 |
|
| Bronchiectasis | Mac Aogain et al. (2018) | Sputum | 238 Patients and 10 controls | ITS |
|
| Bronchiectasis | Mac Aogain et al. (2021) | Sputum | 217 Patients and 30 controls. A validation cohort with 166 patients | 16S V3–V4, ITS, viral qPCR, metagenomics |
|
| Cystic fibrosis | Zemanick et al. (2017) | BAL | 146 Patients and 45 controls | 16S V1–V2 |
|
| IPF | Molyneaux et al. (2017) | BALF, blood | 60 Patients and 20 controls, patients sampled longitudinally | 16S V3–V5, human transcriptome |
|
| Pneumonia | Kitsios et al. (2018) | Endotracheal aspirates | 56 Patients | 16S V4 |
|
| Sepsis/ARDS | Dickson et al. (2016) | BALF | 100 Samples from 68 patients | 16S V4 |
|
| ARDS | Kyo et al. (2019) | BALF | 40 Patients, 7 controls | 16S V5–V6 |
|
| ARDS | Panzer et al. (2018) | Endotracheal aspirate | 74 On ICU admission, 30 at 48 h after admission | 16S V4 |
|
| COVID‐19 | Sulaiman et al. (2021) | Bronchoscopies | 142 Patients | Metagenomics, metatranscriptomics, host transcriptome |
|
| COVID‐19 | Zhong et al. (2021) | Sputum, nasal and throat swab, anal swab and feces | 8 Mildly and 15 severely ill patients, 47 airway samples, 20 anal swab and feces | Metatranscriptomics |
|
| COVID‐19 | Ren et al. (2021) | Oropharyngeal swab | 588 Samples, 192 patients and 95 controls | Metatranscriptomics |
|
| Lung cancer | Tsay et al. (2018) | Airway brushings | 39 Lung cancer, 36 noncancer, and 10 healthy controls | 16S V4, human transcriptome |
|
| Lung cancer | Tsay et al. (2021) | Bronchoscope, airway brushing | 83 Lung cancer patients | 16S V4, human transcriptome |
|
| Lung transplantation | Combs et al. (2021) | BALF | 134 Patients | 16S, droplet digital PCR |
|
| Lung transplantation | Bernasconi et al. (2016) | BALF | 203 Samples from 112 patients postlung transplantation | 16S V1–V2 |
|
| Lung transplantation | Watzenboeck et al. (2022) | BALF | 78 Lung donors and recipients | 16S V1–V2, lipidomics, and metabolome |
|
| HIV | Lozupone et al. (2013) | BALF | 82 HIV‐positive, 77 HIV‐negative | 16S, metagenomics |
|
| HIV | Twigg 3rd et al. (2016) | BALF | 30 HIV‐positive, 22 HIV‐negative | 16S V1–V3 |
|
| Tuberculosis | Hu et al. (2020) | BALF | 30 MTB+, 30 MTB− | 16S V3–V4, metagenomics |
|
| Tuberculosis | Zhou et al. (2015) | BALF | 32 MTB+, 24 MTB− | 16S V3 |
|
Abbreviations: ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; ERK, extracellular signal‐regulated kinase; HIV, human immunodeficiency virus; IL, interleukin; IPF, idiopathic pulmonary fibrosis; ITS, internal transcribed spacer; PA, pseudomonas aeruginosa; PI3K, phosphatidylinositol 3‐kinase; PPM, potentially pathogenic microorganisms; qPCR, quantitative polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TB, tuberculosis; TNF‐α, tumor necrosis factor alpha.
Chronic lung diseases
One disease area that lung microbiome studies have extensively focused on is chronic respiratory diseases, including COPD, asthma, bronchiectasis, cystic fibrosis, interstitial pulmonary fibrosis, and so on. A key manifestation of these disorders is the chronic airway inflammation that persists throughout disease progression. In a hallmark study by Hilty et al. [2] that challenged the concept of lung sterility, the airway microbiota was found to differ between patients with COPD, asthma, and healthy controls, with elevated Proteobacteria in disease states. This pattern was subsequently supported by numerous studies demonstrating the association of members of Proteobacteria such as Haemophilus, Moraxella, and Pseudomonas with diseases and key clinical features. In our previous study of 476 sputum samples collected longitudinally from 87 COPD patients across stable state, exacerbations, 2‐week posttherapy, and 6‐week recovery, Proteobacteria and specifically Moraxella were found to be elevated in exacerbations, which was reversed posttreatment [42]. Haemophilus was identified as the hub node in the microbiome network and positively correlated with sputum interleukin‐8 (IL‐8) [42]. These results were further supported by our subsequent larger COPD microbiome studies on 775 sputum samples collected over 2 years from 287 COPD patients across three centers in United Kingdom [43]. The elevation of Proteobacteria was also associated with increased long‐term mortality and resistance to antimicrobial therapy for COPD patients [44, 45]. In our recent large‐scale microbiome meta‐analysis using 1666 public samples, enrichment of Haemophilus, Streptococcus, Moraxella, and Lactobacillus was found in COPD versus healthy controls [46]. In our pilot COPD multiomic study, Haemophilus and Moraxella were associated with different components of host immune and inflammatory patterns, implying their differential roles in the pathogenesis of COPD [47]. Increased Proteobacteria was also observed in asthmatic patients with the elevation of non‐Proteobacteria taxa such as Porphyromonas, Fusobacterium, and Sphingomonadaceae [48], and associated with worse clinical outcome of severe asthma [49], as well as expression of human Th17‐related genes [50]. As the key pathogenic agent, Pseudomonas was markedly elevated in bronchiectasis in particular in Asian populations [51, 52], whereas altered mycobiome was also found in bronchiectasis with increased abundance of Aspergillus, Penicillium, and Cryptococcus [53]. A recent seminal study by Mac Aogain et al. [54] delineated the integrated microbiomics in bronchiectasis by coprofiling bacteriome, mycobiome, and virome, and suggested that their mutual interactions were associated with key clinical features such as exacerbation frequency and antibiotic treatment. Haemophilus and Pseudomonas are also implicated in cystic fibrosis [55] and IPF [56, 57], with other pathogenic taxa such as Staphylococcus and Stenotrophomonas also commonly associated with both diseases [58, 59, 60, 61].
An important feature of the chronic respiratory diseases such as asthma and COPD are the inherent nature of heterogeneity, underpinned by different clinical phenotypes, inflammatory endotypes (the inflammatory pattern underlying a specific phenotype), and pathophysiology processes. Such heterogeneity has led to the proposal of a new paradigm for disease management not by disease “labels,” but according to “treatable traits” [62]. The lung microbiome differs substantially according to the specific phenotype or endotype of a disease, rendering difficulty in identifying disease‐specific microbiome features. In terms of clinical phenotype, the increase of Proteobacteria was most pronounced in a subgroup of COPD exacerbations with clinical evidence of bacterial infections, compared to the other exacerbation phenotypes such as those driven by viral infection or eosinophilic inflammation [42, 43]. In terms of inflammatory endotype, both neutrophilic inflammation and eosinophilic inflammation are evident in asthma and COPD with distinct airway microbiota. Haemophilus was predominant in neutrophilic inflammation, whereas certain less abundant taxa such as Gemella, Granulicatella, and Campylobacter were elevated in eosinophilic inflammation [63, 64, 65]. Differential mycobiome was also evident according to asthma endotypes, with Fusarium, Cladosporium, and Aspergillus specifically enriched in T2‐high asthma [66]. Our recent large‐scale integrative microbiome analysis on 1706 sputum samples from 510 patients has subdivided neutrophilic COPD into two subgroups based on the airway microbiome: the “Haemophilus‐predominant” and “balanced‐microbiome” subgroups. We found that these two subgroups have distinct inflammatory profiles, temporal variability, and microbiome–host interaction patterns, providing a novel framework for COPD “microbiome–host” cophenotyping [64]. Our recent study has further shown a differential airway resistome, a collection of antimicrobial‐resistant genes, in neutrophilic and eosinophilic COPD, suggesting the need to consider the inflammatory endotype for targeted antibiotic therapy [67].
Acute lung diseases
Acute lung diseases are the pulmonary manifestation of an acute inflammation caused by local or systemic pathogenic infections such as pneumonia, sepsis, and the most recent COVID‐19. Acute lung injury (ALI) and the more severe ARDS are the primary syndromes for acute lung diseases in which lung microbiome is implicated. In a pioneer study by Dickson et al., [68] alteration of lung microbiota with enrichment of gut‐specific bacteria (i.e., Bacteroides spp.) was found in BAL samples of sepsis and ARDS patients, which was correlated with alveolar tumor necrosis factor‐α providing evidence for a potential role of gut–lung translocation in critically ill patients. In a follow‐up study by the same team on BAL samples of 91 critically ill patients, enrichment of gut‐specific taxa including Lachnospiraceae and Enterobacteriaceae was associated with poor clinical outcome including fewer ventilation‐free days and progression to ARDS [69]. Consistently, Panzer et al. [10] found that progression of critical ill patients to ARDS was associated with enrichment of Enterobacteriaceae, as well as taxa such as Prevotella and Fusobacterium that were related to smoking. By BAL sampling of 47 mechanically ventilated patients with or without ARDS, Kyo et al. [70] showed that Staphylococcus, Streptococcus, and Enterobacteriaceae were positively correlated with serum IL‐6 and mortality. Likewise, Kitsios et al. [71] found that enrichment of Staphylococcus and Pseudomonadaceae in tracheal aspirates was associated with worse clinical outcome of ventilated patients. Collectively, these results point to a consensus that airway dysbiosis with enrichment of gut‐related or other pathogenic taxa is characteristic of ALI/ARDS patients and is associated with poor clinical outcomes.
