Abstract
Objective:
A substantial gap exists between patients and their mental health providers about patient’s perceived barriers, facilitators, and motivators (BFMs) for taking antipsychotic medications. This paper describes how we used an Intervention Mapping (IM) framework coupled with qualitative and quantitative item-selection methods to develop an intervention to bridge this gap with the goal of improving antipsychotic medication adherence.
Methods:
IM is a stepwise method for developing and implementing health interventions. A previous study conducted in-depth qualitative interviews with patients diagnosed with schizophrenia and identified 477 BFMs associated with antipsychotic medication adherence. This paper reports the results of using a variety of qualitative and quantitative item reduction and intervention development methods to transform the qualitative BFM data into a viable checklist and intervention.
Results:
The final BFM checklist included 76 items (28 barriers, 30 facilitators, and 18 motivators). An electronic and hard copy of the adherence progress note included a summary of current adherence, top three patient-identified barriers and top three facilitators and motivators, clarifying questions, and actionable adherence tips to address barriers during a typical clinical encounter.
Discussion:
The IM approach supplemented with qualitative and quantitative methods provided a useful framework for developing a practical and potentially sustainable antipsychotic medication adherence intervention. A similar approach to intervention development may be useful in other clinical situations where a substantial gap exists between patients and providers regarding medication adherence or other health behaviors.
Keywords: medication adherence, schizophrenia, intervention studies, qualitative research
INTRODUCTION
Recent meta-analyses and reviews confirm that antipsychotic medications are efficacious treatments for schizophrenia (Johnsen & Jorgensen, 2008; Leucht et al., 2009). However, efficacious antipsychotic regimens frequently fail because non-adherence rates approach 50% or more (Cramer & Rosenheck, 1998; Lacro, Dunn, Dolder, Leckband, & Jeste, 2002; Lieberman et al., 2005). Non-adherence to antipsychotic medication treatment leads to a variety of clinical and economic problems, including psychotic relapse, increased clinic and emergency room visits, and rehospitalization (Byerly, Nakonezny, & Lescouflair, 2007; Terkelsen & Menikoff, 1995; Weiden & Olfson, 1995). In a naturalistic study, previously stable patients with schizophrenia who chose to discontinue their medication experienced a 93% rate of relapse within one year (Wistedt, 1981). To address the problem of non-adherence to antipsychotic medications, we used Intervention Mapping (IM) as a framework to develop a practical, sustainable, patient-centered intervention that could be implemented in time- and resource-limited “real-world” clinical settings.
Intervention Mapping (IM) is a stepwise method for developing and implementing of health interventions that has been successfully applied to a wide range of health issues (Alewijnse, Mesters, Metsemakers, & van den Borne, 2002; Hoelscher, Evans, Parcel, & Kelder, 2002; Koekkoek, van Meijel, Schene, & Hutschemaekers, 2010; Murray, Kelder, Parcel, & Orpinas, 1998; Schmid, Andersen, Kent, Williams, & Damush, 2010). The IM process for this study included six steps: (1) a needs and capacities assessment of the at-risk group (patients) and the capacities of patients and providers, (2) definition of proximal intervention objectives and the behavioral and environmental changes necessary to meet them, (3) review of available interventions and practical strategies to address the problem, (4) intervention development, (5) intervention implementation, and (6) intervention evaluation (Bartholomew, Parcel, & Kok, 1998; Bartholomew, Parcel, Kok, & Gottleib, 2000). This paper will describe IM steps 1–5.
METHODS
-
Step 1:
Needs and capacities assessment. Step 1 included reviewing the literature on medication non-adherence in schizophrenia, reviewing adherence models/theories, and “going to the source” by asking patients and providers about patients’ experience with adherence. Qualitative “going to the source” interviews were conducted with 15 VA and 11 non-VA patients diagnosed with schizophrenia or schizoaffective disorder and their mental health providers. (Pyne et al., 2006) Patient qualitative interviews were conducted in-person, audio-taped, and transcribed verbatim within one week of the interview. Patient qualitative interviews included questions about medication adherence barriers, facilitators, and motivators (BFMs). Patients’ mental health providers completed open-ended hard-copy questionnaires which included questions about an individual patient’s BFMs. We found substantial disagreement between patients and providers regarding the patient’s predominant barriers, facilitators, and motivators for taking antipsychotic medication; suggesting the need for an intervention to improve patient/provider communication about antipsychotic medication adherence (Pyne et al., 2006). In keeping with the patient-centered approach of this project, only the patient data were used to identify barriers, facilitators, and motivators for the intervention.
-
Step 2:
Intervention objectives. Step 2 included defining the intervention goals and how the intervention would achieve these goals. We also specified behavioral and environmental intervention objectives, the theoretical personal and external changes required to achieve them, and the target populations for the intervention.
-
Step 3:
Review of intervention methods and strategies. Step 3 involved reviewing lessons learned from previous antipsychotic medication adherence trials, identifying theory-based intervention methods, and identifying practical strategies to improve patient/provider communication around antipsychotic medication adherence.
-
Step 4:
Intervention design and pilot testing. Step 4 included defining the components of the intervention, designing and developing intervention tools, and pilot testing those tools. The main intervention tools were a BFM checklist, a computer-interface for collecting checklist data, and adherence tips for providers.
BFM checklist – Item reduction and reliability
To transform the qualitative BFM data from Step 1 into a viable checklist, we (a) reduced and refined the BFM items, (b) tested the reliability of checklist responses, and (c) (c) conducted cognitive debriefing interviews with patients to assess the clarity of the BFM checklist and ranking methods.
