Abstract
Introduction:
Early-onset sepsis (EOS) and late-onset Sepsis (LOS) are common diagnoses entertained in sick newborns treated in neonatal intensive care units (NICUs), and antibiotics are the medications most prescribed in NICUs. Antibiotic stewardship programs have an important impact on limiting unnecessary antibiotic use.
Methods:
Following the Model for Improvement, between 2/1/16 and 1/31/17, at a level 3 NICU, a multidisciplinary team implemented PDSA cycles to promote antibiotic stewardship practices for newborns at risk of EOS and LOS. The main goal was to decrease the antibiotic usage rate (AUR) safely. Primary strategies included discontinuing antibiotics within 24 hours of life if the newborn was stable, and the blood culture was negative for EOS and implementing an “antibiotic time-out” during rounds.
Results:
For all newborns admitted to our NICU, the AUR decreased, for EOS from 137 to 32 days per 1000 patient days (77% reduction) and for LOS from 277 to 121 days per 1000 patient days (56% reduction). We demonstrated the sustainability of both EOS-AUR and LOS-AUR during the 2 years postcompletion of the intervention period. There were no adverse effects of reducing the AUR.
Conclusion:
Interventions that reduce unnecessary antibiotic use in the NICU are safe and prevent excessive antibiotic exposure.
INTRODUCTION
Antibiotics are the most commonly prescribed medications in the neonatal intensive care units (NICU).1 Their use varies widely among NICUs, as reported by a study that demonstrated a 40-fold difference in the use of antibiotics regardless of the level of care and the complexity of patients.2 A significant side effect of early antibiotic exposure during the perinatal period is disruption of the normal development of intestinal microbiota in the newborn.3,4 Also, using antibiotics in premature newborns makes them more susceptible to late-onset sepsis (LOS) (sepsis occurring at or after 72 hours of life),5,6 necrotizing enterocolitis (NEC),7 fungal infections,8 bronchopulmonary dysplasia,9 severe retinopathy of prematurity, and higher mortality.10 Antibiotic exposure during infancy is associated with increased childhood obesity,11 asthma,12 allergic disorders,13 and inflammatory bowel disease.14 Overuse of antibiotics induces the selection of multi-drug-resistant organisms. Each year in the United States, approximately 2.8 million people have infections with antibiotic-resistant bacteria or fungi, and more than 35,000 people die.15
There are multiple reasons why antibiotics are prescribed excessively in the NICU. First, providers commonly initiate antibiotic treatment in the presence of a symptomatic newborn in whom sepsis cannot initially be ruled out. It often occurs in premature newborns admitted shortly after birth16 and for those infants that present with clinical deterioration while being treated in the NICU. Second, clinicians often hesitate to discontinue antibiotics despite a negative blood culture due to persistent abnormal symptomatology or laboratory values (culture-negative sepsis).17 However, Cantey et al18 concluded that (1) adequately drawn blood cultures have excellent sensitivity even at low-level bacterial counts (1–4 CFU/mL); (2) blood cultures are reliable after maternal intrapartum antibiotic prophylaxis; and (3) a significant majority of ill-appearing newborns evaluated for sepsis are uninfected.
Various international and national campaigns have addressed the utility of antibiotic stewardship. The Centers for Disease Control and Prevention (CDC) has recently updated the Core Elements of Hospital Antibiotic Stewardship Programs in the United States.19 Many institutions have formed local antibiotic stewardship committees and collaboratives. The “Choosing Antibiotics Wisely Collaborative” from the Vermont Oxford Network (VON) represents one of these efforts. A few participating NICUs in this collaborative have already published their results.20–22 This quality improvement (QI) project aimed to safely reduce the AUR in our NICU population by 20% over 12 months (February 2016 to January 2017).
METHODS
Context:
The NICU at Saint Joseph Hospital in Denver, Colarado is a 40-bed, level 3 unit with approximately 500 admissions per year, and 80 newborns are very low birth weight. Most of our NICU patients are inborn, and we transfer to a level 4 unit only those newborns with congenital heart disease that require surgery or candidates for ECMO therapy. The unit’s staff includes 8 neonatologists, 10 neonatal nurse practitioners, approximately 100 nurses, 3 pharmacists, 6 respiratory therapists, and other ancillary staff, such as occupational and physical therapists. Since 2001, our institution has participated in various QI collaboratives with VON. Our team has developed a culture of evidence-based practice and collaboration through a well-standardized process23 for guideline implementation that involves timely dissemination of the guidelines, approval at the monthly Neonatal Quality Assurance meeting, and education of the guidelines by the NICU nurse educator.
