Abstract
Background:
Current national guidelines recommend against chest X-rays (CXRs) for patients with acute asthma exacerbation (AAE). The overuse of CXRs in AAE has become a concern, prompting the need for a quality improvement (QI) project to decrease CXR usage through guideline-based interventions. We aimed to reduce the percentage of CXRs not adhering to national guidelines obtained for pediatric patients presenting to the Emergency Department (ED) with AAE by 50% within 12 months of project initiation.
Methods:
We conducted this study at a New York City urban level-2 trauma center. The team was composed of members from the ED and pediatric departments. Electronic medical records of children aged 2 to 18 years presenting with AAE were evaluated. Monthly data on CXR utilization encompassing instances where the ordered CXR did not adhere to guidelines was collected before and after implementing interventions. The interventions included provider education, visual reminders, printed cards, grand-round presentations, and electronic medical records modifications.
Results:
The study encompassed 887 eligible patients with isolated AAE. Baseline data revealed a mean preintervention CXR noncompliance rate of 37.5% among children presenting to the ED with AAE. The interventions resulted in a notable decrease in unnecessary CXR utilization, reaching 16.7%, a reduction sustained throughout subsequent months.
Conclusions:
This QI project successfully reduced unnecessary CXR utilization in pediatric AAE. A multi-faceted approach involving education, visual aids, and electronic reminders aligned clinical practice with evidence-based guidelines. This QI initiative is a potential template for other healthcare institutions seeking to curtail unnecessary CXR usage in pediatric AAE.
INTRODUCTION
Asthma is a common condition with exacerbations and remissions that result in more than 500,000 emergency department (ED) visits per year in children under 18 years of age in the United States.1 Chest X-rays (CXRs) should not be routinely performed in acute asthma exacerbation (AAE) as endorsed by the National Asthma Education and Prevention Program, Global Initiative for Asthma (GINA), American Academy of Pediatrics, and American Thoracic Society.2–5 Reducing CXRs during AAE has important implications: it avoids exposure to unnecessary radiation, lowers the risk of discovering clinically nonsignificant findings, mitigates health care costs, and reduces ED length of stay. Despite the well-documented guidelines, a concerning issue has emerged regarding the excessive utilization of CXRs in cases of AAE. The overutilization of CXRs in AAE stems from many factors, including a lack of awareness among healthcare providers about the guidelines, perceived CXR benefits, clinical uncertainty, defensive medicine practices, historical practices, and patient expectations. In the existing literature, a growing number of studies have successfully used effective approaches to diminish CXR utilization in pediatric AAE, encompassing evidence-based CPGs, concise clinical pathways, and targeted Quality improvement (QI) interventions.6–9
Our baseline data indicated that the mean preintervention rate of CXRs not aligning with guidelines among children with AAE presenting to our ED was 37.5%. We aimed to achieve a 50% reduction in the proportion of CXRs not conforming to national guidelines for pediatric patients with AAE in the ED within twelve months.
METHODS
Setting and Population:
The study was conducted at a safety net acute care facility in New York City, serving as an urban level 2 trauma center and handling an annual volume of 26,000 pediatric visits to the ED. The ED is equipped with a specialized care area dedicated to pediatrics, overseeing nearly 500 cases of pediatric asthma exacerbation every year. This pediatric care area in the ED boasts a team of providers, including ED physicians, ED residents, nurse practitioners, physician assistants, pediatricians, and pediatric residents.