COVID‐19 has infected more than 500 million people worldwide and remains an ongoing global health crisis [72]. Acute infection of SARS‐CoV‐2 results in an uncontrolled inflammatory response and cytokine storm leading to ALI and ARDS [73, 74]. Respiratory dysbiosis could be a prominent feature of COVID‐19. By sampling the lower respiratory tract of critically ill patients with COVID‐19, Sulaiman et al. [75] found that poor clinical outcome was associated with lower airway enrichment with an oral commensal Mycoplasma salivarium and suggested that secondary bacterial infections may not drive mortality in COVID‐19. By a metatranscriptomic characterization of serial clinical specimens (sputum, nasal and throat swab, anal swab, and feces), Zhong et al., [76] identified Burkholderia cepacia, Staphylococcus epidermidis, and Mycoplasma spp. to be predominant in severely ill patients with codetection of other human respiratory viruses that were not identified in mildly affected patients suggesting the need to prevent the spread of antimicrobial resistance for hospitalized, severely ill COVID‐19 patients. Through a metatranscriptomic survey on 588 oropharyngeal swab specimens collected longitudinally from 192 COVID‐19 patients and 95 controls, Ren et al. [77] characterized the upper airway dysbiosis in COVID‐19 patients with a Streptococcus‐dominant microbiota specifically present in recovered patients. Specifically, Streptococcus parasanguinis in the upper airway could be a marker for the prognosis of non‐severe COVID‐19 patients.
Other lung diseases
The lung microbiota is also implicated in other immune‐related lung diseases, including lung cancer, complications postlung transplantation, HIV, and tuberculosis. As chronic airway inflammation increases the susceptibility of lung cancer, the airway dysbiosis may be involved as a pathogenic mechanism [78]. In a pilot study, airway commensals Veillonella and Megasphaera were found to be enriched in BALF of patients with lung adenocarcinoma [79]. These findings are further supported by Huang et al., [80] who showed that the same two taxa were enriched in bronchial washing fluid in patients with lung adenocarcinoma versus squamous cell lung carcinoma. Studies have further associated the lung microbiome with key mutations and signaling pathways in lung cancer. Greathouse et al. [81] showed that increased lung Acidovorax was associated with the TP53 mutation. Tsay et al. [82] found that enrichment of oral taxa such as Streptococcus and Veillonella in the lower airways was associated with extracellular signal‐regulated kinase (ERK) and phosphatidylinositol 3‐kinase (PI3K) signaling. In a further mechanistic study, the same team showed that lung dysbiosis was associated with progression and poorer prognosis of lung cancer, and specifically, enriched oral taxa Veillonella parvula led to decreased survival, increased tumor burden, IL‐17 inflammation, and upregulated checkpoint inhibitor markers in a murine model of lung cancer [83]. Microbiome is associated with response to cancer immunotherapy [84]. Jang et al. [85] showed that Veillonella dispar was dominant in lung cancer patients with high PD‐L1 and responsive to immunotherapy, whereas Neisseria perflava was dominant in nonresponders, providing preliminary evidence for the implication of lung microbiome in lung cancer immunotherapy.
Lung transplantation is the last therapeutic option for patients with end‐stage lung disease. The most common complications postlung transplantation include acute and chronic lung allograft dysfunction. On analyzing BAL collected from 134 patients during 1‐year posttransplantation, Combs et al. [86] found that increased lung bacterial burden was predictive of chronic rejection and mortality, highlighting lung microbiome as a risk factor for lung allograft dysfunction. By combined amplicon sequencing and culture efforts, Das et al. [87] identified distinct “pneumotypes” in lung transplant recipients and established a link between microbiome, lung function, and clinical status post‐transplantation. Mechanistically, the same team further demonstrated that airway dysbiosis led to an imbalanced inflammatory and remodeling profiles of macrophages in the transplanted lung, which determined the airway immunologic tones [88]. In a multiomic study on BAL from lung donors and recipients, Watzenbock et al. [89] showed that the collective lung microbiome, metabolome, and lipidome are predictive of future lung function changes after transplantation.
Initiated by the Lung HIV Microbiome project, HIV is probably one disease area in which lung microbiome was first studied. One important early finding was the detection of Tropheryma whipplei in the lower airways of HIV patients, which was decreased after highly active antiretroviral therapy (HAART) [90]. Later studies showed increased Prevotella and Veillonella in HIV patients after 1 year of HAART treatment [91]. Mycobiome was also shown to be altered in HIV with the outgrowth of Pneumocystis jirovecii observed in both human and nonhuman primate models [92, 93]. For tuberculosis, Mycobacterium tuberculosis, its causative agent, was elevated in BAL of tuberculosis patients [94], although its detection rate by sequencing varies among studies [95]. Other taxa positively associated with tuberculosis include Cupriavidus, Porphyromonas, and Streptococcus [96, 97]. Aspergillus and Candida were also enriched in tuberculosis patients [97].
MECHANISTIC INSIGHTS ON THE LUNG MICROBIOME
The field of microbiome is rapidly advancing from correlations to causations between microbiome and diseases. Compared with the gut microbiome, whose mechanistic roles are increasingly well characterized, the effects and functions of the lung microbiome are only now beginning to be elucidated [98, 99]. Microbiome may contribute to chronic lung diseases through regulating host immunity and inflammation (Figure 3). By comparing germ‐free mice with special pathogen‐free mice with allergic airway inflammation, Herbst et al. [100] showed that the presence of commensal bacteria is critical for normal host immune function and control of allergic airway inflammation. By intranasally administering lipopolysaccharide (LPS) and elastase to establish a murine disease model that mimics key features of COPD, the same team further showed that the microbiota contributed to host IL‐17A inflammation and autoantibodies [101]. The lung microbiome also contributes to IPF progression. In a murine experiment by O'Dwyer et al., [102] lung dysbiosis was found to precede peak lung injury and persist afterwards. The microbiome's role in IPF was further examined in detail by Yang et al., [103] who showed that lung dysbiosis drove IL‐17B production and fibrosis through TLR‐Myd88 signaling. Inhaled corticosteroid (ICS) is a standard therapy for eosinophilic COPD patients, while it has the major risk of subsequent bacterial infection. By integrating human, cellular, and mouse data, Singanayagam et al. [104] showed that ICS suppressed a host defense protein named cathelicidin and resulted in airway dysbiosis with streptococci expansion, providing a mechanistic explanation for the risk of pneumonia after ICS use. While the effects of respiratory pathogens are mostly well established, the roles of commensal members of lung microbiota remain poorly understood. In a recent study by Rigauts et al., [105] Rothia mucilaginosa, a commensal member of airway microbiota, was found to alleviate airway inflammation by inhibiting the nuclear factor kappa B pathway. Other than inflammation, lung microbiota plays a role in regulating key host pathophysiological processes, such as oxidative stress, epithelial apoptosis, collagen deposition, mucus hypersecretion, and airway remodeling. D'Alessandro‐Gabazza et al. [106] found that a peptide corisin secreted by Staphylocccus induced lung epithelial apoptosis and collagen deposition toward acute exacerbations in IPF. Mouraux et al. [107] showed that airway microbiota was differentially related to airway anabolic or catabolic remodeling postlung transplantation, suggesting that the host–microbe interplay may determine remodeling activities in the transplanted lung. Lung microbiota is also essential in shaping host immune tolerance [108]. A hallmark study by Gollwitzer et al. [109] showed that lung microbiota promoted the development of Tregs, leading to tolerance to allergens in neonatal mice via PD‐L1. The host responds to microbial colonization through the secretion of immunoglobulins (i.e., IgA, IgG, IgM), which is implicated in respiratory diseases. Collin et al. [110] reported increased IgA production in response to Pseudomonas aeruginosa infection in cystic fibrosis lung. Richmond et al. [111] reported that IgA deficiency in the airways resulted in persistent activation of innate immune response to lung microbiota, leading to a progressive COPD‐like phenotype.
Figure 3.
