Abstract
BACKGROUND
Delays in treating anaesthesia-induced malignant hyperthermia increase risks of complications and death. NPJ5008 is a novel formulation of the indicated treatment, dantrolene sodium, developed to shorten preparation and administration times compared with the reference formulation Dantrium®. The two formulations have been compared preclinically.
OBJECTIVES
Assess bioequivalence of overall dantrolene (free acid) exposure of NPJ5008 versus Dantrium® and ascertain similarities in their pharmacokinetics and safety/tolerability profiles. Evaluate preparation/administration time savings for the new formulation.
DESIGN
Part 1 of this open-label trial in humans was a 1 : 1 randomised crossover study; part 2 was a single-arm study. Trial pharmacy data and laboratory simulations assessed preparation/administration step timings.
SETTING
Single clinical centre in the UK, April to July 2021.
PARTICIPANTS
Twenty-one healthy male and female individuals.
INTERVENTIONS
Part 1: single intravenous 60 mg dose of NPJ5008 or Dantrium®, sequentially. Part 2: single intravenous 120 mg dose of NPJ5008. Simulation: five vials per formulation using paediatric and adult cannulas.
MAIN OUTCOME MEASURES
Overall drug exposure to last measurable concentration (AUC0 to last) and extrapolated to infinity (AUC0 to ∞) were primary endpoints. Other pharmacokinetic, clinical and muscle-function parameters, and adverse events, were monitored.
RESULTS
Adjusted geometric mean ratios of NPJ5008 versus Dantrium® were 90.24 and 90.44% for AUC0 to last and AUC0 to ∞, respectively, with the 90% confidence intervals (CI) within the 80 to 125% acceptance interval, establishing bioequivalence. No new safety issues emerged: any adverse events were of a similar magnitude across treatments and related to pharmacological properties of dantrolene. Pharmacy and simulation data revealed that every step in preparation and administration was 26 to 69% faster for NPJ5008 than Dantrium®.
CONCLUSION
NPJ5008 showed comparable pharmacokinetic and safety profiles to Dantrium®, while reducing dantrolene dose preparation/administration times, potentially reducing patient complications/healthcare resourcing in malignant hyperthermia.
TRIAL REGISTRATION
EudraCT Number: 2020-005719-35, MHRA approval.
KEY POINTS
A novel rapidly prepared intravenous formulation of dantrolene sodium, NPJ5008, was compared with Dantrium® in a phase 1 healthy volunteer study.
The pharmacokinetic profile of NPJ5008 was comparable to Dantrium®, with bioequivalence in terms of overall drug exposure to dantrolene (free acid) demonstrated.
The safety profile of NPJ5008 was comparable to Dantrium® in this study, with adverse events of similar frequency and magnitude.
NPJ5008 requires a smaller total fluid volume and is faster to prepare and administer, potentially reducing patient complications and healthcare resource utilisation in malignant hyperthermia.
Introduction
Malignant hyperthermia, a life-threatening pharmacogenetic condition triggered by volatile anaesthetics and/or succinylcholine, occurs in 1 : 10 000 to 1 : 250 000 procedures, with half of all cases reported in children and young people.1,2 Onset of malignant hyperthermia can be sudden and progression rapid, with potentially fatal outcomes.1 Dantrolene sodium is an effective emergency treatment for malignant hyperthermia; however, the risk of complications, their severity and the length of stay in intensive care increase with every 15 to 30 min delay between the first signs of malignant hyperthermia and dantrolene administration.3–5 European Malignant Hyperthermia Group (EMHG) guidelines recommend rapidly administering 2 to 2.5 mg kg−1 intravenous (i.v.) dantrolene upon recognising the condition, with repeat dosing every ∼10 min until the reaction is controlled, noting that doses of greater than 10 mg kg−1 may be required.6 These guidelines advise precautionary dantrolene stocking based on the DANTRIUM® IV (also marketed as DANTROLEN IV; Norgine, Harefield, UK) formulation available in Europe.6 Dantrium® is supplied in vials containing 20 mg dantrolene for reconstitution in 60 ml water by vigorous shaking; because of the poor solubility of dantrolene, preparation of a single vial can take more than 4 min.7,8 For a 60 kg patient, effective doses may require 8 to greater than 30 vials, requiring the effort of at least three clinical staff and potentially contributing to critical delays.6–8 To shorten the preparation and administration time and facilitate rapid interventions in emergency situations, a novel intravenous formulation of dantrolene, NPJ5008, has been developed using different excipients [2-hydroxypropyl-beta-cyclodextrin (HP-β-CD) and polyethylene glycol (PEG)]. With NPJ5008, the same dose of dantrolene can be reconstituted more rapidly and in smaller volumes than with Dantrium® (unpublished observations). Standard preclinical assessment of NPJ5008 in rats revealed no significant differences to Dantrium® (unpublished observations).
We report a phase 1 clinical safety trial in healthy adult volunteers, evaluating the pharmacokinetics, including bioequivalence, of NPJ5008 versus Dantrium®, and assessing the safety and tolerability profile of NPJ5008. To evaluate the time-saving benefits of the novel formulation, trial pharmacy product preparation data were analysed; however, as the measured conduct of the volunteer trial necessarily differed from the emergency administration of dantrolene in malignant hyperthermia, only timings for discrete processes were considered representative. A laboratory simulation focusing on speed, thus more reflective of a malignant hyperthermia emergency situation, directly compared preparation and administration times for the two formulations.
The Consolidated Standards of Reporting Trials (CONSORT) guidelines were followed in this study: Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials.
Methods
Ethics
This single-centre study received approval with the protocol number NPJ5008-01/2020 from The Office for Research Ethics Committees Northern Ireland, Lisburn BT28 2RF (Chairman Dr Alastair Walker) on 2 February 2021. A substantial protocol amendment was approved (before the commencement of the study) by ORECNI Chairman Barry Mimnagh. Authorisation from the UK's Medicines and Healthcare products Regulatory Agency was received on 12 March 2021. The study followed relevant regulatory requirements and the ethical standards of Directive 2001/20/EC (www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice-scientific-guideline; www.legislation.gov.uk/uksi/2004/1031/contents/made; www.legislation.gov.uk/uksi/2006/2984/contents/made; www.legislation.gov.uk/uksi/2006/1928/contents/made; www.legislation.gov.uk/uksi/2008/941/pdfs/uksi_20080941_en.pdf; www.legislation.gov.uk/uksi/2019/744/made). The trial took place at Quotient Sciences (Ruddington, UK). All participants provided written informed consent in accordance with the Declaration of Helsinki (www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects).