Study Planning:
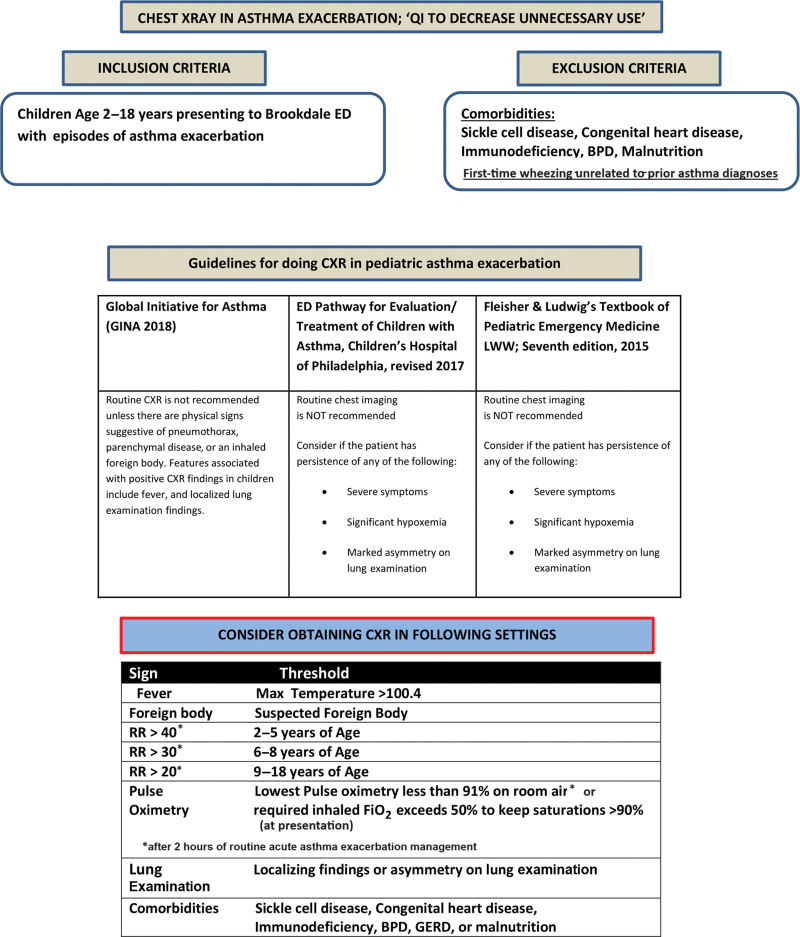
A collaborative effort involving members from the ED and pediatric departments was instrumental in identifying strategies to curtail unnecessary CXR utilization in pediatric patients with AAE cases. The study evaluated electronic medical records (EMR) of children aged 2–18 years with a preexisting asthma diagnosis presenting with AAE to the pediatric ED. A systematic approach involved obtaining monthly medical record numbers for pediatric ED visits bearing the ICD-10 code for asthma (J45). Manual chart reviews were then performed, including a stringent selection process adhering to specific criteria. The inclusion of patients was based on confirmation of AAE diagnosis. The research team meticulously extracted data encompassing age, diagnosis, temperature, respiratory rate, oxygen saturation, localized chest findings, and comorbidities. Exclusion criteria involved patients with concurrent comorbidities or other medical conditions, including sickle cell disease, congenital heart disease, bronchopulmonary dysplasia (BPD), gastroesophageal reflux disease, immunodeficiency, cystic fibrosis, malnutrition, and first-time wheezing unrelated to prior asthma diagnoses.
Data from September 2018 to February 2019 were taken as the baseline for comparison with postintervention results. These 6 months were selected just before the project’s initiation to ensure the same provider group was targeted for CXR orders. Interventions commenced in March 2019. The study’s primary outcome involved analyzing the percentage of chest X-rays not adhering to guidelines and comparing noncompliant X-rays to the total number of X-rays performed. The secondary outcome looked at the overall percentage of children with AAE who received X-rays during their ED visits, irrespective of discharge status (discharged or hospitalized).
