Significance
In an era of predicted recurring pandemics, the ability to produce highly effective vaccines that provide long-lived protection from pathogens and their mutant variants will be critical. A highly successful vaccine will induce memory B cell (MBC) subsets that play distinct yet complementary roles in response to pathogen challenge, differentiating into plasma cells ensuring the immediate production of high-affinity antibodies to homologous and closely related pathogens and the generation of variant-specific MBCs through mutation and selection. The human MBC compartment is heterogeneous, and it is not yet known which MBC subsets function to control homologous versus mutant pathogens. In our manuscript, we show that the MBC’s expression of B cell receptors of the IgG versus IgM type dictates pathogen-driven fates.
Keywords: memory B cells, affinity thresholds, B cell receptor isotype
Abstract
Memory B cells (MBCs) play a critical role in protection against homologous and variant pathogen challenge by either differentiating to plasma cells (PCs) or to germinal center (GC) B cells. The human MBC compartment contains both switched IgG+ and unswitched IgM+ MBCs; however, whether these MBC subpopulations are equivalent in their response to B cell receptor cross-linking and their resulting fates is incompletely understood. Here, we show that IgG+ and IgM+ MBCs can be distinguished based on their response to κ-specific monoclonal antibodies of differing affinities. IgG+ MBCs responded only to high-affinity anti-κ and differentiated almost exclusively toward PC fates. In contrast, IgM+ MBCs were eliminated by apoptosis by high-affinity anti-κ but responded to low-affinity anti-κ by differentiating toward GC B cell fates. These results suggest that IgG+ and IgM+ MBCs may play distinct yet complementary roles in response to pathogen challenge ensuring the immediate production of high-affinity antibodies to homologous and closely related challenges and the generation of variant-specific MBCs through GC reactions.
The adaptive human immune system is remarkable in its ability to provide lifelong protection against pathogen reinfection in individuals who either survived an initial infection or were exposed to a less virulent or attenuated form of the pathogen through vaccination.
Immunity confers protection to homologous antigen challenge, and protection can extend to mutant variants of the original pathogen depending on exposure history and antigenic contexts of infection versus vaccination as observed in the current COVID pandemic (1). Antibodies play an essential effector role in immunity to pathogens, and we now understand that at the cellular level, antibody immunity is dependent on the antigen-driven acquisition of both long-lived plasma cells (LLPCs) and memory B cells (MBCs) (2–5). Both LLPCs and MBCs differentiate from naive B cell precursors in highly specialized microenvironments of germinal centers (GCs) in secondary lymphoid tissues in which GC B cells undergo somatic hypermutation (SHM) and T cell–dependent affinity selection (6, 7). As recently reviewed, most of our present knowledge of the composition and function of the mammalian immune system has come from studies of mice (8, 9) including molecular details of MBC reactivation upon secondary challenge (4). It is not yet clear how results from mice studies can be reliably translated to results of human studies, but it would not be unexpected that contradiction would arise. Consequently, it will be important to appreciate studies done both in mice and humans in interpreting new data.
LLPCs are generated late in GC responses and are highly antigen selected and capable of producing high-affinity, isotype-switched antibodies specific for the homologous pathogen (10).
LLPCs take up residency in the bone marrow and secrete large amounts of antibodies into the circulation that persist for years, potentially for an individual’s lifetime, in the absence of antigen or a secondary challenge (11, 12). Thus, LLPC-produced antibodies act as a first line of defense against homologous or closely related pathogen challenge (2, 4).
Immune individuals also acquire long-lived MBCs, but as compared to LLPCs, MBCs are generated early in GC responses and appear to be less highly somatically mutated and less selected by antigen and as a consequence maintain broadly reactive B cell repertoires (3, 5, 13). Indeed, the repertoire of MBCs and LLPCs does not appear to fully overlap (10, 14, 15), leading to the hypothesis that the greater diversity of the MBCs compartment enables MBCs to respond to variant heterologous pathogens that escape recognition by LLPC-produced antibodies (15–17).
In both mice and humans, upon antigen challenge by vaccine boosting or pathogen reinfection, MBCs appear to predominantly undergo three fates: either differentiating into PCs that produce high-affinity neutralizing antibodies to homologous antigen challenge; differentiating toward GC B cells capable of reentering GCs and undergoing SHM and antigen-affinity selection providing additional long-lasting protection against heterologous variant antigens; or undergoing apoptosis (3, 18, 19). The ability of MBCs generated through primary immunization to rapidly and efficiently differentiate into PCs upon homologous antigen challenge has been shown to contribute to the hallmark increases in both the magnitude and affinity of antibodies in secondary responses (3–5). In the case of heterologous antigen challenge by mutant pathogens, the ability of MBCs to reenter GCs has been proposed to play a critical role in the control of heterologous variant pathogens that escape recognition by the highly specific LLPC-produced antibodies (2, 13). Indeed, recent studies in mouse models showed that MBCs protected against challenge by variants of influenza virus and flavivirus (20, 21).
Understanding that MBCs undergo different fates upon challenge raises a central question, namely, what dictates those fate decisions? MBC production begins early after vaccination when antigen-activated naive B cells rapidly differentiate into GC B cells and form GCs in which B cells expand by proliferation and subsequently undergo antigen-affinity selection in mice and in humans (2, 3, 5). IgM-expressing MBCs (IgM+ MBCs) that have accumulated only a relatively small number of SHMs emerge from GCs first followed by rounds of emergence of isotype-switched MBCs that have acquired larger numbers of affinity-enhancing mutations (10, 22, 23). Thus, the MBC compartment is heterogeneous and composed of B cells of diverse origins. The heterogeneity in the MBC compartment appears to be linked to function as studies in mice and in humans provided evidence that different subsets of MBCs primarily defined by isotype switching have different fates upon antigen challenge. In mice, in response to antigen challenge IgG+ MBCs have been reported to differentiate toward antibody-secreting PCs, whereas IgM+ MBCs were fated to differentiate toward GC B cells (24, 25). In addition, in mice, immunization with a model protein antigen induced both IgM+ MBCs and IgG+ MBCs, and even though the IgM+ MBCs were more numerous, the IgG+ MBCs dominated the secondary response, in part, because the IgG+ MBCs were refractory to the presence of neutralizing antibodies (25). However, subsequent studies in mice suggested that BCR isotype did not predict MBC fates but rather that different fates were restricted to MBC subpopulations defined by the expression of CD80 and PD-L2, CD80– PD-L2– MBCs tended to differentiate primarily toward a GC cell fate and CD80+ PD-L2+ MBCs differentiated primarily toward a PC cell fate (26). Activated human IgM+ MBCs were shown to respond to CCL19/CCL21 and differentiate toward a GC B cell fate in vitro, whereas activated IgG+ MBCs preferentially differentiated toward a PC fate (27). Additional evidence for a role for class switching in determining the fate and function of human MBCs was provided by a recent study using single-cell transcriptomes and antibody repertoire analysis of human tonsil B cells (28) that showed that antibody class-dependent gene expression in MBCs was linked to distinct transcriptional profiles consistent with IgG+ MBCs being more likely to differentiate into PCs and IgM+ MBCs more likely to participate in GC responses. However, of note, recent studies in mice have challenged the notion that MBCs participate in secondary GC responses at all. In a model antigen system in mice, MBCs were reported to be infrequent in secondary GCs and that secondary GCs were likely derived from naive B cells, suggesting that the function of secondary GCs is to initiate affinity maturation of naive B cells de novo rather than to refine the affinity of previously matured MBCs (29). It is possible that naive and MBCs are recruited to GC under different conditions of antigen challenge. A recent study provided critical insights into the mechanisms governing recruitment of antigen-specific naive B cells to GCs following secondary challenge (30). In SARS-CoV and HIV mouse models, antibodies elicited during the primary antibody response were shown to either enhance naive B cell GC participation (for broad-binding, low-affinity, low titer primary antibodies) or conversely restrict naive B cell GC participation (for mono-epitope-binding, high-affinity, high titer primary antibodies). In addition, the secondary antibody response to heterologous flavivirus infections was not affected by the conditional deletion of AID, eliminating SHM, or by abrogation of GCs, providing evidence that recall responses were confined by preexisting clonal diversity (21). Clearly, the development of effective vaccines would benefit from a better understanding of antigen-driven fates of human MBCs and how such fate decisions are made.