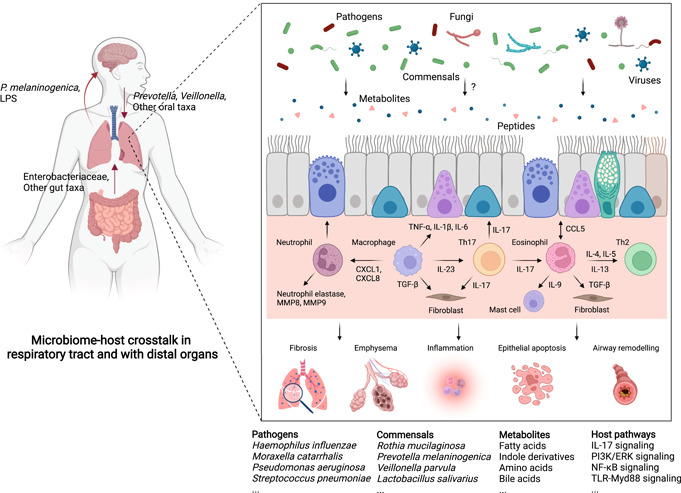
Microbiome–host crosstalk in the local respiratory tract and between the lung and distal organs. In the local respiratory tract, the pathogens or commensal bacteria, fungi, and viruses interact with each other and together interact with the host by producing or consuming metabolites or peptides, which are further involved in the molecular pathways underlying key pathophysiological processes such as fibrosis, emphysema, inflammation, epithelial apoptosis, and airway remodeling. The pathogens, commensals, their potential metabolites, and the host pathways modulated by microbiota based on evidence from mechanistic studies are shown below the pathway map. In between the lung and distal organs, enrichment of oral microbes (i.e., Prevotella and Veillonella) in the lower respiratory tract is shown to have complex effects on lung pathology. Enrichment of Enterobacteriaceae and other gut‐related taxa in the lower respiratory tract suggests the existence of a “gut–lung” axis. Prevotella melaninogenica and LPS from the lung microbiome are shown to regulate brain autoimmunity, implying a potential “lung–brain” axis. LPS, lipopolysaccharide.
The lung microbiome is also implicated in the crosstalk between the lung and distal organs (Figure 3). For example, multiple lines of evidence suggest that enrichment of oral taxa in the lower respiratory tract is a common phenomenon and exerts complex effects on lung pathology, by enhancing lung Th17 inflammation [112, 113], upregulating ERK and PI3K signaling [82], and increasing lung tumor burden [83]. The enrichment of Enterobacteriaceae and other gut‐related taxa in the lower respiratory tract during ALI suggests the existence of a “gut–lung” axis [114]. In support of the “gut–lung” axis, emerging evidence suggests the role of gut dysbiosis in respiratory diseases. Lai et al. [115] showed significantly altered gut microbiota in a COPD murine model and identified a commensal gut bacterium Parabacteroides goldsteinii that ameliorated COPD through LPS‐mediated antagonism of host TLR4 signaling. Li et al. [116] reported elevated lung inflammation, airway remodeling, and mucus hypersecretion in mice receiving fecal transplantation from COPD patients. Obese individuals have a higher risk of developing asthma, in which gut dysbiosis is also implicated. Michalovich et al. [117] showed that asthma severity was negatively associated with the fecal Akkermansia muciniphila level, and administration of this bacterium in an asthma murine model ameliorated airway hyperreactivity and inflammation. A recent ground‐breaking study by Hosang et al. [118] showed that lung dysbiosis with depletion of LPS‐enriched phyla increased the susceptibility of brain autoimmune diseases, proposing the first concept of a “lung–brain” axis.
CURRENT CHALLENGES OF THE LUNG MICROBIOME
Notwithstanding these advances, the field of lung microbiome is still facing a myriad of challenges. First, as described previously, the low microbial biomass and excessive host contamination limit the application of metagenomics and metatranscriptomics that are fundamental to elucidating the microbiome functions. An efficient sample processing and sequencing procedure capable of capturing the airway metaomics with sufficient coverage and reasonable cost is required. Second, similar to other chronic diseases, most chronic lung diseases are heterogeneous, with different clinical manifestations, disease pathobiology, and airway microbiota. Disentangling the intricate relationships between microbiome and disease phenotypes and endotypes is a prerequisite to precisely define the microbiome's role in diseases. Third, microbiome produces metabolites or peptides that serve as ligands to interact with host receptors and trigger downstream signaling. Generally, little is known regarding the molecules specifically produced by the lung microbiome and their functions, as compared to those that are well characterized in the gut (i.e., short‐chain fatty acids, indole derivatives, amino acids, bile acids). A systems biology approach is required to generate an airway “microbial–host” multiomic landscape that delineates what airway microbes are capable of producing or consuming what molecules, and how these molecules interact with host proteins, pathways, and processes. Fourth, being able to precisely manipulate the airway microbiota in animal studies is crucial to assessing its functional impacts. Compared with techniques such as fecal microbiota transplantation, which is widely applied in gut microbiome studies, there is a lack of a standard procedure for manipulating the airway microbiota. Fifth, despite the power of next‐generation sequencing, being able to culture the microbes from the respiratory tract is instrumental for translational research. Although culturing respiratory pathogens is standard in clinical laboratories, little is known regarding the culturability of commensal lung microbiota. It is noteworthy that, using a culturomic strategy, Whelan et al. [119] showed that 82.1% of the operational taxonomic units in cystic fibrosis sputum were culturable.
FUTURE AVENUES OF RESEARCH ON THE LUNG MICROBIOME
In light of these challenges, innovative experimental and analytical strategies tailored for the lung microbiome are paramount in moving the field forward. Longitudinal, interventional, and mechanistic studies are required to address causality. With these studies, it may be possible to answer more fundamental scientific questions in terms of the lung microbiome: What is the baseline status of healthy lung microbiome? How does lung microbiome respond to environmental stimuli? What are the roles of lung microbiome in different biological endotypes of respiratory diseases? How does lung microbiome differ in patients with different radiological features? Can microbiome be harnessed as a marker to facilitate the diagnosis, phenotyping, and prognosis of patients with respiratory diseases? What are the topographic structure and spatial dynamics of microbial communities in the lung? How do respiratory bacteria, fungi, and viruses interact with each other and how do they modulate host immunity? What are the key microbial metabolites that regulate host inflammation or other processes in the respiratory tract? What are the distal organs that can be influenced by lung microbiota and what are the mechanisms? Being able to answer these questions will eventually lead to a step closer toward our fundamental goal—to monitor the airway microbiome as a biomarker, and to manipulate the microbiome as a therapeutic target, toward precision medicine in respiratory and broader human diseases.
AUTHOR CONTRIBUTIONS
Xinzhu Yi performed the literature review and wrote the manuscript. Jingyuan Gao performed the literature review. Zhang Wang supervized the project, and wrote and revised the manuscript. All authors have read the final manuscript and approved it for publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant Nos. 31970112, 32170109, 41907211) and the Science and Technology Foundation of Guangdong Province (Grant No. 2019A1515011395).
Yi, Xinzhu , Gao Jingyuan, and Wang Zhang. 2022. “The Human Lung Microbiome—A Hidden Link Between Microbes and Human Health and Diseases.” iMeta 1, e33. 10.1002/imt2.33
DATA AVAILABILITY STATEMENT
No new data and script were used in this paper. Supporting Information Materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.