INTERVENTIONS
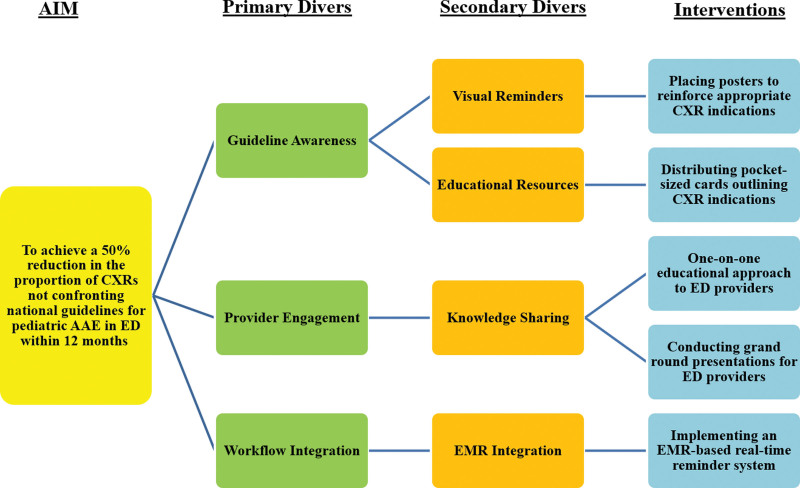
The team created a comprehensive set of interventions to enhance compliance with the latest guidelines from GINA 2018. These interventions were strategically designed based on the key driver diagram (Fig. 1), which played a pivotal role in shaping our intervention strategy. A comparison was made between these guidelines and widely recognized resources to determine CXR indications during an asthma exacerbation.3,5,10,11 The interventions were executed over 5 monthly cycles, using the Plan-Do-Study-Act (QI methodology. The series of interventions included:
Fig. 1.
Key Driver Diagram.
Educational Initiative: A one-on-one educational approach was adopted to align ED providers with CXR indications per prevailing guidelines.
Informative Posters: Strategically placed posters (Fig. 2) within the ED highlighted appropriate CXR indications for AAE.
Distribution of Guidance Cards: Every pediatric ED provider received laminated, pocket-sized, colored cards outlining CXR indications.
Grand Round Presentation: An inclusive grand round presentation was jointly organized by pediatric ED faculty and residents, encouraging interactive knowledge sharing.
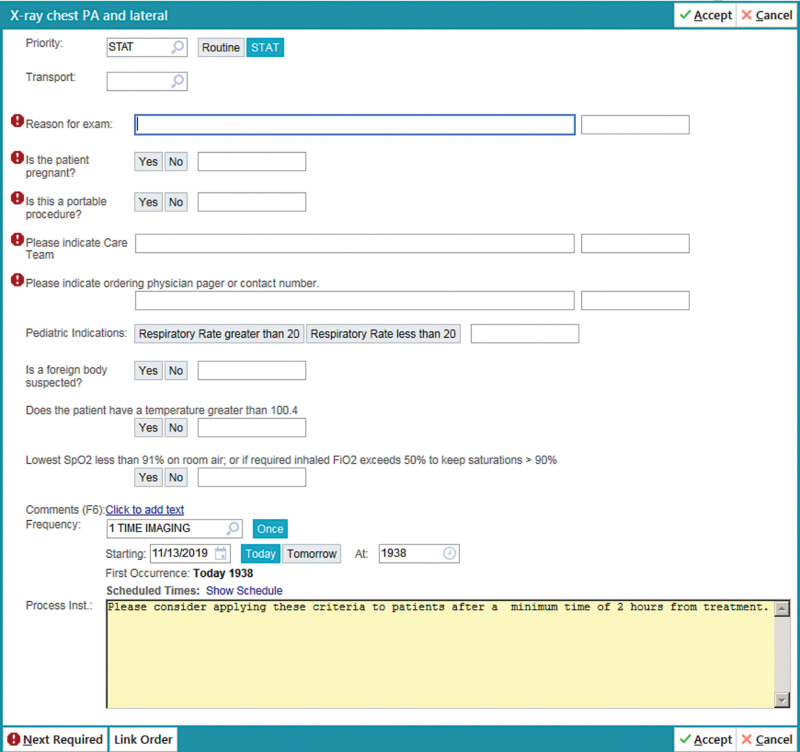
EMR Reminder Integration: An EMR-based real-time reminder system (Fig. 3) for CXR indications was incorporated during the CXR ordering process within the ED.
Fig. 2.
ED Poster of CXR indications in pediatric asthma exacerbation.
Fig. 3.
Electronic Medical System drop-down reminder of CXR order in ED.
There was ongoing education and orientation of the new house staff in the ED throughout the project.
