Abstract
The purpose of this study was to define the relationship between herpes simplex virus (HSV) latency and in vivo ganglionic reactivation. Groups of mice with numbers of latently infected neurons ranging from 1.9 to 24% were generated by varying the input titer of wild-type HSV type 1 strain 17syn+. Reactivation of the virus in mice from each group was induced by hyperthermic stress. The number of animals that exhibited virus reactivation was positively correlated with the number of latently infected neurons in the ganglia over the entire range examined (r = 0.9852, P < 0.0001 [Pearson correlation]).
Herpes simplex virus (HSV) is a neurotrophic pathogen of humans that establishes latent infections in the sensory ganglia innervating the site of primary disease. The latent virus periodically reactivates, producing infectious virus which can result in recurrent surface lesions (for reviews, see references 23 and 35). The frequency with which infected individuals experience clinically manifested reactivation of HSV is quite variable, ranging from 0 to 12 or more episodes per year (30; see reference 35 for a review). Why this is the case is not understood, but the number of latent sites in the ganglia is one factor that may be important.
The correlation between the amount of HSV latency and the frequency of reactivation both in vitro and in vivo has been examined by a number of investigators. In these studies, latency was quantified on the basis of (i) the total amount of viral DNA in latently infected ganglia (1, 2, 4, 8, 10, 13–16, 34), (ii) the number of latency-associated transcript (LAT) RNA-positive or LAT promoter reporter-positive neurons (5, 7, 26), or (iii) the number of neurons containing the viral genome as determined by PCR-based approaches (16, 33). Steiner et al. reported a direct correlation between input PFU and cocultivation reactivation of HSV in the trigeminal ganglia (TG) of mice inoculated with a VP16-negative mutant (32). In all but one of these reports, mutant virus and/or wild-type strains differing in their ability to reactivate were compared. Leib et al. examined the correlation between input titers of HSV strain KOS, the amount of viral DNA in the latently infected ganglia, and cocultivation reactivation (15). A reduction of cocultivation reactivation was observed only with very low input titers. The level of establishment could not be quantified in these ganglia because of the insensitivity of the slot blot hybridization method employed (15).
These studies have extended our understanding of the link between acute infection, the establishment of latency, and subsequent reactivation. However, a basic issue remains unresolved. Does the number of latently infected neurons influence the in vivo HSV reactivation potential of ganglia infected with a given virus strain? If so, what is the nature of the relationship? Recent estimates made by using PCR-based assays for the viral genome indicate that as many as 10 to 30% of the neurons can be latently infected, a number significantly larger than previously concluded on the basis of in situ detection of LAT RNA-expressing sites (16, 18, 21, 22, 24). Delineating the relationship between this number and reactivation would provide clues to the mechanism of reactivation and aid in establishing clinical treatment goals. In this study, the ability of HSV type 1 (HSV-1) strain 17syn+ to reactivate from ganglia containing different numbers of latently infected neurons was determined. A recently developed method, contextual analysis of DNA (CXA-D), was used to quantify virus latency at the single-cell level. Using this assay, the percentage of ganglionic neurons containing viral DNA can be determined (24).
Correlation among input titer, PIN, and in vivo reactivation.
Groups of male Swiss Webster mice (18 to 20 g) obtained from Harlan Laboratories (Indianapolis, Ind.) were infected on scarified corneas with input titers of wild-type strain 17syn+ (obtained from J. Subak-Sharpe of the Medical Research Council Virology Unit in Glasgow, Scotland) ranging from ∼5 × 102 to ∼5 × 105 PFU. Preliminary experiments demonstrated that this 3-log span of inoculum titers resulted in significant differences in the numbers of latently infected neurons in the ganglia. Higher input titers resulted in unacceptable levels of mortality, and lower input titers increased the probability that the mice would not be infected. At >30 days postinoculation, six ganglia from each group were processed and then analyzed by CXA-D (24). In brief, perfusion-fixed trigeminal ganglia were removed and dissociated into single-cell suspensions, and enriched neuron populations were obtained by using Percoll (Pharmacia) gradients. The percentage of infected neurons (PIN) in the latently infected ganglia was determined by a single-neuron PCR assay as described previously (24). Consistent with our previous report (24), reducing the input titer resulted in ganglia containing fewer latently infected neurons (Table 1). Input titers of ∼105 PFU resulted in >20% of the ganglionic neurons harboring HSV DNA, while only ∼2% of the neurons were positive when the input titer was reduced to 500 PFU.
TABLE 1.