Study population
Healthy adult volunteers aged 18 to 55, weighing at least 55 kg, with a body mass index (BMI) between 19.0 and 32.0 kg (m2)−1 were eligible provided they passed preliminary screening. Only women of nonchildbearing potential were enrolled (as data on dantrolene use in pregnant women are limited) and male individuals were required to adhere to contraception directives (www.medicines.org.uk/emc/product/1097/smpc#gref).9 Key exclusion criteria included: exposure to any investigational medicinal product within defined time frames; drug abuse; alcohol abuse; smoking; history or symptoms of significant medical conditions, including estimated glomerular filtration rate of less than 30 ml min−1 (1.73 m2)−1 or impairments in respiratory/airway function; abnormal blood parameters; history of adverse reaction to any drug/allergen and taking any prohibited concomitant medication/herbal remedies within specified periods.
Study design, objectives and endpoints
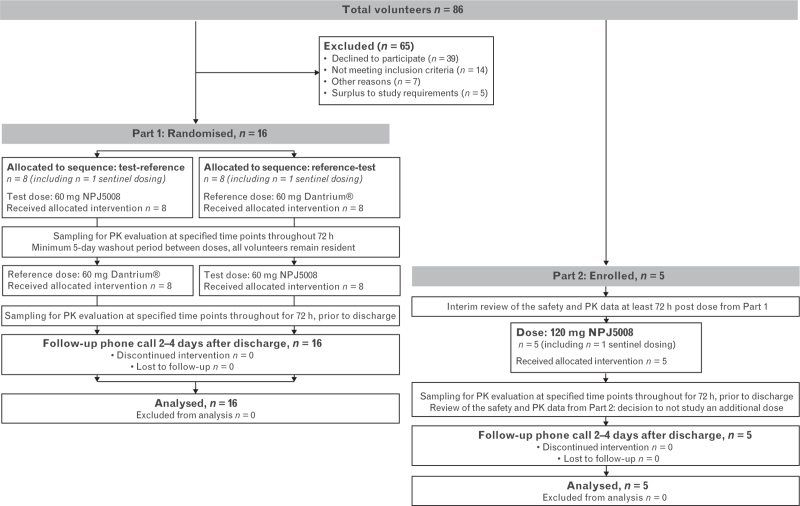
The study comprised two open-label, single-dose parts (see Fig. 1 for participant flow). Doses of 60 and 120 mg NPJ5008 were selected equating to 1 and 2 mg kg−1, respectively, for a 60 kg person (rounded average adult weight worldwide),10 i.e. the initial dose of dantrolene recommended in some countries (e.g. Japan) and the lower end of EMHG dose recommendation, respectively.6,11 These doses were set below the maximum tolerability threshold for healthy volunteers reported previously (www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205579Orig1s000SumR.pdf).
Fig. 1.
Part 1 and Part 2 participant flow diagram.
Part 1 was a two-period crossover study with participants randomised 1 : 1 to the two treatment sequences using computer-generated randomisation. The primary objective was to assess the bioequivalence, in terms of overall exposure, of the NPJ5008 (test) formulation (administered as 11.3 ml of a 5.3 mg ml−1 solution over at least 1 min) versus Dantrium® (the reference product; administered as 186 ml of a 0.32 mg ml−1 solution over at least 5 min), both yielding a dose of 60 mg dantrolene, following standard methods,12 using an Alaris CC infusion pump (Becton Dickinson, Wokingham, UK). The rate of administration of Dantrium® in a clinical trial setting is limited by the speed at which the volume can be delivered by available infusion pumps. In a previous study investigating higher concentration formulations of i.v. dantrolene in healthy volunteers, severe adverse events related to the mode of action (MoA) of dantrolene occurred when 1.75 mg kg−1 was administered over 30 s. No severe adverse events occurred when slower infusion times were used with doses up to 2.5 mg kg−1 (www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205579Orig1s000SumR.pdf). Therefore, infusions in this study were to have a minimum duration of 1 min.
The primary endpoints included area under the concentration–time curve (AUC) from time zero to last measurable concentration AUC0 to last and extrapolated to infinity AUC0 to ∞, but excluded the maximum observed drug concentration in plasma (Cmax), because Cmax values were expected to vary by product infusion rate (60 mg min−1 for NPJ5008 and 12 mg min−1 for Dantrium®).
Secondary objectives of Part 1 assessed the i.v. pharmacokinetics and the safety and tolerability profile of NPJ5008. Secondary plasma pharmacokinetic endpoints included: Cmax; truncated AUCs (time zero to 6 and 72 h post dose); time prior to the first measurable concentration (Tlag); time from drug administration to maximum observed analyte concentration (Tmax); terminal rate constant (λz); terminal half-life of the analyte (T1/2); total body clearance calculated after a single i.v. administration (dantrolene only); and volume of distribution based on the terminal phase calculated using AUC0 to ∞ after a single administration (Vz, dantrolene only), including statistical assessment of relative bioavailability.
Part 2 started at least 72 h post dose of Part 1, following a data review, and assessed the safety and tolerability profile of a 120 mg dose of NPJ5008 administered as 22.6 ml of a 5.3 mg ml−1 solution (Alaris CC infusion pump). The relative bioavailability of 60 versus 120 mg NPJ5008 was an exploratory objective.
Pharmacokinetic parameters were estimated at selected times before dosing and at 20 time points until 72 h post dose by noncompartmental analysis using Phoenix WinNonlin software (v8.0 and v8.3, Certara USA, Inc., Princeton, New Jersey, USA).
Bioanalytical methods
The plasma concentrations of dantrolene (free acid) and 5-hydroxydantrolene (a pharmacologically active metabolite) were determined using a validated analytical method using liquid chromatography with tandem mass spectrometry detection at Labcorp Drug Development (Norgine Pharmaceuticals Ltd data on file). The lower limit of quantification was 10 ng ml−1 for dantrolene and 1 ng ml−1 for 5-hydroxydantrolene.
