Abstract
Aim:
The authors aimed to conduct a meta-analysis to determine if acetylcholinesterase inhibitors may pose a direct threat, increasing the incidence of fractures in dementia patients.
Methods:
PubMed, Scopus, and Cochrane Library were searched. Inclusion criteria were any original studies that demonstrated the link between acetylcholinesterase inhibitors and the incidence of fracture in patients with dementia. RevMan(5.4) was used.
Results:
Seven observational studies were included. The total number of patients included in the acetylcholinesterase inhibitors group is 274 332 and 290 347 in the control group. The pooled analysis showed that the risk of bone fracture was not statistically different between dementia patients who received acetylcholinesterase inhibitors and those who did not receive them (odds ratio=1.44, CI 0.95, 2.19, P=0.09). Subgroup analysis showed no statistically significant difference between dementia patients who took acetylcholinesterase inhibitors, and those who didn’t take acetylcholinesterase inhibitors in those more than or equal to 80 years old and those less than 80 years old (P=0.44) and (P=0.34) respectively. However, our results showed a statistically significant association between dementia patients who received acetylcholinesterase inhibitors and decreased fracture risk in those receiving the treatment for more than or less than 2 years (risk ratio=0.48, CI= 0.45, 0.51, P<0.00001) and (risk ratio=0.84, CI 0.70, 0.99, P=0.04), respectively.
Conclusion:
Our study revealed no role for acetylcholinesterase inhibitors in increasing the risk of fracture compared with controls. Hence, based on our analysis, they might have a protective role against fracture when used for long periods considering their positive action on bone growth and development. Therefore, Acetylcholinesterase inhibitors could be considered a safe option for improving cognitive functions in elderly demented patients without carrying any additional risks.
Keywords: acetylcholinesterase inhibitors, dementia, fracture
Introduction
Highlights
Closed-box approach was associated with less bleeding compared to open-box approach.
Both approaches yielded comparable findings in other parameters.
Closed-box prostheses should be favored, particularly in patients with risk factors for transfusion and bleeding.
Acetylcholinesterase inhibitors are a class of medications that prevent acetylcholine breakdown and thereby improve cholinergic signalling1. Since the mid-1990s, these medications have been widely utilized in the management of Alzheimer’s disease (AD) and other dementias. Degeneration of neuronal circuits and poor neurotransmission in the afflicted brain regions are thought to be the causes of the symptoms of all varieties of dementia2. Acetylcholine levels in the brain, notably in the temporal and parietal neocortex and hippocampus, are decreasing as a result of the gradual death of cholinergic neurons that occurs in individuals with probable AD3,4. Hence, both AD and vascular dementia individuals have cholinergic impairments in their brains5,6. These findings imply that cholinergic function impairment contributes to the symptoms of all three types of dementia and that all dementia patients, regardless of the ultimate autopsy diagnosis, may benefit from cholinergic replacement treatment. Doctors have first implemented cholinergic medications as a first line of treatment for AD since the release of the first acetylcholinesterase inhibitor in 19977. These drugs include donepezil, galantamine, and rivastigmine. According to research, they all can modestly slow the decline in cognitive functions in those with mild to severe Alzheimer’s disease7. These medications act by inhibiting the acetylcholine breakdown which is a crucial neurotransmitter linked to memory. As an illustration, some Alzheimer’s patients who routinely used one of these drugs reported having easier recalls. More specifically, using galantamine as an example, around 14 out of every 100 users report improvements in their memory and cognitive abilities8.
The activity of the autonomic nervous system’s two arms, the adrenergic and cholinergic systems, appears to govern bone remodelling9. The adrenergic system’s activation has a catabolic effect on bone10–13. Therefore, drugs that block the adrenergic signal enhance bone mineral density in humans14, consequently lowering the incidence of fractures in humans15. The cholinergic system, on the other hand, has an anabolic impact on bone growth9. Laboratory research has revealed that cholinergic agonists (e.g. nicotinic and muscarinic) may promote the growth of osteocytes16,17. Investigations on mouse models have shown that the loss of certain cholinergic receptors (muscarinic-3 receptor and nicotinic subtype-2) is linked to bone loss18,19. Indeed, mice with deletion nicotinic subtype-2 receptors are osteoporotic because the interleukin-1 signal in the central nervous system is reduced, resulting in very low levels of vesicular acetylcholine transporter and an increase in the number of osteoclasts18. Mice with knockout muscarinic-3 receptors, on the other hand, are osteoporotic due to an increase in the number of osteoclasts and a decrease in the number of osteoblasts19. These findings show that cholinergic agonists, such as acetylcholinesterase inhibitors, may have a beneficial effect on bone mass in people20. Additionally, Studies conducted on mice treated with donepezil demonstrate an increase in total bone mass and overall energy20 perhaps by preventing osteoclast differentiation (Fig. 1) brought on by receptor activator of NF-B ligand21. In light of these findings, a small number of studies in humans reported that acetylcholinesterase inhibitors may provide protection against fractures, speed up bone healing, and decrease post-hip fracture sequelae and all-cause mortality22–24. By the inhibition of osteoclasts and bone resorption, pyridostigmine, a peripherally active acetylcholinesterase inhibitors, increases acetylcholine levels and increases trabecular bone density in mice25. Therefore, acetylcholinesterase inhibitors usage may be associated with better bone health26. However, independently, dementia doubles the risk of hip fracture27. We found conflicting evidence from studies on the relationship between dementia and the likelihood of fractures at various locations, such as the wrist and vertebra28. In 2020, a case-control study found that acetylcholinesterase inhibitors use did not correlate with reducing the likelihood of osteoporotic fractures in patients with AD; rather, it was associated with a mildly elevated risk of osteoporotic fractures29. As a result, we aimed to perform this meta-analysis to resolve the conflict and determine the actual association between acetylcholinesterase inhibitors and the risk of fracture in demented patients.