We recently provided evidence that several human B cell subpopulations, in particular naive B cells and GC B cells, differed in their responses to membrane-associated anti-κ monoclonal antibodies (anti-κ) that differed in their affinity for Igκ (31, 32). Consistent with the role of GC B cells in antigen affinity selection, GC B cells responded only to high-affinity anti-κ in contrast to naive B cells that responded to both low- and high-affinity anti-κ. Remarkably, the responses of naive and GC B cells were strikingly different involving not only quantitative and qualitative difference in BCR signaling and antigen gathering and processing but also in the architecture of the cells themselves and how the architecture influenced the behavior of the BCR. Naive B cells formed large flat contact areas that concentrated signaling active BCRs and maintained active ruffling around the cell’s periphery on both low- and high-affinity anti-κ. In contrast, GC B cells bound anti-κ through highly dynamic, actin and ezrin-rich pod-like structures that concentrated BCRs. Low-affinity anti-κ triggered continuous engagement and disengagement of pod-like structures as the B cells searched for antigen, whereas high-affinity anti-κ induced stable synapse formation and B cell activation. Thus, the affinity of the BCR anti-κ engagements appeared to trigger predetermined functional behaviors of the naive and GC B cells.
Here, we provide evidence that in humans, antigen-affinity thresholds for activation of IgG+ MBCs are set considerably higher as compared to IgM+ MBCs as judged by B cell responses to high- versus low-affinity anti-κ in terms of clustering and IS formation, phosphorylation of key kinases in the BCR signaling pathway, expression of a variety of activation markers, and cellular proliferation. Using a recently described flow cytometry–based classification scheme that segregates all human peripheral blood (PB) MBCs into five unique subsets (33) two of which contained only either IgM- or IgG-expressing MBCs, we determined that independently of the MBC subset characteristics, the IgM MBCs showed low-affinity thresholds for activation and the IgG MBCs high-affinity thresholds. Importantly, IgM+ MBCs and IgG+ MBCs underwent different fates following high- versus low-affinity anti-κ challenge in vitro. IgG+ MBCs responded only poorly to low-affinity anti-κ but robustly differentiated toward PC fates in response to high-affinity anti-κ. In contrast, IgM+ MBC responded to low-affinity anti-κ by differentiating toward GC B cell fates but underwent apoptosis in response to high-affinity anti-κ. Thus, our studies reveal a compartmentalization of MBC function based on the antigen affinity threshold of the MBC subset that correlated with the isotype of the expressed BCR.
Results
Human IgG+ and IgM+ MBCs Have Distinct Affinity Thresholds for Antigen-Induced Activation.
In two recent studies, we described differences in antigen-affinity thresholds for BCR-mediated activation of human tonsil naive and GC B cells and in subpopulations of PB atypical B cells using as surrogate antigens anti-human Igκ LC antibodies (anti-κ) that differed 100-fold in their affinity for ĸ chains and were presented to B cells on planar lipid bilayers (PLBs) and imaged by a variety of microscopy techniques (31, 32, 34). A description of the physical properties of the PLBs, the engagement of B cells with the membrane-associated antigens, and the microscopy techniques are detailed in SI Appendix and reviewed separately (35). In addition, two applications of the techniques to define intrinsic properties of BCRs in B cell activation are provided (36, 37). From the results of these studies of GC and naive B cells, we concluded that although the affinity of the anti-κ engaged was critical to the differences observed in the initiation of the responses, intrinsic features of the naive and GC B cells including the wiring of the signaling and antigen processing pathways and the architecture of the B cells themselves may also influence the outcome of anti-κ binding. Here, using this approach, we compared the affinity thresholds for activation of human IgG+ Igκ + MBCs, IgM+ Igκ + MBCs, and IgM+ Igκ + naive B cells. We isolated IgG+ MBCs (CD19+ Igλ− CD27+ CD21+ IgG+) and IgM+ MBCs (CD19+ Igλ− CD27+ CD21+ IgM+) as well as IgM+ naive B cells (CD19+ Igλ− CD27− CD21+ IgM+) from human PBMCs by the strategy shown (SI Appendix, Fig. S1). Aliquots of the purified B cell subpopulations were stained to allow detection of IgM+ or IgG+ using fluorescently labeled Fab fragments of Ig specific for either human IgM or IgG and placed on PLBs to which either high-affinity or low-affinity human κ-specific antibodies were attached, referred to as high-affinity PLBs versus low-affinity PLBs. As shown, the high- and low-affinity-κ specific antibodies bind similarly to the majority of IgM+ and IgG+ MBCs suggesting the possible heterogeneities between the IgM+ and IgG+ MBCs in BCR density or surface distribution or organization did not impact anti-κ binding (SI Appendix, Fig. S2A). Cells were incubated on the PLBs for eight min, fixed, and stained with fluorescently labeled antibodies specific for phosphorylated Syk (pSyk) and PLCγ2 (pPLCγ2). Cells were imaged by total internal reflection fluorescence (TIRF) microscopy, and the fluorescent images quantified. Representative TIRF images for BCR, pSyk, pPLCγ2, and their overlay for IgG+ MBCs, IgM+ MBCs, and IgM+ naive B cells placed on either high-affinity or low-affinity PLBs are shown (Fig. 1A). Quantification of the images are given for the BCR, pSyk, and pPLCγ2 for a large number of IgG+ MBCs, IgM+ MBCs, and IgM+ naive B cells (Fig. 1 B−D).
Fig. 1.
Human IgG+ and IgM+ MBCs have distinct activation thresholds. B cells were isolated from PBMCs or tonsils using negative magnetic selection. Naive B cells (CD21+ CD27− Igλ−) and MBCs (CD21+ CD27+ Igλ−) were FACS-sorted from peripheral B cells. Alternatively, naive B cells (IgD+ CD10− Igλ−), GC B cells (IgD− CD10+ Igλ−), and MBCs (IgD− CD10− Igλ−) were FACS-sorted from tonsil B cells. FACS-sorted cells were stained with Alexa Fluor 594–conjugated Fab fragments of either anti-IgM or anti-IgG and placed on either high- or low-affinity PLBs for 8 min, fixed and stained with antibodies specific for pSyk and pPLCγ2, and imaged by TIRF microscopy. (A) Representative TIRF microscopy images indicating accumulation of the BCR (IgM+ or IgG+) (red), pSyk (green), or pPLCγ2 (blue) and the overlay of the three images in the IS formed by IgG+ MBCs, IgM+ MBCs, and naive B cells from PB activated on high- or low-affinity PLBs (Scale bar, 2 μm). (B–D) Quantification of gMFI of BCR (B) pSyk (C) and pPLCγ2 (D) accumulated in the IS of IgG+ MBCs, IgM+ MBCs, and naive B cells from PB incubated on either high-affinity PLBs (red dots) or on low-affinity PLBs (blue dots). Data are from three independent experiments, each analyzing 25 to 50 cells. (E) Quantification of gMFI of BCR (Left), pSyk (Middle), and pPLCγ2 (Right) accumulated in the IS of IgG+ MBCs, IgM+ MBCs, IgG+ GC B cells, and naive B cells from tonsils incubated on either high-affinity PLBs (red dots) or on low-affinity PLBs (blue dots). Data are from two independent experiments, each analyzing 25 to 50 cells. The error bars indicate SEM. Data were analyzed using the unpaired t test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant.
All three B cell subpopulations formed immune synapses (ISs) on high-affinity PLBs visualized by the accumulation of either IgG+ or IgM+ BCRs and the early BCR signaling molecules, pSyk and pPLCγ2 in the area of contact of the B cells with the antigen-containing PLB (Fig. 1A). The IgM+ MBCs and IgM+ naive B cells each showed similar levels of accumulation of IgM+ BCR, pSyk, and pPLCγ in the IS in response to high- versus low-affinity PLB (Fig. 1 B–D). In contrast, IgG+ MBCs showed considerable reductions in the levels of BCR, pSyk, and pPLCγ2 in responses to low- versus high-affinity PLB (Fig. 1 B–D) indicating that low-affinity PLBs were sufficient to trigger BCR signaling in IgM+ naive and IgM+ MBCs but insufficient to induce BCR signaling in IgG+ MBCs. Notably, when placed on high-affinity PLB, the levels of pSyk and pPLCγ in IgG+ MBCs were lower than those of IgM+ MBCs and naive B cells (Fig. 1 C and D). However, as described below these differences were not observed in comparing pSyk and pPLCγ2 levels IgG+ MBCs and IgM+ MBCs obtained from tonsil tissue (Fig. 1E) and may be tissue-specific signaling adaptations.
