Abstract
Traumatic colon injury (TCI) is a typical injury with high mortality. Prolongation of the intervention time window is a potentially useful approach to improving the outcomes of TCI casualties. This study aimed to identify the pathological mechanisms of TCI and to develop effective strategies to extend the survival time. A semicircular incision was made to prepare a TCI model using C57BL/6 mice. An overview of microbiota dysregulation was achieved by metagenome sequencing. Protein expression reprogramming in the intestinal epithelium was investigated using proteomics profiling. The mice that were subjected to TCI died within a short period of time when not treated. Gut symbiosis showed abrupt turbulence, and specific pathogenic bacteria rapidly proliferated. The protein expression in the intestinal epithelium was also reprogrammed. Among the differentially expressed proteins, SERPINA3N was overexpressed after TCI modeling. Deletion of Serpina3n prolonged the posttraumatic survival time of mice with TCI by improving gut homeostasis in vivo. To promote the translational application of this research, the effects of melatonin (MLT), an oral inhibitor of the SERPINA3N protein, were further investigated. MLT effectively downregulated SERPINA3N expression and mitigated TCI‐induced death by suppressing the NF‐κB signaling pathway. Our findings prove that preventive administration of MLT serves as an effective regimen to prolong the posttraumatic survival time by restoring gut homeostasis perturbed by TCI. It may become a novel strategy for improving the prognosis of patients suffering from TCI.
Keywords: gut homeostasis, melatonin, microbiota dysbiosis, posttraumatic survival time, SERPINA3N, traumatic colon injury
Traumatic colon injury leads to significant disruption of gut homeostasis within a short period of time, indicated by inflammatory responses, intestinal barrier hyperpermeability, and microbiota dysregulation. Melatonin administration can restore gut homeostasis by suppressing SERPINA3N expression in the intestinal epithelium, subsequently prolonging the survival time of mice with traumatic colon injury.

Highlights
SERPINA3N expression is significantly upregulated in the intestinal epithelium of traumatic colon injury (TCI) mice.
Restoration of ectopic SERPINA3N expression prolongs the survival time by attenuating the dysregulation of gut homeostasis in TCI mice.
Preventive administration of melatonin, as an oral inhibitor of SERPINA3N expression, is a potentially useful approach to treat patients with TCI.
INTRODUCTION
Traumatic colon injury (TCI) is a type of abdominal trauma that poses a major threat to human health. Due to the nature of warfare, the incidence of TCI has dramatically increased in disaster‐hit areas and battlefields, and for a large number of patients, TCI leads to poor prognosis and quality of life [1, 2, 3]. Compared with the convenience of casualty hospitalization during peacetime, disaster‐hit and battlefield areas are complicated and unpredictable. The evacuation speeds and stability cannot be guaranteed. TCI patients may die or show rapid disease progression within a short period of time [4, 5]. Current advances in first‐aid equipment and skills have reached an impasse, which hinders the progress of military medical services [6]. Therefore, prolonging posttraumatic survival time and buying time for evacuation to station hospitals could serve as effective approaches to increase the survival rates of TCI patients. Considering the organizational discipline of the army and the foreseeability of warfare, the development of preventive medical regimens may be a promising approach. However, research on this scientific issue is still lacking.
Melatonin (MLT) is the main neuroendocrine product of the pineal gland, synthesized from tryptophan and predominantly secreted during the night. This drug plays an important role in regulating circadian rhythms. The administration of exogenous MLT has been used for the adjuvant treatment of insomnia [7]. With the development of relevant research, accumulating evidence has revealed its other properties, including antioxidation, induction of cancer apoptosis, immunomodulation, and alleviation of senescence [8, 9, 10, 11]. Importantly, MLT has shown the potential to restore intestinal functions. For instance, MLT can ameliorate sepsis‐induced intestinal injury through inhibition of oxidative stress, mitochondrial‐function protection, and autophagic induction [12]. The progression of necrotizing enterocolitis is also alleviated by MLT by activation of the AMPK/SIRT1 signaling pathway and prevention of Th17/Treg imbalance [13]. Hence, MLT may serve as an effective drug to, at least partly, restore gut homeostasis when the intestinal barrier is damaged.
SERPINA3N, also referred to as α‐1‐antichymotrypsin, is a member of the serpin family of protease inhibitors [14]. SERPINA3N is highly expressed in multiple organs, such as the brain, lung, muscle, and kidney [15]. The pathophysiological functions of SERPINA3N protein have been widely investigated. For instance, dysregulation of SERPINA3N expression is closely associated with neuropathological disorders [16, 17]. The overexpression of SERPINA3N has been identified in various types of cancers and may become a biomarker for cancer diagnosis [18]. Moreover, SERPINA3N upregulation contributes to the progression of dilated myocarditis in response to inflammatory responses [19]. However, the role of SERPINA3N during the response to TCI is not well characterized.
In this study, microbiome and proteomics analyses were jointly conducted to explore the mechanisms underlying TCI‐induced death based on a TCI mouse model. A series of in vivo experiments were further performed to elucidate the critical functions of SERPINA3N in the intestinal epithelium during the rapid progression of TCI. The efficacy of MLT, as an effective inhibitor of SERPINA3N, was examined to facilitate the translational application of this research. This preventive medical regimen may provide a novel strategy for managing TCI casualties during disaster rescue and military operations.
RESULTS
The abundance of pathogenic bacteria is abruptly increased after TCI modeling
To gain insights into the effects of TCI on intestinal microbiota, we first induced TCI in mice and performed survival follow‐up (Figure 1A). The mortality rate of TCI mice reached 100% within 96 h, while there were no deaths in the sham group. These data demonstrate that TCI can be a lethal injury for mice without medical intervention (Figure 1B). Next, to investigate microbiome alterations induced by TCI, we set up another batch of experimental groups, including sham and TCI mice, and samples were collected after 24 h of modeling. Metagenome profiling was conducted to compare differences in gut microbiota compositions between the sham and TCI groups. There were no significant differences in alpha diversity, as shown by the Shannon and Chao indices (Figure 1C). Nevertheless, the principal coordinates analysis indicated an obvious separation in the intestinal microbiota structure between these two groups (Figure 1D). These results prove that the damage to colonic integrity induces a rapid shift in dominant bacteria, whereas species evenness and the amounts are unchanged in the short term.
Figure 1.
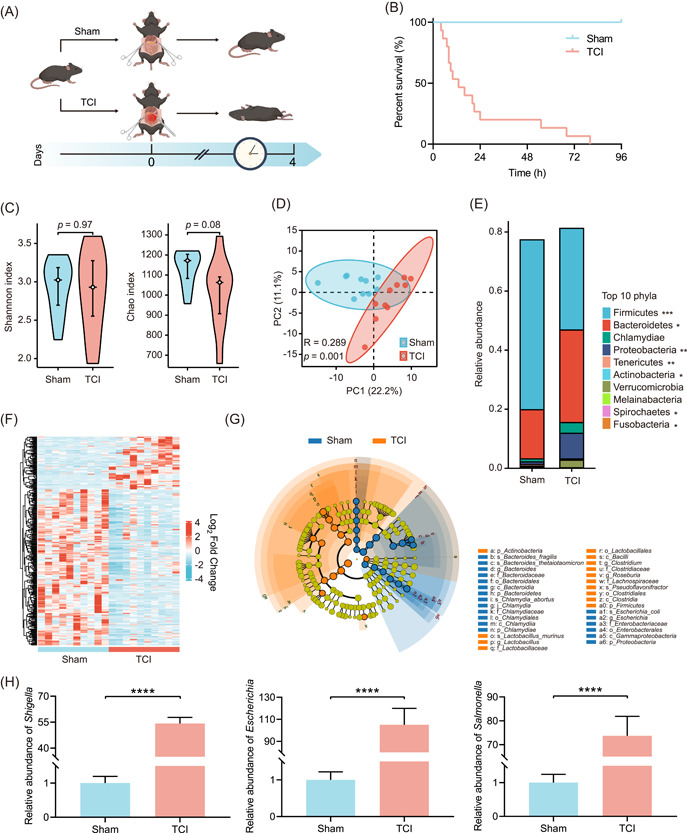
The abundance of pathogenic bacteria is abruptly increased after TCI modeling. (A) Flow diagram used to establish sham and TCI mouse models. TCI was induced in mice by the incision of the colon that was 3 cm from the cecum end. Sham mice were only subjected to laparotomy without colon incision. (B) Survival curve showing the survival differences between sham and TCI groups (n = 15 per group). (C) Violin plots comparing alpha diversities of gut microbiota in the sham and TCI groups. (D) Scatter plot comparing the beta diversity of gut microbiota as in (C). (E) Stacked histogram showing the relative abundances of top 10 phyla of gut microbiota as in (C). (F) Heatmap showing the bacterial genera with different abundances as in (C) (p < 0.05). (G) Cladogram showing the differential bacteria as in (C). The circles from the inside to the outside indicate the taxonomic ranks from phylum to genus. The blue and orange points indicate the bacteria with important functions in the corresponding groups. The yellow points indicate the species without significant differences in relative abundances. (H) qRT‐PCR analysis to determine the relative abundances of Shigella, Escherichia, and Salmonella in intestinal contents of sham and TCI mice (n = 15 per group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. qRT‐PCR, quantitative real‐time polymerase chain reaction; TCI, traumatic colon injury.