Figure 1.
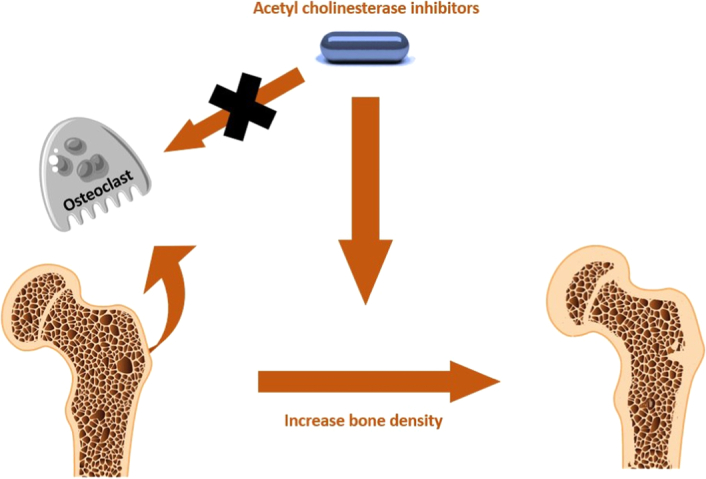
The effect of acetylcholinesterase inhibitors on improving bone density.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook were followed to perform this meta-analysis30. The Meta-analysis has been rated as high quality according to the AMSTAR 2 (Assessing the methodological quality of systematic reviews) Guidelines.
Study design
This is a meta-analysis study that aimed to investigate the association between acetylcholinesterase inhibitors medication and fracture risk in patients with dementia.
Search strategy
The protocol of the review was published on PROSPERO (CRD42023439441) before the literature search https://www.crd.york.ac.uk/prospero/#recordDetails. A literature search of the following databases (PubMed, Scopus, and Web of Science) on the 11th of November 2022, using key terms such as (“Acetylcholinesterase Inhibitors” OR “anticholinesterase”) AND (“dementia” OR “Alzheimer disease”), (“Acetylcholinesterase Inhibitors” OR “anticholinesterase”) AND (“dementia” OR “Alzheimer disease”), (“Acetylcholinesterase Inhibitors”) AND (“dementia”).
Eligibility criteria
Any randomized control trials and controlled observational studies, such as cross-sectional, prospective, or retrospective cohort, and case-control studies that demonstrated the link between acetylcholinesterase inhibitors and the incidence of fracture in people with dementia as compared to a control group taking a placebo or no medication at all. So, the PICO was:
Population: Patients with dementia.
Intervention: Acetylcholinesterase inhibitors.
control: placebo or no drug at all.
outcome: Fracture.
Exclusion criteria
Animal studies, post-mortem studies, case reports, case series, editorials, and reviews were all dismissed.
Study selection process
Two independent authors screened the titles and abstracts of the studies according to our criteria. If a consensus is not achieved, the first author resolved any disagreements among authors.
Data extraction and management
Members of our team were each assigned several studies for data extraction, where each study was extracted by two reviewers independently. The data were then compared to confirm accuracy.
For the baseline and summary, the following data were extracted from the eligible studies: the first author of the study, year of publication, study design, duration of the study, number of participants, age of participants, sex of participants, BMI of participants, their smoking status and alcohol abuse, history of falls, other comorbidities, and co-administered medications.
For the outcomes, the following data were extracted: the overall fracture risk. Additional subgroup analysis of fracture risk by age and treatment duration was conducted.
Data extraction for the results was done by two authors, and the first author resolved any disagreements.
Quality assessment
Newcastle–Ottawa scale tool (NOS) was used to assess the quality, with a total score of eight points for case-control studies and five, six, seven, and nine points for cohort studies, to evaluate the quality of observational studies. Each study was ranked as good, fair, or poor quality according to its score.
Data synthesis
Data were analyzed using RevMan software, version 5.4. Dichotomous data were presented as risk ratio (RR) and odds ratio (OR) with a 95% CI. If no heterogeneity was observed, results were presented in a fixed effect model, and a random effect model was used if significant heterogeneity was observed. Sensitivity analysis (leave-one-out test and subgroup analysis) will be used to resolve the heterogeneity if detected. Results were considered significant if the P value was less than 0.05.
Results
Literature search
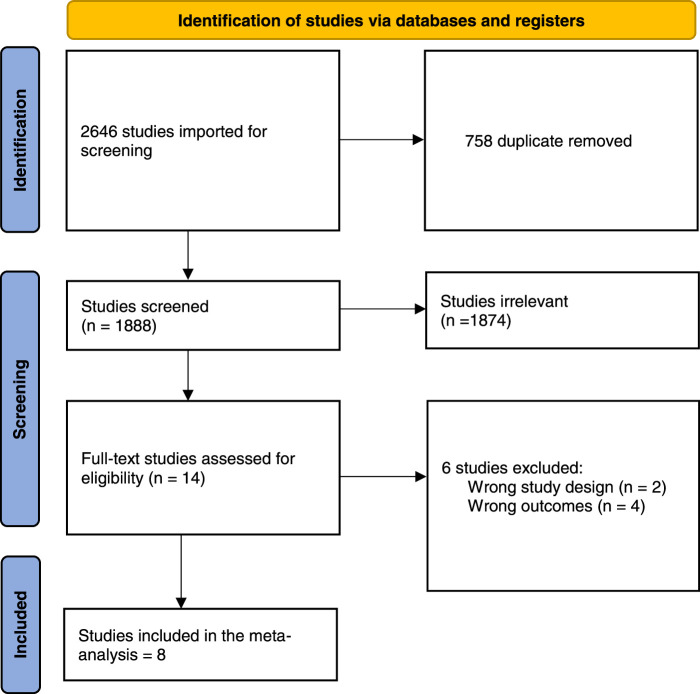
After a comprehensive search of the literature, 2646 studies resulted and then 1888 became eligible for title and abstract screening after removal of duplicates. Of the 1888, 1874 were irrelevant and 14 studies were eligible for full-text screening. Finally, eight studies were included in the meta-analysis after the full-text screening23,24,29,31–35, as shown in the PRISMA in Fig. 2.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
The overall quality was good in seven studies and fair in one study included in the analysis as shown in Table 1.