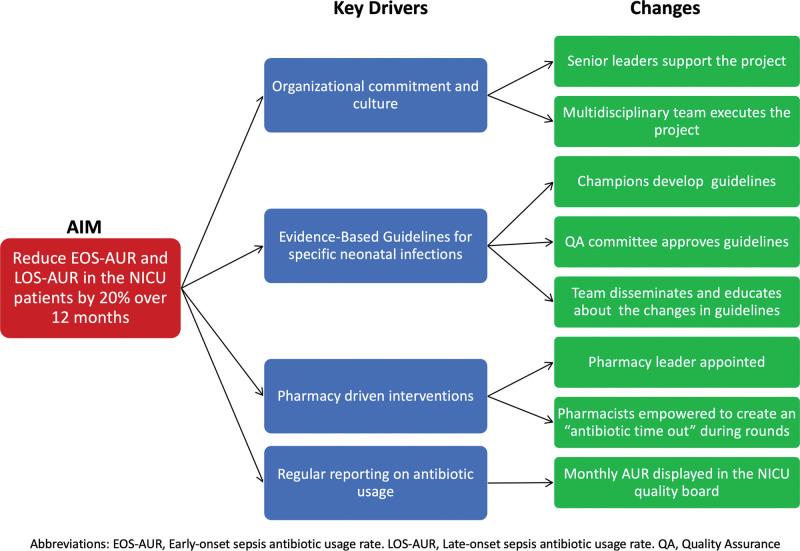
By the end of 2015, our unit accepted the invitation from VON to participate in the “Choosing Antibiotic Wisely Collaborative.” A multidisciplinary team formed by two neonatologists, two pharmacists, two NNPs, and a NICU educator guided this QI project. We began the project by creating a key driver diagram (Fig. 1).
Fig. 1.
Antibiotic Stewardship Project Key Driver Diagram.
PRIMARY DRIVERS
Primary Driver #1:
Organizational commitment and culture. The chief nursing officer, the NICU medical director, and the NICU head nurse provided support. In addition, our existing multidisciplinary team to prevent nosocomial infections led the antibiotic stewardship project.
Primary Driver #2:
Guidelines for specific neonatal infections. The VON Audit identified the need to update or create evidence-based clinical practice guidelines for diagnosing and treating early-onset sepsis (EOS; sepsis occurring before 72 hours of life), LOS, NEC, and urinary tract infections.
Primary Driver #3:
We considered that pharmacy-driven interventions were essential for the success of this project. Our team empowered NICU pharmacists to implement the “antibiotic time-out” during daily multidisciplinary rounds. A NICU pharmacist, one of the project’s leaders, calculated the AUR.
Primary Driver #4:
The NICU pharmacist published monthly reports of the AUR in the “Quality Board” located in a visible area of the NICU and the NICU weekly electronic newsletter.
Measures:
We used the monthly AUR to monitor our progress. AUR is the total number of days infants are exposed to antibacterial agents, given intravenously or intramuscularly, per 1000 patient days (for all NICU patients).24 Multiple antibiotics administered to the same infant on a single day were considered 1 antibiotic day. Our pharmacist obtained the total number of days of exposure to antibacterial agents from the Electronic Medical Record (EMR) (Epic, Epic Systems, Verona, Wis.) using the clinical surveillance platform VigiLanz (VigiLanz Corporation, Chicago). A neonatologist obtained the monthly patient days from the NICU database (Neodata, Isoprime Corp. Lisle, Ill.). EOS-AUR is the number of antibiotic days administered in patients during the first 3 days of life per 1000 patient days in the NICU. Similarly, LOS-AUR is the number of antibiotic days administered in patients after the third day of life per 1000 patient days in the NICU.
As balancing measures, we followed the yearly incidence of LOS and readmissions after NICU discharge with bacterial sepsis. In addition, a neonatologist retrieved the patient’s data from the NICU database (Neodata).