Statistical methods
The sample sizes were based on regulatory guidance and the possibility of drop-out for Part 1, and on previous experience with similar studies for Part 2. The sample size for Part 1 exceeded that calculated to have at least 80% power to conclude equivalence based on two one-sided t tests with a type I error rate of 0.05 [resulting in 90% confidence intervals (CIs)], assuming a ratio of means of 1.05, intra-individual variability of 11% and standard acceptance limits for equivalence. The assumed variability was based on data for Dantrium® (www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205579Orig1s000ClinPharmR.pdf).
The pharmacokinetic parameters were log-transformed prior to analysis. Statistical analyses utilised analysis of variance with mixed-effects modelling, which included terms for period, sequence and regimen as fixed effects and individual within sequence as a random effect. The ratios of the geometric means (GMR) of test versus reference and their two-sided 90% CIs were calculated for all primary and secondary pharmacokinetic endpoints. Bioequivalence was evaluated using the average bioequivalence method: if the 90% CI for the GMRs for each of the primary endpoints fell within the prespecified standard 80 to 125% acceptance range on formal statistical analysis, then the null hypothesis of nonequivalence was rejected.12 This analysis strategy follows relevant guidelines (www.fda.gov/regulatory-information/search-fda-guidance-documents/statistical-approaches-establishing-bioequivalence; www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf). Assessment of relative bioavailability was performed on secondary pharmacokinetic parameters with no correction for nominal dose received (D) using the same model as for bioequivalence (Part 1 only) and corrected for D between the 60 and 120 mg NPJ5008 dose levels, analysed as above, in a model that included terms for regimen as a fixed effect and body weight as a covariate. Pharmacokinetic parameters were analysed descriptively, with geometric coefficients of intra-individual variability (CVw) measuring dispersion. The model was fitted using SAS Software PROC MIXED (version 9.4, SAS Ltd, Marlow, UK) specifying the restricted maximum likelihood method.
More details are available in the Supplemental Digital Content.
Safety assessment
Safety evaluations comprised analysis of adverse events, laboratory variables (haematology, clinical chemistry and urinalysis) and vital signs monitoring at specified intervals, including continuous peripheral oxygen saturation (pulse oximeter), 12-lead ECGs, hand grip tests (Jamar Hand Dynamometer, Williams Medical Supplies, Rhymney, UK), physical examinations and spirometry, using published methods to calculate and adjust predicted values.13 Adverse events were coded and classified according to seriousness, severity and relationship to the study drug, determined primarily in terms of the temporal relationship with dosing and the known MoA of the drug (www.ema.europa.eu/en/ich-e2a-clinical-safety-data-management-definitions-standards-expedited-reporting-scientific; www.meddra.org/news-and-events/news/english-meddra-version-240-now-available-download).
Pharmacy report analysis
Data were gathered on discrete ‘start’ and ‘end’ time points within the preparation and administration process of NPJ5008 and Dantrium® from trial pharmacy records. Only interval timings with an identical and discrete process for all three regimens were analysed.
Laboratory simulation of preparation and administration
Five vials each of the two dantrolene formulations were reconstituted and delivered as swiftly as possible via suitable syringes, and either a 16-gauge or 22-gauge cannula, to simulate an i.v. push in adults and children, respectively. Infusion pumps were not used. Data were gathered on preparation [of water for injection (WFI), reconstitution, draw up] and administration times. Additional study details are available in the Supplemental Digital Content.
Results
Participants
Enrolment started on 2 April 2021 and the study continued until July 2021 (see Fig. 1 for participant flow).
For Part 1, 16 healthy volunteers (one female) were enrolled, dosed per the assigned treatment sequence and completed the study. All were non-Hispanic white, aged 23 to 55 years and weighed 61.5 to 105.5 kg (median 82.05 kg), with a BMI of 20.4 to 29.5 (median 25.25). The mean age was higher in the treatment sequence test-reference than in the reverse sequence (44.3 vs. 34.6 years), but there were no other notable group differences.
For Part 2, five participants (one female) aged 20 to 55 years who enrolled completed the study. All were non-Hispanic white, weighed 83.2 to 92.4 kg (median 85.8 kg), with a BMI of 25.1 to 29.4 (median 27.90).
Bioequivalence results (primary objective)
On average, overall dantrolene (free acid) exposure following 60 mg NPJ5008 administration was 90.24 and 90.44% of that following administration of 60 mg Dantrium®, as measured by AUC0 to last and AUC0 to ∞, respectively (see Table 1). Formal statistical analysis indicated 90% CIs of the ratios were within the prespecified 80 to 125% bioequivalence limits, demonstrating bioequivalence of NPJ5008 and Dantrium® in terms of overall exposure.
Table 1.
Results of bioequivalence assessment for dantrolene (free acid) following single intravenous infusion of 60 mg Dantrium® or 60 mg NPJ5008 in healthy volunteers (n = 16)
| Parameter | Adjusteda geometric mean NPJ5008 (test) (n = 16) | Adjusteda geometric mean Dantrium® (reference) (n = 16) | Ratio (test : reference) | 90% CI | Largest P value from two one-sided tests | CVw |
| AUC0 to last (ng h ml−1) | 11 900 | 13 200 | 90.24% | 85.94 to 94.76% | <0.001 | 7.85% |
| AUC0 to ∞ (ng h ml−1) | 12 300 | 13 600 | 90.44% | 85.97 to 95.14% | <0.001 | 8.16% |
AUC0 to ∞, area under the curve from time zero extrapolated to infinity; AUC0 to last, area under the curve from time zero to last measurable concentration; CI, confidence interval; CVw, geometric coefficient of intra-individual variability.
The model was adjusted for fixed effects of period, regimen and sequence, and individual within sequence as a random effect. Of note there was a significant period effect, P = 0.021 for AUC0 to last and P = 0.044 for AUC0 to ∞, but no significant sequence effect was seen, therefore, the balanced crossover design meant period had no material impact on the results.