Analysis:
SPSS software facilitated data analysis, with numerical and frequency metrics used for descriptive purposes. We used the preinterventional period to establish the baseline mean utilizing monthly percentages of CXRs not adhering to guidelines. We used real-time run-charts and statistical process control (SPC) charts for project data analysis. SPC charts had control limits set at three SDs from the mean. Sustained Special Cause Variation detection, such as a point beyond control limits or over eight consecutive values surpassing the prior centerline, prompted SPC chart centerline (CL) adjustments. The institute’s institutional review board approved the study.
RESULTS
The QI project spanned from September 2018 to February 2020, involving a total of 83 providers in the Pediatric ED. Of the 1058 patients presenting with AAE in the pediatric ED, 1001 cases were eligible for analysis after excluding 35 patients with comorbidities and 22 patients lacking a prior asthma history. During the preintervention phase, 226 eligible patients were included; 118 (52%) underwent CXRs. The monthly data for CXRs not adhering to guidelines (total nonconforming CXRs/total CXRs) showed rates that varied from 34% in September 2018 to 44% in February 2019, establishing a baseline mean of 37.5%. Table 1 summarizes the impact of sequential interventions on the percentage of chest X-rays not adhering to guidelines, presenting results over 12 months, along with corresponding P values for statistical significance.
Table 1.
Summary of Interventions and Impact on NonCompliant CXRs
| Month | Description | Percentage of CXR not Adhering To Guidelines (%) |
P value* |
|---|---|---|---|
| September 2018–February 2019 | Baseline | 37.5% | NA |
| March 2019 | Postintervention 1 | 25% | 0.067 |
| Apr 2019 | Postintervention 2 | 23% | 0.045 |
| May 2019 | Postintervention 3 | 19% | 0.007 |
| June 2019 | Postintervention 4 | 18% | 0.004 |
| July 2019 | 2nd month postintervention 4 | 20% | 0.012 |
| August 2019 | 3rd month postintervention 4 | 19% | 0.007 |
| September 2019 | 4th month postintervention 4 | 15% | 0.001 |
| October 2019 | PostIntervention 5 | 12% | <0.001 |
| November 2019 | 2nd month postintervention 5 | 12% | <0.001 |
| December 2019 | 3rd month postintervention 5 | 13% | <0.001 |
| January 2020 | 4th month postintervention 5 | 12% | <0.001 |
| February 2020 | 5th month postintervention 5 | 12% | <0.001 |
P value—comparing the values to the baseline mean of 37.5%
Intervention 1: One-to-one education (31% reduction).
Intervention 2: Poster reminder (5.5% reduction).
Intervention 3: Providing printed cards (11% reduction).
Intervention 4: Grand round (11% reduction).
Intervention 5: Epic drop-down (8% reduction).
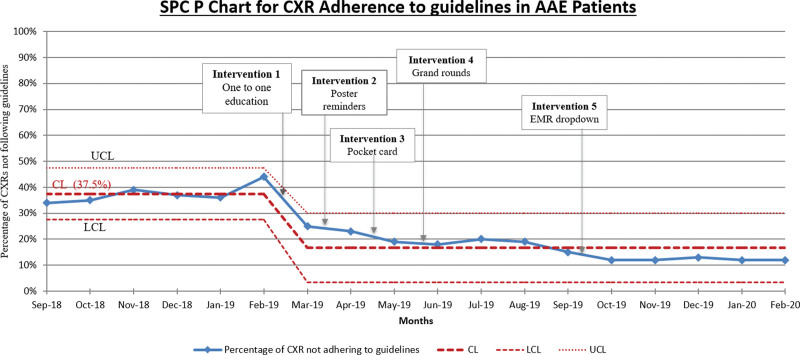
Displayed in Figure 4 is the SPC chart depicting the primary outcome measure, which is the analysis of CXR adherence to guidelines for AAE patients across a designated time frame. The chart visually represents the percentage of CXRs not following guidelines alongside the initial Central Line (CL) set at 37.5%. Around March, a notable Special Cause Variation prompted a shift of the CL to 16.7%, indicating process improvement.