We continued this project past the original end date of January 2017 until December 2019 to assess the sustainability of the improvement. However, we did not report AUR after 2019 since the leading author of this article (AP) retired as a neonatologist in July 2020.
PDSA CYCLES
Following the Model for Improvement,25 we implemented our strategies sequentially using Plan, Do, Study, Action (PDSA) cycles (Table 1).
Table 1.
PDSA Cycles
| PDSA Cycle | Description | |
|---|---|---|
| Feb 2015 | VON Audit | Our team discussed the survey results and designated the PDSA cycles to achieve our aim. |
| Apr 2015 | Update EOS and LOS guidelines, followed by the creation of standardized orders in the EMR | On admission, newborns at risk for EOS were to receive two doses of ampicillin (50 mg/kg IV q 12 h) and one dose of gentamicin. If the blood culture was negative and the infant’s clinical condition had improved, providers did not need to prescribe further doses of antibiotics.For LOS, the initial antibiotic treatment included nafcillin and gentamicin. Attending physicians decided the duration of antibiotic therapy.EOS and LOS guidelines did not include the determination of CRP or other inflammatory markers to aid in diagnosing sepsis or prolonging the duration of antibiotic treatment. |
| May 2015 | Antibiotic “Time-Out” | During daily rounds, pharmacists questioned continued antibiotic usage in the presence of a negative blood culture. Providers documented in the EMR the reasons for continuing antibiotic treatment in newborns with a negative blood culture. |
| Jun 2015–Dec 2019 | AUR Display in the NICU | We displayed run charts and SPCC in the NICU Quality Board and the unit’s electronic newsletter. |
| Aug 2017 | Parents’ participation in rounds | Parents began participating in daily rounds as part of the Integrated Family Care initiative. |
| Dec 2017 | Defer CBC after 4th hour of life | The team modified the admission order set to obtain a CBC after completing the 4th hour of life. |
PDSA, plan do study act; VON, Vermont-Oxford Network; CRP, c-reactive protein; SPCC, statistical process control chart.
PDSA #1:
VON Audit Day One: A Neonatologist performed the VON audit on a random day during February 2016. We shared the audit results with the multidisciplinary team during our monthly meetings to reflect on our current performance using the 7 CDC core elements and general antibiotic stewardship program practices.26
PDSA #2:
Guideline Development and Implementation: A designated “champion” member of the multidisciplinary team updated or developed a clinical practice guideline for diagnosing and treating EOS and LOS. The team reviewed the relevant literature in our monthly evidence-based journal club.
By April 2016, we standardized the therapy component of the EOS guideline developing an order set in the EMR for prescribing antibiotics for newborns admitted with the presumptive diagnosis of EOS. For infants with a gestational age ≥ 34 weeks gestation, we used the neonatal sepsis calculator to aid in diagnosing EOS.27 More premature newborns with a relatively low risk of EOS28 received antibiotics based on their clinical condition. On admission, these newborns were to receive 2 doses of ampicillin (50 mg/kg IV q 12 hours) and 1 dose of gentamicin adjusted to their gestational age (Neofax micromedexsolutions.com, Merative, Apple, Inc. Cupertino, Calif.). If the blood culture was negative and the infant’s clinical condition had improved, providers did not need to prescribe further doses of antibiotics. However, close observation of the newborns continued, looking for clinical signs associated with an infection.
Our microbiology department has used the BACTEC 9240 fluorometric detection system to detect positive blood cultures timely since December 2013. A review of all the positive blood cultures of EOS in our NICU from 2014 and 2015 revealed that all the cultures were positive within the first 24 hours. We used this information to update our Clinical Practice Guidelines for EOS. Furthermore, our team continued documenting the time of positivity of the blood cultures throughout this project, and we validated that all positive cultures grew within 24 hours.
For LOS, the order set in the EMR standardized the antibiotic treatment to use nafcillin and gentamicin. However, we did not limit the initial number of antibiotic doses due to the possibility of positive blood cultures of Coagulase Negative Staphylococcus (CONS) and other organisms that may take longer to grow in blood cultures. Instead, the attending physician decided on the duration of the antibiotic treatment.
EOS and LOS guidelines did not include the determination of c-reactive Protein (CRP) or other inflammatory markers to aid in diagnosing sepsis or prolonging the duration of antibiotic treatment.