We next analyzed the alterations in specific bacteria induced by TCI. The histogram shows the compositions of the bacterial communities of the sham and TCI mice (Figure 1E). The heatmap showed that the abundances of more than 300 genera showed appreciable changes (Figure 1F). Moreover, linear discriminant analysis effect size (LEfSe) analysis was carried out to identify operational taxonomic units (OTUs) and taxa with different abundances in sham and TCI mice. The dominant bacteria at different levels were affected by TCI (Figure 1G). We found that the abundances of three typically pathogenic bacteria, Shigella, Escherichia, and Salmonella, abruptly increased in the intestines of TCI mice. Quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis was performed to confirm these results as shown by metagenome profiling. The abundances of Shigella, Escherichia, and Salmonella increased by 54.194 ± 3.581, 105.067 ± 14.860, and 73.763 ± 8.102 fold, respectively (Figure 1H). Moreover, the abundances of two kinds of opportunistic pathogens, Cronobacter and Proteus, were also upregulated according to the high‐throughput data, which was then confirmed by qRT‐PCR analysis (Cronobacter: 29.914 ± 1.894; Proteus: 14.847 ± 1.122, Supporting Information: Figure S1). These data collectively prove that TCI can disrupt gut microbiota symbiosis and that pathogenic bacteria rapidly proliferate during this pathological process.
TCI reprograms the protein expression profile of the intestinal epithelium following TCI challenge
The TCI‐induced alterations in the protein profile of the intestinal epithelium were not previously investigated. Herein, we used proteomics profiling to reveal the mechanisms underlying protein expression reprogramming, followed by TCI modeling. The results showed 71 upregulated proteins and 40 downregulated proteins in the epithelium of TCI mice (log2FC < −1 or >1, p < 0.05). Among all the significantly altered proteins, SERPINA3N was found to be upregulated by over 10‐fold in the intestinal epithelium (Figure 2A). Furthermore, these differentially expressed proteins were found to be enriched by Gene Ontology (GO) analysis, and can be classified into biological process (BP), cellular component (CC), and molecular function (MF) categories. According to the top 10 enriched categories in BP analysis, differentially expressed proteins detected among the sham and TCI groups were primarily involved in inflammatory responses (Figure 2B). Extracellular processes and lipid metabolism were the representative terms in CC (Figure 2C). As shown by the MF analysis, protein modification and catabolism were mostly enriched, and the impact of TCI on lipid metabolism was also confirmed (Figure 2D). To specifically identify the pathological processes during TCI progression, we reviewed the literature on the functions of the differentially expressed proteins. Most of them could be clustered into three aspects: inflammation, infection defense, and barrier integrity. These related proteins are shown in the heatmap (Figure 2E). IHC examination was then performed to validate the upregulated expression of SERPINA3N in the intestinal epithelium (Figure 2F).
Figure 2.

TCI reprograms the protein expression profile of the intestinal epithelium following TCI challenge. (A) Volcano plot showing the differential proteins in intestinal epithelium (n = 10 per group, log2FC < −1 or >1, p < 0.05). (B–D) Bubble plots showing the top enriched 10 items of BP, CC, and MF categories by GO analysis. (E) Heatmap showing differential proteins reported to be associated with inflammation, infection defense, and barrier integrity. (F) IHC examination used to detect the SERPINA3N expression in the intestinal epithelium of sham and TCI groups. The histogram is displayed below the IHC pictures. Scale bar: 100 μm. ****p < 0.0001. BP, biological process; CC, cellular component; GO, Gene Ontology; IHC, immunohistochemistry; MF, molecular functional; TCI, traumatic colon injury.
Knockout of Serpina3n protein prolongs the survival time of TCI mice
Regarding the ectopic expression of SERPINA3N during TCI progression, it was speculated that restoration of a low level of SERPINA3N expression might alleviate TCI aggravation and prolong posttraumatic survival time. We first established Caco‐2 cells with stable knockdown and overexpression of Serpina3n for in vitro experiments. The interfering effects were verified by WB analysis (Supporting Information: Figure S2A). Transwell assays showed that downregulation of Serpina3n expression enhanced the migration of Caco‐2 cells and that Serpina3n overexpression led to significant suppression of cell migration (Supporting Information: Figure S2B,C). To further detect its regulatory functions in vivo, we generated Serpina3n KO mice and conducted IHC to confirm the loss of SERPINA3N in the KO mice (Supporting Information: Figure S3). TCI modeling was again performed using wild‐type (WT) and Serpina3n KO mice (Figure 3A). Posttraumatic survival analysis showed that Serpina3n deletion contributed to survival prolongation in TCI mice (TCI+Serpina3n KO versus TCI+WT: hazard ratio [HR] = 0.361, 95% confidence interval [CI] 0.160–0.815, p = 0.004) (Figure 3B). However, SERPINA3N is expressed in multiple organs. To further explore the regulatory role of SERPINA3N in the gut, we generated conditional knockout (CKO) mice with the absence of Serpina3n expression in the gut epithelium (Supporting Information: Figure S4A). The TCI model was subsequently established using Serpina3n CKO, Serpina3n KO, and control mice. The posttraumatic survival time of Serpina3n CKO mice was significantly extended compared to the control mice (TCI+Serpina3n CKO versus TCI: HR = 0.325, 95% CI 0.139–0.756, p = 0.009). There were no statistical differences between the survival time of Serpina3n CKO mice and Serpina3n KO mice (TCI+Serpina3n CKO versus TCI+Serpina3n KO: HR = 1.186, 95% CI 0.520–2.704, p = 0.686) (Figure S4B). These results suggest that deletion of Serpina3n in the gut alleviated TCI progression, rather than knockout of Serpina3n in other organs. Taken together, both in vitro and in vivo experiments indicate that abrupt upregulation of Serpina3n plays an important role in TCI progression toward death. Elimination of Serpina3n efficaciously extended the posttraumatic survival time of TCI mice.
Figure 3.

Knockout of SERPINA3N protein prolongs the survival time and gut homeostasis of TCI mice. (A) Flow diagram used to establish the Serpina3n KO mice that were subjected to sham or TCI operation and WT mice that were subjected to the TCI operation (n = 15 per group). (B) Survival curve comparing the survival time of mice as in (A). (C) ELISA assay used to detect the concentrations of IL‐6 and TNF‐α in the plasma of WT or Serpina3n KO mice that were subjected to sham or TCI operation (n = 15, 15, 11, and 13, respectively). (D) ELISA assay used to detect the concentrations of IL‐6 and TNF‐α in the intestines of mice as in (C). (E) IHC examination used to determine ZO‐1 and occludin expression in the intestinal epithelium of mice as in (C). The histogram is displayed on the right of the pictures. Scale bar: 100 μm. (F) qRT‐PCR analysis used to determine the relative abundances of Shigella, Escherichia, and Salmonella in the intestinal contents of mice as in (C). ***p < 0.001, ****p < 0.0001. ELISA, enzyme‐linked immunosorbent assay; IHC, immunohistochemistry; IL‐6, interleukin‐6; KO, knockout; n.s, not significant; qRT‐PCR, quantitative real‐time polymerase chain reaction; TCI, traumatic colon injury; TNF‐α, tumor necrosis factor‐α; WT, wild type.
