Abstract
Subcutaneous administration in mice of recombinant Sindbis viruses expressing a class I major histocompatibility complex-restricted 9-mer epitope of the Plasmodium yoelii circumsporozoite protein or the nucleoprotein of influenza virus induces a large epitope-specific CD8+ T-cell response. This immunization also elicits a high degree of protection against infection with malaria or influenza A virus.
Alpha-RNA viruses such as Sindbis, Semliki Forest, and Venezuelan equine encephalitis viruses are attractive vectors for gene therapy and vaccine development because of their capacities to express large amounts of foreign proteins (7, 10, 24). Sindbis virus offers certain additional advantages, such as a broad range of susceptible host cells (23) and the relative ease of engineering and manipulating recombinant (RE) RNA molecules (10). It is also important that, in spite of its small size of approximately 11.8 kb, this virus is capable of expressing foreign inserts of up to 3.2 kb (18).
Earlier data demonstrated the potential of RE Sindbis viruses, expressing foreign antigen, to induce immunity against a variety of intracellular pathogens (10, 14, 18). We have determined the capacity of these RE viruses expressing the corresponding foreign antigen or epitope to generate CD8+ T-cell responses and protection against infection by the malaria parasite Plasmodium yoelii and the influenza A virus, respectively. In both systems, CD8+ T cells were shown to play major roles in mediating protective immunity (2, 8, 13, 19, 22).
RE Sindbis viruses expressing a minigene coding only for the CD8+ T-cell epitope SYVPSAEQI of the circumsporozoite (CS) protein of P. yoelii (SIN.Mal), recognized by H-2d mice, or TYQRTRALV from the nucleoprotein (NP) of the influenza A virus (SIN.Flu) were generated by using infectious mRNA transcripts, as described elsewhere (12).
Six- to 8-week-old BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) were immunized by various routes, namely, subcutaneously (s.c.), intramuscularly (i.m.), intravenously (i.v.), intraperitoneally (i.p.), and intranasally (i.n.) (17). The magnitudes of the epitope-specific CD8+ T-cell responses were measured 12 days after immunization with an ELISPOT assay. This assay has been described in detail by us (15, 20) and other investigators (4, 5, 11, 16) using various experimental models. In all of these systems it was shown that the results of the ELISPOT correlate closely with the chromium release assay, the ELISPOT being considerably more sensitive and quantitative.
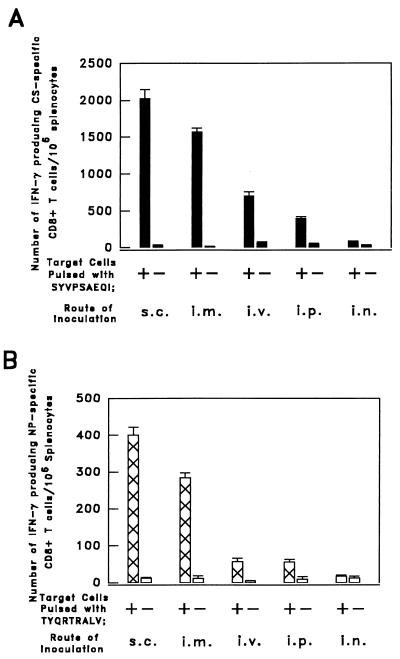
The largest numbers of CS-specific CD8+ T cells, detected 12 days after immunization, were elicited in mice immunized s.c. with 5 × 106 PFU of SIN.Mal (∼2 × 103 CS-specific CD8+ T cells/106 spleen cells) (Fig. 1A). The i.m. immunization was only slightly less efficient. Thirty-two days after immunization, the total number of epitope-specific CD8+ T cells had decreased by approximately 50%. As observed at day 12, the magnitude of the CD8+ T-cell response closely depended on the route of immunization, and the greatest response also resulted from s.c. or i.m. inoculation (not shown). It is noteworthy that the magnitude of the anti-SYVPSAEQI CD8+ T-cell response, induced by the RE Sindbis virus, is the greatest we have observed so far, compared to past immunization with other RE vaccinia, influenza, or adenoviruses (17, 19).
FIG. 1.
Results of ELISPOT performed on spleen cells of mice immunized by different routes with RE Sindbis viruses expressing a CS-specific or an NP-specific CD8+ T-cell epitope. (A) Groups of three BALB/c mice were immunized by inoculation with 5 × 106 PFU of SIN.Mal administered s.c., i.m., i.v., i.p., or i.n. (B) Groups of three BALB/c mice were immunized by inoculation with 5 × 106 PFU of SIN.Flu administered by various routes. The numbers of CS- and NP-specific CD8+ T cells in the spleens of the immunized mice were established 12 days after RE virus inoculation by an ELISPOT assay, which detects epitope-specific gamma interferon (IFN-γ) secretion by single cells. The corresponding results are expressed as averages ± standard errors of triplicate cultures.