REFERENCES
- 1. Cho, Ilseung , and Blaser Martin J.. 2012. “The Human Microbiome: At the Interface of Health and Disease.” Nature Reviews Genetics 13: 260–70. 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hilty, Markus , Burke Conor, Pedro Helder, Cardenas Paul, Bush Andy, Bossley Cara, Davies Jane, et al. 2010. “Disordered Microbial Communities in Asthmatic Airways.” PLoS One 5: e8578. 10.1371/journal.pone.0008578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paggiaro, Pierluigi , Chanez Pascal, Holz Olaf, Ind Philip W., Djukanovic Ratko, Maestrelli Piero, and Sterk Peter J.. 2002. “Sputum Induction.” European Respiratory Journal 37: 3s–8s. 10.1183/09031936.02.00000302 [DOI] [PubMed] [Google Scholar]
- 4. An, Shi‐Qi , Adilia Warris, and Steve Turner. 2018. “Microbiome Characteristics of Induced Sputum Compared to Bronchial Fluid and Upper Airway Samples.” Pediatric Pulmonology 53: 921–8. 10.1002/ppul.24037 [DOI] [PubMed] [Google Scholar]
- 5. Murdoch, David R. , Morpeth Susan C., Hammitt Laura L., Driscoll Amanda J., Watson Nora L., Baggett Henry C., Brooks W. Abdullah, et al. 2017. “Microscopic Analysis and Quality Assessment of Induced Sputum from Children with Pneumonia in the PERCH Study.” Clinical Infectious Diseases 64: S271–9. 10.1093/cid/cix083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sulaiman, Imran , Wu Benjamin G., Li Yonghua, Scott Adrienne S., Malecha Patrick, Scaglione Benjamin, Wang Jing, et al. 2018. “Evaluation of the Airway Microbiome in Nontuberculous Mycobacteria Disease.” European Respiratory Journal 52: 1800810. 10.1183/13993003.00810-2018 [DOI] [PubMed] [Google Scholar]
- 7. Durack, Juliana , Huang Yvonne J., Nariya Snehal, Christian Laura S., Ansel K. Mark, Beigelman Avraham, Castro Mario, et al. 2018. “Bacterial Biogeography of Adult Airways in Atopic Asthma.” Microbiome 6: 104. 10.1186/s40168-018-0487-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Denner, Darcy R. , Sangwan Naseer, Becker Julia B., Hogarth D. Kyle, Oldham Justin, Castillo Jamee, Sperling Anne I., et al. 2016. “Corticosteroid Therapy and Airflow Obstruction Influence the Bronchial Microbiome, which is Distinct from that of Bronchoalveolar Lavage in Asthmatic Airways.” The Journal of Allergy and Clinical Immunology 137: P1398–405. 10.1016/j.jaci.2015.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalantar, Katrina L. , Moazed Farzad, Christenson Stephanie C., Wilson Jenny, Deiss Thomas, Belzer Annika, Vessel Kathryn, et al. 2019. “Metagenomic Comparison of Tracheal Aspirate and Mini‐Bronchial Alveolar Lavage for Assessment of Respiratory Microbiota.” American Journal of Physiology‐Lung Cellular and Molecular Physiology 316: L578–84. 10.1152/ajplung.00476.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panzer, Ariane R. , Lynch Susan V., Langelier Chaz, Christie Jason D., McCauley Kathryn, Nelson Mary, Cheung Christopher K., Benowitz Neal L., Cohen Mitchell J., and Calfee Carolyn S.. 2018. “Lung Microbiota is Related to Smoking Status and to Development of Acute Respiratory Distress Syndrome in Critically Ill Trauma Patients.” American Journal of Physiology‐Lung Cellular and Molecular Physiology 197: 621–31. 10.1164/rccm.201702-0441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charlson, Emily S. , Bittinger Kyle, Haas Andrew R., Fitzgerald Ayannah S., Frank Ian, Yadav Anjana, Bushman Frederic D., and Collman Ronald G.. 2011. “Topographical Continuity of Bacterial Populations in the Healthy Human Respiratory Tract.” American Journal of Respiratory and Critical Care 184: 957–63. 10.1164/rccm.201104-0655OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salter, Susannah J. , Cox Michael J., Turek Elena M., Calus Szymon T., Cookson William O., Moffatt Miriam F., Turner Paul, Parkhill Julian, Loman Nicholas J., and Walker Alan W.. 2014. “Reagent and Laboratory Contamination can Critically Impact Sequence‐Based Microbiome Analyses.” BMC Biology 12: 87. 10.1186/s12915-014-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marsh, Robyn L. , Nelson Maria T., Pope Chris E., Leach Amanda J., Hoffman Lucas R., Chang Anne B., and Smith‐Vaughan Heidi C.. 2018. “How Low can we Go? The Implications of Low Bacterial Load in Respiratory Microbiota Studies.” Pneumonia 10: 7. 10.1186/s41479-018-0051-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carney, Sharon M. , Clemente Jose C., Cox Michael J., Dickson Robert P., Huang Yvonne J., Kitsios Georgios D., Kloepfer Kirsten M., et al. 2020. “Methods in Lung Microbiome Research.” American Journal of Respiratory Cell and Molecular Biology 62: 283–99. 10.1165/rcmb.2019-0273TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson, Jethro S. , Spakowicz Daniel J., Hong Bo‐Young, Petersen Lauren M., Demkowicz Patrick, Chen Lei, Leopold Shana R., et al. 2019. “Evaluation of 16S rRNA Gene Sequencing for Species and Strain‐Level Microbiome Analysis.” Nature Communications 10: 5029. 10.1038/s41467-019-13036-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang, Zhang , Liu Haiyue, Wang Fengyan, Yang Yuqiong, Wang Xiaojuan, Chen Boxuan, Stampfli Martin R., et al. 2020. “A Refined View of Airway Microbiome in Chronic Obstructive Pulmonary Disease at Species and Strain‐Levels.” Frontiers in Microbiology 11: 1758. 10.3389/fmicb.2020.01758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Toma, Ian , Siegel Marc O., Keiser John, Yakovleva Anna, Kim Alvin, Davenport Lionel, Devaney Joseph, et al. 2014. “Single‐Molecule Long‐Read 16S Sequencing to Characterize the Lung Microbiome from Mechanically Ventilated Patients with Suspected Pneumonia.” Journal of Clinical Microbiology 52: 3913–21. 10.1128/JCM.01678-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silverman, Justin D. , Bloom Rachael J., Jiang Sharon, Durand Heather K., Dallow Eric, Mukherjee Sayan, and David Lawrence A.. 2021. “Measuring and Mitigating PCR Bias in Microbiota Datasets.” PLoS Computational Biology 17: e1009113. 10.1371/journal.pcbi.1009113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kembel, Steven W. , Wu Martin, Eisen Jonathan A., and Green Jessica L.. 2012. “Incorporating 16S Gene Copy Number Information Improves Estimates of Microbial Diversity and Abundance.” PLoS Computational Biology 8: e1002743. 10.1371/journal.pcbi.1002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knight, Rob , Vrbanac Alison, Taylor Bryn C., Aksenov Alexander, Callewaert Chris, Debelius Justine, Gonzalez Antonio, et al. 2018. “Best Practices for Analysing Microbiomes.” Nature Reviews Microbiology 16: 410–22. 10.1038/s41579-018-0029-9 [DOI] [PubMed] [Google Scholar]
- 21. Ahsanuddin, Sofia , Afshinnekoo Ebrahim, Gandara Jorge, Hakyemezoglu Mustafa, Bezdan Daniela, Minot Samuel, Greenfield Nick, and Mason Christopher E.. 2017. “Assessment of REPLI‐g Multiple Displacement Whole Genome Amplification (WGA) Techniques for Metagenomic Applications.” Journal of Biomolecular Techniques 28: 46–55. 10.7171/jbt.17-2801-008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marotz, Clarisse A. , Sanders Jon G., Zuniga Cristal, Zaramela Livia S., Knight Rob, and Zengler Karsten. 2018. “Improving Saliva Shotgun Metagenomics by Chemical Host DNA Depletion.” Microbiome 6: 42. 10.1186/s40168-018-0426-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nelson, Maria T. , Pope Christopher E., Marsh Robyn L., Wolter Daniel J., Weiss Eli J., Hager Kyle R., Vo Anh T., et al. 2019. “Human and Extracellular DNA Depletion for Metagenomic Analysis of Complex Clinical Infection Samples Yields Optimized Viable Microbiome Profiles.” Cell Reports 26: 2227–40. 10.1016/j.celrep.2019.01.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mac Aogain, Micheal , Lau Kenny J. X., Cai Zhao, Narayana Jayanth Kumar, Purbojati Rikky W., Drautz‐Moses Daniela I., Gaultier Nicolas E., et al. 2020. “Metagenomics Reveals a Core Macrolide Resistome Related to Microbiota in Chronic Respiratory Disease.” American Journal of Respiratory and Critical Care Medicine 202: 433–47. 10.1164/rccm.201911-2202OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang, Jie , Iliev Iliyan D., Brown Jordan, Underhill David M., and Funari Vincent A.. 2015. “Mycobiome: Approaches to Analysis of Intestinal Fungi.” Journal of Immunological Methods 421: 112–21. 10.1016/j.jim.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu, Haiyue , Liang Zhenyu, Cao Nannan, Yi Xinzhu, Tan Xilan, Liu Zuheng, Wang Fengyan, et al. 2020. “Airway Bacterial and Fungal Microbiome in Chronic Obstructive Pulmonary Disease.” Medicine in Microecology 7: 100035. [Google Scholar]