Ablation of Serpina3n restores gut homeostasis perturbed by TCI
Since there are close associations between gut homeostasis and the survival time of TCI mice, as indicated by proteomics profiling, we then investigated the effects of Serpina3n knockout on inflammation, intestinal hyperpermeability, and bacterial dysregulation. Another batch of experimental groups was established and sample collection was conducted after 24 h of modeling. ELISA assay was first used to determine the concentrations of tumor necrosis factor‐α (TNF‐α) and interleukin‐6 (IL‐6) in the WT and Serpina3n KO mice that were subjected to sham or TCI modeling. The results showed that TCI induced a marked increase in TNF‐α and IL‐6 in both plasma and intestines. Deletion of Serpina3n had no significant impacts on these indicators in sham mice, whereas it partly reversed the increase of TNF‐α and IL‐6 induced by TCI (Figure 3C,D). Moreover, C‐reactive Protein (CRP) is an early indicator of inflammatory conditions. TCI led to a significant increase of plasma CRP, and Serpina3n deletion partly mitigated the effects of TCI on CRP levels (Supporting Information: Figure S5A).
Plasma diamine oxidase (DAO), D‐lactate, and endotoxin were selected as markers of gut barrier permeability. TCI modeling resulted in drastic increases in the three indicators, which were partly inhibited by knockout of Serpina3n in TCI mice (Supporting Information: Figure S5B). ZO‐1 and occludin are tight junction proteins that are widely expressed in the intestinal barrier. IHC showed that TCI reduced ZO‐1 and occludin expression in the epithelium. The expression levels of these molecules were found to be partly restored in Serpina3n KO mice that were subjected to TCI (Figure 3E).
To investigate the effects of Serpina3n on microbiota dysbiosis, we chose five pathogenic or opportunistic pathogenic genera, Shigella, Escherichia, Salmonella, Cronobacter, and Proteus, as representative markers and detected these using qRT‐PCR. Similar to the results of inflammation and barrier permeability, elimination of Serpina3n partly suppressed their proliferation in the intestines (TCI+WT vs. TCI+KO: Shigella: 52.765 ± 12.250 vs. 24.541 ± 6.97; Escherichia: 97.493 ± 13.401 vs. 63.588 ± 8.990; Salmonella: 70.256 ± 12.068 vs. 50.665 ± 8.398; Cronobacter: 30.703 ± 2.237 vs. 16.436 ± 2.264; Proteus: 14.797 ± 1.357 vs. 8.368 ± 0.850. Figures 3F and Supporting Information: Figure S5C). These data collectively demonstrate that deletion of Serpina3n alleviates the dysregulation of gut homeostasis in vivo, characterized by inflammatory attenuation, barrier integrity improvement, and dysbiosis inhibition.
Administration of MLT extends the survival time of TCI mice by downregulation of SERPINA3N expression
MLT has been reported to suppress SERPINA3N expression in vivo [20]. Based on this evidence, we speculated that administration of MLT could prolong the survival time of TCI mice. SERPINA3N expression was measured using WB analysis in Caco‐2 cells pretreated with gradient doses of MLT. With increasing MLT concentrations, SERPINA3N protein levels gradually decreased (Supporting Information: Figure S6A). The migration capabilities of Caco‐2 cells were subsequently inhibited by MLT treatment in a significant dose‐dependent manner (Supporting Information: Figure S6B). Next, in vivo, the mice were orally administered with MLT for 14 days before TCI modeling (Figure 4A). The mice proactively treated with MLT showed better survival time than the TCI group (TCI+MLT vs. TCI+vehicle: HR = 0.374, 95% CI 0.166‐0.842, p = 0.0039, Figure 4B). The suppressive effects of MLT on SERPINA3N expression were confirmed using IHC examinations (Supporting Information: Figure S6C).
Figure 4.

Administration of MLT extends the survival time and gut homeostasis of TCI mice by downregulation of SERPINA3N expression. (A) Flow diagram used to generate the mice in the sham or TCI groups that were administered with vehicle or MLT as indicated (n = 15 per group). (B) Survival curve comparing the survival time of mice as in (A). (C) ELISA assay used to detect the concentrations of IL‐6 and TNF‐α in the plasma of mice in the sham or TCI group that were administered with vehicle or MLT (n = 15, 15, 10, and 13, respectively). (D) ELISA assay used to detect the concentrations of IL‐6 and TNF‐α in the intestines of mice as in (C). (E) IHC examination used to determine ZO‐1 and occludin expression in the intestinal epithelium of mice as in (C). The histogram is displayed on the right of the pictures. Scale bar: 100 μm. (F) qRT‐PCR analysis used to determine the relative abundances of Shigella, Escherichia, and Salmonella in intestinal contents of mice as in (C). **p < 0.01, ***p < 0.001, ****p < 0.0001. ELISA, enzyme‐linked immunosorbent assay; IHC, immunohistochemistry; IL‐6, interleukin‐6; MLT, melatonin; n.s, not significant; qRT‐PCR, quantitative real‐time polymerase chain reaction; TCI, traumatic colon injury; TNF‐α, tumor necrosis factor‐α; WT, wild type.
Furthermore, to confirm that SERPINA3N is a downstream target of MLT, Caco‐2 cells with stable overexpression of SERPINA3N were created and treated with MLT. WB analysis showed that MLT effectively mitigated SERPINA3N expression in both control Caco‐2 cells and Caco‐2 cells overexpressing SERPINA3N (Supporting Information: Figure S7A). For the migratory capability, MLT could also suppress the inhibitory effects of upregulation of SERPINA3N expression (Supporting Information: Figure S7B,C). In vivo, survival analysis demonstrated that MLT and Serpina3n KO prolonged the survival time of TCI mice, which was consistent with previous results. However, no significant survival benefits from MLT were observed in TCI mice with the deletion of Serpina3n (Supporting Information: Figure S7D). The data collectively prove that MLT extends the posttraumatic survival time of TCI mice by targeting SERPINA3N expression.
MLT protects against TCI by restoring the disruption of gut homeostasis
Because of the associations between SERPINA3N and gut homeostasis, we conducted a series of experiments to detect indicators of inflammation, intestinal permeability, and pathogenic bacteria for the determination of homeostasis status. MLT reduced TNF‐α, IL‐6, and CRP, suggesting that the systemic and gut inflammatory responses were effectively inhibited (Figure 4C,D and Supporting Information: Figure S8A). The levels of three barrier permeability indicators, DAO, D‐lactate, and endotoxin, were downregulated (Supporting Information: Figure S8B). ZO‐1 and occludin protein expression was also rescued by MLT administration (Figure 4E). In the examinations of pathogenic bacterial abundances, a significant delay in Shigella, Escherichia, Salmonella, Cronobacter, and Proteus proliferation was observed (TCI+vehicle vs. TCI+MLT: Shigella: 52.705 ± 9.904 vs. 18.503 ± 6.214; Escherichia: 104.730 ± 17.057 vs. 40.217 ± 6.607; Salmonella: 72.386 ± 11.031 vs. 37.927 ± 6.503; Cronobacter: 31.253 ± 3.602 vs. 15.594 ± 2.350; and Proteus: 15.514 ± 1.702 vs. 7.97 ± 1.101, Figure 4F and Supporting Information: Figure S8C).
MLT suppresses SERPINA3N expression by inhibiting the NF‐κB signaling pathway
To investigate the mechanisms underlying MLT‐downregulated SERPINA3N expression in the gut epithelium of TCI mice, we conducted Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis to clarify alterations in the signaling pathways of gut epithelium. The results indicated that the NF‐κB signaling pathway of the gut epithelium ranked first among the altered pathways after TCI modeling (Figure 5A). Thus, we speculated that MLT might prolong the survival time of TCI mice by reducing activation of the NF‐κB signaling pathway. To validate this assumption, we first examined the activity of the NF‐κB signaling pathway in Caco‐2 cells treated with different doses of MLT. The phosphorylation of NF‐κB decreased with increasing MLT doses (Figure 5B).
Figure 5.

