Abstract
The human gastrointestinal (GI) tract harbors diverse microbes, and the family Lachnospiraceae is one of the most abundant and widely occurring bacterial groups in the human GI tract. Beneficial and adverse effects of the Lachnospiraceae on host health were reported, but the diversities at species/strain levels as well as their metabolites of Lachnospiraceae have been, so far, not well documented. In the present study, we report on the collection of 77 human‐originated Lachnospiraceae species (please refer hLchsp, https://hgmb.nmdc.cn/subject/lachnospiraceae) and the in vitro metabolite profiles of 110 Lachnospiraceae strains (https://hgmb.nmdc.cn/subject/lachnospiraceae/metabolites). The Lachnospiraceae strains in hLchsp produced 242 metabolites of 17 categories. The larger categories were alcohols (89), ketones (35), pyrazines (29), short (C2–C5), and long (C > 5) chain acids (31), phenols (14), aldehydes (14), and other 30 compounds. Among them, 22 metabolites were aromatic compounds. The well‐known beneficial gut microbial metabolite, butyric acid, was generally produced by many Lachnospiraceae strains, and Agathobacter rectalis strain Lach‐101 and Coprococcus comes strain NSJ‐173 were the top 2 butyric acid producers, as 331.5 and 310.9 mg/L of butyric acids were produced in vitro, respectively. Further analysis of the publicly available cohort‐based volatile‐metabolomic data sets of human feces revealed that over 30% of the prevailing volatile metabolites were covered by Lachnospiraceae metabolites identified in this study. This study provides Lachnospiraceae strain resources together with their metabolic profiles for future studies on host–microbe interactions and developments of novel probiotics or biotherapies.
Keywords: alcohols, aldehydes, Blautia, Lachnospiraceae, metabolite profiling, phenols, short‐chain fatty acids
The human‐originated Lachnospiraceae biobank included 77 species was constructed. In vitro metabolite profiling of 110 Lachnospiraceae strains yielded 242 metabolites of 17 categories. Many Lachnospiraceae strains produce short‐chain fatty acids, and Agathobacter rectalis strain Lach‐101 and Coprococcus comes strain NSJ‐173 are the top two butyric acid producers in vitro.
Highlights
The human‐originated Lachnospiraceae biobank included 77 species was constructed.
In vitro metabolite profiling of 110 Lachnospiraceae strains yielded 242 metabolites of 17 categories.
Many Lachnospiraceae strains produce SCFAs, and Agathobacter rectalis strain Lach‐101 and Coprococcus comes strain NSJ‐173 are the top two butyric acid producers in vitro.
INTRODUCTION
Members of the family Lachnospiraceae are prevalent and globally distributed in human guts [1, 2, 3, 4, 5]. All the members of Lachnospiraceae are strictly anaerobic, Gram stain positive or negative, and can ferment a variety of substrates, such as cellobiose and fructose, and produce a variety of metabolites, including short‐chain fatty acids (SCFAs) [6, 7, 8]. According to an integrated analysis of 75 different studies on human gut metagenomic data sets, the Lachnospiraceae taxa accounted for approximately 10% of the total gut microbiomes [9]. In addition, Lachnospiraceae was detected in subjects of different age groups, including infants [10, 11], teenagers [12, 13], young and middle‐aged adults [14, 15], and elderly people [16, 17, 18]. The high prevalence and abundance, and lifelong associations with human beings suggest that Lachnospiraceae possibly plays important roles in human health and diseases throughout their lives. Indeed, both beneficial and harmful effects of Lachnospiraceae on host health have been reported: The members of Lachnospiraceae, such as Roseburia homins, Blautia producta, and Roseburia intestinalis, and Anaerobutyricum hallii produce SCFAs and vitamins, and they were reported to have anti‐inflammatory, immunity‐inducing, and homeostasis‐maintaining effects [19]. As one of the most well‐studied SCFAs, butyric acid was reported to be a preferred energy source for colonocytes [20] and affects peripheral organs indirectly by activation of hormonal and nervous systems [21]. Intestinal microbiota produces many aromatic compounds, but only a few of them were well studied, among which equol was reported to reduce the risk of prostate cancer [22], 2,4‐di‐tert‐butylphenol was an antipathogenic compound [23], while some aromatic compounds, including p‐cresol and indole, were reported to be toxic to host health [24, 25]. For the beneficial effects, certain members of Lachnospiraceae were characterized as commercial probiotics, such as R. homins, which was patented for probiotics (the United States, Patent No. US9314489) [26]. Studies based on germ‐free mice revealed that Lachnospiraceae isolates suppressed Clostridium difficile infection [27]. Despite the beneficial effects, metagenomic studies showed that increased abundances of genera Blautia, Dorea, and Mediterraneibacter may contribute to host obesity [28, 29, 30, 31]. Increased abundances of Blautia species and Mediterraneibacter gnavus were observed in subjects with inflammatory bowel disease and primary sclerosing cholangitis [29, 32], although other studies reported contradictory results [33, 34]. Members of Anaerostipes, Blautia, Dorea, Roseburia, and Coprococcus were reportedly associated with the occurrences of major depressive disorder and Crohn's disease [35]. Further culture‐based cause‐and‐effect studies confirmed the functions of Lachnospiraceae members [36, 37, 38, 39]. For example, Roseburia hominis alleviated neuroinflammation via SCFA production, Blautia wexlerae ameliorated obesity and type 2 diabetes via gut microbiota remodeling [40, 41] and Agathobacter rectalis suppressed lymphomagenesis [42] and attenuates HSV‐1 induced systemic inflammation [43]. The different and even controversial effects of Lachnospiraceae members on host well‐being might also be attributed to the differences of Lachnospiraceae members due to their diversities at species/strain levels and/or to their unique metabolisms.
As of the date of writing, the family Lachnospiraceae is comprised of validly published 80 genera and 176 species (https://lpsn.dsmz.de/family/lachnospiraceae) that originated from environments, humans, and animals. Still, many important Lachnospiraceae have neither been successfully cultivated nor described, and the uncultivated Lachnospiraceae members comprised almost 10% of the proposed prioritized 1468 gut microbial taxa [44]. Due to the limited resources of cultivated Lachnospiraceae strains from human guts, the metabolite pools of Lachnospiraceae are rarely explored, although several Lachnospiraceae species were characterized for productions of well‐known beneficial metabolites, such as SCFAs [45, 46, 47], vitamins [48], and pyrazine [49], or productions of harmful metabolites, such as cytotoxic and genotoxic p‐cresol [50].
Here, we report the cultivation and profiling of metabolites of Lachnospiraceae strains from healthy human adults. By modification of culturing methods, we newly cultured 114 Lachnospiraceae strains. Together with our previous Lachnospiraceae culture collections [51, 52], we collected 77 species representing 33 genera of the Lachnospiraceae family, and provided taxonomic descriptions of nine novel species and five genera (human‐originated Lachnospiraceae species [hLchsp], https://hgmb.nmdc.cn/subject/lachnospiraceae). In total 242 metabolites comprised 17 major categories were detected for Lachnospiraceae strains (https://hgmb.nmdc.cn/subject/lachnospiraceae/metabolites). By evaluating the prevalence of the Lachnospiraceae metabolites in human fecal volatile‐metabolomic data sets, we found that 17 Lachnospiraceae metabolites were prevalent in human feces, and two of which were specifically enriched in nonalcoholic fatty liver disease (NAFLD) cohorts.
RESULTS
Cultivation and collection of Lachnospiraceae strains and the establishment of human‐derived Lachnospiraceae (hLchsp) biobank
Matching culture medium components with bacterial physiology is critical to optimize bacterial cultivation. Thus, we explored the cultivation and physiological information of previously cultivated Lachnospiraceae strains. We first referred to the growth medium components of previously successfully cultivated 138 Lachnospiraceae species (Supporting Information Table S1A) that were listed in the Bacterial Diversity Metadatabase [53]. Analysis of the data sets revealed that 66 nonredundant media with different medium components were used to cultivate these 138 Lachnospiraceae strains. The most frequently applied components used as carbon and energy sources were cellobiose, maltose, starch, casitone, trypticase peptone, peptone, and glucose (Supporting Information Figure S1A). Second, we extracted the metabolic features of Lachnospiraceae strains from API 32A test results (Supporting Information Table S1C). Analysis revealed that most of the API 32A test results (n = 89 in total) were positive for α‐galactosidase (n = 58), β‐galactosidase (n = 65), β‐glucosidase (n = 45), and α‐arabinosidase (n = 42) (Supporting Information Figure S1B). Third, we investigated the carbon sources assimilation by 23 Lachnospiraceae species that were cultivated and characterized by BIOLOG test in our previous study [52], and found that the following substrates were frequently used by the Lachnospiraceae strains, that is, d‐galactose, α‐d‐glucose, l‐rhamnose, palatinose, l‐fucose, d‐fructose, d‐galacturonic acid, pyruvic acid, glyoxylic acid, 3‐methyl‐d‐glucose, d‐mannose, dextrin, d‐melibiose, glucose 6‐phosphate, and methyl pyruvate as the preferred carbon sources for the cellular growth (Supporting Information Figure S1C). Integrating the results above, we defined a new growth medium for the cultivation of Lachnospiraceae, namely, Lach‐GAM, by supplementing diet‐fiber‐derived carbohydrates into the Gifu anaerobic medium (GAM) [54]. The Lach‐GAM and additional six media‐ yeast casitone fatty aacids broth (YCFA), X media, Columbia blood agar (CB), fastidious anaerobe broth (FAB), peptone yeast glucose broth (PYG), and 2216E media Supporting Information Table S2) were applied for the cultivation of Lachnospiraceae from five fecal samples of healthy Chinese adults, following our previously established workflow [38]. Bacterial colonies were picked and purified by plate‐streaking, and further phylogenic associations were determined based on sequenced 16S RNA gene identities. In total, we obtained 1116 bacterial isolates (Supporting Information Table S3B) belonging to 32 families, and the top four families were Lachnospiraceae (219 isolates, 19.6%), Bacteriodaceae (164 isolates, 14.7%), Enterobacteriaceae (141 isolates, 13.0%), and Morganellaceae (104 isolates, 9.3%) (Figure 1A). Overall, we recovered diverse bacterial isolates, including over 30 Lachnospiraceae genera, which suggested the effectiveness of the current cultivation strategy regarding taxonomic diversity of Lachnospiraceae isolates. In the current study, by the long period of cultivation (>30 days), we recovered slow‐growing taxa, such as Coprococcus, Exibacter, and Eisenbergiella [55, 56, 57]. There were 57, 52, 46, 28, 6, 17, and 13 Lachnospiraceae isolates recovered from YCFA, Lach‐GAM, FAB, CB, PYG, 2216E, and X media, respectively (Figure 1B). There was overlapping of the species of cultivated Lachnospiraceae isolates from the seven media, and seven species (Anaerofusibacter homins gen. nov. sp. nov., Blautia hydrogenotrophica, Entrocloster clostridiformis, Mediterraneibacter torques, Muricomes intestini, Roseburia faecis, and Mediterraneibacter faecis) were recovered only from the newly defined Lach‐GAM medium. Five species (Blautia stercoris, Faecalicatena contorta, Simiaoa sunii, R. intestinalis, and Sellimonas intestinalis) are only from YCFA medium, and the four species (A. hallii, Blautia faecis, Coprococcus eutactus, and Dorea formicigenerans) only from 2216E medium, two species (Sporofaciens scindens and R. homins) only from PYG medium. Mediterraneibacter intestinihomins gen. nov. sp. nov. and Faecalimonas umbilicata were from FAB and CB media, respectively (Figure 1B). These results demonstrated that the Lach‐GAM medium was effective for growing Lachnospiraceae, and the application of multiple culture media facilitated the recovery of different taxa of Lachnospiraceae.
Figure 1.
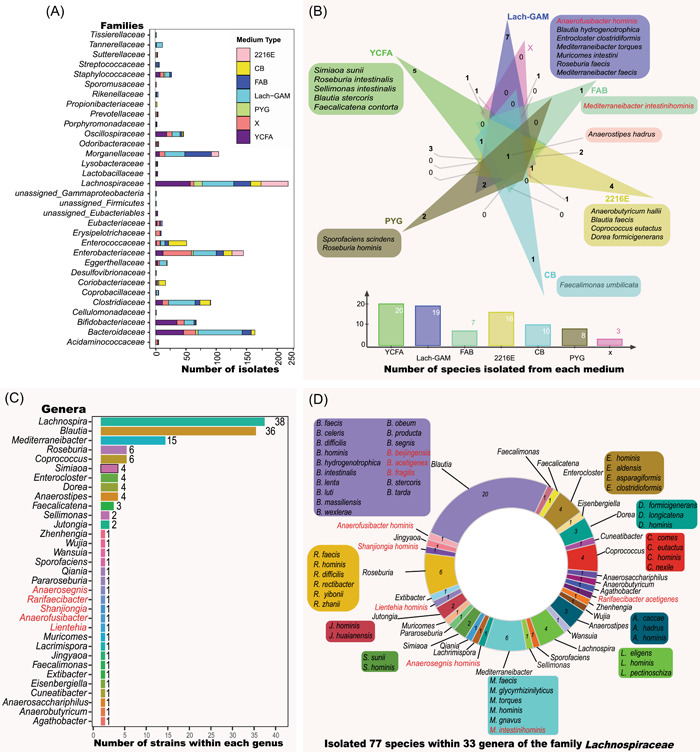
Cultivation and collection of Lachnospiraceae isolates for the hLchsp biobank. (A) Distribution at the family level of 1116 bacterial isolates. (B) Uniqueness at the species level of Lachnospiraceae growth on seven culture media. Panels (C) and (D) describe the features of the established hLchsp biobank. (C) Genus names and number of strains of each genus. (D) Species composition of hLchsp biobank. Numbers in the donut chart represent numbers of the species, and species names are provided outside the donut chart when a genus comprises more than one species. Red names represent novel taxa that are newly described in this study. hLchsp, human‐originated Lachnospiraceae species; Lach‐GAM, Lachnospiraceae Gifu Anaerobic medium.
From the 219 Lachnospiraceae isolates, we successfully maintained and deposited 114 strains at China General Microbiological Culture Collection Centre (CGMCC). We also retrieved 34 strains from the Human Gut Microbiomes [52]. Integrating these Lachnospiraceae strains, we established the hLchsp biobank of 148 human‐derived Lachnospiraceae strains (https://hgmb.nmdc.cn/subject/lachnospiraceae, Supporting Information Table S3A, Figure 1C). The hLchsp biobank was composed of 77 species and 33 genera (including nine novel species and five novel genera that were firstly isolated, identified, and described in this study, see Taxonomic descriptions of novel taxa) within the family Lachnospiraceae (Figure 1D). The genera Lachnospira, Blautia, and Roseburia were prevalent inhabitants of human gastrointestinal (GI) with high abundances [1, 9], and they were well represented in the hLchsp biobank. There were 39 strains of Lachnospira, 36 strains of Blautia, and six strains of Roseburia (Figure 1C). Fifteen strains from genus Mediterraneibacter, including species of M. faecis, Mediterraneibacter glycyrrhizinilyticus, M. torques, Mediterraneibacter hominis, M. gnavus, Mediterraneibacter intestinihominis, were also covered. Additional members were from species of Anaerosacchariphilus hominis, A. hallii, Anaerostipes caccae, Anaerostipes hadrus, Anaerostipes hominis, Coprococcus comes, C. eutactus, Coprococcus hominis, Coprococcus nexile, Cuneatibacter caecimuris, D. formicigenerans, Dorea longicatena, Dorea hominis, Eisenbergiella tayi, Enterocloster hominis, Enterocloster aldensis, Enterocloster asparagiformis, Enterocloster clostridioformis, Extibacter muris, F. contorta, F. umbilicata, Lacrimispora celerecrescens, M. intestini, S. intestinalis, and S. scindens (Figure 1D and Supporting Information Table S3A). So far as we know, this is the first targeted collection of Lachnospiraceae strains.