Input titer, PIN, and total number of latently infected neurons per TG pair
| Input titera | PINb | No. of latently infected neurons per TG pairc |
|---|---|---|
| ∼4 × 105 | 24.0 (40/167) | 9,600 |
| ∼2 × 105 | 20.1 (28/139) | 8,000 |
| ∼2 × 103 | 11.5 (45/392) | 4,600 |
| ∼5 × 102 | 1.9 (33/1780) | 760 |
Total PFU.
Determined from a pool of six ganglia.
Calculated on the basis of 40,000 neurons per TG pair.
Groups of mice, each inoculated with a different input titer, were subjected to hyperthermic stress (HS) to induce HSV reactivation as described previously (25). At 22 h posttreatment, the time at which peak amounts of infectious virus are detected in the ganglia (25), the trigeminal ganglia were removed, homogenized, centrifuged to remove cellular debris, and plated on rabbit skin cell (RSC) monolayers. The plates were monitored for 5 days, but all primary plaques became evident within 24 to 36 h postplating.
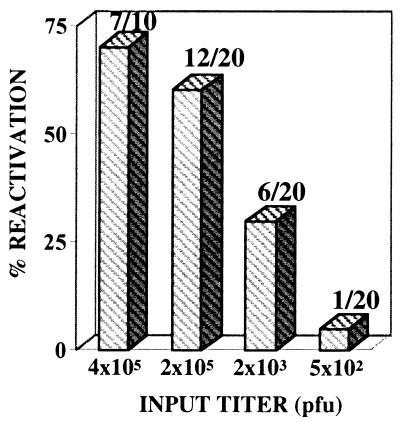
There was a positive correlation between the input titer and the number of mice in the group that exhibited HSV reactivation following HS (Fig. 1). Seventy and 60% of the mice in the two high-input-titer groups demonstrated HSV reactivation. These groups contained the largest number of HSV-positive neurons, 24 and 20%, respectively. With an intermediate input titer, 11.5% of the neurons were latently infected, and 30% of the mice in this group showed viral reactivation. At the lowest input titer, only 1.9% of the neurons were latently infected, and 5% of the mice from this group exhibited HSV reactivation.
FIG. 1.
The percentage of mice undergoing viral reactivation following HS. Groups of latently infected mice inoculated with the input titers indicated were subjected to HS. Shown are the numbers of mice exhibiting viral reactivation over the total number treated. There was no difference between the two groups receiving input titers of >105 PFU (two-sided P = 0.7, Fisher’s exact test). For statistical analysis, these two groups were combined and compared to the groups receiving either 2 or 3 logs less input virus. The differences were significant (P = 0.04 and P < 0.0001, respectively, Fisher’s exact test).
Consistent with previous reactivation experiments, only a few PFU were recovered from a given reactivation event, and this did not vary among the groups (data not shown). The importance of the sensitivity of the reactivation assay for the interpretation of these results warranted examination of this issue. The sensitivity of detection of infectious virus in this assay had been previously approximated by a simple spiking experiment (25). Repeating this experiment under the laboratory conditions used in the present study demonstrated that when 20 PFU was added to uninfected ganglia and the ganglia were then homogenized and plated on RSC monolayers, an average of 6 PFU was detected. This experiment, however, did not evaluate the efficiency of virus recovery when the ganglia were latently infected or in the process of HSV reactivation. To examine this, a human cytomegalovirus immediate-early promoter–lacZ reporter mutant, KZ, kindly provided by J. Mester, was utilized to spike latently infected ganglia removed pre-HS or at 22 h post-HS. The construction and characterization of this mutant have been previously described (20). It was now possible to distinguish input virus from latent virus reactivating in the ganglia on the basis of lacZ expression, detected as blue plaques as described elsewhere (20). A comparison of the number of blue plaques recovered on RSC monolayers when virus was (i) plated in the absence of ganglion homogenate, (ii) homogenized with uninfected ganglia (pre-HS or 22 h post-HS), and (iii) homogenized with latently infected ganglia (pre-HS or 22 h post-HS) was performed. The assay for each group was run in triplicate. The results of this experiment were consistent with those of the previous one in that on average, 16 blue plaques were evident when 50 PFU was added to uninfected ganglia. HS did not alter the recovery. When 50 PFU was added to latently infected ganglia and those ganglia were then homogenized, 10 plaques were observed on average. The reason for this approximately twofold reduction in sensitivity was not examined, but it may represent the presence of immune factors in the latently infected ganglia (29). Interestingly, there was no difference in the efficiencies of exogenous virus recovery from latently infected ganglia pre- and post-HS. This experiment indicates that the assay for the detection of infectious virus in a reactivating ganglia is quite sensitive, but depending on the amount of virus in the ganglia at 22 h post-HS, some reactivation events would go undetected. In addition, it provides further confirmation that the striking difference in the levels of virus recoverable from the ganglia during acute infection (104 PFU on day 4 postinoculation with 17syn+) and during reactivation (1 to 20 PFU) reflects the levels being produced and not merely immune factor interference with detection.