Table 1.
Quality assessment using Newcastle–Ottawa scale.
| Selection | Exposure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NO | Study | Case definition | Representativeness | Selection of controls | Definition of controls | Comparability | Ascertainment | Same method | Non-response rate | Total score | AHRQ standards |
| NOS scale Risk of Bias Assessment for case-control studies | |||||||||||
| 1 | Won 2022 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| 2 | Tamimi et al. 201724 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| 3 | Tamimi et al. 201232 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| Selection | Outcome | ||||||||||
| NO | Study | Representativeness of exposed cohort | Selection of non-exposed cohorts | Ascertainment of the exposure | Demonstration that the outcome of interest was not present at the start of study | Comparability | Assessment of outcome | Was the follow-up long enough | Adequacy of the follow-up | Total score | AHRQ standards |
| NOS scale Risk of Bias Assessment for cohort studies | |||||||||||
| 4 | Tamini 2017 | 1 | 0 | 1 | 0 | 2 | 1 | 1 | 0 | 6 | Good |
| 5 | Seitz et al. 201135 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Good |
| 6 | Niznik 2019 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Good |
| 7 | Ogunwale et al. 202031 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 5 | Fair |
| 8 | Gill et al. 200933 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 | Good |
NOS, Newcastle–Ottawa scale tool AHRQ, Agency for Health Research and Quality.
The total number of patients included in the study is 564 679 patients with a mean age of 79 years old, 274 332 dementia patients who received acetylcholinesterase inhibitors, and 290 347 dementia patients who didn’t receive acetylcholinesterase inhibitors. The most commonly used acetylcholinesterase inhibitors combination was rivastigmine, donepezil, and galantamine. Other baseline data are shown in Table 2 and supplementary material (Supplementary Table S1, http://links.lww.com/MS9/A414) respectively.
Table 2.
Baseline characteristics describing the population of the included studies
| No. patients in each group | Age (years) | Sex (n) | Comorbidites and medical conditions | Medications | BMI | Smoking/alchol | Duration of Alzheimer disease | History of falls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AChEIs users | Non-users | |||||||||||||||||||
| ID | AChEIs users | Non-users | AChEIs users | Non-users | Female | Male | Female | Male | AChEIs users | Non-users | AChEIs users | Non-users | AChEIs users | Non-users | AChEIs users | Non-users | AChEIs users | Non-users | AChEIs users | Non-users |
| Won et al. 202029 | 2385 | 7085 | 77 | 77 | 1719 | 666 | 5104 | 1981 | CVD 987 CKD 35 COPD 401 DM 539 Hyperlipidemia 142 Hypertension 1224 |
CVD 2969 CKDe 161 COPD 940 DM 1421 Hyperlipidemia 226 Hypertension 1224 |
TCA 299 SSRIs 313 Benzodiazepines 1225 Opioids 1176 Corticosteroids 739 Statins 423 Memantine 257 |
TCA 672 SSRIs 679 Benzodiazepines 3040 Opioids 2083 Corticosteroids 1574 Statins 832 Memantine 147 |
NA | NA | 233 | 391 | NA | NA | NA | NA |
| Ogunwale et al. 202031 | 152 274 | 207 741 | 75.8 (6.1) | 75.4 (6.5) | NA | NA | NA | NA | Rheumatoid arthritis (%) 0.5 Malabsorption (%) 0.4 Chronic lung disease (%) 26.1 Chronic liver disease (%) 6.8 Stage 3–4 kidney disease (%) 1.0 Congestive heart failure (%) 16.9 1 Hyperthyroidism (%) 0.9 Diabetes (%) 32.9 Parkinson’s disease (%) 7.1 Osteoarthritis (%) 33.2 Stroke (%) 1.2 |
Rheumatoid arthritis (%) 0.6 Malabsorption (%) 0.6 Chronic lung disease (%) 35.9 Chronic liver disease (%) 11.2 Stage 3–4 kidney disease (%) 1.9 Congestive heart failure (%) 24.9 Hyperthyroidism (%) 1.8 Diabetes (%) 37.2 Parkinson’s disease (%) 5.4 Osteoarthritis (%) 38.9 Stroke (%) 2.2 |