Pharmacokinetic results for NPJ5008 compared with Dantrium® at 60 mg dose level (secondary objective)
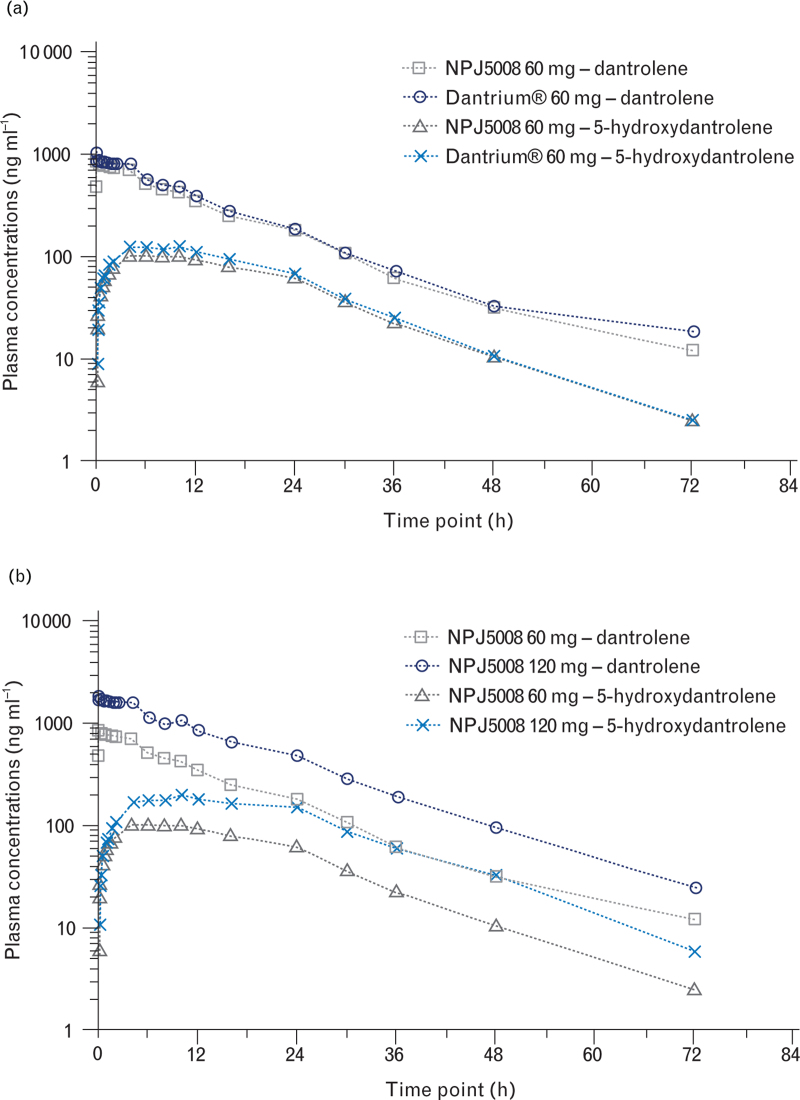
The mean plasma concentration–time profiles for NPJ5008 and Dantrium® were similar (Fig. 2a), and had comparable Tmax ranges, Cmax and resultant T1/2 (Table 2).
Fig. 2.
Mean plasma concentration–time profiles on log10/linear scale for dantrolene (free acid) and the 5-hydroxydantrolene metabolite following single intravenous infusion doses of (a) 60 mg NPJ5008 or 60 mg Dantrium® and (b) 60 mg NPJ5008 or 120 mg NPJ5008.
Table 2.
Plasma pharmacokinetic parameters for dantrolene (free acid) and 5-hydroxydantrolene following single intravenous infusion of 60 mg Dantrium®, 60 mg NPJ5008 and 120 mg NPJ5008 in healthy volunteers
| Analyte | Dantrolene | 5-Hydroxydantrolene | ||||
| Treatment dose level | Dantrium® 60 mgan = 16 | NPJ5008 60 mgan = 16 | NPJ5008 120 mgbn = 5 | Dantrium® 60 mgan = 16 | NPJ5008 60 mgan = 16 | NPJ5008 120 mgbn = 5 |
| Tmax (h) | 0.155 [0.14 to 4.16] | 0.100 [0.01 to 4.01] | 0.149 [0.07 to 0.80] | 10.135 [1.64 to 16.15] | 6.017 [4.00 to 10.02] | 10.064 [10.05 to 16.17] |
| Cmax (ng ml−1) | 1170 (34.8) | 1090 (29.7) | 2090 (24.2) | 138 (34.1) | 114 (25.3) | 208 (22.5) |
| Cmax/Dc (ng ml−1 mg−1) | 24.9 (34.8) | 23.1 (29.7) | 22.1 (24.2) | 2.93 (34.1) | 2.40 (25.3) | 2.20 (22.5) |
| AUC0 to 6 (ng h ml−1) | 4790 (18.8) | 4230 (15.4) | 9400 (25.7) | 581 (40.0) | 497 (27.4) | 765 (17.4) |
| AUC0 to 72 (ng h ml−1) | 13 500 (23.9) | 12 200 (20.8) | 30 500 (31.1) | 3380 (21.2) | 2890 (19.9) | 6150 (23.6) |
| AUC0 to last (ng h ml−1) | 13 200 (23.9) | 11 900 (20.6) | 30 400 (31.7) | 3370 (21.1) | 2880 (19.6) | 6150 (23.6) |
| AUC0 to last/Dc (ng h ml−1 mg−1) | 279 (23.9) | 252 (20.6) | 322 (31.7) | 71.4 (21.1) | 60.9 (19.6) | 65.2 (23.6) |
| AUC0 to ∞ (ng h ml−1) | 13 600 (24.2) | 12 300 (21.1) | 30 900 (31.5) | 3420 (20.8) | 2920 (19.9) | 6270 (23.9) |
| AUC0 to ∞/Dc (ng h ml−1 mg−1) | 287 (24.2) | 260 (21.1) | 327 (31.5) | 72.4 (20.8) | 61.9 (19.9) | 66.4 (23.9) |
| T1/2 (h) | 8.476 (21.8) | 9.041 (27.9) | 10.993 (18.5) | 9.488 (24.0) | 10.171 (20.3) | 11.212 (19.4) |
| λz (h−1) | 0.08178 (21.8) | 0.07667 (27.9) | 0.06305 (18.5) | 0.07306 (24.0) | 0.06815 (20.3) | 0.06182 (19.4) |
| CL (ml min−1) | 58.2 (24.2) | 63.5 (21.1) | 50.5 (31.5) | NC | NC | NC |
| Vz (l) | 42.7 (21.1) | 49.7 (22.7) | 48.0 (29.2) | NC | NC | NC |
| Vss (l) | 46.3 (16.6) | 53.3 (14.9) | 49.2 (24.5) | NC | NC | NC |
| MPR Cmax (ratio) | NC | NC | NC | 0.112 (37.4) | 0.099 (32.8) | 0.095 (35.6) |
| MPR AUC0 to last (ratio) | NC | NC | NC | 0.243 (30.3) | 0.230 (23.3) | 0.193 (24.0) |
| MPR AUC0 to ∞ (ratio) | NC | NC | NC | 0.240 (29.9) | 0.227 (22.9) | 0.193 (23.7) |
Data are median [range] and geomteric mean (geometric coefficient of variation%).