ACV treatment reduces the number of latent infections and subsequent in vivo reactivation.
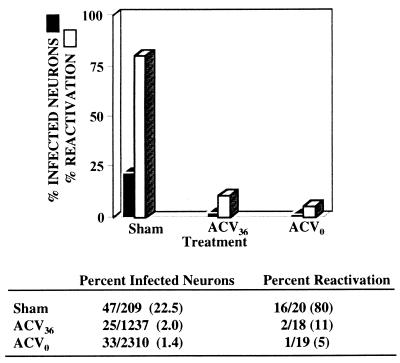
We had previously shown that acyclovir (ACV) administered during acute infection resulted in a reduction of the number of latently infected neurons in the ganglia of treated mice (24). Therefore, an additional experiment was performed to test whether controlling the number of latently infected neurons in this manner has a similar impact on reactivation. This would test the hypothesis that the viral reactivation frequency is determined by the input titer and not by events, such as replication, occurring subsequent to inoculation. Male Swiss Webster mice (18 to 20 g) were inoculated on scarified corneas with 2.6 × 105 PFU of wild-type HSV-1 strain 17syn+. A 50-mg/kg dose of ACV (Glaxo Wellcome) was administered to each mouse intraperitoneally three times per day beginning either at the time of inoculation or following a delay of 36 h and continuing through day 7 postinoculation. Control mice received saline alone. As predicted, acute virus replication was markedly reduced in the eyes and TG of ACV-treated mice, confirming the efficacy of treatment (data not shown). At >30 days postinoculation, the percentage of neurons latently infected in each group was determined by CXA-D (24). For each group, neurons harvested from six ganglia were pooled and analyzed. ACV treatment dramatically reduced the number of latently infected neurons in the ganglia, a finding consistent with our previous report (Fig. 2) (24). Even when ACV treatment was delayed for 36 h, the number of latently infected neurons was reduced >10-fold compared to the number in the ganglia of sham-treated control mice. As described above, HSV reactivation in mice from each of these groups was induced by HS (25). The results showed a clear correlation between the number of latently infected neurons in the ganglia and the number of mice in which HSV reactivation occurred following HS (Fig. 2). In addition, the possibility that the reactivation frequency was determined directly by the input titer was eliminated.
FIG. 2.
The percentage of neurons latently infected and the percentage of mice that exhibited HSV reactivation following HS in ACV- and sham-treated groups. The percentage of latently infected neurons in ganglia of sham-treated mice and mice which had been treated with ACV from the time of inoculation (ACV0) or from 36 h postinoculation (ACV36) through day 7 postinoculation was determined by CXA-D. Viral reactivation was induced in mice from each of these groups. The differences between the number of latently infected neurons and the number of mice exhibiting reactivation in the sham- and ACV-treated groups were both significant (two-tailed P < 0.0001 for both ACV treatment groups, Fisher’s exact test).
Variability in PIN among animals.
In the preceding experiments, the number of latently infected neurons in each of the groups of mice was determined by analyzing a pool of six ganglia from mice representing that group. Thus, there was a possibility that the reactivation data at the lower input titers reflected the reactivation of virus in one animal in the group which had a very high percentage of latently infected neurons. It was therefore important to gain insight into the degree of variability among the individual mice in each input-titer group. To do this, groups of mice were inoculated, via corneal scarification, with either 500, 5,000, or 500,000 PFU of HSV. At >30 days postinoculation, mice were perfusion fixed and ganglia from individual mice were analyzed by CXA-D to determine the number of latently infected neurons. Eight individual mice in the 500-PFU group, six in the 5,000-PFU group, and five in the 500,000-PFU group were analyzed. All of the inoculated mice examined were found to contain latently infected neurons. The PIN was most variable in the 500-PFU input-titer group, ranging from 0.16 to 4.6% (mean, 1.5%), while those of the 5,000- and 500,000-PFU groups ranged from 5 to 18% (mean, 10%) and 24 to 29% (mean, 27%), respectively. The average PIN in the input-titer groups in this experiment were very similar to those obtained in the first experiment, 1.9, 11.5, and 24%, respectively. As presented below, seven mice in the 500-PFU group were also analyzed by whole-ganglion quantitative PCR (QPCR), and none of the TG contained high levels of HSV DNA. Thus, in a total of 15 individual mice and a pool of 3 mice of the lowest-input-titer group, no outliers were detected. It therefore seems unlikely that HSV reactivation in the 500-PFU group was due to the presence of a single animal with a very large number of latently infected neurons.