A convenience sample of 50 patients (25 from the general mental health clinic and 25 from an intensive case-management clinic) completed a hard copy version of the 260-item BFM checklist endorsing those statements that applied to them using a Yes/No response set. Participants had a medical chart diagnosis of schizophrenia or schizoaffective disorder, were currently prescribed outpatient antipsychotic medication (oral or depot), and scored <10 (mild to no cognitive impairment) on the Blessed Orientation-Memory-Concentration (BOMC) Test (Katzman, Holman, & Ashley, 1993). Additional data included sociodemographic information and self-report antipsychotic medication adherence covering the past four weeks using a 5-point scale ranging from ‘I stopped taking the medicine altogether’ to ‘I never missed taking my medicine’ (Fischer et al., 2000; Miklowitz, Goldstein, Nuechterlein, Snyder, & Doane, 1986).
Item reduction methods included: elimination of infrequently (≤10%) endorsed items, Kuder-Richardson Formula 20 (KR20) (Kaplan & Saccuzzo, 2001), Chi-squared Automatic Interaction Detector (CHAID) (Magidson, 1994), and agreement across domains. We used KR20 to identify item-pairs that had a percent agreement of 85% or more and a kappa coefficient of 0.6 or more and deleted the item for which the KR20 formula improved when the item was removed.
CHAID was first used to identify items that best predicted antipsychotic medication adherence. The CHAID approach constructs trees, where each (non-terminal) node identifies a split condition, to yield optimum prediction (of continuous dependent or response variables) or classification (of categorical dependent or response variables). To classify medication adherence categories we used the chi-square test to determine the best next split at each step. Later we used the CHAID approach in separate analyses to identify items that best predicted antipsychotic medication adherence by race (Caucasian versus African-American), cognitive status (based on BOMC), and clinic assignment (general versus intensive case management – a proxy for symptom severity). A BOMC score of 0–2 was used to indicate no cognitive impairment and a BOMC score >2 was used to indicate some cognitive impairment. A 2-sided Fisher’s Exact test (p<0.05) was used to identify items with statistically significant response rates by race, cognition or clinic.
In the final item-reduction step, we calculated agreement across domains defined as percent agreement ≥ 80% and a kappa coefficient ≥0.5. If items met these agreement criteria then we would retain only one item.
Test/re-test reliability of the resulting BFM checklist was conducted in a separate sample of 30 outpatients who met the same inclusion/exclusion criteria as the item-reduction sample. Participants completed a paper and pencil version of the reduced BFM checklist twice, at baseline and again two weeks later. We calculated both the kappa statistic and percent agreement.
Automating data collection and cognitive debriefing
Following automation of data collection (see Results), we conducted cognitive-debriefing interviews with 10 patients who met the same inclusion/exclusion criteria as for previous samples. Interviews assessed the clarity of the BFM checklist and clarity and acceptability of two BFM ranking methods. Visual analogue scale (VAS) and simple ranking approaches were used to identify the three barriers and three facilitators and motivators that were most important for a patient. Participants were asked to describe what they were thinking as they completed the checklist and ranking-method exercises (van Someren, Barnard, & Sandberg, 1994). These interviews were recorded and transcribed.
-
IM step 5:
Intervention adoption and implementation. Step 5 included mental health provider focus groups to review the intervention and logistics of intervention delivery and implementation. Focus group participants were VA mental health providers who had been treating patients with schizophrenia over the past year and included psychiatrists, clinical pharmacists, nurses, psychologists, and social workers. We conducted three focus groups: one with six medication prescribing providers, one with 10 non-prescribing providers, and one combination group (prescribing and non-prescribing providers) with 13 providers. Standard focus group procedures were followed (Morgan, 1997). We audio taped each focus group session and transcribed the tapes verbatim.
RESULTS
-
Step 1:
Needs Assessment. The literature review highlighted the antipsychotic medication adherence communication gap between patients and providers (Santone et al., 2008; Voruganti, Baker, & Awad, 2008); lack of provider time, skills, or resources to adequately address non-adherence problems (Haynes et al., 2008; McDonald, Garg, & Haynes, 2002); and the large number of potential risk factors associated with antipsychotic medication non-adherence (Adams & Howe, 1993; Bebbington, 1995; Budd, Hughes, & Smith, 1996; Fenton, Blyler, & Heinssen, 1997; Hoge et al., 1990; Lacro et al., 2002; Olfson et al., 2000; Perkins, 1999; Rosenheck et al., 2000).
Qualitative “going to the source” qualitative interviews findings were reported elsewhere (Pyne et al., 2006). Briefly, the patients identified 477 barriers, facilitators, and motivators (174, 127, and 176, respectively). The barriers, facilitators, and motivators (BFMs) were organized into eight categories (environment, side effects, patient-provider and patient-family/significant other relationships, insight/knowledge, symptoms/outcomes, substance abuse, stigma, and dosing). We found substantial disagreement between patients and providers regarding the patient’s predominant barriers, facilitators, and motivators for taking antipsychotic medication; suggesting the need for an intervention to improve patient/provider communication about antipsychotic medication adherence (Pyne et al., 2006).