Lachnospiraceae strains produce diverse metabolites
We cultivated all 148 hLchsp Lachnospiraceae strains and found that 110 strains successfully grew in the Lach‐GAM broth. The grown cultures were extracted and proceeded for metabolite profiling, while the metabolites in the sterile Lach‐GAM medium measured together with inoculated bacterial cultures were included as a blank control, and any metabolite detected in the control was removed from the metabolite profiles of bacterial cultures. Totally 242 nonredundant metabolites were identified (for detailed metabolites of each strain, refer to Supporting Information Table S4A) and they were classified into 17 categories according to chemical natures. The top categories were alcohols (89), ketones (35), pyrazines (29), acids (31), phenols (14), and aldehydes (14) (Figure 2A).
Figure 2.
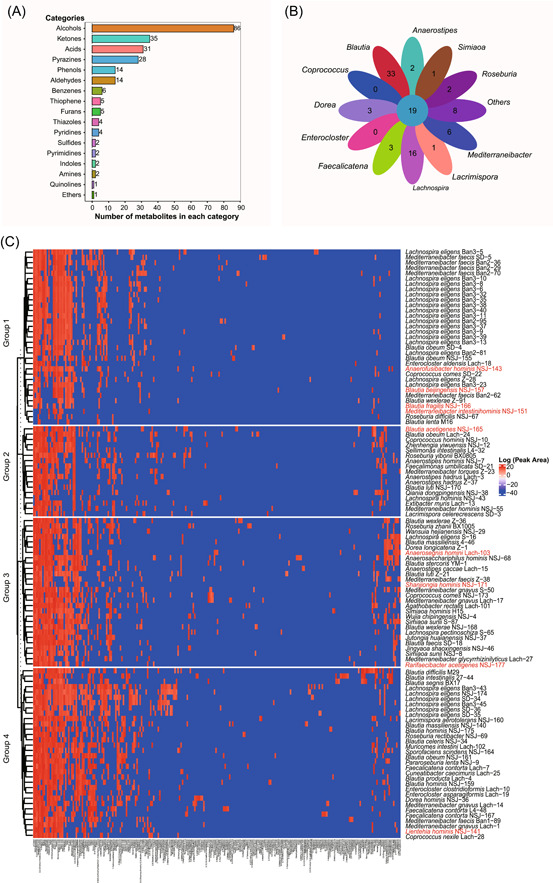
Profiling of metabolites from 110 strains of Lachnospiraceae. (A). Catalogories of 242 metabolites. (B) Shared and unique metabolites among genera of Lachnospiraceae. The inner circle denotes the shared 19 metabolites, and each leaf represents a genus group. The numbers shown on leaves represent genus‐specific metabolites. (C) Heatmap of metabolites from 110 Lachnospiraceae strains. Color describes the relative amounts of metabolites represented with the Log values of peak area; blue to red indicates relative amounts from low to high, strain names in red denoting novel taxa.
We identified metabolites shared or uniquely featured to Lachnospiraceae members at the genus level (Figure 2B). Results showed that there are 19 shared metabolites at the genus level. They were acetic acid, butyric acid, hexadecanoic acid, octadecanoic acid, ethanol, n‐dodecanol, n‐hexanol, n‐tetradecanol, pentanol, skatole, 2,5‐dimethyl‐4‐hydroxy‐3(2H)‐furanone, p‐cresol, 2,5‐dimethylpyrazine, 2,5‐methylethylpyrazine, 2,6‐dimethylpyrazine, 5‐isopentyl‐2,3‐dimethylpyrazine, methylpyrazine, pyrazine, and trimethylpyrazine. Noticeably, 2,5‐dimethyl‐4‐hydroxy‐3(2H)‐furanone, a general plant‐originated antimicrobial agent, was reported to be produced by Zygosaccharomyces rouxii [58, 59], and there has been no report about the production by prokaryotes so far. We would contain the interpretation of genus‐shared metabolites within those Lachnospiraceae strains, as many of the genera were represented by only a couple of strains. Exceptions were the genera Blautia and Lachnospira that were well represented (36 and 38 strains, respectively). We found that the genera Blautia and Lachnospira produced more unique metabolites: The genus Blautia produced 33 (alcohols and acids), and the genus Lachnospira produced 16 (alcohols, ketones, and pyrazines) unique metabolites (Figure 2B).
We tried to correlate the metabolite profiles with bacterial phylogenies. As shown in Figure 2C, Spearman clustering [60] of metabolites yielded four groups. The members of group 1 were mainly Lachnospira strains and a few Mediterraneibacter and Blautia strains, and they mainly produced SCFAs, furanones, and alcohols, and they all produce propionic and butyric acids. The members of group 2 were composed of strains from Blautia, Mediterraneibacter, Agathobacter, and unclassified Lachnospiraceae strains. Bacterial strains of this group 2 mainly produced butyric and hexanoic acids, and the amounts of production were relatively high. Group 3, mainly composed of Blautia and several members of Mediterraneibacter, Anaerostipes, Coprococcus, and Roseburia, was the largest cluster that was metabolically highly active and their metabolites were diverse, including alcohols, acids, ketones, and aldehydes. Group 4 was composed of strains of Enterocloster, Dorea, Faecalicatena, Muricomes, Sporofaciens, and several members of Blautia, and members within this group were metabolically less active.
Lachnospiraceae species are generally productive for SCFAs but are significantly different at the strain level
SCFAs exert important probiotic functions on host health: Butyrate serves as the primary energy source for intestinal epithelial cells [61], and suppresses pathogen colonization [62]. Acetate can mediate fat accumulation [63] and can be converted into butyrate [64, 65]. Propionate was reported to lower the serum cholesterol levels of the host [66]. We examined the capabilities of 110 Lachnospiraceae strains for SCFAs production, and quantified their SCFAs production (Figure 3). Results showed that there were 91, 88, 78, and 73 Lachnospiraceae strains producing acetic, propionic, butyric, and valeric acids, respectively. Twenty‐four and 19 Lachnospiraceae strains produced also isobutyric and isovaleric acids, respectively. These results demonstrated that most of the Lachnospiraceae species particularly Blautia species were able to produce SCFAs. The productions of SCFAs with Lachnospiraceae species and strains are shown in Figure 3. Among the acetic acid producers, the top five strains were B. producta Lac‐4, B. acetigenes NSJ‐165, B. wexlerae NSJ‐168, B. homins NSJ‐175, and B. homins NSJ‐159, and their productions were 991.3, 923.4, 522.5, 498.8, and 459.4 mg/L after 3 days cultivation at 37°C in Lach‐GAM liquid medium, respectively. The top five Lachnospiraceae strains for propionic acid production were B. massiliensis NSJ‐140, B. producta Lach‐4, B. wexlerae NSJ‐168, B. acetigenes NSJ‐165, and B. obeum Lach‐24, and they produced 1165.5 and 547.1, 499.5, 451.0, and 48.9 mg/L, respectively. The top five Lachnospiraceae strains for butyric acid production were A. rectalis Lach‐101, C. comes NSJ‐173, A. homins NSJ‐7, J. huaianensis NSJ‐37, and A. hadrus Z‐37, and they produced 331.5, 310.9, 224.8, 186.9, and171.9 mg/L, respectively. Other strains that yield over than 50 mg/L butyric acids were C. hominis strain NSJ‐10, R. rectibacter strain NSJ‐69, A. hadrus strain Lach‐3, W. hejianensis strain NSJ‐29, B. massiliensis strain NSJ‐140, B. obeum strain Lach‐24, B. hominis strain NSJ‐159, M. gnavus strain Lach‐17, B. wexlerae strain NSJ‐168, and B. producta strain Lach‐4, indicating several few reported taxa also could be potential probiotics. The top five Lachnospiraceae strains for valeric acid production were B. producta Lach‐4, C. comes NSJ‐173, B. acetigenes NSJ‐165, B. producta Lach‐4, and B. massiliensis NSJ‐140, and they produced 21.1, 20.1, 14.9, 13.9, and 12.1 mg/L, respectively. The top five Lachnospiraceae strains for isobutyric acid production were C. homins NSJ‐10, B. intestinalis 27‐44, B. obeum Lach‐24, B. massiliensis NSJ‐140, and B. acetigenes NSJ‐165, and they produced 647.7, 42.1, 16.3, 14.4, and 11.3 mg/L, respectively. The top five Lachnospiraceae strains for isovaleric acid production were B. obeum Lach‐24, B. acetigenes NSJ‐165, M. gnavus Lach‐17, B. homins NSJ‐175, and M. gnavus Lach‐1, and they produced 56.9, 41.7, 33.2, 31.2, and 28.6 mg/L, respectively (Figure 3).
Figure 3.
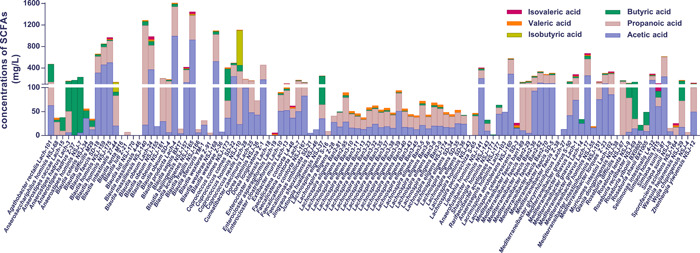
Production of SCFAs by Lachnospiraceae strains. Bars in violet, ochre, green, light green, orange, and red represent acetic, propionic, butyric, isobutyric, valeric, and isovaleric acids, respectively; in this scattered bar chart, the height of each bar in different color represents the amount (mg/L) of SCFAs production. SCFAs, short‐chain fatty acids.
Although Lachnospiraceae strains were generally productive for SCFAs, significant differences occurred at species and strain levels in productivities and composition. Thus, we evaluated further the productions for SCFAs of Blautia (36 strains) and Lachnospira (38 strains). As shown in Figure 3, Blautia generally showed higher SCFAs production than Lachnospira at species and strain levels. Yet, very different productions of SCFAs among strains of Blautia or Lachnospira were observed (Figure 3). For example, B. wexlerae strain NSJ‐168 produced high amounts of acetic and propionic acids, but B. wexlerae strain Z‐36 did not produce significant amounts of SCFAs. L. homins NSJ‐43 did not produce SCFAs. The difference in SCFAs productions was also observed for members of other genera in the hLchsp biobank. The recently discovered S. sunii strain NSJ‐8 produced trace amounts, but S. sunii strain S‐87 produced high amounts, of acetic and propionic acids (Figure 3).
Productions of alcohols (including farnesol derivatives), aldehydes, and ketones by Lachnospiraceae strains
The productions of alcohols (C2–C19) were detected for the 110 Lachnospiraceae strains, and the results showed that strains of Blautia, Roseburia, Lachnospira, Muricomes, Faecalicatena, Mediterraneibacter, Enterocloster, Dorea, and Enterocloster were the major producers (Figure 4A). We observed that more than half of the 110 strains produced ethanol (n = 88), pentanol (n = 86), n‐dodecanol (n = 85), acetol (n = 73), n‐tetradecanol (n = 64), phenylethyl alcohol (n = 63), and n‐hexanol (n = 62). Importantly, certain alcohols were detected with known physiological functions to hosts but were not well investigated for gut microbial production. For example, we detected that 33, 36, and 3 Lachnopiraceae strains produced farnesol, trans‐farnesol, and 2,3‐dihydrofarnesol, respectively. Farnesol and its isomers or derivatives have been reported to be able to regulate host metabolisms and had anti‐inflammatory functions [67, 68]. Antimicrobial and antifungal alcohols were also produced by many of Lachnopiraceae strains, such as geraniol (n = 26) [69], nonyl alcohol (n = 17) [70], 2‐undecanol (n = 6) [71], decyl alcohol (n = 23), and 1‐octanol (n = 3) (Supporting Information Table S4B).
Figure 4.
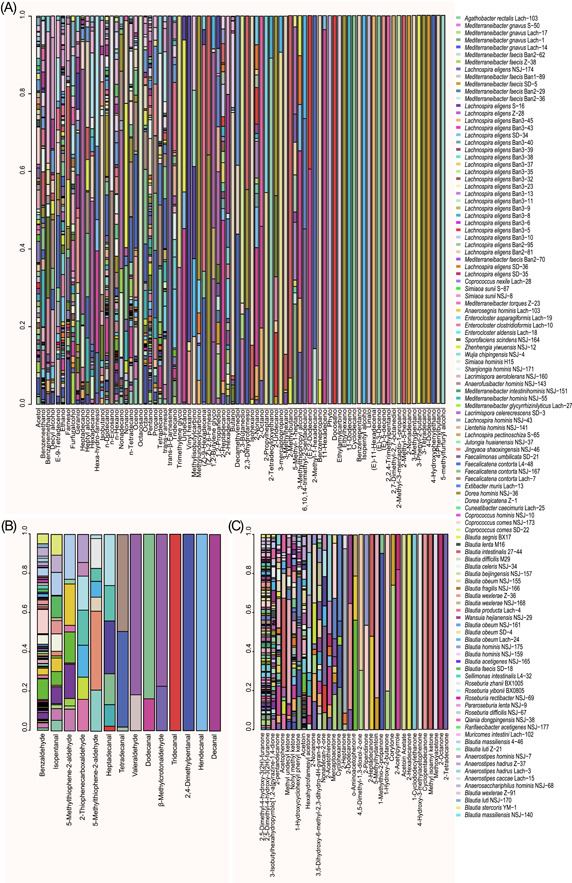
Productions of alcohols, aldehydes, and ketones by 110 Lachnospiraceae strains. (A) Scattered bar chart demonstrating the relative amounts of alcohols (n = 86) produced by 110 strains. (B) Scattered bar chart demonstrating the relative amounts of aldehydes (n = 14) produced by 110 strains. (C) Scattered bar chart demonstrating the relative amounts of ketones (n = 35) produced by 110 strains. And for panels (A)–(C), the relative amounts of each metabolite were represented by the relative percentage of metabolite GC‐MS peak area. GC‐MS, Gas Chromatography–Mass Spectrometry.
Compared with the beneficial effects of alcohols, aldehydes are generally considered to trigger oxidizing stresses and are harmful to hosts [72]. Our results (Figure 4B) showed that Lachnospiraceae strains produced a range of aldehydes, for example, benzaldehyde (n = 91), isopentanal (n = 18), 5‐methyl‐2‐thiophenecarboxaldehyde (n = 10), 3‐methyl‐2‐thiophenecarboxaldehyde (n = 8), cis‐9‐hexadecenal (n = 6), heptadecanal (n = 6), 2,4‐dimethylbenzaldehyde (n = 7), pentanal (n = 3), dodecanal (n = 2), 2,4‐dimethylpentanal (n = 1), (e)‐11‐hexadecenal (n = 1), decanal (n = 1), tridecanal (n = 1), and undecanal (n = 1). The 14 aldehydes produced by Lachnospiraceae strains are presented in Figure 5, and we found that some of the robust alcohol producers were also active in aldehyde production, such as strains of the genera Blautia, Lacrimispora, Roseburia, Anaerostipes, Mediterraneibacter, Sellimonas, Anaerosacchariphilus, Lachnospira, Dorea, and Coprococcus.