Glucocorticoid (%) 1.2 Androgen deprivation therapy (%) 0.0 Antiepileptic drugs (%) 9.8 Proton pump inhibitors (%) 40.7 SSRIs (%) 12.6 Others (%) 10.3 Opiates (%) 2.5 Any psychoactive medication (%) 27.5 |
Glucocorticoid (%) 1.7 Androgen deprivation therapy (%) 0.0 Antiepileptic drugs (%) 13.3 Proton pump inhibitors (%) 40.4 SSRIs (%) 9.7 Others (%) 8.9 Opiates (%) 2.0 Any psychoactive medication (%) 15.3 |
27.5 (4.4) | 28.0 (4.8) | Smoking 16.6 Chronic alcohol use (%) 19.0 |
Smoking 20.7 Chronic alcohol use (%) 20.0 |
NA | NA | NA | NA |
| Niznik 2019 | 93 760 | 15 480 | NA | NA | 71 978 (76.8) | 21 781 (23.2) | 12 223 (79.0) | 3257 (21.0) | Cancer 3,798 (4.1) Heart failure 4451 (15.4) End Stage Renal Disease 18 532 (9.1) Short of breath 5969 (6.4) Poor appetite 11,739 (12.5) Weight loss 5297 (5.7) Swallowing difficulty 3081 (3.3) Mechanically altered diet 49 631 (52.9) IV/parenteral nutrition or feeding tube 2510 (2.7) |
Cancer 664 (4.3) Heart failure 2246 (14.5) End Stage Renal Disease 1428 (9.2) Short of breath 953 (6.2) Poor appetite 2751 (17.8) Weight loss 1655 (10.7) Swallowing difficulty 709 (4.6) Mechanically altered diet 6710 (43.4) IV/parenteral nutrition or feeding tube 451 (2.9) |
Memantine 39 928 (42.6) Benzodiazepine 13 013 (13.9) Antipsychotic use 54 476 (22.6) Antidepressant use 53 096 (56.6) Highly Anticholinergic Drugs (Beers) 13 153 (14.0) |
Memantine 4414 (28.5) Benzodiazepine 1833 (11.8) Antipsychotic use 2,853 (18.4) Antidepressant use 7736 (50.0) Highly Anticholinergic Drugs (Beers) 1623 (10.5) |
NA | NA | NA | NA | NA | NA | NA | NA |
| Tamimi et al. 201824 | 1190 | 4760 | 75 | 75 | 76.4 | 23.6 | 76.4 | 23.6 | COPD 17.9 DM 7.8 Cardiac arrhythmia 7.7 Hemiplegia 0.3 Chronic liver disease 0.6 Ischaemic heart disease 2.9 Peptic ulcer 10.8 Renal diseases 2.6 |
COPD 17.8 DM 8.3 Cardiac arrhythmia 6.7 Hemiplegia 0.3 Chronic liver disease 0.5 Ischaemic heart disease 3.6 Peptic ulcer 10.8 Renal diseases 2.5 |
PPI 18.4 Statins 17.3 SSRI 18.9 Memantine 2.3 |
PPI 19.7 Statins 22.3 SSRI 15.7 Memantine 3.3 |
< 20: 18.0 20–24: 35.7 25–29: 20.3 ≥ 30: 6.2 Unknown: 19.8 |
< 20: 13.2 20–24: 35.6 25–29: 23.2 ≥ 30: 8.7 Unknown: 19.8 |
Current 10.7 Ex-smoker 24.5 |
Current 8.1 Ex-smoker 23.8 |
< 2: 29.4 2–6: 61.3 ≥ 6: 9.3 |
< 2: 29.4 2–6: 61.3 ≥ 6: 9.3 |
0.16±0.47 | 0.10±0.4 |
| Tamimi et al. 201723 | 223 | 309 | 84.0±7.0 | 84.6±6.4 | 78.5 | 21.5 | 73.8 | 26.2 | COPD 14.4 DM 9.4 CVD 9.9 Chronic liver disease 0.5 IHD 83.0 PU 5.4 Renal diseases 2.2 Known Osteoporosis 3.1 |
COPD 18.5 DM 6.5 CVD 13.9 Chronic liver disease 0.3 IHD 86.1 PU 10.0 Renal diseases 3.2 Known Osteoporosis 2.6 |
PPI 21.5 Statins 19.3 SSRI 25.6 Hypnotics 39.5 Diuretics 13.5 ACE inhibitors 17.5 Pre-baseline use of AChEIs 91.3 |
PPI 18.8 Statins 12.3 SSRI 20.4 Hypnotics 38.8 Diuretics 15.5 ACE inhibitors 10.4 Pre-baseline use of AChEIs 40.8 |
<20: 16.6 20–24: 29.2 25–29: 12.1 ≥30: 4.0 Unknown: 38.1 |
<20: 17.5 20–24: 33.0 25–29: 14.2 ≥30: 2.9 Unknown: 32.4 |
Current: 1.8 Ex-smoker: 4.9 |
Current: 1.9 Ex-smoker: 5.5 |
<2: 47.1 2-6: 46.6 ≥6: 6.3 |
<2: 35.9 2-6: 54.1 ≥6: 10.0 |
37.7 | 42.1 |
| Tamimi et al. 201232 | 1771 | 487 | 82.2±4.5 | 83.5±4.2 | 72 (70–74) | 25 (22–28) | 69 (65–73) | 27 (22–33) | NA | NA | SSRI 19 (17–21) | SSRI 26 (20–32) | 26.0±3.5 | 24.6±4.3 | 1 (0–1) | 1 (1–2) | NA | NA | NA | NA |
| Seitz et al. 201135 | 624 | 624 | 83.89 (6.02) | 83.96 (6.53) | 480 (76.92) | NA | 480 (76.92) | NA | Stroke 140 (22.44) 1 PD 39 (6.25) CHF 142 (22.76) Angina 152 (24.36) PrIor myocardial Infarction 180 (28.85) COPD 94 (15.06) Pneumonia 129 (20.67) CKD 52 (8.33) Diabetes 159 (25.48) Cancer 31 (4.97) |