-
Step 2:
Intervention objectives. The overall goal defined for the intervention was to maintain or improve antipsychotic medication adherence. Proximal objectives were increased patient and provider awareness of patient-identified adherence BFMs and increased patient/provider BFM communication. Thus the target populations were both patients with schizophrenia prescribed antipsychotic medications and their mental health providers. The theoretical model which closely reflected the qualitative data and intervention objectives was the Health Decision Model (see Figure 1) (Eraker, Kirscht, & Becker, 1984). The Health Decision Model extends the Health Belief Model in that it explicitly adds patient preferences and experiences to the Health Belief Model. The intervention objectives were divided into patient and provider behavioral objectives and clinic environmental objectives. For example, the patient behavioral objectives were to complete the BFM checklist, receive the adherence progress note and handouts, discuss the progress note and handouts with the provider, and implement the adherence tips associated with patient-identified barriers. The determinants of patient behavior were then linked to the Health Decision Model. For example, personal determinants were derived from the Adherence Beliefs and Experience domains and external determinants were derived from the Social and Environmental Interaction domain of the model (see Figure 1). The adherence tips component of the intervention operationalized the change objectives required to address patient-identified barriers. The patient-identified facilitators and motivators were meant to be acknowledged and reinforced by the mental health provider.
-
Step 3:
Review of intervention methods and strategies. Lessons learned from the literature included the following. First, many antipsychotic medication adherence interventions have been tested but few have been successful and none has been widely adopted (Julius, Novitsky, & Dubin, 2009; Zygmunt, Olfson, Boyer, & Mechanic, 2002). Second, interventions that improved adherence had a strong patient-centered focus: e.g., behavioral tailoring, patient activation, and motivational interviewing (Boczkowski, Zeichner, & DeSanto, 1985; Kelly & Scott, 1990; Kemp, Kirov, Everitt, Hayward, & David, 1998; Morken, Grawe, & Widen, 2007; Valenstein et al., 2011; Velligan et al., 2008). Third, the most likely reasons for the lack of widespread adoption of successful interventions are the significant additional resources/cost required and/or lack of symptom improvement (Julius et al., 2009).
One practical mechanism for addressing the communication gap was an information feedback system in which BFM data were collected from the patient, summarized, and delivered to the provider using the electronic medical record. Successful feedback interventions in the literature included: real-time feedback (Giolas, 1998; Rubenstein et al., 1995; Tierney, Hui, & McDonald, 1986), focus on a defined population (Deyo & Carter, 1992; Kumana et al., 1998), provider and patient input (Thomson O’Brien et al., 2001; Tierney et al., 1986; Wasson, Splaine, Bazos, & Fisher, 1998; Winkens, Pop, Grol, Kester, & Knottnerus, 1992), and commitment and cooperation from all levels of the provider organization (Hershey, Goldberg, & Cohen, 1988; Splaine, Bierman, & Wasson, 1998). A Cochrane Review reported the following specific domains as important for feedback interventions: content (what data and/or recommendations are included), source (where the data come from), recipient (who receives the feedback), timing (when is feedback received), and format (how is the feedback presented) (Thomson O’Brien et al., 2001).
Feedback on BFMs alone is unlikely to generate the desired patient/provider discussions about patient-centered goal setting, self-monitoring, problem solving, skill training, knowledge acquisition, and reinforcement (Bartholomew et al., 1998). Therefore, as discussed below, providers would also receive brief, actionable adherence tips linked to patient-identified barriers to facilitate discussion.
-
Step 4:
Intervention design and pilot testing.
Figure 1.
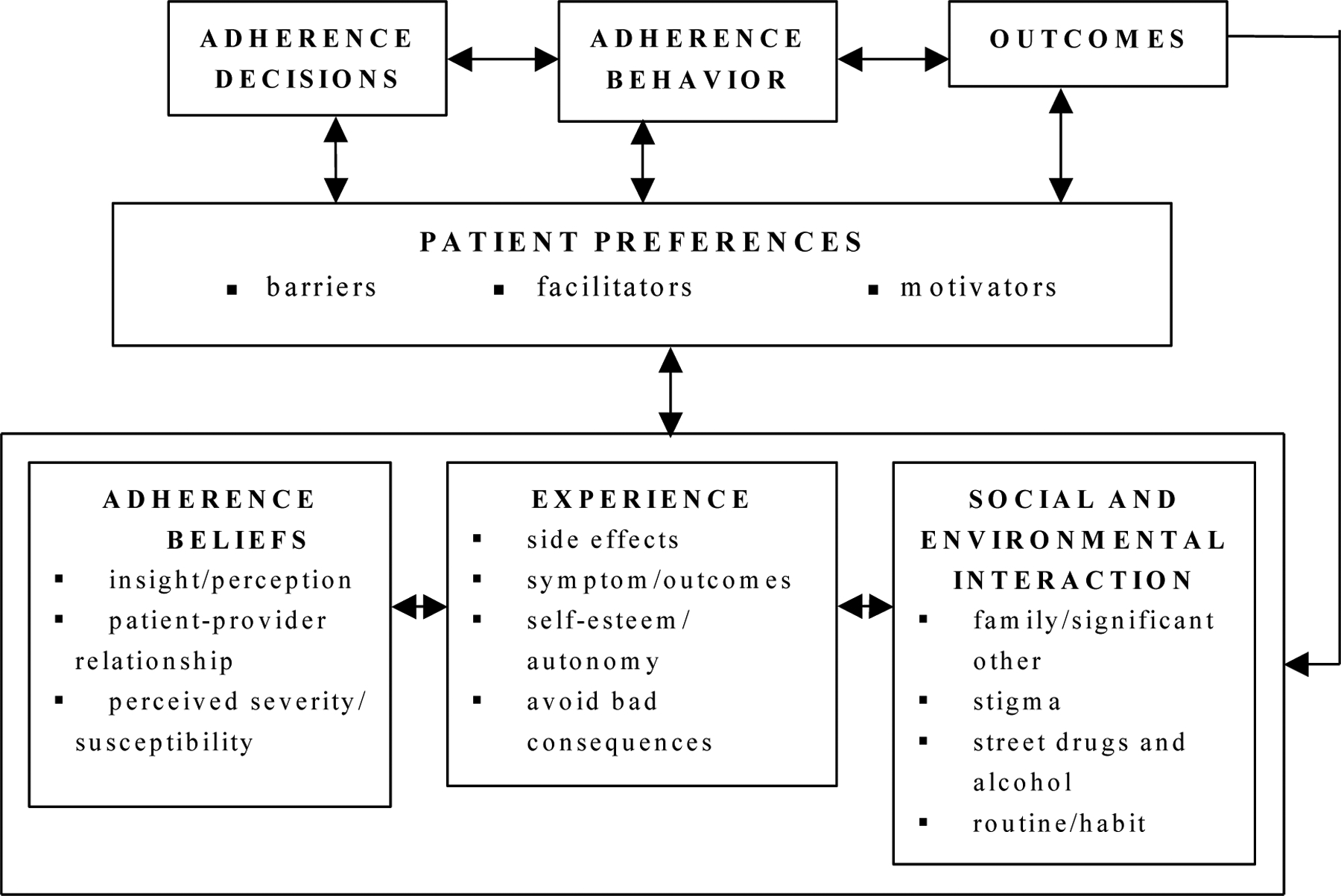
Modified Health Decision Model for Antipsychotic Medication Adherence.
BFM checklist – Item reduction and reliability
