Significance
While evidence from several species supports a trend toward synaptic upscaling during wakefulness, little is known about whether this synaptic accumulation occurs similarly across many brain regions or cell types or whether it is restricted to particular hotspots of plasticity. Here, we quantified presynaptic Bruchpilot (BRP) abundance in 37 regions of the Drosophila melanogaster brain according to sleep history and neurotransmitter identity. We found that BRP increased in excitatory neurons after sleep loss while other cell types showed few changes. Excitatory upscaling occurred across the brain but at different rates in different transmitter systems. This suggests that sleep loss drives a global, dynamic imbalance between excitatory and inhibitory synapses which may contribute to the myriad cognitive consequences of sleep loss.
Keywords: sleep, plasticity, Drosophila
Abstract
Sleep is an evolutionarily conserved state that supports brain functions, including synaptic plasticity, in species across the animal kingdom. Here, we examine the neuroanatomical and cell-type distribution of presynaptic scaling in the fly brain after sleep loss. We previously found that sleep loss drives accumulation of the active zone scaffolding protein Bruchpilot (BRP) within cholinergic Kenyon cells of the Drosophila melanogaster mushroom body (MB), but not in other classes of MB neurons. To test whether similar cell type–specific trends in plasticity occur broadly across the brain, we used a flp-based genetic reporter to label presynaptic BRP in cholinergic, dopaminergic, GABAergic, or glutamatergic neurons. We then collected whole-brain confocal image stacks of BRP intensity to systematically quantify BRP, a marker of presynapse abundance, across 37 neuropil regions of the central fly brain. Our results indicate that sleep loss, either by overnight (12-h) mechanical stimulation or chronic sleep disruption in insomniac mutants, broadly elevates cholinergic synapse abundance across the brain, while synapse abundance in neurons that produce other neurotransmitters undergoes weaker, if any, changes. Extending sleep deprivation to 24 h drives brain-wide upscaling in glutamatergic, but not other, synapses. Finally, overnight male–male social pairings induce increased BRP in excitatory synapses despite male–female pairings eliciting more waking activity, suggesting experience-specific plasticity. Within neurotransmitter class and waking context, BRP changes are similar across the 37 neuropil domains, indicating that similar synaptic scaling rules may apply across the brain during acute sleep loss and that sleep need may broadly alter excitatory–inhibitory balance in the central brain.
Many lines of evidence support a role for sleep in synaptic plasticity (1–3). Sleep is elevated during periods of heightened synaptic organization, such as during early development (4–6), recovery from neural injury (7, 8), and memory consolidation (9, 10). While several studies have found markers of elevated synaptic abundance across whole brains or tissue homogenates following prolonged waking (11–13), other studies have reported synaptic potentiation during sleep (14–16). It is likely that sleep has varying effects on plasticity, depending on developmental stage (4, 17, 18), waking experience (10, 19–23), and cell type (24–26). Our recent studies found that overnight sleep deprivation (SD) leads to diverse patterns of synaptic scaling in the Drosophila mushroom bodies (MB) depending on cell type (26). We found that cholinergic memory-encoding Kenyon cells (KCs), but not other MB cell types, exhibit increased abundance of the presynaptic protein Bruchpilot (BRP) throughout the axonal lobes following SD (26). Complementary studies have found that sleep deprivation enhances cholinergic signaling onto GABAergic interneurons in learning/memory-related circuits, which likely increases inhibition onto memory-encoding cells (24). However, it remains unclear whether the cell type–specific trends in plasticity seen in the MB can be generalized to other neuropil regions. While previous work has described plastic changes with sleep loss in discrete brain regions or cell types (14–16, 24–28), it is unclear whether similar trends in scaling can be generalized across many regions or, alternatively, whether synaptic scaling during sleep deprivation is restricted to localized foci.
To address this issue, genetic tools available in Drosophila allow us to visualize changes in synapse abundance in each neuropil region throughout the central brain. Because SD can differentially affect excitatory vs. inhibitory synapses (12, 16, 24, 25), here we quantify the effects of sleep loss on abundance of BRP of different neurotransmitter types across neuropil regions of the central brain. Using cell type–specific genetic drivers to label endogenous BRP, we find that cholinergic neurons exhibit a net increase in synaptic abundance across each neuropil region in the central brain and that glutamatergic neurons show increased BRP across the brain after 24 h of sleep deprivation. Both of these cell types demonstrate BRP elevations after overnight male–male social exposure relative to male–female pairings, even though mixed-sex socializing results in a more pronounced increase in activity. These results support our previous findings in the MB that cholinergic neurons are particularly sensitive to sleep loss and that different cell types across the central brain can be differentially affected by SD.
Results
To examine the consequences of sleep loss on synaptic abundance throughout the central brain, we used synaptic tagging with recombination (STaR), a flp-based reporter to specifically label the presynaptic protein BRP in genetically defined neuronal populations (29, 30). BRP is a core component of presynaptic active zones (31, 32), and presynaptic BRP protein levels correlate closely with active zone size and release probability (33–35). Using STaR, we were able to use targeted Gal4 genetic drivers to label presynaptic terminals of neurons that produce one of four distinct neurotransmitter signals (schematized in SI Appendix, Fig. S1): acetylcholine [Chat2A-Gal4 (36)], dopamine [TH-Gal4 (37)], glutamate [VGlut-Gal4 (38)], and GABA [Gad1-Gal4 (39)]. Sleep in each genotype was monitored for 2 to 4 baseline days, then flies were either mechanically deprived of sleep for 12 h overnight or allowed ad libitum rest (SI Appendix, Fig. S2 A–D). The next morning, we dissected brains from individuals in each group, stained for STaR-labeled BRP (BRP::V5), and acquired confocal stacks covering the whole central brain. To quantitatively compare BRP::V5 intensity across brains, we registered each brain image to a common template (40–42). In cholinergic neurons labeled by the genetic driver Chat2A-Gal4, brain-wide BRP::V5 mean intensity increased by 27% after a night of sleep loss compared to rested controls (Fig. 1 A and A′ and Movie S1). Next, we quantified BRP::V5 intensity in dopaminergic neurons labeled by TH-Gal4 (Fig. 1B and Movie S2). BRP::V5 intensity was largely unaffected by sleep loss, showing a 2% decrease in intensity after SD. Glutamatergic neurons labeled by VGlut-Gal4 (Fig. 1C and Movie S3) were similarly unaffected by SD, with a 4% decrease in BRP::V5 intensity in sleep-deprived animals compared to controls. Finally, following sleep deprivation, GABAergic neurons labeled by Gad1-Gal4 (Fig. 1D and Movie S4) exhibited a 9% increase in BRP::V5 intensity. Due to the large numbers of pixels in our analyses and their non-normal distribution of intensity values, we used Kolmogorov–Smirnov tests (K–S tests) to compare the cumulative distributions of pixel intensities of rested and sleep-deprived brains within each neurotransmitter class (Fig. 1 A′–D′). K–S tests comparing the effect of sleep loss on pixel intensity of labeled BRP found a higher D value for cholinergic neuron BRP (D = 0.134; Fig. 1A′) than for the other neurotransmitters we tested, indicating that the most significant effect of sleep loss occurred in ChAT-positive neurons. These data indicate that overnight sleep deprivation results in the strongest brain-wide presynaptic expansion in cholinergic neurons. We detected only modest changes in dopaminergic, glutamatergic, and GABAergic neurons across the brain while our regional analysis found a single area with significant GABAergic scaling and none with detectable changes in dopaminergic or glutamatergic BRP plasticity.