The largest numbers of influenza virus NP-specific CD8+ T cells were also observed upon s.c. or i.m. immunization with SIN.Flu (Fig. 1B). The total numbers of NP-specific CD8+ T cells in the spleens of these mice were smaller than those elicited by inoculation of an equal number of PFU of SIN.Mal, but the rankings of results obtained by the different routes of inoculation were the same.
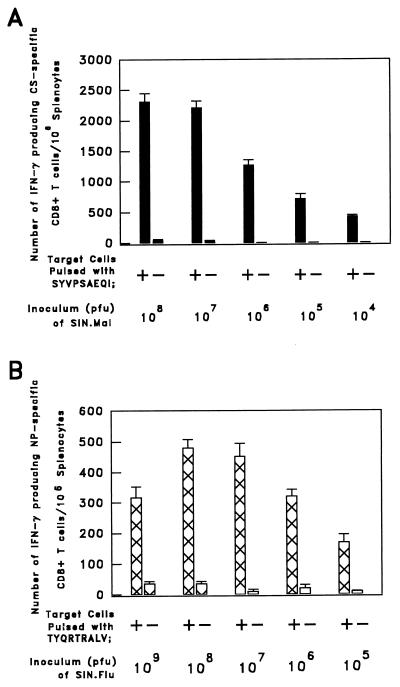
The number of CS-specific CD8+ T cells closely correlated with the viral dose used for immunization. Animals inoculated s.c. with 107 or 108 PFU of SIN.Mal displayed the largest numbers of CS-specific CD8+ T cells (Fig. 2A). Immunization with doses greater than 109 PFU of SIN.Mal failed to increase the frequency of CS-specific T cells (data not shown). The level of the NP-specific CD8+ T-cell response of s.c. immunized mice was also found to correlate closely with the inoculum size of SIN.Flu. Mice immunized with 108 PFU of the RE virus displayed the highest numbers of NP-specific CD8+ T cells in the spleen, while a larger dose of SIN.Flu, 109 PFU, elicited lower numbers of NP-specific CD8+ T cells (Fig. 2B). This close correlation between virus dose and CD8+ T-cell response may reflect a very low replication rate of the Sindbis virus in vivo. Although pTE3′2J-derived RE double-subgenomic Sindbis viruses are replication competent in vitro, as shown by amplification at a low multiplicity of infection without loss of either infectivity or foreign protein expression, the stability significantly depends on the length and protein product of the foreign gene insert (6, 10, 18). Inoculation with up to 109 PFU of double-subgenomic Sindbis virus recombinants causes inapparent infections in 6- to 8-week-old mice, independent of the route of immunization. Furthermore, productive replication in vivo may be very low, as virus titers in the serum were diminished within the first two days after i.p. inoculation with 5 × 107 PFU and were undetectable thereafter (2a).
FIG. 2.
Effect of inoculum size on the numbers of specific splenic CD8+ precursor T cells resulting from immunization. (A) Numbers of gamma interferon (IFN-γ)-secreting CS-specific CD8+ T cells detected in the spleens of groups of three mice immunized by s.c. inoculation with different doses of SIN.Mal (104 to 108 PFU). (B) Numbers of NP-specific CD8+ T cells in the spleens of groups of five mice immunized by s.c. inoculation with different doses of SIN.Flu (105 to 109 PFU). The numbers of IFN-γ-secreting CS- and NP-specific CD8+ T cells in the spleens of these mice were detected by an ELISPOT assay 12 days after immunization. The results reflect two identical experiments and are expressed as averages ± standard errors of triplicate cultures.
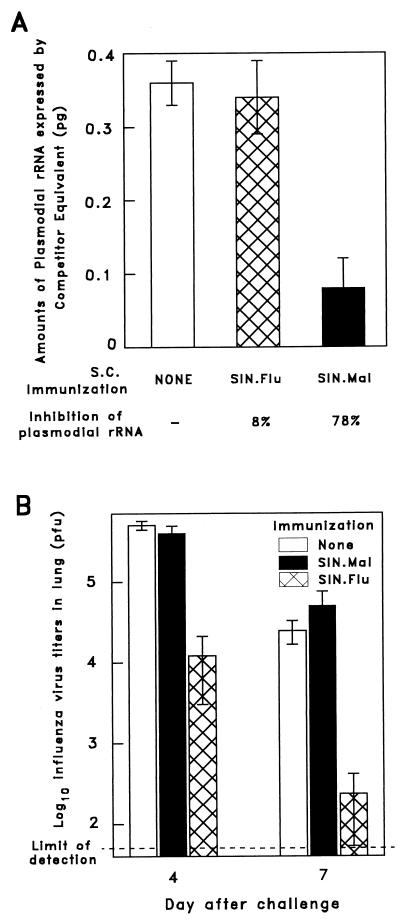
In order to assess the in vivo antiparasite activity of the specific CD8+ T cells elicited by SIN.Mal, mice were injected s.c. with 108 PFU of this RE virus. For specificity control, other mice were given the same dose of RE virus expressing the influenza NP epitope, SIN.Flu. Two weeks after immunization, both groups of mice, as well as naive animals, were challenged i.v. with 2 × 104 P. yoelii sporozoites. Parasite development was determined 42 h later by measuring plasmodial rRNA levels in the livers of the challenged mice with a competitive reverse transcription-PCR assay, as described elsewhere (3). Mice immunized with SIN.Mal displayed a strong inhibition of parasite development in the liver, resulting in an approximate decrease of 80% of hepatic parasite rRNA levels compared to naive controls (Fig. 3A). The epitope specificity of this protective effect was demonstrated by the fact that mice immunized with SIN.Flu displayed a parasite development similar to that of nonimmunized mice. Furthermore, since SIN.Mal expressed only the 9-mer major histocompatibility complex class I-restricted epitope of the P. yoelii CS protein, the observed inhibition of parasite development could be mediated only by epitope-specific CD8+ T cells.