Participants in the item-reduction substudy were 94% men (47/50), 62% African-American (31/50), 16% were married/cohabitating (8/50), and 54% (27/50) had more than high school education. Ninety percent (45/50) completed the 260-item checklist without any assistance. Of those who required assistance, four had vision problems and one had concentration problems.
We first eliminated 20 infrequently endorsed items (≤10%), reducing items to 240. The KR20 approach reduced the number of items to 187 by eliminating items for which the KR20 formula improved when the item was removed.
We next used CHAID to identify items that best predicted antipsychotic medication adherence using the following medication adherence categories: never missed a dose in the past four weeks (N=38), missed only a couple of doses (N=11), and missed several doses but took at least half (N=1). Eliminating poor predictors reduced the total number of items from 187 to 48 (18 barriers, 19 facilitators, and 11 motivators).
Next, we added back those items endorsed by a substantial percentage of participants. This step added back seven barrier items endorsed by at least 40% of participants, one facilitator item endorsed by at least 75% of participants, and five motivator items endorsed by at least 75% of participants. This step increased the total number of items from 48 to 61 (25 barriers, 20 facilitators, and 16 motivators).
We then used expert opinion (study investigators and mental health provider focus groups (see below)) to review the items in the 61-item checklist and items which were previously eliminated. Expert opinion resulted in selected items being combined or modified, a brand new item (“I have negative thoughts about myself because I have to take these meds”), and adding back items eliminated previously. This step increased the total number of items from 61 to 82 items (27 barriers, 29 facilitators, and 26 motivators).
We then conducted separate CHAID analyses to identify items most likely to differentiate between groups based on race (Caucasian versus African-American), cognitive status, and clinic assignment (general mental health versus intensive case management). We used the 187-item checklist that was used to predict medication adherence. As shown in Table 1, there were six items that were added back because there was a significant difference in endorsing a BFM by race, cognitive impairment, or clinic assignment. Reintroducing these items increased the checklist to 88 items (29 barriers, 31 facilitators, and 28 motivators).
Table.
Checklist items differentiated using Chi-squared Automatic Interaction Detector (CHAID) analysis
| Item response by race | |||
|---|---|---|---|
| Item | Caucasian Yes Response | African-American Yes Response | p-value |
| Barrier – I am feeling really good1 | 3/19 (15.8%) | 12/31 (38.7%) | 0.016 |
| Facilitator – I take my meds with my meals1 | 2/19 (10.5%) | 12/31 (38.7%) | 0.05 |
| Motivator – I think something good is going to come out of taking my meds2 | 16/19 (84.2%) | 14/31 (45.2%) | 0.008 |
| Motivator – If I take my meds, I will do a good job at work2 | 3/19 (15.8%) | 15/31 (48.4%) | 0.03 |
| Item response by level of cognitive impairment | |||
| Item | No Cognitive Impairment Yes Response | Some Cognitive Impairment Yes Response | p-value |
| Barrier – My meds remind me that I am sick1 | 4/28 (14.3%) | 9/22 (40.9%) | 0.05 |
| Barrier – My friends tell me that my meds don’t work2 | 6/28 (21.4%) | 0/22 (0%) | 0.03 |
| Facilitator – My doctor or mental health provider gives me frequent pep talks about the importance of taking my meds2 | 10/28 (35.7%) | 15/22 (68.2%) | 0.045 |
| Item response by clinic assignment | |||
| Item | General Clinic Yes Response | Intensive Case Management Yes Response | p-value |
| Barrier – My preacher (minister, priest, or other religious leader) does not understand my mental illness2 | 3/25 (12.0%) | 10/25 (40.0%) | 0.050 |
| Facilitator – My doctor or mental health provider listens to me2 | 19/25 (76.0%) | 11/25 (44.0%) | 0.042 |
| Motivator – I think something good is going to come out of taking my meds2 | 20/25 (80.0%) | 10/25 (40.0%) | 0.009 |
Item was previously selected
Item was added back
In the final item-reduction step, we calculated agreement across domains. After exclusion of redundant items, the final checklist included 76 items (28 barriers, 30 facilitators, and 18 motivators) (see Appendix).