Fig. 1.
Overnight sleep deprivation increases overall brain-wide BRP abundance in cholinergic neurons, but not in dopaminergic, glutamatergic, or GABAergic neurons. (A) Average brains for control (Left) and sleep-deprived (Right) Chat2A-Gal4>STaR flies. (A′) Cumulative distribution plots of total pixel intensities of Chat2A-Gal4>STaR brains for control (gray) and sleep-deprived (maroon) flies. The Kolmogorov–Smirnov test finds D = 0.134, n = 4.63 × 108 to 5.03 × 108 pixels/group. (B) Average brains for control (Left) and sleep-deprived (Right) TH-Gal4>STaR flies. (B′) Cumulative distribution plots of total pixel intensities of TH-Gal4>STaR brains for control (gray) and sleep-deprived (orange) flies. The Kolmogorov–Smirnov test finds D = 0.047, n = 8.25 × 108 to 8.65 × 108 pixels/group. (C) Average brains for control (Left) and sleep-deprived (Right) VGlut-Gal4>STaR flies. (C′) Cumulative distribution plots of total pixel intensities of VGlut-Gal4>STaR brains for control (gray) and sleep-deprived (green) flies. The Kolmogorov–Smirnov test finds D = 0.029, n = 7.85 × 108 to 8.85 × 108 pixels/group. (D) Average brains for control (Left) and sleep-deprived (Right) Gad1-Gal4>STaR flies. (D′) Cumulative distribution plots of total pixel intensities of Gad1-Gal4>STaR brains for control (gray) and sleep-deprived (blue) flies. The Kolmogorov–Smirnov test finds D = 0.056, n = 8.05 × 108 to 8.65 × 108 pixels/group. For panels (A′–D′), dotted lines represent group mean pixel intensity values, and box plots depict quartile ranges with group mean. See also SI Appendix, Fig. S2 A–D for sleep traces and Movies S1–S4 for z-stack animations from experimental groups in (A–D).
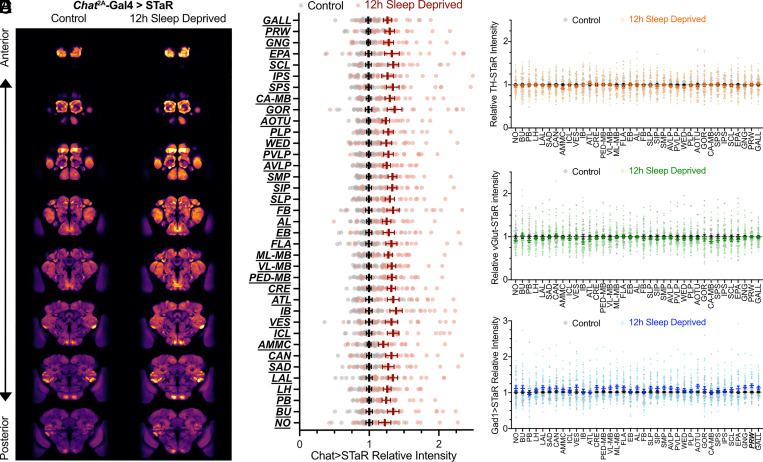
While analysis of pixel intensity distributions found that BRP increased after sleep loss most strongly in cholinergic neurons, this bulk analysis does not assess local trends within individual neuropil regions. To test for region-specific trends in BRP scaling, we used a standard Drosophila brain image template to map pixel locations in our registered images to 37 distinct regions of the central fly brain (42, 43). This enabled us to quantify the effect of sleep loss on BRP abundance in each of the distinct regions. Following overnight SD, STaR labeling in cholinergic neurons using Chat2A-Gal4 increased significantly in each individual neuropil region by ~21 to 39% (Fig. 2 A and B). Regional BRP::V5 intensity was largely unaffected by sleep loss in dopaminergic neurons, with no significant regional changes in intensity (Fig. 2C). Similarly, there were no significant changes in regional BRP::V5 intensity in glutamatergic neurons (Fig. 2D). In GABAergic neurons, we observed a significant increase (~18%) in BRP::V5 within the prow (PRW), but no significant change in other regions following a night of sleep loss (Fig. 2E). The prow is a sparsely studied neuropil region that receives input from gustatory receptor neurons (GRNs) (44). Together, these results suggest that sleep deprivation nearly uniformly affects synapse abundance throughout the entire brain within a neurotransmitter class. However, future region-specific analyses may reveal more nuanced cell type–specific trends in synaptic scaling, as we previously observed in the MB (26).
Fig. 2.
Neuropil regions within each neurotransmitter group are similarly affected by sleep loss. (A) Sections along the z-axis through average brains for control (Left) and sleep-deprived (Right) Chat2A-Gal4>STaR flies. (B) Regional analysis of BRP intensity Chat2A-Gal4>STaR flies after 12 h of overnight sleep loss (maroon) normalized to the average BRP pixel intensity of rested controls (gray). Two-way ANOVA finds a significant effect of SD [F(1,46) = 19.98, P < 0.0001, n = 24 to 25 brains/group]. Pairwise comparisons using Sidak’s multiple comparisons test found significant increases in BRP::V5 intensity in each neuropil region after sleep deprivation relative to rested siblings (P < 0.05 for each test). (C) Regional analysis of BRP intensity TH-Gal4>STaR flies after 12 h of overnight sleep loss (orange) normalized to rested controls (gray). Two-way ANOVA finds no significant effect of SD [F(1,80) = 0.2679, P = 0.6061, n = 41 brains/group]. (D) Regional analysis of BRP intensity VGlut-Gal4>STaR flies after 12 h of overnight sleep loss (green) normalized to rested controls (gray). Mixed-effects analysis finds no significant main effect of SD [F(1,81) = 0.9435, P = 0.3343, n = 36 to 44 flies/group]. Pairwise comparisons using Sidak’s multiple comparisons test found no significant changes in BRP::V5 intensity in each neuropil region after sleep deprivation relative to rested siblings (P > 0.805 for each test). (E) Regional analysis of BRP intensity Gad1-Gal4>STaR flies after 12 h of overnight sleep loss (blue) normalized to rested controls (gray). Two-way ANOVA finds a significant group-by-region interaction [F(36,2875) = 2.713, P < 0.001, n = 40 to 43 brains/group]. Pairwise comparisons using Sidak’s multiple comparisons test found a significant increase in BRP::V5 intensity in the prow after sleep deprivation relative to rested siblings (P = 0.030), but no significant changes in BRP::V5 intensity in other neuropil regions (P > 0.2199 for each test). For (B–E), circles show individual data points; data are normalized to the average BRP pixel intensity of rested controls. Group averages and error bars depict means ± SEM. Pairwise significance is denoted by bolded and underlined region labels.