Stroke 143 (22.92) PD 38 (6.09) CHF 138 (22.12) Angina 152 (24.36) PrIor myocardial Infarction 183 (29.33) COPD 98(15.71) Pneumonia 130(20.83) CKD 50 (8.01) Diabetes 159 (25.48) Cancer 27 (4.33) |
antidepressants 265 (42.47) antipsychotics 177 (28.37) Benzodiazepines 133 (21.31) |
antidepressants 248 (39.74) antipsychotics 169 (27.08) Benzodiazepines 140 (22.44) |
NA | NA | NA | NA | 3.23 (2.31) | 3.33 (2.61) | NA | NA |
| Gill et al. 200933 | 19 803 | 61 499 | 80.4 (6.3) | 80.4 (7.4) | NA | NA | NA | NA | CAD 8363 (42.2) PE 64 (0.3) Aortic valve stenosis 56 AF 4827 (24.4) Conduction disorder 1086 Seizure disorder 608 (3.1) Permanent pacemaker 314 (1.6) Implantable cardioverter defibrillator 15 (0.1) MI 1280 (6.5) CHF 1255 (6.3) PVD 440 (2.2) CVD 1729 (8.7) Chronic pulmonary disease 1341 (6.8) Connective tissue disease 198 (1.0) Ulcer disease 268 (1.4) Mild liver disease 19 (0.1) DM 1581 (8.0) DM with end-organ damage 177 (0.9) Hemiplegia or paraplegia 161 (0.8) Moderate or severe renal disease 415 (2.1) Primary cancer 819 (4.1) Moderate or severe liver disease 16 (0.1) Metastatic cancer 137 (0.7) |
CAD 28 916 (47.0) PE 273 (0.4) Aortic valve stenosis 236 (0.4) AF 17217 (28.0) Conduction disorder 4346 (7.1) Seizure disorder 3578 (5.8) Permanent pacemaker 1091 (1.8) Implantable cardioverter defibrillator 65 (0.1) MI 5670 (9.2) CHF 6921 (11.3) PVD 2212 (3.6) CVD 8706 (14.2) Chronic pulmonary disease 6882 (11.2) Connective tissue disease 906 (1.5) Ulcer disease 1332 (2.2) Mild liver disease 212 (0.3) DM 6782 (11.0) DM with end-organ damage 1123 (1.8) Hemiplegia or paraplegia 1295 (2.1) Moderate or severe renal disease 2529 (4.1) Primary cancer ) 3370 (5.5) Moderate or severe liver disease 222 (0.4) Metastatic cancer 864 (1.4) |
BB 4252 (21.5) NDHP CCB 1493 (7.5) Digoxin 1479 (7.5) Conventional antipsychotics 225 (1.1) Atypical antipsychotics 2352 (11.9) Antiarrhythmics 495 (2.5) Anticonvulsants 530 (2.7) Antidepressants 5177 (26.1) Antimanic agents 78 (0.4) Benzodiazepines 3624 (18.3) |
BB 13 693 (22.3) NDHP CCB 4675 (7.6) Digoxin 5610 (9.1) Conventional antipsychotics1117 (1.8) Atypical antipsychotics 6101 (9.9) Antiarrhythmics 1890 (3.1) Anticonvulsants 3022 (4.9) Antidepressants 14 850 (24.1) Antimanic agents 438 (0.7) Benzodiazepines 14 127 (23.0) |
NA | NA | NA | NA | NA | NA | NA | NA |
AchEI, acetylcholinesterase inhibitor; NA, not applicable.
Outcomes
Fracture risk
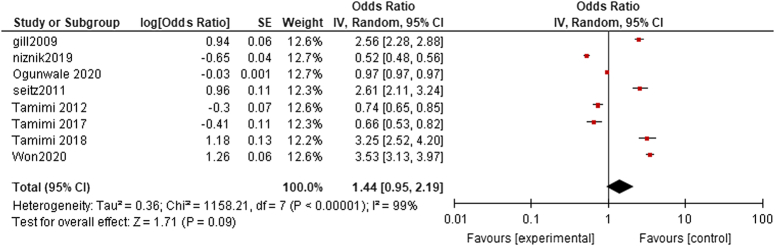
The pooled analysis showed no statistically significant difference between dementia patients who received acetylcholinesterase inhibitors, and dementia patients who didn’t receive acetylcholinesterase inhibitors (OR=1.44, CI=0.95-2.19, P value=0.09). We detected a significant heterogeneity among studies that wasn’t resolved by leave-one-out test (P<0.00001, I²=99%) as shown in Fig. 3.
Figure 3.
Fracture risk.
Fracture risk age subgroup analysis
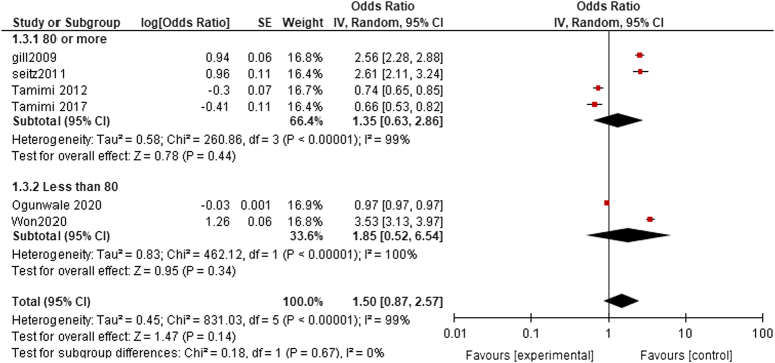
80 or more: The pooled analysis showed no statistically significant difference between dementia patients who received acetylcholinesterase inhibitors, and dementia patients who didn’t receive acetylcholinesterase inhibitors (OR= 1.35, CI 0.63, 2.86, P=0.44). We detected a significant heterogeneity among studies that wasn’t resolved by leave-one-out test (P<0.00001, I²=99%) as shown in Fig. 4.
Figure 4.
Fracture risk age subgroup analysis.
Less than 80: The pooled analysis showed no statistically significant difference between dementia patients who received acetylcholinesterase inhibitors, and dementia patients who didn’t receive acetylcholinesterase inhibitors (OR= 1.85, CI 0.52, 6.54, P=0.34). We detected a significant heterogeneity among studies that wasn’t resolved by leave-one-out test (P<0.00001, I²=100%) as shown in Fig. 4.