We then examined test/re-test reliability of the final 76-item checklist using a separate sample of 30 patients. The mean overall kappa was 0.39; within-category kappas were 0.44 for barriers, 0.36 for facilitators, and 0.36 for motivators. Mean percent agreement was 68.7% overall (73.6% for barriers, 83.5% for facilitators, and 37.1% for motivators).
Automating data collection and cognitive debriefing
The BFM checklist was automated using an audio computer-assisted self-interviewing (ACASI) computer platform which has been found to be a reliable data collection method among seriously mentally ill patients (Chinman, Young, Schell, Hassell, & Mintz, 2004). The ACASI platform also automated identification of patients’ three most highly ranked barriers and three most highly ranked facilitators and motivators, linked the top three barriers to relevant adherence tips for clinicians, and generated an adherence progress note that could be copied and pasted into the electronic medical record. The use of an automated process for collecting BFM data is consistent with clinics moving to automated check-in, outcomes assessment, and the integration of these processes with an electronic health record.
The automated process was piloted with 10 patients and changes made to the system based on their feedback during cognitive debriefing sessions. For example, we found that practice questions were not needed. In addition, participants’ interpretation of the word “currently” ranged from now to the past month. In order to increase precision we changed this wording to “in the past two weeks” for barriers and “over the next 2 weeks” for facilitators and motivators. Participants found the VAS instructions difficult to follow and time consuming. Additionally, during the test/re-test phase, 88% of participants endorsed 10 or more barriers, 100% endorsed 10 or more facilitators, and 77% endorsed 10 or more motivators, making VAS completion for each endorsed item time-consuming and burdensome. Therefore, we dropped the VAS and relied on simple ranking to identify patients’ top three barriers and top three facilitators or motivators. Most patients completed the final 76-item checklist in less than 25 minutes and less than 5% required assistance to use the computer interface.
-
Step 5:
Intervention adoption and implementation. The mental health provider focus groups reviewed and refined the BFM checklist items generated from the patient qualitative interviews, adherence tips generated from literature review, and discussed the logistics of intervention delivery and implementation. Providers did not think simply receiving patient-identified BFMs sufficient to bridge the BFM communication gap. They suggested supplementing the materials providers would receive with suggested questions to generate discussion and clarify what the patient meant when endorsing a given barrier, and actionable adherence “tips” to address each barrier.
Motivational interviewing principles (i.e. expressing empathy, supporting self-efficacy, rolling with resistance, and developing discrepancy) (Miller & Rollnick, 2002) were a common basis for suggested adherence tips (e.g., helping patients generate a list of options for dealing with a particular barrier, discussing the cost and benefit of staying on medications from the patient’s perspective, connecting patient-identified goals with medication adherence, avoiding power struggles, developing discrepancy between patient’s goals and current functional impairments, and focusing on functional impairments related to patient-identified goals). Half of the barriers (14/28) included an adherence tip that was based on one or more of the above motivational interviewing principles.
Specific behavioral strategies (e.g., placing medications near another item used daily, such as a toothbrush, use of pill boxes), environmental supports (e.g., alarms/cues), social supports (e.g. family or friends who could serve as reminders), and cognitive strategies (e.g., reframing schizophrenia as a chronic disease similar to diabetes, linking adherence to times of health or happiness) were also suggested as adherence tips for specific barriers. Additionally, a total of 18 adherence tip handouts were created based on focus group results (e.g., medication side effects, medication adherence reminder signs for patients to place in their home, local VA pharmacy information, medication refill calendar, and sleep hygiene tips). Some adherence tips were not included because they were not feasible within the time constraints of a typical clinical encounter (e.g., skill building using modeling and role play, relaxation training). See Figure 2 for a sample medication adherence progress note and examples of how motivational interviewing principles, behavioral strategies, problem-solving strategies, and patient education were incorporated into adherence tips for the patient-identified barriers.
Figure 2:


Sample Adherence Progress Note
We conducted two additional mental health provider focus groups to guide implementation/delivery of the intervention. Providers suggested that the BFM checklist be completed immediately before a clinic visit and that, in addition to generating an electronic medical record note, the patient be given a hard copy of the BFM results and adherence tips to take into his/her clinic visit. Other suggestions included having all members of the patient’s treatment team sign the electronic adherence note and, at most, monthly completion of the BFM checklist.
DISCUSSION and CONCLUSION
Medication adherence is multi-determined and therefore a variety of adherence strategies tailored to an individual patient’s situation may be needed (Velligan et al., 2009). We used IM to guide development of an antipsychotic medication adherence intervention that addresses each patient’s individual circumstances and would be acceptable and feasible for both patients and providers. The result was a 76-item BFM checklist administered through a computer interface and an adherence note that included clarifying questions and actionable adherence tips based on patient-specific responses.