Figure 5.
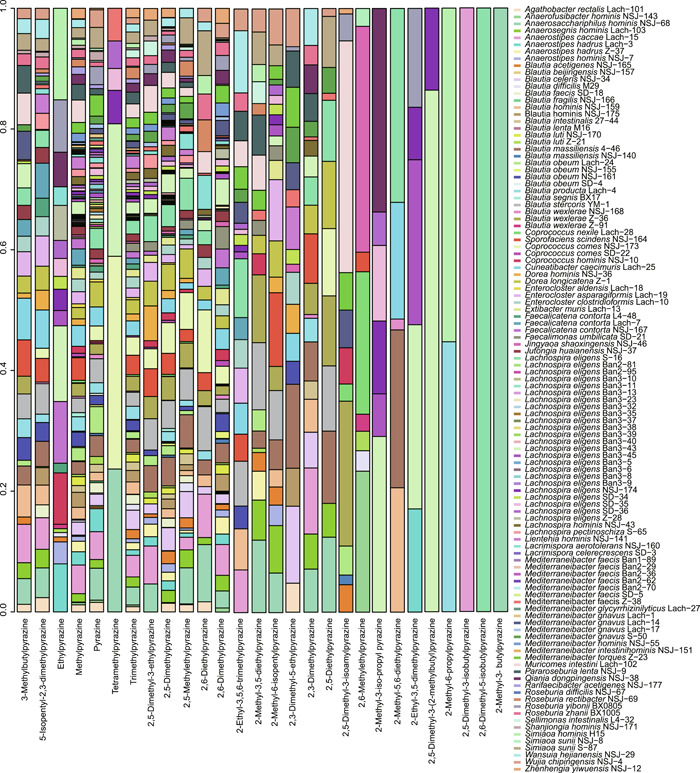
Productions of pyrazine and its derivatives by the 110 Lachnospiraceae strains. The scattered bar chart demonstrates the relative amounts of pyrazines produced by 110 strains (represented by the relative percentage of metabolite GC‐MS peak area). GC‐MS, Gas Chromatography–Mass Spectrometry.
Surprisingly, ketones were also widely produced by Lachnospiraceae strains (Figure 4C). The frequently produced ketones were 2,5‐dimethyl‐4‐hydroxy‐3(2H)‐furanone (n = 98), acetoin (n = 60), and 3‐isobutylhexahydropyrrolo[1,2‐a]pyrazine‐1,4‐dione (n = 45) that was reported to be an antifungal active agent against dermatophytes and filamentous fungi [73, 74, 75, 76]. The following ketones were also detected, they were nonyl methyl ketone (n = 37), 1‐hydroxycyclohexyl phenyl ketone (n = 23), 3,5‐dihydroxy‐6‐methyl‐2,3‐dihydro‐4h‐pyran‐4‐one (n = 16), methyl undecyl ketone (n = 14), acetophenone (n = 12), hexahydrofarnesyl acetone (n = 9), mercaptoacetone (n = 9), 2‐acetothienone (n = 8), 2‐nonanone (n = 8), nonadecan‐2‐one (n = 7), cyclohexanone (n = 5), 2‐heptanone (n = 4), 2‐dodecanone (n = 3), o‐aminoacetophenone (n = 3), 1‐hydroxy‐2‐butanone (n = 2), 1‐methylthio‐2‐propanone (n = 2), 2‐acetylpyrrole (n = 2), 2‐heptadecanone (n = 2), 2‐piperidinone (n = 2), 4,5‐dimethyl‐1,3‐dioxol‐2‐one (n = 2), 5‐methylhydantoin (n = 2), and corylone (n = 2). The top producers of ketones were strains of Roseburia, Lachnospira, Faecalimonas, Zhenhengia, Enterocloster, Lacrimispora, Mediterraneibacter, Wansuia, Qiania, Blautia, Coprococcus, and Extibacter (Figure 4C).
Production of pyrazine and derivatives by Lachnospiraceae strains
Pyrazines and their derivatives are of great pharmaceutical importance and have been developed as antimicrobial and antifungal drugs [77, 78]. To our surprise, the production of pyrazines and their derivatives were frequently observed in the tested 110 Lachnospiraceae strains (Figure 5). For example, 106 strains produced 2,5‐dimethylpyrazine and methylpyrazine, 90 strains produced trimethylpyrazine, and 83 strains produced pyrazine. Interestingly, a previously reported effective metabolite with nervous activity 2,3‐dimethylpyrazine (also named 3DP) [79] was detected in 21 Lachnospiraceae strains. Tetramethylpyrazine, a documented agent with antioxidation and anti‐inflammatory functions and a metabolite that ameliorated hepatic fibrosis [80] and protects mice retinas against oxidative injury, and it was reported to be produced by Bacillus coagulans [81], and we found that it was also produced by seven Lachnospiraceae strains, namely, M. faecis strain Z‐38 and L. eligens strain NSJ‐174, L. eligens strain Ban3‐43, L. eligens strain SD‐36, L. eligens strain SD‐35, C. comes strain NSJ‐173 and A. homins strain NSJ‐68. There were Lachnospiraceae strains (n = 23) produced 2‐ethyl‐3,5‐dimethyl pyrazine that was produced also by E. coli strains [82]. There were 23 Lachnospiraceae strains that produced the flavoring additive 2‐ethyl‐3,5, 6‐trimethyl pyrazine (n = 23), which was reported to be produced by Bacillus [83]. Lachnospiraceae strains L. eligens strain SD‐35 and M. torques strain Z‐23 produced trimethylpyrazine that showed antibacterial activities against the pathogenic Staphylococcus aureus [84].
As shown in Figure 5, many Lachnospiraceae strains produced more than one pyrazine and derivatives. The major productive strains of Lachnospiraceae were from the following genera, Enterocloster, Dorea, Faecalicatena, Blautia, Simiaoa, Lachnospira, Cuneatibacter, Anaerosacchariphilus, Coprococcus, Muricomes, Sporofaciens, and Anaerostipes.
Major metabolites produced by Blautia and Lachnospira strains
Being highly abundant and prevalent bacteria in the gut, Blautia and Lachnospira attracted more attention for their probiotic or harmful roles in host health [46, 85]. We further explored their metabolite productivities. Figure 6A,B shows the top 20 metabolites from Blautia and Lachnospira strains. The major and common metabolites produced by Blautia were butanol, 2,5‐dimethyl‐4‐hydroxy‐3‐2h‐furanone, methylbutyric acid, 2,5‐methylethylpyrazine, propanoic acid, m‐di‐tert‐butylbenzene, benzaldehyde, methylpyrazine, octadecanoic acid, 2,5‐dimethylpyrazine, butyric acid, benzenepropanol, methyl disulfide, isocaproic acid, benzenepropanoic acid, dimethyl trisulphide, hexadecanoic acid, ethanol, p‐cresol, and acetic acid (Figure 6A).
Figure 6.
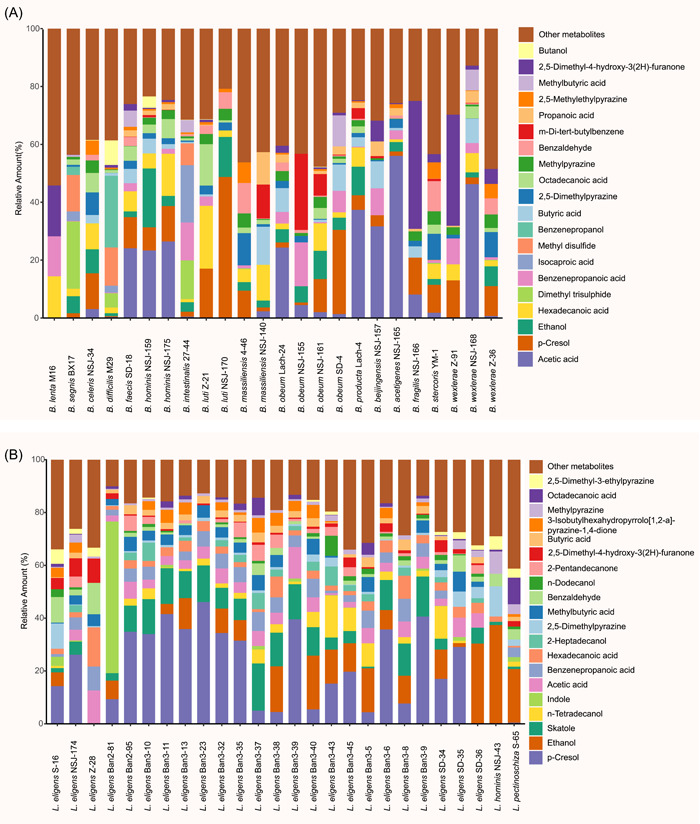
Major metabolites produced by Blautia (A) and Lachnospira (B) strains. The heights of bar segments represent relative amounts of metabolites (represented by GC‐MS peak area), and only the top 20 metabolites were shown. GC‐MS, Gas Chromatography–Mass Spectrometry.
Unlike Blautia, the strains of Lachnospira showed similar features in producing metabolites, but the amounts of metabolites were relatively low, and we found that 2,5‐dimethyl‐3‐ethylpyrazine, octadecanoic acid, methylpyrazine, 3‐isobutylhexahydropyrrolo‐12‐a‐pyrazine‐14‐dione, 2‐pentandecanonene, dodecanol, 2,5‐dimethyl‐4‐hydroxy‐3(2H)‐furanone, benzaldehyde, methylbutyric acid, 2‐heptadecanol, hexadecanoic acid, benzenepropanoic acid, acetic acid, indole, n‐tetradecanol, skatole, ethanol, and p‐cresol were produced by most of the strains within genus Lachnospira (Figure 6B).
Distribution and prevalence of Lachnospiraceae‐derived metabolites in human cohorts
To determine the distribution and prevalence of volatile metabolites produced in vitro by Lachnospiraceae strains in real‐world human GI environments, we extracted and reanalyzed the volatile metabolome data sets of two cohort‐based studies (Cohort study 1 [86] and Cohort study 2 [87]). As shown in Figure 7A, 121 metabolites were identified from 11 fecal samples of the healthy cohort (Cohort study 1), 29 of which were recovered from the Lachnospiraceae‐produced metabolites. For Cohort study 2, 215 volatile metabolites were characterized from fecal samples of 30 NAFLD patients and 30 healthy controls, and 36 of the detected fecal metabolites were recovered from the Lachnospiraceae‐produced metabolites. All recovered metabolites as well as the numbers of Lachnospiraceae producers are displayed in Figure 7B,C. Notably, there were only 56 volatile metabolites shared by both studies, while 17 were covered by the Lachnospiraceae‐produced metabolites, accounting for 30% of the in‐common fecal metabolites (Figure 7A). We then investigated the prevalence of Lachnospiraceae‐produced metabolites in human cohorts. If we define a metabolite with a prevalence >50% among fecal samples in each study as “prevalent,” 58 and 49 metabolites were identified as prevalent fecal metabolites for Cohort studies 1 and 2, respectively. As shown in Figure 7B,C, 33% and 39% of the volatile metabolites prevailing in human feces were produced by Lachnospiraceae strains from this study. Noteworthily, as previous studies reported that Lachnospiraceae were specifically enriched in the gut microbiota of NAFLD patients [87], we further evaluated if the Lachnospiraceae‐produced metabolites were enriched in the NAFLD cohort. There were five metabolites specifically enriched in NAFLD cohort, compared with the healthy control groups, and two of them (1‐propanol and 1,6‐octadien‐3‐ol,3,7‐dimethyl) were produced by Lachnospiraceae species as shown in Figure 7C.
Figure 7.
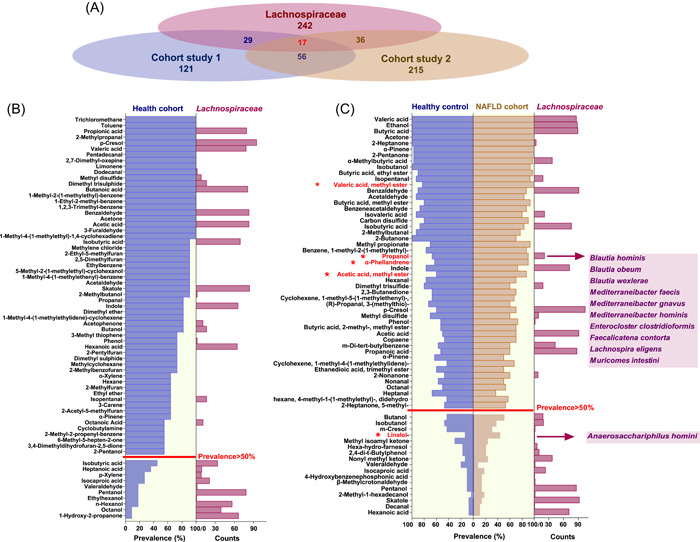
Distribution and prevalence of Lachnospiraceae‐derived metabolites in human fecal samples of different cohorts. (A) The Venn diagram demonstrating the coverage of volatile metabolites in different cohort studies by Lachnospiraceae metabolites from this study. Cohort study 1 comprising fecal samples from healthy humans (n = 11). Cohort study 2 comprising fecal samples from nonalcoholic fatty liver disease (NAFLD) cohort (n = 30) and its healthy counterparts (n = 30). (B) Bar charts displaying the prevalent volatile metabolites in fecal samples of healthy humans from Cohort study 1 (violet bars) and the numbers of Lachnospiraceae producers in this study (wine red bars). (C) Bar charts displaying the prevalent volatile metabolites in fecal samples of Cohort study 2 (healthy control n = 30, violet bars; and NAFLD patients n = 30, ochre bars) and the numbers of Lachnospiraceae producers in this study (wine red bars). The red asterisk marked five metabolites that were significantly enriched in NAFLD cohort, and two of which were identified in this study (the Lachnospiraceae producers are shown in the panel).
DISCUSSION AND CONCLUSIONS
In this study, we established a human Lachnospiraceae (hLchsp) biobank and profiled Lachnospiraceae metabolite. The establishment of the hLchsp biobank benefitted from the improvement of oriented cultivation of Lachnospiraceae species. Previous studies have demonstrated that GAM medium can be used to cultivate many of the prevalent and abundant obligate gut anaerobes, including members of Oscillospiraceae, Clostridiaceae, Bacteroidaceae, and Lachnospiraceae [50, 54], and that YCFA and FAB media are used to cultivate aerointolerant gut bacteria [56, 88, 89, 90]. By integrating current knowledge of cultivation and the physiology of Lachnospiraceae, we developed a new medium, namely, Lach‐GAM, from the GAM medium. By using Lach‐GAM and other six culture media, including YCFA and FAB media, a range of Lachnospiraceae species and strains were cultivated, including four newly nominated genera and nine novel species (refer to the description of novel taxa in the Material and methods section). Considering that there are many slow‐growing microorganisms, we extend the culturing period to 30 days by using a variety of culture media, and as a result, we recovered certain slow‐growing taxa such as Coprococcus, Exibacter, and Eisenbergiella that were rarely isolated during the previous Lachnospiraceae targeted cultivation‐based study [57]. Our efforts on oriented cultivation significantly increased the previous collections of 27 Lachnospiraceae species [57], and the hLchsp biobank has 148 strains of 77 species, covering 33 genera within the family Lachnospiraceae. Our results indicated that the modified culture methods and various culture media can effectively recover diverse intestinal microbiota, which is also consistent with the previous reports [89, 91, 92]. In addition, some of the Lachnospiraceae species covered in this study were reported to affect host health. For example, B. producta showed the ability to inhibit lipid accumulation and effectively ameliorated hyperlipidemia [93]; a strain of R. hominis increases intestinal melatonin level [19]. A. rectalis suppresses lymphomagenesis [42] and attenuates HSV‐1 induced systemic inflammation [43]; and a strain of Anaerostipes was reported to have beneficial roles in renal function [94]. These reports indicate that the Lachnospiraceae biobank we established will provide a broad range of research materials for related studies, and will facilitate any follow‐up researches regarding functions and mechanisms of these species or strains.