Because BRP accumulates proportionally with duration of sleep deprivation (11), we examined whether a longer sleep deprivation period was sufficient to increase BRP abundance in cell types other than cholinergic cells, i.e., those that did not show large responses after 12-h overnight sleep deprivation. We found that 24 h of sleep loss (SI Appendix, Fig. S2 E–G) resulted in little overall BRP::V5 scaling within dopaminergic (decreased ~6.5%) (Fig. 3 A and B and Movie S5) and GABAergic neurons (decreased ~3.7%) (Fig. 3 E and F and Movie S7), but glutamatergic neurons showed a BRP::V5 increase of ~32.4% across the whole brain along with significant increases in nearly every local neuropil region (Fig. 3 C and D and Movie S6). The increased BRP within glutamatergic neurons after 24 h, but not 12 h, of sleep loss suggests that synaptic scaling may not accumulate at the same rate in different neurotransmitter systems across waking time. Instead, BRP may increase in progressive phases in different neurotransmitter systems.
Fig. 3.
Twenty-four hours of sleep loss results in increased BRP across the brain in glutamatergic neurons. (A) Mean confocal projections for control (Left) and 24-h sleep-deprived (Right) TH-Gal4>STaR flies. (B) Cumulative distribution plot of relative TH-Gal4>STaR intensity (Left), and regional mean TH-Gal4>STaR intensities (Right) for control (gray) and 24 h SD (orange) flies. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.030, n = 3.63 × 108 pixels/group. Mixed-effects analysis finds a significant condition-by-region interaction [F(36,1565) = 2.099, P = 0.0002, n = 23 brains/group]. (C) Mean confocal projections for control (Left) and 24-h sleep-deprived (Right) vGlut-Gal4>STaR flies. (D) Cumulative distribution plot of relative vGlut-Gal4>STaR intensity (Left) and regional mean vGlut-Gal4>STaR intensities (Right) for control (gray) and 24-h SD (green) flies. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.0179, n = 3.82 × 108 pixels/group. Mixed-effects analysis finds a significant condition-by-region interaction [F(36,1275) = 1.509, P = 0.0299, n = 19 brains/group). (E) Mean confocal projections for control (Left) and 24-h sleep-deprived (Right) Gad1-Gal4>STaR flies. (F) Cumulative distribution plot of relative Gad1-Gal4>STaR intensity (Left) and regional mean Gad1-Gal4>STaR intensities (Right) for control (gray) and 24-h SD (blue) flies. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.0206, n = 3.82 × 108 pixels/group. Mixed-effects analysis finds no significant effect of condition [F(1,36) = 1.716, P = 0.1985, n = 19 brains/group]. For Left panels in (B, D, and F), dotted lines represent group mean pixel intensity values, and box plots depict quartile ranges with group mean. For regional quantifications in (B, D, and F), circles show individual data points; data are normalized to the average BRP pixel intensity of rested controls. Group averages and error bars depict means ± SEM. Pairwise significance is denoted by bolded and underlined region labels. See also SI Appendix, Fig. S2 E and F for sleep traces and Movies S5–S7 for z-stack animations from experimental groups.
Building from our results on overnight sleep deprivation, we tested the effect of chronic sleep disruption on brain-wide synaptic scaling by measuring BRP::V5 abundance in short-sleeping insomniac mutant male flies (inc2/y) (45, 46) (SI Appendix, Fig. S3 A–D). We observed similar effects of inc2 mutations on brain-wide changes of BRP::V5 in different neurotransmitter types; cholinergic neurons show an overall increase of 8% (Fig. 4A and Movie S8) while dopaminergic, and glutamatergic cells exhibit smaller magnitudes of BRP::V5 scaling (Fig. 4 B and C and Movies S9 and S10). Labeling of GABAergic presynapses using Gad1-Gal4 exhibited reduced signal in inc2 mutants by ~9% compared to genetic controls (Fig. 4D and Movie S11). Next, we examined the region-by-region effects of short-sleeping inc mutations on BRP scaling. As previously reported (47), a majority of brains from inc2/y mutants had missing or abnormal MB lobes (Fig. 4E). These defects were especially visible in inc2 mutants expressing STaR in cholinergic neurons (inc2/y; UAS-flp, brp-FRT-stop-FRT-V5-LexA/+; Chat2A-Gal4/+). While this variability in the MB structure of inc mutants is interesting, we have decided to exclude the MB lobes from our segmented analysis of regional BRP scaling due to the variability of anatomical defects. Regional segmentation of BRP abundance found that increases in STaR signal in cholinergic cell types were more spatially localized than after overnight sleep deprivation with 10 of 35 regions showing a significant increase in tagged BRP (Fig. 5 A and B). Like our 12-h mechanical sleep deprivation results, we detected no significant effect of inc mutations on regional BRP::V5 in glutamatergic or dopaminergic neurons (Fig. 5 C and D). After finding a net decrease in BRP::V5 brain-wide using Gad1-Gal4>STaR in insomniac flies compared to controls, regional neuropil analysis identified a significant BRP loss in only the gall of inc2 mutants (Fig. 5E).
Fig. 4.