During the intervention-mapping process, we learned several lessons that may be useful to others who are developing patient-centered adherence interventions. Using a theory- and evidence-based model for framing the intervention identified key leverage points while the combination of patient and expert input provided a useful check-and-balance throughout the intervention-development process. “Going to the source” and asking patients about their experience with adherence not only provided extremely useful data for intervention development but caught the attention of providers because the data were generated by their patients. Provider input increased intervention utility by making the identification of patient-identified barriers feasible within a busy clinic environment and increased provider “ownership” of the intervention. Cognitive debriefing was critical for minimizing confusion during implementation. Use of a computer platform for the intervention established the basis for future integration with electronic medical record systems. Each of these lessons learned is consistent with the 21 priorities for future patient-centered outcomes research in the area of serious mental illness (Jonas et al., 2011).
CHAID analysis combined with expert opinion also proved an invaluable tool in checklist development. CHAID analyses led to the most substantial reductions in BFM items. However, clinical expertise was required to “rescue” items providers considered essential. That the majority of these items were related to awareness of illness makes sense because decreased awareness of illness is a common problem and unlikely to be recognized by patients with schizophrenia-spectrum disorders (Amador et al., 1994; Pyne, Bean, & Sullivan, 2001). Motivational interviewing approaches have been suggested by others to improve awareness of illness and treatment adherence in patients with schizophrenia (Rusch & Corrigan, 2002) and were frequently suggested by providers for adherence tips.
Test/re-test reliability as measured by kappa statistic (0.36–0.44) was in the fair to moderate range. Between-assessment changes in the number of BFMs endorsed do not appear responsible for the low kappas as the numbers of “yes” responses within each domain at each time were highly correlated (59–82%). Percent agreement for motivators (37%) was approximately half that for barriers and facilitators (74–84%). This suggests that barriers and facilitators may be more stable over time than motivators. Therefore, clinicians may need to reassess motivators more frequently than barriers and facilitators.
Limitations to this intervention development approach included the relatively small number of patients providing data for and the substantial time commitment required. Given the low level of widespread and sustained adoption of antipsychotic medication adherence interventions to date (Julius, Novitsky, & Dubin, 2009; Zygmunt, Olfson, Boyer, & Mechanic, 2002), we believe the time commitment was justified. Outcomes from the intervention evaluation will be reported elsewhere.
An Intervention Mapping approach supplemented with qualitative and quantitative instrument development methods provided a useful framework for developing a practical and potentially sustainable antipsychotic medication adherence intervention. A similar approach may be useful in other clinical situations where a substantial communication gap exists between patients and clinicians.
Supplementary Material
Acknowledgments:
The authors also want to acknowledge the patients who participated in this study, Leslie Bragg for data collection assistance, and Valorie Shue for manuscript production assistance.
Funding:
This study was supported by the Veterans Affairs Health Services Research and Development Service [Grant #IIR 03-257-1], the South Central Mental Illness Research and Education Center, the Center for Mental Health and Outcomes Research, and the National Center for Research Resources [GM103425].
REFERENCES:
- Adams SG Jr., & Howe JT (1993). Predicting medication compliance in a psychotic population. J Nerv Ment Dis, 181(9), 558–560. [DOI] [PubMed] [Google Scholar]
- Alewijnse D, Mesters IE, Metsemakers JF, & van den Borne BH (2002). Program development for promoting adherence during and after exercise therapy for urinary incontinence. Patient Educ Couns, 48(2), 147–160. [DOI] [PubMed] [Google Scholar]
- Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, et al. (1994). Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry, 51(10), 826–836. [DOI] [PubMed] [Google Scholar]
- Bartholomew LK, Parcel GS, & Kok G (1998). Intervention mapping: a process for developing theory- and evidence-based health education programs. Health Educ Behav, 25(5), 545–563. [DOI] [PubMed] [Google Scholar]
- Bartholomew LK, Parcel GS, Kok G, & Gottleib NH (2000). Intervention Mapping: A Process for Designing Theory- and Evidence-Based Education Programs. Mountain View, CA: Mayfield. [Google Scholar]
- Bebbington PE (1995). The content and context of compliance. Int Clin Psychopharmacol, 9 Suppl 5, 41–50. [DOI] [PubMed] [Google Scholar]
- Boczkowski JA, Zeichner A, & DeSanto N (1985). Neuroleptic compliance among chronic schizophrenic outpatients: an intervention outcome report. J Consult Clin Psychol, 53(5), 666–671. [DOI] [PubMed] [Google Scholar]
- Budd RJ, Hughes IC, & Smith JA (1996). Health beliefs and compliance with antipsychotic medication. Br J Clin Psychol, 35(Pt 3), 393–397. [DOI] [PubMed] [Google Scholar]
- Byerly MJ, Nakonezny PA, & Lescouflair E (2007). Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am, 30(3), 437–452. [DOI] [PubMed] [Google Scholar]