λz, first order rate constant associated with the terminal (log-linear) portion of the curve; AUC, area under the curve; AUC0 to ∞, area under the curve from time zero extrapolated to infinity; AUC0 to last, area under the curve from time zero to last measurable concentration; CL, total body clearance calculated after a single i.v. administration; Cmax, maximum observed drug concentration in plasma; D, dose; i.v., intravenous; MPR, metabolite to parent ratio; NC, not calculated; T1/2, terminal half-life of the analyte in plasma; Tmax, time from administration to maximum observed concentration of the analyte in plasma; truncated AUCs, time zero to 6 and 72 h post dose; Vss, predicted volume of distribution at steady state after single i.v. administration; Vz, volume of distribution based on the terminal phase calculated using AUC0 to ∞ after a single i.v. administration.
In Part 1, the actual dose received (based on the products’ Certificate of Analysis, which differed slightly from the nominal dose, and participant weight) ranged from 0.56 to 0.97 mg kg−1 of NPJ5008 and from 0.57 to 0.98 mg kg−1 of Dantrium®.
In Part 2, a range of actual doses of 1.29 to 1.43 mg kg−1 of NPJ5008 were administered. Data presented are median [range]; all other data presented are geometric mean (geometric CV%).
Dose (D)-corrected parameters were normalised by the nominal salt-corrected dose, for example, 120 mg × 0.787 (salt correction factor) = 94.44 mg.
The relative bioavailability for NPJ5008 of dantrolene (free acid) Cmax was comparable to Dantrium®, with an adjusted GMR of 92.76% (90% CI 78.27 to 109.93%, P = 0.45). In terms of truncated AUC assessments, the relative bioavailability of NPJ5008 was significantly lower than for Dantrium®, with adjusted GMRs of 88.35% (90% CI 84.95 to 91.89%, P < 0.001) and 90.27% (90% CI 85.89 to 94.88%, P = 0.003) for AUC0 to 6 and AUC0 to 72, respectively.
The mean plasma concentration–time profiles and resultant pharmacokinetic parameters of the major metabolite 5-hydroxydantrolene were similar for NPJ5008 and Dantrium®, with similar elimination times (T1/2) but different peak times (Tmax) (Fig. 2a, Table 2). The geometric metabolite to parent ratios (MPRs) were similar following administration of NPJ5008 and Dantrium®: 0.099 and 0.112 based on Cmax and 0.230 and 0.243 based on AUC0 to ∞, respectively. Overall exposure of 5-hydroxydantrolene as measured across all AUC or Cmax parameters was significantly lower (P values between <0.001 and 0.006) for NPJ5008 compared with Dantrium® (statistical data in Supplemental Digital Content).
In Part 2, the mean dantrolene (free acid) and metabolite plasma concentration–time profiles and resultant pharmacokinetic parameters for 60 and 120 mg NPJ5008 (Fig. 2b and Table 2) indicate elimination of each analyte was similar at the higher dose (T1/2 ∼11 h), and at both doses, dantrolene exposure was higher than metabolite exposure (MPRs of ∼0.10 for Cmax).
Exploratory assessment of the relative bioavailability of 60 and 120 mg NPJ5008 suggested it was comparable in terms of salt-corrected and dose-corrected Cmax, with an adjusted GMR of 99.26% (90% CI 77.51 to 127.11%, P = 0.96), but was significantly lower for 60 mg than 120 mg for dose-corrected AUC parameters, with adjusted GMRs of 75.21% (90% CI 61.40 to 92.13%, P = 0.026) and 76.15% (90% CI 62.06 to 93.45%, P = 0.033) for AUC0 to last and AUC0 to ∞, respectively.
Safety and tolerability of NPJ5008 at low and high doses (primary/secondary objectives)
No deaths or serious adverse events were reported and no participant withdrawal or study stopping criteria were met. The incidence and severity of the adverse events were comparable between 60 mg NPJ5008 and Dantrium®, and in Part 2 were consistent with an increased dose of NPJ5008 (Table 3). All but one participant experienced adverse events (TEAEs), consistent with the MoA of dantrolene. Overall, only one participant (in Part 2) experienced a severe TEAE, which was attributed to 120 mg NPJ5008: the muscle weakness intensity improved to moderate in less than 1.5 h without intervention and resolved within 24 h. The majority of TEAEs were mild, and most were classified as adverse drug reactions (ADR). One participant receiving Dantrium®, and two participants receiving 60 mg NPJ5008, experienced moderate muscle weakness, and of these, one also experienced a moderate headache considered unrelated to NPJ5008. Four of the five participants in Part 2 experienced moderate TEAEs of muscle weakness (n = 2) and dizziness (n = 3); none required medical intervention.
Table 3.