Our team also developed new guidelines for the treatment of NEC and urinary tract infections.
PDSA #3:
Antibiotic Time-Out: A NICU pharmacist implemented the “antibiotic time-out” during daily multidisciplinary rounds. By May 2016, our team empowered the unit pharmacists to raise the question of continued antibiotic usage in the presence of a negative blood culture during daily rounds. Moreover, we encouraged providers to document in the EMR the reasons for continuing antibiotic treatment in newborns with a negative blood culture. If the blood culture was positive, providers adjusted the dose, duration, and type of the antibiotic according to the antibiogram and the patient’s clinical condition.
PDSA #4:
AUR Display in the NICU: We calculated the monthly AUR for 2015 before initiating the project. Initially, we plotted EOS-AUR and LOS-AUR in run charts. After at least 18 months of monthly AUR values (June 2016), we created a Statistical Process Control Chart. We displayed all charts in the NICU Quality Board and the unit’s electronic newsletter.
PDSA #5:
Parents in Rounds: Since August 2017, parents of infants have been allowed to be present and participate in daily rounds. Providers informed parents of the reasons for initiating, continuing, or discontinuing antibiotics.
PDSA #6:
Defer CBC after the 4th hour of life: Literature evidence suggests that CBCs obtained within the first 4 hours of life have low sensitivity and specificity for EOS.29 The team modified the admission order set in December 2017 to obtain a CBC after completing the 4th hour of life.
DATA ANALYSIS
Our team plotted monthly AUR in annotated statistical process control charts (SPC p’ Laney charts) created by QIMacros for Excel starting in 2015. We recalculated the centerline and control limits as indicated. We performed descriptive statistics and logistic regression analysis using Stata/BE 17.0 for Mac (College Station, Tex.). We set LOS as the dependent variable for the logistic regression analysis. We added the independent variables birth weight, gestational age, and year of birth in a stepwise manner.
Ethical considerations:
The Saint Joseph Hospital Institutional Review Board reviewed the project and qualified it as QI.
RESULTS
Between January 1, 2015, and December 31, 2019, there were 2629 newborns admitted to our unit. The average number of admissions per year was 525 [range 447–575], with an average of 83 VLBW infants per year [range 66–102] (Table 2).
Table 2.
Incidence of EOS and LOS by Birth Weight and Year of Birth
| <1500 g (N = 417) | ≥1500 g (N = 2212) | |||||
|---|---|---|---|---|---|---|
| N | EOS (%) | LOS (%) | N | EOS (%) | LOS (%) | |
| 2015 | 66 | 2 (3.0) | 4 (6.1) | 381 | 1 (0.3) | 0 (0) |
| 2016 | 85 | 2 (2.3) | 5 (5.9) | 431 | 0 (0) | 0 (0) |
| 2017 | 102 | 1 (0.9) | 9 (8.8) | 423 | 0 (0) | 0 (0) |
| 2018 | 85 | 3 (3.5) | 3 (3.5) | 490 | 1 (0.2) | 3 (0.6) |
| 2019 | 79 | 1 (1.3) | 5 (6.3) | 487 | 4 (0.8) | 1 (0.2) |
| Total | 417 | 9 (2.2) | 26 (6.2) | 2212 | 6 (0.3) | 4 (0.2) |
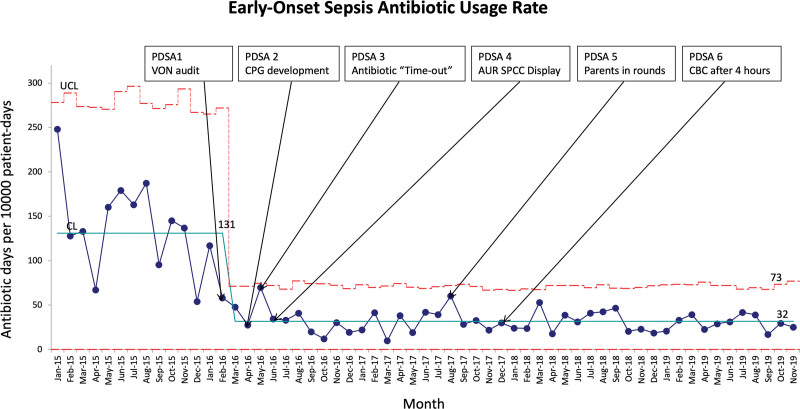
The baseline AUR-EOS in 2015 was 137. We realized a 77% decrease (from 137 to 32 days) in this metric shortly after performing the VON audit in February 2016 (Fig. 2). This AUR remained stable for the following 2 years.