Of note, we wanted to rule out the possibility that the differences in affinity thresholds of the PB B cell subpopulations were due to differences in the levels of expression of the BCRs. Because the antibodies used to detect the BCR IgM and IgG isotypes were different, it was not possible to directly compare the MFI of the BCR in the IS for IgM+ MBCs and IgG+ MBCs in Fig. 1. However, we were able to quantify and compare the levels of surface BCR for each subpopulation indirectly by flow cytometry using IgM-, IgD-, IgG-, and Igκ-specific mAbs (SI Appendix, Fig. S2 B–D). The expression of Igκ differed between B cell subpopulations by approximately twofold and was highest for IgM+ IgD+ naive B cells, lower for both IgM+ IgD+ MBCs and IgG+ MBCs (between which Igκ expression was not significantly different), and still lower for IgM+ MBCs (SI Appendix, Fig. S2C). Due to the contribution of both IgM and IgD to the surface levels of Igκ in IgM+IgD+ MBCs, the levels of surface IgG+ BCRs and IgM+ BCRs could not be directly compared for IgG+ MBCs and IgM+IgD+ MBCs. However, given that the levels of expression of IgM on IgM+ MBCs and IgM+IgD+ MBCs were not significantly different (SI Appendix, Fig. S2D), we can conclude that IgG+ BCR expression is not lower than that of IgM+ BCR expression in MBCs and that the high-affinity threshold for activation observed for IgG+ MBCs cannot be attributed simply to lower expression levels of IgG.
We previously showed that IgG+ GC B cells obtained from human tonsils had higher antigen affinity thresholds for activation as compared to IgM+ naive tonsil B cells (31). Thus, we compared the antigen-affinity thresholds for IgM+ MBCs, IgG+ MBCs, IgM+ naive B cells, and IgG+ GC B cells obtained from human tonsils (Fig. 1E). IgM+ MBCs and IgM+ naive B cells from tonsils each accumulated similar levels of BCR, pSyk, and pPLCγ2 in the IS in response to high-affinity versus low-affinity PLB. In contrast, IgG+ MBCs and IgG+ GC B cells accumulated BCRs, pSyk, and pPLCγ2 in the IS only in response to high-affinity but not low-affinity PLBs. For tonsil IgG+ MBCs and IgG+ GC B cells the levels of IgG (geometric mean fluorescence intensity-gMFI) accumulated in the IS could be directly compared because both were quantified using the same IgG-specific antibodies. IgG+ MBCs accumulated approximately twofold more IgG in the IS as compared to IgG+ GC B cells. Of note, the levels of pSyk and pPLCγ2 accumulated within the IS of the tonsil IgG+ MBCs placed on high-affinity PLB were similar to levels of pSyk and pPLCγ2 that accumulated for tonsil IgM+ MBCs and naive B cells (Fig. 1E) in contrast to the observation for PB IgG+ MBCs that accumulated less pSyk and pPLCγ2 as compared to PB IgM+ MBCs and naive B cells. These findings suggest that there may be tissue-specific differences in PB versus tonsils in the outcomes of high antigen-affinity activation for IgG+ and IgM+ MBCs.
Taken together, these results provide strong evidence that isotype expression is a critical factor in setting affinity thresholds for early events in antigen-dependent activation in B cell subsets from both human PB and tonsils. The BCR isotype appears required but not necessarily sufficient for setting MBC affinity thresholds. In addition, the PLB-Ag system is an in vitro approach, and it is possible that B cell responses in vivo may be influenced by environmental factors including soluble cytokines as well as cell–cell interactions between B cells and regulatory cells.
To explore the correlation of BCR isotype and affinity thresholds within MBC subsets, we used a recently defined flow cytometry-based scheme that segregates all human PB B cells into 10 unique subsets, including five MBC subsets, based on the expression of over 350 B cell surface markers (33) by the strategy shown (SI Appendix, Fig. S3). We isolated all five MBC subsets (II-VI) as well as subset I (CD19Hi CD11C+ CD27−) that includes what we termed atypical MBCs (32) and analyzed their antigen-affinity thresholds. We determined the response of IgG+ and IgM+ B cells within each of the subsets (subpopulations I-VI) to high-affinity versus low-affinity PLBs as quantified by accumulation of BCRs (Fig. 2A), pSyk (Fig. 2B) and pPLCγ2 (Fig. 2C) in the IS in TIRF images. Subpopulation III contained only IgG+ MBCs, subpopulation IV contained only IgM+ MBCs, whereas the remaining subpopulations (I, II, V, and VI) contained both IgM+ and IgG+ MBCs (Fig. 2). IgG+ MBCs within each of the subpopulations responded to high- but not low-affinity PLBs, whereas the IgM+ MBCs responded to both high- and low-affinity PLBs, providing evidence that the isotype of the MBCs correlated with antigen affinity thresholds for activation for all MBC subsets. For IgM+ MBCs in subset V, the accumulation of pSyk and in subsets IV, V, and VI the accumulation of pPLCγ2 were somewhat lower in response to low- versus high-affinity PLB, but in all cases, the levels of accumulation of pSyk and pPLCγ2 were significantly greater than that of pSyk and pPLCγ2 in IgG+ MBCs responding to low-affinity PLB.
Fig. 2.
IgG+ and IgM+ B cells within six distinct subpopulations of MBCs have similar activation thresholds. B cells were isolated from PBMCs using negative magnetic selection and stained using fluorescently labeled antibodies specific for CD19, CD38, CD27, CD11c, CD45RB, CD95, and CD73, followed by FACS into MBC subpopulations I (CD19hi CD11c+), II (CD95+), III (CD45RB+ CD27+ CD73+), IV (CD45RB+ CD27−), V (CD45RB+ CD27+ CD73−), and VI (CD45RB− CD27+). MBC subpopulations were stained with Alexa Fluor 594–conjugated Fab of either IgM−- or IgG−-specific antibodies and DyLight 405-conjugated Fab of IgD-specific antibodies (for subpopulation I) and placed on either high- or low-affinity PLBs for 8 min, fixed and stained with antibodies specific for pSyk and pPLCγ2, and imaged by TIRF microscopy. (A–C) Quantification of gMFI of BCR (A) pSyk (B) and pPLCγ2 (C) accumulated in the IS of MBC subpopulations incubated on either high-affinity PLBs (red dots) or on low-affinity PLBs (blue dots). Data are from two independent experiments, each analyzing 25 to 50 cells. The error bars indicate SEM. Data were analyzed using the unpaired t test. ****P < 0.0001; ns, not significant.
The IgD+ IgMlow MBCs in subpopulation I were of particular interest as they were unique in expressing CD11c, a feature of a subpopulation of B cells that are greatly expanded in chronic infectious diseases and in autoimmune diseases that we termed atypical MBCs (34). We recently provided evidence that atypical MBCs represent a separate B cell lineage in these different diseases with a common driver, namely IFNγ (32). We observed that IgD+ IgM+ atypical MBCs had low antigen affinity thresholds for activation in contrast to IgG+ atypical MBCs and IgMlow IgD+ atypical MBCs that had high-affinity thresholds for activation (32). Based on this observation, we speculated that not only did isotype switching contribute to high-affinity thresholds for antigen activation but that the reduction in expression of IgM on atypical MBCs coexpressing IgM and IgD was sufficient to result in increases in antigen affinity thresholds. Here, we observed that IgG+ and IgMlow IgD+ MBCs in subpopulation I have high-affinity thresholds for antigen resulting in greater accumulation of BCR, pSyk, and pPLCγ2 in response to high-affinity versus low-affinity PLBs. In contrast, IgM+ IgD+ MBCs in subpopulation I responded similarly to both high-affinity and low-affinity PLB indicating low-affinity thresholds (Fig. 2). Of note the amount of IgD BCR that accumulated in the IS of IgMlow IgD+ MBCs appeared unusually low that may be attributed, in part, to the fact that IgD was detected using IgD-specific antibodies labeled with a relatively weak fluorophore, DyLight 405. Taken together these results provide evidence that affinity thresholds for activation are linked to either isotype switching in MBC of diverse phenotypes or in the case of atypical MBCs to low IgM expression (IgMlow IgD+).