MLT suppresses SERPINA3N expression by inhibiting the NF‐κB signaling pathway. (A) Histogram showing the signaling pathways that ranked top 10 according to KEGG analysis based on proteomics profiling. (B) WB analysis used to determine the p‐NF‐κB, NF‐κB, and SERPINA3N expression in Caco‐2 cells treated with 0.01, 0.1, and 1 μM MLT for 24 h. (C) WB analysis used to determine the p‐NF‐κB, NF‐κB, and SERPINA3N expression in Caco‐2 cells treated with vehicle, MLT, 100 μM JSH‐23, or MLT plus 100 μM JSH‐23. (D and E) Transwell assay used to determine cell migration capabilities of cells as in (C). The histogram is displayed on the right. Scale bar: 100 μm. (F) Survival curve comparing the survival time of TCI mice treated with vehicle, MLT, 3 mg/kg JSH‐23, and MLT plus 3 mg/kg JSH‐23 (n = 15 per group). (G) IHC examination used to determine SERPINA3N, p‐NF‐κB, and NF‐κB expression in the intestinal epithelium of mice that were subjected to the same treatment as in (F). Scale bar: 100 μm. (H) Histogram showing IHC scores as in (G). ***p < 0.001, ****p < 0.0001. KEGG, Kyoto Encyclopedia of Genes and Genomes; IHC, immunohistochemistry; MLT, melatonin; n.s not significant; TCI, traumatic colon injury; WB, western blot.
To further confirm the causality, we used JSH‐23, an inhibitor targeting the NF‐κB signaling pathway, to treat Caco‐2 cells. WB analysis showed that MLT or JSH‐23 reduced the phosphorylation of NF‐κB and SERPINA3N expression. There were no differences in the effects of MLT and JSH‐23. However, MLT could not further downregulate SERPINA3N expression on the basis of JSH‐23 treatment (Figure 5C). Likewise, MLT or JSH‐23 effectively promoted Caco‐2 cell migration. The effects of these two drugs were statistically consistent. MLT did not further facilitate the migratory capabilities of Caco‐2 cells after treatment with JSH‐23 (Figure 5D,E). Next, in vivo experiments were performed to validate these results. Mice were pretreated with 1 mg/kg JSH‐23 before 14 days of TCI modeling. A single treatment of JSH‐23 significantly extended the survival time of TCI mice, which was comparable to MLT (TCI+JSH‐23 vs. TCI+MLT: HR = 0.826, 95% CI 0.357–1.911, p = 0.8263). Moreover, combined use of JSH‐23 and MLT did not further promote prolongation of survival time (TCI+JSH‐23+MLT vs. TCI+JSH‐23: HR = 0.726, 95% CI 0.299–1.765, p = 0.4797, Figure 5F). IHC examination also showed that JSH‐23 significantly suppressed SERPINA3N expression and NF‐κB phosphorylation. No more significant effects of combinational treatment of JSH‐23 and MLT were observed (Figure 5G,H). Taken together, these data demonstrate that preventive administration of MLT can downregulate SERPINA3N expression by suppressing the NF‐κB signaling pathway, subsequently extending the posttraumatic survival time of TCI mice (Figure 6).
Figure 6.

Schematic illustration of the mechanisms by which MLT administration prolongs the posttraumatic survival time of TCI mice. TCI leads to significant disruption of gut homeostasis within a short period of time, indicated by inflammatory responses, intestinal barrier hyperpermeability, and microbiota dysregulation. MLT administration can restore gut homeostasis by suppressing SERPINA3N expression in the intestinal epithelium, subsequently prolonging the survival time of TCI mice. MLT, melatonin; TCI, traumatic colon injury.
DISCUSSION
Intestinal injury can be caused by multiple mechanical and nonmechanical factors. In the past few decades, the disease spectrum and therapeutic regimens of intestinal injury have significantly changed due to alterations in the social environment. Most related studies aimed to reveal the mechanisms of nonmechanical intestinal injuries, such as radiation enteritis, inflammatory bowel disease, and intestinal dysfunctions after hemorrhagic shock [21, 22, 23]. Several novel regimens have emerged due to a better understanding of intestinal injury pathogenesis. For instance, a recent clinical trial reported that dietary glutamine supplements could normalize intestinal hyperpermeability [24]. Tranexamic acid, a serine protease inhibitor, is able to attenuate gut barrier failure caused by hypoxia, ischemia–reperfusion, and hemorrhagic shock [25, 26]. Guo et al. identified the pivotal role of specific gut microbiota and metabolites in radioprotection [21]. Research on nonmechanical intestinal injuries has made tremendous progress, whereas breakthroughs in intervention strategies against traumatic intestinal injury are absent.
To the best of our knowledge, this is the first study to conduct proteomics profiling to explore critical molecules during the progression of TCI. Damage to intestinal wall integrity led to significant reprogramming of protein expression in the intestinal epithelium. Clustering analysis showed that molecules involved in inflammatory responses, metabolic disorders, tissue repair, and bacterial defense were significantly dysregulated. These findings suggested similarities in physiopathological processes between the intestines and other organs in response to traumatic injury [27, 28]. The landscape of protein reprogramming can reveal the molecular mechanisms of TCI progression and provide data support for subsequent research.
In our previous study, we generated a TCI animal model using Sprague–Dawley rats and found that disruption of gut homeostasis contributed to injury‐induced death [29]. This finding is consistent with the data based on experiments using C57BL/6 mice, further confirming the reliability of our modeling methods. A series of experiments demonstrated that TCI acutely induced gut homeostasis, indicated by inflammation, intestinal hyperpermeability, and dysbiosis. It is noteworthy that microbiota symbiosis is essential for human health, and dysregulation of microbiota has been proven to be a contributor to many diseases [30, 31]. Based on the bioinformatic analysis of metagenome data, there were no differences in alpha diversities between the sham and TCI groups, whereas beta diversity was significantly altered. These results imply that although species evenness of the gut microbiota TCI is unchanged within a short period of time after TCI modeling, the bacterial community undergoes a huge disruption and the displacement of dominant bacteria is induced.
Shigella, Salmonella, and Escherichia are the classical pathogenic bacteria, and their abundances are under rigid regulation in human intestines. Their rapid proliferation can induce intestinal inflammation, electrolyte disturbances, and even sepsis [32, 33, 34]. The abundances of the three pathogenic bacterial genera were significantly upregulated after damage to intestinal integrity. These above‐mentioned pathological alterations are not limited to TCI progression. Many other types of intestinal diseases, such as inflammatory bowel diseases, radiation enteritis, and metabolic disorders, also show similar pathological mechanisms [35, 36, 37]. According to the metagenome data, there may be other events of microbiota dysregulation contributing to TCI progression. For instance, the Cronobacter genus has been proven to be a pathogen that can cause a large number of diseases, such as meningitis, necrotizing enterocolitis, sepsis, and brain abscesses [38]. The relative abundance of Cronobacter was significantly upregulated in the gut of TCI mice, which may be another reason for TCI‐induced rapid death. The Proteus genus, another putative gastrointestinal pathogen, was found to typically proliferate in the gut [39]. The balance of Coriobacterium, Lyngbya, and many other bacteria with unknown functions was also damaged. These data suggest that the associations between TCI progression and microbiota dysregulation are complicated. More investigations should be conducted to identify important events and mechanisms of TCI and develop more effective intervention regimens.
The role of SERPINA3N in regulating intestinal disorders has not been investigated in depth and remains controversial. Zhang et al. [40] used bioinformatic analysis and a mouse model in combination to reveal its potential value in the diagnosis and therapy of ulcerative colitis (UC). SERPINA3N serves as a biomarker of active UC. Knockdown of Serpina3n expression attenuates inflammatory responses of intestinal epithelial cells. Nevertheless, another study used single‐cell transcriptomics to investigate the interactions of intestinal cells in an animal model of chemically induced colitis. SERPINA3N participates in the resolution of inflammation and the amelioration of colitis symptoms [41]. Our study demonstrated that SERPINA3N expression was upregulated in the intestinal mucosa after TCI modeling. Ablation of SERPINA3N effectively enhanced the migratory capability of intestinal epithelial cells in vitro and prolonged the posttraumatic survival time of TCI mice by improving gut homeostasis. The contradictions may be attributed to the characteristics of different intestinal diseases and model generation methods. SERPINA3N may act as a positive regulator of chronic inflammation. Nevertheless, the outburst of SERPINA3N expression upregulation may aggravate dysfunctions of the intestinal epithelium during the early stage of TCI progression. The delayed inhibition of SERPINA3N upregulation may prevent the progression of acute gut disorders.