Fig. 4.
SPC P Chart for CXR Adherence to Guidelines in AAE Patients.
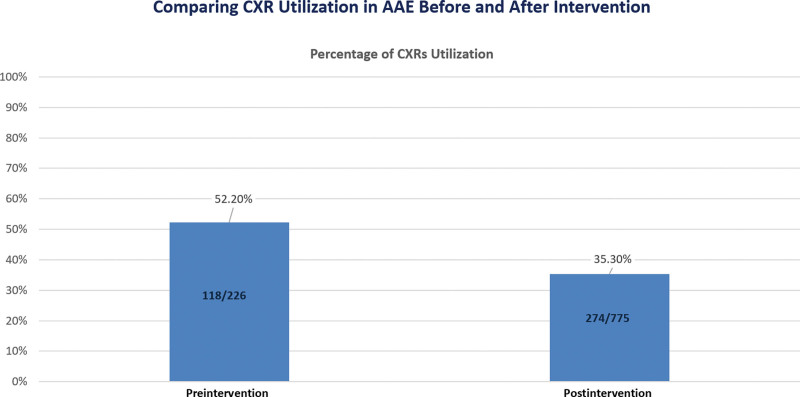
Figure 5 displays the shift in CXR utilization among children with AAE, with the percentage of children undergoing CXRs decreasing from 52.2% in the preintervention period to 35.3% in the postintervention period. This shift serves as a secondary outcome measure in evaluating the intervention’s impact on diagnostic practices.
Fig. 5.
Comparing CXR Utilization in AAE Before and After Intervention.
DISCUSSION
Our QI project successfully led to a substantial reduction in the utilization of CXR that did not adhere to guidelines for pediatric patients with AAE in the ED. This reduction was particularly noteworthy, with a decline from a baseline mean of 37.5%–16.7%, representing a 55% reduction. This achievement is significant and sustained, demonstrating results surpassing recent national data, as published studies indicate.12–15
The American Academy of Pediatrics, the National Heart, Lung, and Blood Institute (NHLBI), and the GINA generally advise against routinely ordering CXR for children with asthma, as these patients seldom exhibit concurrent bacterial pneumonia.16–18 Studies show that only 2%–5% of patients experience radiographic pneumonia during an asthma exacerbation; however, one-third of all exacerbation cases undergo chest radiography.19,20 The possibility of inconclusive radiographs might potentially lead to unjustified antibiotic treatment.21
To adhere to best practices, we integrated modified CXR indication criteria adapted from established pediatric asthma guidelines and pathways.3,5,10 Our findings align with previous research conducted in community hospitals, which examined the effectiveness of pediatric asthma pathways. Pediatric asthma pathways typically include instructions for assessing a child’s clinical severity and offering treatment recommendations, yielding several benefits for children with asthma in the ED and inpatient settings.8,22–24 Desai et al and Bekmezian et al reported decreased CXR usage when they introduced pathways incorporating specific criteria for ordering CXRs.8,25 In our case, we carried out pathway implementation and educational sessions, providing clinicians with the requirements for CXR indications for patients with asthma exacerbation.
In the context of AAE, various QI studies present distinct criteria for CXR utilization. Buckmaster et al7 deemed CXRs unnecessary for known asthmatics responding to medication with no pneumothorax evidence and no ICU admission. Bekmezian et al.8 used the “4 F’s” (fever, focal findings, foreign body concern, and failure to improve) as indications for obtaining a CXR, and Gildenhuys et al6 focused on focal findings, lack of initial treatment response, and suspected pneumothorax. Watnick et al9 considered CXR for focal exam findings, persistent respiratory distress, and suspected pneumothorax. The criteria we used seek to achieve a nuanced balance between comprehensiveness and specificity, considering the goals of our QI project and the unique characteristics of our study population, including a significant prevalence of sickle cell disease patients. The criteria encompassed factors such as fever, concerns about foreign body presence, localized lung examination findings, persistent respiratory distress after 2 hours of routine AAE management, defined by tachypnea or hypoxia < 91% in room air or requiring FiO2 > 0.5 at presentation and the presence of comorbid conditions such as sickle cell disease, BPD, and immunodeficiency. We ensured that these criteria were communicated to healthcare providers and also visually displayed in posters for easy reference.