With Gas Chromatography–Mass Spectrometry (GC‐MS) and solid‐phase microextraction (SPME)/GC‐MS methods, we determined 242 metabolites from 110 Lachnospiraceae strains, and we quantified the productions of the SCFAs (C2–C5). Our results showed that besides the members of previously well‐acknowledged probiotic genera Roseburia [95, 96, 97], strains of genera Coprococcus, Blautia, Anaerostipes, Agathobacter, and Jutongia also produce considerably high amounts of butyric acid, a metabolite that could improve host immunity and regulate tissue inflammation [98], and thus these strains could be considered novel and potential probiotics for further exploration. Blautia was reported to exert beneficial and harmful impacts on host health by different studies [46, 99, 100]. We searched for the previous studies about SCFAs production of Lachnospiraceae, and found that C. nexile KCTC 5578, M. torques ATCC 27756, Faecalicatena fissicatena KCTC, and some Blautia and F. umbilicata members produce acetate [101, 102, 103, 104], while C. comes ATCC 27758 and some Roseburia and Enterocloster members produce butyrate [105, 106, 107]. In this study, we found that different Blautia strains produced diverse metabolites of unknown bioactivities, and exerted different abilities in the production of butyric acids. We noticed that butyrate production by the Roseburia also strain‐specific. Our study would provide bacterial resources and metabolites that support future investigations of host–microbiome interactions at either bacterial species or strain levels.
In addition, it is noteworthy that even though there are numerous reports about the beneficial effects of SCFAs mentioned above, the potentially adverse or contradictory effects of SCFAs on host health were also reported [108, 109]. High concentrations of SCFAs inhibited the growth of pathogens, such as Salmonella and S. aureus [110, 111, 112], but low levels of propionate as a carbon source facilitated the growth of Salmonella [113]. Therefore, the role of SCFAs should be evaluated carefully, as the conclusions reached in different studies may be SCFAs type and concentration dependent.
Besides SCFAs, we also detected many other metabolites from Lachnospiraceae strains, which might play important roles in the human intestines. Hexadecanoic and octadecanoic acids, both bactericidal active compounds [114], were produced by 106 Lachnospiraceae strains. Farnesol and its isomers or derivatives have been reported to have antipathogenic, anti‐inflammatory, and antifungal functions, which are critical to host health [115, 116, 117, 118]. Our results showed that the Mediterraneibacter and Lachnospira strains produced farnesol of different amounts, and these strains could be prioritized in further studies concerning host–microbiome and microbe–microbe interactions. Geraniol has anti‐Candida activity via disruption of cell membrane integrity and function [119, 120]. We found that geraniol was produced by the abundant gut inhabitants including Blautia, Lachnospira and Mediterraneibacter strains in this study, which clued their potential roles in the modulation of pathogenic fungi.
Correlating to the reported harmful effects of Lachnospiraceae on host health, we also detected Lachnospiraceae metabolites that are toxic or trigger host dysbiosis. p‐Cresol, a reported toxin with cytotoxicity and genotoxicity and reduced endothelial barrier function [121, 122, 123], was frequently detected in this study (n = 109). Phenol was another frequently detected metabolite in this study, and it was a reported tumor‐promoting agent [124]. Skatole, being a gut microbial catabolite of tryptophan and able to elicit AhR‐mediated death of intestinal epithelial cells [125, 126], was also frequently detected in this study. There were many functionally unknown metabolites from the 110 Lachnospiraceae strains. Benzaldehyde (n = 91), trimethylpyrazine (n = 90), pentanol (n = 86), and n‐dodecanol (n = 85) were the frequently detected ones. These metabolites are apparently harmful at higher concentrations to host health, but their involvement in interactions of host–microbiome and microbe–microbes would be worthy of further investigations.
However, our present study also has some limitations. We determined SCFAs both by referring to the NIST11 library and standards, while other substances were determined by referencing the NIST11 library only. We have detected that the Lachnospiraceae produced diverse metabolites, including pyrazines, ketones, and phenols in vitro, which were seldom reported to be produced by gut microbes in previous studies [127, 128]. For further interests concerning these metabolites, further standards‐based validations are necessary. Especially for those metabolites that were rarely identified as microbial products, such as pyrazine derivatives, including 2,3‐dimethylpyrazine and 2,5‐dimethyl‐4‐hydroxy‐3(2H)‐furanone, both of which were reported to be generated by the Maillard reaction of plant‐based substances [79, 129], further GC‐MS analysis with standards as well as in silico analysis of the potential genes or pathways involved in their production at genome level would enable a better understanding of the metabolism and functional potentials of Lachnospiraceae in gut microbiota.
MATERIALS AND METHODS
Human feces sample collection and pretreatment
The whole project was approved by the Research Ethics Committee of the Institute of Microbiology, Chinese Academy of Science (ethical approval No. APIMCAS2017049). All the donors of fecal samples were enquired about their health conditions, history of clinical visits for the last half‐year, and history of antibiotic treatments for the last 2 months in person before a consent form was signed for the donation of feces. Five adults (ages ranging from 24 to 33) from Beijing, China without any clearly diagnosed chronic and malignant disease were considered healthy donors, and their feces samples were collected using sterile tubes and placed onto ice packs, transferred into the Electrotek Anaerobic Workstation (AW 400SG) filled with CO2/H2/N2 (5%/10%/85%) gas mix for further use.
Culture media
The growth factors, medium components, and carbon source utilization of the previously cultured Lachnospiraceae strains were collected from publications and public data sources (Supporting Information Table S1A). We obtained the growth medium component data of nonrepeated 66 media used for culturing 138 strains from 66 species of 18 genera within the family Lachnospiraceae, and 32A enzyme data sets of 103 bacterial species. The media used in this study and their components are listed in Supporting Information Table S1C. The transfer and distribution of broth and agar media were conducted at anaerobic conditions under 100% nitrogen flow and media were autoclaved at 115°C for 25 min.
Bacterial isolation, cultivation, and storage
The bacterial isolation, cultivation, and storage were performed as described in our previous studies [52]. Briefly, the fecal samples were washed, pelleted, and suspended three times with 0.01 M of phosphate buffer solution (PBS, pH 7.4) (Cat No. P1022, Solarbio Com. Ltd.) before filtration using 40 μm cell sieves (FALCON) for removal of insoluble particles. The filtration was serially diluted (10−1−10−7) with anoxic PBS supplemented with peptone (0.2% w/v) and l‐cysteine, and the appropriate dilutions (10−4–10−7) were spread on the agar plates of different culture media for incubation at 37°Cfor 2–30 days, anaerobically. The reason for incubation for such a long period is to recover the slow‐growing and less‐abundant, and any yet‐to‐be cultured bacteria taxa [55, 56]. Single colonies that appeared on the plates were picked, and 16S rRNA genes were sequenced by Tianyi Huiyuan Co. Ltd., and the targeted pure cultures were transferred into the liquid broth for further experimentation and onto agar slopes in Hungate tubes for long‐term storage. Colonies on agar slope were washed with 15% (v/v) glycerite for cryopreservation at −80°C. All operations were conducted in the anaerobic workstation unless otherwise indicated.
Bacterial identification and characterization of novel taxa
The cultured bacterial strains were sequenced by Tianyi Huiyuan Co. Ltd. for 16S rRNA gene using the universal primers 27f and 1492r [19], and searched for close relatives using EzBioCloud [20]. For all Lachnospiraceae strains, nearly full‐length 16S rRNA gene sequences were generated and are listed in Supporting Information Table S3A. The delineations of novel taxa were based on the analysis of each type of strain in terms of phylogenetic, genomic, physiological, and morphological characteristics as described in our previous works [62, 130], and the criteria used for the proposal of novel species/genus/family described in our previous publication [52]. In brief, thresholds of 98.7% and 94.5% 16S rRNA gene sequence identities were considered as indications for novel species and genera, respectively [39]. The digital DNA: DNA hybridization (dDDH) values <70% and average nucleotide identity (ANI) values <95% were considered as an indication for separate species [64, 69]. The percentage of conserved proteins (POCPs) values <50% was considered as an indication for separate genera [66]. The dDDH values were calculated with Genome‐to‐Genome Distance Calculator 2.0 at http://ggdc.dsmz.de [64]. The ANI values were calculated with OrthoANI [65]. The POCPs were determined using BLASTP (thresholds for delineation of aligned sequences: E‐value <1e−5, identity >40%, and query coverage of >50%) [66]. The 16S rRNA gene‐based phylogenetic trees and genome‐based phylogenomic trees of newly isolated strains and their related type species were created using UBCG [67], and presented in Supporting Information Figures S2A–8A and Figures S2B–8B. The bacterial cell morphology was observed using a transmission electron microscope JEM‐1400 (JOEL) (Supporting Information Figures S2C–S8C). The nomenclature of each characterized novel taxa was proposed according to the rules of the International Code of Nomenclature of Prokaryotes [131] The descriptions of novel taxa were presented below and in Supporting Information Data 1.
Descriptions of novel taxa
Anaerofusibacter gen. nov. (An.ae.ro.fu.si.bac'ter. Gr. pref. an‐, not; Gr. masc. n. aer (gen. aeros), air; n. fusus, a spindle; N.L. masc. n. bacter, rod; N.L. masc, anaerobic spindle‐shaped rod bacteria). The genus Anaerofusibacter is a member of the Lachnospiraceae family. The type species is A. homins.
A. homins sp. nov. (ho'mi.nis. L. gen. masc. n. homins, of human origin, refers to the type strain isolated from human fecal samples). The type strain NSJ‐143 (=CGMCC 1.17904) was isolated from the feces of a healthy adult. The genome size of the type strain is 2.67 Mbp, and the G + C content is 41.51 mol%. Cells are spindle‐shaped (0.6–1.2 µm wide by 1.2–2.6 µm long), Gram‐positive, non‐spore‐forming, and nonmotile. Cells grow under strictly anaerobic conditions and appear singly or in pairs. After 72 h of anaerobic incubation at 37°C, tiny, smooth white colonies appeared on the Lach‐GAM medium. Cells utilize d‐cellobiose, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, d‐glucosamine, α‐d‐glucose, glucose‐6‐phosphate, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, uridine, lactulose, and maltose for growth. The major cellular fatty acids are C16:0 and C18:0. The major polar lipids are unknown phospholipids, lipids, and glycolipids.
Anaerosegnis gen. nov. (A.nae.ro.sge.nis. Gr. pref. an‐, not; Gr. masc. n. aer (gen. aeros), air; L. masc./fem. adj. segnis, slow. a slow [growing] anaerobic organism). The genus Anaerosegnis is a member of the Lachnospiraceae family. The type species is Anaerosegnis homins.
A. homins sp. nov. (ho'mi.nis. L. gen. masc. n. homins, of a man, the host from which the species was first isolated). The type strain Lach‐103 (=CGMCC 1.46159) was isolated from the feces of a healthy Chinese adult. The genome size of the type strain is 3.03 Mbp and the G + C content is 42.49 mol%. Cells are rod‐shaped, Gram‐negative, straight rods, nonmotile, and non‐spore‐forming. Cells grow under strictly anaerobic conditions and appear singly. Growth occurs at the pH range of 6.5–7.5 (optimum 7.0), a temperature range of 35–40°C (optimum 37°C). Tiny, slightly raised, transparent colonies appear on Lach‐GAM agar plates after 6 days of incubation at 37°C. Cells utilize d‐cellobiose, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, d‐glucosaminic acid, α‐d‐glucose, glucose‐6‐phosphate, maltose, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, uridine, α‐d‐lactose, lactulose, and propionic acid for growth.
B. acetigenes sp. nov. (a.ce.ti'ge.nes.L. neut. n. acetum, acetic acid; Gr. suff.‐genes, forming; from L. v. gigno, to form; N.L. part. adj. acetigenes, acetogenic, refers to the bacterium produces acetic acid). The type strain NSJ‐165 (CGMCC 1.17923) was isolated from the feces of a healthy adult. The genome size of the type strain is 6.46 Mb, and the G + C content is 46.00 mol%. Cell size is 0.4–0.8 µm × 0.6–1.5 µm. Cells grow under strictly anaerobic conditions and are neither spore‐forming nor motile. The optimal growth pH is 6.5–7.5, and the optimal growth temperature is 30–37°C. After 2 days at 37°C, round (0.5–1.1 mm in diameter), dry, flat, yellow to white, rough‐edged colonies appear on Lach‐GAM agar plates. Cells utilize d‐cellobiose, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, d‐glucosamine, α‐d‐glucose, glucose‐6‐phosphoric acid, maltose, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, and uridine, α‐d‐lactose, lactose, fructose, and glyoxylic acid for growth. The major cellular fatty acid components are C16:0, C14:0, and C17:0 2OH.
Blautia beijingensis sp. nov. (bei.jing.en'sis. N.L. fem. adj. beijingensis, of Beijing, in Beijing, specifically refers to the strain isolated in Beijing). Type strain NSJ‐157 (=CGMCC 1.17918) was isolated from the feces of a healthy adult. The genome size of the type strain is 3.61 Mb, and the G + C content of genomic DNA is 44.08 mol%. Cells are oval to short rod‐shaped (0.2–0.6 µm × 0.5–1.2 µm), non‐spore‐forming and nonmotile. Cells grow under strictly anaerobic conditions and mostly occur in pairs or single. The colony is round, raised, and moist on Lach‐GAM agar plates after 2 days of incubation at 37°C. Growth occurs at a pH of 6.5–7.5 (optimum 7.0), and a temperature of 30–45°C (optimum 37°C). Cells utilize d‐arabitol, d‐cellobiose, erythritol, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, d‐gluconic acid, d‐glucosamine, α‐d‐glucose, 6‐phosphoglucose, inositol, lactulose, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, glyoxylic acid, pyruvate and methyl pyruvate, dextrin, and succinic acid for growth.
Blautia fragilis sp. nov. (fra'gi.lis. L. fem. adj. fragilis, fragile, Cells are fragile and hard to culture). The type strain NSJ‐166 (CGMCC 1.46116) was isolated from the feces of a healthy adult. The genome size of the type strain is 3.61 Mb and genomic DNA G + C content is 44.03 mol%. Cells are oval to short rod‐shaped (0.7–1.1 µm × 1.4–2.1 µm), non‐spore‐forming and nonmotile. Cells grow anaerobically and appear singly or in pairs. The growth occurs at pH of 6.5–7.5 (optimum 6.5), and a temperature of 30–37°C (optimum 7.0). After 4 days of anaerobic incubation at 37°C, tiny, slightly raised clear colonies (0.8–1.2 mm in diameter) appeared on Lach‐GAM agar plates. Cells utilize d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, d‐glucosamine, α‐d‐glucose, Lactulose, d‐mannose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, glyoxylic acid, pyruvate, glucose‐6‐phosphate, m‐inositol, α‐ketobutyric acid, α‐ketovaleric acid, and methyl pyruvate for growth.