Oral administration is generally recognized as the most convenient and acceptable route for medication, especially in complicated environments such as disaster‐hit areas and battlefields. Because of the critical role of SERPINA3N in the development of TCI, we searched for a highly potent inhibitor to block SERPINA3N upregulation and validate its curative effects on posttraumatic survival prolongation. Xi et al. reported that MLT can significantly inhibit SERPINA3N upregulation induced by neuroinflammation [20]. We speculated that MLT could serve as a drug for the restoration of intestinal dysregulations induced by TCI. This assumption was confirmed from our experimental data. Oral administration of MTL mitigated inflammatory responses, intestinal hyperpermeability, and rapid proliferation of pathogenic bacteria, subsequently prolonging the survival time of TCI mice. Otherwise, the safety of long‐term MLT use has already been confirmed [42]. The other properties of MLT may further improve the physical and mental health of recipients, such as sleep promotion, analgesia, and immunity enhancement [43, 44, 45]. These features confer unique advantages to MLT application for individuals under threat from TCI. MLT has excellent potential as a preventive regimen for survival extension of TCI causalities.
However, there are some limitations to the current study. First, we only proved that MLT suppresses SERPINA3N expression in the intestinal epithelium. The precise mechanisms by which MLT suppresses SERPINA3N expression still need to be investigated. Second, MLT has been reported to be a multitarget drug [46]. Our findings prove that SERPINA3N is a regulatory target in the intestinal epithelium. Whether there are other underlying targets of posttraumatic survival prolongation is unknown. Third, a mouse model was used to simulate TCI casualties. Notably, there are significant differences between animal models and humans. The value of MLT administration should be carefully evaluated by clinical trials.
CONCLUSION
Our findings demonstrate that SERPINA3N is overexpressed in the intestinal epithelium of TCI mice, which contributes to the aggravation of inflammation, intestinal hyperpermeability, and microbiota dysbiosis. Preventive administration of MLT, a SERPINA3N inhibitor, effectively restored gut homeostasis, further prolonging the posttraumatic survival time of TCI mice. MLT medication may serve as a novel strategy of delayed treatment for high‐risk populations.
METHODS
Cell culture
Caco‐2, a human colon epithelial cancer cell line, was purchased from the American Type Culture Collection (ATCC, #BFN60800651). Cell lines were cultivated using Dulbecco's modified Eagle's medium (DMEM; Gibco, #C11995500BT, Thermo Fisher Scientific) with 10% fetal bovine serum (FBS; Excell Bio, #FCS500) and 1% penicillin‐streptomycin solution (100×; Gibco, #15140163). The incubator was invariably set at 37°C and 5% CO2.
Pharmaceutical preparations
In the in vitro experiments, MLT solution was prepared using dimethyl sulfoxide (DMSO; MedChemExpress, #HY‐Y0320, USA) according to the indicated doses, which ensured that the volumes of DMSO that were used to treat cells did not exceed 0.5% of the volume of the cell culture medium. 100 μM JSH‐23 was also prepared with DMSO. DMSO served as the reference substance. In the in vivo experiments, the vehicle consisted of 15% Cremophor EL (MedChemExpress, #HY‐Y1890) and 85% sterile water. The oral administration doses of MLT (MedChemExpress, #HY‐B0075) and JSH‐23 (MedChemExpress, #HY‐13982) were 0.2 mg/mL and 3 mg/kg, respectively.
Basic animal experiment
Six‐week‐old male C57BL/6 mice were purchased from SPF Biotechnology. After admission to the animal center, a 14‐day acclimatization process was followed to reduce the potential bias caused by the environment. The mice were fed under a specific pathogen‐free environment with 40%–70% relative humidity, 22 ± 2°C, and a 12 h/12 h illumination period. Mice were provided pure water and sterile standard fodder ad libitum (SPF Biotechnology). Each C57BL/6 mouse was fed in one cage.
For administration of MLT or JSH‐23, 3 mL of vehicle supplemented with drugs was added in the feeding bottle. This volume did not exceed the daily water intake of mice, which guaranteed the full administration of drugs within one day. This dose of MLT was based on the methods reported by a previous study [47]. The reference substance, namely, vehicle, was sterile water. After the mice drank the solution, they were given sterile water for the rest of the day. For prevention of MLT degradation induced by light, an aluminum foil was used to wrap the bottle surface. The euthanasia of mice was performed by gradient elevation of the CO2 atmosphere until confirmation of death. The samples collected from the mice were stored at −80°C if not immediately used. It is noteworthy that the sample collection was performed after 24 h of modeling. The mice used were different from the batch used for observation of survival time.
Preparation of the TCI mouse model
Anesthesia was administered to the mice by an intraperitoneal injection of 40 mg/kg pentobarbital sodium (Hnalt Biotechnology). The mice were fixed on the operating table for small animals placed on a clean bench. The fur on the abdomen was shaved, and the skin was disinfected. The anatomical layer of the abdomen was successively incised. For simulation of TCI conditions, the incision of the colon was 3 cm from the cecum end. It was cut open semicircularly, and the wound was perpendicular to the long axis of the intestinal tract. For the mice in the sham group, incision of the colon was not performed, but the other operation processes were conducted. The wound in the abdominal wall was closed using stitches.
Generation of Serpina3n KO and CKO mice
Mice with Serpina3n KO were established using the CRISPR‐Cas9 gene‐editing system (Cyagen Biosciences, USA). The sgRNA target sequences were introduced as follows: gRNA‐A1: GCGTTCAACTATAGTCCATT‐AGG; gRNA‐A2: CTGCAGCATCTAGGACTACT‐TGG; gRNA‐B1: AGGGGTCCAGGTTACATTAT‐TGG; gRNA‐B2: CTGGGACTGAGCTTACTTGA‐TGG. Cas9 mRNA with specific sgRNAs was injected into the fertilized eggs of C57BL/6 mice. The eggs were implanted into a pseudopregnant female surrogate mouse. We used the Cre/LoxP system (Cyagen Biosciences, USA) to cross‐breed Serpina3n flox/flox and Vil1‐Cre genotyped mice for multiple generations until sufficient numbers of Serpina3n flox/flox; Vil1‐Cre mice and Serpina3n flox/flox mice were obtained. The Loxp primers were as follows: Loxp1: F1: 5′‐GGGGCATATGGTATGAATCTGGAA‐3′; R1: 5′‐ATATCGGATCAGTTCGTAATCCCC‐3′. Loxp2:F2:5′‐CTTTATAGCCAAGATAGCCAACCC‐3′; R2:5′‐CAAATTTCAGGCTCAGGAACATGG‐3′.
Metagenome sequencing
For elucidation of TCI‐induced alterations of intestinal symbiosis, total genomic DNA was extracted from intestinal content samples using a PowerSoil DNA Isolation kit (MoBio Laboratories, #12888) according to the manufacturer's protocol. The quality and quantity of the extracted DNA were examined using a Qubit dsDNA HS Assay Kit on a Qubit 3.0 Fluorometer (Life Technologies, #Q33216). A VAHTS Universal Plus DNA Library Prep Kit for Illumina (Vazyme Biotech, #ND617‐01) was used to construct paired‐end libraries. The library was sequenced on an Illumina NovaSeq. 6000 platform (Biomarker Technology) using the 150‐bp paired‐end sequencing mode. After trimming the adaptors and filtering the low‐quality reads, the clean sequence data were further analyzed using the software R package.
Proteomics profiling
Label‐free quantitative proteomics was used to investigate the effects of TCI on protein expression in the intestinal epithelium. The intestinal mucosae of sham and TCI mice were collected and lysed with grinders and lysis buffer. The tissue debris was removed by centrifugation. The protein concentrations of the samples were determined using a Pierce ECL Plus Kit (Thermo Scientific, #32132). An EASY‐nLC 1000 System (Thermo Scientific) and an Orbitrap Fusion Mass Spectrometer (Thermo Scientific, #IQLAAEGAAPFADBMBHQ) were used in combination to record the protein spectra of the samples. Further data analysis was performed using MaxQuant software.