FIG. 3.
Protective immunity elicited by SIN.Mal and SIN.Flu immunization. (A) Inhibition of liver stage development upon s.c. immunization of five BALB/c mice with 108 PFU of SIN.Mal. As controls for the specificity of protection, five mice were inoculated with SIN.Flu expressing the CD8+ T-cell epitope of the influenza A virus NP, while a third group of five mice was left untreated. Two weeks later, all mice were challenged i.v. with 2 × 104 viable P. yoelii sporozoites and the levels of parasite rRNA present in their livers were determined 42 h after challenge. The results are expressed as averages ± standard deviations of the values obtained from individual mice. (B) Inhibition of viral titers in the lungs of a group of five BALB/c mice immunized by s.c. inoculation with 108 PFU of SIN.Flu. Five additional mice were inoculated s.c. with SIN.Mal, while a third group of mice was left untreated. Two weeks later, all mice were challenged i.n. with 103 PFU of influenza A virus and the viral titers in the lungs were determined on day 4 and, in others, day 7 after challenge. The results are expressed as average virus titers of five mice ± standard deviations. The viral titers of mice immunized with SIN.Flu were significantly lower than those of control groups (P < 0.001 [Student’s t test]) both on day 4 and on day 7.
To determine whether immunization with SIN.Flu expressing the influenza NP epitope could elicit protection against influenza virus infection, mice were inoculated s.c. with 108 PFU of SIN.Flu. Other mice were given a comparable dose of SIN.Mal for use as a specificity control. Two weeks after immunization, these and nonimmunized mice were challenged i.n. with 103 PFU of influenza A/WSN/33 virus (subtype H1N1) (13). Viral titers in the lungs of these mice were measured either on day 4 or on day 7 after challenge by using a standard plaque assay to determine the numbers of PFU on Madin-Darby canine kidney cell monolayers (21). Mice immunized with SIN.Flu and challenged with the influenza virus developed much lower virus titers in their lungs than did naive mice or mice immunized with SIN.Mal (Fig. 3B). On day 4, and particularly on day 7, after challenge, the influenza virus titers in the lungs of SIN.Flu-immunized mice were nearly 2 log10 units lower than those of control mice. This supported the view that NP-specific CD8+ T cells, induced by the RE Sindbis virus, were effective in mediating protection against influenza virus infection.
Taken together, these findings reveal that immunization with RE Sindbis virus expressing a single CD8+ epitope of the influenza virus NP or malaria CS protein elicits strong CD8+ T-cell responses and protective immunity against these infections. Previous immunization studies with RE vaccinia viruses expressing the entire malaria CS antigen (13) or the influenza virus NP (1, 9) report the induction of apparently similar or less-efficient protective immunity against these infectious pathogens.
Although Sindbis virus infects humans, this infection is mostly limited to the local skin site, causing a minor rash. Moreover, recent advances in genetic engineering make it feasible to generate replication-defective Sindbis viruses which appear to be nonpathogenic (12). If the immunogenicity of these defective Sindbis viruses remains unaltered, they might be very attractive candidates for the development of safer vaccines against several infectious diseases.
Acknowledgments
We thank Chui Ng for excellent technical assistance.
This work was supported by National Institutes of Health grants AI-27458, AI-36526, and AI-33314.
REFERENCES
- 1.Andrew M E, Coupar B E H, Boyle D B, Ada G L. The roles of influenza virus haemagglutinin and nucleoprotein in protection: analysis using vaccinia virus recombinants. Scand J Immunol. 1987;25:21–28. doi: 10.1111/j.1365-3083.1987.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 2.Bender B S, Rowe C A, Taylor S F, Wyatt L S, Moss B, Small P A., Jr Oral immunization with a replication-deficient recombinant vaccinia virus protects mice against influenza. J Virol. 1996;70:6418–6424. doi: 10.1128/jvi.70.9.6418-6424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Bergmann, C. Unpublished observations.