Lientehia gen. nov. (Lien.teh'i.a. N.L. fem. n. Lientehia, named in honor of Wu Lienteh, a famous Chinese microbiologist and medical scientist who has made great contributions to the fight against the plague). Genus Lientehia is a member of the family Lachnospiraceae. The type species is Lientehia homins.
L. homins sp. nov. (ho'mi.nis. L. gen. masc. n. homins, of a human being, referring to the human gut habita). The type strain NSJ‐141 (=CGMCC 1.17902=KCTC 25345) was isolated from the feces of a healthy Chinese adult. The genome size of the type strain is 3.05 Mbp and the G + C content is 48.7 mol%. Cells are fusiform (0.5–0.8 µm wide and 0.9–1.2 µm long), Gram‐negative, nonmobile and non‐spore‐forming. Cells grow anaerobically and appear in dividing pairs or in dividing chains. Growth occurs at the temperature of 30–45°C (optimum 37°C), pH of 6–7.5 (optimum pH, 6.5), and NaCl pressure of 0%–3.0% (w/v). After incubation at 37°C for 72 h, yellow, smooth, circular, or irregular, flat colonies with semitransparent extended margins in a diameter of 0.9–1.5 mm appear on modified gifu anaerobic medium agar medium. Cells metabolize adonitol, d‐cellobiose, dextrin, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, α‐d‐glucose, glucose‐6‐phosphate, lactulose, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, glyoxylic acid, pyruvic acid and pyruvic acid methyl ester, d‐arabitol, d‐gluconic acid, d‐glucosaminic acid, m‐inositol, and maltose for growth. The predominant cellular fatty acids are C16:0 and C18:0ω7c. The major polar lipids are diphosphatidylglycerol, phosphatidylglycerol, phosphatidylglycolipids, unidentified phospholipids, and glycolipids.
M. intestinihomins sp. nov. (in.tes.ti.ni.ho'mi.nis. L. gen. neut. n. intestini, intestinal; L. gen. masc. n. homins, of human, N.L. gen. masc. n. intestinihomins, human intestinal, refers to type strains isolated from human intestinal). The type strain NSJ‐151 (=CGMCC 1.17913) was isolated from the feces of an adult. The genome size is 3.41 Mbp, and the G + C content of the genomic DNA is 39.0 mol%. Cells are fusobacterium (0.6–1.0 µm × 1.2–2.3 µm), Gram‐positive, non‐spore‐forming, and nonmotile. Cells utilize d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, α‐d‐glucose, 6‐phosphoglucose, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, glyoxylic acid, pyruvate, d‐cellobiose, d‐glucosamine, lactulose, turanose, and l‐asparagine for growth. The major cellular fatty acids are C16:0, C17:0 2OH, and C14:0. Glucose metabolism produces acetate, propionate, isobutyrate, and butyrate.
Rarifaecibacter gen. nov. (Ra.ri.fae.ci.bac.ter L. masc. adj. rarus, rare; fae.ci. L. masc. n. faex (gen. faecis), pertaining to feces; N.L. masc. n. bacter, a rod, a rarely studied bacterium isolated from stool, named because this species is rarely isolated). The genus Rarifaecibacter is a member of the Lachnospiraceae family. The type species is Rarifaecibacter acetigenes.
R. acetigenes sp. nov. (a.ce.ti'ge.nes. L. neut. n. acetum, vinegar; Gr. suff.‐genes, producing; from Gr. ind. v. gennaô, to produce; N.L. part. adj. acetigenes, vinegar‐ or acetic acid‐producing). Type strain NSJ‐177 (=CGMCC 1.17905) was isolated from the feces of a healthy adult. The genome size of the type strain is 4.28 Mbp and the G + C content is 52.08 mol%. Cells are rod‐shaped (0.6–0.9 µm × 1.8–2.4 µm), Gram‐positive, non‐spore‐forming, and nonmobile. After 48 h of anaerobically incubation at 37°C, tiny, raised gray colonies were formed on the Lach‐GAM medium. Cells utilize ribitol, d‐cellobiose, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, α‐d‐glucose, 6‐phosphate glucose, α‐d‐lactose, lactulose, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, glyoxylic acid, pyruvate, methyl pyruvate, d‐arabitol, and dextrin for growth. The major cellular fatty acids are C16:0 and C14:0. The major polar lipids of cells are diphosphatidylglycerol, phosphatidylglycerol, and phosphatidylethanolamine as well as unknown lipids.
Shanjiongia gen. nov. (Shan.jiong.i'a. N.L. fem. n. Shanjiongia, named in honor of the famous microbiologist Shen Shanjiong). The genus Shanjiongia is a member of the Lachnospiraceae family. The type species is Shanjiongia homins.
S. homins sp. nov. (ho'mi.nis. L. gen. masc. n. homins, human, referring to the type strain was isolated from human fecal samples). Type strain NSJ‐171 (=CGMCC 1.17927) was isolated from the feces of a healthy adult. The genome size is about 2.35 Mbp, and the G + C content is about 33.0 mol%. Cells are oval‐shaped, Gram‐negative, non‐spore‐forming, and nonmotile. Cells grow under strictly anaerobic conditions and appear singly or in pairs. Cells utilize erythritol, d‐fructose, l‐fucose, d‐galactose, d‐galacturonic acid, gentiobiose, d‐glucosamine, α‐d‐glucose, lactulose, maltose trisaccharide, d‐mannose, d‐melibiose, 3‐methyl‐d‐glucose, palatinose, l‐rhamnose, turanose, pyruvate, glucose‐6‐phosphate, glyoxylic acid, α‐ketobutyric acid, and methyl pyruvate for growth. The major cellular fatty acids are C14:0 and C16:1.
Determination of SCFAs
The concentrations of SCFAs (including acetate, propionate, butyrate, valerate, isobutyrate, and isovalerate) were determined using GC‐MS. Bacterial cells were incubated at 37°C anaerobically in Lach‐GAM broth for 72–168 h until OD600 nm reached 1.0–1.2, then the cells were collected. For in vitro detection of SCFAs production, no SCFA was added in the liquid medium. The cell cultures were measured for each strain, and the sterile liquid medium was used as a blank control in which no SCFA peak was detected by GC‐MS analysis. According to Sumner et al., the SCFAs identified in this study belonged to level 1‐identified compounds, which referenced both standards and the NIST library [132]. For each sample, 1 ml cell culture was extracted with 1 ml ethyl acetate, and the supernatant was prepared for GC‐MS analysis performed on a GCMS‐QP2010 Ultra with an autosampler (SHIMADZU) and the DB‐wax capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness, SHIMADZU). Standard curves of SCFAs were achieved by pure chemical agents of corresponding chemicals, purchased from Aladdin, diluted in ethyl acetate of chromatographic purity, dilution rate, and corresponding peak area data were detailed in Supporting Information Table S4A. The temperature of the oven was programmed from 35°C to 130°C at 5°C/min gradients, to 230°C at 30°C/min gradients, with 16 min hold. Injection of 2 μl samples was performed at 230°C. The carrier gas, helium, flowed at 1.0 ml/min. Ion source and interface temperature were both set at 230°C. The electronic impact was recorded at 70 eV.
Profiling of metabolites with SPME and GC‐MS
All 110 Lachnospiraceae strains were profiled for metabolites with GC‐MS after SPME. Sterile, noninoculated Lach‐GAM medium was used as control. The SPME fiber, 50/30 μm DVB/CAR/PDMS, Stableflex (Supelco), was inserted through the septum of cell‐culture tubes and exposed in the headspace of the vial for 60 min, to allow complete absorption of the volatile compounds onto the SPME fiber. The SPME fiber was then introduced into the injector port of the gas chromatograph for 1 min in splitless mode, injection temperature was set at 240°C, to desorb the volatile compounds. Helium was used as a carrier gas with a flow of 1.0 ml/min and the oven temperature was programmed as follows: 40°C for 3 min, then ramped at 5°C/min to 240°C, held for 15 min. Ion source and interface temperature were both set at 240°C. The metabolites were identified by searching the obtained mass spectrum in the National Institute for Technology Standards (NIST11; www.nist.gov) mass spectral library with a threshold match score >85 rather than comparison with standards, and according to Sumner et al., the metabolites identified in this study belonged to level 2‐identified compounds, which referenced the NIST library only [132]. Data were reported as the peak area for each compound detected. Spectrum search encompasses baseline subtraction and averaging over a peak. Similar to the determination of SCFAs, the sterile Lach‐GAM liquid medium was taken as blank control, and peaks detected in the corresponding blanks were eliminated from the metabolite profiles of bacterial cultures, for the obtainment of signals attributed solely to bacterial metabolic activity. Relative amounts of metabolites were presented by the relative percentages of peak areas of metabolites produced by each strain. Average peak intensities were mean‐centered and unit‐scaled. All the processes of data analysis and visualization were conducted by using the ggplot2 package [23], RColorBrewer [68], and complex‐heatmap package in R [24].
AUTHOR CONTRIBUTIONS
Shuang‐Jiang Liu and Chang Liu designed and supervised the entire study. Rashidin Abdugheni, Wen‐Zhao Wang, Yu‐Jing Wang, Meng‐Xuan Du, Feng‐Lan Liu, Cheng‐Ying Jiang, Nan Zhou, and Chang‐Yu Wang conducted the experiments. Linhuan Wu and Juncai Ma constructed the websites for hLchsp. Rashidin Abdugheni drafted the manuscript. Shuang‐Jiang Liu and Chang Liu revised, edited, and finalized the manuscript. All the authors confirmed the final version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The ethics application (APIMCAS2017049) was approved by the Research Ethics Committee of the Institute of Microbiology, Chinese Academy of Sciences.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
This study was funded by the National Key Research and Development Program of China (Grant No. 2021YFA0717002) and by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB38020300).
Abdugheni, Rashidin , Wang Wen‐Zhao, Wang Yu‐Jing, Du Meng‐Xuan, Liu Feng‐Lan, Zhou Nan, Jiang Cheng‐Ying, Wang Chang‐Yu, Wu Linhuan, Ma Juncai, Liu Chang, and Liu Shuang‐Jiang 2022. “Metabolite profiling of human‐originated Lachnospiraceae at the strain level.” iMeta 1, e58. 10.1002/imt2.58
Rashidin Abdugheni and Wen‐Zhao Wang equally contributed to this study.
Contributor Information
Chang Liu, Email: liu.c@sdu.edu.cn.
Shuang‐Jiang Liu, Email: liusj@im.ac.cn.
DATA AVAILABILITY STATEMENT
The data were available at the National Microbiology Data Center (NMDC, https://nmdc.cn/) under accessions NMDC10018179. Moreover, the information of all 148 strains and their 16S rRNA gene sequences were accessible via the hLchsp website (https://hgmb.nmdc.cn/subject/lachnospiraceae), and the in vitro metabolite profiles of 110 Lachnospiraceae strains were available at https://hgmb.nmdc.cn/subject/lachnospiraceae/metabolites. Supporting Information materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.
REFERENCES
- 1. Almeida, Alexandre , Nayfach Stephen, Boland Miguel, Strozzi Francesco, Beracochea Martin, Shi Zhou Jason, Pollard Katherine S., et al. 2021. “A Unified Catalog of 204,938 Reference Genomes from the Human Gut Microbiome.” Nature Biotechnology 39: 105–14. 10.1038/s41587-020-0603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arumugam, Manimozhiyan , Raes Jeroen, Pelletier Eric, Le Paslier Denis, Yamada Takuji, Mende Daniel R., Fernandes Gabriel R., et al. 2011. “Enterotypes of the Human Gut Microbiome.” Nature 473: 174–80. 10.1038/nature09944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozupone, Catherine A. , Stombaugh Jesse I., Gordon Jeffrey I., Jansson Janet K., and Knight Rob. 2012. “Diversity, Stability and Resilience of the Human Gut Microbiota.” Nature 489: 220–30. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Truong, Duy Tin , Tett Adrian, Pasolli Edoardo, Huttenhower Curtis, and Segata Nicola. 2017. “Microbial Strain‐Level Population Structure and Genetic Diversity from Metagenomes.” Genome Research 27: 626–38. 10.1101/gr.216242.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tyakht, Alexander V. , Kostryukova Elena S., Popenko Anna S., Belenikin Maxim S., Pavlenko Alexander V., Larin Andrey K., Karpova Irina Y., et al. 2013. “Human Gut Microbiota Community Structures in Urban and Rural Populations in Russia.” Nature Communications 4: 2469. 10.1038/ncomms3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vacca, Mirco , Celano Giuseppe, Calabrese Francesco Maria, Portincasa Piero, Gobbetti Marco, and De Angelis Maria. 2020. “The Controversial Role of Human Gut Lachnospiraceae.” Microorganisms 8, 573. 10.3390/microorganisms8040573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li, Wei , Wu Xiaoli, Hu Xu, Wang Tao, Liang Shan, Duan Yunfeng, Jin Feng, and Qin Bin. 2017. “Structural Changes of Gut Microbiota in Parkinson's Disease and Its Correlation with Clinical Features.” Science China Life Sciences 60: 1223–33. 10.1007/s11427-016-9001-4 [DOI] [PubMed] [Google Scholar]