Establishment of cell lines with stable interference of SERPINA3N expression
The plasmids carrying shRNA targeting Serpina3n or Serpina3n overexpression sequences were designed and synthesized by JTS Scientific. A lentiviral packaging kit (Yeasen, #41102ES40 was used to prepare lentiviruses carrying stable interference sequences according to the protocol. Caco‐2 cells were subsequently cultivated with lentivirus for cell infection. Puromycin (5 μg/mL) was used to chemically select cells that were successfully infected.
qRT‐PCR
To determine the relative abundances of specific pathogenic bacteria, the colon contents were harvested from mice and total bacterial DNA was extracted using a QIAamp FAST DNA Stool Mini Kit (Qiagen; Dusseldorf, #51604). The quality of the extract was measured by Microdrop (BIO‐DL). The DNA samples were amplified using SYBR Premix Ex Taq II (TaKaRa, #RR820A). The 2−ΔΔCt method was performed to calculate the relative abundances of specific genera. The primer sequences for qRT‐PCR are listed in Supporting Information: Table S1.
Western blot analysis (WB) analysis
The collected samples were washed with cold phosphate‐buffered saline and lysed using radioimmunoprecipitation assay buffer (Biorigin, #BN25012) with protease inhibitor cocktail (Biorigin, #BN25002). The sample debris was removed by high‐speed centrifugation. Next, the proteins were subjected to gel electrophoresis according to the protein sizes and transferred onto polyvinylidene difluoride membranes (Millipore, #V515818, Merk). Then, 5% defatted milk was prepared to block the membranes for 1 h at room temperature. The membranes were then incubated with primary antibodies at 4°C overnight and then with secondary antibodies at room temperature for 2 h. The antibodies and the corresponding dilutions are as follows: anti‐SERPINA3N antibody (Bioss, #bs‐0094R, 1:1000), anti‐NF‐κB antibody (Abcam, #ab32536, 1:1000), anti‐p‐NF‐κB antibody (Abcam, #ab86299, 1:1000), anti‐β‐actin antibody (Abcam, #ab32536, 1:1000), and anti‐rabbit IgG (Abcam, #ab6721, 1:3000). The blots were imaged using a Pierce ECL Plus Kit.
Immunohistochemistry (IHC) examination
An IHC assay was conducted to determine ZO‐1 and occludin expression in situ in the intestinal mucosa. Briefly, the tissues were fixed using 4% paraformaldehyde (Servicebio, #G1101, Beijing, China) and embedded in paraffin. A slicer was used to prepare sections that were mounted on sample slides. After deparaffinization, the slides were stained using specific primary and secondary antibodies. The used antibodies and their corresponding dilutions are as follows: anti‐ZO‐1 antibody (Abcam, #ab221547, 1:200), anti‐Occludin antibody (Abcam, #ab216327, 1:200), anti‐SERPINA3N antibody (Bioss, #bs‐0094R, 1:200), anti‐NF‐κB antibody (Abcam, #ab32536, 1:200), anti‐p‐NF‐κB antibody (Abcam, #ab86299, 1:200), and anti‐rabbit IgG (Abcam, #ab6721, 1:1000). The IHC score evaluation was also conducted as described in our previous study [29].
Transwell assay
To analyze the effects of SERPINA3N and MLT on the migratory capabilities of intestinal epithelial cells, 5 × 104 treated Caco‐2 cells were harvested and cultivated in FBS‐free DMEM. Then, they were seeded in the upper chamber of transwells (Corning, #3460) and the bottom chamber was filled with 600 μL of DMEM with 20% FBS. 24‐well plates loaded with transwells were placed in the incubator. After 24 h, the transwells were fixed with 4% paraformaldehyde and cells that migrated to the lower chamber were stained with 0.1% crystal violet (Biorigin, #BN24042). The cells were counted under an optical microscope (Leica).
Enzyme‐linked immunosorbent assay (ELISA)
ELISA was conducted to measure the levels of indicators of inflammation and intestinal permeability. Peripheral blood was harvested and centrifuged at 1800g for 10 min to prepare plasma. ELISA kits for the detection of endotoxin (Enzyme‐linked Biotechnology, #ml‐E6008, Shanghai, China), D‐lactate (Nanjing Jiancheng, #A019), DAO (Enzyme‐linked Biotechnology, #ml‐E4175), IL‐6 (Abbkine, #KTE7009), TNF‐α (Abbkine, #KTE7015), and CRP (Multi Sciences, #EK294/2) were used according to the manufacturers' protocols. The absorbances of the samples were determined using a microplate reader (BioTek). The relative level of each group was calibrated based on the control group.
Statistical analysis
The statistical analysis, survival comparison, and data presentation were performed using Prism 8.0 and SPSS 25.0. The Shapiro–Wilk test was conducted to confirm data normality. Data are presented as the mean ± standard deviation (SD). Student's t‐test was used to compare differences between two groups of data containing one variable with normal distributions. One‐way analysis of variance (ANOVA) was used to compare differences between three or more groups of data containing one variable with normal distributions. For the data containing two variables, two‐way ANOVA was used to compare statistical differences. The Kruskal–Wallis test was used to compare the differences between groups without normal distributions. Comparisons of survival time were conducted using the log‐rank (Mantel‐Cox) test. Two‐sided p < 0.05 was considered significant.
AUTHOR CONTRIBUTIONS
Bo Cao, Jing‐Wang Gao, Qing‐Peng Zhang, and Xing‐Ming Xu performed the in vivo experiments. Bo Cao, Jing‐Wang Gao, Rui‐Yang Zhao, and Hang‐Hang Li conducted basic and in vitro experiments. Bo Cao, Qing‐Peng Zhang, and Xing‐Ming Xu wrote the manuscript and interpreted the data. Bo Wei designed the project, supervised the experimental processes, and revised the manuscript. All authors read and approved the final version of the manuscript. Co‐first authorship was decided based on crucial contributions to experiments and leadership.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The animal studies were approved by the Ethics Committee of Animal Centre of Chinese PLA General Hospital (2020‐X6‐117).
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (82273231, 82073192, 81773135). The pictures of the experimental flow chart and mechanistic illustration were created with BioRender.com.
Cao, Bo , Gao Jing‐Wang, Zhang Qing‐Peng, Xu Xing‐Ming, Zhao Rui‐Yang, Li Hang‐Hang, and Wei Bo. 2023. “Melatonin Supplementation Protects Against Traumatic Colon Injury by Regulating SERPINA3N Protein Expression.” iMeta 2, e141. 10.1002/imt2.141
Bo Cao, Jing‐Wang Gao, and Qing‐Peng Zhang contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request. The raw data reported in this study are available in Science Data Bank (https://www.scidb.cn/en/s/fuiAJn). Supplementary materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) can be found in the online DOI or iMeta Science http://www.imeta.science/.