Effects of insomniac2 mutation on brain-wide BRP abundance across neurotransmitter identities. (A) Projection of mean Chat2A-Gal4>STaR signal in wild-type (Left) and inc2 mutants (right). (A′) Cumulative distribution plots of pixel intensities from Chat2A-Gal4>STaR brains for control (gray) and inc2 mutant (maroon) flies. The Kolmogorov–Smirnov test finds D = 0.0416, n = 4.02 × 108 pixels/group. (B) Mean TH-Gal4>STaR intensity in wild-type (Left) and inc2 mutant (Right) brains. (B′) Cumulative distribution plot of pixel intensities for TH-Gal4>STaR signal from wild-type (gray) and inc2 mutant (orange) flies. The Kolmogorov–Smirnov test finds D = 0.02719, n = 5.03 × 108 to 5.43 × 108 pixels/group. (C) Average VGlut2A-Gal4>STaR intensity from wild-type (Left) and inc2 (Right) brains. (C′) Cumulative distribution plot of pixel intensities for VGlut2A-Gal4>STaR signal from wild-type (gray) and inc2 mutant (green) flies. The Kolmogorov–Smirnov test finds D = 0.0131, n = 6.64 × 108 to 7.45 × 108 pixels/group. (D) Average Gad1-Gal4>STaR signal intensity in wild-type (Left) and inc2 mutant (Right) brains. (D′) Cumulative distribution of voxel intensities for Gad1-Gal4>STaR signal from wild-type (gray) and inc2 mutant (blue) flies. The Kolmogorov–Smirnov test finds D = 0.08844, n = 6.84 × 108 to 8.04 × 108 pixels/group. (E and E′) Chat2A-Gal4>STaR signal reveals variable loss of Mushroom Body lobes in inc2 mutants with only 5/20 brains showing all intact MB lobes. Examples of inc2; Chat2A-Gal4>STaR brains with missing vertical lobes are shown in (E′). For panels (A′, B′, C′, and D′), dotted lines represent group mean pixel intensity values, and box plots depict quartile ranges with group mean. See also SI Appendix, Fig. S3 A–D for sleep traces and Movies S8–S11 for animations of mean registered z-stacks from experimental groups in (A–D).
Fig. 5.
Regional scaling of BRP across neurotransmitter systems in short-sleeping inc2 flies. (A) Montage of individual coronal slices showing group average Chat2A-Gal4>STaR signal in wild-type (Left) and insomniac2 mutant (Right) flies. (B) Mean Chat2A-Gal4>STaR signal across neuropil regions in wild-type (gray) and inc2 mutants (red), normalized to mean BRP pixel intensity from the wild-type control group. Two-way repeated-measures ANOVA finds a significant genotype-by-region interaction [F(34,1292)=5.609, P < 0.0001, n = 20 brains/group]. Bold and underlined labels on the vertical axis represent regions with a significant effect of genotype by post hoc Holm–Šídák's multiple comparisons test. (C) Average normalized TH-Gal4>STaR signal across neuropil regions in wild-type (gray) and inc2 mutants (orange). Mixed-effects analysis finds a significant genotype-by-region interaction [F(34,2443)=1.648, P < 0.0107, n = 33 to 40 brains/group]. (D) Mean relative VGlut2A-Gal4>STaR signal across neuropil regions in wild-type (gray) and inc2 mutants (green). Two-way repeated-measures ANOVA finds a significant genotype-by-region interaction [F(34,2312) = 5.084, P < 0.0001, n = 33 to 37 brains/group]. (E) Mean normalized Gad1-Gal4>STaR signal segmented by neuropil region in wild-type (gray) and inc2 (blue). Mixed-effects analysis finds a significant genotype-by-region interaction [F(34,2343) = 2.161, P < 0.0001, n = 31 to 40 brains/group]. For (B–E), circles show individual data points; data are normalized to the average BRP pixel intensity of wild-type controls. Group averages and error bars depict means ± SEM. Pairwise significance is denoted by bolded and underlined region labels.
To further explore whether synaptic scaling trends can be generalized across additional conditions that elicit sleep loss, we next tested the responses of male flies to overnight social pairings. While sleep loss is typically followed by a homeostatic recovery period of elevated sleep drive, a few external conditions can result in an acute decrease in sleep with no subsequent rebound. For example, Drosophila melanogaster who experience overnight courtship opportunities or food deprivation can suppress sleep for a night with little or no increase in recovery sleep afterward (20–22, 48). To test whether BRP scaling correlates most closely with recent sleep history, we dissected and imaged the brains of male flies after overnight social pairings either with another male fly (male–male pairings) or with a female fly (male–female pairings). Assessing these two social conditions allowed us to directly compare the combined locomotor activity of paired flies and to control for sensory and behavioral enrichment from social experience. Similar to previous reports (21, 48), we found that male–female pairings resulted in a significant loss of quiescence compared to male–male pairings, most likely due to high levels of male courtship (SI Appendix, Fig. S3 E–H). Interestingly, males that were paired with females showed an overall BRP::V5 reduction of ~12.7% in cholinergic (Fig. 6 A and B and Movie S12) and ~18.7% glutamatergic (Fig. 6 E and F and Movie S14) neurons compared to siblings that were housed in male–male tubes, with weaker decreases observed in BRP::V5 within dopaminergic neurons (~9.3%) (Fig. 6 C and D and Movie S13) and little change in BRP::V5 of GABAergic cells (decreased by ~1.1%) (Fig. 6 G and H and Movie S15). Regional segmentation found that the loss of BRP in cholinergic neurons was associated with significant decreases in 13 regions (Fig. 6 B, Right) while glutamatergic cells exhibited significant BRP::V5 loss in 22 regions after male–female pairings (Fig. 6 F, Right). Interestingly, while dopaminergic cells showed little BRP::V5 change with brain-wide analysis, housing in male–female pairs was associated with reduced BRP::V5 in four local regions (Fig. 6 D, Right). The differing effects of mechanical sleep deprivation and overnight male–female pairings on cholinergic and glutamatergic BRP scaling are consistent with the hypothesis that presynaptic scaling might correlate with acute sleep need/pressure and not necessarily with recent sleep history in some contexts (13).
Fig. 6.