- 8. Zhang, Jindong , Song Lijin, Wang Yujing, Liu Chang, Zhang Lu, Zhu Shiwei, Liu Shuangjiang, and Duan Liping. 2019. “Beneficial Effect of Butyrate‐Producing Lachnospiraceae on Stress‐Induced Visceral Hypersensitivity in Rats.” Journal of Gastroenterology and Hepatology 34: 1368–76. 10.1111/jgh.14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almeida, Alexandre , Mitchell Alex L., Boland Miguel, Forster Samuel C., Gloor Gregory B., Tarkowska Aleksandra, Lawley Trevor D., and Finn Robert D.. 2019. “A New Genomic Blueprint of the Human Gut Microbiota.” Nature 568: 499–504. 10.1038/s41586-019-0965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliphant, Kaitlyn , Ali Mehneez, D'Souza Mark, Hughes Patrick D., Sulakhe Dinanath, Wang Annie Z., Xie Bingqing, et al. 2021. “Bacteroidota and Lachnospiraceae Integration into the Gut Microbiome at Key Time Points in Early Life Are Linked to Infant Neurodevelopment.” Gut Microbes 13: 1997560. 10.1080/19490976.2021.1997560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sagheddu, Valeria , Patrone Vania, Miragoli Francesco, Puglisi Edoardo, and Morelli Lorenzo. 2016. “Infant Early Gut Colonization by Lachnospiraceae: High Frequency of Ruminococcus gnavus .” Frontiers in Pediatrics 4: 57. 10.3389/fped.2016.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma, Bingjie , Liang Jingjing, Dai Meixia, Wang Jue, Luo Jingyin, Zhang Zheqing, and Jing Jin. 2019. “Altered Gut Microbiota in Chinese Children with Autism Spectrum Disorders.” Frontiers in Cellular and Infection Microbiology 9: 40. 10.3389/fcimb.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Méndez‐Salazar, Eder Orlando , Ortiz‐López María Guadalupe, Granados‐Silvestre María de los Ángeles, Palacios‐González Berenice, and Menjivar Marta. 2018. “Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children.” Frontiers in Microbiology 9: 2494. 10.3389/fmicb.2018.02494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen, Lea B. S. , Roager Henrik M., Søndertoft Nadja B., Gøbel Rikke J., Kristensen Mette, Vallès‐Colomer Mireia, Vieira‐Silva Sara, et al. 2018. “A Low‐Gluten Diet Induces Changes in the Intestinal Microbiome of Healthy Danish Adults.” Nature Communications 9: 4630. 10.1038/s41467-018-07019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kushida, Mamoru , Sugawara Saeko, Asano Masaki, Yamamoto Kazushi, Fukuda Shinji, and Tsuduki Tsuyoshi. 2019. “Effects of the 1975 Japanese Diet on the Gut Microbiota in Younger Adults.” The Journal of Nutritional Biochemistry 64: 121–7. 10.1016/j.jnutbio.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 16. Biagi, Elena , Franceschi Claudio, Rampelli Simone, Severgnini Marco, Ostan Rita, Turroni Silvia, Consolandi Clarissa, et al. 2016. “Gut Microbiota and Extreme Longevity.” Current Biology 26: 1480–5. 10.1016/j.cub.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 17. Cox, Natalie J. , Bowyer Ruth C. E., Ni Lochlainn Mary, Wells Philippa M., Roberts Helen C., and Steves Claire J.. 2021. “The Composition of the Gut Microbiome Differs Among Community Dwelling Older People with Good and Poor Appetite.” Journal of Cachexia, Sarcopenia and Muscle 12: 368–77. 10.1002/jcsm.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Toole, Paul W. , and Jeffery Ian B.. 2018. “Microbiome–Health Interactions in Older People.” Cellular and Molecular Life Sciences 75: 119–28. 10.1007/s00018-017-2673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song, Lijin , He Meibo, Sun Qinghua, Wang Yujing, Zhang Jindong, Fang Yuan, Liu Shuangjiang, and Duan Liping. 2021. “ Roseburia hominis Increases Intestinal Melatonin Level by Activating p‐CREB‐AANAT Pathway.” Nutrients 14: 117. 10.3390/nu14010117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bultman, Scott J. 2014. “Molecular Pathways: Gene–Environment Interactions Regulating Dietary Fiber Induction of Proliferation and Apoptosis via Butyrate for Cancer Prevention.” Clinical Cancer Research 20: 799–803. 10.1158/1078-0432.CCR-13-2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murugesan, Selvasankar , Nirmalkar Khemlal, Hoyo‐Vadillo Carlos, García‐Espitia Matilde, Ramírez‐Sánchez Daniela, and García‐Mena Jaime. 2018. “Gut Microbiome Production of Short‐Chain Fatty Acids and Obesity in Children.” European Journal of Clinical Microbiology & Infectious Diseases 37: 621–5. 10.1007/s10096-017-3143-0 [DOI] [PubMed] [Google Scholar]
- 22. Sugiyama, Yukiko , Masumori Naoya, Fukuta Fumimasa, Yoneta Akihiro, Hida Tokimasa, Yamashita Toshiharu, Minatoya Machiko, et al. 2013. “Influence of Isoflavone Intake and Equol‐Producing Intestinal Flora on Prostate Cancer Risk.” Asian Pacific Journal of Cancer Prevention 14: 1–4. 10.7314/apjcp.2013.14.1.1 [DOI] [PubMed] [Google Scholar]
- 23. Pelyuntha, Wattana , Chaiyasut Chaiyavat, Kantachote Duangporn, and Sirilun Sasithorn. 2020. “Organic Acids and 2,4‐Di‐tert‐Butylphenol: Major Compounds of Weissella Confusa WM36 Cell‐Free Supernatant Against Growth, Survival and Virulence of Salmonella Typhi.” PeerJ 8: e8410. 10.7717/peerj.8410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito, Shunsuke , and Yoshida Masayuki. 2014. “Protein‐Bound Uremic Toxins: New Culprits of Cardiovascular Events in Chronic Kidney Disease Patients.” Toxins 6: 665–78. 10.3390/toxins6020665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iizuka, Ryoko , Kawakami Koji, Izawa Naoki, and Chiba Katsuyoshi. 2009. “Phenols Produced by Gut Bacteria Affect the Skin in Hairless Mice.” Microbial Ecology in Health and Disease 21: 50–6. 10.1080/08910600802688910 [DOI] [Google Scholar]
- 26. Kelly, Denise , and Mulder Imke. 2016. “Bacterium for Use As a Probiotic for Nutritional and Medical Applications.” WO2013050792A1[P]. Patent no. 9314489. https://www.freepatentsonline.com/9314489.html
- 27. Reeves, Angela E. , Koenigsknecht Mark J., Bergin Ingrid L., and Young Vincent B.. 2012. “Suppression of Clostridium difficile in the Gastrointestinal Tracts of Germfree Mice Inoculated with a Murine Isolate from the Family Lachnospiraceae.” Infection and Immunity 80: 3786–94. 10.1128/IAI.00647-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Del Chierico, Federica , Nobili Valerio, Vernocchi Pamela, Russo Alessandra, De Stefanis Cristiano, Gnani Daniela, Furlanello Cesare, et al. 2017. “Gut Microbiota Profiling of Pediatric Nonalcoholic Fatty Liver Disease and Obese Patients Unveiled by an Integrated Meta‐Omics‐Based Approach.” Hepatology 65: 451–64. 10.1002/hep.28572 [DOI] [PubMed] [Google Scholar]
- 29. Hall, Andrew Brantley , Yassour Moran, Sauk Jenny, Garner Ashley, Jiang Xiaofang, Arthur Timothy, Lagoudas Georgia K., et al. 2017. “A Novel Ruminococcus gnavus Clade Enriched in Inflammatory Bowel Disease Patients.” Genome Medicine 9: 103. 10.1186/s13073-017-0490-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ottosson, Filip , Brunkwall Louise, Ericson Ulrika, Nilsson Peter M., Almgren Peter, Fernandez Céline, Melander Olle, and Orho‐Melander Marju. 2018. “ Connection Between BMI‐related Plasma Metabolite Profile and Gut Microbiota.” The Journal of Clinical Endocrinology & Metabolism 103: 1491–501. 10.1210/jc.2017-02114 [DOI] [PubMed] [Google Scholar]
- 31. Zeng, Qiang , Li Dongfang, He Yuan, Li Yinhu, Yang Zhenyu, Zhao Xiaolan, Liu Yanhong, et al. 2019. “Discrepant Gut Microbiota Markers for the Classification of Obesity‐Related Metabolic Abnormalities.” Scientific Reports 9: 13424. 10.1038/s41598-019-49462-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaughn, Byron P. , Kaiser Thomas, Staley Christopher, Hamilton Matthew J., Reich Jon, Graiziger Carolyn, Singroy Stephanie, et al. 2019. “A Pilot Study of Fecal Bile Acid and Microbiota Profiles in Inflammatory Bowel Disease and Primary Sclerosing Cholangitis.” Clinical and Experimental Gastroenterology 12: 9–19. 10.2147/CEG.S186097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen, Liping , Wang Wei, Zhou Rui, Ng Siew C., Li Jin, Huang Meifang, Zhou Feng, et al. 2014. “Characteristics of Fecal and Mucosa‐Associated Microbiota in Chinese Patients with Inflammatory Bowel Disease.” Medicine (Baltimore) 93: e51. 10.1097/md.0000000000000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suchodolski, Jan S. , Markel Melissa E., Garcia‐Mazcorro Jose F., Unterer Stefan, Heilmann Romy M., Dowd Scot E., Kachroo Priyanka, et al. 2012. “The Fecal Microbiome in Dogs with Acute Diarrhea and Idiopathic Inflammatory Bowel Disease.” PLoS ONE 7: e51907. 10.1371/journal.pone.0051907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baumgart, Martin , Dogan Belgin, Rishniw Mark, Weitzman Gil, Bosworth Brian, Yantiss Rhonda, Orsi Renato H., et al. 2007. “Culture Independent Analysis of Ileal Mucosa Reveals a Selective Increase in Invasive Escherichia coli of Novel Phylogeny Relative to Depletion of Clostridiales in Crohn's Disease Involving the Ileum.” The ISME Journal 1: 403–18. 10.1038/ismej.2007.52 [DOI] [PubMed] [Google Scholar]
- 36. Zhang, Chenhong , Zhang Menghui, Wang Shengyue, Han Ruijun, Cao Youfang, Hua Weiying, Mao Yuejian, et al. 2010. “Interactions Between Gut Microbiota, Host Genetics and Diet Relevant to Development of Metabolic Syndromes in Mice.” The ISME Journal 4: 232–41. 10.1038/ismej.2009.112 [DOI] [PubMed] [Google Scholar]
- 37. Zhao, Liping . 2013. “The Gut Microbiota and Obesity: From Correlation to Causality.” Nature Reviews Microbiology 11: 639–47. 10.1038/nrmicro3089 [DOI] [PubMed] [Google Scholar]
- 38. Zhai, Rui , Xue Xinhe, Zhang Liying, Yang Xin, Zhao Liping, and Zhang Chenhong. 2019. “Strain‐Specific Anti‐Inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice.” Front Cell Infect Microbiol 9: 239. 10.3389/fcimb.2019.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Savignac, H. M. , Kiely B., Dinan T. G., and Cryan J. F.. 2014. “Bifidobacteria Exert Strain‐Specific Effects on Stress‐Related Behavior and Physiology in BALB/c Mice.” Neurogastroenterology & Motility 26: 1615–27. 10.1111/nmo.12427 [DOI] [PubMed] [Google Scholar]
- 40. Song, Lijin , Sun Qinghua, Zheng Haonan, Zhang Yiming, Wang Yujing, Liu Shuangjiang, and Duan Liping. 2022. “ Roseburia hominis Alleviates Neuroinflammation via Short‐Chain Fatty Acids Through Histone Deacetylase Inhibition.” Molecular Nutrition & Food Research 66: 2200164. 10.1002/mnfr.202200164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hosomi, Koji , Saito Mayu, Park Jonguk, Murakami Haruka, Shibata Naoko, Ando Masahiro, Nagatake Takahiro, et al. 2022. “Oral Administration of Blautia wexlerae Ameliorates Obesity and Type 2 Diabetes Via Metabolic Remodeling of the Gut Microbiota.” Nature Communications 13: 4477. 10.1038/s41467-022-32015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu, Haiyang , Xu Xiaoqiang, Fu Di, Gu Yubei, Fan Rong, Yi Hongmei, He Xiangyi, et al. 2022. “Butyrate‐Producing Eubacterium rectale Suppresses Lymphomagenesis by Alleviating the TNF‐induced TLR4/MyD88/NF‐κB.” Cell Host & Microbe 30: 1139–50. 10.1016/j.chom.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 43. Islam, S. M. Shamsul , Ryu Hye‐Myung, Sayeed Hasan M., Byun Hae‐Ok, Jung Ju‐Yang, Kim Hyoun‐Ah, Suh Chang‐Hee, and Sohn Seonghyang. 2021. “ Eubacterium rectale Attenuates HSV‐1 Induced Systemic Inflammation in Mice by Inhibiting CD83.” Frontiers in Immunology 12 : 712312 10.3389/fimmu.2021.712312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fodor, Anthony A. , Desantis Todd Z., Wylie Kristine M., Badger Jonathan H., Ye Yuzhen, Hepburn Theresa, Ping Ping, et al. 2012. “The “Most Wanted” Taxa from the Human Microbiome for Whole Genome Sequencing.” PLoS ONE 7: e41294. 10.1371/journal.pone.0041294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boesmans, Leen , Valles‐Colomer Mireia, Wang Jun, Eeckhaut Venessa, Falony Gwen, Ducatelle Richard, Van Immerseel Filip, Raes Jeroen, and Verbeke Kristin. 2018. “Butyrate Producers as Potential Next‐Generation Probiotics: Safety Assessment of the Administration of Butyricicoccus pullicaecorum to Healthy Volunteers.” mSystems 3: e00094–18. 10.1128/mSystems.00094-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu, Xuemei , Mao Bingyong, Gu Jiayu, Wu Jiaying, Cui Shumao, Wang Gang, Zhao Jianxin, Zhang Hao, and Chen Wei. 2021. “Blautia—A New Functional Genus with Potential Probiotic Properties?” Gut Microbes 13: 1875796. 10.1080/19490976.2021.1875796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Louis, Petra , and Flint Harry J.. 2009. “Diversity, Metabolism and Microbial Ecology of Butyrate‐Producing Bacteria from the Human Large Intestine.” FEMS Microbiology Letters 294: 1–8. 10.1111/j.1574-6968.2009.01514.x [DOI] [PubMed] [Google Scholar]
- 48. Belzer, Clara , Chia Loo Wee, Aalvink Steven, Chamlagain Bhawani, Piironen Vieno, Knol Jan, and de Vos Willem M.. 2017. “Microbial Metabolic Networks at the Mucus Layer Lead to Diet‐Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts.” mBio 8: e00770‐17. 10.1128/mBio.00770-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo, Chun‐Jun , Chang Fang‐Yuan, Wyche Thomas P., Backus Keriann M., Acker Timothy M., Funabashi Masanori, Taketani Mao, et al. 2017. “Discovery of Reactive Microbiota‐Derived Metabolites That Inhibit Host Proteases.” Cell 168: 517–26. 10.1016/j.cell.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saito, Yuki , Sato Tadashi, Nomoto Koji, and Tsuji Hirokazu. 2018. “Identification of Phenol‐ and p‐Cresol‐Producing Intestinal Bacteria by Using Media Supplemented with Tyrosine and Its Metabolites.” FEMS Microbiology Ecology 94: fiy125. 10.1093/femsec/fiy125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abdugheni, Rashidin , Wang Yu‐Jing, Li Dan‐Hua, Du Meng‐Xuan, Liu Chang, Zhou Nan, and Liu Shuang‐Jiang. 2022. “ Pararoseburia lenta gen. nov., sp. nov. Isolated from Human Faeces.” International Journal of Systematic and Evolutionary Microbiology 72: 005371. 10.1099/ijsem.0.005371 [DOI] [PubMed] [Google Scholar]