We also explored the impact of antigen-affinity thresholds of IgM- and IgG-expressing MBCs in a critical late BCR-mediated event, namely antigen internalization (SI Appendix, Fig. S4). Antigen internalization determines the outcome of B cell responses by controlling the number and repertoire of peptide-MHC II molecules presented to helper T cells. One of the best-characterized mechanisms of BCR internalization from the cell surface is clathrin-mediated endocytosis (38). Antigen binding to the BCR induces signaling, antigen gathering, and extraction and internalization of BCR-antigen in clathrin-coated pits. We first examined the transcriptional changes accompanying the response of IgM+ MBCs and IgG+ MBCs to high- versus low-affinity antigens, carrying out RNA-sequencing (RNA-seq) of IgM+ and IgG+ MBCs exposed to either low- or high-affinity antigens (SI Appendix, Fig. S4A). Gene Set Enrichment Analysis (GSEA) of IgM+ and IgG+ MBCs treated with either high- or low-affinity PLB from the Molecular Signature Database (MSigDB) C5 gene ontology (GO): Biological processes (BP) collection revealed that a clathrin-dependent endocytosis signature was enriched in IgG+ MBCs activated on high-affinity PLB as compared to IgM+ MBCs. Conversely, this gene set was enriched in IgM+ MBCs activated on low-affinity antigens as compared to IgG+ MBCs (SI Appendix, Fig. S4A). This observation suggests the possibility of inherent differences in the accumulation and organization of clathrin in IgM+ MBCs and IgG+ MBCs. To investigate this possibility we also imaged clathrin by TIRFM in IgG+ MBCs and IgM+ MBCs placed on high- versus low-affinity PLB. Shown are representative images of the BCR and clathrin heavy chain with time after IgM+ and IgG+ MBCs were placed on high- versus low-affinity PLB or PLB alone (SI Appendix, Fig. S4B). Also given is the quantification of clathrin in the contact site (SI Appendix, Fig. S4C) as well as the colocalization of the BCR and clathrin (SI Appendix, Fig. S4D) for a large number of B cells. On high-affinity PLB IgG+ MBCs accumulated more clathrin in the contact area as compared to IgM+ MBCs (SI Appendix, Fig. S4C) and the IgG BCR showed greater and more rapid colocalized with clathrin as compared to IgM+ MBCs (SI Appendix, Fig. S4D).
To determine whether the IgG subclass of the MBC BCR influenced the antigen affinity threshold for activation we carried out similar analyses of PB MBCs expressing IgG1, IgG2, IgG3, or IgG4 to respond to high- versus low-affinity PLB. PB IgG+ MBCs were first labeled with DyLight594-conjugated Fab of IgG-specific antibodies and placed on high- versus low-affinity PLB. Following activation, cells were fixed, stained with Alexa Fluor 647–conjugated mAbs specific for IgG1, IgG2, IgG3 or IgG4 and the accumulation of BCRs in the IS determined (SI Appendix, Fig. S5). In each case, the amount of BCR accumulated in the IS was higher for MBCs placed on high- versus low-affinity PLB indicating that the IgG subclass of the BCR did not alter the antigen-affinity threshold of the IgG+MBCs.
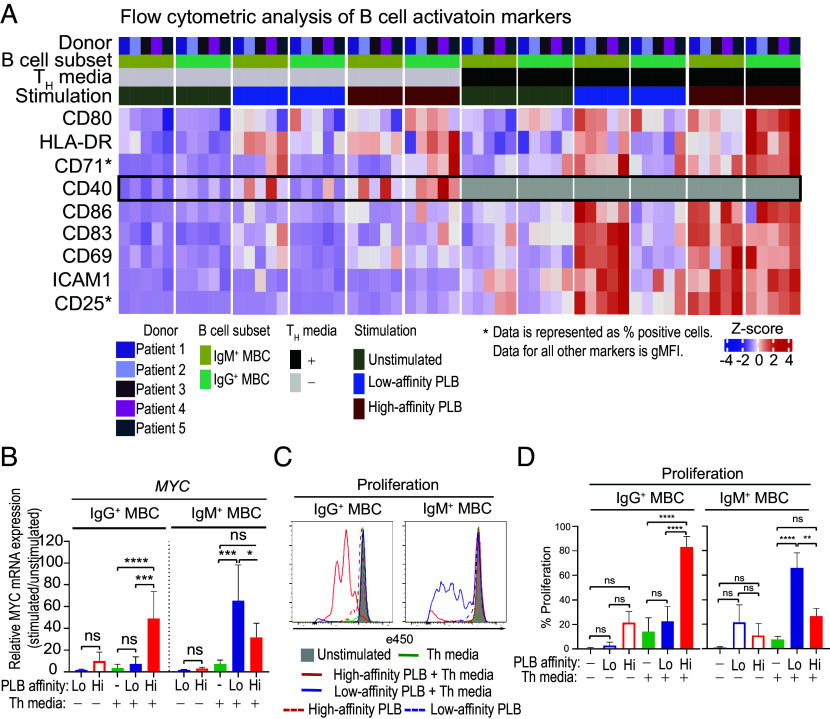
High- versus Low-Affinity PLBs Induce Distinct Patterns of Expression of B Cell Activation Markers in IgG+ versus IgM+ MBCs.
We determined the antigen-affinity dependence of the expression of several markers associated with T cell-dependent B cell activation downstream of BCR signaling. These included markers that reflect the activation state of the B cells and are associated with a number of important functions including responses to cytokines (CD25), nutrient uptake (CD71), antigen presentation to helper T cells (HLA-DR), and interactions with activated helper T cells (CD80, CD83, CD86, ICAM-1, and CD40) (39, 40). MBCs were placed on high-affinity or low-affinity PLBs and incubated in vitro for 24 h in the presence or absence of IL- 4, IL-21, and anti-CD40 antibodies to mimic T cell help (Th media). Cells were harvested and analyzed by flow cytometry for the expression of IgG and IgM and the activation markers listed. Flow cytometry profiles are given (SI Appendix, Fig. S6), and the data are expressed as a heat map (Fig. 3A). In the absence of Th media, there was little induced expression of most of the markers analyzed in either IgM+ or IgG+ MBCs in response to either high-affinity or low-affinity PLB with the exception of CD40 and HLA-DR the control of which appeared somewhat Th-independent. In the presence of Th media, IgM+ MBCs were activated by both low-affinity and high-affinity PLBs, whereas IgG+ MBCs responded only to high-affinity PLB. Taken together these results provide evidence that the high-affinity antigen thresholds set for IgG+ MBCs and low-affinity thresholds for IgM+ MBCs are both sufficient to drive events downstream of early BCR signaling and that the presence of Th does not alter the antigen affinity windows in which IgG+ and IgM+ MBCs responded. We also tested the response of IgM− IgG− MBC, presumed to be IgA+ MBCs, to high- versus low-affinity PLB measuring increases in expression of a variety of activation markers as an outcome. We observed that these MBCs discriminated high- versus low-affinity PLB and showed higher levels of expression of cell surface activation markers in response to high-affinity versus low-affinity PLB (SI Appendix, Fig. S7).
Fig. 3.
The impact of antigen-affinity thresholds on induced expression of activation markers and MYC and proliferation of IgG+ and IgM+ MBCs. MBCs were isolated from PBMCs using positive magnetic selection and activated in vitro with high- or low-affinity PLBs and IL-4, IL-21, and anti-CD40 antibodies to mimic T cell help (Th media) for 24 h (n = 5). Expression of activation markers CD69, CD25, and CD71 and B cell–T cell interaction molecules HLA-DR, CD80, CD40, ICAM-1, CD83, and CD86 was assessed in IgG+ MBCs and IgM+ MBCs by flow cytometry. (A) Heatmap showing scaled and centered cell surface expression levels of the indicated markers in either IgM+ MBCs or IgG+ MBCs under different stimulation conditions. Data are represented as gMFI or percentage positive cells, as indicated. The color scale represents the Z-score. (B) Relative mRNA expression level of MYC in FACS-sorted IgG+ MBCs and IgM+ MBCs after in vitro activation with high (Hi)- or low (Lo)-affinity PLBs and Th media for 72 h. The error bars indicate SD of data from five independent experiments. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant. (C) Representative histograms of the expression of eFluor 450 by IgG+ MBCs and IgM+ MBCs following culture in vitro for 6 d without stimulation (gray shaded area) or stimulated under the indicated conditions. (D) Comparison of the decrease in expression of eFluor 450 (%) by IgG+ MBCs and IgM+ MBCs. FACS-sorted IgG+ MBCs and IgM+ MBCs from three donors were stained with Cell Proliferation Dye eFluor 450 and activated in vitro with high (Hi)- or low (Lo)-affinity PLBs and Th media for 6 d. The error bars indicate SD. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant.
The Impact of High- versus Low-Affinity Antigens on the Expression of MYC and Cellular Proliferation.