Total-ganglion QPCR.
To compare the efficiency of detection of low levels of latency by total-ganglion QPCR with that of single-neuron PCR, DNA was prepared from TG of seven mice belonging to the 500-PFU inoculation group (described above) and was analyzed by the method of Katz et al. (11). HSV DNA was detected in only three of the seven ganglion pairs; one contained an estimated 26,500 viral genomes, and the other two had levels too low to accurately quantify. The standards in this assay demonstrated that in the background of 100 ng of mouse DNA, 50 HSV genomes were detectable but 5 HSV genomes were not. This meant that ganglion pairs containing significantly fewer than 50 HSV genomes per 100 ng of mouse ganglion DNA could appear to be negative. Thus, in line with the sensitivity of the QPCR assay, HSV was detected in those ganglia containing the largest number of latently infected neurons but not in those containing the fewest. Two previous studies have demonstrated that estimates of total HSV DNA obtained by whole-ganglion QPCR are consistent with those obtained by CXA-D single-neuron PCR (24, 28).
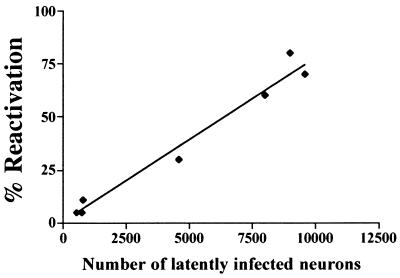
The total number of latently infected neurons per TG pair was calculated for each group by multiplying the percentage of neurons latently infected by 40,000 (total neurons per TG pair) (3, 24) (Table 1). This number was then used to determine the covariation or correlation between a detectable reactivation event and the numbers of latently infected neurons present in the groups of mice infected with different HSV titers. In the group of mice receiving the smallest amount of virus, a detectable reactivation event (i.e., a positive animal) was detected once in every 15,200 latently infected neurons. In those animals that received the most virus, this number was once in every 13,700 latently infected neurons. It should be emphasized that this represents the minimum number of reactivation events which occurred in vivo. An additional assumption is that all latently infected neurons have an equal probability of HSV reactivation. The relationship between the number of latently infected neurons in the ganglia and in vivo reactivation frequency is shown graphically in Fig. 3. For this analysis, data from both the input-titer and the ACV experiments were included. Although there is a strong correlation between the frequency of HSV reactivation and the number of latently infected neurons (r = 0.9852, P < 0.0001), this does not indicate that there is a causal link between these two parameters. Unfortunately, there is currently no method for quantification of the number of latently infected neurons and assessment of viral reactivation in the same ganglia. These data do suggest, however, that large numbers of latently infected neurons in the ganglia do not necessarily lead to detectable HSV reactivation in all animals and, further, that some animals with relatively few latently infected neurons undergo viral reactivation.
FIG. 3.
Plot of the number of latently infected neurons per TG pair versus the percentage of mice exhibiting reactivation. Data from both the input-titer experiment and the ACV experiment were included. The number of latently infected neurons per TG pair was determined from the PIN for each input titer group. There is a significant correlation between these two variables (r = 0.9852, P < 0.0001 [Pearson correlation test]).
Human HSV infection has not been explored at this level of detail. However, it is reasonable to assume that, as in the mouse, the number of latently infected neurons resulting from primary infection in the human could be a significant determinant of the frequency of HSV reactivation. In humans, the frequency of reactivation events resulting in recurrent disease is related to the severity of primary infection (for a review, see reference 35). This is consistent with the impact of the input titer on the number of latently infected neurons reported here as well as the reduction in the number of latent sites in ganglia of mice treated with ACV during acute infection. Thus, while the complete prevention of latent infections may not be possible, a practical and achievable clinical goal would be minimization of the number of neurons in which establishment of latency occurs. Empirical evidence that transmission of an alphaherpesvirus can be reduced in a population through the control of viral replication and shedding is provided by the success of immunization strategies against pseudorabies virus in pig herds (19, 31).