- 52. Liu, Chang , Du Meng‐Xuan, Abuduaini Rexiding, Yu Hai‐Ying, Li Dan‐Hua, Wang Yu‐Jing, Zhou Nan, et al. 2021. “Enlightening the Taxonomy Darkness of Human Gut Microbiomes with a Cultured Biobank.” Microbiome 9: 119. 10.1186/s40168-021-01064-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reimer, Lorenz Christian , Vetcininova Anna, Carbasse Joaquim Sardà, Söhngen Carola, Gleim Dorothea, Ebeling Christian, and Overmann Jörg. 2019. “Bac Dive in 2019: Bacterial Phenotypic Data for High‐Throughput Biodiversity Analysis.” Nucleic Acids Research 47: D631–6. 10.1093/nar/gky879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gotoh, Aina , Nara Misaki, Sugiyama Yuta, Sakanaka Mikiyasu, Yachi Hiroyuki, Kitakata Aya, Nakagawa Akira, et al. 2017. “Use of Gifu Anaerobic Medium for Culturing 32 Dominant Species of Human Gut Microbes and Its Evaluation Based on Short‐Chain Fatty Acids Fermentation Profiles.” Bioscience, Biotechnology, and Biochemistry 81: 2009–17. 10.1080/09168451.2017.1359486 [DOI] [PubMed] [Google Scholar]
- 55. Adamberg, Kaarel , and Adamberg Signe.. 2018. “Selection of Fast and Slow Growing Bacteria from Fecal Microbiota Using Continuous Culture with Changing Dilution Rate.” Microbial Ecology in Health and Disease 29: 1549922. 10.1080/16512235.2018.1549922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hou, Fengyi , Chang Yuxiao, Huang Zongyu, Han Ni, Bin Lei, Deng Huimin, Li Zhengchao, et al. 2019. “Application of LpxC Enzyme Inhibitor to Inhibit Some Fast‐Growing Bacteria in Human Gut Bacterial Culturomics.” BMC Microbiology 19: 308. 10.1186/s12866-019-1681-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sorbara, Matthew T. , Littmann Eric R., Fontana Emily, Moody Thomas U., Kohout Claire E., Gjonbalaj Mergim, Eaton Vincent, et al. 2020. “Functional and Genomic Variation Between Human‐Derived Isolates of Lachnospiraceae Reveals Inter‐ and Intra‐Species Diversity.” Cell Host & Microbe 28: 134–46, e134. 10.1016/j.chom.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dahlen, Thomas , Hauck Tobias, Wein Martina, and Schwab Wilfried. 2001. “2,5‐Dimethyl‐4‐Hydroxy‐3 (2H)‐Furanone as a Secondary Metabolite from d‐Fructose‐1,6‐Diphosphate Metabolism by Zygosaccharomyces rouxii .” Journal of Bioscience and Bioengineering 91: 352–8. 10.1016/S1389-1723(01)80150-X [DOI] [PubMed] [Google Scholar]
- 59. Hauck, Tobias , Brühlmann Fredi, and Schwab Wilfried. 2003. “4‐Hydroxy‐2,5‐Dimethyl‐3 (2H)‐Furanone Formation by Zygosaccharomyces rouxii: Effect of the Medium.” Journal of Agricultural and Food Chemistry 51: 4753–6. 10.1021/jf026062l [DOI] [PubMed] [Google Scholar]
- 60. Spearman, Charles. 2010. “The Proof and Measurement of Association Between Two Things.” International Journal of Epidemiology 39: 1137–50. 10.1093/ije/dyq191 [DOI] [PubMed] [Google Scholar]
- 61. Elce, A. , Amato F., Zarrilli F., Calignano A., Troncone R., Castaldo G., and Canani R. B.. 2017. “Butyrate Modulating Effects on Pro‐Inflammatory Pathways in Human Intestinal Epithelial Cells.” Beneficial Microbes 8: 841–7. 10.3920/BM2016.0197 [DOI] [PubMed] [Google Scholar]
- 62. Walker, Alyssa C. , Bhargava Rohan, Vaziriyan‐Sani Alfonso S., Pourciau Christine, Donahue Emily T., Dove Autumn S., Gebhardt Michael J., et al. 2021. “Colonization of the Caenorhabditis elegans Gut with Human Enteric Bacterial Pathogens Leads to Proteostasis Disruption That Is Rescued by Butyrate.” PLoS Pathogens 17: e1009510. 10.1371/journal.ppat.1009510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hu, Jiamiao , Lin Shaoling, Zheng Baodong, and Cheung Peter C. K.. 2018. “Short‐Chain Fatty Acids in Control of Energy Metabolism.” Critical Reviews in Food Science and Nutrition 58: 1243–9. 10.1080/10408398.2016.1245650 [DOI] [PubMed] [Google Scholar]
- 64. Stoeva, Magdalena K. , Garcia‐So Jeewon, Justice Nicholas, Myers Julia, Tyagi Surabhi, Nemchek Madeleine, McMurdie Paul J., Kolterman Orville, and Eid John. 2021. “Butyrate‐Producing Human Gut Symbiont, Clostridium butyricum, and Its Role in Health and Disease.” Gut Microbes 13: 1907272. 10.1080/19490976.2021.1907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Koh, Ara , De Vadder Filipe, Kovatcheva‐Datchary Petia, and Bäckhed Fredrik. 2016. “From Dietary Fiber to Host Physiology: Short‐Chain Fatty Acids As Key Bacterial Metabolites.” Cell 165: 1332–45. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 66. Hosseini, Elham , Grootaert Charlotte, Verstraete Willy, and Van de Wiele Tom. 2011. “Propionate As a Health‐Promoting Microbial Metabolite in the Human Gut.” Nutrition Reviews 69: 245–58. 10.1111/j.1753-4887.2011.00388.x [DOI] [PubMed] [Google Scholar]
- 67. Sell, Lacey B. , Ramelow Christina C., Kohl Hannah M., Hoffman Kristina, Bains Jasleen K., Doyle William J., Strawn Kevin D., et al. 2022. “Farnesol Induces Protection Against Murine CNS Inflammatory Demyelination and Modifies Gut Microbiome.” Clinical Immunology 235: 108766. 10.1016/j.clim.2021.108766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lopes, Alyne Pereira , de Oliveira Castelo Branco Renata Rodrigues, de Alcântara Oliveira Felipe Araújo, Santana Campos Marina Alicea, de Carvalho Sousa Bianca, Rossana Costa Agostinho Ítala, Gonçalves Martins Gonzalez Alice, et al. 2021. “Antimicrobial, Modulatory, and Antibiofilm Activity of tt‐Farnesol on Bacterial and Fungal Strains of Importance to Human Health.” Bioorganic & Medicinal Chemistry Letters 47: 128192. 10.1016/j.bmcl.2021.128192 [DOI] [PubMed] [Google Scholar]
- 69. Pontes, Eveline Kelle Ursulino , Melo Hider Machado, Nogueira José Walter Araújo, Firmino Nairley Cardoso Sá, de Carvalho Mário Geraldo, Catunda Júnior Francisco Eduardo Aragão, and Cavalcante Theodora Thays Arruda. 2019. “Antibiofilm Activity of the Essential Oil of Citronella (Cymbopogon nardus) and Its Major Component, Geraniol, on the Bacterial Biofilms of Staphylococcus aureus .” Food Science and Biotechnology 28: 633–9. 10.1007/s10068-018-0502-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang, Shuai‐Bing , Qin Yu‐Liang, Li Sheng‐Fa, Lv Yang‐Yong, Zhai Huan‐Chen, Hu Yuan‐Sen, and Cai Jing‐Ping. 2021. “Antifungal Mechanism of 1‐nonanol Against Aspergillus flavus Growth Revealed by Metabolomic Analyses.” Applied Microbiology and Biotechnology 105: 7871–88. 10.1007/s00253-021-11581-8 [DOI] [PubMed] [Google Scholar]
- 71. Villa‐Ruano, Nemesio , Pacheco‐Hernández Yesenia, Zárate‐Reyes José Alejo, Cruz‐Durán Ramiro, and Lozoya‐Gloria Edmundo. 2019. “Volatile Composition and Biological Activities of the Leaf Essential Oil from Zanthoxylum limoncello Grown in Oaxaca, México.” Chemistry & Biodiversity 16: e1800498. 10.1002/cbdv.201800498 [DOI] [PubMed] [Google Scholar]
- 72. Ahmed Laskar, Amaj , and Younus Hina. 2019. “Aldehyde Toxicity and Metabolism: The Role of Aldehyde Dehydrogenases in Detoxification, Drug Resistance and Carcinogenesis.” Drug Metabolism Reviews 51: 42–64. 10.1080/03602532.2018.1555587 [DOI] [PubMed] [Google Scholar]
- 73. Devadas, Suganthi Martena , Nayak Usha Y., Narayan Reema, Hande Manjunath H., and Ballal Mamatha. 2019. “2,5‐Dimethyl‐4‐Hydroxy‐3 (2h)‐Furanone as an Anti‐Biofilm Agent Against Non‐Candida albicans Candida Species.” Mycopathologia 184: 403–11. 10.1007/s11046-019-00341-y [DOI] [PubMed] [Google Scholar]
- 74. Meena, Himani , Mishra Rashmi, Ranganathan Sampathkumar, Sarma V. Venkateswara, Ampasala Dinakara Rao, Kalia Vipin Chandra, Lee Jung‐Kul, and Siddhardha Busi. 2020. “ Phomopsis tersa as Inhibitor of Quorum Sensing System and Biofilm Forming Ability of Pseudomonas aeruginosa .” Indian Journal of Microbiology 60: 70–7. 10.1007/s12088-019-00840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mirzaee, Hooman , Ariens Emily, Blaskovich Mark A. T., Clark Richard J., and Schenk Peer M.. 2021. “Biostimulation of Bacteria in Liquid Culture for Identification of New Antimicrobial Compounds.” Pharmaceuticals 14: 1232. 10.3390/ph14121232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gotor‐Vila, Amparo , Teixidó Neus, Di Francesco Alessandera, Usall Josep, Ugolini L., Torres Rosario, and Mari Marta. 2017. “Antifungal Effect of Volatile Organic Compounds Produced by Bacillus amyloliquefaciens CPA‐8 Against Fruit Pathogen Decays of Cherry.” Food Microbiology 64: 219–25. 10.1016/j.fm.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 77. Hassan, Nayera W. , Saudi Manal N., Abdel‐Ghany Yasser S., Ismail Azza, Elzahhar Perihan A., Sriram Dharmarajan, Nassra Rasha, Abdel‐Aziz Marwa M., and El‐Hawash Soad A.. 2020. “Novel Pyrazine Based Anti‐Tubercular Agents: Design, Synthesis, Biological Evaluation and In Silico Studies.” Bioorganic Chemistry 96: 103610. 10.1016/j.bioorg.2020.103610 [DOI] [PubMed] [Google Scholar]
- 78. Verbitskiy, Egor V. , Slepukhin Pavel A., Kravchenko Marionella A., Skornyakov Sergey N., Evstigneeva Natal'ya P., Kungurov Nikolay V., Zil'berberg Natal'ya V., et al. 2015. “Synthesis, Antimycobacterial and Antifungal Evaluation of Some New 1‐Ethyl‐5‐(Hetero)aryl‐6‐Styryl‐1,6‐Dihydropyrazine‐2,3‐Dicarbonitriles.” Bioorganic & Medicinal Chemistry Letters 25: 524–8. 10.1016/j.bmcl.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 79. Ohata, Motoko , Zhou Lanxi, Yada Yukihiro, Yokoyama Issei, and Arihara Keizo. 2020. “2,3‐Dimethylpyrazine (3dp) and 2,5‐Dimethyl‐4‐Hydroxy‐3(2h)‐Furanone (DMHF) Generated by the Maillard Reaction in Foods Affect Autonomic Nervous Activity and Central Nervous Activity in Human.” Bioscience, Biotechnology, and Biochemistry 84: 1894–902. 10.1080/09168451.2020.1775066 [DOI] [PubMed] [Google Scholar]
- 80. Yan, Shuai , Yue Yin‐zi, Zong Yang, and Zeng Li. 2019. “Tetramethylpyrazine Improves Postoperative Tissue Adhesion: A Drug Repurposing.” Chinese Journal of Integrative Medicine 25: 554–60. 10.1007/s11655-018-3021-3 [DOI] [PubMed] [Google Scholar]
- 81. Zhong, Haoxuan , Shen Jie, Meng Zhe, Zhao Jing‐yi, and Xiao Zijun. 2020. “Tetramethylpyrazine Production from Edible Materials by the Probiotic Bacillus coagulans .” Preparative Biochemistry & Biotechnology 50: 935–42. 10.1080/10826068.2020.1774777 [DOI] [PubMed] [Google Scholar]
- 82. Ratiu, Ileana Andreea , Ligor Tomasz, Bocos‐Bintintan Victor, Al‐Suod Hossam, Kowalkowski Tomasz, Rafińska Katarzyna, and Buszewski Bogusław. 2017. “The Effect of Growth Medium on Escherichia coli Pathway Mirrored into GC/MS Profiles.” Journal of Breath Research 11: 036012. 10.1088/1752-7163/aa7ba2 [DOI] [PubMed] [Google Scholar]
- 83. Vibhuti, Munjal , Nadakkakath Agisha Valiya, Sheoran Neelam, Kundu Aditi, Venugopal Vibina, Subaharan Kesavan, Rajamma Suseelabhai, Eapen Santhosh J., and Kumar Aundy. 2016. “Genotyping and Identification of Broad Spectrum Antimicrobial Volatiles in Black Pepper Root Endophytic Biocontrol Agent, Bacillus megaterium BP17.” Biological Control 92: 66–76. 10.1016/j.biocontrol.2015.09.005 [DOI] [Google Scholar]
- 84. Yamanishi, Yasuhito , and Kawasaki Nobuyuki. 1967. “Syntheses of Trimethyl Pyrazine Derivatives and Their Antibacterial Properties.” Journal of the Pharmaceutical Society of Japan 87: 105–7. 10.1248/yakushi1947.87.1_105 [DOI] [PubMed] [Google Scholar]
- 85. Stiemsma, Leah T. , Arrieta Marie‐Claire, Dimitriu Pedro A., Cheng Jasmine, Thorson Lisa, Lefebvre Diana L., Azad Meghan B., et al. 2016. “Shifts in Lachnospira and Clostridium sp. in the 3‐month Stool Microbiome Are Associated with Preschool Age Asthma.” Clinical Science 130: 2199–207. 10.1042/CS20160349 [DOI] [PubMed] [Google Scholar]
- 86. De Preter, Vicky , Van Staeyen Greet, Esser Diederik, Rutgeerts Paul, and Verbeke Kristin. 2009. “Development of a Screening Method to Determine the Pattern of Fermentation Metabolites in Faecal Samples Using On‐Line Purge‐and‐Trap Gas Chromatographic‐Mass Spectrometric Analysis.” Journal of Chromatography A 1216: 1476–83. 10.1016/j.chroma.2008.12.095 [DOI] [PubMed] [Google Scholar]
- 87. Raman, Maitreyi , Ahmed Iftikhar, Gillevet Patrick M., Probert Chris S., Ratcliffe Norman M., Smith Steve, Greenwood Rosemary, et al. 2013. “Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans with Nonalcoholic Fatty Liver Disease.” Clinical Gastroenterology & Hepatology 11: 868. 10.1016/j.cgh.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 88. Browne, Hilary P. , Forster Samuel C., Anonye Blessing O., Kumar Nitin, Neville B. Anne, Stares Mark D., Goulding David, and Lawley Trevor D.. 2016. “Culturing of ‘Unculturable’ Human Microbiota Reveals Novel Taxa and Extensive Sporulation.” Nature 533: 543–6. 10.1038/nature17645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lagier, Jean‐Christophe , Khelaifia Saber, Alou Maryam Tidjani, Ndongo Sokhna, Dione Niokhor, Hugon Perrine, Caputo Aurelia, et al. 2016. “Culture of Previously Uncultured Members of the Human Gut Microbiota by Culturomics.” Nature Microbiology 1: 16203. 10.1038/nmicrobiol.2016.203 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 90. Soro, Valeria , Dutton Lindsay C., Sprague Susan V., Nobbs Angela H., Ireland Anthony J., Sandy Jonathan R., Jepson Mark A., et al. 2014. “Axenic Culture of a Candidate Division TM7 Bacterium from the Human Oral Cavity and Biofilm Interactions with Other Oral Bacteria.” Applied and Environmental Microbiology 80: 6480–9. 10.1128/AEM.01827-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rettedal, Elizabeth A. , Gumpert Heidi, and Sommer Morten O. A.. 2014. “Cultivation‐Based Multiplex Phenotyping of Human Gut Microbiota Allows Targeted Recovery of Previously Uncultured Bacteria.” Nature Communications 5: 4714. 10.1038/ncomms5714 [DOI] [PubMed] [Google Scholar]