B cell activation through the BCR and CD40 induces the expression of the transcription factor Myc through the MAPK/ERK pathway and the NF-κB pathway, respectively (41). In B cells, Myc controls the expression of several genes involved in the regulation of cell growth, cell proliferation, cell cycle, and apoptosis (42). To determine the antigen affinity dependence of the induction of MYC in IgG+ MBCs and IgM+ MBCs we incubated purified MBCs in vitro with high-affinity or low-affinity PLBs, in the presence or absence of Th media, for 72 h and determined the relative expression of MYC mRNA (Fig. 3B). Proliferation was measured 6 d later (Fig. 3 C and D). IgG+ MBCs increased transcription of MYC, but only in response to high-affinity PLB and in the presence of Th media. For IgM+ MBCs, MYC transcripts were detectable in response to both low- and high-affinity PLB (Fig. 3B), but the level of MYC transcription in response to high-affinity PLB was lower than that induced by low-affinity PLB. Examples of cell proliferation are given in flow cytometry plots of the intensity of the proliferation dye, e450 (Fig. 3C), and as the percentage of proliferating cells averaged from several experiments (Fig. 3D). IgG+ MBCs proliferated in response to high-affinity PLB and Th media but did not proliferate to low-affinity PLB above that resulting from addition of Th media alone (Fig. 3 C and D). In contrast, IgM+ MBCs proliferated in response to both high-affinity and low-affinity PLB but showed more robust proliferation to low-affinity as compared to high-affinity PLB (Fig. 3 C and D). These data were consistent with the RNAseq analysis of MYC, AHR (43), and CD81 (44) which are known to be related with the proliferation in B cells (SI Appendix, Fig. S8A). The reduced responses of IgM+ MBCs to high-affinity PLB as compared low-affinity PLB observed for both MYC transcription and proliferation were unexpected given the equivalent increases in BCR accumulation and phosphorylation of B cell signaling molecules (Fig. 1) and activation markers (Fig. 3A) to low-affinity and high-affinity PLB. This observation suggests the possibility that IgM+ MBCs have both a low antigen affinity threshold for B cell activation as well as a upper ceiling on the affinity of antigens that can trigger Myc expression and proliferation.
High- and Low-Affinity Antigens Induce Distinct Fates in IgG+ and IgM+ MBCs.
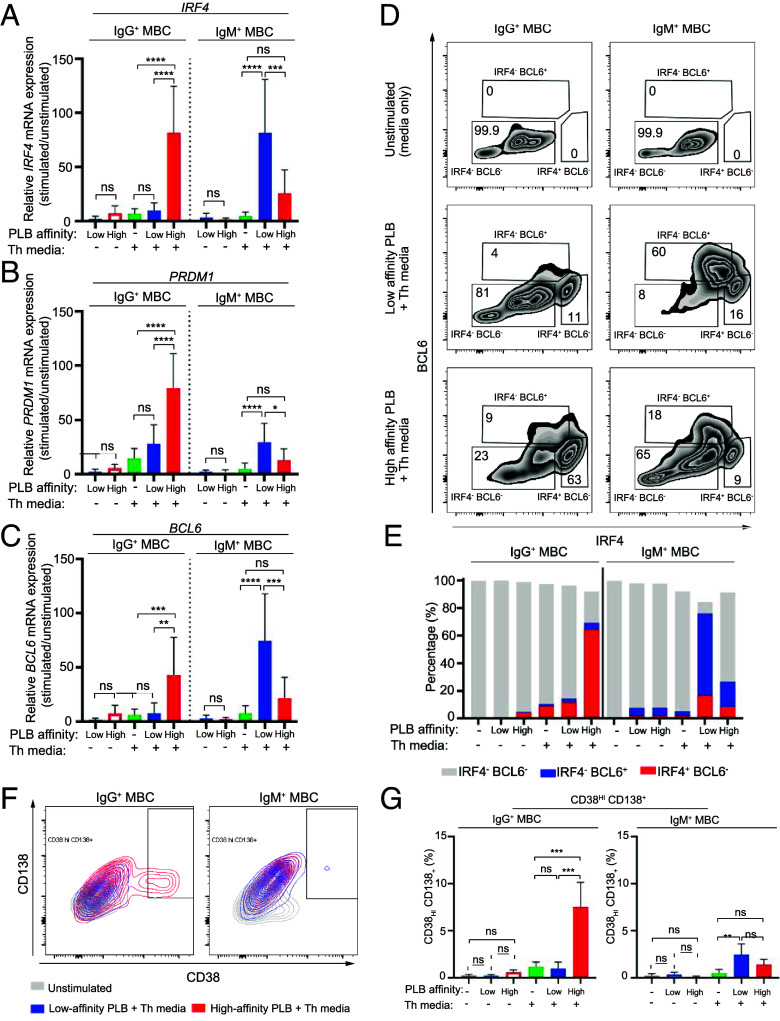
To explore the possibility that high-affinity and low-affinity antigens induced distinct fates in IgG+ and IgM+ MBCs we first determined the impact of antigen affinity on the expression of transcription factors that regulate the differentiation of B cells toward PCs, including IRF4 and PRDM1, versus toward GC B cells, including BCL6 (41, 45, 46). We quantified the mRNA levels of IRF4, PRDM1, and BCL6 in sorted PB IgG+ MBCs and IgM+ MBCs in response to high-affinity or low-affinity PLBs after 72-h incubation in the presence or absence of Th media. Relative levels of expression of IRF4, PRDM1, and BCL6 mRNA were determined by quantitative real-time PCR (RT-PCR) and quantified by the 2−ΔΔCt method. Data are expressed as fold changes in expression of stimulated cells as compared to cells cultured 72 h in media alone. We observed that IgG+ MBCs expressed IRF4, PRDM1, and BCL6 mRNAs but only in response to high-affinity PLBs in the presence of Th media (Fig. 4 A–C). In contrast, IgM+ MBCs expressed IRF4, PRDM1, and BCL6 mRNA in response to both low-affinity and high-affinity PLBs in the presence of Th media and the relative response to low-affinity PLBs was significantly greater than to high-affinity PLB (Fig. 4 A–C). Similar results were obtained using IgG+ and IgM+ MBCs from tonsils (SI Appendix, Fig. S9 A–C).
Fig. 4.
High- and low-affinity antigens induce distinct fates among IgG+ and IgM+ MBCs. B cells were isolated from PBMCs using negative magnetic selection. IgG+ MBCs and IgM+ MBCs were FACS-sorted from B cells and activated in vitro with high- or low-affinity PLBs in the presence or absence of Th media for 72 h (for analysis of IRF4, PRDM1, and BCL6 mRNA expression by quantitative RT-PCR) or 6 d (for analysis of protein expression of IRF4, BCL6, CD38, and CD138 by flow cytometry). (A–C) Relative mRNA expression level of IRF4 (A), PRDM1 (B), and BCL6 (C) in FACS-sorted IgG+ MBCs and IgM+ MBCs after activation in vitro with high- or low-affinity PLBs in the presence or absence of Th media for 72 h (n = 8). The error bars indicate SD. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant. (D) Flow cytometry plots indicating expression of IRF4 and BCL6 by IgG+ MBCs and IgM+ MBCs following culture for 6 d in vitro without stimulation (Top) or stimulation with low-affinity PLB and Th media (Middle) or high-affinity PLB and Th media (Bottom). Data are representative of two experiments. (E) Comparison of the proportion (%) of IgG+ MBCs and IgM+ MBCs differentiating toward PC fate (IRF4+BCL6−) (red), GC fate (IRF4−BCL6+) (blue) or undifferentiated (IRF4−BCL6−) (gray) as in (D). (F) Representative flow cytometry plots indicating expression of CD38 and CD138 by IgG+ MBCs and IgM+ MBCs following culture for 6 d in vitro without stimulation (gray), stimulated with low-affinity PLB and Th media (blue) or high-affinity PLB and Th media (red) (n = 3). (G) Comparison of CD38hi CD138+ cells (%) within IgG+ MBCs and IgM+ MBCs as in (F). The error bars indicate SD. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant (n = 3).