BRP scaling after overnight social pairings. (A) Mean confocal projections of Chat2A-Gal4>STaR brains from male flies that were paired overnight with another male (Left) or with a female fly (Right). (B) Cumulative pixel intensity distribution (Left) and mean regional Chat2A-Gal4>STaR intensity (Right) after overnight male–male (gray) or male–female (red) social pairings. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.0845, n = 3.82 × 108 to 4.02 × 108 pixels/group. Mixed-effects analysis finds a significant condition-by-region interaction [F(36,1317) = 7.030, P < 0.0001, n = 19 to 20 brains/group]. (C) Mean confocal projections of TH-Gal4>STaR male brains following overnight social pairings. Brains from male–male groupings are shown on left, male–female on right. (D) Cumulative pixel distribution (Left) and mean regional intensity (Right) for TH-Gal4>STaR males after overnight male–male (gray) or male–female (orange) pairings. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.0625, n = 4.22 × 108 to 4.42 × 108 pixels/group. Mixed-effects analysis finds a significant main effect of condition [F(1,41) = 8.353, P = 0.0056, n = 21 to 22 brains/group]. (E) Mean confocal projections of vGlut-Gal4>STaR male brains after overnight male–male (Left) or male–female (Right) pairings. (F) Cumulative pixel distribution (Left) and mean regional intensity (Right) for vGlut-Gal4>STaR males after overnight male–male (gray) or male–female (green) pairings. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.1477, n = 3.02 × 108 to 3.62 × 108 pixels/group. Mixed-effects analysis finds a significant main effect of condition [F(1,31) = 16.33, P = 0.0003, n = 15 to 18 brains/group]. (G) Mean confocal projections of Gad1-Gal4>STaR male brains after overnight male–male (Left) or male–female (Right) pairings. (H) Cumulative pixel distribution (Left) and mean regional intensity (Right) for Gad1-Gal4>STaR males after overnight male–male (gray) or male–female (blue) pairings. The Kolmogorov–Smirnov test for cumulative distribution plot finds D = 0.0289, n = 3.62 × 108 to 3.82 × 108 pixels/group. Mixed-effects analysis finds no significant main effect of condition [F(1,35) = 0.005296, P = 0.9424, n = 18 to 19 brains/group]. For Left panels in (B, D, F, and H), dotted lines represent group mean pixel intensity values, and box plots depict quartile ranges with group mean. For regional quantifications in (B, D, F, and H), circles show individual data points; data are normalized to the average BRP pixel intensity of brains from male–male pairs. Group averages and error bars depict means ± SEM. Pairwise significance is denoted by bolded and underlined region labels. See also SI Appendix, Fig. S3 E–H for sleep traces and Movies S12–S15 for animations of mean registered z-stacks from experimental groups.
Discussion
While sleep’s basic functions remain poorly understood, several lines of evidence support a trend for synaptic upscaling across waking experiences (11, 13, 26, 49–51). This upscaling has been hypothesized to impact the capacity for future plasticity and memory (3), but previous studies did not systematically examine the brain-wide distribution of synaptic scaling or test whether particular classes of neurons contribute to synaptic upscaling across distinct brain regions. To address these gaps, we used a genetic reporter to quantify the effects of sleep deprivation on the presynaptic active zone marker BRP in different neurotransmitter cell types throughout the Drosophila central brain. BRP abundance in cholinergic neurons increases consistently throughout each neuropil region examined, whereas BRP abundance of dopaminergic, glutamatergic, and GABAergic cells remains largely unchanged following a night of sleep loss. These findings are consistent with our previous study, which found that cholinergic Kenyon cells, but not other MB cell types, exhibit increased synapse abundance after SD (26). Both this current and that previous study find a ~30% increase in BRP intensity in cholinergic neurons with overnight sleep deprivation. Although we saw little net change in BRP abundance in dopaminergic, glutamatergic, and GABAergic cells after 12 h of mechanically imposed sleep loss, extending sleep deprivation to 24 h elicited substantial, global upscaling in glutamatergic cells. This suggests that different neurotransmitter systems may contain distinct thresholds of plasticity. It is possible that smaller subpopulations of these cell types undergo synaptic scaling that we are unable to detect when examining large groups of neurons. While the consequences of sleep loss appear to elevate BRP in cholinergic, then glutamatergic neurons across the brain, it is not clear whether changes in glutamatergic neurons after 24 h of sleep loss represent plasticity that might offset cholinergic upscaling or marks synaptic changes that further degrade circuit function.
In contrast to the consistent, brain-wide increase of BRP in cholinergic neurons following overnight sleep deprivation or in glutamatergic neurons after 24 h of sleep deprivation, we observed more localized upscaling in cholinergic neurons of chronically short-sleeping inc mutants. It is possible that these structural differences could be influenced by the daily magnitude of sleep loss during these conditions, as flies harboring genomic inc lesions experience only partial sleep loss. In contrast, 24 h of near-total sleep deprivation causes globally uniform upscaling in glutamatergic circuits, echoing the regional similarities in cholinergic plasticity after 12 h of enforced wakefulness. Alternatively, chronic sleep disruption in inc mutants might lead to alternative mechanisms to contain synaptic scaling (52), or developmental changes in circuit wiring might influence the effects of sleep disruptions on plasticity (47). Because different contexts and durations of sleep loss can elicit varying behavioral or synaptic consequences (22, 26, 52, 53), whole-brain imaging strategies may present opportunities to explore whether differences in the distribution of synaptic scaling might correlate with subsequent behavioral impairments.
Results from overnight social pairings reveal that not all waking experiences are equally potent in their capacity to elicit synaptic restructuring. Surprisingly, 12 h of overnight male–female pairings increase nighttime waking but induce a net downscaling of cholinergic and glutamatergic BRP compared to brains from male–male pairings. Because previous studies found that male flies exhibit a significant sleep rebound after male–male but not male–female pairings (21), our findings are consistent with the hypothesis that broad increases in BRP may correlate with sleep need and modulate restorative sleep rebound (13, 54). These findings also indicate that specific behavioral experiences differentially drive synaptic scaling during waking. For instance, cholinergic circuitry mediates aggressive behaviors between males (55–57). Cholinergic synaptic potentiation after male–male pairing, enriched in neuropil regions associated with visual and olfactory processing, may thus result from elevated activity during aggressive behavior.
Interestingly, thermogenetic activation of all cholinergic neurons in Drosophila almost completely suppresses sleep and is followed by homeostatic rebound sleep, indicating that some cholinergic neurons may play a role in maintaining sleep homeostasis (58). Previous work in flies and rodents suggests that cholinergic neurons may become increasingly active with extended waking (24, 59–61). It is possible that elevated activity of cholinergic cells drives the increase in BRP abundance we observe after some forms of sleep loss. As acetylcholine is the primary excitatory neurotransmitter in the Drosophila central brain (62–64), our findings of elevated BRP abundance in cholinergic neurons after SD may be consistent with reports of increased cortical excitability seen in humans and rodents (50, 65). After sleep loss, excitatory neurons may increase their drive onto inhibitory neurons (24, 26), perhaps in a compensatory effort to prevent runaway excitation (66, 67). Indeed, sleep deprivation can increase the duration and frequency of seizures in mutant flies or mice with seizure phenotypes (68, 69) and patients with epilepsy (70). Because a recent study found that excitation of local sleep-promoting circuits contributes to increased seizure risk (71), further investigation is required to draw a direct connection between brain-wide synaptic scaling and seizure susceptibility in flies.