- 92. Lagier, Jean‐Christophe , Hugon Perrine, Khelaifia Saber, Fournier Pierre‐Edouard, La Scola Bernard, and Raoult Didier. 2015. “The Rebirth of Culture in Microbiology Through the Example of Culturomics to Study Human Gut Microbiota.” Clinical Microbiology Reviews 28: 237–64. 10.1128/CMR.00014-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wu, Chongming , Xu Wenyi, Yu Jiaqi, Li Zhuanyu, Zhang Yinghui, Zhang Fang, Yang Yanan, et al. 2021. “Strain‐Level Screening of Human Gut Microbes Identifies Blautia producta as a Novel Anti‐Hyperlipidemic Probiotic Via the Production of 12‐methylmyristic Acid.” Research Square. 10.21203/rs.3.rs-989302/v1 [DOI] [Google Scholar]
- 94. Mazidi, Mohsen , Shekoohi Niloofar, Covic Adrian, Mikhailidis Dimitri P., and Banach Maciej. 2020. “Adverse Impact of Desulfovibrio spp. and Beneficial Role of Anaerostipes spp. on Renal Function: Insights from a Mendelian Randomization Analysis.” Nutrients 12: 2216. 10.3390/nu12082216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tamanai‐Shacoori, Zohreh , Smida Imen, Bousarghin Latifa, Loreal Olivier, Meuric Vincent, Fong Shao Bing, Bonnaure‐Mallet Martine, and Jolivet‐Gougeon Anne. 2017. “Roseburia spp.: A Marker of Health?” Future Microbiology 12: 157–70. 10.2217/fmb-2016-0130 [DOI] [PubMed] [Google Scholar]
- 96. Patterson, Angela M. , Mulder Imke E., Travis Anthony J., Lan Annaig, Cerf‐Bensussan Nadine, Gaboriau‐Routhiau Valerie, Garden Karen, et al. 2017. “Human Gut Symbiont Roseburia hominis Promotes and Regulates Innate Immunity.” Frontiers in Immunology 8: 1166. 10.3389/fimmu.2017.01166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bultman, Scott J. 2018. “Bacterial Butyrate Prevents Atherosclerosis.” Nature Microbiology 3: 1332–3. 10.1038/s41564-018-0299-z [DOI] [PubMed] [Google Scholar]
- 98. Wu, Liuting , Tang Zhiru, Chen Huiyuan, Ren Zhongxiang, Ding Qi, Liang Kaiyang, and Sun Zhihong. 2021. “Mutual Interaction Between Gut Microbiota and Protein/Amino Acid Metabolism for Host Mucosal Immunity and Health.” Animal Nutrition 7: 11–6. 10.1016/j.aninu.2020.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ozato, Naoki , Saito Shinichiro, Yamaguchi Tohru, Katashima Mitsuhiro, Tokuda Itoyo, Sawada Kaori, Katsuragi Yoshihisa, et al. 2019. “Blautia Genus Associated with Visceral Fat Accumulation in Adults 20–76 Years of Age.” NPJ Biofilms and Microbiomes 5: 28. 10.1038/s41522-019-0101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Benítez‐Páez, Alfonso , Gómez del Pugar Eva M., López‐Almela Inmaculada, Moya‐Pérez Ángela, Codoñer‐Franch Pilar, and Sanz Yolanda. 2020. “Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening.” mSystems 5: e00857–19. 10.1128/mSystems.00857-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu, Chengxu , Finegold Sydney M., Song Yuli, and Lawson Paul A.. 2008. “Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and Description of Blautia wexlerae sp. nov., Isolated from Human Faeces.” International Journal of Systematic and Evolutionary Microbiology 58: 1896–902. 10.1099/ijs.0.65208-0 [DOI] [PubMed] [Google Scholar]
- 102. Shin, Na‐Ri , Kang Woorim, Tak Euon Jung, Hyun Dong‐Wook, Kim Pil Soo, Kim Hyun Sik, Lee June‐Young, et al. 2018. “ Blautia hominis sp. nov., Isolated from Human Faeces.” International Journal of Systematic and Evolutionary Microbiology 68: 1059–64. 10.1099/ijsem.0.002623 [DOI] [PubMed] [Google Scholar]
- 103. Hitch, Thomas C. A. , Riedel Thomas, Oren Aharon, Overmann Jörg, Lawley Trevor D., and Clavel Thomas. 2021. “Automated Analysis of Genomic Sequences Facilitates High‐Throughput and Comprehensive Description of Bacteria.” ISME Communications 1: 16. 10.1038/s43705-021-00017-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sakamoto, Mitsuo , Iino Takao, and Ohkuma Moriya. 2017. “ Faecalimonas umbilicata gen. nov., sp. nov., Isolated from Human Faeces, and Reclassification of Eubacterium contortum, Eubacterium fissicatena and Clostridium oroticum as Faecalicatena contorta gen. nov., comb. nov., Faecalicatena fissicatena comb. nov. and Faecalicatena orotica comb. nov.” International Journal of Systematic and Evolutionary Microbiology 67: 1219–27. 10.1099/ijsem.0.001790 [DOI] [PubMed] [Google Scholar]
- 105. Duncan, Sylvia H. , Aminov Rustam I., Scott Karen P., Louis Petra, Stanton Thaddeus B., and Flint Harry J.. 2006. “Proposal of Roseburia faecis sp. nov., Roseburia hominis sp. nov. and Roseburia inulinivorans sp. nov., Based on Isolates from Human Faeces.” International Journal of Systematic and Evolutionary Microbiology 56: 2437–41. 10.1099/ijs.0.64098-0 [DOI] [PubMed] [Google Scholar]
- 106. Haas, Kelly N. , and Blanchard Jeffrey L.. 2020. “Reclassification of the Clostridium clostridioforme and Clostridium sphenoides clades as Enterocloster gen. nov. and Lacrimispora gen. nov., Including Reclassification of 15 Taxa.” International Journal of Systematic and Evolutionary Microbiology 70: 23–34. 10.1099/ijsem.0.003698 [DOI] [PubMed] [Google Scholar]
- 107. Seo, Boram , Yoo Ju Eun, Lee Yung Mi, and Ko GwangPyo. 2016. “ Sellimonas intestinalis gen. nov., sp. nov., Isolated from Human Faeces.” International Journal of Systematic and Evolutionary Microbiology 66: 951–6. 10.1099/ijsem.0.000817 [DOI] [PubMed] [Google Scholar]
- 108. Nafday, Suhas M. , Chen Wei, Peng Luying, Babyatsky Mark W., Holzman Ian R., and Lin Jing. 2005. “Short‐Chain Fatty Acids Induce Colonic Mucosal Injury in Rats with Various Postnatal Ages.” Pediatric Research 57: 201–4. 10.1203/01.PDR.0000150721.83224.89 [DOI] [PubMed] [Google Scholar]
- 109. Lin, Jing , Nafday Suhas M., Chauvin Sara N., Magid Margret S., Pabbatireddy Sudha, Holzman Ian R., and Babyatsky Mark W.. 2002. “Variable Effects of Short Chain Fatty Acids and Lactic Acid in Inducing Intestinal Mucosal Injury in Newborn Rats.” Journal of Pediatric Gastroenterology and Nutrition 35: 545–50. 10.1097/00005176-200210000-00016 [DOI] [PubMed] [Google Scholar]
- 110. Wang, Y. , Dai A., Huang S., Kuo S., Shu M., Tapia C. P., Yu J., et al. 2014. “Propionic Acid and Its Esterified Derivative Suppress the Growth of Methicillin‐Resistant Staphylococcus aureus USA300.” Beneficial Microbes 5: 161–8. 10.3920/BM2013.0031 [DOI] [PubMed] [Google Scholar]
- 111. Van Immerseel, F. 2003. “Invasion of Salmonella enteritidis in Avian Intestinal Epithelial Cells In Vitro Is Influenced by Short‐Chain Fatty Acids.” International Journal of Food Microbiology 85: 237–48. 10.1016/s0168-1605(02)00542-1 [DOI] [PubMed] [Google Scholar]
- 112. Ormsby, Michael J. , Johnson Síle A., Carpena Nuria, Meikle Lynsey M., Goldstone Robert J., McIntosh Anne, Wessel Hannah M., et al. 2020. “Propionic Acid Promotes the Virulent Phenotype of Crohn's Disease‐Associated Adherent‐Invasive Escherichia coli .” Cell Reports 30: 2297–305. 10.1016/j.celrep.2020.01.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Horswill, Alexander R. , and Escalante‐Semerena Jorge C.. 1999. “ Salmonella typhimurium LT2 Catabolizes Propionate Via the 2‐Methylcitric Acid Cycle.” Journal of Bacteriology 181: 5615–23. 10.1128/JB.181.18.5615-5623.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cartron, Michaël L. , England Simon R., Chiriac Alina Iulia, Josten Michaele, Turner Robert, Rauter Yvonne, Hurd Alexander, et al. 2014. “Bactericidal Activity of the Human Skin Fatty Acid cis‐6‐Hexadecanoic Acid on Staphylococcus aureus .” Antimicrobial Agents and Chemotherapy 58: 3599–609. 10.1128/AAC.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jadhav, Ashwini Khanderao , and Karuppayil Sankunny Mohan. 2020. “Farnesol: From Perfumery to Quorum Sensing.” In New and Future Developments in Microbial Biotechnology and Bioengineering, 71–7. Elsevier. 10.1016/B978-0-12-821008-6.00007-4 [DOI] [Google Scholar]
- 116. Khan, Rehan , and Sultana Sarwat. 2011. “Farnesol Attenuates 1,2‐Dimethylhydrazine Induced Oxidative Stress, Inflammation and Apoptotic Responses in the Colon of Wistar Rats.” Chemico‐Biological Interactions 192: 193–200. 10.1016/j.cbi.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 117. Jabra‐Rizk, M. A. , Meiller T. F., James C. E., and Shirtliff M. E.. 2006. “Effect of Farnesol on Staphylococcus aureus Biofilm Formation and Antimicrobial Susceptibility.” Antimicrobial Agents and Chemotherapy 50: 1463–9. 10.1128/AAC.50.4.1463-1469.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang, Chonglong , Kim Jae‐Yean, Choi Eui‐Sung, and Kim Seon‐Won. 2011. “Microbial Production of Farnesol (FOH): Current States and Beyond.” Process Biochemistry 46: 1221–9. 10.1016/j.procbio.2011.02.020 [DOI] [Google Scholar]
- 119. Sharma, Y. , Khan L. A., and Manzoor N.. 2016. “Anti‐Candida Activity of Geraniol Involves Disruption of Cell Membrane Integrity and Function.” Journal de Mycologie Médicale/Journal of Medical Mycology 26: 244–54. 10.1016/j.mycmed.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 120. Lira, Maria Helena Pereira de , Andrade Júnior Francisco Patricio de, Moraes Gustavo Fernandes Queiroga, Macena Girlene da Silva, Pereira Fillipe de Oliveira, and Lima Igara Oliveira. 2020. “Antimicrobial Activity of Geraniol: An Integrative Review.” Journal of Essential Oil Research 32: 187–97. 10.1080/10412905.2020.1745697 [DOI] [Google Scholar]
- 121. Passmore, Ian J. , Letertre Marine P. M., Preston Mark D., Bianconi Irene, Harrison Mark A., Nasher Fauzy, Kaur Harparkash, et al. 2018. “Para‐Cresol Production by Clostridium difficile Affects Microbial Diversity and Membrane Integrity of Gram‐Negative Bacteria.” PLoS Pathogens 14: e1007191. 10.1371/journal.ppat.1007191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Andriamihaja, Mireille , Lan Annaïg, Beaumont Martin, Audebert Marc, Wong Ximena, Yamada Kana, Yin Yulong, et al. 2015. “The Deleterious Metabolic and Genotoxic Effects of the Bacterial Metabolite p‐Cresol on Colonic Epithelial Cells.” Free Radical Biology and Medicine 85: 219–27. 10.1016/j.freeradbiomed.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 123. Guerrero, Fatima , Carmona Andres, Obrero Teresa, Jiménez Maria Jose, Soriano Sagrario, Moreno Juan Antonio, Martín‐Malo Alejandro, and Aljama Pedro. 2020. “Role of Endothelial Microvesicles Released by p‐Cresol on Endothelial Dysfunction.” Scientific Reports 10: 10657. 10.1038/s41598-020-67574-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Smith, E. A. , and Macfarlane G. T.. 1997. “Formation of Phenolic and Indolic Compounds by Anaerobic Bacteria in the Human Large Intestine.” Microbial Ecology 33: 180–8. 10.1007/s002489900020 [DOI] [PubMed] [Google Scholar]
- 125. Roager, Henrik M. , and Licht Tine R.. 2018. “Microbial Tryptophan Catabolites in Health and Disease.” Nature Communications 9: 3294. 10.1038/s41467-018-05470-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kurata, Koichi , Kawahara Hideaki, Nishimura Kohji, Jisaka Mitsuo, Yokota Kazushige, and Shimizu Hidehisa. 2019. “Skatole Regulates Intestinal Epithelial Cellular Functions Through Activating Aryl Hydrocarbon Receptors and p38.” Biochemical and Biophysical Research Communications 510: 649–55. 10.1016/j.bbrc.2019.01.122 [DOI] [PubMed] [Google Scholar]
- 127. Krautkramer, Kimberly A. , Fan Jing, and Bäckhed Fredrik. 2021. “Gut Microbial Metabolites as Multi‐Kingdom Intermediates.” Nature Reviews Microbiology 19: 77–94. 10.1038/s41579-020-0438-4 [DOI] [PubMed] [Google Scholar]
- 128. Parker, Aimée , Fonseca Sonia, and Carding Simon R.. 2020. “Gut Microbes and Metabolites as Modulators of Blood–Brain Barrier Integrity and Brain Health.” Gut Microbes 11: 135–57. 10.1080/19490976.2019.1638722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wang, Yu , Rodolfo Juliani Héctor, Simon James E., and Ho Chi‐Tang. 2009. “Amino Acid‐Dependent Formation Pathways of 2‐Acetylfuran and 2,5‐Dimethyl‐4‐hydroxy‐3 [2H]‐furanone in the Maillard Reaction.” Food Chemistry 115: 233–7. 10.1016/j.foodchem.2008.12.014 [DOI] [Google Scholar]
- 130. Li, Danhua , Liu Chang, Abuduaini Rexiding, Du Mengxuan, Wang Yujing, Zhu Haizhen, Chen Honghe, et al. 2022. “The Monkey Microbial Biobank Brings Previously Uncultivated Bioresources for Nonhuman Primate and Human Gut Microbiomes.” mLife 1: 210–7. 10.1002/mlf2.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Garrity, George M. , Parker Charles T., and Tindall Brian J.. 2019. “International Code of Nomenclature of Prokaryotes.” International Journal of Systematic and Evolutionary Microbiology 69: S1–S111. 10.1099/ijsem.0.000778 [DOI] [PubMed] [Google Scholar]
- 132. Sumner, Lloyd W. , Amberg Alexander, Barrett Dave, Beale Michael H., Beger Richard, Daykin Clare A., Fan Teresa W. M., et al. 2007. “Proposed Minimum Reporting Standards for Chemical Analysis.” Metabolomics 3: 211–21. 10.1007/s11306-007-0082-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data were available at the National Microbiology Data Center (NMDC, https://nmdc.cn/) under accessions NMDC10018179. Moreover, the information of all 148 strains and their 16S rRNA gene sequences were accessible via the hLchsp website (https://hgmb.nmdc.cn/subject/lachnospiraceae), and the in vitro metabolite profiles of 110 Lachnospiraceae strains were available at https://hgmb.nmdc.cn/subject/lachnospiraceae/metabolites. Supporting Information materials (figures, tables, scripts, graphical abstract, slides, videos, Chinese translated version, and update materials) may be found in the online DOI or iMeta Science http://www.imeta.science/.