Adverse events experienced by more than 10% of participants, coded according to preferred terma
| Study Part 1 | Study Part 2 | |||||
| Dantrium® 60 mg (N = 16) | NPJ5008 60 mg (N = 16) | NPJ5008 120 mg (N = 5) | ||||
| Adverse event (AE) | n (%) | Total number of events | n (%) | Total number of events | n (%) | Total number of events |
| AEb (TEAE) | 15 (93.8) | 49 | 16 (100) | 61 | 5 (100) | 35 |
| Serious TEAE | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe TEAE | 0 | 0 | 0 | 0 | 1 (20) | 1c (one muscular weakness event) |
| Moderate TEAE | 1 (6.3) | 1c (one muscular weakness) | 2 (12.5) | 3c (one headache; two muscular weakness) | 3 (60) | 5c (two muscular weakness; three dizziness) |
| Adverse drug reactionsd (ADR) | 15 (93.8) | 45 | 16 (100) | 56 | 5 (100) | 35 |
| Dizziness | 14 (87.5) | 14 | 14 (87.5) | 14 | 5 (100) | 8 |
| Muscular weakness | 9 (56.3) | 9 | 8 (50.0) | 8 | 5 (100) | 8 |
| Dysarthria | 6 (37.5) | 6 | 6 (37.5) | 6 | 5 (100) | 5 |
| Blurred vision | 6 (37.5) | 7 | 9 (56.3) | 10 | 4 (80) | 4 |
| Dyspnoea | 2 (12.5) | 2 | 3 (18.8) | 3 | 2 (40) | 2 |
| Somnolence | 2 (12.5) | 2 | 1 (6.3) | 1 | 1 (20) | 1 |
| Headache | 0 | 0 | 2 (12.5) | 3 | 1 (20) | 1 |
| Dysphagia | 0 | 0 | 0 | 0 | 3 (60) | 3 |
| Balance disorder | 0 | 0 | 2 (12.5) | 2 | 0 | 0 |
| Infusion site discomfort | 1 (6.3) | 1 | 1 (6.3) | 1 | 0 | 0 |
| Nausea | 0 | 0 | 1 (6.3) | 1 | 1 (20) | 1 |
| Flushing | 0 | 0 | 2 (12.5) | 2 | 0 | 0 |
| Dysphonia | 0 | 0 | 0 | 0 | 1 (20) | 1 |
Mild adverse events of catheter site-related reactions, flatulence, abdominal discomfort and feeling cold were considered unrelated to the study drugs. N is the total number of participants in the group, n is the number of participants reporting at least one event, % is percentage of total participants in group.
A TEAE was any untoward medical occurrence in a participant that occurred either before dosing (referred to as a predose adverse event) or once a medicinal product had been administered, including occurrences that were not necessarily caused by or related to that product.
All moderate or severe adverse events were considered related to the study drugs (i.e. ADRs) apart from one moderate adverse event of headache that was considered unrelated to the test drug. Counts of number of participants are by maximum severity, that is, individuals experiencing more than one episode of a TEAE are counted only once within each preferred term category using the most severe episode.
An ADR was any TEAE where a causal relationship with the administered medicinal product was at least a reasonable possibility.
In Part 1, the most commonly reported ADR was dizziness (87.5% of participants), followed by muscle weakness, blurred vision and dysarthria. In Part 2, all participants experienced dizziness, muscle weakness and dysarthria. The majority of ADRs began within 1 min to 2 h after infusion commencement and resolved within 24 h.
No clinically important changes in urinalysis, vital signs, peripheral oxygen saturation or ECG results in either part of the study occurred; a few blood laboratory parameters shifted to outside the normal reference range in at least five participants, but as the majority were only marginally abnormal, these were not considered clinically relevant.
In Part 1, mean hand grip strength decreased from baseline at every time point similarly for equal doses of NPJ5008 and Dantrium®. The largest mean percentage change occurred at 16 h post dose (14.96% for NPJ5008 and 11.70% for Dantrium®). In Part 2, with the 120 mg dose of NPJ5008, the largest decrease occurred 1 h post dose (24.94%). The mean percentage decreases with 120 mg of NPJ5008 were approximately twice those seen with the 60 mg dose.
Pharmacy data results
The time taken to reconstitute one vial of product was measurable from the phase 1 study pharmacy records (Table 4). On average, a vial of NPJ5008 was 40 s (44%) faster to reconstitute than a vial of Dantrium®. Comparing reconstitution speeds to account for the differing dose in a vial, NPJ5008 was on average 10.8 times faster to reconstitute than an equivalent dose of Dantrium®.
Table 4.
Reconstitution time and speed per milligram for a single vial of NPJ5008 and Dantrium® in pharmacy record of phase 1 study
| NPJ5008 120 mg per vial (n = 25) | Dantrium® 20 mg per vial (n = 62) | |
| Mean (min to max) reconstitution time of single vial (s) | 50 (20 to 94) | 90 (47 to 141) |
| Difference in mean reconstitution times (Dantrium® – NPJ5008 time) 40 s | ||
| Mean dantrolene reconstitution rate per second (mg s-1) | 2.4 | 0.22 |
| Relative rate to reconstitute an equivalent dose of dantrolene (NPJ5008 / Dantrium® rate) 10.8× faster |
||
Reconstitution time interval measured from the discrete start time of adding water for injection to drug product vial to the end time of having the drug product fully reconstituted after vigorous shaking (a process that is standardised across all regimens tested).
Laboratory clinical simulation results
Laboratory simulation timings (Table 5) showed that for NPJ5008 it was 12 s (26%) faster to prepare the WFI, 41 s (56%) faster to reconstitute, 24 s (39%) faster to draw up, 6 s (50%) faster to administer through a 16-gauge cannula and 33 s (69%) faster to administer through a 22-gauge cannula compared with Dantrium®.
Table 5.
Summary results for the laboratory simulation of preparation, reconstitution and administration times in cannulas representative of an adult patient and a paediatric patient
| NPJ5008 120 mg per vial | Dantrium® 20 mg per vial | Time difference (Dantrium® – NPJ5008 time) | |||
| Mean (min to max) preparation of water for injection (WFI)a time (n = 10) (s) | 34 (29 to 51) | 46 (38 to 62) | 12 | ||
| Mean (min to max) reconstitutionb time (n = 10) (s) | 32 (29 to 40) | 73 (55 to 86) | 41 | ||
| Mean (min to max) draw upc time (n = 10) (s) | 38 (30 to 49) | 62 (51 to 98) | 24 | ||
| Mean (min to max) total drug preparationd time (n = 10) (seconds) | 104 (88 to 140) | 181 (144 to 246) | 77 | ||
| Cannula gauge | 16 (adult) | 22 (child) | 16 (adult) | 22 (child) | |
| Mean (min to max) administratione time (n = 5) (s) | 6 (5 to 7) | 15 (14 to 18) | 12 (11 to 13) | 48 (44 to 55) | 16 gauge: 6 22 gauge: 33 |
| Average total time (n = 5)f | 1 min 53 s | 1 min 57 s | 3 min 0 s | 4 min 2 s | |
Preparation for NPJ5008 is: remove the cap from the NPJ5008 vial; remove the 20 ml syringe from its packaging; remove a sterile needle from its packaging and add to the syringe; open the bottle of WFI and withdraw 20 ml of the WFI into the syringe. Preparation for Dantrium® is: remove the cap from the Dantrium® vial; remove the 60 ml syringe from its packaging; remove a sterile needle from its packaging and add to the syringe; open the bottle of WFI and withdraw 60 ml of WFI into the syringe.