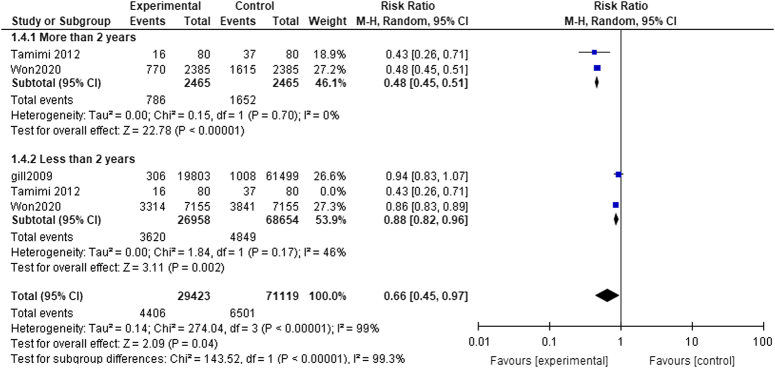
Period of treatment subgroup analysis
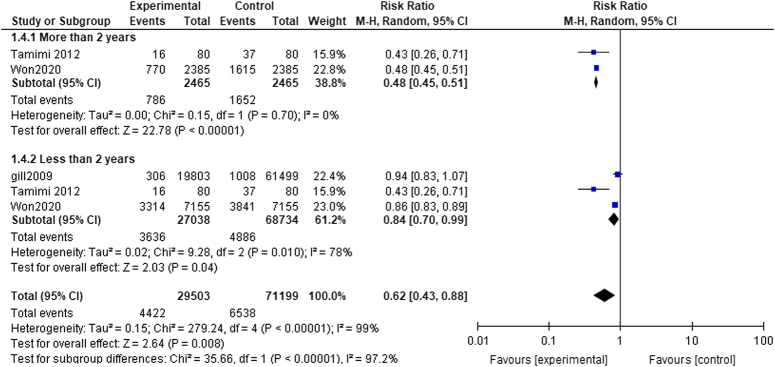
More than 2 years: The pooled analysis showed a statistically significant association between dementia patients who received acetylcholinesterase inhibitors and decreased fracture risk (RR= 0.48, CI= 0.45, 0.51, P<0.00001). We observed no significant heterogeneity among studies (P=0.70, I²=0%) as shown in Fig. 5.
Figure 5.
Period of treatment subgroup analysis.
Less than 2 years: The pooled analysis showed a statistically significant association between dementia patients who received acetylcholinesterase inhibitors and decreased fracture risk (RR= 0.84, CI 0.70, 0.99, P=0.04). We detected a significant heterogeneity among studies (P=0.010, I²=78%) as shown in Figure 4, so we performed leave-one-out test by removing the study (Tamimi2012), and the heterogeneity was solved (P=0.17, I²=46%) and the results showed a statistically significant association between dementia patients who received acetylcholinesterase inhibitors, and decreased fracture risk (RR=0.88, CI=0.82, 0.96, P=0.002) as shown in Fig. 6.
Figure 6.
Less than 2 years.
Discussion
Dementia is characterized as a syndrome that includes any decline in cognition notable enough to interfere with independent, daily functioning. It is a serious health issue that affects elderly people all over the world and has a wide range of effects on a person’s life and the wider community36. To address the cholinergic hypothesis of cognitive decline, acetylcholinesterase inhibitors were developed and used. Numerous randomized controlled trials have assessed these therapies’ efficacy in the functional, global, cognitive, and neuropsychiatric domains37. Notably, it showed that acetylcholinesterase inhibitors are associated with reduced fracture risk among demented patients, in particular, male patients31. In contrast, a cohort study conducted by Gill et al.33 found that the use of acetylcholinesterase inhibitors was associated with an increased risk of hip fractures. These inconsistent results led to an unclear association between increased cholinergic activation by acetylcholinesterase inhibitors and the risk of fractures in dementia patients. Therefore, a careful systematic review and meta-analysis were conducted to assess the risk of bone fractures in dementia patients receiving acetylcholinesterase inhibitors with dementia patients not receiving acetylcholinesterase inhibitors. This meta-analysis included eight studies and showed that the risk of bone fracture was not statistically different between dementia patients who received acetylcholinesterase inhibitors and those who did not receive them (P=0.09). For the treatment of dementia, acetylcholinesterase inhibitors, such as donepezil, rivastigmine, and galantamine, are used to slow the deterioration of cognitive function. These medications work by preventing acetylcholinesterase from breaking down synaptic acetylcholine, hence raising synaptic acetylcholine levels. The cholinergic system and acetylcholinesterase inhibitors are thought to play a role in the process of bone remodelling, according to several studies38. Based on data from animal-based research, giving mice pyridostigmine, a peripherally active acetylcholinesterase inhibitor, increases acetylcholine levels and increases trabecular bone mass by decreasing osteoclasts and bone resorption18. The use of centrally acting acetylcholinesterase inhibitors, such as donepezil and rivastigmine, is linked to reduced risk of hip fracture in older patients with dementia32. A cohort research looking at the use of acetylcholinesterase inhibitors in dementia patients with hip fractures reported a 56% decrease in all-cause mortality and a 41% decrease in the risk of a second hip fracture23. Acetylcholinesterase inhibitor users demonstrated better radiographic union at fracture sites, fewer healing complications, and overall better bone quality in a retrospective study that looked at the impact of acetylcholinesterase inhibitor use on fracture healing among 49 dementia patients39. We also examined the impact of age and duration of acetylcholinesterase inhibitor treatment on the risk of bone fractures. The pooled analysis showed no statistically significant difference in the risk of bone fractures between dementia patients less than 80 years old and those who were 80 years old or above. However, we detected a statistically significant difference in the risk of bone fractures between dementia patients who received treatment less than 2 years and more than 2 years.