Our QI project achieved a low utilization rate of 16.7% for CXRs that did not adhere to guidelines among pediatric patients with AAE in the ED. This accomplishment stands out as a positive achievement compared with the results of many other QI initiatives. Gildenhuys et al6 documented a reduction in the frequency of ordered CXRs for AAE, dropping from 48% to 31% with the implementation of an evidence-based asthma clinical practice guideline (CPG) worksheet. Buckmaster et al7 achieved a decrease in the rate of unnecessary CXR for AAE from 45.3% to 28.4% using a quality assurance project. Bekmezian et al,8 by using a concise clinical pathway aligned with the National Institutes of Health Guidelines, observed a decline in CXR utilization among AAE from 42% to 27%. Additionally, Watnick et al9 introduced asthma CPG, which initially led to a decrease in CXR use among AAE from 29.3% to 23.0% and, through a QI-targeted intervention, further reduced it to 16%.
It’s crucial to highlight that Gildenhuys et al, Bekmezian et al, and Watnick et al’s cited statistics related to the total number of CXRs per AAE, and Buckmaster et al’s cited statistics focused on unnecessary CXRs per AAE.6–9 Buckmaster et al also assessed unnecessary CXRs as a proportion of total CXRs but did not observe any changes with interventions, with the rate remaining consistent at 81% versus 78%.7 In contrast, our QI initiative centered on evaluating the ratio of unnecessary CXRs to total CXRs for AAE as the primary outcome, and postintervention, we observed a significant decline from 37.5% (preintervention) to 16.7% (postintervention). This distinction underscores the effectiveness of our intervention in specifically targeting and reducing the rate of unnecessary CXRs in the overall CXR utilization. We also observed a decline in the percentage of children with AAE for whom CXRs were obtained, with 52.2% (preintervention) and 35.3% (postintervention).
Our analysis revealed that the decrease in noncompliant CXRs was immediately evident after implementing targeted QI interventions, strongly suggesting a causal relationship between the interventions and the observed outcomes. The education initiative and integration of an EMR reminder list when ordering CXRs played a crucial role, resulting in a substantial drop to 16.7%, which persisted during subsequent cycles of the Plan-Do-Study-Act methodology (Fig. 4). This outcome remained well below our predetermined benchmark of 18%, demonstrating the efficacy of EMR reminder integration and ongoing educational initiatives.
Our multifaceted interventions achieved collective impact. The most influential, a 31% reduction (Fig. 5), was an educational initiative for healthcare providers, focusing on CXR indications in AAE through one-on-one discussions. Repeated one-on-one education and email reminders reinforced our commitment to change. Integrating ongoing quarterly education ensures sustained impact. Printed guidance cards and grand round presentations contributed to an 11% reduction, posters on ED walls led to a 5.5% reduction, and an EMR modification yielded an additional 8% reduction (Fig. 5). The combination of ongoing education and the EMR drop-down menu is crucial for maintaining adherence to CXR utilization guidelines.
Our QI initiative significantly reduced CXR utilization for pediatric AAE in the ED, a trend sustained for several months postimplementation. Our adaptable interventions offer feasibility for resource-limited institutions, particularly emphasizing educational efforts. Unlike previous QI endeavors6–9 which often relied on billing codes susceptible to inaccuracies,26 our study used detailed manual chart reviews for precise validation of CXR adherence to guidelines. Implementing our QI interventions did face challenges, including the need for repeated one-on-one education and email reminders and a time-consuming approval process for integrating the EMR drop-down menu.