- 27. Rolling, Thierry , Zhai Bing, Frame John, Hohl Tobias M., and Taur Ying. 2022. “Customization of a DADA2‐based Pipeline for Fungal Internal Transcribed Spacer 1 (ITS1) Amplicon Data Sets.” JCI Insight 7: e151663. 10.1172/jci.insight.151663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wylie, Kristine M. 2017. “The Virome of the Human Respiratory Tract.” Clinics in Chest Medicine 38: 11–9. 10.1016/j.ccm.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi, Sungmi , Sohn Kyoung‐Hee, Jung Jae‐Woo, Kang Min‐Gyu, Yang Min‐Suk, Kim Sujeong, Choi Jeong‐Hee, Cho Sang‐Heon, Kang Hye‐Ryun, and Yi Hana. 2021. “Lung Virome: New Potential Biomarkers for Asthma Severity and Exacerbation.” Journal of Allergy and Clinical Immunology 148: 1007–15. 10.1016/j.jaci.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 30. Koskinen, Kaisa , Pausan Manuela R., Perras Alexandra K., Beck Michael, Bang Corinna, Mora Maximilian, Schilhabel Anke, Schmitz Ruth, and Moissl‐Eichinger Christine. 2017. “First Insights into the Diverse Human Archaeome: Specific Detection of Archaea in the Gastrointestinal Tract, Lung, and Nose and on Skin.” mBio 8: e00824–17. 10.1128/mBio.00824-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jansson, Janet K. , and Baker Erin S.. 2016. “A Multi‐Omic Future for Microbiome Studies.” Nature Microbiology 1: 16049. 10.1038/nmicrobiol.2016.49 [DOI] [PubMed] [Google Scholar]
- 32. Pedersen, Helle Krogh, Valborg Gudmundsdottir, Henrik , Nielsen Bjorn, Hyotylainen Tuulia, Nielsen Trine, Jensen Benjamin A. H., Forslund Kristoffer, et al. 2016. “Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity.” Nature 535: 376–81. 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- 33. Lloyd‐Price, Jason , Arze Cesar, Ananthakrishnan Ashwin N., Schirmer Melanie, Avila‐Pacheco Julian, Poon Tiffany W., Andrews Elizabeth, et al. 2019. “Multi‐Omics of the Gut Microbial Ecosystem in Inflammatory Bowel Diseases.” Nature 569: 655–62. 10.1038/s41586-019-1237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed, Naseer , Bezabeh Tedros, Ijare Omkar B., Myers Renelle, Alomran Reem, Aliani Michel, Nugent Zoann, et al. 2016. “Metabolic Signatures of Lung Cancer in Sputum and Exhaled Breath Condensate Detected by (1)H Magnetic Resonance Spectroscopy: A Feasibility Study.” Magnetic Resonance Insights 9: 29–35. 10.4137/MRI.S40864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu, Tao , Li Shanqun, Wang Jiajia, Liu Chunfang, Gao Lei, Zeng Yuzhen, Mao Ruolin, Cui Bo, Ji Hong, and Chen Zhihong. 2020. “Induced Sputum Metabolomic Profiles and Oxidative Stress are Associated with Chronic Obstructive Pulmonary Disease (COPD) Severity: Potential use for Predictive, Preventive, and Personalized Medicine.” EPMA Journal 11: 645–59. 10.1007/s13167-020-00227-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hardouin, Pauline , Chiron Raphael, Marchandin Helene, Armengaud Jean, and Grenga Lucia. 2021. “Metaproteomics to Decipher CF Host‐Microbiota Interactions: Overview, Challenges and Future Perspectives.” Genes 12(6), 892. 10.3390/genes12060892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thuy‐Boun, Peter S. , Mehta Subina, Gruening Bjoern, McGowan Thomas, Nguyen An, Rajczewski Andrew T., Johnson James E., Griffin Timothy J., Wolan Dennis W., and Jagtap Pratik D.. 2021. “Metaproteomics Analysis of SARS‐CoV‐2‐Infected Patient Samples Reveals Presence of Potential Coinfecting Microorganisms.” Journal of Proteome Research 20: 1451–4. 10.1021/acs.jproteome.0c00822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jagtap, Pratik D. , Viken Kevin J., Johnson James, McGowan Thomas, Pendleton Kathryn M., Griffin Timothy J., Hunter Ryan C., Rudney Joel D., and Bhargava Maneesh. 2018. “BAL Fluid Metaproteome in Acute Respiratory Failure.” American Journal of Respiratory Cell and Molecular Biology 59: 648–52. 10.1165/rcmb.2018-0068LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickson, Robert P. , Martinez Fernando J., and Huffnagle Gary B.. 2014. “The Role of the Microbiome in Exacerbations of Chronic Lung Diseases.” The Lancet 384: 691–702. 10.1016/S0140-6736(14)61136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dickson, Robert P. , Erb‐Downward John R., Freeman Christine M., McCloskey Lisa, Falkowski Nicole R., Huffnagle Gary B., and Curtis Jeffrey L.. 2017. “Bacterial Topography of the Healthy Human Lower Respiratory Tract.” mBio 8(1): e02287–16. 10.1128/mBio.02287-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whiteside, Samantha A. , McGinniss John E., and Collman. Ronald G. 2021. “The Lung Microbiome: Progress and Promise.” Journal of Clinical Investigation 131: e150473. 10.1172/JCI150473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang, Zhang , Bafadhel Mona, Haldar Koirobi, Spivak Aaron, Mayhew David, Miller Bruce E., Tal‐Singer Ruth, et al. 2016. “Lung Microbiome Dynamics in COPD Exacerbations.” European Respiratory Journal 47: 1082–92. 10.1183/13993003.01406-2015 [DOI] [PubMed] [Google Scholar]
- 43. Wang, Zhang , Singh Richa, Miller Bruce E., Tal‐Singer Ruth, Van Horn Stephanie, Tomsho Lynn, Mackay Alexander, et al. 2018. “Sputum Microbiome Temporal Variability and Dysbiosis in Chronic Obstructive Pulmonary Disease Exacerbations: An Analysis of the COPDMAP Study.” Thorax 73: 331–8. 10.1136/thoraxjnl-2017-210741 [DOI] [PubMed] [Google Scholar]
- 44. Dicker, Alison J. , Huang Jeffrey Tj, Lonergan Mike, Keir Holly R., Fong Christopher J., Tan Brandon, Cassidy Andrew J., et al. 2020. “The Sputum Microbiome, Airway Inflammation and Mortality in Chronic Obstructive Pulmonary Disease.” Journal of Allergy and Clinical Immunology 147: 158–67. 10.1016/j.jaci.2020.02.040 [DOI] [PubMed] [Google Scholar]
- 45. Liu, Haiyue , Zheng Daowen, Lin Yanxia, Liu Zuheng, Liang Zhenyu, Su Jin, Chen Rongchang, Zhou Hongwei, and Wang Zhang. 2020. “Association of Sputum Microbiome with Clinical Outcome of Initial Antibiotic Treatment in Hospitalized Patients with Acute Exacerbations of COPD.” Pharmacological Research 160: 105095. 10.1016/j.phrs.2020.105095 [DOI] [PubMed] [Google Scholar]
- 46. Wang, Zhang , Yang Yuqiong, Yan Zhengzheng, Liu Haiyue, Chen Boxuan, Liang Zhenyu, Wang Fengyan, et al. 2020. “Multi‐Omic Meta‐Analysis Identifies Functional Signatures of Airway Microbiome in Chronic Obstructive Pulmonary Disease.” ISME Journal 14: 2748–65. 10.1038/s41396-020-0727-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang, Zhang , Maschera Barbara, Lea Simon, Kolsum Umme, Michalovich David, Van Horn Stephanie, Traini Christopher, Brown James R., Hessel Edith M., and Singh Dave. 2019. “Airway Host‐Microbiome Interactions in Chronic Obstructive Pulmonary Disease.” Respiratory Research 20: 113. 10.1186/s12931-019-1085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chung, Kian Fan . 2017. “Airway Microbial Dysbiosis in Asthmatic Patients: A Target for Prevention and Treatment?” Journal of Allergy and Clinical Immunology 139: 1071–81. 10.1016/j.jaci.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 49. Abdel‐Aziz, Mahmoud I. , Brinkman Paul, Vijverberg Susanne J. H., Neerincx Anne H., Riley John H., Bates Stewart, Hashimoto Simone, et al. 2021. “Sputum Microbiome Profiles Identify Severe Asthma Phenotypes of Relative Stability at 12 to 18 Months.” Journal of Allergy and Clinical Immunology 147: 123–34. 10.1016/j.jaci.2020.04.018 [DOI] [PubMed] [Google Scholar]