Reconstitution is: reconstitute the vial with the filled syringe; shake until the powder has been reconstituted.
Draw up is: using the same syringe and needle, collect all the solution from within the vial. Introduce 20 ml of air via the needle in order to equilibrate pressure within the vial; attach the filled syringe to the cannula via the Luer lock. For Dantrium® only draw up time also includes preparation of the filter needle spike device.
Total drug preparation time is the sum of the previous three steps (preparation, reconstitution, draw up).
Administration is: administer the solution via the cannula into a labelled glass beaker.
The average total time is not a sum of the average data per step, but represents the average total time taken for the n = 5 that used each cannula gauge type.
Further details are available in the Supplemental Digital Content.
Discussion
This study demonstrated the bioequivalence, in terms of overall exposure, of NPJ5008 to Dantrium® (the 90% CIs for the adjusted GMRs of test/reference AUC fell within the prespecified standard acceptance range). The generated 90% CIs were narrow because of lower dispersion in intra-individual variability (CVw ∼8%) than expected from past Dantrium® data (CVw ∼11%) (www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205579Orig1s000ClinPharmR.pdf). Although a period effect was observed, and a slight imbalance in participant age between the treatment sequence groups existed, the randomised crossover design, along with no participant withdrawals, had a negligible impact on the validity of the bioequivalence result. The study confirmed comparable relative bioavailability between NPJ5008 and Dantrium® when considering peak exposure levels, with a Cmax mean ratio of ∼93%. As dantrolene doses are titrated to effect, there is no clinical concern with differences seen in the truncated AUC assessments of NPJ5008 and Dantrium® IV. Both formulations exhibited similar MPRs; concordant with the truncated AUC data for the parent dantrolene (free acid), the relative bioavailability of the 5-hydroxydantrolene metabolite was lower with NPJ5008. The mean plasma concentration–time profiles and resultant pharmacokinetic parameters of dantrolene, and the metabolite, were similar between NPJ5008 and Dantrium® in terms of Tmax ranges and comparable elimination T1/2. In contrast to the ∼10 h T1/2 of dantrolene or the metabolite in NPJ5008, HP-β-CD is cleared by renal filtration much faster, with a half-life of ∼1 to 2 h.14 For both Dantrium® and NPJ5008, formation of the metabolite was delayed, and dantrolene was more abundant than the metabolite (based on the Tmax of dantrolene vs. the metabolite, and the MPR for the Cmax and AUC parameters, respectively).
The metabolite was formed consistently with both formulations (similar MPR), which indicates the HP-β-CD–dantrolene complex in NPJ5008 dissociated sufficiently to not limit this conversion. This suggests that in both formulations dantrolene should be available to act on its target receptor, consistent with the similar plasma protein binding potential observed for the two formulations in vitro (unpublished observations).
The exploratory assessment of the relative bioavailability of 60 and 120 mg NPJ5008 suggested a dose-proportional increase in maximum exposure (Cmax) and a supra-proportional (33%) increase in overall exposure (AUC). Larger sample size and data on additional doses are warranted to confirm dose proportionality results.
The pharmacy study and laboratory simulation showed similar vial reconstitution time differences and demonstrated that NPJ5008 is substantially faster to reconstitute than Dantrium®. In the simulation, speed improvements occurred at other processing steps, and were greater for paediatric than adult administration because of the reduced volume – and hence time – needed for administration through a small-bore cannula. Extrapolation of the results from a single vial suggests that in a real-world example, such as a 2.5 mg kg−1 initial dose for a 48 kg patient, a single operative preparing vials end to end would prepare and administer a 120 mg dose of NPJ5008 (using one vial in 20 ml water) more than 16 min (90%) faster through an adult cannula and over 22 min (92%) faster through a paediatric cannula than the same dose of Dantrium®, which would require six vials and 360 ml of water. Although the time-saving benefit appears greater for children than adults, weight-based dosing means further time savings are likely in the adult population. This may reduce the morbidity and mortality associated with treatment delay, and reduce healthcare resource utilisation for a malignant hyperthermia event, including the clinical staff numbers needed, which could be beneficial at times of low staffing.4–7,15 Savings in consumables such as syringes, needles and filtration devices are expected.
The safety profiles of NPJ5008 and Dantrium® were similar, and the TEAEs reported were consistent with the known MoA of dantrolene as a skeletal muscle relaxant.16–18 Together with the observed dose-related decrease in hand grip strength, the MoA-related safety profiles suggested expected efficacy in both formulations, with free drug reaching the target receptors in the skeletal muscle similarly for NPJ5008 and Dantrium®. The number and severity of TEAEs per individual appeared to increase with increasing dose of NPJ5008. All those considered ADRs had resolved by the end of the study without medical intervention.
The excipients used in NPJ5008 are present in numerous pharmaceuticals, thus no significant safety issues were expected, although safety data for cyclodextrin in patients aged under 2 years are sparse, and neither excipient has previously been used in malignant hyperthermia treatments.19–22 A standard rat toxicology study of 14-day NPJ5008 repeat administration found no unexpected safety signals (unpublished observations). Clinical testing of i.v. HP-β-CD for the chronic treatment of Niemann–Pick disease type C has reported cases of audiometrically detected high-frequency hearing loss with repeated doses of 1500 to 2500 mg kg−1.23 There is no experience with NPJ5008 at these levels, and this dose level is not expected to be used in the treatment of malignant hyperthermia. The relevance to the acute use of NPJ5008 in malignant hyperthermia is unclear; therefore, ototoxicity is not considered a substantial safety risk.