Fig. 2.
Annotated monthly p’ Laney chart for AUR for all infants admitted with possible EOS diagnosis. The solid light blue line represents the mean AUR. Broken red lines represent the upper control limits (UCL) and the lower control limits (LCL).
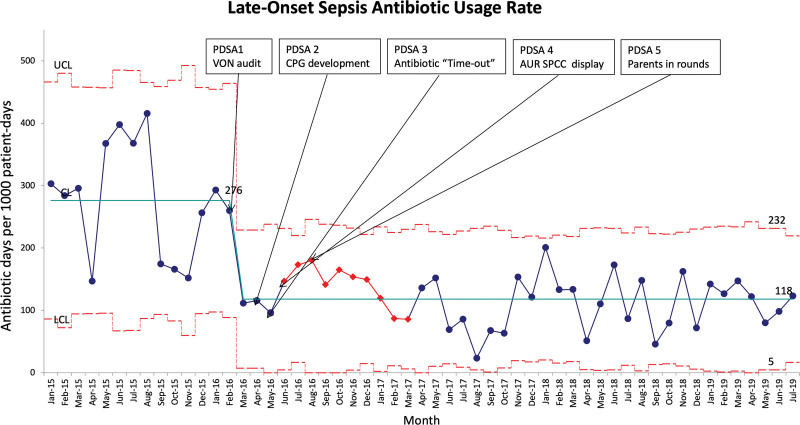
The baseline AUR-LOS in 2015 was 277 days. Similarly to what we reported for EOS, we realized a reduction of 56% in AUR-LOS (from 277 to 121 days) after performing the VON audit but showing more variability than the AUR-EOS (Fig. 3). While we also achieved sustainability for AUR-LOS, monthly data showed more variability than AUR-EOS. The most prevalent organism isolated in both EOS and LOS was E. coli (See table 1, Supplemental Digital Content 1, which shows positive blood cultures 2015–2019. http://links.lww.com/PQ9/A493).
Fig. 3.
Annotated monthly p’ Laney chart for AUR for all infants diagnosed with possible LOS. The solid light blue line represents the mean AUR. Broken red lines represent the upper control limits (UCL) and the lower control limits (LCL). The seven red points represent successive consecutive points above the mean and may constitute a “trend”; however, the Institute for Health Care Improvement uses the rule of 8 to prevent a type I error.
During the baseline period in 2015, 40% of the infants admitted to the NICU received at least 1 day of antibiotics during their NICU stay compared with only 25% for the duration of the QI project (P < 0.001; Table 3). There was also a significant reduction in the number of infants that received 2 or more days of antibiotic treatment (24.8%–6%) (P < 0.001) (Table 3).
Table 3.
Days on Antibiotics
| 0 days | 1 day N (%) | ≥2 d | Total | |
|---|---|---|---|---|
| 2015 | 268 (60.0) | 68 (15.2) | 111 (24.8) | 447 (100) |
| 2016–2019 | 1645 (75.4) | 406 (18.6) | 130 (6.0) | 2181 (100) |
| Total | 1913 | 474 | 241 | 2628 |
0 days versus ≥ 1-day χ2 = 44.8 P < 0.001.
0–1 days versus ≥ 2 days χ2 = 158.6 P < 0.001.
Stepwise logistic regression analysis showed that only gestational age at birth was statistically significant (P < 0.001) for the development of LOS (See table 2, Supplemental Digital Content 2, which shows stepwise logistic regression for late-onset sepsis. http://links.lww.com/PQ9/A494). This result suggests that the temporal change in the approach to the antibiotic treatment for possible EOS did not increase the occurrence of LOS. There were no cases of newborns readmitted to the NICU for treatment of LOS.