- 50. Huang, Yvonne J. , Nariya Snehal, Harris Jeffrey M., Lynch Susan V., Choy David F., Arron Joseph R., and Boushey Homer. 2015. “The Airway Microbiome in Patients with Severe Asthma: Associations with Disease Features and Severity.” Journal of Allergy and Clinical Immunology 136: 874–84. 10.1016/j.jaci.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang, Hung‐Yu , Chung Fu‐Tsai, Lo Chun‐Yu, Lin Horng‐Chyuan, Huang Yu‐Tung, Yeh Chih‐Hsin, Lin Chang‐Wei, Huang Yu‐Chen, and Wang Chun‐Hua. 2020. “Etiology and Characteristics of Patients with Bronchiectasis in Taiwan: A Cohort Study from 2002 to 2016.” BMC Pulmonary Medicine 20: 45. 10.1186/s12890-020-1080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guan, Wei‐Jie , Yuan Jing‐Jing, Li Hui‐Min, Gao Yong‐Hua, Chen Chun‐Lan, Huang Yan, Chen Rong‐Chang, and Zhong Nan‐Shan. 2018. “Altered Community Compositions of Proteobacteria in Adults with Bronchiectasis.” International Journal of Chronic Obstructive Pulmonary Disease 13: 2173–82. 10.2147/COPD.S159335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mac Aogain, Micheal , Ravishankar Chandrasekaran, Albert Yick Hou Lim , Teck Boon Low, Gan Liang Tan , Tidi Hassan, Thun How Ong , et al. 2018. “Immunological Corollary of the Pulmonary Mycobiome in Bronchiectasis: The CAMEB Study.” European Respiratory Journal 52: 1800766. 10.1183/13993003.00766-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mac Aogain, Micheal , Narayana Jayanth Kumar, Tiew Pei Yee, Ali Nur A'tikah Binte Mohamed, Yong Valerie Fei Lee, Jaggi Tavleen Kaur, Lim Albert Yick Hou, et al. 2021. “Integrative Microbiomics in Bronchiectasis Exacerbations.” Nature Medicine 27: 688–99. 10.1038/s41591-021-01289-7 [DOI] [PubMed] [Google Scholar]
- 55. Zemanick, Edith T. , Wagner Brandie D., Robertson Charles E., Ahrens Richard C., Chmiel James F., Clancy John P., Gibson Ronald L., et al. 2017. “Airway Microbiota across Age and Disease Spectrum in Cystic Fibrosis.” European Respiratory Journal 50: 1700832. 10.1183/13993003.00832-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Molyneaux, Philip L. , Saffron A. G. Willis‐Owen, Cox Michael J., James Phillip, Cowman Steven, Loebinger Michael, Blanchard Andrew, et al. 2017. “Host‐Microbial Interactions in Idiopathic Pulmonary Fibrosis.” American Journal of Respiratory and Critical Care Medicine 195: 1640–50. 10.1164/rccm.201607-1408OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Valenzi, Eleanor , Yang Haopu, Sembrat John C., Yang Libing, Winters Spencer, Nettles Rachel, Kass Daniel J., et al. 2021. “Topographic Heterogeneity of Lung Microbiota in End‐Stage Idiopathic Pulmonary Fibrosis: The Microbiome in Lung Explants‐2 (MiLEs‐2) Study.” Thorax 76: 239–47. 10.1136/thoraxjnl-2020-214770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Koff, Emma M. , de Winter‐de Groot Karin M., and Bogaert Debby. 2016. “Development of the Respiratory Tract Microbiota in Cystic Fibrosis.” Current Opinion in Pulmonary Medicine 22: 623–8. 10.1097/MCP.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 59. Waters, Valerie , Yau Yvonne, Prasad Sudha, Lu Annie, Atenafu Eshetu, Crandall Ian, Tom Stephanie, Tullis Elizabeth, and Ratjen Felix. 2011. “ Stenotrophomonas maltophilia in Cystic Fibrosis: Serologic Response and Effect on Lung Disease.” American Journal of Respiratory and Critical Care Medicine 183: 635–40. 10.1164/rccm.201009-1392OC [DOI] [PubMed] [Google Scholar]
- 60. Wolter, Daniel J. , Julia C. Emerson, McNamara Sharon, Buccat Anne M., Qin Xuan, Cochrane Elizabeth, Houston Laura S., et al. 2013. “ Staphylococcus aureus Small‐Colony Variants are Independently Associated with Worse Lung Disease in Children with Cystic Fibrosis.” Clinical Infectious Diseases 57: 384–91. 10.1093/cid/cit270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Molyneaux, Phillip L. , Michael J. Cox, Willis‐Owen Saffron A. G., Mallia Patrick, Russell Kirsty E., Russell Anne‐Marie, Murphy Elissa, et al. 2014. “The Role of Bacteria in the Pathogenesis and Progression of Idiopathic Pulmonary Fibrosis.” American Journal of Respiratory and Critical Care Medicine 190: 906–13. 10.1164/rccm.201403-0541OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McDonald, Vanessa M. , Fingleton James, Agusti Alvar, Hiles Sarah A., Clark Vanessa L., Holland Anne E., Marks Guy B, et al. 2019. “Treatable Traits: A New Paradigm for 21st Century Management of Chronic Airway Diseases: Treatable Traits Down under International Workshop Report.” European Respiratory Journal 53 1802058. 10.1183/13993003.02058-2018 [DOI] [PubMed]
- 63. Taylor, Steven L. , Leong Lex E. X., Choo Jocelyn M., Wesselingh Steve, Yang Ian A., Upham John W., Reynolds Paul N., et al. 2018. “Inflammatory Phenotypes in Patients with Severe Asthma are Associated with Distinct Airway Microbiology.” Journal of Allergy and Clinical Immunology 141: 94–103. 10.1016/j.jaci.2017.03.044 [DOI] [PubMed] [Google Scholar]
- 64. Wang, Zhang , Locantore Nicholas, Haldar Koirobi, Yavari Ramsheh Mohammadali, Beech Augusta S., Ma Wei, Brown James R., et al. 2021. “Inflammatory Endotype‐Associated Airway Microbiome in Chronic Obstructive Pulmonary Disease Clinical Stability and Exacerbations: A Multicohort Longitudinal Analysis.” American Journal of Respiratory and Critical Care Medicine 203: 1488–502. 10.1164/rccm.202009-3448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beech, Augusta S. , Lea Simon, Kolsum Umme, Wang Zhang, Miller Bruce E, Donaldson Gavin C., Wedzicha Jadwiga A., Brightling Christopher E., and Singh Dave. 2020. “Bacteria and Sputum Inflammatory Cell Counts; a COPD Cohort Analysis.” Respiratory Research 21: 289. 10.1186/s12931-020-01552-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sharma, Anukriti , Laxman Bharathi, Naureckas Edward T., Hogarth D. Kyle, Sperling Anne I., Solway Julian, Ober Carole, Gilbert Jack A., and White Steven R.. 2019. “Associations Between Fungal and Bacterial Microbiota of Airways and Asthma Endotypes.” Journal of Allergy and Clinical Immunology 144: 1214–27.E7. 10.1016/j.jaci.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yi, Xinzhu , Li Yanjun, Liu Haiyue, Liu Xiaomin, Yang Junhao, Gao Jingyuan, Yang Yuqiong, et al. 2022. “Inflammatory Endotype‐Associated Airway Resistome in Chronic Obstructive Pulmonary Disease.” Microbiology Spectrum 10: e0259321. 10.1128/spectrum.02593-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dickson, Robert P. , Singer Benjamin H., Newstead Michael W., Falkowski Nicole R., Erb‐Downward John R., Standiford Theodore J., and Huffnagle Gary B.. 2016. “Enrichment of the Lung Microbiome with Gut Bacteria in Sepsis and the Acute Respiratory Distress Syndrome.” Nature Microbiology 1: 16113. 10.1038/nmicrobiol.2016.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dickson, Robert P. , Schultz Marcus J., Poll Tom van der, Schouten Laura R., Falkowski Nicole R., Luth Jenna E., and Sjoding Michael W., et al. 2020. “Lung Microbiota Predict Clinical Outcomes in Critically Ill Patients.” American Journal of Respiratory and Critical Care Medicine 201: 555–63. 10.1164/rccm.201907-1487OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kyo, Michihito , Nishioka Keisuke, Nakaya Takaaki, Kida Yoshiko, Tanabe Yuko, Ohshimo Shinichiro, and Shime Nobuaki. 2019. “Unique Patterns of Lower Respiratory Tract Microbiota are Associated with Inflammation and Hospital Mortality in Acute Respiratory Distress Syndrome.” Respiratory Research 20: 246. 10.1186/s12931-019-1203-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kitsios, Georgios D. , Yang Haopu, Yang Libing, Qin Shulin, Fitch Adam, Wang Xiao‐Hong, Fair Katherine, et al. 2020. “Respiratory Tract Dysbiosis is Associated with Worse Outcomes in Mechanically‐Ventilated Patients.” American Journal of Respiratory and Critical Care Medicine 202: 1666–77. 10.1164/rccm.201912-2441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guan, Wei‐Jie , Ni Zheng‐Yi, Hu Yu, Liang Wen‐Hua, Ou Chun‐Quan, He Jian‐Xing, Liu Lei, et al. 2020. “Clinical Characteristics of Coronavirus Disease 2019 in China.” New England Journal of Medicine 382: 1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li, Hui , Liu Liang, Zhang Dingyu, Xu Jiuyang, Dai Huaping, Tang Nan, Su Xiao, and Cao Bin. 2020. “SARS‐CoV‐2 and Viral Sepsis: Observations and Hypotheses.” Lancet 395: 1517–20. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li, Liyang , Huang Qihong, Wang Diane C., Ingbar David H., and Wang Xiangdong. 2020. “Acute Lung Injury in Patients with COVID‐19 Infection.” Clinical and Translational Medicine 10: 20–7. 10.1002/ctm2.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sulaiman, Imran , Chung Matthew, Angel Luis, Tsay Jun‐Chieh J., Wu Benjamin G., Yeung Stephen T., Krolikowski Kelsey, et al. 2021. “Microbial Signatures in the Lower Airways of Mechanically Ventilated COVID‐19 Patients Associated with Poor Clinical Outcome.” Nature Microbiology 6: 1245–58. 10.1038/s41564-021-00961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhong, Huanzi , Yanqun Wang, Zhun Shi , Lu Zhang, Huahui Ren , Weiqun He, Zhaoyong Zhang , et al. 2021. “Characterization of Respiratory Microbial Dysbiosis in Hospitalized COVID‐19 Patients.” Cell Discovery 7: 23. 10.1038/s41421-021-00257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ren, Lili , Wang Yeming, Zhong Jiaxin, Li Xia, Xiao Yan, Li Jie, Yang Jing, et al. 2021. “Dynamics of the Upper Respiratory Tract Microbiota and its Association with Mortality in COVID‐19.” American Journal of Respiratory and Critical Care Medicine 204: 1379–90. 10.1164/rccm.202103-0814OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee, Gina , Walser Tonya C., and Dubinett Steven M.. 2009. “Chronic Inflammation, Chronic Obstructive Pulmonary Disease, and Lung Cancer.” Current Opinion in Pulmonary Medicine 15: 303–7. 10.1097/MCP.0b013e32832c975a [DOI] [PubMed] [Google Scholar]
- 79. Lee, Sang Hoon , Sung Ji Yeon, Yong Dongeun, Chun Jongsik, Kim Song Yee, Song Joo Han, Chung Kyung Soo, et al. 2016. “Characterization of Microbiome in Bronchoalveolar Lavage Fluid of Patients with Lung Cancer Comparing with Benign Mass Like Lesions.” Lung Cancer 102: 89–95. 10.1016/j.lungcan.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 80. Huang, Danhui , Su Xiaofang, Yuan Man, Zhang Shujia, He Jing, Deng Qiuhua, Qiu Wenjun, Dong Hangming, and Cai Shaoxi. 2019. “The Characterization of Lung Microbiome in Lung Cancer Patients with Different Clinicopathology.” American Journal of Cancer Research 9: 2047–63. https://www.ncbi.nlm.nih.gov/pubmed/31598405 [PMC free article] [PubMed] [Google Scholar]
- 81. Leigh Greathouse, K., White James R., Vargas Ashely J., Bliskovsky Valery V., Beck Jessica A., von Muhlinen Natalia, Polley Eric C., et al. 2018. “Interaction between the Microbiome and TP53 in Human Lung Cancer.” Genome Biology 19: 123. 10.1186/s13059-018-1501-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tsay, Jun‐Chieh J. , Wu Benjamin G., Badri Michelle H., Clemente Jose C., Shen Nan, Meyn Peter, Li Yonghua, et al. 2018. “Airway Microbiota is Associated with Upregulation of the PI3K Pathway in Lung Cancer.” American Journal of Respiratory and Critical Care Medicine 198: 1188–98. 10.1164/rccm.201710-2118OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsay, Jun‐Chieh J. , Benjamin G. Wu, Sulaiman Imran, Gershner Katherine, Schluger Rosemary, Li Yonghua, Yie Ting‐An, et al. 2021. “Lower Airway Dysbiosis Affects Lung Cancer Progression.” Cancer Discovery 11: 293–307. 10.1158/2159-8290.CD-20-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bullman, Susan , Eggermont Alexander, Johnston Christopher D., and Zitvogel Laurence. 2021. “Harnessing the Microbiome to Restore Immunotherapy Response.” Nature Cancer 2: 1301–4. 10.1038/s43018-021-00300-x [DOI] [PubMed] [Google Scholar]
- 85. Jang, Hye Jin , Choi Ji Yeon, Kim Kangjoon, Hyun Yong Seung, Wook Kim Yeon, Yee Kim Song, Young Kim Eun, et al. 2021. “Relationship of the Lung Microbiome with PD‐L1 Expression and Immunotherapy Response in Lung Cancer.” Respiratory Research 22: 322. 10.1186/s12931-021-01919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Combs, Michael P. , Wheeler David S., Luth Jenna E., Falkowski Nicole R., Walker Natalie M., Erb‐Downward John R, Lama Vibha N., and Dickson Robert P.. 2021. “Lung Microbiota Predict Chronic Rejection in Healthy Lung Transplant Recipients: A Prospective Cohort Study.” Lancet Respiratory Medicine 9: 601–12. 10.1016/S2213-2600(20)30405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Das, Sudip , Bernasconi Eric, Koutsokera Angela, Wurlod Daniel‐Adrien, Tripathi Vishwachi, Bonilla‐Rosso German, Aubert John‐David, et al. 2021. “A Prevalent and Culturable Microbiota Links Ecological Balance to Clinical Stability of the Human Lung after Transplantation.” Nature Communications 12: 2126. 10.1038/s41467-021-22344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bernasconi, Eric , Pattaroni Celine, Koutsokera Angela, Pison Christophe, Kessler Romain, Benden Christian, Soccal Paola M., et al. 2016. “Airway Microbiota Determines Innate Cell Inflammatory or Tissue Remodeling Profiles in Lung Transplantation.” American Journal of Respiratory and Critical Care Medicine 194: 1252–63. 10.1164/rccm.201512-2424OC [DOI] [PubMed] [Google Scholar]
- 89. Watzenboeck, Martin L. , Gorki Anna‐Dorothea, Quattrone Federica, Gawish Riem, Schwarz Stefan, Lambers Christopher, Jaksch Peter, et al. 2022. “Multi‐Omics Profiling Predicts Allograft Function after Lung Transplantation.” European Respiratory Journal 59: 2003292. 10.1183/13993003.03292-2020 [DOI] [PubMed] [Google Scholar]
- 90. Lozupone, Catherine , Cota‐Gomez Adela, Palmer Brent E., Linderman Derek J., Charlson Emily S., Sodergren Erica, Mitreva Makedonka, et al. 2013. “Widespread Colonization of the Lung by Tropheryma whipplei in HIV Infection.” American Journal of Respiratory and Critical Care Medicine 187: 1110–7. 10.1164/rccm.201211-2145OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Twigg, Homer L. 3rd , Knox Kenneth S., Zhou Jin, Crothers Kristina A., Nelson David E., Toh Evelyn, Day Richard B, et al. 2016. “Effect of Advanced HIV Infection on the Respiratory Microbiome.” American Journal of Respiratory and Critical Care Medicine 194: 226–35. 10.1164/rccm.201509-1875OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cui, Lijia , Lucht Lorrie, Tipton Laura, Rogers Matthew B., Fitch Adam, Kessinger Cathy, Camp Danielle, et al. 2015. “Topographic Diversity of the Respiratory Tract Mycobiome and Alteration in HIV and Lung Disease.” American Journal of Respiratory and Critical Care Medicine 191: 932–42. 10.1164/rccm.201409-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shipley, Timothy W. , Kling Heather M., Morris Alison, Patil Sangita, Kristoff Jan, Guyach Siobhan E., Murphy Jessica E., et al. 2010. “Persistent Pneumocystis Colonization Leads to the Development of Chronic Obstructive Pulmonary Disease in a Nonhuman Primate Model of AIDS.” Journal of Infectious Diseases 202: 302–12. 10.1086/653485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hu, Yongfeng , Cheng Min, Liu Bo, Dong Jie, Sun Lilian, Yang Jian, Yang Fan, Chen Xinchun, and Jin Qi. 2020. “Metagenomic Analysis of the Lung Microbiome in Pulmonary Tuberculosis—A Pilot Study.” Emerging Microbes & Infections 9: 1444–52. 10.1080/22221751.2020.1783188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ticlla, Monica R. , Hella Jerry, Hiza Hellen, Sasamalo Mohamed, Mhimbira Francis, Rutaihwa Liliana K., Droz Sara, et al. 2021. “The Sputum Microbiome in Pulmonary Tuberculosis and its Association with Disease Manifestations: A Cross‐Sectional Study.” Frontiers in Microbiology 12: 633396. 10.3389/fmicb.2021.633396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou, Yuhua , Lin Feishen, Cui Zelin, Zhang Xiangrong, Hu Chunmei, Shen Tian, Chen Chunyan, Zhang Xia, and Guo Xiaokui. 2015. “Correlation between either Cupriavidus or Porphyromonas and Primary Pulmonary Tuberculosis Found by Analysing the Microbiota in Patients' Bronchoalveolar Lavage Fluid.” PLoS One 10: e0124194. 10.1371/journal.pone.0124194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Botero, Luz Elena , Delgado‐Serrano Luisa, Lucia Cepeda Martha, Bustos Jose Ricardo, Manuel Anzola Juan, Del Portillo Patricia, Robledo Jaime, and Zambrano Maria Mercedes. 2014. “Respiratory Tract Clinical Sample Selection for Microbiota Analysis in Patients with Pulmonary Tuberculosis.” Microbiome 2: 29. 10.1186/2049-2618-2-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ubags, Niki D. J. , and Marsland Benjamin J.. 2017. “Mechanistic Insight into the Function of the Microbiome in Lung Diseases.” European Respiratory Journal 50: 1602467. 10.1183/13993003.02467-2016 [DOI] [PubMed] [Google Scholar]