This study presents a unique and comprehensive approach to address the overutilization of chest X-rays (CXRs) in pediatric AAE cases in real-world healthcare settings. Our study implemented a distinctive, year-long, multi-faceted strategy to align clinical practice with evidence-based guidelines, facilitating a longitudinal analysis of the sustained impact of our intervention. The integration of Real-Time EMR Reminders played a pivotal role in shaping CXR ordering practices and enhancing the sustainability of interventions. We believe this study contributes valuable insights to the ongoing efforts to reduce unnecessary CXRs in pediatric AAE. It has potential applicability as a model for healthcare institutions aiming to align their practices with evidence-based guidelines and enhance patient care outcomes.
LIMITATIONS
Our study has limitations. Firstly, the study was confined to a single urban level 2 trauma center ED, potentially limiting the generalizability of results to other healthcare settings. Secondly, the study focused on reducing CXR utilization in pediatric AAE cases without assessing broader clinical outcomes or patient experiences. We did not stratify our patients’ pool according to disposition, whether discharged home or admitted. Moreover, children with known asthma treated with antibiotics for possible pneumonia seen in a CXR may not have been coded as asthma and, therefore, may have been missed in our dataset. Our study did not explicitly track ED bounce-backs and instances of missed or delayed diagnoses related to the decision against obtaining a CXR. This omission is a notable limitation of our study. Finally, our population has a high prevalence of sickle cell disease patients who may have presented to the emergency room with the sole complaint of asthma exacerbation but were excluded from our study.
CONCLUSIONS
Our QI project successfully addressed the overutilization of CXRs in pediatric AAE. We significantly reduced the percentage of ordered CXRs not adhering to evidence-based guidelines through a multi-faceted approach involving targeted interventions. The interventions, including education, visual aids, and electronic reminders, effectively aligned clinical practice with the recommended guidelines. The results highlight the importance of tailored interventions in promoting evidence-based practices and optimizing resource utilization. Our study contributes valuable insights to the ongoing efforts to enhance the quality of care for pediatric patients with AAE by demonstrating the feasibility and efficacy of incorporating guideline-based interventions in a real-world healthcare setting. This study can serve as a model for other healthcare institutions aiming to align their practices with evidence-based guidelines and improve patient care outcomes.
Footnotes
Published online April 3, 2024.
The content of this article was initially presented at the HVPAA National Conference in 2021.
To cite: Sakr M, Al Kanjo M, Balasundaram P, Kupferman F, Al-Mulaabed S, Scott S, Viswanathan K, Basak RB. A Quality Improvement Initiative to Minimize Unnecessary Chest X-Ray Utilization in Pediatric Asthma Exacerbations. Pediatr Qual Saf 2024;9:e721.
REFERENCES
- 1.Reed JE, Davey N, Woodcock T. The foundations of quality improvement science. Future Hosp J. 2016;3:199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren CL, Esther CR, Jr, Debley JS, et al. ; ATS Ad Hoc Committee on Infants with Recurrent or Persistent Wheezing. Official American Thoracic Society clinical practice guidelines: diagnostic evaluation of infants with recurrent or persistent wheezing. Am J Respir Crit Care Med. 2016;194:356–373. [DOI] [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007 [published correction appears in J Allergy Clin Immunol. 2008 Jun;121(6):1330]. J Allergy Clin Immunol. 2007;120:S94–S138. [DOI] [PubMed] [Google Scholar]
- 4.Reddel HK, Bacharier LB, Bateman ED, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Eur Respir J. 2021;59:2102730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cloutier MM, Dixon AE, Krishnan JA, et al. Managing asthma in adolescents and adults: 2020 asthma guideline update from the national asthma education and prevention program. JAMA. 2020;324:2301–2317. [DOI] [PubMed] [Google Scholar]