To quantify on a per-cell basis the percent of IgG+ MBCs versus IgM+ MBCs that differentiated toward a PC fate (IRF4+ BCL6−) versus a GC fate (IRF4− BCL6+), we determined the levels of intracellular expression of IRF4 and BCL6 proteins using specific antibodies for intracellular labeling (47). Sorted IgG+ MBC and IgM+ MBCs were placed on high-affinity or low-affinity PLBs for 5 d in vitro in the presence or absence of Th media. Shown are examples of the flow plots for IRF4 and BCL6 (Fig. 4D) and the percentages of IRF4− BCL6−, IRF4+ BCL6+, and IRF4+ BCL6− MBCs quantified from two experiments (Fig. 4E). The majority of IgG+ MBCs (>70%) only responded to high-affinity PLB, and the majority of these differentiated toward IRF4+BCL6− PCs rather than IRF4−BCL6+ GC B cells (Fig. 4E). In contrast, the majority of IgM+ MBCs (>80%) responded to low-affinity PLB, and the majority of these differentiated toward IRF4-BCL6+ GC B cells rather than IRF4+BCL6− PCs. Only a small minority of IgM+ MBCs responded to high-affinity PLB, and these IgM+ MBCs differentiated toward both GC B cell and PC fates. These results are consistent with the results of our RNAseq analysis that showed the enrichment of a gene signature corresponding to plasmablast differentiation from MBC in IgG+ MBC on high-affinity PLB, and an enrichment of a gene signature of GC differentiation from MBC in IgM+ MBC on low-affinity PLB (SI Appendix, Fig. S8B). Additionally, IRF4 and PRDM1 transcripts were strongly up-regulated in IgG+ MBC on high-affinity PLB as compared to other subsets, both with and without Th media while BCL6 was somewhat up-regulated in IgM+ MBC on low-affinity PLB (SI Appendix, Fig. S8C). This was consistent with results obtained for sorted populations of PBMC IgG+ and IgM+ MBCs activated in vitro with high- or low-affinity PLB and Th media for 72 h and analyzed for IRF4, PRDM1, and BCL6 mRNA by quantitative RT-PCR (Fig. 4 A–C).
To confirm the selective differentiation of IgG+ MBCs toward PCs, sorted IgG+ MBCs and IgM+ MBCs were incubated for 6 d on high-affinity or low-affinity PLBs in the presence or absence of Th media, cells were recovered from culture and analyzed by flow cytometry to determine the percent of IgG+ and IgM+ cells that expressed high levels of CD38 (CD38Hi) and were heterogeneous in expression of CD138 (CD138+) termed CD38Hi CD138+. Stimulation of IgG+ MBCs resulted in approximately 10% CD38Hi CD138+ cells but only in response to high-affinity PLB and Th (Fig. 4 F and G). In contrast, the IgM+ MBCs response to high-affinity PLB and Th was not significantly above the response to Th alone, whereas the response to low-affinity PLB and Th resulted in approximately 5% CD38Hi CD138+ PCs, thus confirming the selective differentiation of IgG+ MBCs to PCs.
Cross-linking of BCR in the absence of Th induces activation-induced cell death by apoptosis in B cells (48). Since we observed reduced proliferation and differentiation of IgM+ MBCs to high-affinity versus low-affinity PLB, we speculated that high-affinity antigen may induce apoptosis in IgM+ MBCs. We analyzed the surface expression of Annexin-V by flow cytometry in purified MBCs in response to high-affinity or low-affinity PLBs after 24-h incubation in the presence or absence of Th media. A small percentage of IgG+ MBC underwent apoptosis (<5%) which was not significantly different between conditions (Fig. 5 A and B). For IgM+ MBCs, high-affinity antigen induced significantly more apoptosis as compared to low-affinity antigen, and the apoptosis induced by high-affinity antigens was not prevented in the presence of Th (Fig. 5 A and B).
Fig. 5.
High- and low-affinity antigens induce distinct levels of apoptosis among IgG+ MBCs and IgM+ MBCs. MBCs were isolated from PBMCs using positive magnetic selection and activated in vitro with high- or low-affinity PLBs in the presence or absence of Th media for 24 h. Expression of Annexin-V, 7-AAD, and active caspases (indicated by FAM-FLICA staining) was analyzed in IgG+ MBCs and IgM+ MBCs by flow cytometry. (A) Representative histograms of the expression of Annexin-V with gate for Annexin-V high population by IgG+ MBCs and IgM+ MBCs following culture in vitro for 24 h without stimulation (gray shaded area) or stimulated with Th media alone (solid green line), high-affinity PLB (dotted red line), high-affinity PLB plus Th media (solid red line), low-affinity PLB (dotted blue line), and low-affinity PLB plus Th media (solid blue line). (B) Comparison of the expression of high levels of Annexin-V (%) by IgG+ MBCs and IgM+ MBCs as in (A). The error bars indicate SD. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant (C) Representative flow cytometry plots indicating expression of 7-AAD and FAM-FLICA by IgG+ MBCs and IgM+ MBCs following culture in vitro for 24 h without stimulation (Top) or stimulation with low-affinity PLB plus Th media (Middle) or high-affinity PLB plus Th media (Bottom). (D) Comparison of the proportion (%) of IgG+ MBCs and IgM+ MBCs in early-stage apoptosis (7-AAD− FAM-FLICA+) as in (C). The error bars indicate SD. Data were analyzed using a one-way ANOVA and Tukey’s multiple comparison test. *P < 0.05; **P < 0.005; ***P < 0.001; ****P < 0.0001; ns, not significant. Data are from three independent experiments.
We also analyzed caspase activation using Fluorescent Labeled Inhibitors of Caspases (FLICA). MBCs were recovered from culture and analyzed by flow cytometry to determine the proportion of early apoptotic cells (FLICA+ 7AAD−), late apoptotic cells (FLICA+ 7AAD+), and necrotic cells (FLICA− 7AAD+). For IgG+ MBCs little apoptosis was detected under any conditions (Fig. 5 C and D). We started detecting early apoptotic cells at 3 h in IgM+ MBCs stimulated with high-affinity antigens even though the response was not statistically significant (SI Appendix, Fig. S10A). In contrast, few late apoptotic cells were detected among IgM+ MBCs even at 24 h (SI Appendix, Fig. S10 B and C) that might reflect the rapid induction of early apoptosis or an inefficient progression from early to late apoptosis that could be due to a variety of factors including inadequate ATP production (49) or oxidative stress (50). We also determined that IgM+ MBCs responding to high-affinity antigen in the presence of Th media also expressed the highest levels of genes associated with apoptotic mitochondrial changes and regulation of apoptotic signaling pathways (SI Appendix, Fig. S8D). Of interest, the expression of the anti-apoptotic genes, BCL2 and BCL2L1 (BCL-XL) (51) was lower in IgM+ MBCs and higher in IgG+ MBCs in response to high-affinity antigen relative to low-affinity antigen. The expression of the pro-apoptotic gene, BAX was the opposite, higher in IgM MBCs and lower in IgG MBCs in response to high-affinity antigen, especially in the presence of TH media (SI Appendix, Fig. S8A). Taken together, these observations support the conclusion that the fate of IgM+ MBCs stimulated with high-affinity antigens is apoptosis. However, because the data are exclusively from studies in vitro in which case B cell apoptosis can be altered by the absence of a variety of factors including BAFF (52) and spontaneous GPR174-dependent activation (53), it will be important to verify the finding in vivo.
Discussion
Here, we explored the antigen-affinity dependence of responses of human IgM+ MBCs and IgG+ MBCs to secondary challenge. Protective immunity induced by primary responses to pathogen-targeted vaccines or pathogen infection relies on the function of both LLPC that secrete highly selected antibodies specific for the pathogen and pathogen-specific IgG+ MBCs and IgM+ MBCs. IgG+ MBCs in mouse models and in humans have been shown to have acquired large numbers of SHMs and also appear to have been highly selected by antigen resulting in a narrow pathogen-specific BCR repertoire (10, 22, 23). In contrast, IgM+ MBCs have been shown to accumulate fewer SHMs and appear to have been less selected by antigen and thus maintain a broad antigen-specific BCR repertoire. Reinfection by the homologous or a highly related pathogen should be controlled by the circulating LLPC antibodies. However, if the pathogen breaches this antibody wall (due to the antibodies being of insufficient affinity or magnitude for effective neutralization, for example) the pathogen will next be confronted by IgG+ MBCs that can be stimulated to differentiate into plasma cells providing a boosted response to the pathogen. However, for pathogens that have accumulated mutations that allow escape from both the LLPC wall but also IgG+ MBCs, the response of IgM+ MBCs specific for the mutant pathogen becomes critical for protection.