Recent studies suggest that sleep is associated with the maintenance of excitatory/inhibitory (E/I) balance in the brain (24, 25, 72, 73). Previous work in mammals has demonstrated that global cortical excitability increases during waking (50, 65, 74, 75), whereas cortical excitability decreases over the course of sleep. Consistent with these findings, our data show that sleep deprivation increases presynaptic abundance across excitatory cholinergic neurons. However, sleep’s effect on E/I balance may vary based on time of day (72, 73, 76), sleep stage (77, 78), and brain region (25). Notably, altered E/I balance is implicated in the pathogenesis of neurological and psychiatric disorders (79–84). Elevated excitation from cholinergic cells without compensation from inhibitory neurons could, therefore, contribute to behavioral and cognitive consequences of sleep loss. While our results indicate elevated excitatory drive via upscaling of cholinergic presynapses, a comprehensive understanding of the effect on E/I balance will require examination of postsynaptic responses. For instance, postsynaptic enhancement of GABA receptors or degradation of cholinergic receptors could offset an increase in excitatory drive from cholinergic neurons after sleep deprivation. These studies are becoming feasible with the development of cell type–specific genetic reporters for postsynaptic densities (85) or neurotransmitter receptor subunits (86) to explore spatial and temporal patterns of postsynaptic compensation in response to BRP plasticity. Further, while our findings are consistent with net upscaling of BRP with sleep loss, recent studies have also found that compaction of BRP within release sites could also result in elevated BRP immunofluorescence (87). As we identify cell types that are most susceptible to plasticity during sleep loss, follow-up studies can examine local changes in BRP organization after sleep loss to test whether global upscaling reflects increased signal from existing synaptic contacts, the growth of new active zones, or a combination of both.
The consequences of sleep loss on synaptic plasticity are not yet well understood, but previous work has found net changes in synaptic abundance or size across brain regions (12, 26, 51, 88). Here, we find that cholinergic and glutamatergic neurons across the Drosophila central brain are uniquely sensitive to sleep loss. While future studies are required to identify the mechanisms by which prolonged waking drives increased synapse abundance, our results suggest that common plasticity rules may be broadly applied across brain regions. If this case holds, then opportunities may arise for single interventions that generally protect the brain from synaptic scaling consequences of sleep loss.
Methods
Detailed methods are provided in SI Appendix and include descriptions of behavioral assays, immunohistochemistry, confocal imaging, image registration, and statistical analyses.
Experimental Model and Subject Details
Fly Strains and Environment.
Fly stocks were fed standard cornmeal media (per 1L H20: 12 g agar, 29 g Red Star yeast, 71 g cornmeal, 92 g molasses, 16 mL methyl paraben 10% in EtOH, and 10 mL propionic acid 50% in H20) at 25 °C with 60% relative humidity and entrained to a daily 12-h light, 12-h dark schedule. All flies were reared in environmentally controlled chambers at 25 °C and 60% relative humidity on a 12-h light:12-h dark schedule. Chat2A-Gal4 (84618), VGlut-Gal4 (24635), VGlut2A-Gal4 (84697), Gad1-Gal4 (51630), and inc2 flies (18307) were ordered from the Bloomington Drosophila Stock Center. TH-Gal4 was provided by David Krantz (UCLA; also available as BDSC 8848), and STaR effector flies (w-; 20×UAS-RSR.PEST, 79C23S-RSRT-STOP-RSRT-smGFP_V5-2A-LexA/cyo and UAS-flp, brp(FRT.Stop)V5-2A-LexA-VP16) were provided by Orkun Akin (UCLA) and Bloomington Drosophila Stock Center (55751).
Fly stocks were generated by the labs of S. Lawrence Zipursky and Matt Pecot (STaR effectors) (29, 30), Serge Birman (TH-GAL4) (37), Aaron Diantonio (VGlut-Gal4) (38), Yi Rao (Chat2A-Gal4, VGlut2A-Gal4) (36), and Gero Miesenböck (Gad1-Gal4) (36).
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jeffrey M. Donlea (jdonlea@ucla.edu). This study did not generate new reagents.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) Chat2A-Gal4>STaR flies (n=23-25 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) TH-Gal4>STaR flies (n=41 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) vGlut-Gal4>STaR flies (n=38-44 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) Gad1-Gal4>STaR flies (n=40-43 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 24h SD) TH-Gal4>STaR flies (n=41 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 24h SD) vGlut-Gal4>STaR flies (n=38-44 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 24h SD) Gad1-Gal4>STaR flies (n=40-43 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) Chat2A-Gal4>STaR flies (n=20 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) TH-Gal4>STaR flies (n=37-40 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) vGlut2A-Gal4>STaR flies (n=33-37 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) Gad1-Gal4>STaR flies (n=31-40 flies/group).
Z-stack animations of group average images from male Chat2A-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=19-20 brains/group).
Z-stack animations of group average images from male TH-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=21-22 brains/group).
Z-stack animations of group average images from male vGlut-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=15-18 brains/group).
Z-stack animations of group average images from male Gad1-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=18-19 brains/group).
Acknowledgments
We thank all members of the Donlea lab for many valuable discussions and technical advice throughout this project. This work benefited from helpful feedback by Drs. Gina Poe, David Krantz, Larry Zipursky, and Orkun Akin. Fly stocks were generously provided by Drs. Gero Miesenböck (University of Oxford), David Krantz (UCLA), Orkun Akin (UCLA), and the Bloomington Drosophila Stock Center. This project was supported by a Career Development Award from the Human Frontiers Science Program to J.M.D. (CDA00026-2017-C), NIH Grants R01NS105967 and R01NS119905 to J.M.D., a Jessamine K. Hilliard UCLA Neurobiology Graduate Student Grant to J.T.W., and support from the UCLA Undergraduate Research Fellowship Program and Biomedical Research Summer Scholarship to M.Z.B.
Author contributions
J.T.W., M.Z.B., and J.M.D. designed research; J.T.W. and P.S. performed research; M.Z.B. contributed new reagents/analytic tools; J.T.W., M.Z.B., and J.M.D. analyzed data; J.T.W., M.Z.B., P.S., and J.M.D. revised the paper; and J.T.W., M.Z.B., and J.M.D. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Data used to generate figures are available as supporting information. Code generated from this study is available at https://doi.org/10.5061/dryad.q573n5tqv (89). All other data are included in the manuscript and/or supporting information.