The strengths and limitations
This study has several substantial strengths. It is, to the best of our knowledge, the first meta-analysis to look into the relationship between the usage of acetylcholinesterase inhibitors and bone fractures in dementia patients. In addition, by examining the effects of age and treatment duration, this study added to the clinical evidence about the link between acetylcholinesterase inhibitor use and bone fractures in dementia patients. The diverse databases were investigated to select relevant papers.
Despite having several strengths, our study has its share of limitations. First, the number of studies included in this meta-analysis was limited. Second, because this meta-analysis was based on published data, it is possible that publication bias contributed to the irrelevant results being less representative. Third, there was serious inter-study heterogeneity seen for several results, which makes pooled analysis more complicated. In these cases, we performed a subgroup analysis to examine the sources of heterogeneity and we revealed that age was a source of heterogeneity. Substantial heterogeneity, which is expected in meta-analysis studies, can change how results can be interpreted40. As a result, careful consideration must be given to the present work’s findings.
Future implications
The use of acetylcholinesterase inhibitors should not be prohibited in dementia patients as they have a role in stabilizing, and slowing down the progression of the disease without increasing the risk of fracture. This might be attributed to the fact that patients receiving acetylcholinesterase inhibitors have better cognitive functions and correspondingly less risk of falls and fractures or due to their anabolic effect on bones. Moreover, the use of acetylcholinesterase inhibitors should not be subjected to any age restriction. However, doctors who prescribe the medication should pay more attention to the duration of treatment rather than their side effects.
Strengths and limitations
The overall quality is good in most of the studies included in our analysis. A good number of studies were subjected to analysis as eight studies were included. Along with a decent sample size, 564 679 patients were included in our analysis.
Our study shows some limitations. For, example all the studies included were non-randomized observational, not randomized clinical trials, and hence might be subjected to bias. Prospective multicenter studies are needed to further evaluate the relationship between acetylcholinesterase inhibitors and the risk of fracture. Moreover, statistically significant heterogeneity was detected in all the outcomes and it was not resolved by leave-one-out test which is considered a primary limitation of our analysis.
Conclusion
In comparison to controls, our investigations found no statistically significant association between acetylcholinesterase inhibitors and an increased risk of fracture. Owing to their beneficial effect on cognition, which will consequently minimize the likelihood of serious falls, acetylcholinesterase inhibitors prescription is considered to be a safe method of reducing fracture risk in elderly suffering from dementia via enhancing the anabolic effect on bones. More multicenter studies are needed to support our findings.
Ethics approval and consent to participate
Not applicable as it is a meta-analysis study.
Consent for publication
Not applicable as it is a meta-analysis study.
Source of funding
None.
Author contribution
R.H.E.: study design and writing. K.R.M.: supervision and writing. P.C.H.: analysis. N.B., N.H., H.H., and M.A.: writing. H.G., M.B., and J.S.: screening, data extraction. M.A.: quality assessment. All authors approved the manuscript.
Conflicts of interest disclosure
None.
Research registration unique identifying number (UIN)
The protocol is registered on prospero on 6/7/2023 ID: CRD42023439441.
Guarantor
Jaffer Shah and Rowan H. Elhalag.
Availability of data and material
Data and material are available within the manuscript.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Contributor Information
Rowan H. Elhalag, Email: rawan.fathy1801@alexmed.edu.eg.
Pensée Chèbl, Email: pensee.chebl@gmail.com.
Nervana M. Bayoumy, Email: nbayoumy@ksu.edu.sa.
Noheir Ashraf Ibrahem Fathy Hassan, Email: noheirfathy.usmle@gmail.com.
Hanan Hagar, Email: hhagar@uams.edu.
Marwan Abowafia, Email: Marwan.abowafiaa@gmail.com.
Hamed Gaber, Email: ham7d78@gmail.com.
Mohamed Mohamed Belal, Email: Mohamed.abdo1801@alexmed.edu.eg.
Jaffer Shah, Email: jaffer.shah@kateb.edu.af.
Karam R. Motawea, Email: karam.Ahmed1650@alexmed.edu.eg.