Presumably, IgM+ MBCs are able to respond to mutant pathogens that IgG+ MBCs cannot because they maintain a broad, relatively unselected B cell repertoire specific for the primary pathogen. This raises the question: Is the BCR repertoire the only difference between IgG+ MBCs and IgM+ MBCs and are they otherwise equivalent in their ability to discern the affinity of their BCRs for a pathogen? Clearly, the affinity of the BCR for antigen is a key determinant of the responses of MBCs to antigen challenge. However, we recently provided evidence that in addition to the affinity of the BCR itself for antigen, B cells at different differentiated states have different intrinsic affinity thresholds for antigen activation (31, 54). We found that in addition to the antigen affinity of B cells, activation by antigen binding to the BCR is controlled by an inherent affinity threshold of the B cells. We first described differences in intrinsic antigen-affinity thresholds of human tonsil naive B cells and GC B cells in response to membrane-associated antigens. We determined that naive B cells had low intrinsic affinity thresholds for antigen allowing responses to both low-affinity (KD = 4 x 10−7) as well as to high-affinity antigens (KD = 2 x 10−9). In contrast, GC B cells responded only to high- but not to low-affinity antigens. We subsequently observed similar differences in intrinsic antigen-affinity thresholds in a B cell lineage expanded in chronic infectious diseases and in autoimmunity, termed tissue-like MBCs or ABCs (32). We determined that the intrinsic affinity threshold for activation of ABC subpopulations was associated with the isotype of the expressed BCRs. IgD+ IgM+ ABCs had low intrinsic antigen affinity thresholds, whereas IgG+ ABCs and IgMlow IgD+ MBCs had high intrinsic antigen-affinity thresholds.
Here, we confirmed the correlation of isotype expression with intrinsic antigen affinity thresholds for six different human MBC subpopulations present in PB and in tonsils. We showed that MBC subpopulations expressing IgG+ BCRs or IgMlow IgD+ BCRs had high-affinity thresholds for activation, whereas IgM+ MBCs had low-affinity thresholds. In addition, we demonstrated that the intrinsic antigen-affinity thresholds in IgM+ MBCs versus IgG+ MBCs control antigen-driven MBC fates upon secondary antigen challenge in vitro. IgG+ MBCs responded only to high-affinity antigen challenge and differentiate toward PCs in contrast to IgM+ MBCs that responded to low-affinity antigens by differentiating toward GC B cells but underwent apoptosis in response to high-affinity antigens.
Our data predict that in secondary antigen challenge, IgG+ MBCs would only be capable of responding to the homologous or closely related pathogens for which the IgG+ MBCs were selected and would rapidly produce PCs secreting antibodies to control the homologous challenge infection. Critically, if the secondary infection was by pathogens that have mutated sufficiently to escape both the circulating LLPC-produced antibodies and IgG+ MBC high-affinity recognition, IgM+ MBCs, with even low affinity for the variant pathogen would be able to respond and differentiate toward a GC B cell fate and undergo selection for variant-specific LLPC or MBCs in GCs. However, IgM+ MBCs with high affinity for either the homologous or closely related pathogens would presumably be redundant with high-affinity IgG+ MBCs and of little use in the control of the challenging pathogen and would thus be eliminated by apoptosis following high-affinity pathogen antigen binding.
These findings raise a variety of questions the answers to which may be critical to our ability to develop effective long-lived protective vaccines. A key question is what cellular mechanisms underlie the observed intrinsic antigen-affinity thresholds for activation. Our earlier studies suggested that the intrinsic affinity thresholds of naive B cells and GC B cells were “hard wired” and associated with dramatically different cellular structures (31). GC B cells bound antigen through highly dynamic actin-and ezrin-rich pod-like structures in which BCRs were concentrated. For GC B cells low-affinity antigens triggered continuous BCR engagement and disengagement of the antigen, whereas high-affinity antigens induced stable IS formation. In contrast, naive B cells and MBCs immediately formed stable flat contact areas with both low- and high-affinity containing membranes. We observed differences in intrinsic affinity thresholds for both BCR signaling as well as for antigen gathering for antigen processing and presentation to TH cells such that the intrinsic affinity thresholds would affect B cell signaling for activation directly as well as the ability to compete for T cell help. The regulation of cell architecture in B cells is likely to be mediated by the BCR (55) and may be attributable to inherent differences in the signaling capacity of IgM+ and IgG+ BCRs or by the strength of the BCR’s pulling force that is necessary to succeed in antigen internalization. Clearly, a better understanding of the mechanisms at play in setting affinity thresholds for B cells may provide targets for the regulation of affinity thresholds in vaccine design.
Another central question is what “quality” of the primary antigen challenge determines the generating of IgG+ MBCs versus IgM+ MBCs. The relationship between class switch recombination and entry into GCs as recently reported (56) will also be of interest in relationship to the generation of IgM+ versus IgG+ MBCs. In our studies, we assessed the affinity thresholds and fates of human IgG+ and IgM+ MBCs responding to surrogate antigens. Thus, we do not know the “antigen history” of these MBCs. In our studies, PBMCs and tonsils from most donors contained equal numbers of IgM+ MBCs and IgG+ MBCs, but it is possible that infection or vaccination favors antigen-specific MBCs of one isotype over the other. It is also possible that MBCs continue to undergo SHM, isotype switching and differentiation in the periphery or secondary lymphoid organs after exiting the GCs in response to environmental conditions such as inflammation or persistent antigen exposure. A recent study in mice provided evidence that differentiation of naive B cells into GC B cells is dependent on the affinity and titer of LLPC produced circulating antibodies induced in the primary response (30). Thus, it is possible that there is communication between LLPC produced antibodies and MBC differentiation and isotype expression. To address such questions, it will be necessary to analyze antigen-specific naive B cells, MBCs, and circulating antibodies in individuals with time following primary vaccination or infection and challenge. Studies, of individuals vaccinated to COVID19 or infected by COVID have provided valuable information about the generation of MBCs (57–59). Given the relatively short time of protection that results from COVID vaccination or infection, future studies of highly effective vaccines that induce long-lived protection will be of interest. Understanding the drivers of IgM+ MBCs versus IgG+ MBCs could provide insights into the development of effective vaccines and targets for therapies for autoimmune disease in which antigen affinity threshold for activation appears to be set low.
Materials and Methods
Study Participants.
PBMCs were obtained from healthy individuals enrolled at the NIH. Informed consent was obtained from all participants. Fresh human tonsils were obtained from the pathology department of the Children's National Medical Center in Washington, DC, following routine tonsillectomies from children. Use of these tonsils for this study was determined to be exempt from review by the NIH Institutional Review Board in accordance with the guidelines issued by the Office of Human Research Protections and were exempted from review.
Isolation of B Cell Subpopulations.
B cell subpopulations were obtained from PBMCs and tonsils by flow cytometry as described (SI Appendix, Figs. S1 and S3). Details of antibodies used are provided in SI Appendix, Table S1.
Preparation of Antigen-Bound PLB.
The proportion of high-affinity or low-affinity PLBs was prepared as previously described (31, 55) and detailed in SI Appendix, SI Materials and Methods.
BCR Signaling Assay Using the TIRF Microscope.
B cells fluorescently labeled with a variety of BCR and signaling-specific antibodies were activated and analyzed by TIRF microscopy (SI Appendix, SI Materials and Methods).
In Vitro Stimulation of B Cell Subsets, Flow Cytometry, and Reverse Transcription and qRT-PCR.
Sorted B cells (2 × 105 to 5 × 105) were incubated in complete RPMI at 37 °C with 5% CO2 for 24 or 72 h in chambers without any stimulations, with BCR stimulation (high-affinity or low-affinity PLB) or with BCR stimulation (high-affinity or low-affinity PLB) in the presence of IL-4 (25 ng/mL, BioLegend), IL-21 (100 ng/mL, BioLegend), and anti-CD40 antibodies (1 μg/mL, BioLegend) to mimic T cell help (TH media). Following incubation, cells were either analyzed by flow cytometry or lysed for RNA extraction and analyzed by qRT-PCR for mRNA expression as detailed in SI Appendix, SI Materials and Methods.
RNA seq.
RNA sequencing was carried out as described previously (32). Raw counts were converted into an Expression set using the R package Biobase (60). Data were batch corrected with Combat_seq from the sva package in R (61). Differential gene expression analysis was done using the DESeq2 package (62). GSEA was run with a custom function based on the fgseaMultilevel function from the fgsea package (https://github.com/TranLab/ModuleLists) that includes MSigDB gene collections as well as blood transcription modules (63) and other gene sets relevant to immunology and blood transcriptomics (64). Heatmaps of gene expression were generated using the ComplexHeatmap package (65).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases. We specially thank Dr. Ludmila Krymskaya in the Laboratory of Immunogenetics, Flow Cytometry facility for the isolation of B cell subsets by fluorescence-activated cell sorting.