REFERENCES
- 1. Belmont Philip J. Jr., McCriskin Brendan J., Sieg Ryan N., Burks Robert, and Schoenfeld Andrew J.. 2012. “Combat Wounds in Iraq and Afghanistan From 2005 To 2009.” Journal of Trauma and Acute Care Surgery 73: 3–12. 10.1097/TA.0b013e318250bfb4 [DOI] [PubMed] [Google Scholar]
- 2. Owens, Brett D. , Kragh John F. Jr., Wenke Joseph C., Macaitis Joseph, Wade Charles E., and Holcomb John B.. 2008. “Combat Wounds in Operation Iraqi Freedom and Operation Enduring Freedom.” Journal of Trauma 64: 295–299. 10.1097/TA.0b013e318163b875 [DOI] [PubMed] [Google Scholar]
- 3. Arafat, Shawqi , Alsabek Mhd Belal, Ahmad Mousa, Hamo Iman, and Munder Eskander. 2017. “Penetrating Abdominal Injuries During the Syrian War: Patterns and Factors Affecting Mortality Rates.” Injury 48: 1054–1057. 10.1016/j.injury.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 4. Fouda, E. , Emile S., Elfeki H., Youssef M., Ghanem A., Fikry A. A., Elshobaky A., et al. 2016. “Indications for and Outcome of Primary Repair Compared with Faecal Diversion in the Management of Traumatic Colon Injury.” Colorectal Disease 18: O283–O291. 10.1111/codi.13421 [DOI] [PubMed] [Google Scholar]
- 5. Smith, Iain M. , Naumann David N., Marsden Max E. R., Ballard Mark, and Bowley Douglas M.. 2015. “Scanning and War: Utility of FAST and CT in the Assessment of Battlefield Abdominal Trauma.” Annals of Surgery 262: 389–396. 10.1097/sla.0000000000001002 [DOI] [PubMed] [Google Scholar]
- 6. Roman, Pablo , Rodriguez‐Alvarez Amanda, Bertini‐Perez Daniel, Ropero‐Padilla Carmen, Martin‐Ibañez Luis, and Rodriguez‐Arrastia Miguel. 2021. “Tourniquets as a Haemorrhage Control Measure in Military and Civilian Care Settings: an Integrative Review.” Journal of Clinical Nursing: in press. 10.1111/jocn.15834 [DOI] [PubMed] [Google Scholar]
- 7. Sumsuzzman, Dewan Md , Choi Jeonghyun, Jin Yunho, and Hong Yonggeun. 2021. “Neurocognitive Effects of Melatonin Treatment in Healthy Adults and Individuals with Alzheimer's Disease and Insomnia: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials.” Neuroscience & Biobehavioral Reviews 127: 459–473. 10.1016/j.neubiorev.2021.04.034 [DOI] [PubMed] [Google Scholar]
- 8. Xu, Dejun , Liu Lingbin, Zhao Yongju, Yang Li, Cheng Jianyong, Hua Rongmao, Zhang Zelin, and Li Qingwang. 2020. “Melatonin Protects Mouse Testes From Palmitic Acid‐Induced Lipotoxicity By Attenuating Oxidative Stress and DNA Damage In a SIRT1‐dependent Manner.” Journal of Pineal Research 69: e12690. 10.1111/jpi.12690 [DOI] [PubMed] [Google Scholar]
- 9. Wu, Jinpeng , Tan Zengqi, Li Hongjiao, Lin Meixuan, Jiang Yazhuo, Liang Liang, Ma Qilong, et al. 2021. “Melatonin Reduces Proliferation and Promotes Apoptosis of Bladder Cancer Cells By Suppressing O‐GlcNAcylation of Cyclin‐Dependent‐Like Kinase 5.” Journal of Pineal Research 71: e12765. 10.1111/jpi.12765 [DOI] [PubMed] [Google Scholar]
- 10. Lv, Weijie , Liu Cui, Yu Linzeng, Zhou Jiahao, Li Yue, Xiong Ying, Guo Ao, et al. 2020. “Melatonin Alleviates Neuroinflammation and Metabolic Disorder in DSS‐Induced Depression Rats.” Oxidative Medicine and Cellular Longevity 2020: 1–17. 10.1155/2020/1241894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cardinali, Daniel P . 2021. “Melatonin and Healthy Aging.” Vitamins and Hormones 115: 67–88. 10.1016/bs.vh.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 12. Xu, Siqi , Li Lulan, Wu Jie, An Sheng, Fang Haihong, Han Yunyang, Huang Qiaobing, Chen Zhongqing, and Zeng Zhenhua. 2021. “Melatonin Attenuates Sepsis‐Induced Small‐Intestine Injury by Upregulating SIRT3‐Mediated Oxidative‐Stress Inhibition, Mitochondrial Protection, and Autophagy Induction.” Frontiers in Immunology 12: 625627. 10.3389/fimmu.2021.625627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma, Fei , Hao Hu, Gao Xiaoyan, Cai Yao, Zhou Jialiang, Liang Puping, Lv Junjian, et al. 2020. “Melatonin Ameliorates Necrotizing Enterocolitis by Preventing Th17/Treg Imbalance Through Activation Of the AMPK/SIRT1 Pathway.” Theranostics 10: 7730–7746. 10.7150/thno.45862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forsyth, Sharon , Horvath Anita, and Coughlin Paul. 2003. “A Review and Comparison of the Murine α1‐antitrypsin and α1‐antichymotrypsin Multigene Clusters With the Human Clade A Serpins.” Genomics 81: 336–345. 10.1016/s0888-7543(02)00041-1 [DOI] [PubMed] [Google Scholar]
- 15. Horvath, Anita J. , Forsyth Sharon L., and Coughlin Paul B.. 2004. “Expression Patterns of Murine Antichymotrypsin‐Like Genes Reflect Evolutionary Divergence at the Serpina3 Locus.” Journal of Molecular Evolution 59: 488–497. 10.1007/s00239-004-2640-9 [DOI] [PubMed] [Google Scholar]
- 16. Zattoni, Marco , Mearelli Marika, Vanni Silvia, Colini Baldeschi Arianna, Tran Thanh Hoa, Ferracin Chiara, Catania Marcella, et al. 2022. “Serpin Signatures in Prion and Alzheimer's Diseases.” Molecular Neurobiology 59: 3778–3799. 10.1007/s12035-022-02817-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vicuña, Lucas , Strochlic David E., Latremoliere Alban, Bali Kiran Kumar, Simonetti Manuela, Husainie Dewi, Prokosch Sandra, et al. 2015. “The Serine Protease Inhibitor SerpinA3N Attenuates Neuropathic Pain by Inhibiting T Cell‐Derived Leukocyte Elastase.” Nature Medicine 21: 518–523. 10.1038/nm.3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aslam, Mehwish Saba , and Yuan Liudi. 2020. “Serpina3n: Potential Drug and Challenges, Mini Review.” Journal of Drug Targeting 28: 368–378. 10.1080/1061186X.2019.1693576 [DOI] [PubMed] [Google Scholar]
- 19. Coronado, Michael J. , Brandt Jessica E., Kim Eunyong, Bucek Adriana, Bedja Djahida, Abston Eric D., Shin Jaewook, et al. 2012. “Testosterone and interleukin‐1β Increase Cardiac Remodeling During Coxsackievirus B3 Myocarditis Via Serpin A 3n.” American Journal of Physiology‐Heart and Circulatory Physiology 302: H1726–H1736. 10.1152/ajpheart.00783.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xi, Yu , Liu Mengyu, Xu Shuzhen, Hong Huihui, Chen Mengyan, Tian Li, Xie Jia, et al. 2019. “Inhibition of SERPINA3N‐dependent Neuroinflammation Is Essential for Melatonin to Ameliorate Trimethyltin Chloride‐Induced Neurotoxicity.” Journal of Pineal Research 67: e12596. 10.1111/jpi.12596 [DOI] [PubMed] [Google Scholar]
- 21. Guo, Hao , Chou Weichun, Lai Yunjia, Liang Kaixin, Tam Jason W., Brickey W June, Chen Liang, et al. 2020. “Multi‐Omics Analyses of Radiation Survivors Identify Radioprotective Microbes and Metabolites.” Science 370: eaay9097. 10.1126/science.aay9097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franzosa, Eric A. , Sirota‐Madi Alexandra, Avila‐Pacheco Julian, Fornelos Nadine, Haiser Henry J., Reinker Stefan, Vatanen Tommi, et al. 2019. “Gut Microbiome Structure and Metabolic Activity In Inflammatory Bowel Disease.” Nature Microbiology 4: 293–305. 10.1038/s41564-018-0306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duan, Chenyang , Kuang Lei, Xiang Xinming, Zhang Jie, Zhu Yu, Wu Yue, Yan Qingguang, Liu Liangming, and Li Tao. 2020. “Activated Drp1‐mediated Mitochondrial ROS Influence the Gut Microbiome and Intestinal Barrier After Hemorrhagic Shock.” Aging 12: 1397–1416. 10.18632/aging.102690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou, Qiqi , Verne Meghan L., Fields Jeremy Z., Lefante John J., Basra Sarpreet, Salameh Habeeb, and Verne G Nicholas. 2019. “Randomised Placebo‐Controlled Trial of Dietary Glutamine Supplements for Postinfectious Irritable Bowel Syndrome.” Gut 68: 996–1002. 10.1136/gutjnl-2017-315136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diebel, Mark E. , Diebel Lawrence N., Manke Charles W., Liberati David M., and Whittaker John R.. 2015. “Early Tranexamic Acid Administration: A Protective Effect on Gut Barrier Function Following Ischemia/Reperfusion Injury.” Journal of Trauma and Acute Care Surgery 79: 1015–1022. 10.1097/ta.0000000000000703 [DOI] [PubMed] [Google Scholar]