- Chinman M, Young AS, Schell T, Hassell J, & Mintz J (2004). Computer-assisted self-assessment in persons with severe mental illness. J Clin Psychiatry, 65(10), 1343–1351. [DOI] [PubMed] [Google Scholar]
- Clark NM, & Becker MH (1998). Theoretical models and strategies for improving adherence and disease management. In Shumaker SA, Schron EB, Ockene JK & McBee WL (Eds.), The Handbook of Health Behavior Change (2nd ed., pp. 5–32). New York: Springer Publishing Co. [Google Scholar]
- Cramer JA, & Rosenheck R (1998). Compliance with medication regimens for mental and physical disorders.[comment]. [Review] [62 refs]. Psychiatric Services., 49(2), 196–201. [DOI] [PubMed] [Google Scholar]
- Deyo RA, & Carter WB (1992). Strategies for improving and expanding the application of health status measures in clinical settings. A researcher-developer viewpoint. Med Care, 30(5 Suppl), MS176–186; discussion MS196–209. [DOI] [PubMed] [Google Scholar]
- Eraker SA, Kirscht JP, & Becker MH (1984). Understanding and improving patient compliance. Ann Intern Med, 100, 258–268. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Blyler CR, & Heinssen RK (1997). Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull, 23(4), 637–651. [DOI] [PubMed] [Google Scholar]
- Fischer EP, Cuffel BJ, Owen RR, Burns BJ, Hargreaves WA, Karson C, et al. (2000). Schizophrenia Outcomes Module (SCHIZOM). In Handbook of Psychiatric Measures (pp. 218). Washington DC: American Psychiatric Association. [Google Scholar]
- Giolas D (1998). Interweaving outcomes measurement with the clinical process. Behav Healthc Tomorrow, 7(2), 27–28. [PubMed] [Google Scholar]
- Haynes RB, Yao X, Degani A, Kripalani S, Garg A, & McDonald HP (2008). Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews, 1. [DOI] [PubMed] [Google Scholar]
- Hershey CO, Goldberg HI, & Cohen DI (1988). The effect of computerized feedback coupled with newsletter upon outpatient prescribing charges. Med Care, 26(1), 88–94. [DOI] [PubMed] [Google Scholar]
- Hoelscher DM, Evans A, Parcel GS, & Kelder SH (2002). Designing effective nutrition interventions for adolescents. J Am Diet Assoc, 102(3 Suppl), S52–63. [DOI] [PubMed] [Google Scholar]
- Hoge SK, Appelbaum PS, Lawlor T, Beck JC, Litman R, Greer A, et al. (1990). A prospective, multicenter study of patients’ refusal of antipsychotic medication. Arch Gen Psychiatry, 47(10), 949–956. [DOI] [PubMed] [Google Scholar]
- Johnsen E, & Jorgensen HA (2008). Effectiveness of second generation antipsychotics: a systematic review of randomized trials. BMC Psychiatry, 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas D, Mansfield AJ, Curtis P, Gimlmore J, Watson L, Brode S, et al. (2011). Identifying Priorities for Patient-Centered Outcomes Research for Serious Mental Illness. Research White Paper. In RTI-UNC Institute Evidence-based Practice Center (Ed.): Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- Julius RJ, Novitsky MA Jr., & Dubin WR (2009). Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract, 15(1), 34–44. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, & Saccuzzo DP (2001). Psychological Testing: Principle, Applications and Issues (5th ed.). Belmont, CA: Wadsworth. [Google Scholar]
- Katzman e. M., Holman E, & Ashley J (1993). A nurse managed center’s client satisfaction survey. Nursing and Health Care, 14(8), 420–425. [PubMed] [Google Scholar]
- Kelly GR, & Scott JE (1990). Medication compliance and health education among outpatients with chronic mental disorders. Med Care, 28(12), 1181–1197. [DOI] [PubMed] [Google Scholar]
- Kemp R, Kirov G, Everitt B, Hayward P, & David A (1998). Randomised controlled trial of compliance therapy. 18-month follow-up. Br J Psychiatry, 172, 413–419. [DOI] [PubMed] [Google Scholar]
- Koekkoek B, van Meijel B, Schene A, & Hutschemaekers G (2010). Development of an intervention program to increase effective behaviours by patients and clinicians in psychiatric services: Intervention Mapping study. BMC Health Serv Res, 10, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumana CR, Ching TY, Cheung E, Kong Y, Kou M, Chan CK, et al. (1998). Antiulcer drug prescribing in hospital successfully influenced by “immediate concurrent feedback”. Clin Pharmacol Ther, 64(5), 569–574. [DOI] [PubMed] [Google Scholar]
- Lacro JP, Dunn LB, Dolder CR, Leckband SG, & Jeste DV (2002). Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry, 63(10), 892–909. [DOI] [PubMed] [Google Scholar]
- Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, et al. (2009). A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry, 166(2), 152–163. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med, 353(12), 1209–1223. Epub 2005 Sep 1219. [DOI] [PubMed] [Google Scholar]
- Magidson J (Ed.). (1994). The CHAID approach to segmentation modeling: Chi-squared automatic interaction detection. Oxford: Blackwell. [Google Scholar]
- McDonald HP, Garg AX, & Haynes RB (2002). Interventions to enhance patient adherence to medication prescriptions Journal of the American Medical Association, 288(22), 2868–2879. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Goldstein MJ, Nuechterlein KH, Snyder KS, & Doane JA (1986). Expressed emotion, affective style, lithium compliance, and relapse in recent onset mania. Psychopharmacol Bull, 22(3), 628–632. [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational Interviewing: Preparing people for change (2 ed.). New York, NY: Guilford Press. [Google Scholar]