- 3.Briones M R S, Tsuji M, Nussenzweig V. The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes. Mol Biochem Parasitol. 1996;77:7–17. doi: 10.1016/0166-6851(96)02574-1. [DOI] [PubMed] [Google Scholar]
- 4.Busch D H, Pamer E G. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160:4441–4448. [PubMed] [Google Scholar]
- 5.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J P, Miller D, Katow S, Frey T K. Expression of the rubella virus structural proteins by an infectious Sindbis virus vector. Arch Virol. 1995;140:2075–2084. doi: 10.1007/BF01322694. [DOI] [PubMed] [Google Scholar]
- 7.Davis N L, Brown K W, Johnston R E. A viral vaccine vector that expresses foreign genes in lymph nodes and protects against mucosal challenge. J Virol. 1996;70:3781–3787. doi: 10.1128/jvi.70.6.3781-3787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doolan D L, Sedegah M, Hedstrom R C, Hobart P, Charoenvit Y, Hoffman S L. Circumventing genetic restriction of protection against malaria with multigene DNA immunization: CD8+ cell-, interferon gamma-, and nitric oxide-dependent immunity. J Exp Med. 1996;183:1739–1746. doi: 10.1084/jem.183.4.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo A, Itamura S, Iinuma H, Funabashi S, Shida H, Koide F, Nerome K, Oya A. Homotypic and heterotypic protection against influenza virus infection in mice by recombinant vaccinia virus expressing the haemagglutinin or nucleoprotein gene of influenza virus. J Gen Virol. 1991;72:699–703. doi: 10.1099/0022-1317-72-3-699. [DOI] [PubMed] [Google Scholar]
- 10.Hahn C S, Hahn Y S, Braciale T J, Rice C M. Infectious Sindbis virus transient expression vectors for studying antigen processing and presentation. Proc Natl Acad Sci USA. 1992;89:2679–2683. doi: 10.1073/pnas.89.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalvani A, Brooks R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LaStarza M W, Lemm J A, Rice C M. Genetic analysis of the nsP3 region of Sindbis virus: evidence for roles in minus-strand and subgenomic RNA synthesis. J Virol. 1994;68:5781–5791. doi: 10.1128/jvi.68.9.5781-5791.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Rodrigues M, Rodriguez D, Rodriguez J R, Esteban M, Palese P, Nussenzweig R S, Zavala F. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc Natl Acad Sci USA. 1993;90:5214–5218. doi: 10.1073/pnas.90.11.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.London S D, Schmaljon A L, Dalrymple J M, Rice C M. Infectious enveloped RNA virus antigenic chimeras. Proc Natl Acad Sci USA. 1992;89:207–211. doi: 10.1073/pnas.89.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 16.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 17.Murata K, Garcia-Sastre A, Tsuji M, Rodrigues M, Rodriguez D, Rodriguez J R, Nussenzweig R S, Palese P, Esteban M, Zavala F. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell Immunol. 1996;173:96–107. doi: 10.1006/cimm.1996.0255. [DOI] [PubMed] [Google Scholar]
- 18.Pugachev K V, Mason P W, Shope R E, Frey T K. Double-subgenomic Sindbis virus recombinants expressing immunogenic proteins of Japanese encephalitis virus induce significant protection in mice against lethal JEV infection. Virology. 1995;212:587–594. doi: 10.1006/viro.1995.1516. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues E G, Zavala F, Eichinger D, Wilson J M, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- 20.Rodrigues M, Li S, Murata K, Rodriguez D, Rodriguez J R, Bacik I, Bennink J R, Yewdell J W, Garcia-Sastre A, Nussenzweig R S, Esteban M, Palese P, Zavala F. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J Immunol. 1994;153:4636–4648. [PubMed] [Google Scholar]
- 21.Tite J P, Hughes-Jenkines C, O’Callaghan D, Dougan G, Russel S M, Gao X-M, Liew F Y. Anti-viral immunity induced by recombinant nucleoprotein of influenza A virus. II. Protection from influenza infection and mechanism of protection. Immunology. 1990;71:202–207. [PMC free article] [PubMed] [Google Scholar]
- 22.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 23.Xiong C, Levis R, Shen P, Schlesinger S, Rice C, Huang H V. Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science. 1989;243:1188–1191. doi: 10.1126/science.2922607. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Berglund P, Zhao H, Liljeström P, Jondal M. Generation of cytotoxic and humoral immune responses by nonreplicative recombinant Semliki Forest virus. Proc Natl Acad Sci USA. 1995;92:3009–3013. doi: 10.1073/pnas.92.7.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]