Study limitations included: small sample size, limited data in female individuals, lack of higher NPJ5008 doses or repeat dosing, and its restriction to healthy adults. These precluded evaluation of less common safety differences or sex differences, the paediatric population or the malignant hyperthermia condition.
Conclusion
This study demonstrated the bioequivalence, in terms of overall exposure, similar healthy volunteer safety profiles and indicators of similar efficacy, of NPJ5008 and Dantrium®. Improved solubility of NPJ5008 enables faster preparation and administration with a smaller total fluid volume, which may reduce the rate and severity of complications for malignant hyperthermia patients, including mortality rates, and therefore shows a clinically relevant advantage of NPJ5008 over Dantrium® in the treatment of malignant hyperthermia for patients of all ages.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: Norgine Ltd sponsored and funded the studies carried out by Quotient Sciences, UK. Medical writing services were provided by Stephanie Maury and Hannah Jedrey, PhD, of TVF Communications, UK.
Conflicts of interest: RHNKS, LBC, SLS and MJW are employees of Norgine Ltd. LMM, DPC and GW are employees of Quotient Sciences. JGB is a paid advisor to Norgine related to malignant hyperthermia but was not paid for his contributions to this publication.
Presentation: none.
Author contributions: RHNKS and SLS conceived the study, analysed and reviewed data, and wrote the manuscript. LBC, MJW, LMM, DPC and GW analysed and reviewed data, and wrote the manuscript. JGB reviewed the manuscript and provided expert clinical perspective and contributed to the interpretation of the data. All authors read and approved the final manuscript.
This manuscript was handled by Dan Longrois.
Footnotes
Supplemental digital content is available for this article.
References
- 1.Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet J Rare Dis 2015; 10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hopkins PM. Malignant hyperthermia: advances in clinical management and diagnosis. Br J Anaesth 2000; 85:118–128. [PubMed] [Google Scholar]
- 3.Brandom BW, Kang A, Sivak E, et al. Update on dantrolene in the treatment of anesthetic induced malignant hyperthermia. SOJ Anesthesiol Pain Manag 2015; 2:1–6. [Google Scholar]
- 4.Larach MG, Gronert GA, Allen GC, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg 2010; 110:498–507. [DOI] [PubMed] [Google Scholar]
- 5.Riazi S, Larach MG, Hu C, et al. Malignant hyperthermia in Canada: characteristics of index anesthetics in 129 malignant hyperthermia susceptible probands. Anesth Analg 2014; 118:381–387. [DOI] [PubMed] [Google Scholar]
- 6.Glahn KPE, Bendixen D, Girard T, et al. European Malignant Hyperthermia Group. Availability of dantrolene for the management of malignant hyperthermia crises: European Malignant Hyperthermia Group guidelines. Br J Anaesth 2020; 125:133–140. [DOI] [PubMed] [Google Scholar]
- 7.Giraldo-Gutiérrez DS, Arrendo-Verbel MA, Rincón-Valenzuela DA, et al. Dantrolene reconstitution: description of a simulation model in malignant hyperthermia. Colomb J Anestesiol 2018; 46:152–158. [Google Scholar]
- 8.Kugler Y, Russell WJ. Speeding dantrolene preparation for treating malignant hyperthermia. Anaesth Intensive Care 2011; 39:84–88. [DOI] [PubMed] [Google Scholar]
- 9.Strickland RA, Oliver WC, Chantigian RC, et al. Anesthesia, cardiopulmonary bypass, and the pregnant patient. Mayo Clin Proc 1991; 66:411–429. [DOI] [PubMed] [Google Scholar]
- 10.Walpole SC, Prieto-Merino D, Edwards P, et al. The weight of nations: an estimation of adult human biomass. BMC Public Health 2012; 12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuki S, Miyoshi H, Mukaida K, et al. Age-specific clinical features of pediatric malignant hyperthermia: a review of 187 cases over 60 years in Japan. Anesth Analg 2022; 135:128–135. [DOI] [PubMed] [Google Scholar]
- 12.Chow SC. Bioavailability and bioequivalence in drug development. WIREs Comput Stat 2014; 6:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quanjer PH, Stanojevic S, Cole TJ, et al. Multiethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stella VJ, Rao VM, Zannou EA, et al. Mechanisms of drug release from cyclodextrin complexes. Adv Drug Deliv Rev 1999; 36:3–16. [DOI] [PubMed] [Google Scholar]
- 15.Brandom BW, Larach MG, Chen MSA, et al. Complications associated with the administration of dantrolene 1987 to 2006: a report from the North American Malignant Hyperthermia Registry of the Malignant Hyperthermia Association of the United States. Anesth Analg 2011; 112:1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis KO, Bryant SH. Excitation-contraction uncoupling in skeletal muscle by dantrolene sodium. Naunyn Schmiedebergs Arch Pharmacol 1972; 274:107–109. [DOI] [PubMed] [Google Scholar]
- 17.Krause T, Gerbershagen MU, Fiege M, et al. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004; 59:364–373. [DOI] [PubMed] [Google Scholar]
- 18.Ward A, Chaffman MO, Sorkin EM. Dantrolene. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in malignant hyperthermia, the neuroleptic malignant syndrome and an update of its use in muscle spasticity. Drugs 1986; 32:130–168. [DOI] [PubMed] [Google Scholar]
- 19.Gould S, Scott RC. 2-Hydroxypropyl-beta-cyclodextrin (HP-beta-CD): a toxicology review. Food Chem Toxicol 2005; 43:1451–1459. [DOI] [PubMed] [Google Scholar]
- 20.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 2006; 1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 21.Loftsson T. Cyclodextrins in parenteral formulations. J Pharm Sci 2021; 110:654–664. [DOI] [PubMed] [Google Scholar]
- 22.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol 2008; 36:30–42. [DOI] [PubMed] [Google Scholar]
- 23.Hastings C, Liu B, Hurst B, et al. Intravenous 2-hydroxypropyl-β-cyclodextrin (Trappsol® CycloTM) demonstrates biological activity and impacts cholesterol metabolism in the central nervous system and peripheral tissues in adult subjects with Niemann-Pick Disease Type C1: results of a phase 1 trial. Mol Genet Metab 2022; 137:309–319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.