References
- 1.Pepeu G, Giovannini MGJCAR. Cholinesterase inhibitors and beyond. Curr Alzheimer Res 2009;6:86–96. [DOI] [PubMed] [Google Scholar]
- 2.Poirier J. Evidence that the clinical effects of cholinesterase inhibitors are related to potency and targeting of action. Int J Clin Pract Suppl 2002;1:6–19. [PubMed] [Google Scholar]
- 3.Davies P, Maloney A. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976;308:1403. [DOI] [PubMed] [Google Scholar]
- 4.Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 1982;215:1237–1239. [DOI] [PubMed] [Google Scholar]
- 5.Gottfries C-G, Blennow K, Karlsson I, et al. The neurochemistry of vascular dementia. Dement Geriatr Cogn Disord 1994;5:163–167. [DOI] [PubMed] [Google Scholar]
- 6.Tohgi H, Abe T, Kimura M, et al. Cerebrospinal fluid acetylcholine and choline in vascular dementia of Binswanger and multiple small infarct types as compared with Alzheimer-type dementia. J Neural Transmiss 1996;103:1211–1220. [DOI] [PubMed] [Google Scholar]
- 7.Birks JS, Dementia C, Group CI. Cholinesterase inhibitors for Alzheimer’s disease. Cochr Database Syst Rev 1996;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006. Alzheimer's disease: How effective are cholinesterase inhibitors? 2009 Apr 15 [Updated 2017 Jun 29]. https://www.ncbi.nlm.nih.gov/books/NBK279358/
- 9.Eimar H. Cholinergic regulation of bone. McGill University: Canada; 2015. [Google Scholar]
- 10.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000;100:197–207. [DOI] [PubMed] [Google Scholar]
- 11.Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005;434:514–520. [DOI] [PubMed] [Google Scholar]
- 12.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 2006;4:341–348. [DOI] [PubMed] [Google Scholar]
- 13.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002;111:305–317. [DOI] [PubMed] [Google Scholar]
- 14.Pasco JA, Henry MJ, Sanders KM, et al. β‐adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong osteoporosis study. J Bone Miner Res 2004;19:19–24. [DOI] [PubMed] [Google Scholar]
- 15.Schlienger RG, Kraenzlin ME, Jick SS, et al. Use of β-blockers and risk of fractures. JAMA 2004;292:1326–1332. [DOI] [PubMed] [Google Scholar]
- 16.Liu P-S, Chen Y-Y, Feng C-K, et al. Muscarinic acetylcholine receptors present in human osteoblast and bone tissue. Eur J Pharmacol 2011;650:34–40. [DOI] [PubMed] [Google Scholar]
- 17.Sato T, Abe T, Chida D, et al. Functional role of acetylcholine and the expression of cholinergic receptors and components in osteoblasts. FEBS Lett 2010;584:817–824. [DOI] [PubMed] [Google Scholar]
- 18.Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci USA 2012;109:15455–15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Oury F, Yadav VK, et al. Signaling through the M3 muscarinic receptor favors bone mass accrual by decreasing sympathetic activity. Cell Metab 2010;11:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eimar H, Alebrahim S, Manickam G, et al. Donepezil regulates energy metabolism and favors bone mass accrual. Bone 2016;84:131–138. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Enoki Y, Sakamoto Y, et al. Donepezil prevents RANK-induced bone loss via inhibition of osteoclast differentiation by downregulating acetylcholinesterase. Heliyon 2015;1:e00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh J-Y, Yang Y, Jung H. Molecular mechanisms and emerging therapeutics for osteoporosis. Int J Mol Sci 2020;21:7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamimi I, Madathil S, Kezouh A, et al. Effect of acetylcholinesterase inhibitors on post-surgical complications and mortality following a hip fracture: a cohort study. J Musculoskelet Neuronal Interact 2017;17:69. [PMC free article] [PubMed] [Google Scholar]
- 24.Tamimi I, Nicolau B, Eimar H, et al. Acetylcholinesterase inhibitors and the risk of osteoporotic fractures: nested case-control study. Osteop Int 2018;29:849–857. [DOI] [PubMed] [Google Scholar]
- 25.Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci 2012;109:15455–15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eimar H, Perez Lara A, Tamimi I, et al. Acetylcholinesterase inhibitors and healing of hip fracture in Alzheimer’s disease patients: a retrospective cohort study. J Musculoskelet Neuronal Interact 2013;13:454–63. [PubMed] [Google Scholar]
- 27.Melton LJ, Beard CM, Kokmen E, et al. Fracture risk in patients with Alzheimer’s disease. J Am Geriatr Soc 1994;42:614–619. [DOI] [PubMed] [Google Scholar]
- 28.Wang H-K, Hung C-M, Lin S-H, et al. Increased risk of hip fractures in patients with dementia: a nationwide population-based study. BMC Neurol 2014;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Won DY, Byun SJ, Jeong JS, et al. Association between acetylcholinesterase inhibitors and osteoporotic fractures in older persons with Alzheimer’s disease. J Am Med Dir Assoc 2020;21:1128–33.e1. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.0. 2 [updated Sept 2009]. Cochr Collab 2010;2009. [Google Scholar]
- 31.Ogunwale AN, Colon‐Emeric CS, Sloane R, et al. Acetylcholinesterase inhibitors are associated with reduced fracture risk among older veterans with dementia. J Bone Miner Res 2020;35:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamimi I, Ojea T, Sanchez‐Siles JM, et al. Acetylcholinesterase inhibitors and the risk of hip fracture in Alzheimer’s disease patients: a case‐control study. J Bone Miner Res 2012;27:1518–1527. [DOI] [PubMed] [Google Scholar]
- 33.Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med 2009;169:867–873. [DOI] [PubMed] [Google Scholar]
- 34.Niznik JD, Zhao X, He M, et al. Risk for health events after deprescribing acetylcholinesterase inhibitors in nursing home residents with severe dementia. J Am Geriatr Soc 2020;68:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seitz DP, Gill SS, Gruneir A, et al. Effects of cholinesterase inhibitors on postoperative outcomes of older adults with dementia undergoing hip fracture surgery. Am J Geriatr Psychiatry 2011;19:803–813. [DOI] [PubMed] [Google Scholar]
- 36.Gale S. Acar in KR Daffner. Dementia Am J Med 2018;131:1161. [DOI] [PubMed] [Google Scholar]
- 37.Van De Glind EM, Van Enst WA, Van Munster BC, et al. Pharmacological treatment of dementia: a scoping review of systematic reviews. Dement Geriatr Cogn Disord. 2013;36:211–228. [DOI] [PubMed] [Google Scholar]
- 38.Luo X, Lauwers M, Layer PG, et al. Non-neuronal role of acetylcholinesterase in bone development and degeneration. Front Cell Dev Biol 2021;8:620543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eimar H, Perez Lara A, Tamimi I, et al. Acetylcholinesterase inhibitors and healing of hip fracture in Alzheimer's disease patients: a retrospective cohort study. J Musculoskelet Neuronal Interact 2013 2013;13:454–63. [PubMed] [Google Scholar]
- 40.Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3:e1919325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material are available within the manuscript.