There are several lines of evidence which suggest that the HSV in the vast majority of latently infected neurons does not reactivate during any given reactivation event. The most compelling of these is the fact that HSV can reactivate many times over the lifetime of the human host. If one accepts the theory that viral DNA replication is not compatible with cell survival (23) and the evidence that the neuron in which the virus reactivates does not survive (25, 29), then the conclusion that the virus reactivates in very few neurons per episode is reasonable. Animal model studies support this in that (i) the latent-DNA reservoir appears to be stable over long periods of time (9) and (ii) examination of ganglia in which HSV is reactivating has revealed that very few neurons express lytic viral proteins or ICP0 RNA (5, 6, 17, 25, 29). We have found that neurons expressing viral lytic proteins post-HS are rare even in ganglia in which >20% of the neurons contain the latent viral genome (27). If many neurons produced very low (undetectable) levels of viral proteins and a few infectious virions post-HS, one would predict an increased recovery of virus from mice in which many (>20%) of the neurons were latently infected. However, the amount of virus recovered per reactivation did not increase with increasing numbers of latently infected neurons in the ganglia.
PCR-amplifiable segments of RNA related to lytic genes have been detected in latently infected ganglia in which infectious virus cannot be detected, suggesting that there are more neurons in which the latent viral transcriptional program is exited than there are neurons that proceed to detectable infectious virus production (12). Nonetheless, the findings presented here indicate that neuronal and/or viral mechanisms serve to maintain the viral latent state in the vast majority of infected neurons. The viral genome copy number in individual latently infected neurons has been hypothesized to play a critical role in reactivation (23). We have found that while the majority of neurons latently infected with HSV-1 strain 17syn+ contain between 10 and 100 viral genomes (24), extremely rare neurons contain copy numbers in the range of 5,000 to 10,000 (27). It may be that only these very-high-copy-number neurons are reactivation competent in response to HS induction in vivo. The relationship between these rare neurons and reactivation is currently under investigation.
Acknowledgments
I thank R. L. Thompson for helpful discussion and C. S. Tansky for expert technical assistance.
This work was supported by Public Health Service grants AI32121 (from the National Institutes of Allergy and Infectious Diseases) and NS25879 (from the National Institutes of Neurological Communicative Disorders and Stroke) and CHMCC Trustee grant 31-358-639.
REFERENCES
- 1.Bloom D C, Hill J M, Devi-Rao G, Wagner E K, Feldman L T, Stevens J G. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J Virol. 1996;70:2449–2459. doi: 10.1128/jvi.70.4.2449-2459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai W, Astor T L, Liptak L M, Cho C, Coen D M, Schaffer P A. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies A, Lumsden A. Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol. 1984;223:124–137. doi: 10.1002/cne.902230110. [DOI] [PubMed] [Google Scholar]
- 4.Devi-Rao G B, Bloom D C, Stevens J G, Wagner E K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecob-Prince M S, Rixon F J, Preston C M, Hassan K, Kennedy P G E. Reactivation in vivo and in vitro of herpes simplex virus from mouse dorsal root ganglia which contain different levels of latency-associated transcripts. J Gen Virol. 1993;74:995–1002. doi: 10.1099/0022-1317-74-6-995. [DOI] [PubMed] [Google Scholar]
- 6.Fawl R L, Roizman B. Induction of reactivation of herpes simplex virus in murine sensory ganglia in vivo by cadmium. J Virol. 1993;67:7025–7031. doi: 10.1128/jvi.67.12.7025-7031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon Y J, Romanowski E G, Araullo-Cruz T, Kinchington P R. The proportion of trigeminal ganglionic neurons expressing herpes simplex virus type 1 latency-associated transcripts correlates to reactivation in the New Zealand rabbit ocular model. Graefe’s Arch Clin Exp Ophthalmol. 1995;233:649–654. doi: 10.1007/BF00185286. [DOI] [PubMed] [Google Scholar]