- 6.Gildenhuys J, Lee M, Isbister GK. Does implementation of a paediatric asthma clinical practice guideline worksheet change clinical practice? Int J Emerg Med. 2009;2:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckmaster A, Boon R. Reduce the rads: a quality assurance project on reducing unnecessary chest X-rays in children with asthma. J Paediatr Child Health. 2005;41:107–111. [DOI] [PubMed] [Google Scholar]
- 8.Bekmezian A, Fee C, Weber E. Clinical pathway improves pediatrics asthma management in the emergency department and reduces admissions. J Asthma. 2015;52:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watnick CS, Arnold DH, Latuska R, et al. Successful chest radiograph reduction by using quality improvement methodology for children with asthma. Pediatrics. 2018;142:e20174003. [DOI] [PubMed] [Google Scholar]
- 10.Zorc J, Scarfone R, Reardon A, et al. ; Children’s Hospital of Philadelphia. Asthma Emergent Care Clinical Pathway. Available at: https://www.chop.edu/clinical-pathway/asthma-emergent-care-clinical-pathway. Revised May 2023. Accessed August 28, 2023. [Google Scholar]
- 11.Farah MM, Tay KY, Lavelle J. A general approach to ill and injured children. In. Shaw KN, Bachur RG. (Eds.), Fleisher & Ludwig’s Textbook of Pediatric Emergency Medicine. 7th ed. Wolters Kluwer; 2016; pp. 1–8. [Google Scholar]
- 12.Stanley RM, Teach SJ, Mann NC, et al. ;Pediatric Emergency Care Applied Research Network. Variation in ancillary testing among pediatric asthma patients seen in emergency departments. Acad Emerg Med. 2007;14:532–538. [DOI] [PubMed] [Google Scholar]
- 13.Davies G, Paton JY, Beaton SJ, et al. Children admitted with acute wheeze/asthma during November 1998-2005: a national UK audit. Arch Dis Child. 2008;93:952–958. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan S, Magruder T, Walley SC, et al. Relevance of chest radiography in pediatric inpatients with asthma. J Asthma. 2014;51:751–755. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain JM, Teach SJ, Hayes KL, et al. Practice pattern variation in the care of children with acute asthma. Acad Emerg Med. 2016;23:166–170. [DOI] [PubMed] [Google Scholar]
- 16.Mensah GA, Kiley JP, Gibbons GH. Generating evidence to inform an update of asthma clinical practice guidelines: perspectives from the national heart, lung, and blood institute. J Allergy Clin Immunol. 2018;142:744–748. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DP, Arnold DH, Gay JC, et al. Implementation and improvement of pediatric asthma guideline improves hospital-based care. Pediatrics. 2018;141:e20171630. [DOI] [PubMed] [Google Scholar]
- 18.Patel SJ, Teach SJ. Asthma. Pediatr Rev. 2019;40:549–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florin TA, Carron H, Huang G, et al. Pneumonia in children presenting to the emergency department with an asthma exacerbation. JAMA Pediatr. 2016;170:803–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathews B, Shah S, Cleveland RH, et al. Clinical predictors of pneumonia among children with wheezing. Pediatrics. 2009;124:e29–e36. [DOI] [PubMed] [Google Scholar]
- 21.Beyyumi E, Tawil MI, AlDhanhani H, et al. A single-institution experience in the use of chest radiographs for hospitalized children labeled as asthma exacerbation. Front Pediatr. 2021;9:722480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucia D, Cain J, Porter A, et al. Pediatric asthma pathway in the emergency room. Proc (Bayl Univ Med Cent). 2020;34:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCoy EM, Kink RJ, Harrold PL, et al. Implementing a standardized clinical pathway leads to reduced asthma admissions and health care costs. Pediatr Qual Saf. 2018;3:e091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morse RB, Hall M, Fieldston ES, et al. Hospital-level compliance with asthma care quality measures at children’s hospitals and subsequent asthma-related outcomes. JAMA. 2011;306:1454–1460. [DOI] [PubMed] [Google Scholar]
- 25.Desai M, Caldwell K, Gupta N, et al. Effectiveness of pediatric asthma pathways in community hospitals: a multisite quality improvement study. Pediatr Qual Saf. 2020;5:e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Malley KJ, Cook KF, Price MD, et al. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40:1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu B, Hadzi-Tosev M, Liu Y, et al. Accuracy of international classification of diseases, 10th revision codes for identifying sepsis: a systematic review and meta-analysis. Crit Care Explor. 2022;4:e0788. [DOI] [PMC free article] [PubMed] [Google Scholar]