- 26. Peng, Zhanglong , Ban Kechen, LeBlanc Anthony, and Kozar Rosemary A.. 2016. “Intraluminal Tranexamic Acid Inhibits Intestinal Sheddases and Mitigates Gut and Lung Injury and Inflammation In a Rodent Model of Hemorrhagic Shock.” Journal of Trauma and Acute Care Surgery 81: 358–365. 10.1097/ta.0000000000001056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weaver, Jessica L. , Eliceiri Brian, and Costantini Todd W.. 2022. “Fluoxetine Reduces Organ Injury and Improves Motor Function After Traumatic Brain Injury in Mice.” Journal of Trauma and Acute Care Surgery 93: 38–42. 10.1097/ta.0000000000003646 [DOI] [PubMed] [Google Scholar]
- 28. Yang, Mingshi . 2015. “Systemic Inflammation and Multiple Organ Injury in Traumatic Hemorrhagic Shock.” Frontiers in Bioscience 20: 927–933. 10.2741/4347 [DOI] [PubMed] [Google Scholar]
- 29. Cao, Bo , Zhao Ruiyang, Li Hanghang, Xu Xingming, Cui Hao, Deng Huan, Chen Lin, and Wei Bo. 2022. “Oral Administration Of Asparagine and 3‐indolepropionic Acid Prolongs Survival Time of Rats with Traumatic Colon Injury.” Military Medical Research 9: 37. 10.1186/s40779-022-00397-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feuerstadt, Paul , Louie Thomas J., Lashner Bret, Wang Elaine E. L., Diao Liyang, Bryant Jessica A., Sims Matthew, et al. 2022. “SER‐109, an Oral Microbiome Therapy for Recurrent Clostridioides Difficile Infection.” New England Journal of Medicine 386: 220–229. 10.1056/NEJMoa2106516 [DOI] [PubMed] [Google Scholar]
- 31. Lima‐Junior, Djalma S. , Krishnamurthy Siddharth R., Bouladoux Nicolas, Collins Nicholas, Han Seong‐Ji, Chen Erin Y., Constantinides Michael G., et al. 2021. “Endogenous Retroviruses Promote Homeostatic and Inflammatory Responses To the Microbiota.” Cell 184: 3794–3811.e19. 10.1016/j.cell.2021.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huttner, Angela , Hatz Christoph, van den Dobbelsteen Germie, Abbanat Darren, Hornacek Alena, Frölich Rahel, Dreyer Anita M., et al. 2017. “Safety, Immunogenicity, and Preliminary Clinical Efficacy of a Vaccine Against Extraintestinal Pathogenic Escherichia coli in Women with a History of Recurrent Urinary Tract Infection: A Randomised, Single‐Blind, Placebo‐Controlled Phase 1b Trial.” The Lancet Infectious Diseases 17: 528–537. 10.1016/s1473-3099(17)30108-1 [DOI] [PubMed] [Google Scholar]
- 33. Loomis, Wendy P. , Delaney Martha A., Johnson Matthew L., and Cookson Brad T.. 2020. “Failure of CD4 T Cell‐Deficient Hosts to Control Chronic Nontyphoidal Salmonella Infection Leads to Exacerbated Inflammation, Chronic Anemia, and Altered Myelopoiesis.” Infection and Immunity 89: e00417–e00420. 10.1128/iai.00417-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tickell, Kirkby D. , Brander Rebecca L., Atlas Hannah E., Pernica Jeffrey M., Walson Judd L., and Pavlinac Patricia B.. 2017. “Identification and Management of Shigella Infection in Children with Diarrhoea: A Systematic Review and Meta‐Analysis.” The Lancet Global Health 5: e1235–e1248. 10.1016/s2214-109x(17)30392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stephens, Matthew , and von der Weid Pierre‐Yves. 2020. “Lipopolysaccharides Modulate Intestinal Epithelial Permeability and Inflammation In a Species‐Specific Manner.” Gut Microbes 11: 421–432. 10.1080/19490976.2019.1629235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li, Yuan , Dong Jiali, Xiao Huiwen, Zhang Shuqin, Wang Bin, Cui Ming, and Fan Saijun. 2020. “Gut Commensal Derived‐Valeric Acid Protects Against Radiation Injuries.” Gut Microbes 11: 789–806. 10.1080/19490976.2019.1709387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thevaranjan, Netusha , Puchta Alicja, Puchta Alicja, Naidoo Avee, Szamosi J. C., Verschoor Chris P., Loukov Dessi, et al. 2017. “Age‐Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction.” Cell Host & Microbe 21: 455–466.e454. 10.1016/j.chom.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Healy, Brendan , Cooney Shane, O'Brien Stephen, Iversen Carol, Whyte Paul, Nally Jarlath, Callanan John J., and Fanning Séamus. 2010. “Cronobacter (Enterobacter sakazakii): an Opportunistic Foodborne Pathogen.” Foodborne Pathogens and Disease 7: 339–350. 10.1089/fpd.2009.0379 [DOI] [PubMed] [Google Scholar]
- 39. Hamilton, Amy L. , Kamm Michael A., Ng Siew C., and Morrison Mark. 2018. “ Proteus spp. As Putative Gastrointestinal Pathogens.” Clinical Microbiology Reviews 31: e00085–e00117. 10.1128/cmr.00085-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang, Jingwei , Wang Wei, Zhu Shenglong, and Chen Yongquan. 2021. “Increased SERPINA3 Level Is Associated with Ulcerative Colitis.” Diagnostics 11: 2371. 10.3390/diagnostics11122371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ho, Yen‐Ting , Shimbo Takashi, Wijaya Edward, Kitayama Tomomi, Takaki Satoshi, Ikegami Kentaro, Miyashita Kazuya, et al. 2021. “Longitudinal Single‐Cell Transcriptomics Reveals a Role for Serpina3n‐Mediated Resolution of Inflammation in a Mouse Colitis Model.” Cellular and Molecular Gastroenterology and Hepatology 12: 547–566. 10.1016/j.jcmgh.2021.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rzepka‐Migut, Beata , and Paprocka Justyna. 2020. “Efficacy and Safety of Melatonin Treatment in Children with Autism Spectrum Disorder and Attention‐Deficit/Hyperactivity Disorder‐A Review of the Literature.” Brain Sciences 10: 219. 10.3390/brainsci10040219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sletten, Tracey L. , Magee Michelle, Murray Jade M., Gordon Christopher J., Lovato Nicole, Kennaway David J., Gwini Stella M., et al. 2018. “Efficacy of Melatonin With Behavioural Sleep‐Wake Scheduling for Delayed Sleep‐Wake Phase Disorder: A Double‐Blind, Randomised Clinical Trial.” PLoS Medicine 15: e1002587. 10.1371/journal.pmed.1002587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Procaccini, D. , Lobner K., Azamfirei R., and Kudchadkar S. R.. 2021. “Melatonin for Anaesthetic Indications in Paediatric Patients: A Systematic Review.” Anaesthesia 76: 837–849. 10.1111/anae.15249 [DOI] [PubMed] [Google Scholar]
- 45. Crespo, Irene , Fernández‐Palanca Paula, San‐Miguel Beatriz, Álvarez Marcelino, González‐Gallego Javier, and Tuñón María Jesús. 2020. “Melatonin Modulates Mitophagy, Innate Immunity and Circadian Clocks in a Model of Viral‐Induced Fulminant Hepatic Failure.” Journal of Cellular and Molecular Medicine 24: 7625–7636. 10.1111/jcmm.15398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hossain, Md Farhad , Wang Ni, Chen Ruijun, Li Siwen, Roy Jaydeep, Uddin Md Giash, Li Zhong, Lim Lee Wei, and Song Youqiang. 2021. “Exploring the Multifunctional Role of Melatonin In Regulating Autophagy and Sleep to Mitigate Alzheimer's Disease Neuropathology.” Ageing Research Reviews 67: 101304. 10.1016/j.arr.2021.101304 [DOI] [PubMed] [Google Scholar]
- 47. Ren, Wenkai , Wang Peng, Yan Jiameng, Liu Gang, Zeng Benhua, Hussain Tarique, Peng Can, et al. 2018. “Melatonin Alleviates Weanling Stress in Mice: Involvement Of Intestinal Microbiota.” Journal of Pineal Research 64: 64. 10.1111/jpi.12448 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request. The raw data reported in this study are available in Science Data Bank (https://www.scidb.cn/en/s/fuiAJn). Supplementary materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) can be found in the online DOI or iMeta Science http://www.imeta.science/.