Supporting Information
References
- 1.Abel T., Havekes R., Saletin J. M., Walker M. P., Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol. 23, R774–R788 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasch B., Born J., About sleep’s role in memory. Physiol. Rev. 93, 681–766 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tononi G., Cirelli C., Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roffwarg H. P., Muzio J. N., Dement W. C., Ontogenetic development of the human sleep-dream cycle. Science 152, 604–619 (1966). [DOI] [PubMed] [Google Scholar]
- 5.Shaw P. J., Cirelli C., Greenspan R. J., Tononi G., Correlates of sleep and waking in Drosophila melanogaster. Science 287, 1834–1837 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Kayser M. S., Yue Z., Sehgal A., A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science 344, 269–274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P., Donlea J. M., Bidirectional regulation of sleep and synapse pruning after neural injury. Curr. Biol. Cb 30, 1063–1076.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanhope B. A., Jaggard J. B., Gratton M., Brown E. B., Keene A. C., Sleep regulates glial plasticity and expression of the engulfment receptor draper following neural injury. Curr. Biol. Cb 30, 1092–1101.e3 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Walker M. P., Brakefield T., Morgan A., Hobson J. A., Stickgold R., Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron 35, 205–211 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Ganguly-Fitzgerald I., Donlea J. M., Shaw P. J., Waking experience affects sleep need in Drosophila. Science 313, 1775–1781 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Gilestro G. F., Tononi G., Cirelli C., Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 324, 109–112 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diering G. H., et al. , Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S., Piao C., Beuschel C. B., Götz T., Sigrist S. J., Presynaptic active zone plasticity encodes sleep need in Drosophila. Curr. Biol. Cb 30, 1077–1091.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Frank M. G., Issa N. P., Stryker M. P., Sleep enhances plasticity in the developing visual cortex. Neuron 30, 275–287 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Aton S. J., Suresh A., Broussard C., Frank M. G., Sleep promotes cortical response potentiation following visual experience. Sleep 37, 1163–1170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aton S. J., et al. , Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 110, 3101–3106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vienne J., Spann R., Guo F., Rosbash M., Age-related reduction of recovery sleep and arousal threshold in Drosophila. Sleep 39, 1613–1624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mander B. A., Winer J. R., Walker M. P., Sleep and human aging. Neuron 94, 19–36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber R., Ghilardi M. F., Massimini M., Tononi G., Local sleep and learning. Nature 430, 78–81 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Keene A. C., et al. , Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr. Biol. 20, 1209–1215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckwith E. J., Geissmann Q., French A. S., Gilestro G. F., Regulation of sleep homeostasis by sexual arousal. Elife 6, e27445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thimgan M. S., Suzuki Y., Seugnet L., Gottschalk L., Shaw P. J., The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. Plos Biol. 8, e1000466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirszenblat L., Yaun R., van Swinderen B., Visual experience drives sleep need in Drosophila. Sleep 8, 269 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delorme J., et al. , Sleep loss drives acetylcholine- and somatostatin interneuron–mediated gating of hippocampal activity to inhibit memory consolidation. Proc. Natl. Acad. Sci. U.S.A. 118, e2019318118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puentes-Mestril C., et al. , Sleep loss drives brain region-specific and cell type-specific alterations in ribosome-associated transcripts involved in synaptic plasticity and cellular timekeeping. J. Neurosci. 41, 5386–5398 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss J. T., Donlea J. M., Sleep deprivation results in diverse patterns of synaptic scaling across the Drosophila mushroom bodies. Curr. Biol. 31, 3248–3261.e3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDermott C. M., et al. , Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J. Neurosci. 23, 9687–9695 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Appelbaum L., et al. , Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron 68, 87–98 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y., et al. , Cell-type-specific labeling of synapses in vivo through synaptic tagging with recombination. Neuron 81, 280–293 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng J., et al. , Drosophila Fezf coordinates laminar-specific connectivity through cell-intrinsic and cell-extrinsic mechanisms. Elife 7, e33962 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kittel R. J., et al. , Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science 312, 1051–1054 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Wagh D. A., et al. , Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833–844 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Matkovic T., et al. , The Bruchpilot cytomatrix determines the size of the readily releasable pool of synaptic vesiclesAZ cytomatrix regulates readily releasable pool. J. Cell Biol. 202, 667–683 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbergenova Y., Cunningham K. L., Zhang Y. V., Weiss S., Littleton J. T., Characterization of developmental and molecular factors underlying release heterogeneity at Drosophila synapses. Elife 7, e38268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong H., et al. , Structural remodeling of active zones is associated with synaptic homeostasis. J. Neurosci. Offi. J. Soc. Neurosci. 40, 2817–2827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng B., et al. , Chemoconnectomics: Mapping chemical transmission in Drosophila. Neuron 101, 876–893.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Friggi-Grelin F., et al. , Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 54, 618–627 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Daniels R. W., Gelfand M. V., Collins C. A., DiAntonio A., Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J. Comp. Neurol. 508, 131–152 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Ng M., et al. , Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463–474 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Cachero S., Ostrovsky A. D., Yu J. Y., Dickson B. J., Jefferis G. S. X. E., Sexual dimorphism in the Fly brain. Curr. Biol. 20, 1589–1601 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrovsky A., Cachero S., Jefferis G. S. X. E., Clonal analysis of olfaction in Drosophila: Image registration. Cold Spring Harb. Protoc. 2013, 347–349 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Jenett A., et al. , A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ito K., et al. , A systematic nomenclature for the insect brain. Neuron 81, 755–765 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Scheffer L. K., et al. , A connectome and analysis of the adult Drosophila central brain. Elife 9, e57443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stavropoulos N., Young M. W., insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron 72, 964–976 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfeiffenberger C., Allada R., Cul3 and the BTB adaptor insomniac are key regulators of sleep homeostasis and a dopamine arousal pathway in Drosophila. Plos Genet. 8, e1003003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q., Jang H., Lim K. Y., Lessing A., Stavropoulos N., Insomniac links the development and function of a sleep-regulatory circuit. Elife 10, e65437 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machado D. R., et al. , Identification of octopaminergic neurons that modulate sleep suppression by male sex drive. Elife 6, 1717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donlea J. M., Ramanan N., Shaw P. J., Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science 324, 105–108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huber R., et al. , Human cortical excitability increases with time awake. Cereb Cortex 23, 1–7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bushey D., Tononi G., Cirelli C., Sleep and synaptic homeostasis: Structural evidence in Drosophila. Science 332, 1576–1581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bellesi M., et al. , Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J. Neurosci. 37, 5263–5273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zamore Z., Veasey S. C., Neural consequences of chronic sleep disruption. Trends Neurosci. 45, 678–691 (2022), 10.1016/j.tins.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joyce M., et al. , Divergent evolution of sleep homeostasis. bioRxiv [Preprint] (2023). 10.1101/2023.05.27.541573 (Accessed 28 May 2023). [DOI]