- 8.Hill J M, Garza H H, Jr, Su Y-H, Meegalla R, Hanna L A, Loutsch J M, Thompson H W, Varnell E D, Bloom D C, Block T M. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J Virol. 1997;71:6555–6559. doi: 10.1128/jvi.71.9.6555-6559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill J M, Gebhardt B M, Wen R, Bouterie A M, Thompson H W, O’Callaghan R J, Halford W P, Kaufman H E. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J Virol. 1996;70:3137–3141. doi: 10.1128/jvi.70.5.3137-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill J M, Maggioncalda J B, Garza H H, Jr, Su Y-H, Fraser N W, Block T M. In vivo epinephrine reactivation of ocular herpes simplex virus type 1 in the rabbit is correlated to a 370-base-pair region located between the promoter and the 5′ end of the 2.0-kilobase latency-associated transcript. J Virol. 1996;70:7270–7274. doi: 10.1128/jvi.70.10.7270-7274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz J P, Bodin E T, Coen D M. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J Virol. 1990;64:4288–4295. doi: 10.1128/jvi.64.9.4288-4295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer M F, Coen D M. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause P R, Stanberry L R, Bourne N, Connelly B, Kurawadwala J, Patel A, Straus S E. Expression of the herpes simplex virus type 2 latency associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J Exp Med. 1995;181:297–306. doi: 10.1084/jem.181.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leib D A, Bogard C L, Kosz-Vnenchak M, Hicks K A, Coen D M, Knipe D M, Schaffer P A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggioncalda J, Mehta A, Su Y-H, Fraser N W, Block T M. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology. 1996;225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 17.McLennan J L, Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980;51:233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- 18.Mehta A, Maggioncalda J, Bagasra O, Thikkavarapu S, Saikumari P, Valyi-Nagy T, Fraser N W, Block T M. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology. 1995;206:633–640. doi: 10.1016/s0042-6822(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 19.Mengeling W L, Brockmeier S L, Lager K M, Vorwald A C. The role of biotechnologically engineered vaccines and diagnostics in pseudorabies (Aujeszky’s disease) eradication strategies. Vet Microbiol. 1997;55:49–60. doi: 10.1016/s0378-1135(96)01306-5. [DOI] [PubMed] [Google Scholar]
- 20.Mester J C, Pitha P M, Glorioso J C. Antiviral activity of herpes simplex virus vectors expressing murine α1-interferon. Gene Ther. 1995;2:187–196. [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Levine M, Fink D J. PCR-based analysis of herpes simplex virus type 1 latency in the rat trigeminal ganglion established with a ribonucleotide reductase-deficient mutant. J Virol. 1994;68:7083–7091. doi: 10.1128/jvi.68.11.7083-7091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramakrishnan R, Fink D J, Jiang G, Desai P, Glorioso J C, Levine M. Competitive quantitative PCR analysis of herpes simplex virus type 1 DNA and latency-associated transcript RNA in latently infected cells of the rat brain. J Virol. 1994;68:1864–1873. doi: 10.1128/jvi.68.3.1864-1873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippencott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 24.Sawtell N M. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawtell N M, Thompson R L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150–2156. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawtell, N. M. Unpublished data.
- 28.Sawtell N M, Poon D K, Tansky C S, Thompson R L. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol. 1998;72:5343–5350. doi: 10.1128/jvi.72.7.5343-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimeld C, Whiteland J L, Williams N A, Easty D L, Hill T J. Reactivation of herpes simplex virus type 1 in the mouse trigeminal ganglion: an in vivo study of virus antigen and immune cell infiltration. J Gen Virol. 1996;77:2583–2590. doi: 10.1099/0022-1317-77-10-2583. [DOI] [PubMed] [Google Scholar]
- 30.Spruance S L, Overall J C, Jr, Kern E R, Krueger G G, Pliam V, Miller W. The natural history of recurrent herpes simplex labialis. N Engl J Med. 1977;297:69–75. doi: 10.1056/NEJM197707142970201. [DOI] [PubMed] [Google Scholar]
- 31.Stegeman A, de Jong M C, van Nes A, Bauma A. Dynamics of pseudorabies virus infections in vaccinated pig populations: a review. Vet Q. 1997;19:117–122. doi: 10.1080/01652176.1997.9694754. [DOI] [PubMed] [Google Scholar]
- 32.Steiner I, Spivack J G, Deshmane S L, Ace C I, Preston C M, Fraser N W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol. 1990;64:1630–1638. doi: 10.1128/jvi.64.4.1630-1638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang K, Pesnicak L, Straus S E. Mutations in the 5′ end of the herpes simplex virus type 2 latency-associated transcript (LAT) promoter affect LAT expression in vivo but not the rate of spontaneous reactivation of genital herpes. J Virol. 1997;71:7903–7910. doi: 10.1128/jvi.71.10.7903-7910.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, editors. Virology. Philadelphia, Pa: Lippencott-Raven Publishers; 1996. pp. 2297–2342. [Google Scholar]