- Morgan DL (1997). Focus groups as qualitative research. (2nd ed.). Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Morken G, Grawe RW, & Widen JH (2007). Effects of integrated treatment on antipsychotic medication adherence in a randomized trial in recent-onset schizophrenia. J Clin Psychiatry, 68(4), 566–571. [DOI] [PubMed] [Google Scholar]
- Murray N, Kelder SH, Parcel GS, & Orpinas P (1998). Development of an intervention map for a parent education intervention to prevent violence among Hispanic middle school students. J School Health, 68(2), 46–52. [DOI] [PubMed] [Google Scholar]
- Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, & Weiden PJ (2000). Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv, 51(2), 216–222. [DOI] [PubMed] [Google Scholar]
- Perkins DO (1999). Adherence to antipsychotic medications. J Clin Psychiatry, 60(Suppl 21), 25–30. [PubMed] [Google Scholar]
- Pyne JM, Bean D, & Sullivan G (2001). Characteristics of patients with schizophrenia who do not believe they are mentally ill. J Nerv Ment Dis, 189(3), 146–153. [DOI] [PubMed] [Google Scholar]
- Pyne JM, McSweeney J, Kane HS, Harvey S, Bragg L, & Fischer E (2006). Agreement between patients with schizophrenia and providers on factors of antipsychotic medication adherence. Psychiatr Serv, 57(8), 1170–1178. [DOI] [PubMed] [Google Scholar]
- Rodgers BL, & Cowles KV (1993). The qualitative research audit trail: a complex collection of documentation. Research in Nursing and Health, 16, 219–226. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Chang S, Choe Y, Cramer J, Xu W, Thomas J, et al. (2000). Medication continuation and compliance: a comparison of patients treated with clozapine and haloperidol. J Clin Psychiatry, 61(5), 382–386. [PubMed] [Google Scholar]
- Rosenstock IM, Strecher VJ, & Becker MH (1988). Social learning theory and the Health Belief Model. Health Education Quarterly, 15(2), 175–183. [DOI] [PubMed] [Google Scholar]
- Rubenstein L, McCoy J, Cope D, Barrett P, Hirsch S, Messer K, et al. (1995). Improving patient qualty of life with feedback to physicians about functional status. J. Gen. Intern. Med, 10, 607–614. [DOI] [PubMed] [Google Scholar]
- Rusch N, & Corrigan PW (2002). Motivational interviewing to improve insight and treatment adherence in schizophrenia. Psychiatr Rehabil J, 26(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Santone G, Rucci P, Muratori ML, Monaci A, Ciarafoni C, & Borsetti G (2008). Attitudes toward medication in inpatients with schizophrenia: A cluster analytic approach. Psychiatry Research, 158(3), 324–334. [DOI] [PubMed] [Google Scholar]
- Schmid AA, Andersen J, Kent T, Williams LS, & Damush TM (2010). Using intervention mapping to develop and adapt a secondary stroke prevention program in Veterans Health Administration medical centers. Implement Sci, 5, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splaine M, Bierman AS, & Wasson JH (1998). Implementing a strategy for improving care: lessons from studying those age 80 and older in a health system. J Ambulatory Care Manage, 21(3), 56–59. [DOI] [PubMed] [Google Scholar]
- Terkelsen KG, & Menikoff A (1995). Measuring the costs of schizophrenia. Implications for the post-institutional era in the US. [Review] [178 refs]. Pharmacoeconomics, 8(3), 199–222. [DOI] [PubMed] [Google Scholar]
- Thomson O’Brien MA, Oxman AD, Davis DA, Haynes RB, Freemantle N, & Harvey EL (2001, 11/29/00). Audit and feedback versus alternative strategies: effects on professional practice and health care outcomes. [DOI] [PMC free article] [PubMed]
- Tierney WM, Hui SL, & McDonald CJ (1986). Delayed feedback of physician performance versus immediate reminders to perform preventive care. Med Care, 24(8), 659–666. [DOI] [PubMed] [Google Scholar]
- Valenstein M, Kavanagh J, Lee T, Reilly P, Dalack GW, Grabowski J, et al. (2011). Using a pharmacy-based intervention to improve antipsychotic adherence among patients with serious mental illness. Schizophr Bull, 37(4), 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Someren MW, Barnard YF, & Sandberg JAC (1994). The Think Aloud Method: A practical guide to modelling cognitive processes. London: Academic Press Limited. [Google Scholar]
- Velligan DI, Diamond PM, Mintz J, Maples N, Li X, Zeber J, et al. (2008). The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull, 34(3), 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Weiden PJ, Sajatovic M, Scott J, Carpenter D, Ross R, et al. (2009). The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry, 70 Suppl 4, 1–46; quiz 47–48. [PubMed] [Google Scholar]
- Voruganti LP, Baker LK, & Awad AG (2008). New generation antipsychotic drugs and compliance behaviour. Current Opinion in Psychiatry, 21(2), 133–139. [DOI] [PubMed] [Google Scholar]
- Wasson JH, Splaine ME, Bazos D, & Fisher ES (1998). Overview: working inside, outside, and side by side to improve the quality of health care. Jt Comm J Qual Improv, 24(10), 513–517. [DOI] [PubMed] [Google Scholar]
- Weiden PJ, & Olfson M (1995). Cost of relapse in schizophrenia. Schizophr Bull, 21(3), 419–429. [DOI] [PubMed] [Google Scholar]
- Winkens RA, Pop P, Grol RP, Kester AD, & Knottnerus JA (1992). Effect of feedback on test ordering behaviour of general practitioners. BMJ, 304(6834), 1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistedt B (1981). A depot neuroleptic withdrawal study. A controlled study of the clinical effects of the withdrawal of depot fluphenazine decanoate and depot flupenthixol decanoate in chronic schizophrenic patients. Acta Psychiatr Scand, 64(1), 65–84. [DOI] [PubMed] [Google Scholar]
- Zygmunt A, Olfson M, Boyer CA, & Mechanic D (2002). Interventions to improve medication adherence in schizophrenia. Am J Psychiatry, 159(10), 1653–1664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