- 55.Mundiyanapurath S., Chan Y.-B., Leung A. K. W., Kravitz E. A., Feminizing cholinergic neurons in a male Drosophila nervous system enhances aggression. Fly 3, 179–184 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoopfer E. D., Jung Y., Inagaki H. K., Rubin G. M., Anderson D. J., P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, e11346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wohl M. P., Liu J., Asahina K., Drosophila tachykininergic neurons modulate the activity of two groups of receptor-expressing neurons to regulate aggressive tone. J. Neurosci. 43, 3394–3420 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seidner G., et al. , Identification of neurons with a privileged role in sleep homeostasis in Drosophila melanogaster. Curr. Biol. 25, 2928–2938 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Modirrousta M., Mainville L., Jones B. E., Gabaergic neurons with α2-adrenergic receptors in basal forebrain and preoptic area express c-Fos during sleep. Neuroscience 129, 803–810 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Bushey D., Tononi G., Cirelli C., Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc. Natl. Acad. Sci. U.S.A. 112, 4785–4790 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ge F., et al. , Chronic sleep fragmentation enhances habenula cholinergic neural activity. Mol. Psychiatr. 26, 941–954 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Breer H., Sattelle D. B., Molecular properties and functions of insect acetylcholine receptors. J. Insect. Physiol. 33, 771–790 (1987). [Google Scholar]
- 63.Restifo L. L., White K., Molecular and genetic approaches to neurotransmitter and neuromodulator systems in Drosophila. Adv. Insect. Physiol. 22, 115–219 (1990). [Google Scholar]
- 64.Lee D., O’Dowd D. K., Fast excitatory synaptic transmission mediated by nicotinic acetylcholine receptors in Drosophila neurons. J. Neurosci. 19, 5311–5321 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vyazovskiy V. V., et al. , Cortical firing and sleep homeostasis. Neuron 63, 865–878 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng Y.-R., et al. , Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J. Neurosci. 30, 16220–16231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xue M., Atallah B. V., Scanziani M., Equalizing excitation–inhibition ratios across visual cortical neurons. Nature 511, 596–600 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lucey B. P., Leahy A., Rosas R., Shaw P. J., A new model to study sleep deprivation-induced seizure. Sleep 38, 777–785 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konduru S. S., et al. , Sleep deprivation exacerbates seizures and diminishes GABAergic tonic inhibition. Ann. Neurol. 90, 840–844 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattson R. H., Pratt K. L., Calverley J. R., Electroencephalograms of epileptics following sleep deprivation. Arch. Neurol. 13, 310–315 (1965). [DOI] [PubMed] [Google Scholar]
- 71.Cuddapah V. A., et al. , Sleepiness, not total sleep amount, increases seizure risk. bioRxiv [Preprint] (2023). 10.1101/2023.09.30.560325 (Accessed 14 November 2023). [DOI]
- 72.Chellappa S. L., et al. , Circadian dynamics in measures of cortical excitation and inhibition balance. Sci. Rep-uk 6, 33661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bridi M. C. D., et al. , Daily oscillation of the excitation-inhibition balance in visual cortical circuits. Neuron 105, 621–629.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Z.-W., Faraguna U., Cirelli C., Tononi G., Gao X.-B., Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J. Neurosci. Official J. Soc. Neurosci. 30, 8671–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chia C.-H., et al. , Cortical excitability signatures for the degree of sleepiness in human. Elife 10, e65099 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pacheco A. T., Bottorff J., Gao Y., Turrigiano G. G., Sleep promotes downward firing rate homeostasis. Neuron 109, 530–544.e6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niethard N., et al. , Sleep-stage-specific regulation of cortical excitation and inhibition. Curr. Biol. 26, 2739–2749 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Tamaki M., et al. , Complementary contributions of NREM and REM sleep to visual learning. Nat. Neurosci. 23, 1150–1156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rubenstein J. L. R., Merzenich M. M., Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kehrer C., Maziashvili N., Dugladze T., Gloveli T., Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front. Mol. Neurosci. 1, 6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gogolla N., et al. , Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yizhar O., et al. , Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bi D., Wen L., Wu Z., Shen Y., GABAergic dysfunction in excitatory and inhibitory (E/I) imbalance drives the pathogenesis of Alzheimer’s disease. Alzheimer’s Dementia 16, 1312–1329 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Lauterborn J. C., et al. , Increased excitatory to inhibitory synaptic ratio in parietal cortex samples from individuals with Alzheimer’s disease. Nat. Commun. 12, 2603 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parisi M. J., Aimino M. A., Mosca T. J., A conditional strategy for cell-type-specific labeling of endogenous excitatory synapses in Drosophila. Cell Rep. Methods 3, 100477 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanfilippo P., et al. , Mapping of multiple neurotransmitter receptor subtypes and distinct protein complexes to the connectome. Neuron, 10.1016/j.neuron.2023.12.014 (2024). [DOI] [PMC free article] [PubMed]
- 87.Mrestani A., et al. , Active zone compaction correlates with presynaptic homeostatic potentiation. Cell Rep. 37, 109770 (2021). [DOI] [PubMed] [Google Scholar]
- 88.de Vivo L., et al. , Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donlea J. M., Data from “Sleep deprivation drives brain-wide changes in cholinergic pre-synapse abundance in Drosophila melanogaster.” Dryad. 10.5061/dryad.q573n5tqv. Deposited 1 March 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) Chat2A-Gal4>STaR flies (n=23-25 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) TH-Gal4>STaR flies (n=41 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) vGlut-Gal4>STaR flies (n=38-44 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 12h SD) Gad1-Gal4>STaR flies (n=40-43 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 24h SD) TH-Gal4>STaR flies (n=41 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 24h SD) vGlut-Gal4>STaR flies (n=38-44 flies/group).
Z-stack animations of group average images from control (left) and sleep deprived (right, 24h SD) Gad1-Gal4>STaR flies (n=40-43 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) Chat2A-Gal4>STaR flies (n=20 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) TH-Gal4>STaR flies (n=37-40 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) vGlut2A-Gal4>STaR flies (n=33-37 flies/group).
Z-stack animations of group average images from wild-type (left) and inc2 (right) Gad1-Gal4>STaR flies (n=31-40 flies/group).
Z-stack animations of group average images from male Chat2A-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=19-20 brains/group).
Z-stack animations of group average images from male TH-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=21-22 brains/group).
Z-stack animations of group average images from male vGlut-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=15-18 brains/group).
Z-stack animations of group average images from male Gad1-Gal4>STaR flies housed overnight in male-male (left) or male-female (right) pairs (n=18-19 brains/group).
Data Availability Statement
Data used to generate figures are available as supporting information. Code generated from this study is available at https://doi.org/10.5061/dryad.q573n5tqv (89). All other data are included in the manuscript and/or supporting information.