DISCUSSION
Our QI project reduced EOS-AUR and LOS-AUR in newborns admitted to a level 3-B NICU in Colorado. Furthermore, it showed sustainability during the 3 years of observation (2017–2019). Various antibiotic stewardship QI projects in newborns20,22 have reported successful reductions in antibiotic usage associated with implementing strategies like ours. Our QI project differs from others in several ways. First, we separately analyzed the effect of the interventions to reduce the AUR for EOS and LOS. Second, we did not continue antibiotic treatment for newborns with presumptive EOS who were clinically stable with negative blood culture after 24 hours, as opposed to other QI investigators who wait until 36 hours21 or 48 hours30,31 hours for antibiotic discontinuation. Our admission order set included the administration of 2 doses of ampicillin and one dose of gentamicin. Our approach is similar to the one reported recently by Sanchez et al for treating EOS.32 We based our decision to discontinue antibiotics earlier on our initial retrospective blood culture analysis that all the organisms involved in EOS became culture-positive in less than 24 hours. The study by Marks et al found that all but one of their positive blood cultures in EOS became positive within 24 hours.33 It supports this approach. Similarly, Arias-Felipe et al reported that 100% of gram-negative and 97.9% of gram-positive organisms grew within 24 hours in neonatal EOS and LOS.34 Third, by only ordering a few doses of antibiotics for EOS and discussing the results of the blood cultures during the rounds of “time-out,” we compelled our providers to write a new order for antibiotic therapy and to justify further their decision to continue antibiotic treatment in the presence of a negative blood culture. The microbiology department’s immediate reporting of blood culture results in the EMR facilitated the implementation of this strategy. The authors acknowledge that this approach may not be feasible in other NICUs with different medical records, laboratory facilities, or protocols. Fourth, the order set for LOS included using nafcillin and gentamicin as the initial antibiotic choice with no end point specified, recognizing that some organisms causing LOS may take longer than 24 hours to turn positive. Vancomycin was used only in the presence of infection due to documented or suspected MRSA. Fifth, in contrast with other QI projects,31 we did not encourage using inflammation markers in our EOS or LOS clinical practice guidelines to diagnose or continue antibiotic treatment due to their poor specificity and positive predictive value.35,36 Furthermore, guiding therapy by repeated CRP determinations may result in further unnecessary investigations, increased lumbar punctures, longer durations of antibiotic treatment, and hospitalization.37 Sixth, acknowledging the benefits associated with the daily participation of a NICU pharmacist in multidisciplinary rounds in similar projects,38 our NICU pharmacist was present in multidisciplinary rounds 7 days a week. Seventh, we continued to report on the sustainability of our project 36 months after the completion of our initial QI project goal. Eighth, we observed a reduction of EOS-AUR and the LOS-AUR around the time of completion of the VON Audit in February 2016. Our team discussed the survey results and designated the PDSA cycles that we believed would help us achieve our aim. This intervention probably influenced providers’ antibiotic prescription approach before the guidelines were updated. Implementing the other interventions through the PDSA methodology likely contributed to the sustainability of our project.
We did not try to influence providers in their decision to initiate antibiotics. However, a CBC is frequently obtained on admission to aid in the differential diagnosis of EOS. Our last PDSA cycle addressed the optimal postnatal age (in hours) to get a CBC in cases of suspected EOS.29 Implementing this PDSA did not affect the subsequent AUR, as we observed a steady EOS-AUR for the remainder of the observation period.
Other unique factors may have further guided the success of this QI project. First, our NICU has a consistent group of neonatologists, nurse practitioners, nurses, and pharmacists, most of whom had previously participated in other QI projects within our unit. Second, our team has a well-established culture of evidence-based practice23 that facilitated the “buy-in” of providers in adopting new evidence-based clinical practice guidelines.
A limitation of our QI project was the lack of a pediatric infectious disease consultant in our institution. However, pediatric infectious disease consultants from Children’s Hospital Colorado staff were available for consultation.
Reassuringly, no infant needed evaluation for LOS after discontinuing antibiotics, and no infants required readmission after discharge for LOS. The logistic regression showed that the reduction of antibiotics in our unit during the project period did not result in an increased incidence of LOS; however, due to the low incidence of LOS per year, we cannot exclude the existence of a type II error.
Another limitation of our project is that we did not try to quantify the value assessment (quality/cost) of the changes implemented. However, there was a subjective impression from our pharmacists and nurses that this project decreased the hours dedicated to preparing and administering antibiotic doses, specifically for newborns admitted with presumptive EOS.