Author contributions
A.A.A. and S.K.P. designed research; A.A.A., P.H., H.S., and R.G. performed research; P.H. and T.M.T. contributed new reagents/analytic tools; A.A.A., P.H., H.S., and R.G. analyzed data; and A.A.A., P.H., and S.K.P. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
RNAseq data have been deposited in Gene Expression Omnibus (GSE215788) (66). All other data are included in the manuscript and/or SI Appendix.
Supporting Information
References
- 1.Roltgen K., et al. , Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 185, 1025–1040.e14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akkaya M., Kwak K., Pierce S. K., B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 20, 229–238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurosaki T., Kometani K., Ise W., Memory B cells. Nat. Rev. Immunol. 15, 149–159 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Inoue T., Kurosaki T., Memory B cells. Nat. Rev. Immunol. 24, 5–17 (2023). [DOI] [PubMed] [Google Scholar]
- 5.Weisel F., Shlomchik M., Memory B cells of mice and humans. Annu. Rev. Immunol. 35, 255–284 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Victora G. D., Nussenzweig M. C., Germinal centers. Annu. Rev. Immunol. 40, 413–442 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Young C., Brink R., The unique biology of germinal center B cells. Immunity 54, 1652–1664 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Medetgul-Ernar K., Davis M. M., Standing on the shoulders of mice. Immunity 55, 1343–1353 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulendran B., Davis M. M., The science and medicine of human immunology. Science 369, eaay4014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisel F. J., Zuccarino-Catania G. V., Chikina M., Shlomchik M. J., A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 44, 116–130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortnick A., et al. , Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J. Immunol. 188, 5389–5396 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manz R. A., Lohning M., Cassese G., Thiel A., Radbruch A., Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10, 1703–1711 (1998). [DOI] [PubMed] [Google Scholar]
- 13.Cyster J. G., Allen C. D. C., B cell responses: Cell interaction dynamics and decisions. Cell 177, 524–540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavinder J. J., et al. , Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc. Natl. Acad. Sci. U.S.A. 111, 2259–2264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purtha W. E., Tedder T. F., Johnson S., Bhattacharya D., Diamond M. S., Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 208, 2599–2606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgarth N., How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth. Immunol. Rev. 255, 82–94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong R., Bhattacharya D., Basics of memory B-cell responses: Lessons from and for the real world. Immunology 156, 120–129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berard M., et al. , Activation sensitizes human memory B cells to B-cell receptor-induced apoptosis. Immunology 98, 47–54 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viant C., et al. , Antibody affinity shapes the choice between memory and germinal center B cell fates. Cell 183, 1298–1311.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leach S., et al. , Requirement for memory B-cell activation in protection from heterologous influenza virus reinfection. Int. Immunol. 31, 771–779 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Wong R., et al. , Affinity-restricted memory B cells dominate recall responses to heterologous flaviviruses. Immunity 53, 1078–1094.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor J. J., Pape K. A., Jenkins M. K., A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J. Exp. Med. 209, 597–606 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaji T., et al. , Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J. Exp. Med. 209, 2079–2097 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogan I., et al. , Multiple layers of B cell memory with different effector functions. Nat. Immunol. 10, 1292–1299 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Pape K. A., Taylor J. J., Maul R. W., Gearhart P. J., Jenkins M. K., Different B cell populations mediate early and late memory during an endogenous immune response. Science 331, 1203–1207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuccarino-Catania G. V., et al. , CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 15, 631–637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert M., et al. , Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc. Natl. Acad. Sci. U.S.A. 112, E546–E555 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King H. W., et al. , Single-cell analysis of human B cell maturation predicts how antibody class switching shapes selection dynamics. Sci. Immunol. 6, eabe6291 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Mesin L., et al. , Restricted clonality and limited germinal center reentry characterize memory B cell reactivation by boosting. Cell 180, 92–106.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tas J. M. J., et al. , Antibodies from primary humoral responses modulate recruitment of naive B cells during secondary responses. Immunity 55, 1856–1871.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak K., et al. , Intrinsic properties of human germinal center B cells set antigen affinity thresholds. Sci. Immunol. 3, eaau6598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holla P., et al. , Shared transcriptional profiles of atypical B cells suggest common drivers of expansion and function in malaria, HIV, and autoimmunity. Sci. Adv. 7, eabg8384 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass D. R., et al. , An integrated multi-omic single-cell atlas of human B cell identity. Immunity 53, 217–232.e15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holla P., Ambegaonkar A., Sohn H., Pierce S. K., Exhaustion may not be in the human B cell vocabulary, at least not in malaria. Immunol. Rev. 292, 139–148 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Pierce S. K., Liu W., The tipping points in the initiation of B cell signalling: How small changes make big differences. Nat. Rev. Immunol. 10, 767–777 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W., Meckel T., Tolar P., Sohn H. W., Pierce S. K., Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity 32, 778–789 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W., Meckel T., Tolar P., Sohn H. W., Pierce S. K., Antigen affinity discrimination is an intrinsic function of the B cell receptor. J. Exp. Med. 207, 1095–1111 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogeboom R., Tolar P., Molecular mechanisms of B cell antigen gathering and endocytosis. Curr. Top. Microbiol. Immunol. 393, 45–63 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Crotty S., A brief history of T cell help to B cells. Nat. Rev. Immunol. 15, 185–189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corcoran L. M., Tarlinton D. M., Regulation of germinal center responses, memory B cells and plasma cell formation-an update. Curr. Opin. Immunol. 39, 59–67 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Ochiai K., et al. , Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 38, 918–929 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMahon S. B., MYC and the control of apoptosis. Cold Spring Harb. Perspect. Med. 4, a014407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villa M., et al. , Aryl hydrocarbon receptor is required for optimal B-cell proliferation. EMBO J. 36, 116–128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosa D., et al. , Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc. Natl. Acad. Sci. U.S.A. 102, 18544–18549 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro-Shelef M., et al. , Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity 19, 607–620 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Fukuda T., et al. , Disruption of the Bcl6 gene results in an impaired germinal center formation. J. Exp. Med. 186, 439–448 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato Y., et al. , Multifaceted effects of antigen valency on B cell response composition and differentiation in vivo. Immunity 53, 548–563.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akkaya M., et al. , Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat. Immunol. 19, 871–884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyoshi N., Oubrahim H., Chock P. B., Stadtman E. R., Age-dependent cell death and the role of ATP in hydrogen peroxide-induced apoptosis and necrosis. Proc. Natl. Acad. Sci. U.S.A. 103, 1727–1731 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lingappan K., NF-kappaB in oxidative stress. Curr. Opin. Toxicol. 7, 81–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardwick J. M., Soane L., Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 5, a008722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cremasco V., et al. , B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol. 15, 973–981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf E. W., et al. , GPR174 signals via Galphas to control a CD86-containing gene expression program in B cells. Proc. Natl. Acad. Sci. U.S.A. 119, e2201794119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwak K., Akkaya M., Pierce S. K., B cell signaling in context. Nat. Immunol. 20, 963–969 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Tolar P., Cytoskeletal control of B cell responses to antigens. Nat. Rev. Immunol. 17, 621–634 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Roco J. A., et al. , Class-switch recombination occurs infrequently in germinal centers. Immunity 51, 337–350.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodda L. B., et al. , Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell 184, 169–183.e17 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokal A., et al. , mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity 54, 2893–2907.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goel R. R., et al. , Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci. Immunol. 6, eabi6950 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber W., et al. , Orchestrating high-throughput genomic analysis with bioconductor. Nat. Methods 12, 115–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson W. E., Li C., Rabinovic A., Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNAseq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S., et al. , Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat. Immunol. 15, 195–204 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Senkpeil L., et al. , Innate immune activation restricts priming and protective efficacy of the radiation-attenuated PfSPZ malaria vaccine. medRxiv [Preprint] (2021). 10.1101/2021.10.08.21264577 (Accessed 27 June 2022). [DOI] [PMC free article] [PubMed]
- 65.Gu Z., Eils R., Schlesner M., Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Pierce S. K., Ambegaonkar A. A., Holla P., Sohn H., Data from “Isotype switching in human memory B cells sets intrinsic antigen-affinity thresholds that dictate antigen-driven fates.” Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE215788. Deposited 14 October 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
RNAseq data have been deposited in Gene Expression Omnibus (GSE215788) (66). All other data are included in the manuscript and/or SI Appendix.