- 99. Budden, Kurtis F. , Shukla Shakti D., Rehman Saima Firdous, Bowerman Kate L., Keely Simon, Hugenholtz Philip, Armstrong‐James Darius P. H., et al. 2019. “Functional Effects of the Microbiota in Chronic Respiratory Disease.” The Lancet Respiratory Medicine 7: 907–20. 10.1016/S2213-2600(18)30510-1 [DOI] [PubMed] [Google Scholar]
- 100. Herbst, Tina , Sichelstiel Anke, Schar Corinne, Yadava Koshika, Burki Kurt, Cahenzli Julia, McCoy Kathy, Marsland Benjamin J., and Harris Nicola L.. 2011. “Dysregulation of Allergic Airway Inflammation in the Absence of Microbial Colonization.” American Journal of Respiratory and Critical Care Medicine 184: 198–205. 10.1164/rccm.201010-1574OC [DOI] [PubMed] [Google Scholar]
- 101. Yadava, Koshika , Pattaroni Celine, Sichelstiel Anke K., Trompette Aurelien, Gollwitzer Eva S., Salami Olawale, von Garnier Christophe, Nicod Laurent P., and Marsland Benjamin J.. 2016. “Microbiota Promotes Chronic Pulmonary Inflammation by Enhancing IL‐17A and Autoantibodies.” American Journal of Respiratory and Critical Care Medicine 193: 975–87. 10.1164/rccm.201504-0779OC [DOI] [PubMed] [Google Scholar]
- 102. O'Dwyer, David N. , Ashley Shanna L., Gurczynski Stephen J., Xia Meng, Wilke Carol, Falkowski Nicole R., Norman Katy C., et al. 2019. “Lung Microbiota Contribute to Pulmonary Inflammation and Disease Progression in Pulmonary Fibrosis.” American Journal of Respiratory and Critical Care Medicine 199: 1127–38. 10.1164/rccm.201809-1650OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang, Daping , Chen Xi, Wang Jingjing, Lou Qi, Lou Yunwei, Li Li, Wang Honglin, et al. 2019. “Dysregulated Lung Commensal Bacteria Drive Interleukin‐17B Production to Promote Pulmonary Fibrosis through their Outer Membrane Vesicles.” Immunity 50: 692–706.e697. 10.1016/j.immuni.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 104. Singanayagam, Aran , Glanville Nicholas, Cuthbertson Leah, Bartlett Nathan W., Finney Lydia J., Turek Elena, Bakhsoliani Eteri, et al. 2019. “Inhaled Corticosteroid Suppression of Cathelicidin Drives Dysbiosis and Bacterial Infection in Chronic Obstructive Pulmonary Disease.” Science Translational Medicine 11: eaav3879. 10.1126/scitranslmed.aav3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rigauts, Charlotte , Aizawa Juliana, Taylor Steven, Rogers Geraint B., Govaerts Matthias, Cos Paul, Ostyn Lisa, et al. 2021. “ Rothia mucilaginosa is anAnti‐Inflammatory Bacterium in the Respiratory Tract of Patients with Chronic Lung Disease.” European Respiratory Journal 59(5): 2101293. 10.1183/13993003.01293-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. D'Alessandro‐Gabazza, Corina N. , Kobayashi Tetsu, Yasuma Taro, Toda Masaaki, Kim Heejin, Fujimoto Hajime, Hataji Osamu, et al. 2020. “A Staphylococcus Pro‐Apoptotic Peptide Induces Acute Exacerbation of Pulmonary Fibrosis.” Nature Communications 11: 1539. 10.1038/s41467-020-15344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mouraux, Stephane , Bernasconi Eric, Pattaroni Celine, Koutsokera Angela, Aubert John‐David, Claustre Johanna, Pison Christophe, et al. 2018. “Airway Microbiota Signals Anabolic and Catabolic Remodeling in the Transplanted Lung.” Journal of Allergy and Clinical Immunology 141: 718–29. 10.1016/j.jaci.2017.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sommariva, Michele , Noci Valentino Le, Bianchi Francesca, Camelliti Simone, Balsari Andrea, Tagliabue Elda, and Sfondrini Lucia. 2020. “The Lung Microbiota: Role in Maintaining Pulmonary Immune Homeostasis and its Implications in Cancer Development and Therapy.” Cellular and Molecular Life Sciences 77: 2739–49. 10.1007/s00018-020-03452-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gollwitzer, Eva S. , Saglani Sejal, Trompette Aurelien, Yadava Koshika, Sherburn Rebekah, McCoy Kathy D, Nicod Laurent P., Lloyd Clare M., and Marsland Benjamin J.. 2014. “Lung Microbiota Promotes Tolerance to Allergens in Neonates Via PD‐L1.” Nature Medicine 20: 642–7. 10.1038/nm.3568 [DOI] [PubMed] [Google Scholar]
- 110. Collin, Amandine M. , Lecocq Marylene, Noel Sabrina, Detry Bruno, Carlier Francois M., Nana Frank Aboubakar, Bouzin Caroline, et al. 2020. “Lung Immunoglobulin A Immunity Dysregulation in Cystic Fibrosis.” EBioMedicine 60: 102974. 10.1016/j.ebiom.2020.102974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Richmond, Bradley W. , Brucker Robert M., Han Wei, Du Rui‐Hong, Zhang Yongqin, Cheng Dong‐Sheng, Gleaves Linda, et al. 2016. “Airway Bacteria Drive a Progressive COPD‐like Phenotype in Mice with Polymeric Immunoglobulin Receptor Deficiency.” Nature Communications 7: 11240. 10.1038/ncomms11240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Segal, Leopoldo N. , Clemente Jose C., Tsay Jun‐Chieh J., Koralov Sergei B., Keller Brian C., Wu Benjamin G., Li Yonghua, et al. 2016. “Enrichment of the Lung Microbiome with Oral Taxa is Associated with Lung Inflammation of a Th17 Phenotype.” Nature Microbiology 1: 16031. 10.1038/nmicrobiol.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wu, Benjamin G. , Sulaiman Imran, Tsay Jun‐Chieh J., Perez Luisanny, Franca Brendan, Li Yonghua, Wang Jing, et al. 2021. “Episodic Aspiration with Oral Commensals Induces a MyD88‐dependent, Pulmonary T‐Helper Cell Type 17 Response that Mitigates Susceptibility to Streptococcus pneumoniae .” American Journal of Respiratory and Critical Care Medicine 203: 1099–111. 10.1164/rccm.202005-1596OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Budden, Kurtis F. , Gellatly Shaan L., Wood David L. A., Cooper Matthew A., Morrison Mark, Hugenholtz Philip, and Hansbro Philip M.. 2017. “Emerging Pathogenic Links between Microbiota and the Gut–Lung Axis.” Nature Reviews Microbiology 15: 55–63. 10.1038/nrmicro.2016.142 [DOI] [PubMed] [Google Scholar]
- 115. Lai, Hsin‐Chih , Lin Tzu‐Lung, Chen Ting‐Wen, Kuo Yu‐Lun, Chang Chih‐Jung, Wu Tsung‐Ru, Shu Ching‐Chung, Tsai Ying‐Huang, Swift Simon, and Lu Chia‐Chen. 2021. “Gut Microbiota Modulates COPD Pathogenesis: Role of Anti‐Inflammatory Parabacteroides goldsteinii Lipopolysaccharide.” Gut 71: 309–21. 10.1136/gutjnl-2020-322599 [DOI] [PubMed] [Google Scholar]
- 116. Li, Naijian , Dai Zhouli, Wang Zhang, Deng Zhishan, Zhang Jiahuan, Pu Jinding, Cao Weitao, et al. 2021. “Gut Microbiota Dysbiosis Contributes to the Development of Chronic Obstructive Pulmonary Disease.” Respiratory Research 22: 274. 10.1186/s12931-021-01872-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Michalovich, David , Rodriguez‐Perez Noelia, Smolinska Sylwia, Pirozynski Michal, Mayhew David, Uddin Sorif, Van Horn Stephanie, et al. 2019. “Obesity and Disease Severity Magnify Disturbed Microbiome‐Immune Interactions in Asthma Patients.” Nature Communications 10: 5711. 10.1038/s41467-019-13751-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hosang, Leon , Canals Roger Cugota, van der Flier Felicia Joy, Hollensteiner Jacqueline, Daniel Rolf, Flugel Alexander, and Odoardi Francesca. 2022. “The Lung Microbiome Regulates Brain Autoimmunity.” Nature 603: 138–44. 10.1038/s41586-022-04427-4 [DOI] [PubMed] [Google Scholar]
- 119. Whelan, Fiona J. , Waddell Barbara, Syed Saad A., Shekarriz Shahrokh, Rabin Harvey R., Parkins Michael D., and Surette Michael G.. 2020. “Culture‐Enriched Metagenomic Sequencing Enables in‐Depth Profiling of the Cystic Fibrosis Lung Microbiota.” Nature Microbiology 5: 379–90. 10.1038/s41564-019-0643-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data and script were used in this paper. Supporting Information Materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.