CONCLUSIONS
Our project introduces new initiatives that may be adaptable to other NICUs to reduce unnecessary antibiotic usage. EMR antibiotic order sets with limited antibiotic doses, discouraging the use of inflammatory markers to guide the initiation or continuation of antibiotics, and involving a multidisciplinary team approach to antibiotic stewardship in rounds, are some elements that NICU teams can add to create a successful antibiotic stewardship program.
ACKNOWLEDGMENTS
The authors thank the NICU providers, nurses, and pharmacists, particularly Ann Ryan, MD, the NICU medical director, for supporting this project. Thanks to Dr. Leandro Huayanay Falconí from the Universidad Peruana Cayetano Heredia, Lima-Perú, for his advice on some of the project’s statistical aspects.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online June 7, 2023
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Pantoja A, Sveum S, Frost S, Duran A, Burks J, Schernecke C, Feinberg M. New Strategies to Reduce Unnecessary Antibiotic Use in the Neonatal Intensive Care Unit: A Quality Improvement Initiative. Pediatr Qual Saf 2023;8:e659.
REFERENCES
- 1.Clark RH, Bloom BT, Spitzer AR, et al. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–1987. [DOI] [PubMed] [Google Scholar]
- 2.Schulman J, Dimand RJ, Lee HC, et al. Neonatal intensive care unit antibiotic use. Pediatrics. 2015;135:826–833. [DOI] [PubMed] [Google Scholar]
- 3.Azad MB, Konya T, Persaud RR, et al. ; CHILD Study Investigators. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123:983–993. [DOI] [PubMed] [Google Scholar]
- 4.Nogacka A, Salazar N, Suarez M, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome. 2017;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ting JY, Roberts A, Sherlock R, et al. ; Canadian Neonatal Network Investigators. Duration of initial empirical antibiotic therapy and outcomes in very low birth weight infants. Pediatrics. 2019;143:e20182286. 10.1542/peds.2018-2286. [DOI] [PubMed] [Google Scholar]
- 6.Kuppala VS, Meinzen-Derr J, Morrow AL, et al. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten CM, McDonald S, Stoll B, et al. ; National Institute for Child Health and Human Development Neonatal Research Network. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. 2006;118:717–722. [DOI] [PubMed] [Google Scholar]
- 9.Cantey JB, Huffman LW, Subramanian A, et al. Antibiotic exposure and risk for death or bronchopulmonary dysplasia in very low birth weight infants. J Pediatr. 2017;181:289–293.e1. [DOI] [PubMed] [Google Scholar]
- 10.Ting JY, Synnes A, Roberts A, et al. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr. 2016;170:1181–1187. [DOI] [PubMed] [Google Scholar]
- 11.Saari A, Virta LJ, Sankilampi U, et al. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135:617–626. [DOI] [PubMed] [Google Scholar]
- 12.Kummeling I, Stelma FF, Dagnelie PC, et al. Early life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort Study. Pediatrics. 2007;119:e225–e231. [DOI] [PubMed] [Google Scholar]
- 13.Metsala J, Lundqvist A, Virta LJ, et al. Mother’s and offspring’s use of antibiotics and infant allergy to cow’s milk. Epidemiology. 2013;24:303–309. [DOI] [PubMed] [Google Scholar]
- 14.Kronman MP, Zaoutis TE, Haynes K, et al. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130:e794–e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The AMR Challenge. Available at https://www.cdc.gov/drugresistance/intl-activities/amr-challenge.html. Accessed May 21, 2021. [Google Scholar]
- 16.Puopolo KM, Mukhopadhyay S, Hansen NI, et al. ; NICHD Neonatal Research Network. Identification of extremely premature infants at low risk for early-onset sepsis. Pediatrics. 2017;140:e20170925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prusakov P, Goff DA, Wozniak PS, et al. ; Global NEO-ASP Study Group. A global point prevalence survey of antimicrobial use in neonatal intensive care units: the no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMedicine. 2021;32:100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantey JB, Baird SD. Ending the culture of culture-negative sepsis in the neonatal ICU. Pediatrics. 2017;140:e20170044. [DOI] [PubMed] [Google Scholar]
- 19.Core Elements of Hospital Antibiotic Stewardship Programs. Available at https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed May 20, 2021. [Google Scholar]
- 20.Astorga MC, Piscitello KJ, Menda N, et al. Antibiotic stewardship in the neonatal intensive care unit: effects of an automatic 48-hour antibiotic stop order on antibiotic use. J Pediatric Infect Dis Soc. 2019;8:310–316. [DOI] [PubMed] [Google Scholar]
- 21.Bhat R, Custodio H, McCurley C, et al. Reducing antibiotic utilization rate in preterm infants: a quality improvement initiative. J Perinatol. 2018;38:421–429. [DOI] [PubMed] [Google Scholar]
- 22.Makri V, Davies G, Cannell S, et al. Managing antibiotics wisely: a quality improvement programme in a tertiary neonatal unit in the UK. BMJ Open Qual. 2018;7:e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantoja AF, Britton JR. An evidence-based, multidisciplinary process for implementation of potentially better practices using a computerized medical record. Research Support, Non-U.S. Gov’t Review. Int J Qual Health Care. 2011;23:309–316. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim OM, Polk RE. Antimicrobial use metrics and benchmarking to improve stewardship outcomes: methodology, opportunities, and challenges. Infect Dis Clin North Am. 2014;28:195–214. [DOI] [PubMed] [Google Scholar]
- 25.Langley G, Nolan K, Nolan T, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance (2nd edition). Jossey-Bass Publishers; 2009. [Google Scholar]
- 26.Dukhovny D, Buus-Frank ME, Edwards EM, et al. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics. 2019;144:e20190589. [DOI] [PubMed] [Google Scholar]
- 27.Puopolo KM, Draper D, Wi S, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. multicenter study research support, N.I.H., extramural. Pediatrics. 2011;128:e1155–e1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puopolo KM, Benitz WE, Zaoutis TE, Committee on Fetus and Newborn, Committee on Infectious Diseases. Management of neonates born at </=34 6/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics. 2018;142:e20182896. [DOI] [PubMed] [Google Scholar]
- 29.Newman TB, Puopolo KM, Wi S, et al. Interpreting complete blood counts soon after birth in newborns at risk for sepsis. Pediatrics. 2010;126:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berardi A, Zinani I, Rossi C, et al. Antibiotic use in very low birth weight neonates after an antimicrobial stewardship program. Antibiotics (Basel). 2021;10:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitano T, Takagi K, Arai I, et al. A simple and feasible antimicrobial stewardship program in a neonatal intensive care unit of a Japanese community hospital. J Infect Chemother. 2019;25:860–865. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez PJ, Prusakov P, de Alba Romero C, et al. Short-course empiric antibiotic therapy for possible early-onset sepsis in the NICU. J Perinatol. 2023;1–5. [DOI] [PubMed] [Google Scholar]
- 33.Marks L, de Waal K, Ferguson JK. Time to positive blood culture in early onset neonatal sepsis: a retrospective clinical study and review of the literature. J Paediatr Child Health. 2020;56:1371–1375. [DOI] [PubMed] [Google Scholar]
- 34.Arias-Felipe A, Ramirez-Berrios J, Recio-Martinez R, et al. Determining time to positivity of blood cultures in a neonatal unit. J Pediatric Infect Dis Soc. 2022;11:510–513. [DOI] [PubMed] [Google Scholar]
- 35.Tiozzo C, Mukhopadhyay S. Noninfectious influencers of early-onset sepsis biomarkers. Pediatr Res. 2022;91:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JVE, Meader N, Wright K, et al. Assessment of C-reactive protein diagnostic test accuracy for late-onset infection in newborn infants: a systematic review and meta-analysis. JAMA Pediatr. 2020;174:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukherjee A, Davidson L, Anguvaa L, et al. NICE neonatal early onset sepsis guidance: greater consistency, but more investigations, and greater length of stay. Arch Dis Child Fetal Neonatal Ed. 2015;100:F248–F249. [DOI] [PubMed] [Google Scholar]
- 38.Vyas DP, Quinones-Cardona V, Gilfillan MA, et al. Reduction of unnecessary antibiotic days in a level IV neonatal intensive care unit. Antimicrob Steward Health Epidemiol. 2022;2:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.